Abstract
Patients who died from COVID-19 often had comorbidities, such as hypertension, diabetes, and chronic obstructive lung disease. Although angiotensin-converting enzyme 2 (ACE2) is crucial for SARS-CoV-2 to bind and enter host cells, no study has systematically assessed the ACE2 expression in the lungs of patients with these diseases. Here, we analyzed over 700 lung transcriptome samples from patients with comorbidities associated with severe COVID-19 and found that ACE2 was highly expressed in these patients compared to control individuals. This finding suggests that patients with such comorbidities may have higher chances of developing severe COVID-19. Correlation and network analyses revealed many potential regulators of ACE2 in the human lung, including genes related to histone modifications, such as HAT1, HDAC2, and KDM5B. Our systems biology approach offers a possible explanation for increased COVID-19 severity in patients with certain comorbidities.
Keywords: angiotensin converting enzyme 2, COVID-19, SARS-CoV-2, KDM5B
A comprehensive transcriptome analysis of over 700 lung samples revealed that the expression of ACE2, which encodes the entry receptor of SARS-CoV-2, is increased in the lungs of patients with some of the comorbidities associated with severe COVID-19.
Recent studies of the epidemiological characteristics of coronavirus disease 2019 (COVID-19) have revealed that severe infection is more likely in people with an existing chronic medical condition. Two independent studies of infected populations in Wuhan, China found that approximately half the subjects with COVID-19 had an existing comorbidity [1, 2]. In a study of 1099 patients across mainland China, 38.7% of patients with comorbidities progressed to severe infection [3] and in a study of 52 inpatients in Wuhan, 67% of patients with comorbidities died [2]. The most common comorbidities reported in these studies were hypertension, diabetes, cerebrovascular disease, chronic obstructive lung disease, and coronary heart disease [1–3]. Other comorbidities such as carcinoma, chronic kidney disease, chronic liver disease, digestive system disease, and nervous system disease have also been reported in patients with COVID-19 [1, 2, 4]. A better understanding of the link between these conditions and COVID-19 infection is required to inform improved treatment and prevention interventions.
The molecular mechanism responsible for the increased disease severity in patients with these comorbidities is not fully understood, but previous studies suggest a role for angiotensin-converting enzyme 2 (ACE2) [5]. ACE2 is a membrane protein required for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to bind and enter cells [6–8]. After binding, viral entry is facilitated by activation of the viral spike glycoprotein and cleavage of the C-terminal segment of ACE2 by proteases like TMPRSS2 and FURIN that are readily expressed in lung tissue [9–11]. ACE2 is only moderately expressed in healthy lung tissue compared to the heart, kidneys, and testes [12], but staining of lung tissue sections from adults with pulmonary hypertension has revealed increased ACE2 protein in the endothelium of pulmonary arteries compared to healthy controls [13]. A comprehensive analysis of single-cell RNA sequence (RNA-seq) datasets revealed that ACE2 was coexpressed with TMPRSS2 within ileal absorptive enterocytes, nasal goblet secretory cells, and lung type 2 pneumocytes [14]. ACE2 upregulation has also been observed in animal models of liver fibrosis [15]. However, the reason for this upregulation remains unclear, and a link to other COVID-19 comorbidities has not been determined.
Here, we showed that expression of the gene encoding the ACE2 receptor in lung tissue is upregulated in diseases reported to be comorbidities associated with severe COVID-19. We also used systems biology approaches, including coexpression analysis, meta-analysis, and network analysis, to determine a potential cause of the ACE2 upregulation. From this analysis, we found that ACE2 expression could be regulated by enzymes that modify histones, including KDM5B. This identification of a common molecular mechanism of increased COVID-19 severity in patients with diverse comorbidities could direct the development of interventions to reduce the infection risk and disease severity in this population.
