Abstract
Background
Sulfadoxine-pyrimethamine (SP) is used as intermittent preventive therapy in pregnancy (IPTp) for malaria in sub-Saharan Africa. The resistance marker dhps A581G has been associated with reduced IPTp-SP efficacy and enhanced morbidity in SP recipients.
Methods
We measured SP-resistance allele frequencies in Malawian women participating in a trial comparing IPTp with SP against intermittent screening by rapid diagnostic tests (ISTp). We genotyped polymerase chain reaction-detected parasites using deep sequencing of SP-resistance alleles.
Results
Among 125 placental infections, A581G-bearing parasites were associated with reduced birth weight (mean difference [MD], 252 g; 95% confidence interval [CI], 46–457; P = .017). Relative to ISTp, IPTp-SP was associated with higher birth weights in women with wild-type parasites (MD, 116 g; 95% CI, −40 to 272; P = .142) and lower birth weights in women with A581G-bearing parasites (MD, 192 g; 95% CI, −264 to 648; P = .385) (Pinteraction = .033). Similar associations were noted on gestational age (Pinteraction = .075). Amongst only IPTp-SP recipients, relative to women who last received SP > 4 weeks before delivery, recent SP receipt was associated with lower birth weight in women with wild-type parasites (MD, 118 g; 95% CI, −376 to 139; P = .361) and higher birth weight in women with A581G-bearing parasites (MD, 783 g; 95% CI, −20 to 1586; P = .054) (Pinteraction = .005).
Conclusions
The effectiveness in birth weight of IPTp-SP is compromised by A581G-bearing parasites, but there was no evidence that the adverse effects of these parasites are exacerbated by antenatal SP.
ISRCTN Registry
Keywords: malaria, malaria in pregnancy, placental malaria, drug resistance, prevention
In Malawian pregnant women with placental malaria, the presence of parasites with the SP-resistance allele dhps A581G was associated with lower birth weights, but antenatal SP receipt did not exacerbate the adverse consequences of malaria in pregnancy.
Nearly all malaria-endemic African countries provide intermittent preventive therapy to pregnant women (IPTp) with monthly sulfadoxine-pyrimethamine (SP) beginning in the second trimester. Antenatal receipt of SP reduces the risk of anemia, antenatal infections, placental infections, and low birth weight (LBW) [1], and its benefit on LBW is only partially attenuated [2] by widespread Plasmodium falciparum resistance to SP across Africa [3, 4]. Resistance to SP is conferred by mutations in the parasite genes dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) [5]. The rapid spread across Africa of dhfr mutations at codons 51, 59, and 108 rendered SP unsuitable for malaria case management [6]. In contrast, dhps mutations at codons 437, 540, and 581 have been slower to spread across African settings. In East Africa, where the A437G and K540E mutations are largely fixed, SP as IPTp has remained beneficial to protect from LBW [2, 4].
In recent years, the dhps A581G allele has been more commonly reported in East Africa, where it has been directly associated in pregnant women with increased placental parasite densities [7], reduced birth weight [8], and, in one study in northern Tanzania, with enhanced morbidity in women receiving SP [9]. In this observational study, the receipt of SP within 4 weeks of delivery among women with placental malaria was associated with a higher density of placental parasites and a higher fraction of parasites bearing the A581G allele. Also, ecological studies [4, 10] of IPTp-SP use and LBW in areas of varying prevalence of A581G alleles suggest that high prevalences of these resistant parasites may undermine IPTp-SP efficacy as a strategy to improve birth weight [4]. Overall, however, associations between A581G-bearing parasites and SP efficacy have been inconsistent, and there exists an ongoing need to characterize better the effects of A581G alleles on delivery outcomes and how these effects modify SP’s impact on birth weight.
In this study, we investigated interactions between antenatal SP receipt, SP-resistant placental parasites, and delivery outcomes. We used parasites collected in a trial of pregnant Malawian women randomized to either standard IPTp-SP or to intermittent screening during pregnancy (ISTp) with a rapid diagnostic test (RDT) followed by treatment of RDT-positive infections with dihydroartemisinin-piperaquine (DP) (www.isrctn.com/ISRCTN69800930) [11]. All 3 study sites had measurable frequencies of the dhps A581G allele in women at antenatal presentation (1%–3.4%). Owing to the presence of this allele and a large population of SP-unexposed women, our study was uniquely able to analyze modifications of the effect of antenatal SP on delivery outcomes by resistant parasites. We hypothesized that parasites harboring the dhps A581G mutation in placental infections would be associated with higher parasite densities and worse birth outcomes and would also modify the effect of SP on birth weight.
METHODS
Study Cohort
We used specimens collected 2011–2014 from a randomized clinical trial in Malawi [11]. In this study, HIV-seronegative women presenting for antenatal care to 3 sites were randomized (1:1) to ISTp-DP or IPTp-SP and followed through delivery. Overall, the median number of scheduled antenatal care visits and SP doses was 4. As previously reported [11], the prevalence of polymerase chain reaction (PCR)-positive infections was similar at baseline between ISTp-DP (44.4%) and IPTp-SP (43.0%) recipients, but higher in maternal or placental specimens at delivery in ISTp-DP recipients (30.1% vs 22.7%). Outcome assessments were as previously described for maternal, newborn, and placental measurements, and molecular parasite detection [12]. For the latter, parasite densities were measured by reference to a standard curve on each reaction plate and expressed as ng/µL of strain 3D7 genomic DNA [13].
