CASE PRESENTATION
A 21-year-old woman with unknown family history presented with abdominal pain, mild hypercalcemia (10.6 mg/dL), and an elevated CA125 concentration of 170 U/mL. Exploratory laparotomy revealed a 15-cm right adnexal mass, a 6-cm left adnexal mass, and free intrapelvic fluid suggestive of spontaneous mass rupture. Optimal cytoreductive surgery was performed with bilateral salpingo-oophorectomy, pelvic and para-aortic node dissection, and omentectomy. Pathologic examination revealed small-cell carcinoma of the ovary, hypercalcemic type (SCCOHT) with a large-cell component in bilateral ovaries and para-aortic nodes. SMARCA4/BRG1 immunohistochemical expression was lost. Germline genetic testing demonstrated a deleterious SMARCA4 mutation c.1408C>T (pQ470*) and a variant of uncertain significance in SMARCA4 c.1432A>T (p.S478C). Somatic tumor testing by Clinical Laboratory Improvement Amendments–certified next-generation sequencing (Oncopanel1,2) showed a tumor mutational burden of 6 mutations/megabase, mismatch repair proficiency, and both SMARCA4 alterations. Additional variants of uncertain significance were detected in AKT2 c.573+8C>T (); HABP2 c.420C>T (p.H140H); MLH3 c.2837C>T (p.S946F); MUTYH c.1109C>T (p.A370V); NTHL1 c.793G>A (p.A265T); PDGFRA c.1460G>A (p.R487H); RARA c.1301G>A (p.G434E); RECQL4 c.386C>T (p.P129L); and SH2B3 c.1354G>A (p.A452T).
She received six cycles of adjuvant platinum-based therapy with only one focus of residual disease in the pelvis (Fig 1). She proceeded to high-dose chemotherapy followed by autologous stem cell transplant (autoSCT). A focus of disease persisted posttransplant, prompting complete surgical resection of an isolated retroperitoneal rectovaginal nodule. The patient’s disease quickly progressed postoperatively and she underwent consolidative radiation therapy to the pelvis and para-aortic nodes. This was followed by treatment with nivolumab and ipilimumab, with a complete response after four cycles. The patient continued receiving nivolumab maintenance; however, her disease recurred within 2 months, prompting rechallenge of combination nivolumab and ipilimumab. A mixed response was seen after five cycles of therapy, with progression in a pelvic lesion, mixed response in retroperitoneal nodes, and development of a new left supraclavicular lesion.
FIG 1.

Treatment history and imaging correlates. Key images highlight areas of disease, including a left supraclavicular nodal conglomeration, a retroperitoneal node, and a right-side abdominal sidewall mass. Arrowheads highlight active nodal disease. Within the first Mid-Abemaciclib/Nivolumab scan (top row), the sole area of disease is the left supraclavicular nodal mass (arrowhead); the other fluorodeoxyglucose-avid areas are sites of brown fat activation. AutoSCT, autologous stem cell transplant; EBRT, external beam radiotherapy.
Therapy was changed to ponatinib, with continued progression of disease, including a new paracolic mass. Treatment was changed to abemaciclib and nivolumab, with significant response in nodal disease after 2 months. The paracolic mass decreased in size but increased in fluorodeoxyglucose avidity, initially thought to reflect treatment response and possible immunotherapy-associated inflammation. After an additional 3 months of treatment, scans demonstrated progression of the paracolic mass and new external iliac nodes. Notably, all other previously seen sites of disease had resolved. Because of concern for resistance to abemaciclib in the oligoprogressive paracolic mass, therapy was switched to olaparib with continued nivolumab.