METHODS
Literature Curation
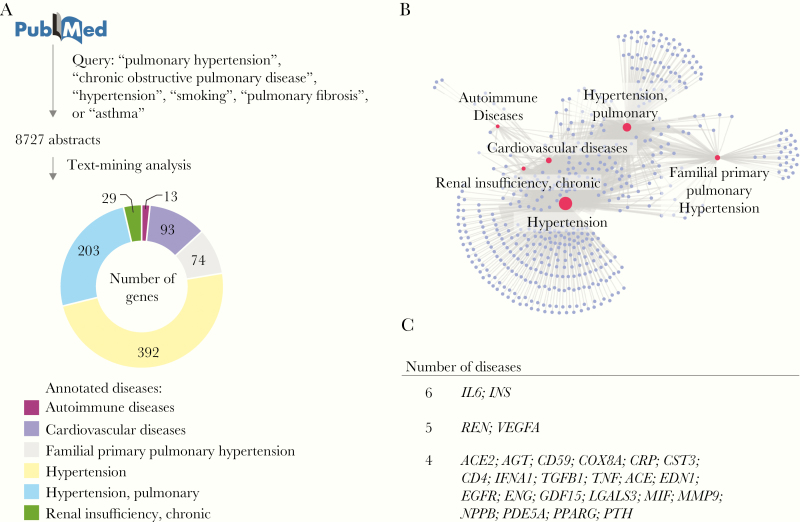
Relevant scientific literature related to key COVID-19 comorbidities was retrieved from PubMed on 16 March 2020 using the query terms “pulmonary hypertension,” “chronic obstructive pulmonary disease,” “hypertension,” “smoking,” “pulmonary fibrosis,” and “asthma.” For terms returning more than 100 000 papers (“hypertension,” “smoking,” and “asthma”), only the most recent 100 000 papers were analyzed. Abstracts were annotated to identify all genes, diseases, and species appearing in the title or abstract using the PubTator Central application programming interface [16]. This open source tool uses TaggerOne for disease annotations, GNormPlus for gene annotations, and SR4GN for species annotations [16]. The data were filtered to retain only papers containing a human species annotation. Annotation of the abstract text identified 6 relevant disease medical subject heading (MeSH) terms: “autoimmune diseases,” “cardiovascular diseases,” “familial primary pulmonary hypertension,” “hypertension,” “hypertension, pulmonary,” and “renal insufficiency, chronic,” as shown in Figure 1. Next, every possible combination of gene and disease annotation within the title and abstract of each paper was generated. Only gene-disease associations supported by at least 4 documents, and those with a proximity less than or equal to the median sentence length of the paper section were retained.
Figure 1.
Literature curation of genes associated with key COVID-19 comorbidities. A, Text-mining of 8727 abstracts identified 6 relevant disease medical subject heading (MeSH) terms associated with a total of 804 genes. The number of genes associated with each disease MeSH term in at least 4 abstracts is shown in the pie chart. B, The knowledge-based network of COVID-19 comorbidities. The network shows the diseases (red nodes) and genes (purple nodes) from (A). The edges represent an association between a disease and a gene. The size of the nodes is proportional to its degree. C, Genes associated with 4 or more COVID-19 comorbidities.
Gene identifiers (IDs) were converted to gene symbols using the biomaRt R package [17, 18], and disease IDs were converted to disease MeSH terms using the Entrez Programming Utilities to query the Entrez database provided by the National Center for Biotechnology Information. The data were then further filtered to retain disease MeSH terms relevant to reported clinical COVID-19 comorbidities [3]. Redundant terms were collapsed using fuzzy string matching. The final gene-disease data set was used to generate a network utilizing Gephi software where the nodes were genes and diseases, and the edge weight was determined by the number of analyzed papers containing the gene-disease combination [19].