Genotyping Procedures
We used a 2-stage genotyping approach: we first genotyped pools of parasites to identify those with nonfixed resistance alleles and then we genotyped individual parasitemias within those pools with heterogeneous resistance alleles. For the first stage of pooled analyses, we defined 18 parasite populations on the basis of study arm (2), study site (3), and timing of specimen (antenatal booking, maternal peripheral blood at delivery, and placental blood) (Supplementary Table 1). The 2 largest populations (207 parasitemias each) were each divided into 2, and therefore we created 20 pooled templates, each consisting of between 19 and 120 parasitemias. For genotyping in the second stage, the same gDNA specimens were used as PCR templates but in unpooled fashion.
We amplified P. falciparum genes dhfr and dhps using nested PCR assays on pooled or individual gDNA templates [14]. Amplicons were prepared as barcoded sequencing libraries using the NEBNext Fast DNA Fragmentation and Library Prep Set for Ion Torrent (New England Biolabs) and Ion Xpress Barcode Adaptors (Thermo Fisher Scientific). Barcoded libraries were mixed in equimolar amounts by gene target and sequenced on an Ion Torrent PGM platform using 318 chips.
Sequence Analyses
All dhfr and dhps reads were analyzed in Galaxy (https://usegalaxy.org) [15–17]: reads were first aligned to 3D7 reference sequences for either dhfr (XM_001351443) or dhps (Z30654) using Bowtie2, and then variants at each position were quantified using MPileup. For quality filtering of allele frequencies, we allowed reads of any quality to be mapped to reference sequences but analyzed within those reads only bases with quality scores > q33 for dhfr or > q29 for dhps [18]. This enforces stringent quality while limiting false discovery, resulting in expected per-base error probabilities of less than 5 × 10−4 (for dhfr) and 1.3 × 10−3 (for dhps). At resistance loci, the mutant allele frequency was defined as the proportion of reads at that locus that harbored the nucleotide substitution encoding the amino acid substitution conferring resistance; we censored frequencies in pooled parasitemias < 1% to mitigate the risk of false discovery [18]. Read processing was performed by personnel masked to study data (B.L.).
Statistical Analyses
We used Poisson regression using robust standard errors to compute prevalence ratios (PRs) on dichotomous outcomes and linear regression to compute mean differences on continuous outcomes and 95% confidence intervals (CIs) for each. Results are presented for crude models, for multivariable models, adjustment for treatment arm, gravidity, maternal underweight (body mass index < 18.5 kg/m2), maternal bed net use the night before enrollment, and conditioned on resistance allele using interaction terms. We estimated the significance of regression interaction terms between treatment arm and resistance allele using the Wald test. We performed sensitivity analyses of models on gestational age and birth weight by recomputing models after resetting an outlier value for each variable to the lowest value of the 99th percentile of the remainder values. We used the Kruskal-Wallis test to compare parasite densities. A P value of < .05 was considered statistically significant. All analyses were conducted in Stata/SE (v14.2, Stata Corp).
Ethics
Written informed consent was obtained from all participants before enrollment. Ethical approval was obtained from the Malawian National Health Science Research Committee and the Liverpool School of Tropical Medicine; molecular testing of parasites was approved by the ethical review boards of the University of North Carolina and Duke University.
RESULTS
Frequencies of dhfr and dhps Mutations in Pooled Parasite Populations
Median read depth at loci of interest was 1251 (interquartile range [IQR], 427–2583) for dhfr and 2397 (IQR, 1351–3781) for dhps (Supplementary Figure 1). Similar to parasites collected at antenatal booking [11], across each study site, study arm, and specimen type, allele frequencies exceeded 92% for the dhfr substitutions N51I, C59R, and S108N, and 97% for the dhps substitutions A437G and K540E (Supplementary Table 2 and Table 3). We did not observe any pools harboring the dhfr I164L or the dhps I431V mutations.
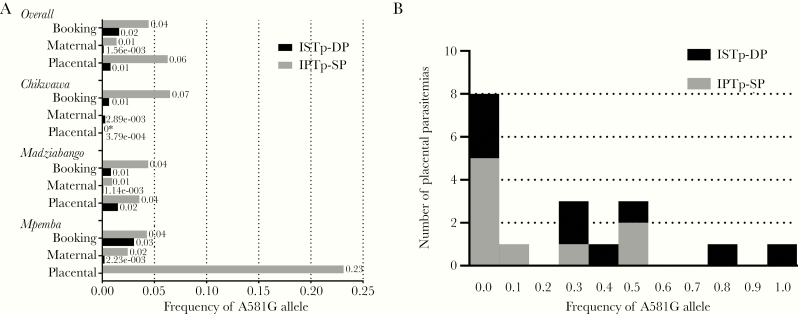
The frequency of the dhps A581G mutation ranged between 0% and 23% across the 20 pools of parasites (Figure 1A) (antenatal booking, 0.6%–6.5%; delivery, 0.1%–2.5% in maternal peripheral parasites and 0%–23.1% in placental parasites).
Figure 1.
