Abstract
Background
Patients with breast cancer (BC) show strong interest in complementary and alternative medicine (CAM), particularly for adverse effects of adjuvant endocrine treatment — e.g., with letrozole. Letrozole often induces myalgia/limb pain and arthralgia, with potential noncompliance and treatment termination. This analysis investigated whether CAM before aromatase inhibitor (AI) therapy is associated with pain development and the intensity of AI-induced musculoskeletal syndrome (AIMSS) during the first year of treatment.
Patients and methods
The multicenter phase IV PreFace study evaluated letrozole therapy in postmenopausal, hormone receptor–positive patients with early BC. Patients were asked about CAM use before, 6 months after, and 12 months after treatment started. They recorded pain every month for 1 year in a diary including questions about pain and numeric pain rating scales. Data were analyzed for patients who provided pain information for all time points.
Results
Of 1396 patients included, 901 (64.5%) had used CAM before AI treatment. Throughout the observation period, patients with CAM before AI treatment had higher pain values, for both myalgia/limb pain and arthralgia, than non-users. Pain increased significantly in both groups over time, with the largest increase during the first 6 months. No significant difference of pain increase was noted regarding CAM use.
Conclusions
CAM use does not prevent or improve the development of AIMSS. Pain intensity was generally greater in the CAM group. Therefore, because of the risk of non-compliance and treatment discontinuation due to the development of higher pain levels, special attention must be paid to patient education and aftercare in these patients.
Keywords: Breast cancer, Endocrine therapy/treatment, Aromatase inhibitors, Letrozole, Integrative medicine, Complementary and alternative medicine, Arthralgia, Myalgia
Highlights
-
•
Pain levels of myalgia/limb pain and arthralgia increase under letrozole intake.
-
•
Within one year pain levels increase in both, CAM users as well as non-CAM users.
-
•
In CAM users pain levels were higher at all time points than in non-CAM users.
-
•
The greatest increase of pain levels was noted in the first six treatment months.
-
•
CAM does not prevent or improve the development of myalgia/limb pain and arthralgia.
1. Introduction
According to current guidelines, postmenopausal women with hormone receptor–positive early breast cancer (BC) should receive adjuvant endocrine treatment — i.e., tamoxifen or an aromatase inhibitor (AI) — in order to reduce the risk of recurrence [1,2]. Since AI therapy is more effective in these patients [[3], [4], [5], [6]], it is a standard treatment in the adjuvant setting [7,8]. However, AIs are known to induce musculoskeletal pain as one of the main side effects [[9], [10], [11], [12], [13]], so that patients often become noncompliant [11] and discontinue treatment [9,[12], [13], [14], [15]]. Noncompliance and early cessation of treatment in turn lead to a poorer prognosis [12,16] — emphasizing the importance of maintaining patients’ compliance and persistence.
Several pharmacological and nonpharmacological methods aimed at improving therapy adherence with AIs have been analyzed [13,[17], [18], [19], [20]]. Analgesics or a switch to another antihormone therapy, for instance, significantly reduced pain [19,21,22]. Providing patients with additional information material does not appear to affect compliance and persistence with anastrozole [23]. Although yoga has been reported to significantly improve musculoskeletal symptoms in various trials [17,19,24,25], there are contradictory results in relation to physical activity [18,19,26,27]; and the same applies to acupuncture [19,20,28,29] and other complementary and alternative medicines (CAM) [19,30,31].
Patients are strongly interested in CAM [32,33] — particularly those who are dissatisfied with the information provided regarding their disease [33]. A cross-sectional study mainly including postmenopausal patients with early breast cancer found that 69% of the patients were physically active, 87% paid attention to nutrition, and 46% used CAM [34]. Almost 50% of postmenopausal BC patients treated with an AI are interested in CAM [33]. An Australian study investigating strategies for managing aromatase inhibitor musculoskeletal syndrome (AIMSS), including CAM, noted only limited effectiveness of CAM in patients who had AIMSS [13].
The aim of this analysis was therefore to investigate whether using CAM before the start of AI treatment is associated with the development of pain and with the severity of AIMSS during the first 12 months of adjuvant letrozole treatment, in postmenopausal patients with hormone receptor–positive breast cancer.
2. Patients and methods
2.1. Patients
The PreFace study is a multicenter, noninterventional and observational phase IV study in which letrozole is being evaluated in postmenopausal, hormone receptor–positive, AI-naïve early BC patients (Clinical Trial Number: NCT01908556). Inclusion criteria were: histologically confirmed hormone receptor–positive, nonmetastatic breast cancer, female patients aged ≥18 years, and postmenopausal status. Patients who had received endocrine treatment for breast cancer with an aromatase inhibitor in the past, or who did not have an indication for letrozole, were excluded.