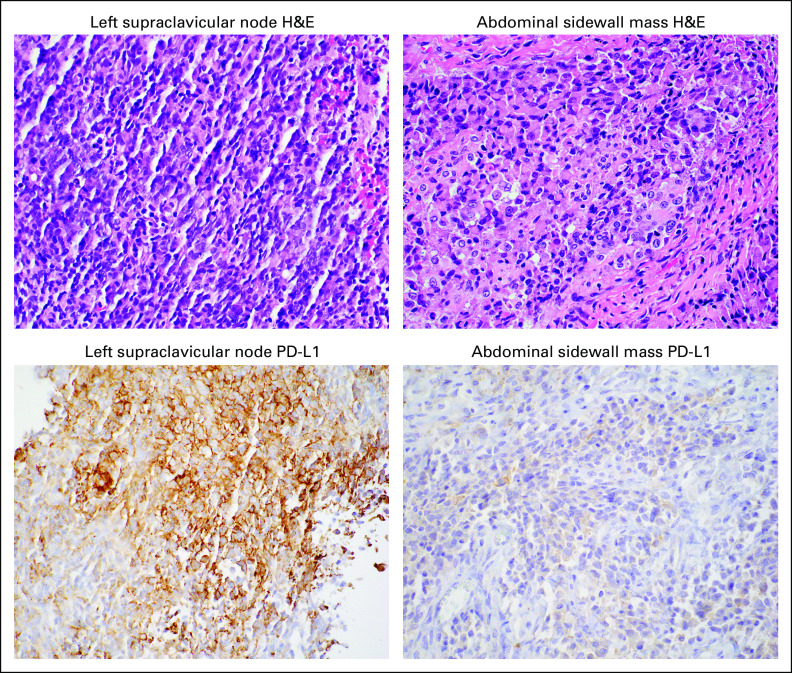
Olaparib was discontinued after 3 weeks because of intolerance, and nivolumab monotherapy was continued. Restaging scans demonstrated resolution of the external iliac nodes but continued progression in the paracolic mass, for which the patient received radiation therapy. Postradiation scans showed marked decrease in the right paracolic mass and no evidence of disease elsewhere. She underwent resection of the mass, which was found to be a retroperitoneal abdominal sidewall mass. There was no gross evidence of disease elsewhere in the abdomen or pelvis. Pathology was consistent with her prior tumor specimens, with marked treatment effect. Tumor cells were negative for PD-L1 and Rb expression was intact (Fig 2).
FIG 2.
PD-L1 loss in oligoprogressive abdominal sidewall mass. (Top row) Representative hematoxylin and eosin (H&E) stains from the left supraclavicular node metastasis, obtained before treatment with combination abemaciclib and nivolumab, and the resected abdominal sidewall mass. (Bottom row) Representative PD-L1 immunohistochemical stains. PD-L1 staining was positive (10% expression in tumor cells) in the left supraclavicular node metastasis and subsequently lost (< 1% expression in tumor cells) in the resected abdominal sidewall mass after abemaciclib/nivolumab treatment.
One month postoperatively, the patient resumed treatment with abemaciclib and remains on combination treatment with nivolumab after 4 months and currently almost 3 years since her initial diagnosis. This patient provided written informed consent allowing discussion of her case and inclusion of pertinent images in this report.
SCCOHT: PATHOGENESIS AND HISTORICAL TREATMENT
SCCOHT is a rare, aggressive malignancy primarily affecting young women; mean age at diagnosis is 24 years.3 Tumors are large, averaging 15.7 cm,4 and hypercalcemia is present in approximately 65% of patients. The hypercalcemia is theorized to be secondary to parathyroid hormone–related protein secretion,3,5 though symptomatic hypercalcemia is less common.6 Approximately one half of patients are diagnosed with advanced-stage disease.3,7-9 Prognosis is poor; one study reported a mean overall survival (OS) declining from 35 months for stage I disease to 3.3 months for stage IV disease, with 75% of all patients experiencing disease relapse.4
SCCOHT is associated with germline and somatic SMARCA4 mutations, with biallelic inactivation occurring in 25% to 100% of patients in small case series.10,11 To date, 96 unique pathogenic SMARCA4 mutations have been described in SCCOHT,11 of which 36% are frameshift mutations, 32% stop/nonsense, 20% splice-site, 5.9% missense, and 5.1% in-frame deletion alterations. SMARCA4 encodes an ATPase required for the formation of the SWI/SNF complex and imparts the complex’s ability to target specific genomic loci.12 The presence of a nonfunctional SMARCA4 is sufficient for localization to some premarked enhancer and transcription sites; however, intact ATPase activity is required for access to remaining sites. SCCOHT is typified by genomic stability, with a low tumor mutational burden and few chromosomal abnormalities.13,14 Despite this, these tumors have immunogenic microenvironments with strong PD-L1 expression and increased tumor-infiltrating lymphocytes (TILs).15
Given the rarity of this malignancy, treatment beyond cytoreductive surgery is heterogeneous and unstandardized. Multiagent chemotherapy regimens vary widely, generally including a platinum agent with taxanes, etoposide, vinblastine, cyclophosphamide, doxorubicin, or bleomycin; external beam radiation may be useful in certain circumstances.4-6,8,16-18 High-dose chemotherapy with autologous stem cell rescue may improve OS. In a prospective SCCOHT trial of 10 patients treated with the PAVEP regimen (ie, cisplatin, doxorubicin, etoposide, and cyclophosphamide), followed by consolidative chemotherapy with autoSCT, seven were long-term survivors.19 In another prospective trial of four patients receiving chemotherapy and autoSCT, all were in complete remission at follow-up of 7 to 73 months.20 A retrospective study reported a 5-year OS of 71% in stage II-IV SCCOHT for those undergoing autoSCT, compared with 25% for those treated with adjuvant chemotherapy alone (P = .002).5 Ultimately, however, there is no treatment regimen specifically approved for SCCOHT.