Frequencies of Plasmodium falciparum dhps A581G alleles in pooled (A) and individual placental (B) parasite templates. A, Frequencies of dhps A581G allele in pooled parasitemias by study site, study arm, and specimen type. * The sequencing of pooled maternal peripheral parasites from Chikwawa IPTp-SP recipients failed to yield analyzable reads. B, Frequency distribution within individual placental parasitemias of dhps A581G alleles in participants receiving ISTp-DP or IPTp-SP in the Mpemba site. Abbreviations: IPTp-SP, intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine; ISTp-DP, intermittent screening during pregnancy and treatment with dihydroartemisinin-piperaquine.
Frequencies of dhfr and dhps Mutations in Individual Placental Parasitemias
We genotyped all individual placental parasites across dhfr and dhps loci from Mpemba, the study site with the highest frequency of dhps A581G alleles in placental parasites. At this site, the frequency of A581G alleles was similar at enrollment between IPTp-SP (4.3%) and ISTp-DP (3.1%) recipients. In placental samples, 144 women had PCR-detectable parasites (IPTp-SP, 65; ISTp-DP, 79); after censoring samples with fewer than 10 reads, we obtained allele frequencies for 121 and 125 parasitemias for loci in dhfr or dhps, respectively. Amongst women with placental infections, compared to those with genotype data, women missing genotype data did not differ significantly in randomization group or gravidity, but did have lower median placental parasite densities (0.14 × 10–3 ng/µL versus 0.02 × 10–3 ng/µL; P = .0037 by Kruskal-Wallis).
In 125 placental infections, mean frequencies were high for the dhfr substitutions N51I (0.993 [SD 0.05]), C59R (0.987 [SD 0.09]), and S108N (0.999 [SD 0.002]), and all less than 0.001 for I164L. Similarly, in 121 placental infections, mean frequencies were high for the dhps substitutions A437G (0.998 [SD 0.01]) and K540E (0.999 [SD 0.011]) and less than 0.001 for I431V. In contrast, the overall mean frequency of the A581G substitution was 0.037 (SD 0.15) (Figure 1B), and the prevalence of the A581G mutation was 14.4% (18/125) among placental infections. Among these 18 placental infections, the median allele frequency was 0.20 (IQR, 0.001–0.464).
Associations of Mutant Alleles and Clinical Factors
Enrollment characteristics were similar in Mpemba between women who ultimately had placental infections with dhps A581G or wild-type parasites (Table 1). The prevalence of the A581G mutation in placental parasites was similar between women who had received IPTp-SP (9/58, 16%) and ISTp-DP (9/67, 13%) (PR = .87; 95% CI, .34–2.18; P = .760). Amongst women infected with placental parasites bearing the A581G allele (n = 18), there was no evidence of differences in median allele frequencies between those who had received IPTp-SP (0.002; IQR, 0.001–0.273) or ISTp-DP (0.311; IQR, 0.001–0.464; P = .354 by Kruskal-Wallis).
Table 1.
Maternal Characteristics at Antenatal Enrollment Among Women in Mpemba With Placental Malaria by dhps Codon 581 Allele
| Characteristic | Placental Parasites Wild-Type A581 (n = 107) | Placental Parasites With A581G (n = 18) | P Valuea |
|---|---|---|---|
| Allocated to IPTp-SP, % (n/N) | 45.8 (49/107) | 50 (9/18) | .741 |
| Paucigravidae, %, (n/N) | 67.3 (72/107) | 83 (15/18) | .171 |
| Maternal age, y, mean (SD) | 21.9 (4.9) | 20.9 (3.9) | .413 |
| Gestational age at enrollment, d, mean (SD) | 146 (23) | 147 (18) | .872 |
| Maternal weight, kg, mean (SD) | 54.0 (6.0) | 53.1 (5.0) | |
| Maternal height, cm, mean (SD) | 153.7 (5.9) | 153.4 (5.9) | .890 |
| Maternal BMI < 18.5 kg/m2, % (n/N) | 1.9 (2/107) | 0 | .559 |
| Used bed net last night, % (n/N) | 4.7 (5/107) | 6 (1/18) | .871 |
| P. falciparum infection at enrollment,b %, (n/N) | 55.1 (59/107) | 39 (7/19) | .201 |
Abbreviation: BMI, body mass index; IPTp-SP, intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine.
aComputed either with χ 2 test or Student t test.
bPositive by either light microscopy or polymerase chain reaction.
Effect of IPTp-SP Versus ISTp-DP by dhps581 Alleles
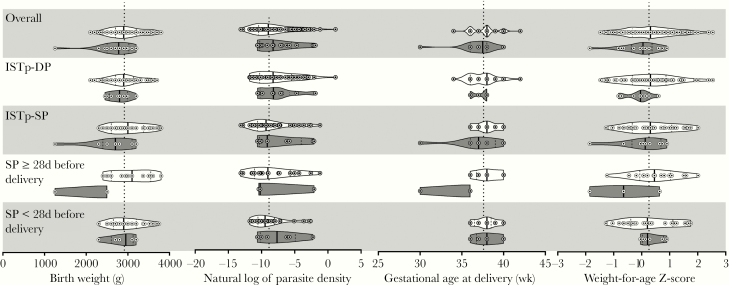
Prior studies have suggested that A581G-bearing parasites modify the effect of antenatal SP to exacerbate placental morbidity [9]. We therefore tested among women with placental infection if the effects of the antenatal prevention strategy on delivery outcomes were modified by A581G-bearing placental parasites. Among these women in whom antenatal strategies failed to prevent placental malaria, compared to women allocated to ISTp-DP, receipt of IPTp-SP resulted in a nonsignificant 63-g increase in birth weight (95% CI, −87 to 213; P = .408) (Table 2). When conditioned on allele, IPTp-SP was associated with a 116-g increase (95% CI, −40 to 272; P = .142) in women with wild-type parasites and a 192-g decrease (95% CI, −648 to 264; P = .385) in women with A581G-bearing parasites (Figure 2) and this difference in effect was statistically significant (Pinteraction = .033). Furthermore, relative to ISTp-DP, IPTp-SP was associated with increased gestational age at delivery in those infected with wild-type parasites (mean difference [MD] = 0.64 weeks; 95% CI, .1–1.2; P = .032) but not among those infected with A581G-bearing parasites (MD = −0.44; 95% CI, −2.7 to 1.8; P = .677) (Pinteraction = .075). We did not observe modification of the effect of antenatal SP receipt by allele on placental parasite density, placental inflammation, maternal hemoglobin concentration, or weight-for-age Z-score (Table 2).