Meta-analysis
We manually curated Gene Expression Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/) on 16 March 2020 to find lung transcriptome datasets related to “pulmonary arterial hypertension” (PAH), “chronic obstructive pulmonary disease” (COPD), and “smoking.” Author-normalized expression values and metadata from these datasets were downloaded using the GEOquery package [20]. We performed differential expression analyses between patients with a disease and the control individuals (Supplementary Table 1) using the limma package [21]. The gene symbol for each probe was obtained from the annotation file [22]. Probes that matched the same gene symbol were collapsed by taking the one with the lowest P value. Meta-analysis was performed with the MetaVolcanoR package [23] by combining the P values using the Fisher method. To adjusting for multiple comparisons, we calculated the false discovery rate (FDR) to identify the differentially expressed genes (FDR < 0.05). For enrichment analyses, we utilized the EnrichR tool [24] with the GO Biological Process 2018 and BioPlanet 2019 databases. We then selected pathways with a P value adjusted for multiple comparisons lower than 0.05. The network was created in Cytoscape [25].
Author-normalized fragments per kilobase of transcript per million mapped reads (FPKM) expression values of ACE2 gene in COPD patients and in subjects with normal spirometry were downloaded from GEO (accession ID, GSE57148). A single t test was performed between COPD patients and controls (P = .000359).
Pearson correlation between the expression of ACE2 and all other genes in each of the 7 lung transcriptome studies was performed. The P values were then combined using the Fisher method, and an FDR correction was applied to adjust for multiple comparisons.
For the epigenetics analysis, we run the EnrichR tool [24] with the ENCODE and ChEA consensus transcription factors from ChIP-X and Epigenomics Roadmap databases on the genes negatively or positively correlated with ACE2. Pathways with a P value adjusted for multiple comparisons lower than 10−10 were selected. We utilized the Encode Roadmap browser (http://www.roadmapepigenomics.org/) from the Roadmap Epigenomics Project database [26] to identify the H3K27ac, H3K4m1, and H3K4m3 markers of histone acetylation and methylation with the corresponding P values in the Lung of ENCODE donor STL002 (Roadmap alias E096).
Ethical approval was not applicable as we utilized publicly available data.
RESULTS
To identify the genes highly associated with key comorbidities of severe COVID-19 [1, 3], we mined all relevant scientific literature of these human diseases. Specifically, over 8000 abstracts were gathered from PubMed by querying titles and abstracts for the terms “pulmonary hypertension,” “chronic obstructive pulmonary disease,” “hypertension,” “smoking,” “pulmonary fibrosis,” or “asthma” (Figure 1A). Several relevant terms, such as “autoimmune diseases” and “cardiovascular diseases” were excluded from the PubMed query because of the breadth of literature published in these fields. Annotation of the abstract text identified 6 relevant disease MeSH terms: “autoimmune diseases,” “cardiovascular diseases,” “familial primary pulmonary hypertension,” “hypertension,” “hypertension, pulmonary,” and “renal insufficiency, chronic” [3] (Figure 1A). Our text-mining analysis revealed 804 genes highly associated with 1 or more COVID-19 comorbidities (Figure 1B). Among those genes, 26 were associated with 4 or more diseases (Figure 1C). Although ACE2 was known to be related to “cardiovascular diseases,” “familial primary pulmonary hypertension,” “hypertension, pulmonary,” and “hypertension,” none of the articles containing this gene-disease association studied how ACE2 expression was altered in the lungs of patients with these diseases.
