Abstract
Aims:
Improved prostate cancer (PCa)-specific biomarkers are urgently required to distinguish between indolent and aggressive disease, in order to avoid overtreatment. In this study, we investigated the prostatic tissue expression of secreted frizzled-related protein (SFRP)-2.
Methods and results:
Following immunohistochemical analysis on PCa tissue microarrays with samples from 216 patients, strong/moderate SFRP-2 expression was observed in epithelial cells of benign prostatic hyperplasia, and negative/weak SFRP-2 expression was observed in the majority of tumour epithelia. However, among Gleason grade 5 carcinomas, 40% showed strong/moderate SFRP-2 expression and 60% showed negative SFRP-2 expression in epithelial cells. Further microscopic evaluation of Gleason grade 5 tumours revealed different morphological patterns, corresponding with differential SFRP-2 expression. The first subgroup (referred to as Type A) appeared to have a morphologically solid growth pattern, whereas the second subgroup (referred to as Type B) appeared to have a more diffuse pattern. Furthermore, 100% (4/4) of Type A patients experienced biochemical recurrence, as compared with 0% (0/6) of Type B patients.
Conclusions:
These results imply: (i) that there is a loss of SFRP-2 expression from benign to malignant prostate glands; and (ii) differential SFRP-2 expression among two possible subgroups of Gleason grade 5 tumours.
Keywords: immunohistochemistry, prostate cancer, SFRP-2, tissue microarrays, Wnt signalling
Introduction
Prostate cancer (PCa) is the second most frequently diagnosed cancer in developed countries and the third most common cause of death from cancer in men.1 The heterogeneous nature of the disease results in a broad range of clinical behaviour, from relatively indolent to aggressive metastatic disease.2 Current detection strategies do not reliably detect the disease at an early stage, and cannot distinguish between aggressive and non-aggressive PCa, leading to potential overtreatment of the disease and associated morbidity.
The Wingless (Wnt) signalling pathway is composed of a complex network of proteins that can regulate the production of Wnt signalling molecules. It is involved in diverse biological processes required for embryonic development and adult homeostasis, including determination, proliferation, migration, and differentiation.1 At least three distinct intracellular pathways induce Wnt signals; the Wnt–β-catenin pathway, the Wnt–Ca2+ pathway, and the Wnt–c-Jun N-terminal kinase (JNK) pathway.2 The Wnt–β-catenin pathway is known as the canonical pathway, and the Wnt–Ca2+ and Wnt–JNK pathways are known as the non-canonical pathways.
The canonical Wnt signalling pathway controls cell fate by regulating gene expression.1 It is activated when Wnt molecules bind to a Frizzled (Fzd) receptor and one of two low-density lipoprotein receptor-related protein coreceptors (LRP5 and LRP6).1 This leads to the phosphorylation of three domains of Dishevelled (Dsh), which is a family of cytosolic signal transducer molecules.3 Activation of Dsh results in the phosphorylation and inhibition of glycogen synthase kinase 3, which leads to stabilization and consequently cytosolic accumulation of β-catenin.3 As a result, the accumulated β-catenin enters the nucleus to interact with members of the T-cell factor (TCF)/lymphoid enhancer factor (LEF) transcription factor family (LEF1, TCF1, TCF3, and TCF4) and transcription initiator p300,1 which ultimately leads to the activation of cell proliferation and prosurvival genes.
Two functional classes of soluble extracellular Wnt antagonists regulate Wnt signalling: the secreted frizzled-related proteins (SFRPs), which include SFRP1–SFRP5, Wnt inhibitory factor 1, and Cerberus; and the Dickkopf (DKK) class, which comprises DKK1–DKK4 and a unique DKK1-related protein, DKKL1. Wnt antagonists inhibit Wnt signalling by binding to Wnt ligands via their cysteine-rich domain, preventing them from binding to Fzd receptors.4 They can also inhibit Wnt signalling by binding directly to the Wnt–receptor complex. The expression levels of SFRPs are altered in different types of disease, such as bone pathologies, retinal degeneration, and hypophosphataemic diseases, which suggests that SFRP activity is fundamental for tissue homeostasis.5
Aberrant activation of the Wnt pathway has been shown to be associated with tumour development, tumour progression and metastatic spread in many types of cancer, including PCa.6 Carcinogenesis can result from the functional loss of Wnt antagonists through dysregulation of cell proliferation.4 In PCa, it has been shown that the major player in canonical Wnt signalling, β-catenin, contributes to PCa cell growth and survival, and its dysregulation is thought to contribute to disease progression.6 SFRP-2 has been shown to increase the intracellular concentration of β-catenin and confer antiapoptotic properties.7 The SFRPs have not been directly linked to cancer; however, it could be speculated that the antiapoptotic activity observed with SFRP-2 could contribute to tumour progression.7 SFRP-2 has been identified as an epigenetic target in many cancers, such as colon cancer,8 oesophageal cancer,9 bladder cancer,10 stomach cancer,11 liver cancer,12 lung cancer,13 renal cell carcinoma,4 and breast cancer.14
The aim of this study was threefold: (i) to investigate the prostatic tissue expression of SFRP-2 at both protein and mRNA levels; (ii) to establish whether there was a difference in the expression of SFRP-2 between prostatic tumour tissue and benign prostatic hyperplasia (BPH); and (iii) to determine whether there was a difference in the expression of SFRP-2 across the different Gleason grades of PCa.