MOLECULAR TUMOR BOARD CASE DISCUSSION AND PERSPECTIVES ON MATCHED THERAPIES
To our knowledge, this is the first report of this SMARCA4 germline mutation in SCCOHT11,21 and the first report of treatment with either ponatinib or combination CDK4/6 inhibition and immune checkpoint blockade (ICB) in SCCOHT. Because SMARCA4 is not a directly druggable target in SCCOHT, therapies aim to either exploit or offset the consequent epigenetic dysregulation of SMARCA4 loss.
ICB
We treated this patient early in her course with off-label ICB on the basis of literature showing increased PD-L1 expression in SCCOHT and an immunologically inflamed tumor microenvironment,15 with anecdotal reports of clinical benefit. In a case series of four patients with SCCOHT,15 three remained disease free for ≥ 1.5 years after treatment with nivolumab. The fourth patient, treated with pembrolizumab, achieved a partial response (PR) of 6 months at the time of case report. Our choice of dual anti–PD-1 and anti-CTLA–4 agents was based on trials in melanoma in which dual ICB was associated with improved outcome compared with ICB monotherapy.22,23
Kinase Inhibition
Our decision to use ponatinib, a multikinase inhibitor approved by the US Food and Drug Administration (FDA) for chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia, was based on in silico screens identifying it as a potent inhibitor of SMARCA4-mutant SCCOHT cell lines with antitumor activity in murine xenograft models.24 Antiproliferative activity is thought to be due, in part, to aberrant transcriptional upregulation of FGFR, a target of ponatinib.25 Furthermore, inhibition of FGFR2, CSF1R, and VEGFR negatively affects the recruitment and survival of myeloid-derived suppressor cells, suggesting a role for ponatinib in modulating the immune microenvironment for antitumor effect.26 Though ponatinib alone was insufficient for disease control in this case, and immunotherapy was not added to ponatinib due to absence of safety data, it is certainly possible that a more immunogenic environment was created by ponatinib, predisposing to increased effectiveness of subsequent immunotherapy with nivolumab and abemaciclib.
Cyclin D1 Deficiency and CDK4/6 Inhibition
Our choice to use abemaciclib, currently FDA approved for the treatment of hormone receptor–positive, HER2-negative advanced breast cancer, was based on preclinical studies showing a SMARCA4-related cyclin D1 deficiency in SCCOHT and opportunity for synthetic lethality with CDK4/6 inhibition.27 Cyclin D1 (CCND11) mRNA and protein expression were significantly reduced in SCCOHT cell lines and patient-derived tumor specimens, concomitant with low inhibitory p16 levels and intact Rb but reduced phospho-Rb levels. In combination with an RNA interference kinome screen, these findings suggested a critical role for CDK4/6. Compromise of CDK4/6 kinase activity through knockdown or palbociclib administration impaired tumor cell growth in vitro and in patient-derived xenograft models. The presence of SMARCA4 at CCND1 promoter regions implicates SMARCA4 in CCND1 transcription (Fig 3). Overall, this work suggests that in SCCOHT, loss of functional SMARCA4 leads to reduced cyclin D1 expression, subsequently impairing the kinase activity of CDK4/6 and exposing an opportunity for synthetic lethality by CDK4/6 inhibition.
