SUMMARY
The Cre-loxP system is invaluable for spatial and temporal control of gene knockout, knockin, and reporter expression in the mouse nervous system. However, we report varying probabilities of unexpected germline recombination in distinct Cre driver lines designed for nervous system-specific recombination. Selective maternal or paternal germline recombination is showcased with sample Cre lines. Collated data reveal germline recombination in over half of 64 commonly used Cre driver lines, in most cases with a parental sex bias related to Cre expression in sperm or oocytes. Slight differences among Cre driver lines utilizing common transcriptional control elements affect germline recombination rates. Specific target loci demonstrated differential recombination; thus, reporters are not reliable proxies for another locus of interest. Similar principles apply to other recombinase systems and other genetically targeted organisms. We hereby draw attention to the prevalence of germline recombination and provide guidelines to inform future research for the neuroscience and broader molecular genetics communities.
In Brief
Luo et al. report variable rates of germline recombination in commonly used mouse Cre driver lines, influenced by sex of Cre-carrying parents and target loci. Guidelines are provided to optimize cell-type-specific recombination in genetically targeted organisms expressing site-specific recombinases.
INTRODUCTION
Advances in modern neuroscience research rely on genetically targeted animal models incorporating site-specific recombinase technology to achieve gene manipulations in a spatially and temporally controlled manner. Among all genetic tools, the Cre-loxP system has arguably been the most frequently used approach since its first discovery in bacteriophage P1 (Sternberg and Hamilton, 1981) and development for genetic manipulations in mammalian cells (Sauer and Henderson, 1988) and in transgenic mice (Gu et al., 1994; Tsien et al., 1996a). Cre recombinase recognizes 34 base pair loxP sites, mediating deletion of DNA fragments between two loxP sites of the same orientation, or flipping of DNA fragments between two inverted loxP sites. Manipulation of genetic material flanked by loxP sites, in floxed genes, has been facilitated by the large-scale generation of mouse Cre driver lines with diverse expression patterns in the nervous system and mice with floxed target genes and reporters (Daigle et al., 2018; Gerfen et al., 2013; Gondo, 2008; Taniguchi et al., 2011). Thus, the Cre-loxP system has become a mainstay for conditional gene knockout (KO), knockin (KI), and reporter gene expression in mice, and recently in rats (Bäck et al., 2019; Witten et al., 2011). However, several caveats have been noted, including Cre-mediated toxicity and metabolic phenotypes due to illegitimate recombination, mosaic and/or inconsistent recombination activity, genetic background effects, and unexpected expression of Cre in undesirable cell types (Gil-Sanz et al., 2015; Harno et al., 2013; Heffner et al., 2012; Murray et al., 2012; Schmidt et al., 2000; Tachibana et al., 2018; Wojcinski et al., 2019).
A particularly under-appreciated and limiting caveat in terms of major undesirable consequences is unintentional germline recombination. When Cre expression and associated recombinase activity occur in germline cells, the Cre-mediated excision of the floxed allele will occur in all cells instead of in the intended region- and cell-type-specific pattern. A recent review describes how unexpected germline recombination could happen and how to detect such events (Song and Palmiter, 2018), but information about affected Cre driver lines has been scarce, with only a few sporadic reports (Choi et al., 2014; Kobayashi and Hensch, 2013; Liput, 2018; Zhang et al., 2013). Awareness of potential germline recombination in Cre driver lines designed to be nervous system specific is essential for correct genotyping and data interpretation. Furthermore, information about parental sex effects on germline recombination and comparisons among related Cre driver lines could guide optimal breeding schemes to save researchers valuable time and resources. Yet, such a meta-analysis has been lacking.
In this report, we used two Cre lines, Dlx5/6-Cre and Gpr26-Cre, expressing Cre recombinase in distinct neuronal populations, as examples to demonstrate undesirable germline recombination occurring selectively in the female or male Cre-carrying parents, respectively. To generate a more comprehensive resource, we compiled information on germline recombination frequencies from a total of 64 different Cre driver lines generally used for nervous system-specific genetic manipulations. We anticipate that our short report will serve as a collective resource to guide the optimal usage of Cre driver lines.
RESULTS AND DISCUSSION
Maternal Germline Recombination in Dlx5/6-CreDriver Mice
The Dlx5/6-Cre line (Tg(dlx5a-cre)1Mekk) (Zerucha et al., 2000) has been used in over 70 papers to specifically target forebrain interneurons (Mouse Genome Informatics [MGI] database; Bult et al., 2019). We combined this transgene with the Clstn3f/f (B6-Clstn3tm1Amcr/J) allele (Pettem et al., 2013) and then crossed Clstn3f/f; Dlx5/6-Cre with Clstn3f/f mice to generate experimental conditional KO animals. We genotyped the offspring with three sets of primers: the first for the Clstn3 floxed and wild-type (WT) alleles, the second for the Cre-recombined Clstn3 KO allele, and the third for Cre. We expected to observe only the Clstn3 floxed allele and no KO allele regardless of Cre. Yet, 88% (73/83) of the offspring expressed a Clstn3 KO allele when the female parent carried Cre, suggesting germline deletion. In contrast, none of the 114 offspring we tested had the KO allele when the Cre recombinase was transmitted from the male parent (Figure 1A). Counting Cre-negative offspring to rule out any potential direct effects of Cre in the offspring, all (54) offspring from paternal Cre crosses were Clstn3f/f but 85.3% (29/34) of the offspring from maternal Cre crosses were Clstn3f/–. These results indicate that a high fraction of female mice carrying the Dlx5/6-Cre and floxed genes apparently expressed Cre in the germ cells resulting in maternal germline recombination and that this unexpected germline deletion could be circumvented by transmitting Cre strictly paternally.
Figure 1. Dlx5/6-Cre Mice Show Maternal Germline Recombination.