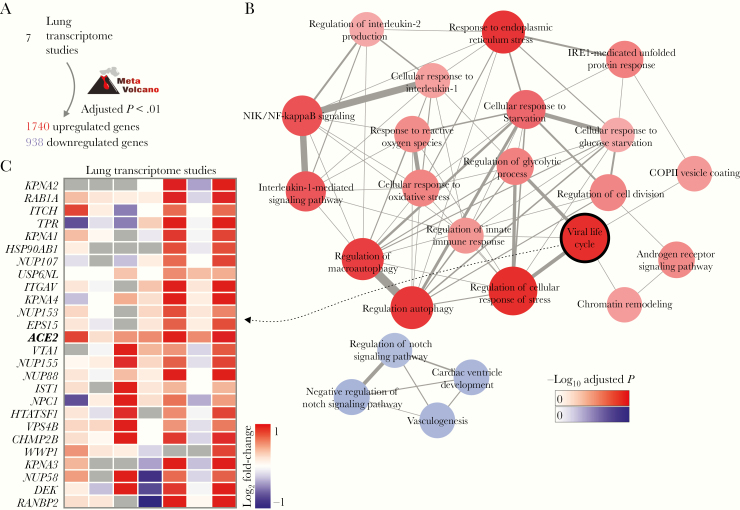
Based on the list of key comorbidities of severe COVID-19 [1, 3], we searched for lung transcriptome datasets available at the GEO repository. We identified 7 lung transcriptome studies of patients with either COPD or PAH, as well as smoking volunteers compared to nonsmoking volunteers, which were downloaded and used in our meta-analysis (Supplementary Table 1). For each study, we performed differential expression analysis between patients and control individuals (Supplementary Table 1). By combining the P values obtained in all the 7 comparisons, we were able to identify 1740 and 938 genes that were, respectively, up- and downregulated in the disease (Figure 2A). Enrichment analysis using these differentially expressed genes revealed several pathways associated with inflammatory processes, metabolism, and endoplasmic reticulum stress. Among the pathways enriched with downregulated genes, were the “vasculogenesis” and “regulation of Notch signaling pathway” (Figure 2B). The “viral life cycle” pathway, which describes the processes utilized by viruses to ensure survival and to attach and enter the host cells, was enriched with upregulated genes (Figure 2B). ACE2 was included in this pathway, as well as 25 other genes (Figure 2C), including RAB1A. Rab GTPases are involved in the replication of many viruses infecting humans [27] but have not been associated with SARS-CoV-2 life cycle yet. Genes encoding both TMPRSS2, which is required for SARS-CoV-2 S protein priming [5], and FURIN, which cleaves SARS-CoV-2 spike glycoprotein [28], were not differentially expressed in most of the lung transcriptome. However, both genes were highly expressed in lung (data not shown), suggesting that the levels of ACE2 may be the limiting factor for viral infection.
Figure 2.