FIG 3.

Proposed model of small-cell carcinoma of the ovary, hypercalcemic type (SCCOHT), CDK4/6 inhibition, and increased immunogenicity. At baseline, SCCOHT is associated with upregulated PD-L1 and T-cell infiltration. SMARCA4 deficiency leads to reduced cyclin D1 expression, decreased CDK4/6 kinase activity, and downstream effects on E2F dissociation from Rb and cell cycle progression. In the tumor cell, CDK4/6 inhibition leads to cytostasis and increased antigen presentation. In immune cells, CDK4/6 inhibition decreases expression of inhibitory receptors and increases secretion of chemokines that promote T-cell infiltration and effector function.
In this case, we elected to combine abemaciclib with ICB to capitalize on the underlying immunogenic tumor microenvironment in SCCOHT and on CDK4/6-induced immunogenicity (Fig 3). CDK4/6 inhibitors increase tumor cell antigen presentation through induction of a type III interferon response and upregulation of β2M and MHC class I.28 Concurrently, regulatory T-cell (Treg) proliferation is suppressed, the Treg to CD8+ T-cell ratio decreases, the number of CD3+ and CD4+ T cells increases, and CD8+ T-cell expression of PD-1, Tim-3, CTLA-4, and LAG-3 decreases, all suggesting promotion of an antitumor immune microenvironment.28-30 Intratumoral T-cell infiltration increased, at least partly due to upregulated T-cell trafficking chemokines, in the setting of CDK4/6 inhibition.29,30 Consequently, combination abemaciclib and anti–PD-L1 therapy induced the greatest degree of tumor reduction in murine models of breast cancer compared with monotherapy.28 A separate study evaluated different dosing schedules, finding that overlapping, rather than sequential, administration of abemaciclib and anti–PD-L1 treatment demonstrated the strongest antitumor synergy. Notably, mice having achieved a complete response were able to resist a rechallenge of a second implanted tumor in the absence of active treatment, suggesting that combination therapy imparted durable immunologic memory.29
Epigenetic Reprogramming
As future therapeutic options, we may consider the use of epigenetic modulators, in view of literature suggesting preclinical efficacy in SCCOHT models. SMARCA4-deficient cells have been shown to be dependent on EZH2, the main histone methyltransferase subunit of PRC2, involved in chromatin remodeling.31 Inhibition of EZH2 induced cancer cell death, suppression of tumor growth, and improved survival in vitro and in murine models of SCCOHT.32-34 However, though tazemetostat gained accelerated FDA approval for the treatment of epithelioid sarcoma, the arm accruing patients with SCCOHT in a phase II multiarm trial (ClinicalTrials.gov identifier: NCT02601950) did not pass futility for continued study.35 Of note, 1 patient with SCCOHT was reported to have a PR of 32 weeks.35 One strategy to improve efficacy may lie in combining EZH2 inhibitors with histone deacetylase inhibitors, such as panobinostat or quisinostat, which, in preclinical models, demonstrated greater synergistic antitumor effect than monotherapy, thought to be due to enhanced “reprogramming” of the proteome.33
A similar approach is used with inhibitors of lysine-specific demethylase-1 (LSD1/KDM1), which is upregulated in SWI/SNF-mutated cancers. The LSD1 inhibitor seclidemstat (SP-2577) demonstrated single-agent antitumor activity in murine models; furthermore, it induced antitumor immunity by upregulating PD-L1 expression, generating a type I interferon response, and increasing TILs, providing a basis for combining LSD1 inhibitors with ICB.36,37 Synergistic antitumor activity was shown with combined anti-PD1 therapy and LSD1 inhibition in vivo, greater than single agent treatment.38 Seclidemstat has received FDA fast-track approval for the treatment of Ewing sarcoma, a disease also driven by aberrant transcriptional activity, and is under active study in a phase I trial (ClinicalTrials.gov identifier: NCT03895684) for advanced solid cancers. Bromodomain and extraterminal motif (BET) inhibitors are another potential therapeutic strategy. In vivo studies showed antiproliferative effects of BET inhibition in SMARCA4-deficient SCCOHT, due to downregulation of oncogenic HER3 and other receptor tyrosine kinases.39 BET inhibitors also promote an antitumor response, with decreased regulatory T cells, increased TILs, and synergism in vivo with anti-PD1 therapy.40