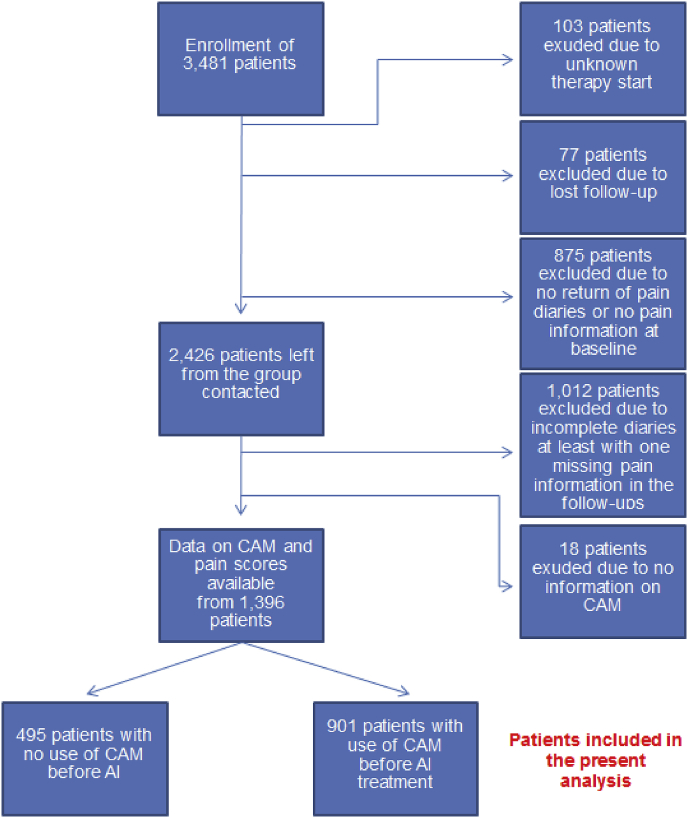
Between 2009 and 2010, 3481 postmenopausal patients were enrolled at 220 sites in Germany. Patients were excluded in the following hierarchical order: unknown documentation of the start of treatment (103 patients excluded); lost to follow-up (77 patients excluded); no return of the pain diary or no pain information at baseline (875 patients excluded); incomplete diary with at least one missing item of pain information during the follow-up (1012 patients excluded); and no information on CAM use (18 patients excluded). A total of 1396 patients with complete datasets were therefore finally included in the analysis (Fig. 1).
Fig. 1.
Patient recruitment algorithm. CAM, complementary and alternative medicine.
Patients received letrozole at 2.5 mg per day. Letrozole treatment was continued for a maximum of 5 years or until recurrence of BC. All of the patients provided written informed consent, and all of the relevant ethics committees approved the study.
2.2. Data acquisition
Data on patient and tumor characteristics were documented in electronic case report forms. The tumor characteristics noted included stage and previous treatments. Patient information included common epidemiological characteristics, comorbidities, and concomitant medication. There were four prespecified study visits after 6, 12, 24, and 60 months from the time of inclusion in the trial. At the follow-up visits, the patients’ disease status was assessed and they were asked about compliance and adverse events. In addition, to assess musculoskeletal side effects during the first year of the treatment, a pain diary including pain maps and numeric rating scales from 0 (no pain) to 10 (very strong pain) was issued to each patient at the time of inclusion. Patients were asked to document any symptoms at monthly intervals. For months 0, 6, and 12 the diary also included questions regarding the use of CAM. The patients were asked each time whether they were currently using or had in the past used vitamins, high-dose vitamin C, food supplements, mistletoe, enzymes, acupuncture, homeopathy, Chinese herbs/tea, mushrooms, meditation, prayer, relaxation techniques, yoga, t’ai chi, qigong, or bioresonance. Only the data at month 0 — i.e. before the start of endocrine treatment — were evaluated for the present analysis.
2.3. Statistical methods
Nonparametric methods were used, as the outcome data were skewed with many zeros. Patients were divided into two groups: one group consisting of patients who had used CAM before the start of AI treatment and the other of patients who were not using CAM.
Statistical tests were performed for patients with complete observations. Myalgia/limb pain and arthralgia were analyzed separately. For each patient, the mean pain score over all assessments (average of 12 values) was calculated. To analyze the influence of CAM on pain during AI treatment, the pain score before the start of AI treatment was subtracted from the mean pain score for all assessments. Both patient groups were then compared using the Wilcoxon rank sum test.
To assess the course of pain, the pain values at month 1 and month 6 were compared within each patient group, as well as the pain values at month 6 and month 12, using Wilcoxon signed rank tests. Significant test results indicated changes in pain during therapy. If both tests within a group were significant, the difference between the pain values at months 1 and 6 was compared with the difference between months 6 and 12 using a Wilcoxon signed rank test. A significant test result shows that the increase or decrease changed during therapy. For each of the two time intervals (the first and second 6-month periods), the difference in the CAM group was compared with the difference in the non-CAM group to assess whether the course of pain development differed between the patient groups, given that both differences had been significant using the Wilcoxon rank sum test.
Mean and median values are shown. Confidence intervals for mean values were determined using 10,000 bootstrap samples. Since the pain values were not symmetrically distributed, the mean values should be interpreted cautiously together with median values.
