Abstract
Background
Zika virus (ZIKV) is an emergent flavivirus initially considered a benign and self-limited exanthematic illness. In 2015, a new epidemic emerged in northeastern of Brazil with increased incidence of a previously rare clinical outcome, microcephaly, in newborns from mothers who were infected during pregnancy. Little is known about the immunopathogenesis of ZIKV-associated microcephaly. Understanding the inflammatory profile and degree of inflammation of persons affected with such condition is an important step towards development of innovative therapeutic strategies.
Methods
A case-control study compared plasma levels of several inflammatory biomarkers from newborns with ZIKV microcephaly, asymptomatic ZKV infection, or uninfected controls. Plasma biomarkers were assessed using Luminex. A series of multidimensional analysis was performed to characterize the systemic immune activation profile of the clinical groups.
Results
We identified an inflammatory signature associated with ZIKV microcephaly that suggested an increased inflammation. Network analysis suggested that ZIKV microcephaly is associated with imbalanced immune activation and inflammation. The cephalic perimeter was inversely proportional with the degree of inflammatory perturbation. Furthermore, a combination of plasma inflammatory biomarkers could discriminate ZIKV with microcephaly from those with ZIKV without microcephaly or uninfected neonates.
Conclusions
An intense inflammatory imbalance that is proportional to the disease severity hallmarks ZIKV microcephaly.
Keywords: inflammation, microcephaly, newborns, Zika virus
The recent Brazilian epidemic of Zika virus in Brazil and its associated microcephaly emerges as a critical public health challenge. Here, we performed immune activation profiling of newborns with Zika-associated microcephaly and demonstrated an inflammatory imbalance that hallmarks this condition.
Zika virus (ZIKV) is an emergent flavivirus that was first detected in Uganda in 1947 [1] and is associated with outbreaks in Asia and the Pacific areas [2–4]. In May 2015, the first case of ZIKV was confirmed in northeastern Brazil [5, 6] and has rapidly spread throughout South America, Central America, and the Caribbean [7], affecting thousands of people in the Americas between 2015 and 2016 [7]. Brazil, with 440 000–1.3 million of affected people, has had the highest number of reported ZIKV cases worldwide [8].
Initially, human ZIKV infection was considered a benign and self-limited exanthematic illness [9]. However, at the end of 2015, an inexplicable and unexpected increase in number of cases of newborns with microcephaly was notified in northeastern Brazil, which was found later to be associated with congenital Zika infection (CZI) [10, 11], culminating with declaration of state of public health emergency in the country.
Microcephaly is defined as a head circumference of at least 2 standard deviations below average for gestational age and gender [12]. It is known that microcephaly is one of manifestations of CZI, which is characterized by a set of birth defects and deficiencies associated with viral neuropathogenesis [13]. The CZI clinical spectrum can range from less severe neurologic abnormalities to neurodevelopment delays in normocephalic. Other documented findings include hearing and ocular abnormalities [14], arthrogryposis, dysphagia, and brain sequels, such as calcifications and ventriculomegaly [13].
The role of immune response to ZIKV in pregnant women and their babies remains poorly understood. A recent analysis suggested that ZIKV microcephaly is linked to increases in concentration of inflammatory markers [15]. Nevertheless, it is not yet known whether systemic immunological mechanisms are involved in the clinical outcome of intrauterine infection caused by ZIKV and the interaction between inflammation and microcephaly development. In this study, we aimed to investigate the degree of inflammatory imbalance linked to ZIKV microcephaly by comparing the expression of several biomarkers of inflammation in peripheral blood of ZIKV newborns with or without microcephaly and well as uninfected controls. The findings elucidate immune pathways possibly associated with microcephaly.
MATERIALS AND METHODS
Ethics Statement
Written informed consent was obtained from legal guardians of all newborns, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of the Gonçalo Muniz Institute, Oswaldo Cruz Foundation (protocol no. 1.935.854/2016).
Study Design
Participants were recruited from a previous neonatal surveillance for CZI [16, 17] in 2016, in a public maternity hospital in Salvador, Northeast Brazil, that was strongly affected by the microcephaly outbreak. We enrolled a total of 50 participants: 14 ZIKV-exposed normocephalic newborns, 22 newborns with ZIKV-associated microcephaly, and 14 healthy newborns from mothers not exposed to ZIKV.
Clinical and epidemiological data of newborns were obtained through interviews with mothers and review of medical records. Data storage and management was performed using the REDCap 6.18.1 (Vanderbilt University, Nashville, TN).
The newborns enrolled were classified according to the International Fetal and Newborn Growth Consortium for the 21st Century charts. Microcephaly was defined as a head circumference below 2 standard deviations from the mean for sex and gestational age, and a normal head circumference would be one whose measurement was within 2 standard deviations from the mean [13]. Biological samples were obtained from umbilical cord blood collected at birth. Ethylenediaminetetraacetic acid (EDTA) plasma was obtained by centrifugation and stored at −80°C. Serological and molecular diagnosis for ZIKV was performed according to previously described methods [16, 17]. Congenital Zika infection was defined as newborns whose serological testing (anti-ZIKV immunoglobulin M) or a qualitative reverse-transcription polymerase chain reaction assay for ZIKV was positive. Healthy controls had negative serological and molecular results for ZIKV.