In summary, this patient with SMARCA4-deficient SCCOHT demonstrated a response to combination abemaciclib and nivolumab, despite having tumor growth through multiple prior regimens, including chemotherapy, radiation therapy, and immunotherapy with both anti–PD-1 and anti–CTLA-4 therapy. Combination CDK4/6 inhibitor and ICB may be an effective alternative option in our otherwise limited armamentarium against SMARCA4-deficient SCCOHT and possibly other malignancies in which cyclin D1 is similarly suppressed, such as in SMARCA4-deficient non–small-cell lung cancer.41 This case underscores the importance of rigorous preclinical investigation, demonstrates the potential to clinically validate biologically sound preclinical data, and highlights the value of access to innovative therapies.
ACKNOWLEDGMENT
We acknowledge nurse practitioner Susanne Menon for her dedicated care of this patient.
SUPPORT
P.A.K. received support from the National Institutes of Health (U10CA180868, PI: Wolmark, and UM1 CA186709, PIs: Shapiro and Kufe).
The patient provided written informed consent allowing discussion of her case and inclusion of pertinent images.
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth K. Lee, Panagiotis A. Konstantinopoulos
Financial support: Panagiotis A. Konstantinopoulos
Administrative support: Panagiotis A. Konstantinopoulos
Provision of study material or patients: Elizabeth K. Lee, Katharine M. Esselen, Panagiotis A. Konstantinopoulos
Collection and assembly of data: Elizabeth K. Lee, Katharine M. Esselen, David L. Kolin, Ursula A. Matulonis, Panagiotis A. Konstantinopoulos
Data analysis and interpretation: Elizabeth K. Lee, Larissa J. Lee, Ursula A. Matulonis, Panagiotis A. Konstantinopoulos
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Larissa J. Lee
Research Funding: AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Methods and Systems for Quantitative Monitoring of In Vivo Tumor Oxygenation; shared patent filing and intellectual property held by Brigham and Women's Hospital and Massachusetts Institute of Technology (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Ursula A. Matulonis
Honoraria: Advaxis
Consulting or Advisory Role: Merck, Immunogen, Novartis
Research Funding: Merck, Novartis, Tesaro, Syndax, Immunogen, Mersana, Leap Therapeutics, Fujifilm, SQZ Biotechnologies
Travel, Accommodations, Expenses: AstraZeneca
Panagiotis A. Konstantinopoulos
Consulting or Advisory Role: Merck, Vertex, AstraZeneca, Pfizer/EMD Serono, Tesaro, Bayer
Research Funding: Pfizer (Inst), Eli Lilly (Inst), Tesaro (Inst), Merck Serono (Inst), AstraZeneca (Inst), Merck (Inst), Bayer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1:e87062. doi: 10.1172/jci.insight.87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia EP, Minkovsky A, Jia Y, et al. Validation of oncopanel: A targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–758. doi: 10.5858/arpa.2016-0527-OA. [DOI] [PubMed] [Google Scholar]
- 3.Estel R, Hackethal A, Kalder M, et al. Small cell carcinoma of the ovary of the hypercalcaemic type: An analysis of clinical and prognostic aspects of a rare disease on the basis of cases published in the literature. Arch Gynecol Obstet. 2011;284:1277–1282. doi: 10.1007/s00404-011-1846-5. [DOI] [PubMed] [Google Scholar]
- 4. Callegaro-Filho D, Gershenson DM, Nick AM, et al: Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): A review of 47 cases. Gynecol Oncol 140:53-57, 2016. [DOI] [PMC free article] [PubMed]