Table 2.
Comparisons of Clinical and Parasitological Variables Among Women With PCR-Positive Placental Malaria Parasites in Mpemba Study Site With and Without dhps A581G Mutation by Study Arm
| Variable | All Women | ISTp-DP | IPTp-SP | Mean Difference (95% CI) | P Valuea | Interaction Termb |
|---|---|---|---|---|---|---|
| Birth weight, No., mean (SD), g | ||||||
| All infected women | 121, 2916 (415) | 66, 2887 (364) | 55, 2950 (471) | 63 (−87 to 213) | .408 | |
| dhps A581 | 103, 2953 (399) | 57, 2901 (379) | 46, 3018 (418) | 116 (−40 to 272) | .142 | |
| dhps A581G | 18, 2702 (454) | 9, 2797 (248) | 9, 2606 (596) | −192 (−648 to 264) | .385 | |
| Mean difference (95% CI) | −252 (−457 to −46) | −104 (−365 to 158) | −412 (−740 to −84) | |||
| P valuea | .017 | .432 | .015 | |||
| 0.033 | ||||||
| Parasite density, No., median (IQR), ng/μL × 10−3 | ||||||
| All infected women | 125, 0.14 (0.03 to 2.5) | 67, 0.27 (0.04 to 4.1) | 58, 0.10 (0.03 to 0.7) | .110 | ||
| dhps A581 | 107, 0.13 (0.02 to 2.5) | 58, 0.26 (0.04 to 4.1) | 49, 0.09 (0.03 to 0.6) | .086 | ||
| dhps A581G | 18, 0.26 (0.03 to 3.0) | 9, 0.27 (0.05 to 0.99) | 9, 0.11 (0.04 to 3.0) | .895 | ||
| P valuea | .288 | .269 | .672 | |||
| 0.306 | ||||||
| Intervillous inflammation leukocytes ≥ 5, % (n/N) | ||||||
| All infected women | 38 (47/124) | 38 (25/66) | 38 (22/58) | .995 | ||
| dhps A581 | 40 (42/106) | 40 (23/57) | 39 (19/49) | .869 | ||
| dhps A581G | 28 (5/18) | 22 (2/9) | 33 (3/9) | .599 | ||
| P valuea | .338 | .297 | .757 | |||
| 0.769 | ||||||
| Maternal hemoglobin at delivery, No., mean (SD), g/dL | ||||||
| All infected women | 125, 12.1 (1.7) | 67, 11.9 (1.8) | 58, 12.2 (1.6) | 0.3 (−.4 to 0.9) | .432 | |
| dhps A581 | 107, 12.0 (1.6) | 58, 11.9 (1.8) | 49, 12.1 (1.4) | 0.2 (−.4 to .8) | .579 | |
| dhps A581G | 18, 12.5 (2.3) | 9, 12.2 (2.2) | 9, 12.8 (2.6) | 0.6 (−1.8 to 3.0) | .610 | |
| Mean difference (95% CI) | 0.5 (−.4 to 1.4) | 0.3 (−1.0 to 1.6) | 0.7 (−.4 to 1.9) | |||
| P valuea | .237 | .643 | .219 | |||
| 0.538 | ||||||
| Gestational age at delivery, No., mean (SD), wk | ||||||
| All infected women | 122, 37.6 (1.6) | 66, 37.3 (1.5) | 56, 37.8 (1.8) | 0.46 (−.1 to 1.0) | .123 | |
| dhps A581 | 104, 37.7 (1.5) | 57, 37.4 (1.5) | 47, 38.0 (1.4) | 0.64 (.1 to 1.2) | .032 | |
| dhps A581G | 18, 37 (2.2) | 9, 37.2 (1.0) | 9, 36.8 (3.0) | −0.44 (−2.7 to 1.8) | .677 | |
| Mean difference (95% CI) | −0.67 (−1.5 to .2) | −0.16 (−1.2 to .9) | −1.24 (−2.5 to .04) | |||
| P valuea | .106 | .756 | .057 | |||
| 0.075 | ||||||
| Weight-for-age Z-score, No., mean (SD) | ||||||
| All infected women | 121, 0.26 (0.87) | 66, 0.23 (0.88) | 55, 0.30 (0.86) | 0.07 (−.24 to .39) | .650 | |
| dhps A581 | 103, 0.31 (0.90) | 57, 0.28 (0.93) | 46, 0.36 (0.87) | 0.08 (−.28 to .43) | .674 | |
| dhps A581G | 18, −0.04 (0.66) | 9, −0.10 (0.45) | 9, 0.02 (0.85) | 0.12 (−.56 to .80) | .706 | |
| Mean difference (95% CI) | −0.35 (−.79 to .08) | −0.23 (−.45 to .25) | −0.30 (−.96 to .30) | |||
| P valuea | .113 | .232 | .292 | |||
| 0.427 |
Abbreviations: CI, confidence interval; IPTp-SP, intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine; IQR, interquartile range; ISTp-DP, intermittent screening during pregnancy and treatment with dihydroartemisinin-piperaquine; PCR, polymerase chain reaction.
aComputed by linear regression or the Kruskall-Wallis test (for continuous variables) or the χ 2 test (for categorical variables).
bComputed by the Wald test.