Immunoassays
We evaluated a panel of 26 soluble markers to examine inflammation and immune activation. The cytokines interleukin (IL)-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-15, IL-17A, interferon (IFN)-α2, IFN-γ, tissue necrosis factor (TNF)-α, granulocyte colony-stimulating factor (GCSF), granulocyte-macrophage colony-stimulating factor (GMCSF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), monocyte chemoattractant protein-1 (MCP-1/CCL2), IFN-γ-induced protein/chemokine (C-X-C motif) ligand 10 (IP-10/CXCL-10), macrophage inflammatory protein-1 alpha (MIP-1α/CCL3), MIP-1 beta (MIP-1β/CCL4), and eotaxin (CCL11) (Millipore, Boston, MA) were measured in duplicate samples using a Luminex-FLEXMAP3D 348-well plate reader (Austin, TX). The quality control of each analyte included the following: the standard concentrations needed to be within 80%−110% of their expected values; and the accepted coefficient of variation between replicated samples was ≤10%. Values that were under the limit of detection were inputted as zero or .01 (to be log2-transformed in heatmaps). No values above the upper limit of detection were obtained.
Data Analysis
Median values with interquartile ranges were used as measures of central tendency and dispersion. The Kruskal-Wallis test with the Dunn’s multiple comparison test were used to compare continuous variables, and the Pearson’s χ 2 was used to compare variables showed as percentage. The Spearman rank test was used to identify associations between different cytokines or between the degree of molecular perturbation of each biomarker and the cephalic perimeter. In the network analysis based on Spearman correlations, nodes represent each given marker and lines represent statistically significant correlations (correlation coefficient [rho] > ±.5 and P < .05).
Hierarchical cluster analyses (Ward’s method) of log10-transformed and z-score-normalized data were used to depict the overall expression profile of indicated biomarkers in the study groups. Dendrograms represent Euclidean distance. Venn diagrams were used to illustrate differentially expressed markers. All comparisons were prespecified and 2-tailed. Differences with P < .05 after Holm-Bonferroni’s adjustment for multiple comparisons were considered statistically significant.
The molecular degree of perturbation (MDP) was calculated to infer the level of inflammatory imbalance associated with ZIKV. This method has been used and detailed previously [18, 19]. Healthy controls were defined as the “reference” group, and the average level and standard deviation of this reference group were calculated for the plasma concentrations of each marker. The MDP score of an individual marker in a given sample “s” was defined by taking the difference in concentration level in sample “s” from the average of the marker in reference group divided by the corresponding standard deviation. The MDP score represents the number of standard deviations from the reference. The statistical analyses were performed using JMP 14.0 (SAS, Cary, NC) and Gephi (version 0.9.2).
RESULTS
A total of 50 participants were enrolled: 14 normocephalic newborns exposed to ZIKV, 22 newborns with ZIKV-microcephaly, and 14 healthy newborns without ZIKV infection. Newborns with ZIKV microcephaly were similar to those who did not developed such clinical presentation and controls with regard to gestational age at delivery, weight, and size at birth (Table 1). There was a high frequency of female individuals in the groups of newborns with ZIKV infection with microcephaly (63.3%) or asymptomatic (57.1%), whereas male individuals were predominant in the control group (71.4%) (Table 1).
Table 1.
Characteristics of Study Participantsa
| Characteristics/Cytokines | All (N = 50) | Healthy Control (n = 14) | ZIKV With Microcephaly (n = 22) | ZIKV Without Microcephaly (n = 14) | P Value |
|---|---|---|---|---|---|
| Female, no. (%) | 26 (52) | 4 (28.6) | 14 (63.6) | 8 (57.1) | .54 |
| Gestational age (week) | 39 (38–40) | 38 (37–39) | 39 (38–40) | 39 (38–40) | .32 |
| Prematurity birth, no. (%) | 10 (20) | 4 (28.6) | 4 (18.2) | 2 (14.3) | .87 |
| Head circumference (cm) | 32 (31–34) | 32.5 (32–34.5) | 31 (30–31) | 34 (33–35.5) | <.01 |
| Weight at birth (g) | 2850 (2328–4130) | 2813 (2393–2930) | 2705 (2209–2929) | 2973 (2755–3540) | .08 |
| Size at birth (cm) | 46.0 (45.0–47.5) | 50.0 (45.0–47.6) | 46.0 (42.0–48.2) | 47.0 (46.0–49.5) | .29 |
| CCL2 | 497.85 (304.57–1214.09) | 475.81 (325.04–533.63) | 1050.17 (377.49–1494.95) | 301.68 (179.63–934.69) | .01 |
| CCL3 | 9.26 (2.82–22.45) | 3.97 (0–10.77) | 20.18 (7.38–80) | 7.85 (3.2–13.62) | .01 |
| CCL4 | 28.42 (20.12–39.6) | 21.86 (19.08–28.22) | 42.06 (26.15–59.2) | 28.22 (20.63–30.19) | .01 |
| CCL11 | 95.45 (66.29–114.58) | 95.45 (46.07–122.04) | 101.6 (71.12–117.63) | 78.69 (66.29–101.76) | .22 |