Meta-analysis of lung transcriptomes of patients with COVID-19 comorbidities. A, Meta-analysis of 7 differential expression analyses. MetaVolcano tool was used to combine the P values of 7 studies (Supplementary Table 1) and to identify the differentially expressed genes (false discovery rate < 0.01). B, Pathway enrichment analysis. Pathways from the GO Biological Process 2018 database with adjusted P value < .05 were selected to create the network. The width of edges is proportional to the number of genes shared by 2 pathways (nodes). The size and color of nodes are proportional to the −log10 adjusted P value. C, Genes from the viral life cycle pathway that were upregulated in human diseases. The colors in the heat map represent the log2 fold-change between patients and control individuals.
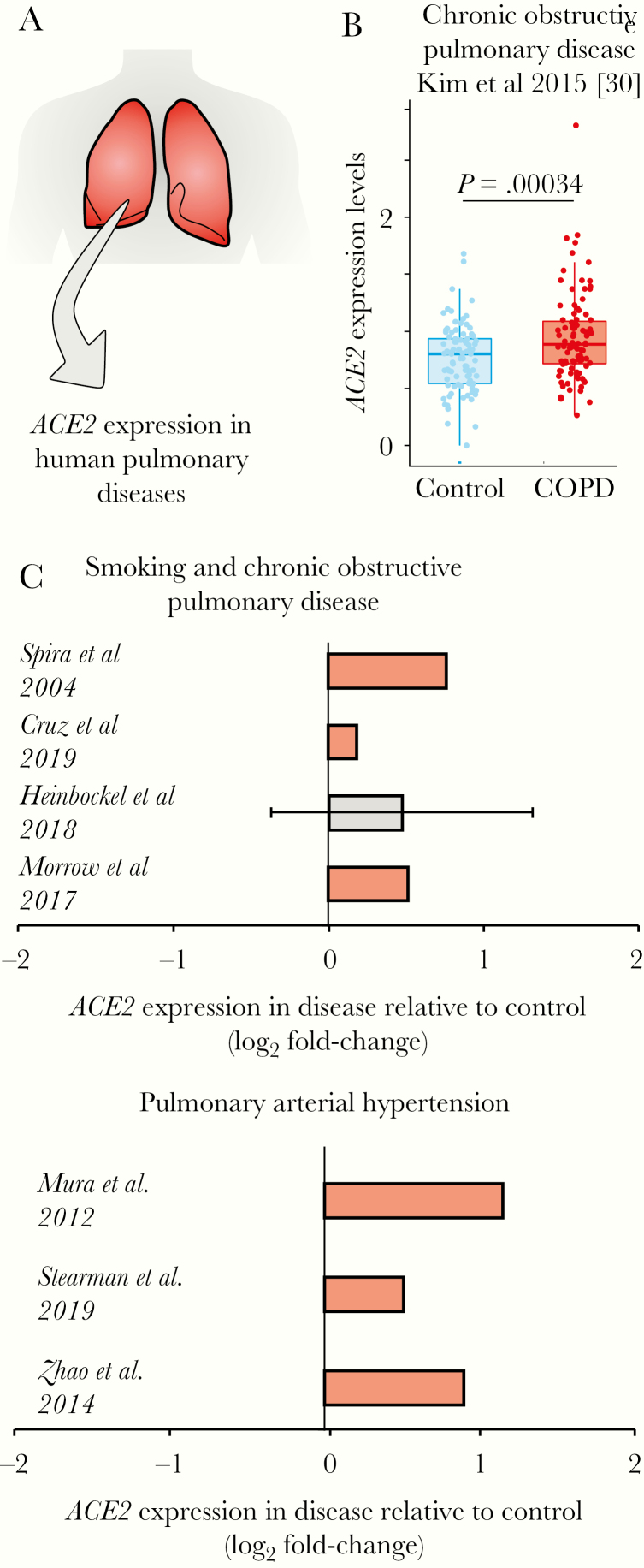
We then investigated whether the gene encoding the ACE2 receptor was specifically upregulated in the lungs of patients having one of these morbidities (Figure 3A). In a lung RNA-seq dataset (Supplementary Table 1), we compared ACE2 expression between patients with COPD and subjects with normal spirometry [29]. Again, the expression of ACE2 was significantly upregulated in the disease compared to controls (Figure 3B). In fact, ACE2 was significantly upregulated in 6 of 7 lung transcriptome studies (Figure 3C), suggesting that patients who have COPD or PAH, and even people who smoke, may have higher chances of developing severe COVID-19.
Figure 3.
ACE2 is upregulated in patients with lung diseases. A, Analysis of ACE2 expression in lung transcriptome datasets of patients with human pulmonary diseases. B, ACE2 expression in patients with chronic obstructive pulmonary disease (COPD). The boxplot on the right shows the difference between COPD patients (red dots) and control individuals (blue dots). Student t test P value is given. C, ACE2 is upregulated in patients with COVID-19 comorbidities. Each bar represents the log2 expression fold-change between patients and control individuals. The error bars indicate the 95% confidence interval. Bars in red represent a P value <.05 and in grey a nonsignificant P value. The original studies are indicated and can be found in Supplementary Table 1.