Materials and methods
ETHICS STATEMENT
Ethical approval for this study was obtained from the ethics committees of St James’s Hospital, Beaumont Hospital and Mater Misericordiae University Hospital, Dublin.
SAMPLE COLLECTION/TISSUE MICROARRAY (TMA) CONSTRUCTION
Two hundred and sixteen PCa cases (age range 43–74 years) were obtained from the Beaumont Hospital, St James’s Hospital and the Mater Misericordiae University Hospital histopathology archives dating from 2002 to 2008. The 216 cases included a variety of Gleason scores, ranging from 5 to 10. Suitable formalin-fixed paraffin-embedded blocks of each case, representing as many Gleason grades as possible, were selected. The corresponding haematoxylin and eosin (H&E)-stained sections were reviewed by a pathologist. Three 1-mm-diameter cores of BPH and Gleason grade 3, 4 and 5 carcinomas were taken from every case where available. The TMAs were assembled with the Beecher Instruments Tissue-Arrayer (Beecher Instruments, Silver Spring, MD, USA).
For control purposes, BPH lesions from 37 men with no diagnosis of PCa who underwent transurethral resection of the prostate (TURP) were also collected.
In order to ensure that the SFRP-2 expression observed on the TMAs was a true representation of SFRP-2 expression on prostate whole sections, whole sections from 13 of the 216 cases were selected for immunohistochemical investigation. Representative whole sections from these cases containing histologically benign glands and every Gleason grade present were selected for analysis, and for five of the 13 cases representative sections from both lobes of the prostate (left and right) were analysed. Postoperative prostate-specific antigen (PSA) data were available for 22 patients from the cohort (10 of these patients had Gleason grade 5 tumours) to assess for biochemical recurrence (BCR).
IMMUNOHISTOCHEMISTRY (IHC)
Deparaffinization, antigen retrieval and IHC were performed on the paraffin-embedded 4-μm TMA sections on an automated IHC platform (Bond Max; Leica Microsystems, Newcastle upon Tyne, UK). A polymer-based detection system (DS9800; Leica Microsystems) was used with 3′, 3-diaminobenzidine as the chromogen, resulting in a brown end colour. Optimal concentrations of primary antibodies for IHC of 1:100, 1:15 000 and 1:20 were determined for SFRP-2 (anti-SFRP-2 polyclonal HPA002652; Atlas Antibodies, Stockholm, Sweden), PSA (anti-PSA polyclonal A0562; Dako, Glostrup, Denmark), and synaptophysin (anti-synaptophysin monoclonal M0776; Dako), respectively, with the appropriate antigen retrieval methods [ER1 protocol for 10 min, ER1 protocol for 20 min and ER1 protocol for 20 min with citrate buffer (for Bond Max)]. Prior to the study, the SFRP-2 antibody was subjected to western blot analysis against PC-3 cells, BPH cells and the SFRP-2 immunogen used to generate the antibody (kindly donated by Atlas Antibodies), which confirmed specificity for SFRP-2 (data not shown). Sections were counterstained with haematoxylin. The immunohistochemical reactivity of SFRP-2 was scored in a blinded fashion with regard to histological diagnosis by two independent observers (G.O.H. and E.K.). Cores that had <10% prostatic epithelium were excluded.
SCORING OF SFRP-2 PROTEIN EXPRESSION
Immunoreactivity for SFRP-2 was assessed in BPH epithelium and tumour epithelium across all Gleason grades. For the purpose of statistical analysis, immunoexpression in 10% or more cells was required for a positive score, and was graded according to the following scale: 0, no staining; 1, faint but clearly detectable staining in >10% of epithelial cells; 2, moderate staining in >10% of epithelial cells; and 3, strong staining in >10% of epithelial cells.
STATISTICAL ANALYSIS
Often, more than one histological grade of PCa was present on a single TMA core. Because of this, individual cores were not scored for immunohistochemical staining as a whole; instead, the different histological areas present on the core representing more than 10% of the core were graded separately. Thus, altogether, 1603 histological areas/Gleason grades were scored for each variable (BPH, 699; Gleason grade 3, 439; Gleason grade 4, 321; and Gleason grade 5, 144).
