TO THE EDITOR—Coronaviruses (CoV) comprise a large family of zoonotic RNA viruses. Among the 7 members known to infect humans, SARS-CoV-2, the causative agent of COVID-19, together with SARS-CoV and MERS-CoV, cause severe respiratory syndrome. The other 4 members, including NL63, HKU1, OC43, and 229E, are widely circulating in humans but predominantly cause mild respiratory tract illness [1]. Thus we call these 4 viruses low-pathogenic human CoVs (LPH-CoV). Two recent studies by Nickbakhsh et al and Monto et al in The Journal of Infectious Diseases have reported the prevalence of LPH-CoV as 4.0% in western Scotland and 8.3%–16.3% in Michigan, United States [2, 3]. Interestingly, both studies detected the highest frequency of infection in children younger than 5 years. This is the opposite to the COVID-19 pandemic where children are less commonly affected by SARS-CoV-2 [4]. These intriguing findings trigger important hypotheses on whether coinfection with LPH-CoV interferes with SARS-CoV-2 or exposure to LPH-CoV confers cross-protective immunity to some extent.
As COVID-19 is currently affecting the global population and research on LPH-CoV has been largely neglected in the past, we attempted to perform a systematic review and meta-analysis to map the global epidemiology of LPH-CoV. LPH-CoV–related studies from 1990 to March 2020 were systematically searched in Medline, Embase, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), and Google scholar. Studies were included and data extracted only if they reported participants with symptoms of acute respiratory tract infections or influenza like illness. A 95% confidence interval (95% CI) was estimated using Wilson score method. Pooled prevalence (detection rate) was calculated using the DerSimonian-Laird random-effects model with Freeman-Tukey double arcsine transformation.
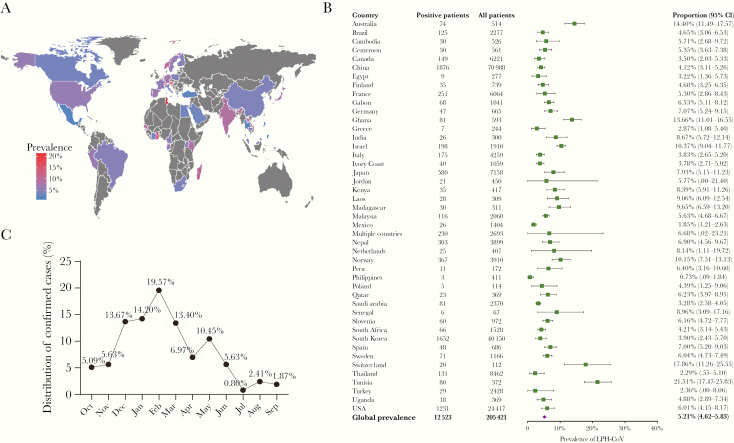
In total, 128 studies with 205 421 individuals were included, and an overall infection rate was estimated as 5.21% (95% CI, 4.62%–5.83%; I2 = 97%). The prevalence of LPH-CoV varied substantially among the reported 44 countries, from 0.73% (Philippines; 95% CI, 0.09%–1.84%) to 21.51% (Tunisia; 95% CI, 17.47%–25.83%) (Figure 1A and 1B). The number of available studies was very limited and many studies had small population sizes. This likely caused bias in prevalence estimations. Furthermore, similar to the studies of Nickbakhsh et al and Monto et al [2, 3], our included studies only detected LPH-CoV in individuals with respiratory illness or symptoms. This suggests that the prevalence rate of LPH-CoV in the general population could be even lower, raising the question of how large the impact could be on COVID-19.
Figure 1.
A, Global prevalence of LPH-CoV. The rate was determined as positive cases in tested populations with respiratory illness or symptoms. B, Forest plot of LPH-CoV prevalence among 44 countries. C, Monthly distribution of confirmed LPH-CoV cases. Abbreviations: CI, confidence interval; LPH-CoV, low-pathogenic human coronavirus.
Monto et al have nicely presented the seasonal distribution of the identified cases in Michigan according to the 4 LPH-CoV types [3]. We performed similar analyses by pooling 5 studies with relevant data, and all these studies were from countries in the northern hemisphere. We confirmed their findings that more cases were detected in the winter season (Figure 1C). However, we are cautious about the interpretation of these seasonal distribution data (Figure 1C) [3] because they only specified the identified cases and not the rate of infection, as the total number of tested cases in each month was not given. More importantly, whether SARS-CoV-2 will develop into a seasonal and/or endemic virus only time will tell [3].
In summary, we have comprehensively estimated the global prevalence of LPH-CoV among 44 countries and mapped their seasonal distribution. Our results further strengthen the epidemiological findings of Nickbakhsh et al and Monto et al, but also raise cautions about the interpretation of existing data. We agree that continued and enhanced monitoring of circulating HLP-CoV is necessary to understand how they may have an impact on the epidemiology and outcome of COVID-19 [5, 6].
Notes
Author contributions. P. L., J. L., and Q. P. designed the project and analyzed the data. P. L. drafted the manuscript. W. M. B. performed database searching. Z. M. and M. P. P. discussed the project and critically revised the manuscript. All authors reviewed the final version of the manuscript and approved for submission.
Disclaimer. Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial support. This work was supported by the Netherlands Organization for Scientific Research (grant number 91719300 to Q. P.); and the China Scholarship Council (grant numbers 201808370170 and 201606240079 PhD fellowships to P. L. and J. L.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis 2002; 185:1338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nickbakhsh S, Ho A, Marques DFP, McMenamin J, Gunson RN, Murcia PR. Epidemiology of seasonal coronaviruses: establishing the context for COVID-19 emergence [published online ahead of print 15 April 2020]. J Infect Dis doi: 10.1093/infdis/jiaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monto AS, DeJonge P, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan [published online ahead of print 4 April 2020]. J Infect Dis doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020; 39:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bedford J, Enria D, Giesecke J, et al. ; WHO Strategic and Technical Advisory Group for Infectious Hazards COVID-19: towards controlling of a pandemic. Lancet 2020; 395:1015–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health 2020; 8:e480. [DOI] [PMC free article] [PubMed] [Google Scholar]