Pain development relative to CAM status before AI treatment and after 6 months of treatment are shown for descriptive purposes, rather than for hypothesis testing.
All of the tests were two-sided, and a p value < 0.05 was regarded as statistically significant. Calculations were carried out using the R system for statistical computing (version 3.0.1; R Development Core Team, Vienna, Austria, 2013).
3. Results
3.1. Patients
The study population consisted of 1396 patients who completed questionnaires about their pain levels at each of the observation time points and also answered the questions about CAM use. Of these, with a total of 901 patients (64.5%) the majority declared that they had already used CAM before AI treatment start, while 495 patients (35.5%) were non-CAM users.
The patients’ average age was 63.5 (standard deviation 7.4) years in the non-CAM group and 62.6 (SD 7.1) years in the CAM group. Their average body mass index (BMI) was 27.5 kg/m2 (SD 5.4) in the CAM group and 26.8 kg/m2 (SD 4.9) in the non-CAM group. Among the patients in the non-CAM group, 30.7% (n = 141) were currently receiving or had formerly received hormone replacement therapy (HRT), in comparison with 39.9% (n = 343) of those in the CAM group. Most of the patients in both groups had a negative nodal status (pN0) and a low tumor stage (pT1). The characteristics of the patients and tumors are shown in Table 1. Additionally, there did not appear to be any major differences between patients who did not return the patient diary, those who returned it but provided incomplete information about pain levels at the required time points, and those who returned the diary with complete information (Supplementary Table 1).
Table 1.
Characteristics of the patients and tumors.
| Characteristic | No use of CAM before AI treatment (n = 495) |
Use of CAM before AI treatment (n = 901) |
||
|---|---|---|---|---|
| Mean or n | SD or % | Mean or n | SD or % | |
| Age (year) | 63.5 | 7.4 | 62.6 | 7.1 |
| Body mass index (kg/m2) | 27.5 | 5.4 | 26.8 | 4.9 |
| Hormone replacement therapy | ||||
| Never | 319 | 69.3 | 517 | 60.1 |
| Former | 95 | 20.7 | 241 | 28.0 |
| Current | 46 | 10.0 | 102 | 11.9 |
| Neoadjuvant chemotherapy | ||||
| No | 453 | 92.6 | 817 | 91.5 |
| Yes | 36 | 7.4 | 76 | 8.5 |
| Adjuvant chemotherapy | ||||
| No | 337 | 69.5 | 574 | 64.4 |
| Yes | 148 | 30.5 | 317 | 35.6 |
| Lymph-node status | ||||
| pN0 | 348 | 70.7 | 640 | 71.7 |
| pN+ | 144 | 29.3 | 253 | 28.3 |
| Tumor stage | ||||
| pT0 | 8 | 1.6 | 6 | 0.7 |
| pT1 | 323 | 65.7 | 587 | 65.7 |
| pT2 | 138 | 28 | 271 | 30.3 |
| pT3 | 18 | 3.7 | 25 | 2.8 |
| pT4 | 5 | 1.0 | 5 | 0.6 |
| Estrogen receptor | ||||
| Negative | 1 | 0.2 | 15 | 1.7 |
| Positive | 490 | 99.8 | 882 | 98.3 |
| Progesterone receptor | ||||
| Negative | 68 | 13.8 | 120 | 13.4 |
| Positive | 424 | 86.2 | 778 | 86.6 |
| Tumor grade | ||||
| G1 | 106 | 21.5 | 160 | 17.9 |
| G2 | 318 | 64.5 | 559 | 62.4 |
| G3 | 69 | 14.0 | 177 | 19.8 |
AI, aromatase inhibitor; CAM, complementary and alternative medicine.
Means and standard deviation (SD) are shown for continuous characteristics, and frequency and percentage for categorical characteristics.
3.2. Myalgia/limb pain
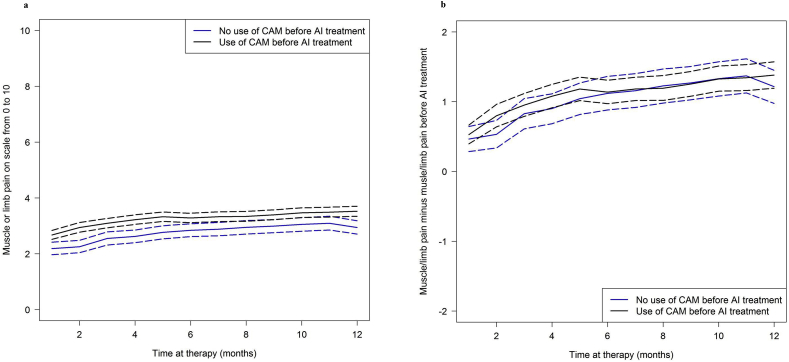
The patient-reported outcomes for myalgia/limb pain during course of treatment are shown in Fig. 2a and summarized in Table 2. Patients who had used CAM before AI treatment had consistently higher pain values than non-users throughout the observation time. The average pain value across all observation time points among the CAM users was 3.3 (95% CI, 3.6 to 3.4; median 3.2), while in the non-CAM users it was 2.8 (95% CI, 2.6 to 3.0; median 2.7). However, the pain values before AI treatment were also higher in the CAM group than in the non-CAM group. The average increase during AI treatment was 1.1 units (95% CI, 1.0 to 1.3) in the CAM group and 1.0 units (95% CI, 0.8 to 1.2) in the non-CAM group (Table 2). No differences in the increases between the two patient groups were found (p = 0.23, Fig. 2b).