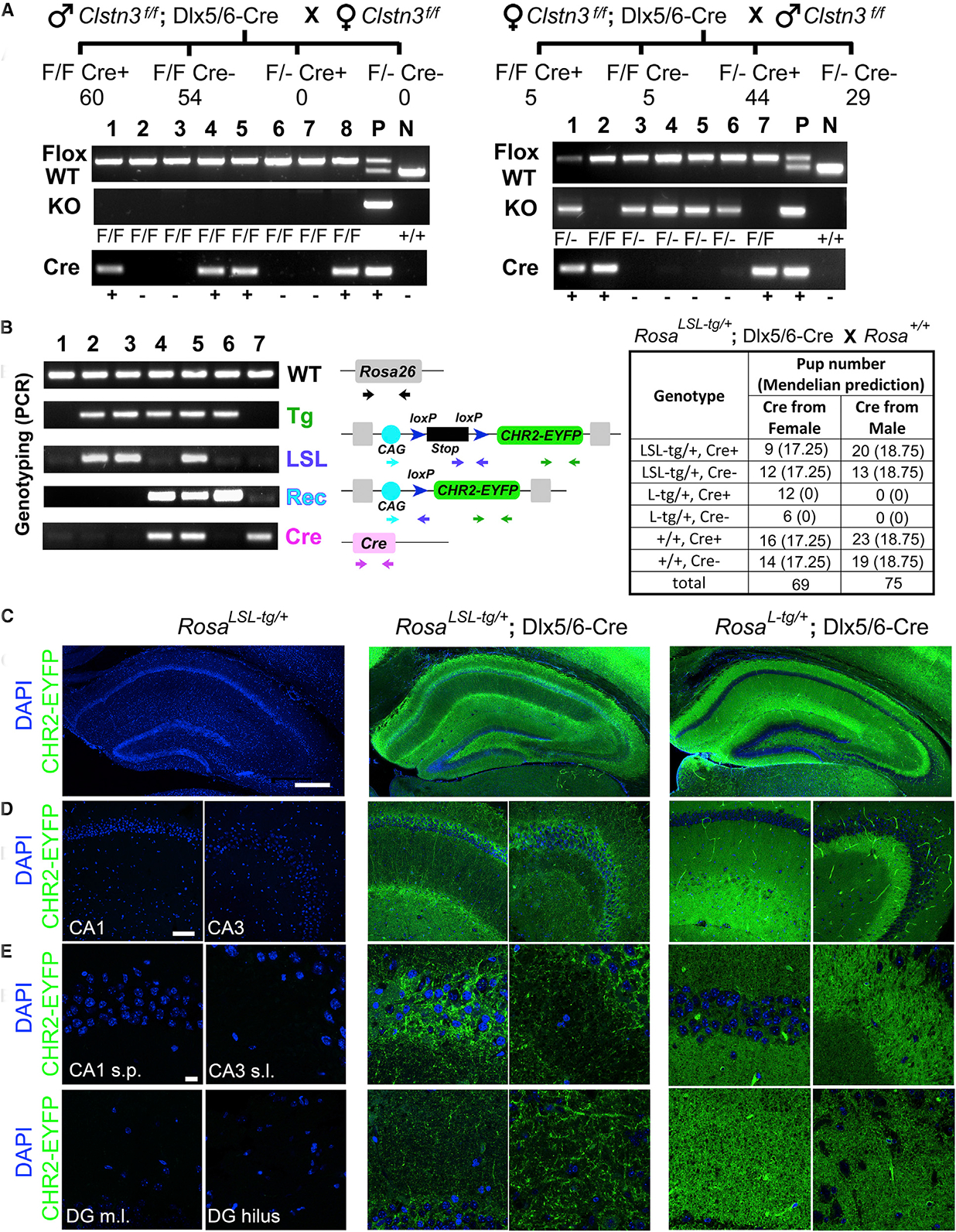
(A) Pedigree and sample genotyping results of Clstn3f/f crosses with Dlx5/6-Cre transmitted from either the male or female parent. F/F Cre+ indicates Clstn3f/f; Dlx5/6-Cre. F/F Cre– indicates Clstn3f/f without Cre. F/–Cre+ indicates Clstn3f/–; Dlx5/6-Cre in which recombination has occurred (this labeling is used for simplicity, but some of these mice may be F/–Cre+ and some may be F/F Cre+ genotypes because mosaic recombination was observed in tail tissue used for genotyping). F/–Cre– indicates Clstn3f/– without Cre in which recombination has occurred. P and N indicate controls (multiple mice were used for P). The numbers below genotypes indicate the total number of offspring obtained with that genotype.
(B) Representative genotyping results and numbers of offspring from Ai32 Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J reporter and Dlx5/6-Cre crosses. LSL-tg indicates the CAG promoter and lox-stop-lox sequences before the transgene channelrhodopsin-2(H134R)-EYFP (CHR2-EYFP) on the Rosa locus. L-tg indicates the transgene after germline recombination by Cre resulting in global transgene expression. Genotypes of the animals are 1, +/+ and Cre–; 2 and 3, LSL-tg/+ and Cre–; 4, L-tg/+ and Cre+; 5, LSL-tg/+ and Cre+ (showing some mosaic recombination); 6, L-tg/+ and Cre–; 7, +/+ and Cre+.
(C–E) Tiled images of the hippocampus (C) and sample CA1 and CA3 regions (D) showing CHR2-EYFP transgene expression and DAPI nuclear stain for selected offspring from (B). (E) Higher-magnification images are shown for hippocampal CA1 stratum pyramidale (s.p.), CA3 stratum lucidum (s.l.), dentate gyrus molecular layer (DG m.l.), and dentate gyrus hilus regions. Note that the laser power used for the EYFP channel in the far right panel for images from Rosa26L-tg/+;Dlx5/6-Cremice was only 15% of that for the rest. Scale bars, 500 mm (C), 100 mm (D), and 20 mm (E).
To test whether this maternal germline recombination is floxed locus specific, we crossed the Dlx5/6-Cre with an Ai32 reporter line (B6;129S-Gt(ROSA)26Sor tm32(CAG-COP4*H134R/EYFP)Hze/J) (Madisen et al., 2012). The Ai32 mouse line contains a LoxP-stop-LoxP-EYFP-channelrhodopsin2 (ChR2) cassette at the Rosa 26 locus. To distinguish the reporter allele before and after recombination, we designed a primer set targeting the loxP-stop-loxP-EYFP region, with the forward primer annealing to the stop cassette that would be deleted by Cre recombinase. Thus, PCR product with these primers is present in the tail tissue of transgenic mice without Cre-dependent recombination (“LSL-tg” in Figure 1B) and absent in mice with germline recombination (“L-tg” in Figure 1B). When female RosaLSL-tg/+; Dlx5/6-Cre mice were crossed with male WT mice, 46.2% (18/39) of offspring with the transgene had a recombined L-tg allele instead of the original LSL-tg, indicating germline Cre-recombination (Figure 1B). In contrast, no recombined allele was detected when male RosaLSL-tg/+; Dlx5/6-Cre mice were crossed with female WT mice (0/33 offspring with the transgene). This germline recombination activity in female but not male germ cells is consistent with the finding from the Clstn3f/f crosses. However, the recombination frequencies differed (33.3% for RosaLSL-tg and 85.3% for Clstn3f per target allele for Cre-negative mice, assuming no recombination in the zygote, which was verified as discussed below).