| CXCL-10 | 239.25 (161.91–410.13) | 131.34 (80.47–152.45) | 517.71 (378.55–631.44) | 202.55 (185.87–235.08) | <.01 |
| EGF | 69.96 (31.81–133.89) | 83.98 (41.92–115.13) | 54.84 (29.1–109.63) | 112.73 (31.81–196.72) | .27 |
| GCSF | 188.28 (105.22–313.06) | 161.42 (100.33–243.39) | 289.62 (141.32–368.29) | 163.76 (97.88–227.44) | .10 |
| GMCSF | 29.47 (20.73–39.9) | 24.64 (10.64–37.08) | 34.25 (20.73–49.2) | 27.07 (22.69–38.02) | .35 |
| IFN-α2 | 90.37 (19.36–127.41) | 11.95 (9.91–13.12) | 101.98 (82.35–141.14) | 114.32 (95.6–136.62) | <.01 |
| IFN-γ | 15.57 (8–31.27) | 3.88 (2.65–7.04) | 31.63 (25.05–51.13) | 11.38 (8.93–15.37) | <.01 |
| IL-1RA | 29.84 (10.26–65.32) | 9.35 (3.95–23.25) | 46.51 (23.25–79.91) | 33.64 (12.1–47.95) | .01 |
| IL-1α | 327.86 (223.75–524.41) | 192.07 (126.62–206.74) | 519.18 (397.8–605.29) | 303.7 (245.63–321.5) | <.01 |
| IL-1β | 1.63 (0.47–2.8) | 1.63 (0.73–2.58) | 1.58 (0.47–2.58) | 1.8 (0.18–3.33) | .95 |
| IL-2 | 2.57 (1.88–4.18) | 2.4 (0.97–2.9) | 2.81 (1.88–4.18) | 3.3 (2.23–4.49) | .20 |
| IL-3 | 0.71 (0.16–1.51) | 0.93 (0.64–1.79) | 0.56 (0–1.65) | 0.86 (0.16–1.37) | .52 |
| IL-4 | 9.47 (5.39–21.1) | 11.45 (5.39–21.1) | 9.47 (3.28–21.1) | 11.45 (5.39–24.87) | .91 |
| IL-6 | 9.4 (3.79–22.42) | 4.92 (0–12.42) | 20.92 (7.01–33.49) | 3.94 (2.05–14.96) | <.01 |
| IL-7 | 10.86 (7.52–13.47) | 10.04 (7.52–11.51) | 9.88 (5.41–14.84) | 10.99 (8.15–12.99) | .65 |
| IL-8 | 12.9 (6.8–23.23) | 6.25 (3.42–13.01) | 16.82 (10.99–27.85) | 14.5 (8.26–23.23) | .01 |
| IL-10 | 16.2 (9.22–30.01) | 9.22 (2.67–11.68) | 28.91 (16.82–55.95) | 12.85 (9.58–19.24) | <.01 |
| IL-12p40 | 6.5 (0–17.34) | 0 (0–12.79) | 7.68 (0–38.48) | 6.5 (0–13.73) | .16 |
| IL-12p70 | 1.38 (0–7.26) | 0 (0–4.8) | 0 (0–6.08) | 2.36 (0–8.37) | .26 |
| IL-15 | 8.38 (5.25–13.77) | 5.64 (4–6.4) | 12.28 (7.32–20.31) | 9.16 (4.84–12.04) | .01 |
| IL-17A | 3.69 (0.67–7.42) | 2.77 (0–4.49) | 4.3 (0–7.71) | 4.06 (1.63–9.62) | .31 |
| TNF-α | 28.47 (7.38–72.38) | 5.71 (4.66–31.7) | 66.93 (21.83–130.72) | 23.26 (7.88–56.03) | <.01 |
| VEGF | 154.36 (100.09–227.18) | 118.2 (89.16–185.53) | 176.75 (110.62–252.75) | 147.34 (89.16–214.98) | .12 |
Bold font indicates statistical significance.
Abbreviations: CCL, chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; EGF, epidermal growth factor; GCSF, granulocyte colony-stimulating factor; GMCSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IL-1RA, IL-1 receptor antagonist; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; ZIKV, Zika virus.
aData are shown as median and interquartile range or frequency (percentage). Plasma markers concentrations are in pg/mL. Data were compared between the clinical groups using the Kruskal-Wallis test (continuous variables) or the Pearson’s χ 2 test (for data on frequency).
Inflammatory Imbalance in Zika Virus-Infected Newborns
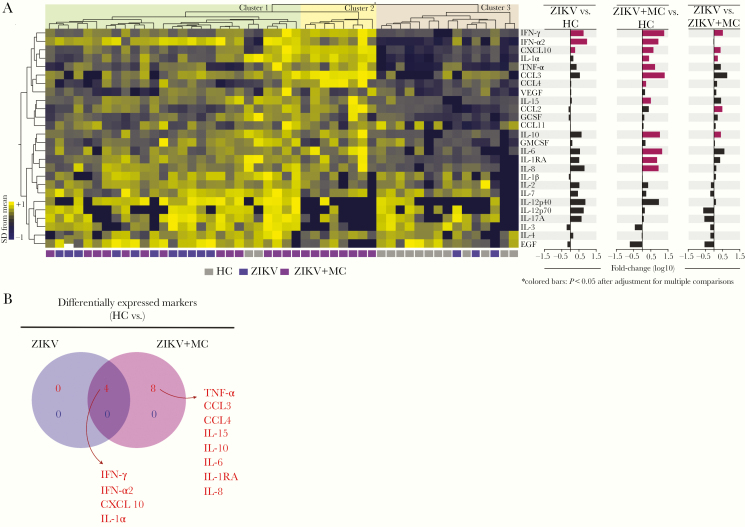
To further evaluate inflammatory profiles in patients with ZIKV, we examined the overall expression of several soluble proteins in cord blood of study participants stratified according to the presence of ZIKV and microcephaly (Table 1). Unsupervised hierarchical cluster analysis of biomarker values identified 3 main groups of individuals (Figure 1A). Although the first cluster included all groups of study participants, with notable tendency of increased expression levels of IL-12p40, IL-12p70, IL-3, IL-4, and EGF, the second cluster showed only those that developed microcephaly, and the third cluster was basically composed by healthy controls. Of note, individuals that presented microcephaly showed a tendency of increase in levels of IFN-γ, IFN-α2, CXCL-10, IL-1α, TNF-α, CCL3, and CCL4 (Figure 1A). Furthermore, we used a fold-difference analysis and identified that concentrations of only IFN-γ, IFN-α2, and CXCL-10 were statistically significant between ZIKV and healthy control groups, whereas microcephalic patients exhibited several markers with significant differences, such as IFN-γ, IFN-α2, CXCL-10, IL-1α, TNF-α, CCL3, CCL4, IL-15, IL-10, IL-6, IL-1RA, and IL-8. It is curious that when we performed the same analysis between patients with only ZIKV infection and those who developed microcephaly, we identified some similarities with the finding described for comparison between microcephaly versus healthy controls, with the addition of CCL2 (Figure 1A). Furthermore, we designed a Venn diagram to summarize the differences between the study groups. As expected, ZIKV infection led to increases in cytokine expression levels compared with the healthy control group independent of presence of microcephaly (Figure 1B). Of note, concentrations of 4 markers were substantially higher in both groups of ZIKV patients, whereas 12 markers were elevated uniquely in those with ZIKV microcephaly compared with controls (Figure 1B).