Fig. 2.
Myalgia/limb pain during the course of treatment. Solid curves show (a) mean pain values and (b) mean pain value changes since the start of aromatase inhibitor (AI) treatment. The corresponding 95% confidence intervals are indicated by the lines with long dashes. CAM, complementary and alternative medicine.
Table 2.
Overall pain across all time points.
| Outcome | No use of CAM before AI treatment (n = 495) |
Use of CAM before AI treatment (n = 901) |
||
|---|---|---|---|---|
| Mean (95% CI) | Median (IQR) | Mean (95% CI) | Median (IQR) | |
| Myalgia/limb pain | ||||
| Average throughout AI treatmenta | 2.8 (2.6, 3.0) | 2.7 (0.5, 4.5) | 3.3 (3.1, 3.4) | 3.2 (1.4, 4.9) |
| Before AI treatment | 1.7 (1.5, 1.9) | 0.0 (0.0, 3.0) | 2.1 (2.0, 2.3) | 1.0 (0.0, 4.0) |
| Differenceb | 1.0 (0.8, 1.2) | 0.8 (0.0, 2.2) | 1.1 (1.0, 1.3) | 0.8 (0.0, 2.6) |
| Arthralgia | ||||
| Average throughout AI treatment | 2.9 (2.7, 3.1) | 3.0 (0.6, 4.9) | 3.4 (3.3, 3.6) | 3.4 (1.5, 5.2) |
| Before AI treatment | 1.8 (1.6, 1.8) | 0.0 (0.0, 3.0) | 2.3 (2.1, 2.4) | 2.0 (0.0, 4.0) |
| Difference | 1.1 (1.0, 1.3) | 0.8 (0.0, 2.3) | 1.2 (1.0, 1.3) | 1.0 (0.0, 2.3) |
AI, aromatase inhibitor; CAM, complementary and alternative medicine; CI, confidence interval(s); IQR, interquartile range.
The mean pain score over 12 pain assessments (one per month) was calculated for each patient. Patients with missing values were excluded.
Difference between the mean pain assessments during AI treatment and the pain assessment before the start of AI treatment.
Myalgia/limb pain values for selected time points are presented in Table 3. In both patient groups myalgia/limb pain values increased over 12 months. However, the strongest increase in both groups was observed within the first 6 months (mean increases 0.7 and 0.6 units, each p < 0.00001, Table 4). Afterwards, the increase was weaker, but still significant, in the CAM group (mean increase 0.2 units, p < 0.001), while in the non-CAM group pain assessments remained almost constant (mean increase 0.1 unit, p = 0.15).
Table 3.
Pain score at selected time points.
| Myalgia/limb pain |
Arthralgia |
|||||||
|---|---|---|---|---|---|---|---|---|
| No use of CAM before AI treatment |
Use of CAM before AI treatment |
No use of CAM before AI treatment |
Use of CAM before AI treatment |
|||||
| Mean (95% CI) | Median (IQR) | Mean (95% CI) | Median (IQR) | Mean (95% CI) | Median (IQR) | Mean (95% CI) | Median (IQR) | |
| Month 1 | 2.2 (2.0, 2.4) | 1 (0, 4) | 2.7 (2.5, 2.8) | 3 (0, 4) | 2.1 (1.9, 2.3) | 1 (0, 4) | 2.7 (2.6, 2.9) | 3 (0, 4) |
| Month 6 | 2.8 (2.6, 3.1) | 3 (0, 5) | 3.3 (3.1, 3.5) | 3 (1, 5) | 3.0 (2.8, 3.2) | 3 (0, 5) | 3.5 (3.3, 3.7) | 3 (1, 5) |
| Month 12 | 2.9 (2.7, 3.2) | 3 (0, 5) | 3.5 (3.3, 3.7) | 3 (1, 6) | 3.2 (3.0, 3.4) | 3 (0, 5) | 3.8 (3.6, 4.0) | 4 (1, 6) |
AI, aromatase inhibitor; CAM, complementary and alternative medicine; CI, confidence interval(s); IQR, interquartile range.
Table 4.