Figure 2.
Comparisons of continuous delivery outcomes among women with PCR-positive placental malaria parasites with and without the dhps A581G mutation by study arm and, among SP recipients, by timing of most recent SP dose. White plots, dhps A581; dark gray plots, dhps A581G. Thick line within each plot indicates median, dotted lines are quartiles. Overlying dots are individual values. Vertical dotted line on each subgraph indicates overall population mean. Abbreviations: IPTp-SP, intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine; ISTp-DP, intermittent screening during pregnancy and treatment with dihydroartemisinin-piperaquine; PCR, polymerase chain reaction; SP, sulfadoxine-pyrimethamine.
Difference in Outcomes by dhps581 Alleles Among SP Recipients
Among the 55 women with placental infections who received IPTp-SP, the mean (SD) birth weight among 9 women infected with parasites carrying the A581G mutation were significantly lower than those among 46 women infected with wild-type parasites: 2606 g (SD 596) vs 3018 g (SD 418); MD = 412 g (95% CI, 84–740); P = .015 (Table 2). This reflected a difference in gestational age (MD = 1.24 weeks, 95% CI, −.04 to 2.52; P = .057) rather than z-score for weight-for-gestational age (MD = 0.30; 95% CI, −.30 to .96; P = .292) (Table 2).
Timing of SP and Outcome by dhps581 Alleles
We next tested if SP receipt within 4 weeks prior to delivery modified the association of the A581G allele with birth outcomes (Table 3). The number of days since the last dose of SP prior to delivery was similar between women infected with wild-type (n = 49, median 20 [IQR, 11–33] days) and A581G-bearing parasites (n = 9, median 23 [IQR, 14–30] days; P = .940 by Kruskal-Wallis). Overall, the timing of SP receipt did not modify the effect of SP on mean birth weight (SP ≥ 28 days, n = 20, 2941 g [SD 610] vs < 28 days, n = 35, 2956 g [SD 379]; MD = 15 g [95% CI, −252 to 282]; P = .911). However, this differed significantly by 581 allele: recent SP receipt < 28 days before delivery was associated with a 783-g increase in mean birth weight (95% CI, −20 to 1586; P = .054) among the 9 women infected with A581G-bearing parasites and a 118-g decrease (95% CI, −139 to 375; P = .361) among the 46 women infected with wild-type parasites (Pinteraction = .005) (Table 3 and Figure 2). Similar effect modification by the timing of SP use and 581 allele was found for gestational age; recent SP intake was associated with longer duration of pregnancies (P = .037) among women infected with parasites carrying the A581G mutation but not among women infected with wild-type parasites (P = .590) (Pinteraction = .001). In addition, we did not observe associations between A581G-bearing parasites and significant increases in parasite density or intervillous inflammation with recent SP use (Table 3). Collectively, these findings suggest that recent SP use was not associated with increased morbidity among women carrying the A581G-bearing parasites at delivery.
Table 3.
Effect of dhps A581G Mutation on the Impact of Recent SP on Birth Outcomes Among Women Receiving IPTp-SP
| Variable | All Women | SP ≥ 28 d Before Delivery | SP < 28 d Before Delivery | Mean Difference (95% CI) | P Valuea | Interaction Termb |
|---|---|---|---|---|---|---|
| Birth weight, No., mean (SD), g | ||||||
| Overall | 55, 2950 (471) | 20, 2941 (610) | 35, 2956 (379) | 15 (−252 to 282) | .911 | |
| dhps A581 | 46, 3018 (418) | 17, 3092 (463) | 29,2974 (390) | −118 (−375 to 139) | .361 | |
| dhps A581G | 9, 2606 (596) | 3, 2083 (722) | 6, 2867 (339) | 783 (−20 to 1586) | .054 | |
| Mean difference (95% CI) | −412 (−740 to −84) | −1009 (−1665 to −353) | −107 (−457 to 242) | |||
| P valuea | .015 | .005 | .536 | |||
| 0.005 | ||||||
| Parasite density, No., median (IQR), ng/µL × 10−3 | ||||||
| Overall | 58, 0.10 (0.03 to 0.70) | 22, 0.09 (0.03 to 3.4) | 36, 0.10 (0.03 to 0.53) | .737 | ||
| dhps A581 | 49, 0.09 (0.03 to 0.60) | 19, 0.11 (0.02 to 3.4) | 30, 0.08 (0.03 to 0.31) | .594 | ||
| dhps A581G | 9, 0.11 (0.04 to 3.0) | 3, 0.04 (0.03 to 125) | 6, 1.10 (0.04 to 3.0) | .796 | ||
| P valuea | .269 | .811 | .188 | |||
| 0.615 | ||||||