Figure 1.
Inflammatory imbalance in Zika virus (ZIKV) infection. Concentrations of several markers of inflammation were assessed in plasma samples from a cohort of 50 children. Data were log10-transformed. (A) Hierarchical cluster analysis using z-scored values of each parameter (Ward’s method) was used to depict the overall biomarker expression profile in the study. Fold differences were calculated, and statistically significant differences are highlighted in colored bars. (B) Venn diagram describes the markers that values were statistically different between ZIKV vs ZIKV + MC. HC, healthy control; MC, microcephaly.
Zika Virus Microcephaly Leads to Consistent Alterations in Correlations Between Plasma Concentrations of Inflammatory Biomarkers
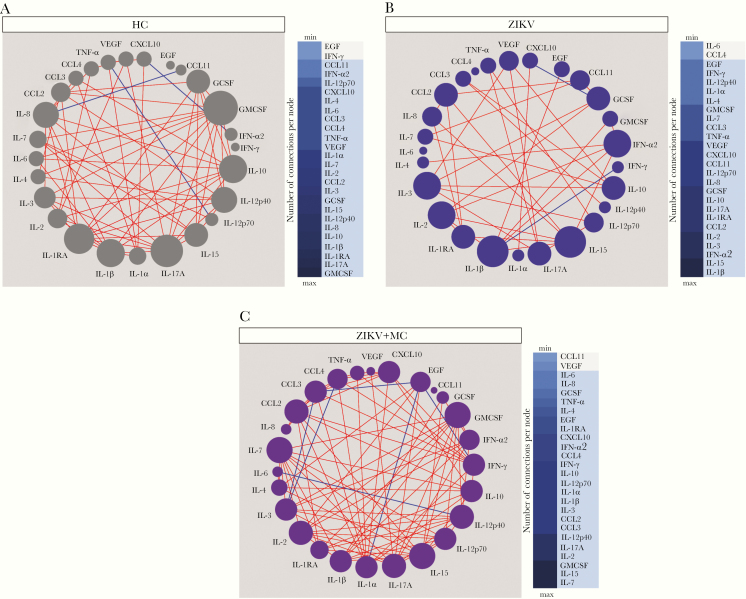
Inflammation is a coordinated process that occurs with synchronized changes in production of inflammatory molecules and cells. To understand these intricate relationships, we have previously used a method based on network analysis using Spearman correlation matrices to identify the dynamicity, degree, and quality of inflammation in several diseases [18–21]. In this study, we extended this approach to infer ZIKV pathophysiology and found that networks from the distinct clinical groups displayed differences in complexity and quality of statistical interactions between the inflammatory markers (Figure 2). The highest density of significant correlations was found in the group of ZIKV microcephaly, whereas the lowest values were detected in those with ZIKV without this condition (Figure 2). Regardless of the clinical groups, most of the significant correlations were positive, meaning that increases in concentrations of a given marker were mostly followed by rises in levels of other inflammatory molecules. The few negative correlations found included TNF-α versus IL-12p70 and IL-8 versus CCL11 in healthy controls (Figure 2A), whereas IFN-γ versus IL-1β and GCSF versus CXCL-10 were observed in ZIKV without microcephaly (Figure 2B). An increased number of negative correlations were found in the group of neonates with ZIKV microcephaly (Figure 2C).
Figure 2.
Zika virus (ZIKV) microcephaly (MC) leads to consistent alterations in correlations between plasma concentrations of inflammatory biomarkers. Network analysis of the biomarker correlation matrices was performed with bootstrap (100×). Significant correlations (P < .05) and Spearman rank (rho) >0.3 are shown. Each node represents a different parameter. The size of each circle (node) is proportional to the number of significant correlations involving such node. Connecting lines represent the Spearman rank coefficient (rho) values. Red color infers positive correlation, whereas blue color denotes negative correlations. Color maps on the right of each network denote the number of significant correlations per parameter (node) per clinical group. A, Healthy controls (HC). B, ZIKV without Microcephaly. C, ZIKV with Microcephaly.
The network analysis highlighted that in healthy controls, GMCSF was the most highly connected marker, followed by IL-17A, IL-1RA, IL-1β, IL-8, and IL-10 (Figure 2A). In ZIKV without microcephaly, IL-1β was the most relevant marker, with positive correlations with EGF, GMSCF, IFN-α2, and negative interactions with IFN-γ. Furthermore, IL-15 was also a significant node in the network of ZIKV without microcephaly, exhibiting positive interactions with CCL2, IL-2, IL-8, IL-12p70, and IL-17A (Figure 2B), and a third significant node detected in this groups was IFN-α2, which displayed only positive correlations with IL-2, IL-4, IL-17A, IL-1β, and IL-15. In patients with ZIKV microcephaly, our analysis indicated that IL-7, IL-15, and GMCSF were the most highly connected markers. It is curious that, in this latter group, we found a number of negative interactions between proinflammatory markers such as IL-1α versus EGF, IL-6 versus IL-12p70, TNF-α versus IL-3, and IFN-γ versus EGF (Figure 2C). Thus, these results argue that ZIKV microcephaly is associated with several alterations in cytokine correlations, including increases in number of negative interactions.