Course of myalgia/limb pain and arthralgia during therapya.
| Myalgia/limb pain | |||||||
| No use of CAM before AI treatment (n = 495) |
Use of CAM before AI treatment (n = 901) |
||||||
| Outcome |
Mean (95% CI) |
Median (IQR) |
p value |
Mean (95% CI) |
Median (IQR) |
p value |
p valueb |
| Difference, month 1 vs. month 6 | 0.7 (0.4, 0.9) | 0 (0, 2) | <0.00001 | 0.6 (0.5, 0.8) | 0 (0, 2) | <0.00001 | 0.98 |
| Difference, month 6 vs. month 12 | 0.1 (−0.1, 0.3) | 0 (−1, 1) | 0.15 | 0.2 (0.1, 0.4) | 0 (−1, 1) | <0.001 | – |
|
p valued |
–c |
0.02 |
|||||
| Arthralgia | |||||||
| No use of CAM before AI treatment (n = 495) |
Use of CAM before AI treatment (n = 901) |
||||||
| Outcome |
Mean (95% CI) |
Median (IQR) |
p value |
Mean (95% CI) |
Median (IQR) |
p value |
p valueb |
| Difference, month 1 vs. month 6 | 0.9 (0.7, 1.1) | 0.0 (0.0, 2.0) | <0.00001 | 0.8 (0.6, 0.9) | 0 (0, 2) | <0.00001 | 0.55 |
| Difference, month 6 vs. month 12 | 0.2 (0.0, 0.4) | 0.0 (−0.5, 1.0) | 0.01 | 0.3 (0.1, 0.4) | 0 (−1, 1) | <0.001 | 0.38 |
| p valued | <0.001 | <0.01 | |||||
AI, aromatase inhibitor; CAM, complementary and alternative medicine; CI, confidence interval(s); IQR, interquartile range.
Positive differences mean increasing pain during the course of therapy, negative differences indicate decreasing pain.
A significant p value shows that pain changes varied between the two patient groups.
No statistical testing was performed, as the prespecified conditions for testing were not fulfilled.
A significant p value shows that the increase in pain became weaker or stronger over the course of time.
3.3. Arthralgia
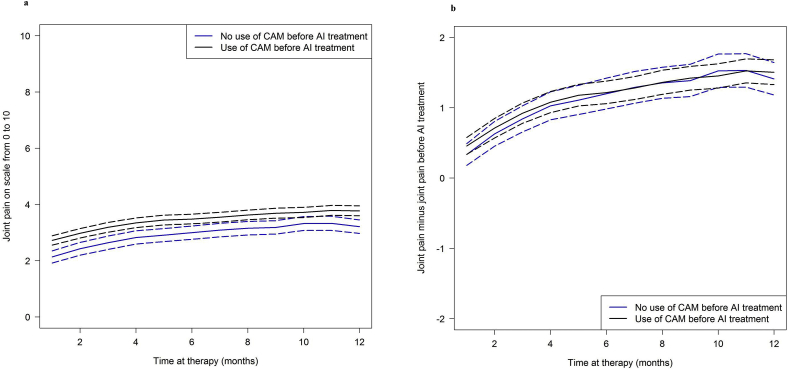
The development of arthralgia was similar to that of myalgia/limb pain. CAM users reported higher pain values than non-CAM users at all time points. Pain values before therapy were also higher among CAM users (Fig. 3a and Table 2). Changes in pain were similar in the two patient groups after taking into account arthralgia assessments before the start of AI treatment (Fig. 3b and Table 2). Pain values of arthralgia also increased in both patient groups over 12 months. The increase in pain was significantly greater in the first 6 months than afterwards in both groups (mean increases 0.9 and 0.8 units, each p < 0.00001, Table 4). Between months 6 and 12 it was also significant, but weaker (mean increase of 0.2 units in non-CAM users and 0.3 units in CAM users, p 0.01 and p < 0.001, respectively, Table 4). There was no evidence of varyingly strong pain increases between the two patient groups, either in the first 6 months or in the second 6 months of the observation period (Table 4).
Fig. 3.
Arthralgia during the course of treatment. Solid curves show (a) mean pain values and (b) mean pain value changes since begin of aromatase inhibitor (AI) treatment. The corresponding 95% confidence intervals are indicated by the lines with long dashes. CAM, complementary and alternative medicine.
3.4. Myalgia/limb pain and arthralgia in the course of therapy relative to CAM status
In an exploratory analysis, the pain levels over time were plotted relative to CAM status before AI treatment and after 6 months (Suppl. Fig. 1a and 1b). In general, patients who did not use CAM before and in the first 6 months of AI treatment showed constantly the lowest levels of pain compared to those who used CAM at some point of time. Patients who did not use CAM before AI treatment, but used it later on, did not experience a serious reduction in myalgia/limb pain or arthralgia. Conversely, patients who first used CAM and later stopped it due to reduced symptoms showed a renewed increase in pain, both in myalgia/limb pain and arthralgia.
4. Discussion
This study shows that patients who were CAM users before AI treatment had generally higher pain values — for myalgia/limb pain as well as for arthralgia — than non-users throughout the observation period. An increase in pain levels of myalgia/limb pain and arthralgia was registered in both patient groups over time, particularly in the first 6 months. Afterwards, pain increase was weaker, but still significant in both groups, except for the non-CAM users regarding myalgia/limb pain. CAM use did not appear to be associated with different changes in pain increase over time. However, a large proportion of the patients, at 64.5%, reported ongoing CAM at the time of diagnosis of breast cancer and before the start of AI treatment.