| Intervillous inflammation leukocytes ≥ 5, % (n/N) | ||||||
| Overall | 38 (22/58) | 36 (8/22) | 39 (14/36) | .847 | ||
| dhps A581 | 39 (19/49) | 42 (8/19) | 37 (11/30) | .703 | ||
| dhps A581G | 33 (3/9) | 0 (0/3) | 50 (3/6) | .134 | ||
| P valuea | .757 | .159 | .541 | |||
| 0.811 | ||||||
| Maternal hemoglobin at delivery, No., mean (SD), g/dL | ||||||
| Overall | 58, 12.2 (1.6) | 22, 12.1 (1.4) | 36, 12.8 (2.6) | 0.35 (−.53 to 1.2) | .427 | |
| dhps A581 | 49, 12.1 (1.4) | 19, 12.0 (1.1) | 30, 12.1 (1.5) | 0.08 (−.73 to .89) | .836 | |
| dhps A581G | 9, 12.8 (2.6) | 3, 11.6 (0.9) | 6, 13.4 (3.1) | 1.75 (−2.7 to 6.2) | .379 | |
| Mean difference (95% CI) | 0.72 (−.44 to 1.9) | −0.39 (−1.8 to .99) | 1.3 (−.40 to 2.9) | |||
| P valuea | .219 | .560 | .130 | |||
| 0.273 | ||||||
| Gestational age at delivery, No., mean (SD), wk | ||||||
| Overall | 56, 37.8 (1.8) | 21, 37.6 (2.3) | 35, 38.0 (1.4) | 0.40 (−.60 to 1.4) | .426 | |
| dhps A581 | 47, 38.0 (1.4) | 18, 38.2 (1.5) | 29, 37.9 (1.4) | 0.24 (−1.1 to .64) | .590 | |
| dhps A581G | 9, 36.8 (3.0) | 3, 34.0 (3.5) | 6, 38.2 (1.6) | 4.2 (.33 to 8.0) | .037 | |
| Mean difference (95% CI) | −1.2 (−2.5 to .04) | −4.2 (−6.6 to −1.8) | 0.24 (−1.1 to 1.5) | |||
| P valuea | .057 | .002 | .714 | |||
| 0.001 | ||||||
| Weight-for-age Z-score, No., mean (SD) | ||||||
| Overall | 55, 0.30 (0.9) | 20, 0.31 (0.9) | 35, 0.30 (0.8) | −0.01 (−.50 to .48) | .967 | |
| dhps A581 | 46, 0.36 (0.9) | 17, 0.47 (0.8) | 29, 0.29 (0.9) | −0.18 (−.72 to .35) | .495 | |
| dhps A581G | 9, 0.02 (0.8) | 3, −0.62 (1.2) | 6, 0.34 (0.4) | 0.96 (−.28 to 2.2) | .109 | |
| Mean difference (95% CI) | −0.33 (−.96 to .30) | −1.1 (−2.3 to .07) | 0.05 (−.71 to .82) | |||
| P valuea | .292 | .064 | .886 | |||
| 0.254 |
Abbreviations: CI, confidence interval; IPTp-SP, intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine; IQR, interquartile range; SP, sulfadoxine-pyrimethamine.
aComputed by linear regression or the Kruskall-Wallis test (for continuous variables) or the χ 2 test (for categorical variables).
bComputed by the Wald test.
DISCUSSION
We investigated the impact of molecular SP resistance among P. falciparum parasites on the effectiveness of IPTp-SP for antenatal malaria prevention in a large cohort of pregnant women in southern Malawi. Consistent with prior studies of antenatal malaria, parasites harboring the dhps A581G allele in placental infections were associated with reduced birth weight in IPTp-SP recipients relative to birth weights among IPTp-SP recipients infected with less-resistant parasites; this observation was consistent with a similar association with a shorter gestational age. However, contrary to the previous trial in northern Tanzania suggesting SP use in the 4 weeks prior to delivery was associated with increased parasite densities and adverse pregnancy outcomes [9], we found no such associations. Therefore, although failure of SP among women infected with parasites harboring the dhps A581G allele may manifest as placental infection with resistant parasites that are associated with worse birth outcomes, there was no evidence that recent SP use exacerbated malaria-associated morbidity.
Amongst recipients of SP with placental malaria, the presence of the dhps A581G allele was associated with a reduction in birth weight of 412 g (Table 2). This association of the dhps A581G allele with reduced birth weight adds to the growing evidence from antenatal studies [8], delivery series [7, 9], and ecological studies [4, 10] that parasites bearing the A581G allele partially undermine the improvements in birth weight following IPTp-SP. Notably, these associations were present despite the relatively low abundance of A581G alleles within placental infections: in 16 of the 18 women infected with A581G-bearing parasites, the A581G allele frequency within the placental infection was less than 50%, indicating minority variants within mixed populations (Figure 1B). Given the more frequent reporting of this mutation in the past decade [3], these results further underscore the need to identify alternatives to IPTp-SP that can mitigate the deleterious effects of antenatal malaria.