For statistical analysis of immunoreactivity of SFRP-2 versus histopathological features, the staining intensity of the epithelial cells was divided into two groups: low expression (immunohistochemical score of 0 or 1) included those with negative or weak staining, and high expression (immunohistochemical score of 2 or 3) included those with moderate or strong reactivity. Chi-square tests were performed on contingency tables with GraphPad instat-3 software version 3.02 (GraphPad Software, San Diego, CA, USA) to test the association of the PCa samples, BPH samples and tumour grade with immunohistochemical score [positive (2/3) and negative (0/1)].
LASER CAPTURE MICRODISSECTION
To validate the immunohistochemical results, eight of the 216 PCa cases were selected for mRNA analysis. These cases were selected because areas of BPH, and all Gleason grades of tumour (Gleason grades 3, 4, and 5) were present in each of the eight cases selected. Seven-micrometre whole sections were mounted on uncharged slides, dewaxed, and H&E-stained. Pure cell populations from areas of BPH and Gleason grades 3, 4 and 5 were laser capture microdissected (PixCell II System; Acturus Engineering, Mountain View, CA, USA) according to a standard protocol: laser spot size, 30 μm; pulse power, 40 mW; pulse width, 1.5 ms; and threshold voltage, 285 MV.
TOTAL RNA EXTRACTION AND TAOMAN POLYMERASE CHAIN REACTION (PCR) ANALYSIS
Total RNA was extracted from the captured cells with the RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions, and quantified with a Nanodrop Spectrophotometer (ND-1000; Labtech International, East Sussex, UK). By use of a high-capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA), RNA was reverse transcribed to single-stranded cDNA in 25-μl reactions. The context sequence for SFRP-2 used for real-time PCR was CAGCCACCGAGGAAGCTCCAAAGGT. An Applied Biosystems TaqMan PreAmp Master Mix Kit was used to increase the quantity of cDNA for the gene expression study.
TaqMan gene expression assays were performed on the ABI Prism 7000 sequence detection system (Applied Biosystems), with the following cycles: 2 min at 50°C, 10 min at 95°C, and 40 cycles each at 95°C for 15 s and 60°C for 1 min. The 2−ΔΔCt method was used for analysis of the relative gene expression data, with the gene for cyclin-dependent kinase inhibitor 1B as the endogenous control gene. Box plots of the fold increase or decrease in gene expression from the calibrator sample (BPH sample 1) were plotted for the usable data with SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
SFRP-2 IS EXPRESSED IN THE CYTOPLASM OF BPH EPITHELIUM AND LOST IN LOW-GRADE PROSTATE TUMOURS
In total, 13 TMAs were constructed from 216 PCa patients. Immunohistochemical expression of SFRP-2 was first analysed by histological area: either BPH or tumour. SFRP-2 expression was observed in the cytoplasm of prostate epithelial cells. The majority (77%) of BPH areas displayed moderate/strong (score 2/3) expression (Figure 1). In comparison, only 23% of tumour areas stained positively (P < 0.0001). Further analysis across a spectrum of tumour grades revealed significant differences in expression between different Gleason grades. Acini of Gleason grades 3 and 4 largely showed negative/weak (score 0/1) expression, whereas 40% of Gleason grade 5 tumour epithelial cells showed moderate/strong expression (P < 0.0001) (Table 1).