Zika Virus Infection Is Associated With Increased Systemic Molecular Degree of Inflammatory Perturbation
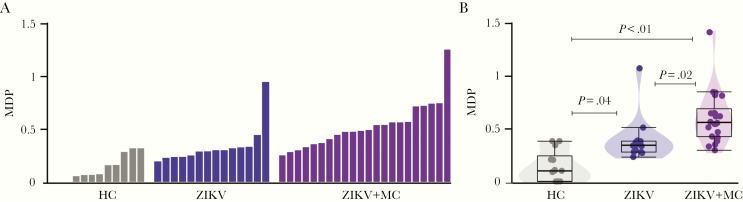
To access the analyses of systemic inflammatory activity, we used an MDP (Figure 3A), a new statistical approach proposed recently by us to evaluate the degree and intensity of inflammatory disturbance in patients with a variety of infectious diseases [18, 19, 22]. As expected, individuals from the healthy control group exhibited diminished MDP values, whereas patients with ZIKV microcephaly exhibited substantial increase in MDP score values compared with controls and those with ZIKV without microcephaly (Figure 3B). In addition, MDP values of patients with ZIKV without microcephaly were also significantly higher than those from uninfected controls (Figure 3B).
Figure 3.
Zika virus (ZIKV) infection is associated with increased systemic molecular degree of inflammatory perturbation. (A) Histograms show the single sample molecular degree of perturbation (MDP) score values relative to each study group as indicated in y-axis. (B) Box plots represent the distribution of the MDP between study groups. Values were compared between control, ZIKV, and ZIKV microcephaly groups using the Kruskal-Wallis test. HC, healthy control; MC, microcephaly.
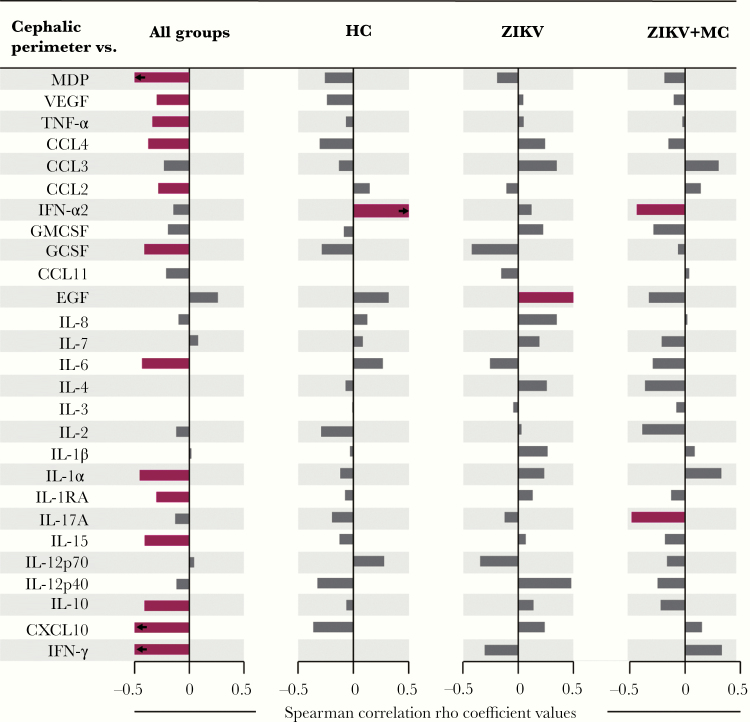
We considered that the microcephaly diagnosis is based on cephalic perimeter and performed a correlation analysis to identify possible relationships between the cephalic perimeter measurement values and overall MDP expression as well as the perturbation values for each individual biomarker. This approach was designed to test whether the severity of microcephaly was proportional to increases in inflammatory perturbation. Our findings revealed that the measures of cephalic perimeter were inversely correlated with molecular perturbation values of several of inflammatory parameters (Figure 4). Furthermore, when the clinical groups were tested individually, a dramatic decrease in statistically significant correlations were detected. It is interesting to note that the cephalic perimeter values exhibited different correlations with inflammatory markers depending on the clinical group. In healthy neonates, the only significant finding was a positive correlation between antiviral cytokine IFN-α2 and cephalic perimeter (Figure 4). In those with ZIKV without microcephaly, another single significant correlation was found, but now with EGF. It is worth noting that, in ZIKV microcephaly, the cephalic perimeter was inversely correlated with both IFN-α2 and IL17A. Our findings suggest that the changes in cephalic perimeter are associated with specific and gradual modification in the degree of inflammatory perturbation in ZIKV-infected neonates.
Figure 4.
Associations between cephalic perimeter and plasma markers and molecular degree of perturbation (MDP) value in study groups. Spearman correlation analysis was used to test association between cephalic perimeter and plasma markers and overall inflammatory profile assessed by the MDP in all study participants (left panel), healthy control (HC) group, patients only with Zika virus (ZIKV) infection, and patients that present ZIKV and microcephalia (ZIKV + MC). Bars represent the Spearman rank (rho) values. Colored bars indicate statistically significant correlations (P < .05) after adjustment for multiple measurements. Highlighting color infers significant correlations.