- 5.Witkowski L, Goudie C, Ramos P, et al: The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol Oncol 141:454-460, 2016. [DOI] [PMC free article] [PubMed]
- 6. doi: 10.1016/j.ygyno.2005.10.024. Harrison ML, Hoskins P, Du Bois A, et al: Small cell of the ovary, hypercalcemic type - analysis of combined experience and recommendation for management. A GCIG study. Gynecol Oncol 100:233-238, 2006. [DOI] [PubMed]
- 7.Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol. 1994;18:1102–1116. doi: 10.1097/00000478-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Nasioudis D, Chapman-Davis E, Frey MK, et al. Small cell carcinoma of the ovary: A rare tumor with a poor prognosis. Int J Gynecol Cancer. 2018;28:932–938. doi: 10.1097/IGC.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 9.Jamy O, Yaghmour G, Hare F, et al. Population-based analysis of the clinical features of primary small cell carcinoma of the ovary. Anticancer Res. 2015;35:3091–3095. [PubMed] [Google Scholar]
- 10. doi: 10.1038/ng.2928. Ramos P, Karnezis AN, Craig DW, et al: Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet 46:427-429, 2014. [DOI] [PMC free article] [PubMed]
- 11. doi: 10.7150/jca.26978. Lu B, Shi H: An in-depth look at small cell carcinoma of the ovary, hypercalcemic type (SCCOHT): Clinical implications from recent molecular findings. J Cancer 10:223-237, 2019. [DOI] [PMC free article] [PubMed]
- 12. Pan J, McKenzie ZM, D’Avino AR, et al: The ATPase module of mammalian SWI/SNF family complexes mediates subcomplex identity and catalytic activity–independent genomic targeting. Nat Genet 51:618-626, 2019. [DOI] [PMC free article] [PubMed]
- 13.Witkowski L, Carrot-Zhang J, Albrecht S, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. 2014;46:438–443. doi: 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- 14. doi: 10.1186/1750-1172-8-33. Gamwell LF, Gambaro K, Merziotis M, et al: Small cell ovarian carcinoma: Genomic stability and responsiveness to therapeutics. Orphanet J Rare Dis 8:33, 2013. [DOI] [PMC free article] [PubMed]
- 15.Jelinic P, Ricca J, Van Oudenhove E, et al. Immune-active microenvironment in small cell carcinoma of the ovary, hypercalcemic type: Rationale for immune checkpoint blockade. J Natl Cancer Inst. 2018;110:787–790. doi: 10.1093/jnci/djx277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pressey JG, Kelly DR, Hawthorne HT. Successful treatment of preadolescents with small cell carcinoma of the ovary hypercalcemic type. J Pediatr Hematol Oncol. 2013;35:566–569. doi: 10.1097/MPH.0b013e318282cca8. [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.1002/1097-0142(19890915)64:6<1183::aid-cncr2820640603>3.0.co;2-n. Senekjian EK, Weiser PA, Talerman A, et al: Vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin, and etoposide in the treatment of small cell carcinoma of the ovary. Cancer 64:1183-1187, 1989. [DOI] [PubMed]
- 18.Wallbillich JJ, Nick AM, Ramirez PT, et al. Vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin, and etoposide (VPCBAE) in the management of three patients with small-cell carcinoma of the ovary. Gynecol Oncol Case Rep. 2012;2:58–60. doi: 10.1016/j.gynor.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pautier P, Ribrag V, Duvillard P, et al. doi: 10.1093/annonc/mdm376. Results of a prospective dose-intensive regimen in 27 patients with small cell carcinoma of the ovary of the hypercalcemic type. Ann Oncol 18:1985-1989, 2007. [DOI] [PubMed] [Google Scholar]
- 20. doi: 10.1002/cncr.22213. Distelmaier F, Calaminus G, Harms D, et al: Ovarian small cell carcinoma of the hypercalcemic type in children and adolescents: A prognostically unfavorable but curable disease. Cancer 107:2298-2306, 2006. [DOI] [PubMed]
- 21. doi: 10.1080/15360280801989377. https://www.ncbi.nlm.nih.gov/clinvar/?term=SMARCA4+c.1408C%3ET National Library of Medicine: ClinVar. [DOI] [PubMed]