To support the PCR results with Dlx5/6-Cre and the Ai32 reporter, we assessed germline versus forebrain interneuron-specific Cre recombination by fluorescence imaging for the Cre-dependent expression of EYFP-ChR2; Figures 1C–1E). RosaLSL-tg/+ mice showed no EYFP signal above background levels. RosaLSL-tg/+; Dlx5/6-Cre mice showed a pattern of EYFP-ChR2 consistent with expression in axons and dendrites of interneurons, as expected. For example, the signal was strong in the hippocampal CA1 and CA3 stratum pyramidale regions that are rich in inhibitory inputs but weak in the CA3 stratum lucidum that is rich in excitatory synapses. The germline-recombined RosaL-tg/+; Dlx5/6-Cre offspring showed a broad EYFP-ChR2 expression pattern spanning all brain regions, consistent with expression in all cell types, neurons, glia cells, and blood vessels (Figures 1C–1E). Note that the excitation laser power for the EYFP channel used to image the germline Cre-recombined RosaL-tg/+; Dlx5/6-Cre mouse hippocampus was only 15% of that for the other two genotypes in Figures 1C–1E. Cre-negative RosaL-tg/+mice showed the same phenotype as RosaL-tg/+; Dlx5/6-Cre (data not shown).
Mosaic Cre Expression in Genotyping Tissue
Of note, the presence of Cre-recombined alleles in the tissue used for PCR genotyping could be due to limited Cre expression and recombination in peripheral nerve or non-neural cell types in the genotyping tissue rather than germline recombination. This phenomenon typically involves mosaic rather than ubiquitous recombination, as indicated by the presence of both intact floxed and recombined products from one target allele. Such mosaic recombination occurred in the tail tissue used for PCR genotyping of Ai32 RosaLSL-tg/+; Dlx5/6-Cre mice. For example, in Figure 1B, lane 5 shows bands for both the recombined RosaL-tg/+and the non-recombined RosaLSL-tg/+ forms for a single RosaLSL-tg locus, indicating a mouse genotype of RosaLSL-tg/+ with some local recombination. Such mosaic recombination was observed in 22/26 RosaLSL-tg/+; Dlx5/6-Cre offspring whereas no RosaLSL-tg/+ Cre-negative offspring (0/20) showed any recombined RosaL-tg/+ product. For mice with two target alleles, it can be difficult based solely on PCR genotyping of the target locus to distinguish germline ubiquitous recombination at one allele from somatic mosaic recombination. A definitive approach to distinguish germline recombination from such local mosaic recombination is the detection of the recombined allele in Cre-negative offspring. Another definitive approach is cellular resolution imaging for the expression of RNA or protein products from both the recombined and non-recombined loci. Additionally, germline recombination is likely to display a parental sex bias, as was the case for most lines reported here, whereas mosaic recombination is typically independent of sex except in some situations involving epigenetic modification.
The use of cells that developmentally diverge considerably from nerve cells for PCR genotyping, such as blood cells, may help distinguish between germline versus local recombination. For example, in offspring from crossing female Emx1-Cre;Wwp1f/f;Wwp2f/f with male Wwp1f/f;Wwp2f/f mice, recombination at Wwp2 alleles was observed in tail tissue of most Cre-positive (14/15) but not Cre-negative (0/16) mice. PCR from blood revealed no recombination in any offspring (0/14), altogether indicating that the recombination observed in tail tissue was due to mosaic rather than germline recombination. However, this approach is not universally useful, as the RosaLSL-tg/+; Dlx5/6-Cre mice showed mosaic recombination in blood as well as in tail tissue.
Paternal Germline Recombination in Gpr26-CreDriver Mice
Given the experience with Dlx5/6-Cre and the recommendations on the Jackson Labs website—”For many cre strains, but not all, using cre-positive males for breeding avoids potential germline deletion of your loxP-flanked allele.” (https://www.jax.org/news-and-insights/jax-blog/2016/may/are-your-cre-lox-mice-deleting-what-you-think-they-are)—we adopted a general breeding strategy of transmitting Cre recombinase paternally. However, we found that Gpr26-Cre showed selective paternal germline recombination.
Gpr26-Cre (Tg(Gpr26-cre)KO250Gsat/Mmucd) was generated by the GENSAT project (http://gensat.org/index.html) and displays abundant expression in the hippocampal CA1 region and sparse expression in other brain regions, including layer V cortex and thalamus (Gerfen et al., 2013; Harris et al., 2014). We chose this line for its specific expression in CA1 pyramidal neurons (Figure 2), which actually turned out to be only in the deep sublayer (near stratum oriens) but not the superficial sublayer of CA1 stratum pyramidale in our characterization (data not shown; Figure 2C). In order to delete Clstn3 in the CA1 region conditionally, we crossed Clstn3f/f; Gpr26-Cre and Clstn3f/f mice and genotyped offspring by PCR. As shown by the presence of KO PCR bands in Figure 2A, we observed Cre-mediated recombination in the tail tissue when male Clstn3f/f; Gpr26-Cre were crossed with female Clstn3f/f but not vice versa. Further germline transmission of this KO allele and the absence of local recombination in tail tissue confirmed selective paternal germline recombination of floxed Clstn3.
Figure 2. Gpr26-Cre Mice Show Paternal Germline Recombination.