Figure 1.
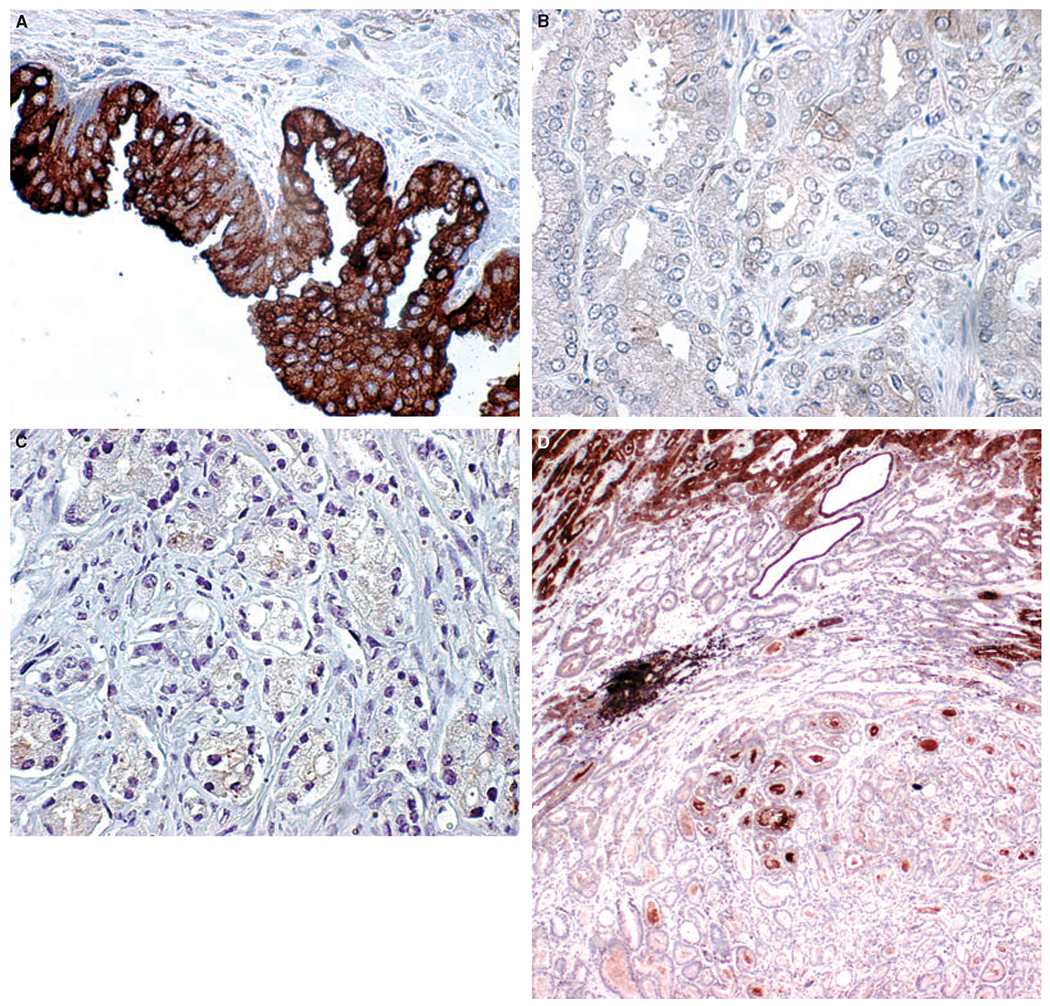
Secreted frizzled-related protein 2 (SFRP-2) protein expression assessed by immunohistochemistry on prostate tissue. A, BPH epithelium showing strong cytoplasmic SFRP-2 expression (×400 magnification). B, Tumour epithelium (Gleason grade 3) with negative SFRP-2 expression (×400 magnification). C, Tumour epithelium (Gleason grade 4) with negative SFRP-2 expression in epithelial cells (×400 magnification). D, Prostate whole section showing Gleason grade 3 carcinoma with negative SFRP-2 expression surrounded by high-grade tumour and two histologically benign glands showing moderate SFRP-2 expression (×40 magnification).
Table 1.
Two-way contingency tables comparing secreted frizzled-related protein 2S (SFRP-2) immunohistochemical scores (0/1, negative; 2/3, positive)
| Test | Histology | Score of SFRP-2 no. (%) | Score of SFRP-2 no. (%) |
|---|---|---|---|
| Histologically benign versus tumour (G3, G4 or G5) | Histologically benign (n = 699) | 161 (23) | 538 (77) |
| Chi-square, P < 0.0001 | Cancer (n = 902) | 696 (77) | 208 (23) |
| Gleason grade 5 versus Gleason grade 3 or 4 | Gleason grade <5 (n = 760) | 610 (80) | 150 (20) |
| Chi-square, P < 0.0001 | Gleason grade 5 (n = 144) | 58 (40) | 86 (60) |
| Histologically benign versus Gleason grade 3 | Histologically benign (n = 699) | 161 (23) | 538 (77) |
| Chi-square, P < 0.0001 | Gleason grade 3 (n = 439) | 371 (85) | 68 (15) |
The immunohistochemical score for the three TMA cores taken from each histological feature (BPH and Gleason grade 3, 4 and 5 tumours) per case were next averaged to produce one score for each diagnostic entity/patient. The results were consistent with the analysis of individual areas. Eighty-five per cent of patients with moderate/strong SFRP-2 expression in their BPH glands showed negative/weak expression in adjacent Gleason grade 3 acini. Ninety-three per cent of patients with negative/weak expression in their Gleason grade 5 acini also showed negative/weak SFRP-2 expression in Gleason grade 3 and/or 4 acini. It is of note that 79% of patients who had positive immunostaining in their Gleason grade 5 acini had negative/weak immunostaining in their adjacent Gleason grade 3 and/or 4 acini. Strong/moderate SFRP-2 expression was observed in epithelial cells of BPH glands on 95% of TURPs from patients with no evidence of PCa.
To ensure that the SFRP-2 expression observed on the TMAs was truly representative of the entire prostate gland, whole sections from 13 patients were also analysed. In support of the TMA data, moderate/strong expression was observed in BPH epithelial cells, negative/weak immunoreactivity was detected in Gleason grade 3 and 4 acini, and Gleason grade 5 acini had moderate/strong expression in six of 12 sections. The immunohistochemical scores for SFRP-2 on the whole sections showed 100% concurrence with those obtained for the corresponding TMAs for every case. In addition, identical SFRP-2 immunoexpression in corresponding histological grades in both left and right lobes of the prostate was noted in five cases selected for multifocal analysis.