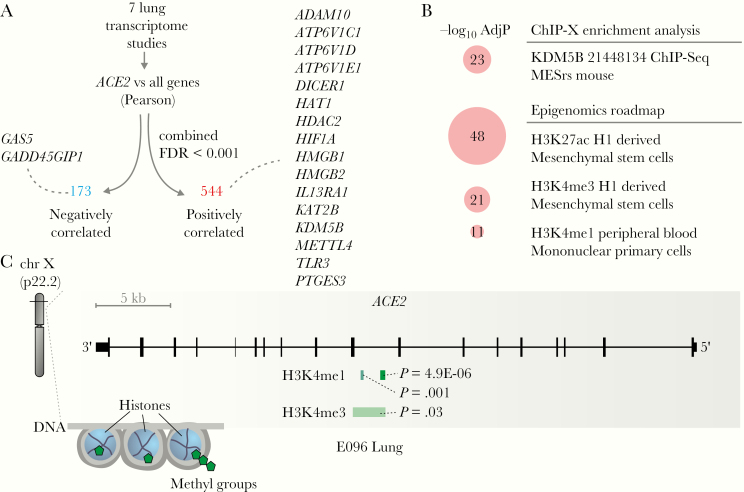
Coexpression analyses can provide useful insights about the functional role of genes and their regulatory mechanisms [30]. We performed Pearson correlation between the expression of ACE2 and all other genes in each of the 7 lung transcriptome studies (Supplementary Table 1), combined the P values using the Fisher method, and applied an FDR correction (Figure 4A). This approach identified 544 and 173 genes with positive and negative correlation with ACE2, respectively (Figure 4A). Several of these genes were related to histone modifications, such as HAT1, HDAC2, KDM5B, among others (Figure 4A). Among the positively correlated genes, we identified ADAM10, which regulates ACE2 cleavage in human airway epithelia [31], and TLR3, which plays a key role in the innate response to SARS-CoV and MERS-CoV infection [32].
Figure 4.
Insights into ACE2 regulation in the lung. A, Genes whose expression is correlated with ACE2 in the lung. Selected genes that were negatively (blue) or positively (red) correlated with ACE2 are highlighted. B, Pathway enrichment analysis using the ACE2-positively correlated genes. Pathways from the ChIP-X Enrichment Analysis and Epigenomics Roadmap databases with adjusted P value <10−10 were selected. The size of the circles is proportional to the −log10 adjusted P value of the enrichment. C, ACE2 locus contains markers of histone acetylation and methylation. The plot was modified from the WashU EpiGenome Browser using E096 lung. The peaks corresponding to each histone modification and the P values of the markers are indicated. Abbreviations: AdjP, adjusted P value; FDR, false discovery rate.
Pathway enrichment analysis revealed that several of the genes positively associated with ACE2 were regulated by KDM5B, and by specific histone acetylation (H3K27ac) and histone methylation (H3K4me1 and H3K4me3) (Figure 4B). In fact, KMD5B demethylates lysine 4 of histone H3 (ie, H3K4) and is involved in transcriptional regulation and DNA repair [33]. We then checked in the Roadmap Epigenomics Project database [26] to see whether the ACE2 locus contained ChIP-seq information for these histone markers. In the human lung, peaks for H3K4me1 and H3K4me3, as well as H3K27ac, were identified in the ACE2 locus (Figure 4C), suggesting that ACE2 may be epigenetically regulated in the lung.
DISCUSSION
We showed here that patients with comorbidities that have very distinct mechanisms have increased expression of ACE2 in the lungs. Although our findings did not include COVID-19 infection data, we suggest that the higher expression of ACE2 in the lungs is associated with higher chances of developing the severe form of COVID-19, by facilitating the SARS-CoV-2 entry into lung cells during the infection. In fact, COVID-19 patients classified as severe cases displayed higher viral loads in nasopharyngeal swab samples during the early stages of disease onset compared to mild patients [34].
The current diabetes pandemic [35] could be worsening the SARS-CoV-2 pandemic by increasing the comorbidities associated with severe COVID-19. As we did not find lung transcriptome samples from patients with type 2 diabetes, we could not directly test whether ACE2 expression is increased in patients with diabetes compared to healthy controls. However, our text-mining approach revealed that interleukin-6 (IL-6) and INS genes were associated with all the diseases we searched. The INS gene encodes the insulin hormone, and insulin is associated with the NAD-dependent histone deacetylase sirtuin 1 (SIRT1) [36]. We found that SIRT1 was upregulated in the lung of patients with severe COVID-19 comorbidities in 4 of 7 studies (data not shown). Clarke et al [37] have demonstrated that, under conditions of cell energy stress, SIRT1 can epigenetically regulate ACE2. Others too have shown that nonsteroidal anti-inflammatory drugs may inhibit the SIRT1 deacetylase activity [38], which in turn could impact ACE2 expression.