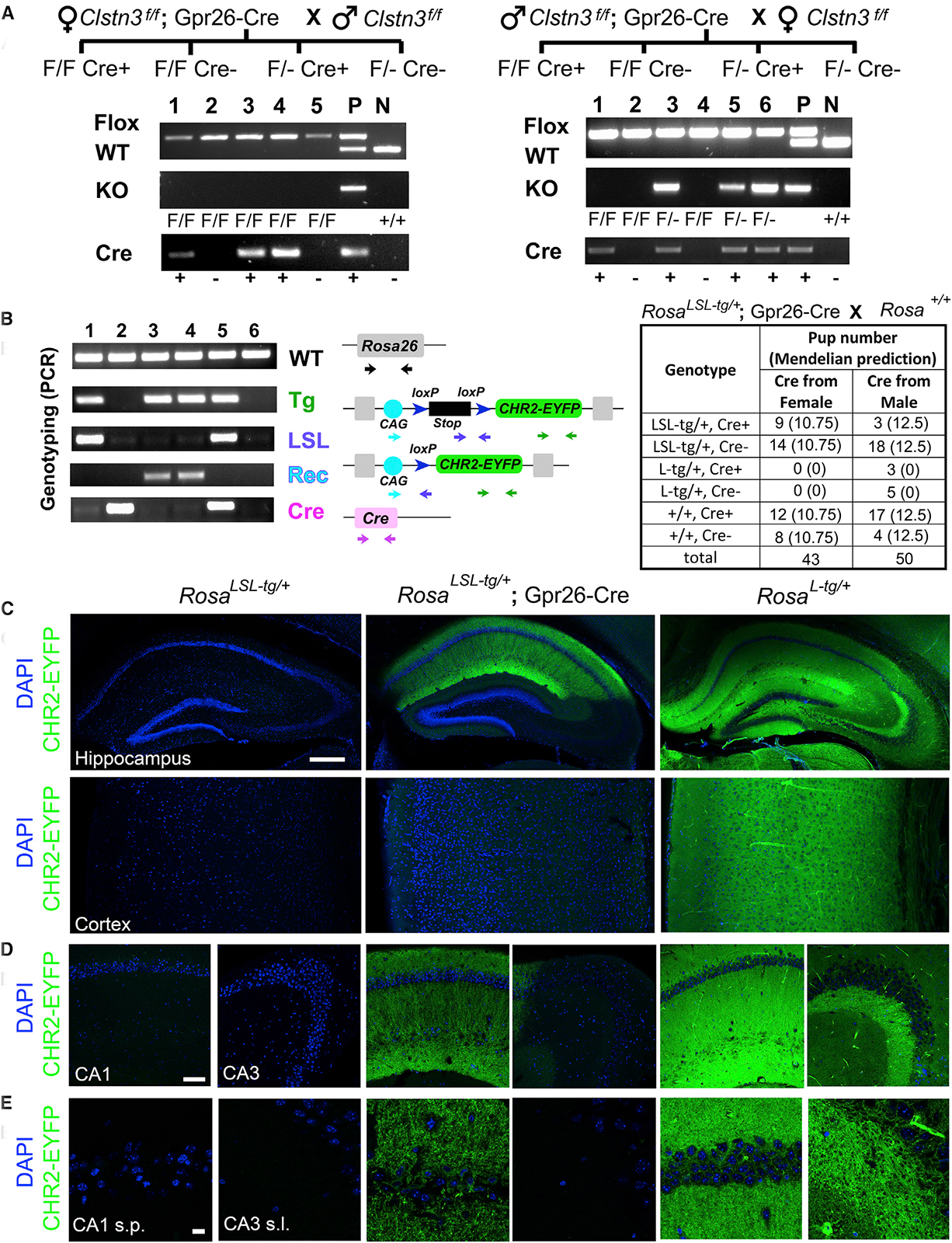
(A) Pedigree and sample genotyping results of Clstn3f/f crosses with Gpr26-Cre from either the male or female parent. F/F Cre+ indicates Clstn3f/f;Gpr26-Cre. F/F Cre– indicates Clstn3f/f without Cre. F/Cre+ indicates Clstn3f/–;Gpr26-Cre in which recombination has occurred. This was confirmed to be germline deletion by the absence of a KO band in tail tissue from Clstn3f/f;Gpr26-Cre mice (n = 46 mice generated from maternal Cre crosses) indicating the absence of local recombination in the tissue used for genotyping and by transmission of the KO allele to offspring. F/–Cre– indicates Clstn3f/– without Cre in which recombination has occurred. P and N indicate controls (multiple mice were used for P).
(B) Representative genotyping results and numbers of offspring from Ai32 Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J reporter and Gpr26-Cre crosses. LSL-tg indicates the CAG promoter and lox-stop-lox sequences before the transgene channelrhodopsin-2(H134R)-EYFP (CHR2-EYFP) on the Rosa locus. L-tg indicates the transgene after germline recombination by Cre resulting in global transgene expression. Genotypes of the animals are 1, LSL-tg/+ and Cre–; 2, +/+ and Cre+; 3 and 4, L-tg/+ and Cre–; 5, LSL-tg/+ and Cre+; 6, +/+ and Cre–.
(C–E) Tiled images of the hippocampus and cortex (C) and sample CA1 and CA3 regions (D) showing CHR2-EYFP transgene expression and DAPI nuclear stain for selected offspring from (B). Higher-magnification images are shown for hippocampal CA1 stratum pyramidale (s.p.) and CA3 stratum lucidum (s.l.) (E). Note that the laser power used for the EYFP channel in the far right panel for images from Rosatg/+ mice was only 15% of that for the rest. Scale bars, 500 mm (C), 100 mm (D), and 20 mm (E).
To test whether selective paternal germline recombination also occurs with another target floxed locus, we crossed Gpr26-Cre mice with the Ai32 reporter line and assessed genotypes of F2 progeny by PCR using tail tissue and by imaging of EYFP-ChR2 in brain sections. When male RosaLSL-tg/+; Gpr26-Cre mice were crossed with WT females, 27.6% (8/29) of the offspring with the transgene had only a recombined allele regardless of the presence of Cre, indicating germline recombination. In contrast, when Cre was transmitted through the female parent, the loxP-stop-loxP sequences remained largely intact (Figure 2B; no ubiquitous germline recombination was observed but 2/17 RosaLSL-tg/+; Gpr26-Cre mice showed mosaic recombination in tail tissue). As expected, RosaLSL-tg/+mice showed no EYFP signal above background levels and RosaLSL-tg/+; Gpr26-Cre mice expressed EYFP-ChR2 prominently in the hippocampal CA1 region with weak expression in the cortex (Figures 2C–2E). In contrast, for animals with germline recombination, i.e., RosaL-tg/+ mice, EYFP-ChR2 was expressed globally. In the hippocampus, RosaLSL-tg/+; Gpr26-Cre mice had strong EYFP-ChR2 expression in the CA1 stratum radiatum and oriens layers but not in CA3, while RosaL-tg/+ mice showed EYFP-ChR2 expression in both regions in a pattern consistent with expression in all cell types (Figures 2C–2E).