Few data are available concerning the association between CAM use before the onset of AIMSS and the development of musculoskeletal pain afterwards, in order to support the treatment of postmenopausal breast cancer patients who are receiving adjuvant aromatase inhibitor therapy. To the best of our knowledge, the PreFace study is the first that has examined this association during the first 12 months of adjuvant letrozole therapy. The level of CAM use, at about 64.5% of the patients included, lies within the range of what other analyses have reported [35]. Prevalence rates of CAM use in breast cancer range from 63% to 83% [33,36,37].
It needs to be investigated why patients who use CAM have higher pain levels before the beginning of AI therapy as well as in the course of the first treatment year. It might be hypothesized that CAM users a priori have a greater susceptibility to pain, leading to a larger percentage of CAM users in this patient population. CAM users might also perhaps have more precise self-perception and be more attentive to themselves and their body — so that these patients might be more attracted to integrative therapy methods in case of myalgia/limb pain and arthralgia than patients without pain. CAM users might therefore be patients with a specific character profile and personality traits who are liable to use CAM in order to improve their quality of life. There are few data in the literature on this aspect [[38], [39], [40], [41], [42], [43]]. A large study including 3032 adults aged 25–74 in the USA found that CAM use was associated with the diagnosis of mental disorders, such as major depression and panic disorders [38]. A cohort study have shown a positive association between alternative medicine and depression, fear of recurrence of cancer, mental distress, sexual dissatisfaction and physical complaints [44]. These aspects all show that there is a need for further research on this topic.
We could show that CAM use before AI therapy does not prevent the incidence of AIMSS or improve the time-related development of pain. Since the expectations of many CAM users that CAM might reduce AIMSS remain unsatisfied, these women are at a higher risk of non-compliance, therapy discontinuation and an associated worse prognosis [45]. In a large retrospective observational study patients with CAM use were more likely to refuse conventional cancer treatment and had therefore a higher mortality risk [46]. In view of that, patients using CAM should receive an additional education and the offer of other therapeutic support. As the same standards of antihormonal therapy applies in Western industrialized countries in general and the use of CAM is also common there, the results of this study can be transferred to other industrialized countries.
This study has several strengths and limitations. One strength is the large number of patients included and recruited throughout Germany. However, a substantial number of patients were excluded for various reasons; approximately 32.8% of the patients were excluded by the study team for several reasons. This may have led to a selection bias. In addition, usage of analgesics was not included in the analysis, and this could have influenced the pain scores. Furthermore, while our patients were enrolled between 2009 and 2010, CAM use gained even more attention in the following years. Searching for the terms “complementary and alternative medicine” and “breast cancer” in PubMed, it delivers results from 1998 to 2019, with the majority of them being published in the past decade. Therefore, considering the gap of ten years between our patient recruitment and publication of results, a similar investigation today may lead to other results due the increased popularity of CAM over the last years. But this remains a hypothesis. A benefit is that the diary was able to collect detailed and extensive information from the patients over the study period. The use of a diary to obtain a history of patient information is unique in medical research. Pain cards and pain scales were used as validated instruments to record pain locations and scores.
5. Conclusion
In conclusion, this study demonstrated that the intensity of pain was generally greater in the CAM group than in the non-CAM group. However, changes in pain increase appeared to be similar in the two patient groups within the first 12 months. Thus, CAM use does not prevent or improve the development of AIMSS. But a large proportion of postmenopausal breast cancer patients (64%) make use of ongoing CAM after a diagnosis of breast cancer. Therefore, because of the risk of non-compliance and treatment discontinuation due to the development of higher pain levels, special attention must be paid to patient education and aftercare in these patients.
Funding
This work was supported in part by Novartis Pharma GmbH Germany (no grant number).
Declaration of competing interest
S·Y.B. has received honoraria from Pfizer and Novartis. W.J. has received honoraria and research grants from Novartis. A.D.H. has received honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene, and Pfizer. R.W. has received honoraria and research funds from Novartis. S.K. has received honoraria from Roche, Celgene, Amgen, and AstraZeneca and funding support from Roche. C.T. has received honoraria from Novartis, Pfizer, and AstraZeneca. M.P.L. has participated on advisory boards for AstraZeneca, MSD, Novartis, Pfizer, Genomic Health, and Roche and has received honoraria for lectures from Lilly, Roche, Novartis, Pfizer, Genomic Health, AstraZeneca, medac, and Eisai. P.G. has received honoraria from Novartis and financial support for symposia from Novartis, Roche, and PharmaMar. H.-C.K. has received honoraria from Carl Zeiss meditec, TEVA, Theraclion, Novartis, Amgen, Astra Zeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, Roche, and Genomic Health. D.S.-B. has received honoraria from Novartis. M.W.B.‘s institution has received research grants from Novartis. T.F. has received honoraria from Pfizer, Novartis, Roche, and Amgen. P.A.F. has received honoraria from Roche, Pfizer, Novartis, and Celgene. His institution conducts research for Novartis. N.N. has received honoraria from Janssen-Cilag, Novartis and Teva.