DIFFERENTIAL SFRP-2 EXPRESSION IDENTIFIES SUBCATEGORIES OF GLEASON GRADE 5 TUMOURS
Further microscopic evaluation of Gleason grade 5 tumours revealed differences in morphological patterns, which corresponded with differential SFRP-2 expression (Figure 2). The first subgroup (referred to as Type A) appeared to be morphologically solid, and showed moderate/strong expression. The second subgroup (referred to as Type B) were morphologically more diffuse than Type A, and did not express SFRP-2 (Table 2). Type A comprised approximately 40% of the Gleason grade 5 tumours, and Type B the remaining 60%. To test whether Type A tumours were neuroendocrine tumours, synaptophysin expression was assessed by IHC, but no correlation was found. PSA expression in the Gleason grade 5 tumours was also examined. However, no correlation between the expression of PSA in the tumour epithelial cells and SFRP-2 expression in the subgroups of Gleason grade 5 tumours was noted.
Figure 2.
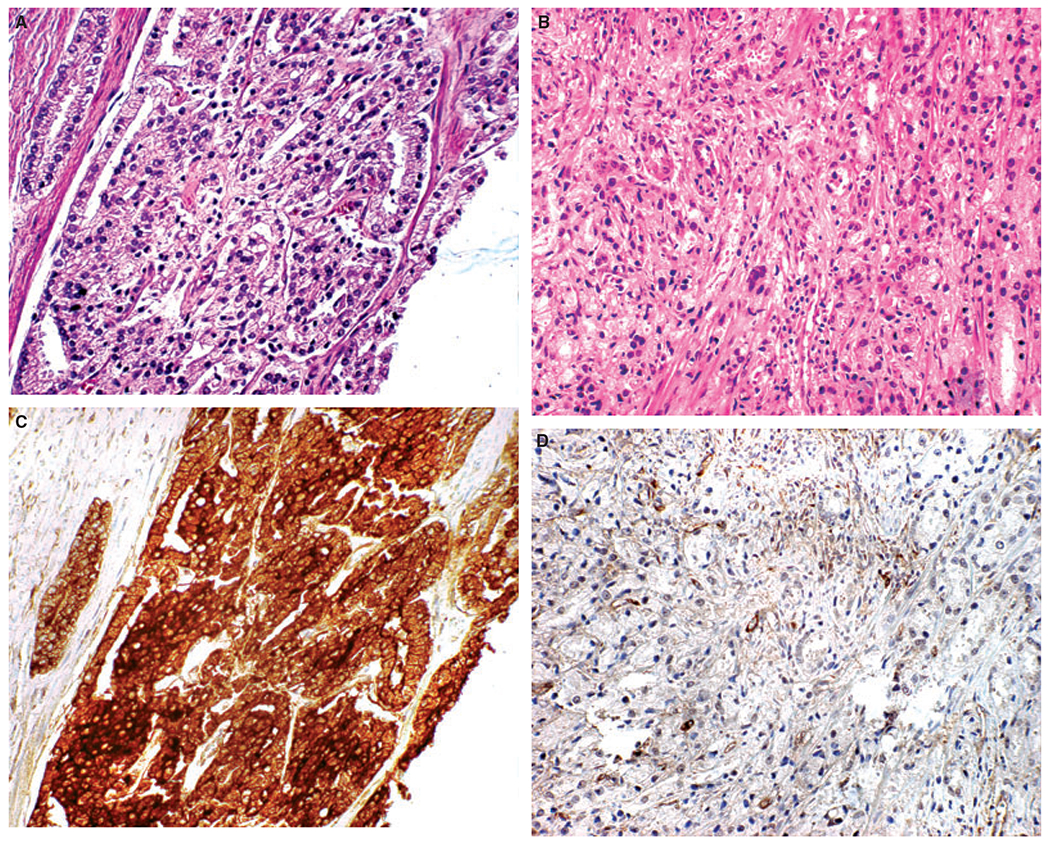
Secreted frizzled-related protein 2 (SFRP-2) expression in Gleason grade 5 tumours. A–C, Examples of Type A Gleason grade 5 tumours. B–D, Examples of Type B Gleason grade 5 tumours. A, B, Haematoxylin and eosin-stained tissue microarray (TMA) cores of the Gleason grade 5 subgroups. C, D, SFRP-2 immunohistochemistry TMA cores of Gleason grade 5 subgroups.
Table 2.