Our study design, which included an SP-unexposed population, allowed us to investigate if the A581G allele modified the effect of SP use on birth weight among women with placental infections. Compared with SP-unexposed women in the ISTp arm, the effect of IPTp-SP on mean birth weight differed significantly between women infected with wild-type parasites (a 116-g increase relative to the ISTp arm) and those infected with A581G-bearing parasites (a 192-g decrease) (P = .033) (Table 2). Additionally, we observed similar interactions between the allele and SP receipt on gestational age at delivery (Pinteraction = .075). Although this agrees with a prior reported study [19], these results were largely driven by a single outlier birth of a 1250-g viable infant at 30 weeks gestation to a mother who received only a single dose of SP; in a sensitivity analysis of these interactions in which this participant’s birth weight and gestational age were set to the lower limit of the 99th percentile of the overall distribution of each variable, these interactions remained but were not statistically significant (Supplementary Table 4). Taken together, these observations suggest that the lower birth weights observed in women infected with parasites harboring the A581G mutation in the SP recipients was driven by shorter gestation in these women rather than intrauterine growth retardation.
We did not observe the previously reported phenomenon whereby the receipt of SP in the 4 weeks prior to delivery exacerbated the pathology of A581G-bearing parasites (Table 3) [9]. On the contrary, we found that recent SP use was associated with higher mean birth weight and longer gestation among women infected with parasites harboring the dhps A581G allele at delivery, and these findings were maintained in sensitivity analyses (Supplementary Table 5). In addition, there was no evidence that recent SP use modified the effect of SP on parasite density, intervillous inflammation, malaria pigment, or maternal hemoglobin concentrations (Table 3) in women harboring A581G-bearing parasites, each of which could provide a mechanism for the previously observed association with reduced birth weight. Collectively therefore, there was no evidence that SP use exacerbates the adverse effect of malaria infections in women infected with highly resistant parasites.
Several other observations were notable. Firstly, other than the Mpemba site, we did not observe consistent increases from enrollment to delivery in the frequency of SP-resistance alleles. This is surprising, given the presence at baseline of parasites bearing the A581G mutation, the large mean number of SP doses received per woman (3.3) in the IPTp-SP group, suboptimal efficacy of SP to clear parasites in Malawi [2], the high prevalence of “breakthrough” parasites at delivery, and reported selection during pregnancy for drug resistance mutations in dhfr by SP [20, 21] and pfmdr1 by mefloquine [22]. This lack of clear increase in A581G alleles may be the result of our ecological approach to genotyping, in which we compared pairings of allele frequencies between only 3 sites, or more mild selection on the A581G allele by sulfadoxine compared with that on dhfr alleles by pyrimethamine. Additionally, there was an absence of the dhfr I164L substitution, which confers a higher degree of pyrimethamine resistance and, for obscure reasons, has remained rare in African settings, as well as the lack of appearance of the dhps I431V substitution, which has been reported in West Africa in association with the A581G substitution [23, 24], but is of uncertain clinical significance.
Why did pooled genotyping fail to detect any A581G alleles in the Mpemba placental specimens from ISTp-DP recipients (Figure 1A)? When tested individually, we identified A581G-bearing parasites in 9/66 of these placental specimens. To explore this, for each parasitemia, we computed the product of the molecular parasite density and the proportion of wild-type and mutant allele at codon 581; we then summed these absolute allele densities for each parasitemia within the ISTp group and computed an estimated proportion of alleles. Using this approach, we estimated that parasites bearing the A581G allele comprised 8.62% of the pooled population of placental parasites amongst IPTp recipients, but only 0.04% of those parasites pooled from women who received ISTp. In prior applications [18, 25], pooled amplification and sequencing has not been demonstrated to be sensitive to minority variants comprising below 1% of a mixed infection.
Our study has several limitations. Common dhfr and dhps mutant alleles are nearly fixed in Malawi, and we were able to analyze the effect of SP resistance only as a function of A581G allele frequency and only in 1 study site. However, the epidemiology of SP resistance markers in Malawi is similar to that in other settings in East and Southern Africa, enhancing the generalizability of our findings in this part of Africa. As noted above, some subgroup analyses were limited by the very few women infected with the A581G allele and with the specified coincident outcomes. Therefore we analyzed a range of delivery outcomes to assess for consistency between effects. Finally, errors in Ion Torrent sequencing could have biased results, particularly if error rates are associated with template abundance; to mitigate this known risk, we only interrogated known resistance loci and analyzed only high-quality base calls.
Our results indicate that the effectiveness of IPTp with SP is compromised in women infected with A581G-bearing parasites. However, there was no evidence that SP use exacerbates the adverse effect of malaria infections in women infected with these resistant parasites. These findings, together with reports of the increased spread of this allele [3] and of its association in ecological studies with a loss of IPTp-SP efficacy to prevent LBW [4], underscore the necessity to identify alternative strategies to IPTp-SP for the prevention of malaria-associated LBW in Africa.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to members of the study team: research nurses Mrs Ebbie Chaluluka, Ms Colleta Mphasa, Mrs Alice Luwanda, Ms Edna Pemba, Ms Milness Mangani, and Mrs Elizabeth Kapenuka; data manager Mr Alfred Malili; and laboratory technicians Mr Humphries Malata and Mr Kelvin Kaneka. We also thank the District Health Officers of the Blantyre and Chikwawa districts and their management teams for their continued support and assistance in the conduct of the study within their health facilities; the investigators from the ISTp trial: Anna M. van Eijk, Doreen Ali, Cheryl Pace, James Smedley, Duolao Wang, Arthur Kang’ombe, and Brian Faragher; and Scott Langdon for his technical assistance with parasite genotyping. Ultimately, we are indebted to the pregnant women who participated in the clinical study.