All of the remaining authors have declared that they have no conflicts of interest.
Acknowledgments
We are grateful to all of the patients, participating study sites, and the study personnel involved. Novartis GmbH Germany provided financial support for the conduct of the clinical study and the publication. All of the analyses, as well as the writing of the manuscript, were performed independently of Novartis. The data for the analysis are completely owned by the authors and investigators. The contribution of L. Willer to this publication was made in partial fulfillment of the requirements for obtaining the doctoral degree “Dr. med.“. Parts of the research published here have been used for her doctoral thesis in the Medical Faculty of Friedrich Alexander University of Erlangen–Nuremberg (FAU).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.12.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.The German Guideline Programme in Oncology (German Cancer Society, German Cancer Aid, AWMF). S3-Guideline on Diagnostics, Therapy and Follow-up of Breast Cancer, long version 4.0 (2017), AWMF registration number: 032–045OL, http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom.
- 2.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer (version 4.2017). http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed on February 13, 2017).
- 3.Early Breast Cancer Trialists’ Collaborative G, Dowsett M., Forbes J.F. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 4.Breast International Group 1-98 Collaborative Group, Thurlimann B., Keshaviah A. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 5.Baum M., Budzar A.U., Cuzick J. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 6.Colleoni M., Giobbie-Hurder A., Regan M.M. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29:1117–1124. doi: 10.1200/JCO.2010.31.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mobus V., Hell S., Schmidt M. Assessing the clinical benefit of systemic adjuvant therapies for early breast cancer. Geburtshilfe Frauenheilkd. 2017;77:1079–1087. doi: 10.1055/s-0043-119542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Untch M., Huober J., Jackisch C. Initial treatment of patients with primary breast cancer: evidence, controversies, consensus: spectrum of opinion of German specialists at the 15th international st. Gallen breast cancer conference (Vienna 2017) Geburtshilfe Frauenheilkd. 2017;77:633–644. doi: 10.1055/s-0043-111601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stearns V., Chapman J.A., Ma C.X. Treatment-associated musculoskeletal and vasomotor symptoms and relapse-free survival in the NCIC CTG MA.27 adjuvant breast cancer aromatase inhibitor trial. J Clin Oncol. 2015;33:265–271. doi: 10.1200/JCO.2014.57.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckwee D., Leysen L., Meuwis K., Adriaenssens N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Support Care Cancer. 2017;25:1673–1686. doi: 10.1007/s00520-017-3613-z. [DOI] [PubMed] [Google Scholar]
- 11.Hadji P., Jackisch C., Bolten W. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann Oncol. 2014;25:372–377. doi: 10.1093/annonc/mdt513. [DOI] [PubMed] [Google Scholar]
- 12.Chirgwin J.H., Giobbie-Hurder A., Coates A.S. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34:2452–2459. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lombard J.M., Zdenkowski N., Wells K. Aromatase inhibitor induced musculoskeletal syndrome: a significant problem with limited treatment options. Support Care Cancer. 2016;24:2139–2146. doi: 10.1007/s00520-015-3001-5. [DOI] [PubMed] [Google Scholar]
- 14.Henry N.L., Azzouz F., Desta Z. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabieva N., Kellner S., Fehm T. Influence of patient and tumor characteristics on early therapy persistence with letrozole in postmenopausal women with early breast cancer: results of the prospective Evaluate-TM study with 3941 patients. Ann Oncol. 2018;29:186–192. doi: 10.1093/annonc/mdx630. [DOI] [PubMed] [Google Scholar]
- 16.Scharl A., Salterberg A. Significance of ovarian function suppression in endocrine therapy for breast cancer in pre-menopausal women. Geburtshilfe Frauenheilkd. 2016;76:516–524. doi: 10.1055/s-0042-106389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peppone L.J., Janelsins M.C., Kamen C. The effect of YOCAS(c)(R) yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy. Breast Canc Res Treat. 2015;150:597–604. doi: 10.1007/s10549-015-3351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin M.L., Cartmel B., Gross C.P. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104–1111. doi: 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts K., Rickett K., Greer R., Woodward N. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early Breast cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;111:66–80. doi: 10.1016/j.critrevonc.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Chen L., Lin C.C., Huang T.W. Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast. 2017;33:132–138. doi: 10.1016/j.breast.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Thorne C. Management of arthralgias associated with aromatase inhibitor therapy. Curr Oncol. 2007;14(Suppl 1):S11–S19. doi: 10.3747/co.2007.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briot K., Tubiana-Hulin M., Bastit L. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Canc Res Treat. 2010;120:127–134. doi: 10.1007/s10549-009-0692-7. [DOI] [PubMed] [Google Scholar]