Secreted frizzled-related protein 2 (SFRP-2) immunohistochemical score within the proposed subgroups of Gleason grade 5
| SFRP-2 immunohistochemical score | Gleason grade 5 Type A | Gleason grade 5 Type B | Total |
|---|---|---|---|
| 0/1 | 7 | 79 | 86 |
| 2/3 | 42 | 16 | 58 |
| Total | 49 | 95 | 144 |
SFRP-2 GENE EXPRESSION
The presence of SFRP-2 was confirmed by TaqMan Real-Time PCR, as shown in Figure 3. Although no significant fold change in SFRP-2 gene expression was noted, the trend in SFRP-2 gene expression correlated with what was observed at the protein level; a decrease in SFRP-2 gene expression was noted in Gleason grade 3 and 4 cell populations as compared with histologically benign and Gleason grade 5 cell populations.
Figure 3.
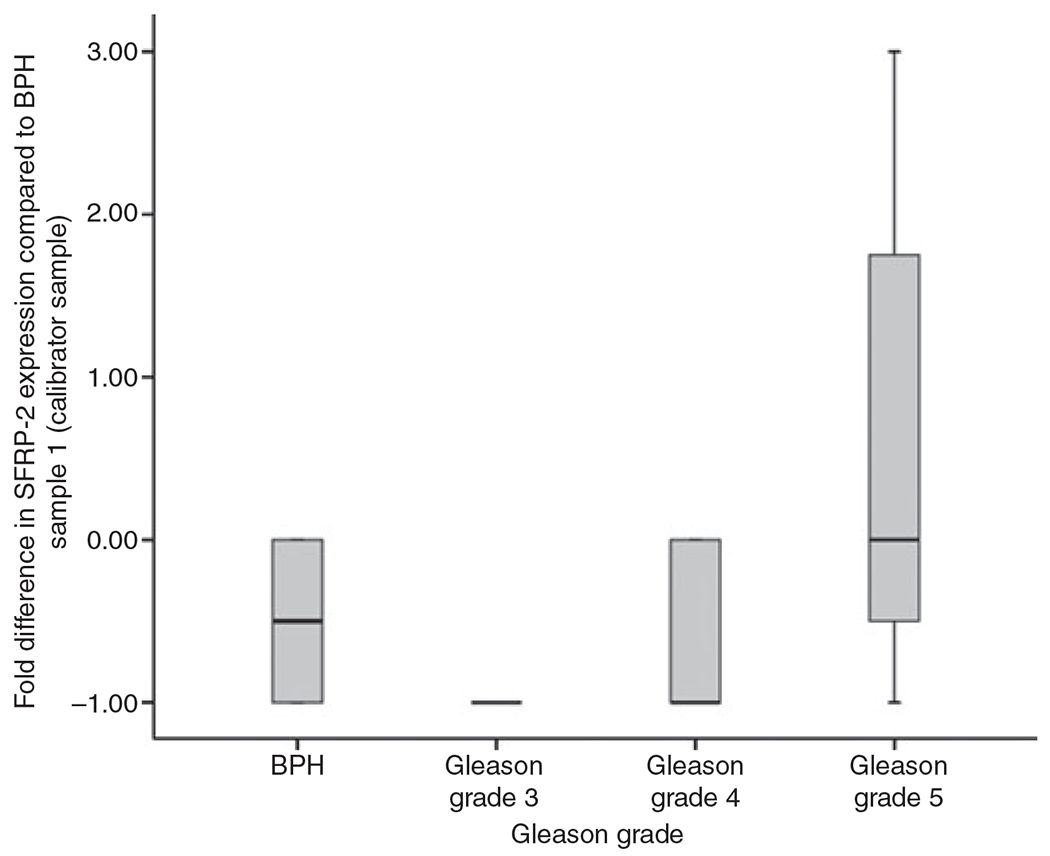
Secreted frizzled-related protein 2 (SFRP-2) gene expression. Real-time quantitative TaqMan data (2−ΔΔCT method), graphically represented as a box plot. The box plot compares SFRP-2 gene expression in benign prostatic hyperplasia (BPH) and Gleason grade 3, 4 and 5 as fold difference from the gene expression of the calibrator sample (BPH from case/sample 1).
SFRP-2 EXPRESSION IN GLEASON GRADE 5 TUMOURS IS ASSOCIATED WITH BCR
Five-year postoperative PSA data were available for 22/216 patients; 9/22 patients experienced BCR and 10/22 patients had a Gleason grade 5 tumour. Consistent with the overall study cohort, 4/10 Gleason grade 5 tumours showed moderate/strong SFRP-2 expression, and all of these patients experienced BCR. In contrast, there was no evidence of BCR in any of the six patients with Gleason grade 5 negative/weak SFRP-2 expression. No correlation between SFRP-2 expression in Gleason grade 3 and 4 tumours and BCR was noted.