- 22.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang JD, Hendricks WPD, Orlando KA, et al. Ponatinib shows potent antitumor activity in small cell carcinoma of the ovary hypercalcemic type (SCCOHT) through multikinase inhibition. Clin Cancer Res. 2018;24:1932–1943. doi: 10.1158/1078-0432.CCR-17-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wöhrle S, Weiss A, Ito M, et al. Fibroblast growth factor receptors as novel therapeutic targets in SNF5-deleted malignant rhabdoid tumors. PLoS One. 2013;8:e77652. doi: 10.1371/journal.pone.0077652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review) Int J Mol Med. 2016;38:3–15. doi: 10.3892/ijmm.2016.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue Y, Meehan B, Macdonald E, et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat Commun. 2019;10:558. doi: 10.1038/s41467-018-06958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. doi: 10.1038/nature23465. Goel S, DeCristo MJ, Watt AC, et al: CDK4/6 inhibition triggers anti-tumour immunity. Nature 548:471-475, 2017. [DOI] [PMC free article] [PubMed]
- 29. Schaer DA, Beckmann RP, Dempsey JA, et al: The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep 22:2978-2994, 2018. [DOI] [PubMed]
- 30. doi: 10.1158/2159-8290.CD-17-0915. Deng J, Wang ES, Jenkins RW, et al: CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov 8:216-233, 2018. [DOI] [PMC free article] [PubMed]
- 31.Kim KH, Kim W, Howard TP, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med. 2015;21:1491–1496. doi: 10.1038/nm.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Chen SY, Karnezis AN, et al. The histone methyltransferase EZH2 is a therapeutic target in small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2017;242:371–383. doi: 10.1002/path.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Chen SY, Colborne S, et al. Histone deacetylase inhibitors synergize with catalytic inhibitors of EZH2 to exhibit antitumor activity in small cell carcinoma of the ovary, hypercalcemic type. Mol Cancer Ther. 2018;17:2767–2779. doi: 10.1158/1535-7163.MCT-18-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan-Penebre E, Armstrong K, Drew A, et al. Selective killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: In vitro and in vivo preclinical models. Mol Cancer Ther. 2017;16:850–860. doi: 10.1158/1535-7163.MCT-16-0678. [DOI] [PubMed] [Google Scholar]
- 35.J ones R, Blay J, Agulnik M, et al: A phase 2, multicenter study of the EZH2 inhibitor tazemetostat in adults (rhabdoid tumor cohort) ( NCT02601950). Ann Oncol 29:viii576–viii595, 2018.
- 36. doi: 10.1371/journal.pone.0235705. Soldi R, Ghosh Halder T, Weston A, et al: The novel reversible LSD1 inhibitor SP-2577 promotes anti-tumor immunity in SWItch/Sucrose-NonFermentable (SWI/SNF) complex mutated ovarian cancer [Internet]. bioRxiv 2020.01.10.902528, 2020. [DOI] [PMC free article] [PubMed]
- 37. Sheng W, LaFleur MW, Nguyen TH, et al: LSD1 Ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 174:549-563.e19, 2018. [DOI] [PMC free article] [PubMed]
- 38. doi: 10.1038/s41388-018-0451-5. Qin Y, Vasilatos SN, Chen L, et al: Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade [Internet]. Oncogene 38:390-405, 2019. [DOI] [PMC free article] [PubMed]
- 39. doi: 10.1158/0008-5472.CAN-18-1545. Shorstova T, Marques M, Su J, et al: SWI/SNF-compromised cancers are susceptible to bromodomain inhibitors. Cancer Res 79:2961-2774, 2019. [DOI] [PubMed]
- 40. doi: 10.1158/2326-6066.CIR-18-0077. Adeegbe DO, Liu S, Hattersley MM, et al: BET bromodomain inhibition cooperates with PD-1 blockade to facilitate antitumor response in kras-mutant non-small cell lung cancer. Cancer Immunol Res 6:1234-1245, 2018. [DOI] [PMC free article] [PubMed]
- 41.Xue Y, Meehan B, Fu Z, et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat Commun. 2019;10:557. doi: 10.1038/s41467-019-08380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]