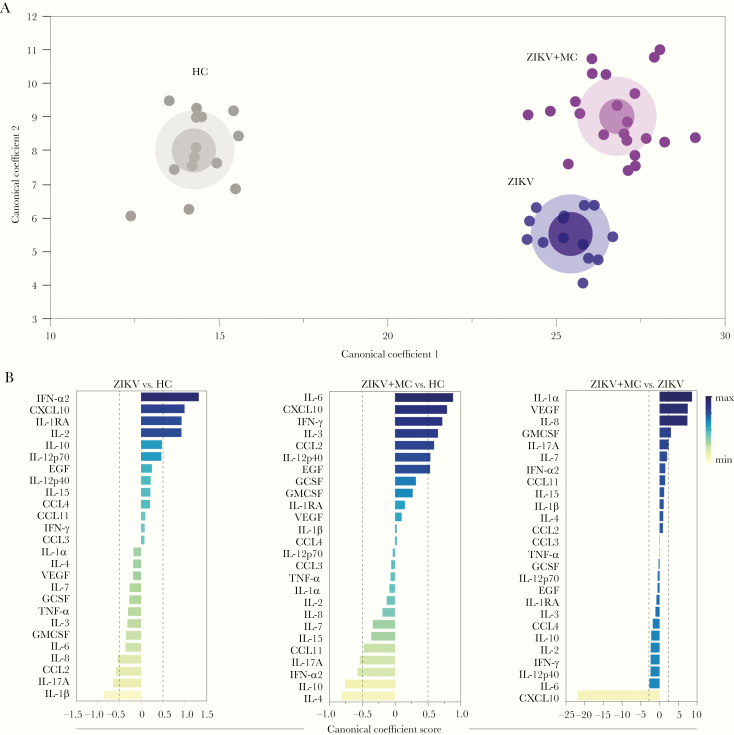
Plasma Concentrations of Inflammatory Biomarkers Can Distinguish Zika Virus Infection With or Without Microcephaly From Uninfected Controls
We finally tested whether simultaneous measurements of the plasma biomarkers of inflammation could be used to distinguish the clinical groups. Given that we observed a distinct correlation profile between the concentrations of the biomarkers (Figure 2), we used a discriminant model using canonical correlation analysis, which takes into account the following: (1) number, (2) strength, and (3) parameters involved in correlations between the biomarker concentrations [19]. We found that ZIKV infection with or without microcephaly could be completely distinguished from healthy controls (area under the curve [AUC] of the receiver operator characteristics curve = 1.0 [100% accuracy]; P < .0001) (Figure 5A). In addition, among neonates with ZIKV infection, those presenting with microcephaly could also be distinguished from the infected individuals who did not have such condition (AUC = 1.0, P < .0001) (Figure 5A). Moreover, we plotted the canonical coefficient scored of each biomarker included in the model to assess the top markers responsible for the discrimination between groups. In the comparison between ZIKV without microcephaly and uninfected healthy controls, we identified IFN-α2, CXCL-10, IL-1RA, IL-2, IL-1β, IL-17, and CCL2 as the top markers in our model (Figure 5B). When the groups of neonates with ZIKV microcephaly and controls, IL-6, CXCL-10, IFN-γ, IL-4, and IL-10 contributed the most for the discrimination (Figure 5B). Furthermore, when ZIKV groups were compared directly, CXCL-10, IL-1α, VEGF, and IL-8 were identified as the top canonical markers. These findings suggest that the infection with ZIKV and occurrence of microcephaly lead to unique disturbances in systemic inflammation and the immune activation profile that hallmark this condition.
Figure 5.
Plasma concentrations of inflammatory biomarkers can distinguish Zika virus (ZIKV) infection with or without microcephaly (MC) from uninfected controls. (A) In an exploratory approach, a sparse canonical correlation analysis (sCCA) was used to test whether experimental groups could be distinguished based on the overall expression profile of all the markers measured. (B) Canonical coefficient scores were calculated to identify the biomarkers responsible for the difference between groups in the sCCA model. HC, healthy control.
DISCUSSION
In the present study, we performed a detailed investigation of the immunological profile in blood of newborns with CZI. Our exploratory results delineated an inflammatory biosignature that could distinguish ZIKV neonates from uninfected controls and also hallmarked infected patients who presented with microcephaly. Such signatures included biomarkers derived from both innate and adaptive immune responses, and the findings presented here advanced the field by describing intricate relationships between these cytokines and growth factors. Recent studies have described a cytokine storm in Flavivirus infection model, with the profile of the immune activation imbalance being associated with the phase of the viral infection [23, 24]. Hence, in acute phase of infection, a higher expression of several biomarkers, such as IL-1β, IL-2, IL-4, IL-6, and IL-10 and also CCL3 and VEGF, was found [23]. In our investigation, newborns with ZIKV but without microcephaly exhibited specific increases of concentrations of IFN-γ, IFN-α2, and CXCL-10, which are biomarkers from the IFN pathway and infer antiviral immunity [25–27]. The involvement of IFN pathways in ZIKV infection has been studied in both human [25] and experimental murine models [28]. Thus, ZIKV infection may cause substantial activation of IFN-related genes that leads to substantial increases in circulating levels of the cytokines described here. Of note, markers derived from the arachidonic acid were reported to hallmark ZIKV microcephaly [15]. The interplay between IFN pathways and lipid mediators is well described in other infectious diseases such as tuberculosis, in which the balance between such players dictates disease outcomes [29]. Curiously, when neonates with ZIKV microcephaly were tested, we found that, in addition to high levels of the IFN-related cytokines described above, unique increases in several markers, including CCL3, IL-6, and IL-10. These latter cytokines have been previously described to be induced in ZIKV-infected placental macrophages [26]. Thus, we propose that ZIKV infection resulting in microcephaly may be caused by disturbances in the IFN pathway linked to other myeloid activation signals that results in this clinical outcome. Further studies are warranted to specifically address this hypothesis.
The inflammatory pathways controlling the development of microcephaly remains unclear, and the magnitude of the influence of immune activation in such process is not yet known. Several studies have tried to explain the pathophysiology of this outcome in ZIKV patients and reported that the presence of infection leads to an altered regulation of genes associated with immune response, cell cycle, differentiation, and apoptosis in neural progenitor cells [30, 31]. These modifications are then associated with reduced cell growth, which culminates in neurological malformations such as microcephaly [31]. Despite this elucidation, the interplay between cytokines, chemokines, and microcephaly development remains unclear. Understanding the specific immune mechanisms linked to microcephaly may aid development of potential interventions that could either prevent or minimize the effects of ZIKV infection and microcephaly. Our results revealed that not only are the concentration values of inflammatory markers altered in ZIKV microcephaly, but also the relationships between them, which was read by our network analyses.