The “viral life cycle” pathway that was enriched with upregulated genes in patients with severe COVID-19 comorbidities contains several genes in addition to ACE2 that could be potentially important for SARS-CoV-2 cell cycle and invasion/attachment. These include RAB1A gene, whose product promotes the replication of vaccinia virus [39]. Also, RAB1A is important for herpes simplex virus 1 secondary envelopment [40] and is required for assembly of classical swine fever virus particles [41]. It is possible that SARS-CoV-2 utilizes RAB1A as well.
The fact that ACE2 gene is located in the X chromosome, and initial findings show that older men with comorbidities are more likely to be have severe COVID-19 compared to women [1], indicate that ACE2 expression in the lung may be sex biased. Although no significant sex difference was found in the activity of ACE2 in mouse lung [42], in rats, the levels of ACE2 were dramatically reduced with aging in both sexes, but with significantly higher ACE2 expression in old female rats than male [43].
Although the mechanisms by which ACE2 is upregulated in patients with severe COVID-19 comorbidities were not addressed, our analysis may shed some light on the subject. Among the genes whose expression was positively correlated with ACE2, we detected genes associated with epigenetic regulation of gene transcription. For instance, HAT and HDAC modulate chromatin and DNA condensation by changing histone acetylation status, thus permitting gene transcription. This could occur in lung tissue, facilitating ACE2 expression, as observed during lung cancer and COPD.
KDM5B is associated with hepatitis B virus infection [44]. In breast cancer cells, blockage of KDM5 triggers a robust interferon response that results in resistance to infection by DNA and RNA viruses [45]. This finding suggests that KDM5 demethylases are potential targets for preventing SARS-CoV-2 infection.
COVID-19 may kill between 5.6% and 15.2% of people infected with SARS-CoV-2 [46]. Drug treatments that lower this mortality rate may save many thousands of lives. Our systems biology approach offers putative gene targets for treating and preventing severe COVID-19 cases.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Tiago Lubiana for his valuable inputs.
Author contributions. B. G. G. P., A. E. R. O., Y. S., L. J., A. N. A. G., R. L. T. O., R. C., and H. I. N. performed the analyses. J. P. S. P. and H. I. N. interpreted the results. All authors helped with the writing of the manuscript.
Financial support. This work was supported by Brazilian National Council for Scientific and Technological Development (grant number 313662/2017-7 to H. I. N.); the São Paulo Research Foundation (grant numbers 2017/50137-3, 2012/19278-6, 2018/14933-2, 2018/21934–5, and 2013/08216-2 to H. I. N.); R. C. was supported by a National Science Foundation (NSF) Graduate Research Fellowship (grant number DGE-1256082), NSF Graduate Research Opportunities Worldwide Fellowship, and the National Institutes of Health STD and AIDS Research Training Fellowship Program (grant number 2T32AI007140-41).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Zhong NS. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med 2020; 382:1861–2. [DOI] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11:875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367:1444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaarala MH, Porvari KS, Kellokumpu S, Kyllönen AP, Vihko PT. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J Pathol 2001; 193:134–40. [DOI] [PubMed] [Google Scholar]
- 10. Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol 2011; 85:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simmons G, Bertram S, Glowacka I, et al. Different host cell proteases activate the SARS-coronavirus spike-protein for cell-cell and virus-cell fusion. Virology 2011; 413:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000; 275:33238–43. [DOI] [PubMed] [Google Scholar]
- 13. Orte C, Polak JM, Haworth SG, Yacoub MH, Morrell NW. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J Pathol 2000; 192:379–84. [DOI] [PubMed] [Google Scholar]
- 14. Ziegler CGK, Allon SJ, Nyquist SK, et al. ; HCA Lung Biological Network. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181:1016–35.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang ML, Li X, Meng Y, et al. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol 2010; 37:e1–6. [DOI] [PubMed] [Google Scholar]
- 16. Wei CH, Allot A, Leaman R, Lu Z. PubTator central: automated concept annotation for biomedical full text articles. Nucleic Acids Res 2019; 47:W587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 2009; 4:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Durinck S, Moreau Y, Kasprzyk A, et al. BioMart and bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 2005; 21:3439–40. [DOI] [PubMed] [Google Scholar]
- 19. Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. Presented at: International AAAI Conference on Weblogs and Social Media, San Jose, CA, 17–20 May 2009.