Discussion
The evaluation of SFRP-2 as a putative marker of PCa has not previously been reported. In this study, we evaluated the tissue expression profile of SFRP-2 in PCa. The aim of this study was to characterize SFRP-2 expression in the different Gleason grades of prostate cancer (3, 4, and 5) and BPH glands with the use of immunohistochemical analysis, TMA technology and Taqman real-time quantitative PCR to determine its role as a potential biomarker in PCa.
The immunohistochemical results for SFRP-2 show cytoplasmic SFRP-2 expression in epithelial cells of the prostate, with overall strong to moderate SFRP-2 expression observed in BPH epithelial cells, and negative to weak SFRP-2 expression observed in tumour epithelium, particularly Gleason grade 3 and 4. However, in Gleason grade 5 carcinoma, there was a 40:60 split in the immunoexpression of SFRP-2, where 40% (58/144) had strong to moderate SFRP-2 expression and 60% (86/144) had negative SFRP-2 expression in epithelial cells. Chi-square tests performed on the contingency tables in Table 1 supported the hypotheses that there are differences in SFRP-2 protein expression between histologically benign epithelium and tumour epithelium, and in SFRP-2 expression between Gleason grade 5 tumours and Gleason grade 3 and 4 tumours. The gene expression profile of SFRP-2 confirmed the presence of the gene, and the trend of SFRP-2 gene expression across BPH and Gleason grade 3, 4 and 5 tumours correlated with what was observed at the protein level, supporting the immunohistochemical results. Immunohistochemical analysis of BPH glands from patients with no evidence of PCa revealed that BPH epithelium had strong/moderate SFRP-2 expression in 95% of TURPs analysed. Immunohistochemical analysis of SFRP-2 on 13 whole sections confirmed that the evaluation of SFRP-2 expression on TMAs was a valid model of the overall SFRP-2 immunoexpression in prostate tissue, as identical immunoexpression was observed on the whole sections and the corresponding TMA cores. Analysis of SFRP-2 immunoexpression in both lobes of the prostate from five patients showed that SFRP-2 immunoexpression was identical for a particular histological area on the left and right lobe of the prostate, implying that there is uniform SFRP-2 expression for particular histological areas throughout the prostate.
Although the majority of prostate tumours overall showed reduced/absent SFRP-2 staining, an interesting observation was made for Gleason grade 5 adenocarcinoma. Within this specific cohort, 40% of Gleason grade 5 tumours showed strong positivity, as compared with only 15% and 25% of Gleason grade 3 and 4 acini, respectively. It is not clear why a significant proportion of Gleason grade 5 carcinomas either retain or regain SFRP-2 expression. Further histological evaluation revealed notable morphological differences between Gleason grade 5 acini that corresponded with SFRP-2 staining patterns, leading to a distinction between Type A Gleason grade 5 (solid tumours, strong SFRP-2 expression) and Type B Gleason grade 5 (diffuse tumours, weak/negative SFRP-2 expression). It was considered that the Gleason grade 5 subgroup Type A (nests of solid tumour) may represent a neuroendocrine subgroup of Gleason grade 5 tumour. However, no correlation between the subgroup and synaptophysin expression was observed. Very aggressive, high Gleason score tumours often lose the ability to secrete PSA.15 Therefore, they often escape detection for long periods of time, as the PSA level in serum appears to be in the normal range. Some of these aggressive tumours have reverted to such a primitive state that they completely stop secreting PSA into the serum. A possible relationship between differential SFRP-2 expression and the ability to secrete PSA was also considered. Evaluation of PSA tissue expression in Gleason grade 5 tumours was assessed by IHC, and PSA serum levels of the patients were documented. However, no correlation between the tumours’ ability to secrete PSA and SFRP-2 expression in the subgroups of Gleason grade 5 tumours was noted.
This pattern of SFRP-2 expression in morphologically dissimilar Gleason grade 5 tumours identifies SFRP-2 as a possible surrogate marker of disease progression. Variation in tumour morphology and association with prognosis has previously been described in PCa by Veltri et al.16 They reported that quantitative nuclear morphometry signatures illustrate alterations in nuclear structure, based on nuclear morphometry within each Gleason grade pattern, that might signify potential variations in PCa risk of disease progression.16
Another interesting result regarding Gleason grade 5 tumours and their subgroups was that Type A tumours with strong/moderate SFRP-2 expression were found to be associated with BCR (4/4). However, follow-up numbers with Gleason grade 5 tumours were small, so further evaluation on larger patient numbers with longer follow-up will show whether SFRP-2 expression has prognostic relevance.
The expression patterns of SFRP-2 are not well defined in the literature. Similar loss of SFRP-2 expression pattern has been reported in the kidney by Kawamoto et al.4 These authors reported strong membranous expression in normal epithelium and loss of expression resulting from DNA methylation and histone modifications in renal cell carcinoma.4 It is possible that the loss of SFRP-2 expression noted in PCa in this study is related to methylation of the SFRP-2 gene, and that the 40% of Gleason grade 5 carcinomas that either retain or regain SFRP-2 expression may do so because of demethylation of the gene.