Using correlation matrices, we demonstrated that the cephalic perimeter values displayed unique statistical relationships with plasma biomarkers depending on the ZIKV infection status and occurrence of microcephaly. Our network analysis showed that the presence of ZIKV infection leads to important differences in connectivity profile, involving the number of correlations, direction of the association, as well as the main markers representing the most significant nodes. Indeed, the group of ZIKV microcephaly displayed a network with increased density of connections and changes in strength and directionality of the relationships, including appearance of several negative correlations. A similar analysis performed by our group in several clinical scenarios, such as tuberculosis [18, 19], human immunodeficiency virus [32], malaria [33], and leishmaniasis [34], suggests that changes in the correlation network profile indicates alteration of the systemic inflammatory status. In this setting, increased connectivity usually infers augmented inflammation, whereas a decreased number of significant correlations compared with the baseline control group may indicate uncoupling of the inflammatory response. We hypothesize that ZIKV infection itself leads to an immune homeostatic disturbance associated with uncoupling of the systemic inflammation, whereas development of microcephaly may be due to a probable secondary factor that then intensifies the coordination of the inflammatory processes, which is demonstrated by an increased number of significant connections.
Another important contribution of our study was the assessment of the molecular degree of inflammatory perturbation and its association with the cephalic perimeter of neonates infected with ZIKV. To our knowledge, no previous study has estimated the global inflammatory disturbance in blood of ZIKV neonates. We demonstrated that ZIKV infection is indeed linked to an overall higher inflammatory perturbance, which was shown to be even more significant in the presence of microcephaly. We extended these observations to show that, in the entire study population, decreases in the cephalic perimeter measures were directly associated with increases in perturbance of several inflammatory biomarkers. In addition, microcephaly led to unique inverse relationships between cephalic perimeter values and degree of perturbance of key inflammatory cytokines such as IFN-α2 and IL-17A. Interferon-α2 is known to play an important role in promoting antiviral immune responses [25], whereas IL-17A has also been described to play a significant role in pathogenesis of several lung-associated viral infection [35]. Whether manipulation of these 2 important cytokines in experimental models results in alleviation of the pathological immune activation observed in ZIKV microcephaly is a potential topic of interest for further investigations.
Of note, the discriminant analyses based on a canonical model described here identified candidate biomarkers likely responsible for the distinction between uninfected neonates and ZIKV infection with or without microcephaly. Hence, this approach indicated that, compared with uninfected neonates, in the group of ZIKV without microcephaly, IFN-α2, CXCL-10, and IL-1β were the most significant markers with discriminatory power, whereas in the presence of microcephaly, IL-6, CXCL-10, and IL-4 were the top parameters implicated in the discrimination. This finding suggests that the immune activation profile detected in ZIKV infection was so substantially different that it could distinguish infection and microcephaly with a high degree of accuracy. It is possible that the top markers identified play a significant role in the pathogenesis of the clinical outcomes described here. It is reasonable to hypothesize that upon exposure to ZIKV during pregnancy, the degree of inflammatory disturbance may directly cause immune-mediated organic dysfunction that promotes development of microcephaly. Such hyperinflammation could alter permeability of the blood-brain barrier [36]. In the central nervous system, ZIKV infection could then drive tissue damage mediated by cytotoxicity [15, 37] that leads to microcephaly. This hypothesis requires direct testing in future mechanistic studies.
CONCLUSIONS
Our study has some limitations. We performed a cross-sectional investigation and examined samples obtained from a single time point, which precludes us from making conclusions about the dynamicity of the inflammatory process before birth. The number of individuals investigated in this study was relatively small, but the groups were carefully matched to reduce the influence of potential confounding factors. Regardless of such limitations, our finds extend the current information about ZIKV infection and immunological chances in human model, with delineation of immune profile and inflammatory changes and its association with microcephaly.
Notes
Acknowledgments. We thank the study participants and their legal guardians.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Financial support. The study was funded by the intramural research program from Fundação Oswaldo Cruz, Conselho Nacional de Desenvolvimento Científico e Tecnológico ([CNPq] award numbers 443875/2018-9 and 425380/2016) and Fundação de Amparo à Pesquisa do Estado da Bahia ([FAPESB] award number PPSUS/BA-FAPESB003/2017/SESAB/CNPq/MS 5125/2017). L. C. J. A., M. G., and B. L. d. A. were supported by CNPq/Ministério da Ciência, Tecnologia e Inovação (MCTI), Departamento de Ciência e Tecnologia (DECIT)/Secretaria de Ciência, Tecnologia e Insumos Estratégicos (SCTIE)/Ministério da Saúde (440685/2016–8), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (88887.130716/2016-00). L. C. J. A. and I. C. d. S. were supported by the European Union’s Horizon 2020 Programme through ZIKAlliance (Grant No. PRES-005- FEX-17-4-2-33). The work from B. B. A. was supported by the National Institutes of Health (U01AI115940) and by CNPq (senior fellowship). M. B. A. received a fellowship from the FAPESB. C. L. V. is a scientific initiation fellow from CNPq.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 2. Duffy MR, Chen TH, Hancock WT, et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 3. Cao-Lormeau VM, Roche C, Teissier A, et al. . Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roth A, Mercier A, Lepers C, et al. . Concurrent outbreaks of dengue, chikungunya and Zika virus infections - an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill 2014; 19:20929. [DOI] [PubMed] [Google Scholar]
- 5. Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 2015; 21:1885–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan American Health Organization. World Health Organization. Epidemiological Update. Neurological syndrome, congenital anomalies and Zika virus infection. January 17, 2016. Available at: https://www.paho.org/hq/dmdocuments/2016/2016-jan-17-cha-epi-update-zika-virus.pdf. Acessed 19 December 2019.