Germline Recombination in Mouse Cre Driver Lines Designed for Cell-Type-Specific Expression
As significant germline recombination happened in both Dlx5/6-Cre and Gpr26-Cre lines designed for recombination in specific neuron types, we wondered about the prevalence of this phenomenon in other Cre lines. To the best of our knowledge, there are only a handful of papers focused on germline recombination in Cre driver lines intended for nervous system-specific recombination (Kobayashi and Hensch, 2013; Liput, 2018; Weng et al., 2008; Zhang et al., 2013) and several other papers that mention this issue, typically in the methods (Table 1). Lines reported to undergo significant germline recombination include the widely used Nestin-Cre, GFAP-Cre, CaMKIIa-Cre, and Synapsin1-Cre lines (Choi et al., 2014; Rempe et al., 2006; Zhang et al., 2013), which collectively have been used in over 1,500 published papers according to the MGI database (Bult et al., 2019). Furthermore, in these data collected from the literature, most (9/10) of the Cre driver lines tested showed a parental sex effect. We suspect that these data represent the tip of an iceberg, as for the majority of Cre driver lines information has not been readily available on either the extent of germline recombination or parental sex bias.
Table 1.
Prevalence of Germline Recombination in Mouse Cre Driver Lines Designed for Nervous System-Specific Recombination
| Cre line Common Name | Full Cre Line Name/Source | Target Gene/Reporter | Breeding Strategya | Germline Recombination Efficiency, Cre from Fatherb | Germline Recombination Efficiency, Cre from Motherb | Germline Recombindation Efficiency, Parental Sex Effects Unknownb |
Reference/Associated Publicationc | Contributorsd |
|---|---|---|---|---|---|---|---|---|
| 799-CreER-IRES-GFP | Tg(hs799-cre/ERT2,-GFP)405Jlr | Mafbtrn1.1Good | H | 0 (from >20 litters) | observed (from >20 litters) | - | Pai et al., 2019; Silberberg et al., 2016 | Emily Ling-Lin Pai, John L.R. Rubenstein |
| Tg(hs799-cre/ERT2,-GFP)405Jlr | Maftrn.1Cbm | H | 0 (from >20 litters) | observed (from >20 litters) | - | Pai et al., 2019; Silberberg et al., 2016 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| Tg(hs799-cre/ERT2,-GFP)405Jlr | Ai14e | H | 0 (from >20 litters) | observed (from >20 litters) | - | Pai et al., 2019; Silberberg et al., 2016 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| A2a-Cre | B6-Tg(Adora2a-Cre) KG139GSat | Ai14e | E or G | 0 (from >3 years breeding) | 0 (from >3 years breeding) | - | - | Kevin T. Beier |
| B6.FVB(Cg)-Tg (Adora2a-cre) KG139Gsat/Mmucd/GENSAT | Gt(ROSA) 26Sortrn2(CAG-tdTomato)Fawa | F | 0(0/15) | ND | - | - | Hisashi Umemori | |
| Bhlhb5-Cre | Bhlhe22trn3.1(cre)Meg | Ai9e | B | 0 (from >10 litters) | 0 (from >10 litters) | - | - | Wenjia You, Constance L. Cepko |
| CaMKIIα-Cre | Tg(Camk2a-cre) 159Kln |
Fdft1trn1Kan | C | 16.2% (12/74) | 6.3% (3/48) | - | Funfschilling et al., 2012; Minichiello et al., 1999 | Gesine Saher, Klaus A. Nave |
| CaMKIIα-Cre | Tg(Camk2a-cre)93Kln | Gnaol | B | 72.1% (31/43) | ND | - | Choi et al., 2014 | - |
| Tg(Camk2α-cre)93Kln | B6;129S4-Gt(ROSA) 26Sortrn1Sor/J | B | 98.5% (64/65) | ND | - | Choi et al., 2014 | - | |
| CaMKIIα-Cre | B6.Cg-Tg(Camk2a-cre)2Szi/J | Leprtrn1.1Chua | A | observed | 0 | - | McMinn et al., 2005 | - |
| B6.Cg-Tg(Camk2a-cre)2Szi/J | Chat/Slc18a3trn1.2Vpra | A or C | observed | ND | - | de Castro et al., 2009 | - | |
| CaMKIIa-Cre (T29–1) | Tg(Camk2a-cre) T29–1Stl | Khdrbs3trn1.1Schei/J | C | 31.3% (5/16) | 0% (0/7) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele |
| Tg(Camk2a-cre) T29–1Stl | Rpl22trn1.1Psam/J | C | 21.4% (6/28) | 0% (0/21) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| Tg(Camk2a-cre) T29–1Stl | Trpm7trn1Clph | C | 25.0% (33/132) | ND | - | Liu et al., 2018b | Cui Chen, Wei Li, Nashat Abumaria | |
| Chat-Cre | B6;129S6-Chattrn2(cre)Lowl/J | Megf10trn1c(KOMP)Jrs | A or C | 0 (from 16 litters) | 0 (from 15 litters) | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay |
| B6;129S6-Chattrn2(cre)Low/J | Tgfb3trn1Moaz | A or C | 0 (from 33 litters) | 0 (from 35 litters) | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay | |
| B6;129S6-Chattrn2(cre)Lowl/J | ROSAmT/mG5 | A or C | 0 (from 17 litters) | 0 (from 19 litters) | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay | |
| B6;129S6-Chattrn2(cre)Lowl/J | Ai14e | A or C | 0 (from 15 litters) | 0 (from 9 litters) | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay | |
| Cux2-Cre | B6.Cg-Cux2trn2.1(cre)Mull | Ai9e | B | - | - | observed | Gil-Sanz et al., 2015 | - |
| B6.Cg-Cux2trn2.1(cre)Mull | Gt(ROSA) 26Sortrn1(CAG-lacZ,-EGFP)Glh/J | B | - | - | observed | Gil-Sanz et al., 2015 | - | |
| Cux2-CreERT2 | B6.Cg-Cux2trn3.1(cre/ERT2)Mull/Mmmh | Rpl22trn1.1PsamU | B | ND | 0(0/15) | - | - | Susanne Falkner, Peter Scheiffele |
| B6.Cg-Cux2trn3.1 (cre/ERT2)Mull/Mmmh | Rpl22trn1.1Psam/J | E | 0 (0/23) | 0 (0/23) | - | - | Susanne Falkner, Peter Scheiffele | |
| CX3CR1-CreER | Cx3cr1trn2.1(cre/ERT2)Litt/WganJ | Syktrn1.2Tara | E | 0 (from 32 litters) | 0 (from 26 litters) | - | Punal et al., 2019 | Ariane Pereira, Jeremy N. Kay |
| Cx3cr1trn2.1(cre/ERT2)Litt/WganJ | Gt(ROSA) 26Sortrn1(HBEGF)Awai/j | A or C | 0 (from 8 litters) | 0 (from 6 litters) | - | Punal et al., 2019 | Ariane Pereira, Jeremy N. Kay | |
| Cx3cr1trn2.1(cre/ERT2)Litt/WganJ | Csf1rtrn1.2Jwp/J | A or C | 0 (from 10 litters) | 0 (from 8 litters) | - | Punal et al., 2019 | Ariane Pereira, Jeremy N. Kay | |
| D2-Cre | Tg(Drd2-cre) ER44Gsat/Mmucd | Chat/Slc18a3trn1.2Vpra | C | 0 (0/55) | 0 (0/44) | - | Guzman et al., 2011 | Marco A.M. Prado, Vania F. Prado |
| DAT-Cre | Slc6a3trn1.1(cre)Bkmn/J | Gria1trn2Rsp | A or C | 0 (from >3 years breeding) | ND | - | Hutchison etal., 2018 | Mary Anne Hutchison, Wei Lu |
| Slc6a3trn1.1(cre)Bkmn/J | Gria2trn3Rsp | A or C | 0 (from >3 years breeding) | ND | - | Hutchison etal., 2018 | Mary Anne Hutchison, Wei Lu | |
| Slc6a3trn1.1(cre)Bkmn/J | Gria3trn1Rsp | A or C | 0 (from >3 years breeding) | ND | - | Hutchison etal., 2018 | Mary Anne Hutchison, Wei Lu | |
| Slc6a3trn1.1(cre)Bkmn/J | Grin1trn2Stl | A or C | 0 (from >3 years breeding) | ND | - | Hutchison etal., 2018 | Mary Anne Hutchison, Wei Lu | |
| Slc6a3trn1.1(cre)Bkmn/J | Ai14e | A or C | 0 (from >3 years breeding) | ND | - | Hutchison et al., 2018 | Mary Anne Hutchison, Wei Lu | |
| B6.SJL-SLc6a3trn1.1(cre)Bkmn/J | Ai14e | E or G | 0 (from >6 years breeding) | 0 (from >6 years breeding) | - | - | Kevin T. Beier | |
| B6.SJL-SLc6a3trn1.1(cre)Bkmn/J | Gt(ROSA) 26Sortrn2(CAG-tdTomato)Fawa | E, F | 0 (0/26) | 0(0/15) | - | - | Hisashi Umemori | |
| B6.SJL-SLc6a3trn1.1(cre)Bkmn/J | Rims1trn3Sud/J | A or C | 0 (from >1 year of breeding) | 0 (from >1 year of breeding) | - | Liu et al., 2018a | Jiexin Wang, Pascal S. Kaeser | |
| B6.SJL-SLc6a3trn1.1(cre)Bkmn/J | Rims2trn1.1Sud/J | A or C | 0 (from >1 year of breeding) | 0 (from >1 year of breeding) | - | Liu et al., 2018a | Jiexin Wang, Pascal S. Kaeser | |
| B6.SJL-SLc6a3trn1.1(cre)Bkmn/J | Ai34e | A or C | 0 (from >19 litters) | 0 (from >14 litters) | - | Liu et al., 2018a | Jiexin Wang, Pascal S. Kaeser | |
| Dlx5/6-Cre | B6-Tg(dlx5a-cre) 1Mekk/J | Prox1trn2Gco | B | 0 or less than female | observed | - | Miyoshi et al., 2015 | - |
| B6-Tg(dlx5a-cre) 1Mekk/J | Clstn3trn1Amcr/J | C | 0(0/52) | 85.3% (29/34) of Cre negative offspring | - | - | Lin Luo, Ann Marie Craig | |
| B6-Tg(dlx5a-cre) 1Mekk/J | Ai32e | B | 0 (0/33) | 33.3% (6/18) of Cre negative offspring | - | - | Lin Luo, Ann Marie Craig | |
| DlxI12B-Cre | Tg(I12b-cre)1Jlr | Mafbtrn1.1Good | H | observed (from >10 litters) | ND | - | Potter et al., 2009 | Emily Ling-Lin Pai, John L.R. Rubenstein |
| Tg(I12b-cre)1Jlr | Mafm2.1Cbm | H | observed (from >10 litters) | ND | - | Potter et al., 2009 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| Tg(I12b-cre)1Jlr | Ai14e | H | observed (from >10 litters) | ND | - | Potter et al., 2009 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| Drd1-Cre | B6-Tg(Drd1-Cre) EY262GSat | Ai14e | E or G | 0 (from >3 years breeding) | 0 (from >3 years breeding) | - | - | Kevin T. Beier |