The results of this study show that there is loss of SFRP-2 from benign to malignant glands. The results also suggest SFRP-2 may be a marker of a subgroup of Gleason grade 5 tumours that may predict prognosis and BCR. However, further immunohistochemical analysis is required on more Gleason grade 5 tumours, with follow-up to confirm these results. The immunohistochemical results observed here prompt methylation analysis of the SFRP-2 gene in PCa to determine whether the loss of SFRP-2 gene expression observed in PCa is caused by hypermethylation of the gene. These findings of discrete differences in SFRP-2 expression and morphological patterns of Gleason grade 5 tumours could provide a useful clinical marker with which to substratify high-risk patients and tailor more appropriate treatment.
Acknowledgements
The authors would like to thank the Prostate Cancer Research Consortium (PCRC) and the Irish Cancer Society, who support and fund this work. The authors would also like to thank all surgeons and research nurses from participating hospitals within the PCRC, including Mr Thomas Lynch and Mr Richard Power.
Abbreviations:
- BCR
biochemical recurrence
- BPH
benign prostatic hyperplasia
- DKK
Dickkopf
- Dsh
Dishevelled
- Fzd
frizzled
- H&E
haematoxylin and eosin
- IHC
immunohistochemistry
- JNK
c-Jun N-terminal kinase
- LEF
lymphoid enhancer factor
- PCa
prostate cancer
- PCR
polymerase chain reaction
- PSA
prostate-specific antigen
- SFRP
secreted frizzled-related protein
- TCF
T-cell factor
- TMA
tissue microarray
- TURP
transurethral resection of the prostate
- Wnt
wingless
References
- 1.Verheyen EM, Gottardi CJ. Regulation of Wnt/beta-catenin signaling by protein kinases. Dev. Dyn 2010; 239; 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JR. The Wnts. Genome Biol 2002; 3; reviews 3001.1-3001.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir. Res 2006; 7; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamoto K, Hirata H, Kikuno N, Tanaka Y, Nakagawa M, Dahiya R. DNA methylation and histone modifications cause silencing of Wnt antagonist gene in human renal cell carcinoma cell lines. Int. J. Cancer 2008; 123; 535–542. [DOI] [PubMed] [Google Scholar]
- 5.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci 2008; 121; 737–746. [DOI] [PubMed] [Google Scholar]
- 6.Emami KH, Corey E. When prostate cancer meets bone: control by wnts. Cancer Lett. 2007; 253; 170–179. [DOI] [PubMed] [Google Scholar]
- 7.Polakis P Wnt signaling and cancer. Genes Dev. 2000; 14; 1837–1851. [PubMed] [Google Scholar]
- 8.Suzuki H, Gabrielson E, Chen W et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat. Genet 2002; 31; 141–149. [DOI] [PubMed] [Google Scholar]
- 9.Zou H, Molina JR, Harrington JJ et al. Aberrant methylation of secreted frizzled-related protein genes in esophageal adenocarcinoma and Barrett’s esophagus. Int. J. Cancer 2005; 116; 584–591. [DOI] [PubMed] [Google Scholar]
- 10.Marsit CJ, Karagas MR, Andrew A et al. Epigenetic inactivation of SFRP genes and TP53 alteration act jointly as markers of invasive bladder cancer. Cancer Res. 2005; 65; 7081–7085. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YY, Yu J, Wong YP et al. Frequent epigenetic inactivation of secreted frizzled-related protein 2 (SFRP2) by promoter methylation in human gastric cancer. Br. J. Cancer 2007; 97; 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomoto S, Kinoshita T, Kato K et al. Hypermethylation of multiple genes as clonal markers in multicentric hepatocellular carcinoma. Br. J. Cancer 2007; 97; 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukui T, Kondo M, Ito G et al. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene 2005; 24; 6323–6327. [DOI] [PubMed] [Google Scholar]
- 14.Veeck J, Noetzel E, Bektas N et al. Promoter hypermethylation of the SFRP2 gene is a high-frequent alteration and tumor-specific epigenetic marker in human breast cancer. Mol. Cancer 2008; 7; 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokoloff MH, Yang XJ, Fumo M, Mhoon D, Brendler CB. Characterizing prostatic adenocarcinomas in men with a serum prostate specific antigen level of < 4.0 ng/mL. BJU Int. 2004; 93; 499–502. [DOI] [PubMed] [Google Scholar]
- 16.Veltri RW, Marlow C, Khan MA, Miller MC, Epstein JI, Partin AW. Significant variations in nuclear structure occur between and within Gleason grading patterns 3, 4, and 5 determined by digital image analysis. Prostate 2007; 67; 1202–1210. [DOI] [PubMed] [Google Scholar]