- 8. Ministry of Health. Surveillance and response protocol for occurrence of microcephaly related to Zika Virus infection [in Portuguese]. [Internet]. Brazil 2015. Available at: https://portalarquivos2.saude.gov.br/images/pdf/2015/dezembro/09/Microcefalia—Protocolo-de-vigil–ncia-e-resposta—vers–o-1—09dez2015-8h.pdf. Accessed 19 December 2019. [Google Scholar]
- 9. Heang V, Yasuda CY, Sovann L, et al. . Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012; 18:349–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mlakar J, Korva M, Tul N, et al. . Zika virus associated with microcephaly. N Engl J Med 2016; 374:951–8. [DOI] [PubMed] [Google Scholar]
- 11. de Araujo TVB, Rodrigues LC, de Alencar Ximenes RA, et al. . Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 2016; 16:1356–63. [DOI] [PubMed] [Google Scholar]
- 12.Villar J, Ismail LC, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384:857–68. [DOI] [PubMed] [Google Scholar]
- 13. Moore CA, Staples JE, Dobyns WB, et al. . Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017; 171:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Linden V, Pessoa A, Dobyns W, et al. . Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth - Brazil. MMWR Morb Mortal Wkly Rep 2016; 65:1343–8. [DOI] [PubMed] [Google Scholar]
- 15. de Oliveira DN, Lima EO, Melo CFOR, et al. . Inflammation markers in the saliva of infants born from Zika-infected mothers: exploring potential mechanisms of microcephaly during fetal development. Sci Rep 2019; 9:13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira JV, Carvalho TCX, Giovanetti M, et al. Neonatal surveillance for congenital Zika infection during the 2016 microcephaly outbreak in Salvador, Brazil: Zika virus detection in asymptomatic newborns. Int J Gynaecol Obstet 2020; 148 Suppl 2:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duarte AO, Oliveira JV, Carvalho TCX, et al. . Maternal and congenital infections arising from Zika, dengue and Chikungunya arboviruses in Salvador, Brazil. Trans R Soc Trop Med Hyg 2020; 114:222–5. [DOI] [PubMed] [Google Scholar]
- 18. Oliveira-de-Souza D, Vinhaes CL, Arriaga MB, et al. . Molecular degree of perturbation of plasma inflammatory markers associated with tuberculosis reveals distinct disease profiles between Indian and Chinese populations. Sci Rep 2019; 9:8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vinhaes CL, Oliveira-de-Souza D, Silveira-Mattos PS, et al. . Changes in inflammatory protein and lipid mediator profiles persist after antitubercular treatment of pulmonary and extrapulmonary tuberculosis: a prospective cohort study. Cytokine 2019; 123:154759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernandes CD, Arriaga MB, Costa MCM, et al. . Host inflammatory biomarkers of disease severity in pediatric community-acquired pneumonia: a systematic review and meta-analysis. Open Forum Infect Dis 2019; 6:ofz520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cruz LAB, Moraes MOA, Queiroga-Barros MR, Fukutani KF, Barral-Netto M, Andrade BB. Chronic hepatitis B virus infection drives changes in systemic immune activation profile in patients coinfected with Plasmodium vivax malaria. PLoS Negl Trop Dis 2019; 13:e0007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prada-Medina CA, Fukutani KF, Pavan Kumar N, et al. . Systems immunology of diabetes-tuberculosis comorbidity reveals signatures of disease complications. Sci Rep 2017; 7:1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tappe D, Pérez-Girón JV, Zammarchi L, et al. . Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol 2016; 205:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Routhu NK, Byrareddy SN. Host-virus interaction of ZIKA virus in modulating disease pathogenesis. J Neuroimmune Pharmacol 2017; 12:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bayer A, Lennemann NJ, Ouyang Y, et al. . Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016; 19:705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quicke KM, Bowen JR, Johnson EL, et al. . Zika virus infects human placental macrophages. Cell Host Microbe 2016; 20:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierson TC, Graham BS. Zika virus: immunity and vaccine development. Cell 2016; 167:625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yockey LJ, Jurado KA, Arora N, et al. . Type I interferons instigate fetal demise after Zika virus infection. Sci Immunol 2018; 3:eaao1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayer-Barber KD, Andrade BB, Oland SD, et al. . Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014; 511:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faizan MI, Abdullah M, Ali S, Naqvi IH, Ahmed A, Parveen S. Zika virus-induced microcephaly and its possible molecular mechanism. Intervirology 2016; 59:152–8. [DOI] [PubMed] [Google Scholar]
- 31. Tang H, Hammack C, Ogden SC, et al. . Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 2016; 18:587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manion M, Andrade BB, DerSimonian R, et al. . Country of residence is associated with distinct inflammatory biomarker signatures in HIV-infected patients. J Virus Erad 2017; 3:24–33. [PMC free article] [PubMed] [Google Scholar]
- 33. Mendonça VR, Queiroz AT, Lopes FM, Andrade BB, Barral-Netto M. Networking the host immune response in Plasmodium vivax malaria. Malar J 2013; 12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Araújo-Santos T, Andrade BB, Gil-Santana L, et al. . Anti-parasite therapy drives changes in human visceral leishmaniasis-associated inflammatory balance. Sci Rep 2017; 7:4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mebratu YA, Tesfaigzi Y. IL-17 plays a role in respiratory syncytial virus-induced lung inflammation and emphysema in elastase and LPS-injured mice. Am J Respir Cell Mol Biol 2018; 58:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neal JW. Flaviviruses are neurotropic, but how do they invade the CNS? J Infect 2014; 69:203–15. [DOI] [PubMed] [Google Scholar]
- 37. Wen Z, Song H, Ming GL. How does Zika virus cause microcephaly? Genes Dev 2017; 31:849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]