| B6.FVB(Cg)-Tg(Drd1-cre)EY262Gsat/Mmucd/GENSAT | Gt(ROSA) 26Sortrn2(CAG-tdTomato)Fawa | F | 0 (0/20) | ND | - | - | Hisashi Umemori | |
| E3-CreN | Grin2ctrn2(icre)Mwa | Cacna1atrn1Kano | C | 41.2% (7/17) of Cre negative offspring | 0 (0/20) | - | - | Junko Motohashi, Michisuke Yuzaki |
| Emx1-Cre | Emx1trn1(cre)Ito | B6.129(FVB)− Gabra1trn1Geh/J | A | 36% | 36% | - | Zeller et al., 2008 | - |
| Emx1-Cre | B6.129S2-Emx1trn1(cre)Krj/J | Syngap1trn1.1Geno | Not specified | observed | 0 or less than male | - | Ozkan et al., 2014 | - |
| B6.129S2-Emx1trn1(cre)Krj/J | Ai93e | Not specified | - | - | observed | Steinmetz et al., 2017 | - | |
| B6.129S2-Emx1trn1(cre)Krj/J | Wwp2trn1.1Hkb | C | 33.3% (4/12) of Cre negative offspring | 0 (0/20) | - | Ambrozkiewicz etal., 2018 | Mateusz C. Ambrozkiewicz, Fritz Benseler, Nils Brose, Hiroshi Kawabe | |
| B6.129S2-Emx1trn1(cre)Krj/J | Rai1trn2.1Luo/J | A | 40.5% (64/158) | ND | - | - | Wei-Hsiang Huang, Liqun Luo | |
| En1-Cre | En1trn2(cre)Wrst/J | Chat/Slc18a3trn1.2Vpra | A or C | 54.6% (95/174 including 17 Cre negative offspring) | 36.2% (54/149 including 3 Cre negative offspring) | - | Janickova et al., 2017 | Marco A.M. Prado, Vania F. Prado |
| Foxd1-Cre | B6;129S4-Foxd1trn1(GFP/cre)Amc/J | Ai9e | B | 0 (from >7 litters) | 0 (from >7 litters) | - | - | Wenjia You, Constance L. Cepko |
| Foxg1-Cre | 129(Cg)-Foxg1trn1(cre)Skm/J | Gt(ROSA)26Sortrn1Sor | B | 68.8% (11/16) | ND | - | Weng et al., 2008 | - |
| Gad2-IRES-Cre | B6.Cg-Gad2trn2(cre)Zjh/J | stxbp1trn1Mver | E or G | - | - | ~50% (from >17 litters) | Kovacevic et al., 2018 | Matthijs Verhage |
| B6N.Cg-Gad2trn2(cre)Zjh/J | Rai1trn2.1Luo/J | A | 0 (0/26) | ND | - | - | Wei-Hsiang Huang, Liqun Luo | |
| B6-Gad2trn2(cre)Zjh/J | Ai14e | E or G | 0 (from >6 years breeding) | 0 (from >6 years - breeding) | - | - | Kevin T. Beier | |
| GFAP-Cre | Tg(GFAP-cre)25Mes | Gja1trn1Kwi | C | 16.7% (7/42) of Cre negative offspring | 50% (8/16) of Cre negative offspring | - | Zhang et al., 2013 | - |
| Tg(GFAP-cre)25Mes | Epas1trn1Mcs/J | A or C | 50% (9/18) | 42.9% (6/14) | - | - | Ariane Pereira, Jeremy N. Kay | |
| GFAP-Cre | B6.Cg-Tg(Gfap-cre) 77.6Mvs/2J | Slc16a1lox/lox | C | observed (100% from a few litters) | <1% (from >35 litters) | - | - | Thomas Phillips, Jeffrey Rothstein |
| GLAST-CreERT2 | Tg(Slc1a3-cre/ERT) 1Nat/J | Nlgn2trn1.1Sud/J | A | - | - | 0(0/160) | Stogsdill et al., 2017 | Jeff Stogsdill, Cagla Eroglu |
| Tg(Slc1a3-cre/ERT) 1Nat/J | Gt(ROSA) 26Sortrn14(CAG-tdTomato)Hze/J | A | - | - | 0(0/160) | Stogsdill et al., 2017 | Jeff Stogsdill, Cagla Eroglu | |
| Gpr26-Cre | B6-Tg(Gpr26-cre) K0250Gsat/Mmucd | Clstn3trn1Amcr/J | C | observed | 0(0/92) | - | - | Lin Luo, Ann Marie Craig |
| B6-Tg(Gpr26-cre) K0250Gsat/Mmucd | Ai32e | B | 27.6% (8/29 including 5 Cre negative offspring) | 0 (0/23) | - | - | Lin Luo, Ann Marie Craig | |
| Grik4-Cre | B6-Tg(Grik4-cre) G32–4Stl/J | Khdrbs3trn1.1Schei/J | E | - | - | 37.5% (12/32) | - | Lisa Traunmuuller, Andrea Gomez, Peter Scheiffele |
| B6-Tg(Grik4-cre) G32–4Stl/J | Khdrbs3trn1.1Schei/J | C | 0% (0/42) | ND | - | - | Lisa Traunmuuller, Andrea Gomez, Peter Scheiffele | |
| B6-Tg(Grik4-cre) G32–4Stl/J | Rpl22trn1.1Psam/J | C | 0% (0/10) | ND | - | - | Lisa Traunmuuller, Andrea Gomez, Peter Scheiffele | |
| B6-Tg(Grik4-cre) G32–4Stl/J | Fgf22trn1a(EUCOMM)Hmgu | A, B, C, F, G | - | - | 0(0/16) | Terauchi et al., 2017 | Hisashi Umemori | |
| Grik4-Cre | Grik4trn1(cre)Ksak | Grin2btrn1Ksak | Not specified | - | - | observed | Akashi et al., 2009 | - |
| Grik4trn1(cre)Ksak | Ai9e | D | 71.4% (5/7) of Cre negative offspring | 48.3% (14/29) of Cre negative offspring | - | - | Yu Itoh-Maruoka, Tomohiko Maruo, Kenji Sakimura, Kenji Mandai, Yoshimi Takai | |
| Grik4trn1(cre)Ksak | Grik2trn1.1Ksak | C | 95% (38/40) of Cre negative offspring | 0(0/31) | - | - | Junko Motohashi, Michisuke Yuzaki | |
| Htr3a-Cre | Tg(Htr3a-cre) N0152Gsat/Mmucd | Ai14e | multiple | - | - | ~20%−50% (from >60 litters) | - | Kenneth Pelkey, Chris J. McBain |
| Isl1-Cre | lsHtrn1(cre)Sev/J | Ptf1atrn3Cvw | A | 0 (from 2 litters) | 0 (from 2 litters) | - | - | Ariane Pereira, Jeremy N. Kay |