- 23.Hadji P., Blettner M., Harbeck N. The Patient’s Anastrozole Compliance to Therapy (PACT) Program: a randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol. 2013;24:1505–1512. doi: 10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen P.B., Muchnick S., Marcus S. Pilot study of Iyengar yoga for management of aromatase inhibitor-associated arthralgia in women with breast cancer. Psycho Oncol. 2015;24:1578–1580. doi: 10.1002/pon.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galantino M.L., Greene L., Archetto B. A qualitative exploration of the impact of yoga on breast cancer survivors with aromatase inhibitor-associated arthralgias. Explore. 2012;8:40–47. doi: 10.1016/j.explore.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Lash B.W., Katz J., Gilman P. Feasibility of a defined exercise program for aromatase inhibitor-related arthralgia (AIRA) in breast cancer survivors. J Clin Oncol. 2011;29 [Google Scholar]
- 27.Lohrisch C.A., McKenzie D., Truong P. A randomized trial of exercise versus control for musculoskeletal symptoms from adjuvant anastrozole (A) for postmenopausal early breast cancer (PEBC) J Clin Oncol. 2011;29 [Google Scholar]
- 28.Bao T., Cai L., Giles J.T. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Canc Res Treat. 2013;138:167–174. doi: 10.1007/s10549-013-2427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crew K.D., Capodice J.L., Greenlee H. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28:1154–1160. doi: 10.1200/JCO.2009.23.4708. [DOI] [PubMed] [Google Scholar]
- 30.Prieto-Alhambra D., Javaid M.K., Servitja S. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Canc Res Treat. 2011;125:869–878. doi: 10.1007/s10549-010-1075-9. [DOI] [PubMed] [Google Scholar]
- 31.Hershman D.L., Unger J.M., Crew K.D. Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol. 2015;33:1910–U1978. doi: 10.1200/JCO.2014.59.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fremd C., Hack C.C., Schneeweiss A. Use of complementary and integrative medicine among German breast cancer patients: predictors and implications for patient care within the PRAEGNANT study network. Arch Gynecol Obstet. 2017;295:1239–1245. doi: 10.1007/s00404-017-4348-2. [DOI] [PubMed] [Google Scholar]
- 33.Hack C.C., Fasching P.A., Fehm T. Interest in integrative medicine among postmenopausal hormone receptor-positive breast cancer patients in the EvAluate-TM study. Integr Cancer Ther. 2016;16(2):165-175 doi: 10.1177/1534735416668575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Templeton A.J., Thurlimann B., Baumann M. Cross-sectional study of self-reported physical activity, eating habits and use of complementary medicine in breast cancer survivors. BMC Canc. 2013;13:153. doi: 10.1186/1471-2407-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horneber M., Bueschel G., Dennert G. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11:187–203. doi: 10.1177/1534735411423920. [DOI] [PubMed] [Google Scholar]
- 36.DiGianni L.M., Garber J.E., Winer E.P. Complementary and alternative medicine use among women with breast cancer. J Clin Oncol. 2002;20:34S–38S. [PubMed] [Google Scholar]
- 37.Molassiotis A., Fernadez-Ortega P., Pud D. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 38.Honda K., Jacobson J.S. Use of complementary and alternative medicine among United States adults: the influences of personality, coping strategies, and social support. Prev Med. 2005;40:46–53. doi: 10.1016/j.ypmed.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Sirois F.M., Purc Stephenson R.J. Personality and consultations with complementary and alternative medicine practitioners: a five-factor model investigation of the degree of use and motives. J Altern Complement Med. 2008;14:1151–1158. doi: 10.1089/acm.2007.0801. [DOI] [PubMed] [Google Scholar]
- 40.Toivonen K.I., Tamagawa R., Speca M. Open to exploration? Association of personality factors with complementary therapy use after breast cancer treatment. Integr Cancer Ther. 2018;17(3):785-792 doi: 10.1177/1534735417753539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connor C., Braun Y., Nota S.P. The association of complementary Health approaches with mood and coping strategies among orthopedic patients. Hand (N Y) 2016;11:295–302. doi: 10.1177/1558944715620798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson R., Geoghegan L., McLaughlin L., Woodward R. Psychological characteristics of cancer patients who use complementary therapies. Psycho Oncol. 2005;14:187–195. doi: 10.1002/pon.834. [DOI] [PubMed] [Google Scholar]
- 43.Moschen R., Kemmler G., Schweigkofler H. Use of alternative/complementary therapy in breast cancer patients--a psychological perspective. Support Care Cancer. 2001;9:267–274. doi: 10.1007/s005200000208. [DOI] [PubMed] [Google Scholar]
- 44.Burstein H.J., Gelber S., Guadagnoli E., Weeks J.C. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med. 1999;340:1733–1739. doi: 10.1056/NEJM199906033402206. [DOI] [PubMed] [Google Scholar]
- 45.Johnson S.B., Park H.S., Gross C.P., Yu J.B. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4(10):1375-1381 doi: 10.1001/jamaoncol.2018.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson S.B., Park H.S., Gross C.P., Yu J.B. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4:1375–1381. doi: 10.1001/jamaoncol.2018.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.