Disclaimer. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the European and Developing Countries Clinical Trials Partnership (grant number IP.2007.31080.003 to F. O. t. K.); the Malaria in Pregnancy Consortium, which was funded through a grant from the Bill and Melinda Gates Foundation (grant number 46099 to F. O. t. K., Liverpool School of Tropical Medicine); the US Centers for Disease Control and Prevention (CDC) cooperative agreement between the CDC Division of Parasitic Diseases and Malaria and the Malaria Epidemiology Unit of the Liverpool School of Tropical Medicine (grant number U01CK000146 to F. O. t. K.); the National Institute of Allergy and Infectious Diseases (grant number K08AI100924 to S. M. T.); and the National Institutes of Health, National Center for Advancing Translational Sciences (grant number UL1TR001117 to S. M. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society of Tropical Medicine and Hygiene Annual Meeting, 13–17 November 2016, Atlanta, GA.
References
- 1. Radeva-Petrova D, Kayentao K, ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst Rev 2014; (10):CD000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desai M, Gutman J, Taylor SM, et al. Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clin Infect Dis 2016; 62:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okell LC, Griffin JT, Roper C. Mapping sulphadoxine-pyrimethamine-resistant Plasmodium falciparum malaria in infected humans and in parasite populations in Africa. Sci Rep 2017; 7:7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Eijk AM, Larsen DA, Kayentao K, et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. Lancet Infect Dis 2019; 19:546–56. [DOI] [PubMed] [Google Scholar]
- 5. Sibley CH, Hyde JE, Sims PF, et al. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol 2001; 17:582–8. [DOI] [PubMed] [Google Scholar]
- 6. Picot S, Olliaro P, de Monbrison F, Bienvenu AL, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J 2009; 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gutman J, Kalilani L, Taylor SM, et al. The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroate synthetase reduces the effectiveness of sulfadoxine-pyrimethamine preventive therapy in Malawian pregnant women. J Infect Dis 2015; 211:1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minja DT, Schmiegelow C, Mmbando B, et al. Plasmodium falciparum mutant haplotype infection during pregnancy associated with reduced birthweight, Tanzania. Emerg Infect Dis 2013; 19:1446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrington WE, Mutabingwa TK, Muehlenbachs A, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci U S A 2009; 106:9027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chico RM, Chaponda EB, Ariti C, Chandramohan D. Sulfadoxine-pyrimethamine exhibits dose-response protection against adverse birth outcomes related to malaria and sexually transmitted and reproductive tract infections. Clin Infect Dis 2017; 64:1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madanitsa M, Kalilani L, Mwapasa V, et al. Scheduled intermittent screening with rapid diagnostic tests and treatment with dihydroartemisinin-piperaquine versus intermittent preventive therapy with sulfadoxine-pyrimethamine for malaria in pregnancy in Malawi: an open-label randomized controlled trial. PLoS Med 2016; 13:e1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor SM, Madanitsa M, Thwai KL, et al. Minimal impact by antenatal subpatent Plasmodium falciparum infections on delivery outcomes in Malawian women: a cohort study. J Infect Dis 2017; 216:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rantala AM, Taylor SM, Trottman PA, et al. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J 2010; 9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor SM, Antonia A, Feng G, et al. Adaptive evolution and fixation of drug-resistant Plasmodium falciparum genotypes in pregnancy-associated malaria: 9-year results from the QuEERPAM study. Infect Genet Evol 2012; 12:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giardine B, Riemer C, Hardison RC, et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res 2005; 15:1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blankenberg D, Von Kuster G, Coraor N, et al. Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol 2010; Chapter 19:Unit 19.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goecks J, Nekrutenko A, Taylor J; Galaxy Team . Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol 2010; 11:R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor SM, Parobek CM, DeConti DK, et al. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis 2015; 211:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrington WE, Mutabingwa TK, Kabyemela E, Fried M, Duffy PE. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis 2011; 53:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cisse M, Awandare GA, Soulama A, Tinto H, Hayette MP, Guiguemdé RT. Recent uptake of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine is associated with increased prevalence of Pfdhfr mutations in Bobo-Dioulasso, Burkina Faso. Malar J 2017; 16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruizendaal E, Tahita MC, Geskus RB, et al. Increase in the prevalence of mutations associated with sulfadoxine-pyrimethamine resistance in Plasmodium falciparum isolates collected from early to late pregnancy in Nanoro, Burkina Faso. Malar J 2017; 16:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huijben S, Macete E, Mombo-Ngoma G, et al. Counter-selection of antimalarial resistance polymorphisms by intermittent preventive treatment in pregnancy. J Infect Dis 2020; 221:293–303. [DOI] [PubMed] [Google Scholar]
- 23. Oguike MC, Falade CO, Shu E, et al. Molecular determinants of sulfadoxine-pyrimethamine resistance in Plasmodium falciparum in Nigeria and the regional emergence of dhps 431V. Int J Parasitol Drugs Drug Resist 2016; 6:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chauvin P, Menard S, Iriart X, et al. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J Antimicrob Chemother 2015; 70:2566–71. [DOI] [PubMed] [Google Scholar]
- 25. Taylor SM, Parobek CM, Aragam N, et al. Pooled deep sequencing of Plasmodium falciparum isolates: an efficient and scalable tool to quantify prevailing malaria drug-resistance genotypes. J Infect Dis 2013; 208:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.