- 20. Davis S, Meltzer PS. GEOquery: a bridge between the gene expression omnibus (GEO) and BioConductor. Bioinformatics 2007; 23:1846–7. [DOI] [PubMed] [Google Scholar]
- 21. Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Lima DS, Cardozo LE, Maracaja-Coutinho V, et al. Long noncoding RNAs are involved in multiple immunological pathways in response to vaccination. Proc Natl Acad Sci U S A 2019; 116:17121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prada C, Lima D, Nakaya HI.. Bioconductor, 2019. https://bioconductor.org/packages/devel/bioc/html/MetaVolcanoR.html. [Google Scholar]
- 24. Chen EY, Tan CM, Kou Y, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 2013; 14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou X, Maricque B, Xie M, et al. the human epigenome browser at Washington University. Nat Methods 2011; 8:989–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spearman P. Viral interactions with host cell Rab GTPases. Small GTPases 2018; 9:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020; 181:281–92.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim WJ, Lim JH, Lee JS, Lee SD, Kim JH, Oh YM. Comprehensive analysis of transcriptome sequencing data in the lung tissues of COPD subjects. Int J Genomics 2015; 2015:206937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cardozo LE, Russo PST, Gomes-Correia B, et al. webCEMiTool: co-expression modular analysis made easy. Front Genet 2019; 10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jia HP, Look DC, Tan P, et al. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 2009; 297:L84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Totura AL, Whitmore A, Agnihothram S, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio 2015; 6:e00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pippa S, Mannironi C, Licursi V, et al. Small molecule inhibitors of KDM5 histone demethylases increase the radiosensitivity of breast cancer cells overexpressing JARID1B. Molecules 2019; 24:1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toniolo A, Cassani G, Puggioni A, et al. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev Med Microbiol 2019; 30:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol 2009; 5:367–73. [DOI] [PubMed] [Google Scholar]
- 37. Clarke NE, Belyaev ND, Lambert DW, Turner AJ. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci (Lond) 2014; 126:507–16. [DOI] [PubMed] [Google Scholar]
- 38. Dell’Omo G, Crescenti D, Vantaggiato C, et al. Inhibition of SIRT1 deacetylase and p53 activation uncouples the anti-inflammatory and chemopreventive actions of NSAIDs. Br J Cancer 2019; 120; 537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pechenick Jowers T, Featherstone RJ, Reynolds DK, et al. RAB1A promotes vaccinia virus replication by facilitating the production of intracellular enveloped virions. Virology 2015; 475:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zenner HL, Yoshimura S, Barr FA, Crump CM. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J Virol 2011; 85:8012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin J, Wang C, Liang W, et al. Rab1A is required for assembly of classical swine fever virus particle. Virology 2018; 514:18–29. [DOI] [PubMed] [Google Scholar]
- 42. Liu J, Ji H, Zheng W, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 2010; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie X, Xudong X, Chen J, et al. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 2006; 78:2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X, Oishi N, Shimakami T, et al. Hepatitis B virus X protein induces hepatic stem cell-like features in hepatocellular carcinoma by activating KDM5B. World J Gastroenterol 2017; 23:3252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu L, Cao J, Cai WL, et al. KDM5 histone demethylases repress immune response via suppression of STING. PLoS Biol 2018; 16:e2006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baud D, Qi X, Nielsen-Saines K, et al. Real estimates of mortality following COVID-19 infection [published online ahead of print 12 March 2020]. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.