| Klf3-CreERT2 | B6;129P-Klf3trn1(cre/ERT2)Pzg/J | Ai9e | B | 0 (from >15 litters) | 0 (from >15 litters) | - | - | Wenjia You, Constance L. Cepko |
| Nestin-Cre | Tg(Nes-cre)1Kln/J | Gja1trn8Kwi | Not specified | - | - | 28.6% (4/14) of Cre negative offspring | Zhang et al., 2013 | - |
| Tg(Nes-cre)1Kln/J | Ai34e | B | 12.5% (1/8) | 20% (2/10) | - | - | Jiexin Wang, Pascal S. Kaeser | |
| Tg(Nes-cre)1Kln/J | Rai1trn2.1Luo/J | A | 79.1% (117/148) | ND | - | Huang et al., 2016, 2018 | Wei-Hsiang Huang, Liqun Luo | |
| Nestin-Cre | Tg(Nes-cre)1Atp | Runx1trn1Buch | D | - | - | observed in Cre negative offspring | uchholz et al., 2000 | - |
| Tg(Nes-cre)1Atp | Fgf8trn1.3Mrt | A | ~100% | observed | - | Dubois et al., 2006; Trumpp et al., 1999 | - | |
| Tg(Nes-cre)1Atp | Ntf3trn2Jae | F | ~100% | ND | - | Bates et al., 1999 | - | |
| Tg(Nes-cre)1Atp | Smad4trn2.1Cxd | A | ~100% | 0 or less than male | - | Zhou et al., 2003 | - | |
| Tg(Nes-cre)1Atp | Rb1trn3Tyj | A | ~100% | ND | - | MacPherson et al., 2003 | - | |
| NEX-Cre | Neurod6trn1(cre)Kan | Wwp1trn1.1Hkb | C | 0 (0/30) | 0 (0/30) | - | - | Hiroshi Kawabe |
| Neurod6trn1(cre)Kan | Wwp2trn1.1Hkb | C | 0 (0/30) | 0 (0/30) | - | - | Hiroshi Kawabe | |
| Neurod6trn1(cre)Kan | Gt(ROSA)26Sortrn1Sor | multiple | 0 (from >5 litters) | 0 (from >5 litters) | - | Goebbels et al., 2006 | Sandra Goebbels, Klaus A. Nave | |
| Ngn2-CreER | Neurog2trn1(cre/Esr1*)And | Ai9e | B | 0 (from >25 litters) | 0 (from >25 litters) | - | - | Wenjia You, Constance L. Cepko |
| Nkx2.1-Cre | C57BL/6J-Tg(Nkx2–1-cre)2Sand/J | RCE:loxpe | multiple | - | - | ~10%−30% - (from >60 litters) | - | Kenneth Pelkey, Chris J. McBain |
| C57BL/6J-Tg(Nkx2–1-cre)2Sand/J | Ai14e | multiple | - | - | ~10%−30% - (from >60 litters) | - | Kenneth Pelkey, Chris J. McBain | |
| C57BL/6J-Tg(Nkx2–1-cre)2Sand/J | Chat/Slc18a3trn1.2Vpra | C | 5.4% (12/224) | 12.5% (24/192 including 5 Cre negative offspring) | - | Kolisnyk et al., 2017 | Marco A.M. Prado, Vania F. Prado | |
| B6.CD1-Tg(Nkx2–1-cre)2Sand | Mafbttrn1.1Good | H | observed (from >100 litters) | observed (from >100 litters) | - | Pai et al., 2019 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| B6.CD1-Tg(Nkx2–1-cre)2Sand | Maftrn2.1Cbm | H | observed (from > 100 litters) | observed (from > 100 litters) | - | Paietal.,2019 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| B6.CD1-Tg(Nkx2–1-cre)2Sand | Ai14e | H | observed (from >100 litters) | observed (from > 100 litters) | - | Paietal.,2019 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| Ntsr1-Cre | B6.FVB(Cg)-Tg(Ntsri-cre) GN220Gsat/Mmucd | Ai93e | Not specified | - | - | observed | Steinmetz et al., 2017 | - |
| B6.Cg-Tg(Ntsr1-cre) GN220Gsat/Mmucd | Rpl22trn1.1Psam/J | C | ND | 0 (0/21) | - | - | Susanne Falkner, Peter Scheiffele | |
| B6.Cg-Tg(Ntsr1-cre) GN220Gsat/Mmucd | Rpl22trn1.1Psam/J | - | - | - | 8.1 % (3/37) | - | Susanne Falkner, Peter Scheiffele | |
| Nos1-Cre | Nos1trn1(cre)Mgmj | Leprtrn1.1Chua | C | ND | observed | - | Rupp et al., 2018 | - |
| Pcp2/L7-Cre | B6.129-Tg(Pcp2-cre) 2Mpin/J | Tsc1trn1.1DJk | AorE | - | - | ~5% | Tsai et al., 2012 | - |
| B6.129-Tg(Pcp2-cre) 2Mpin/J | Adgrb3trm1Ksak | C | 84% (63/75) of Cre negative offspring | 0 (0/90) | - | Kakegawa etal., 2015 | Junko Motohashi, Michisuke Yuzaki | |
| B6.129-Tg(Pcp2-cre) 2Mpin/J | Atgtrn1Myok | C | 14.3% (3/21) of Cre negative offspring | 0 (0/50) | - | Nishiyama et al., 2007 | Junko Motohashi, Michisuke Yuzaki | |
| B6.129-Tg(Pcp2-cre) 2Mpin/J | PhotonSABER-LSL | c | 69% (58/84) of Cre negative offspring | 0 (0/256) | - | Kakegawa etal., 2018 | Junko Motohashi, Michisuke Yuzaki | |
| Pcp2/L7-Cre | B6.129-Pcp2trn1(cre)Nobs | Rpl22trn1.1Psam/J | c | 0(0/11) | ND | - | - | Elisabetta Furlanis, Peter Scheiffele |
| B6.129-Pcp2trn1(cre)Nobs | Rpl22trn1.1Psam/J | G | 0(0/4) | 0(0/4) | - | - | Elisabetta Furlanis, Peter Scheiffele | |
| Pou4f2-Cre | Pou4f2trn1(cne)Bnt/J | Ai9e | B | ~100% | 0 | - | Simmons etal., 2016 | - |
| Pvalb-2A-Cre | B6.Cg-Pvalbtrn1.1(cre)Aibs/J | B6.129S4-Clocktrn1Rep/J | F | 50% (24/48) | <5% | - | Kobayashi and Hensch, 2013 | - |
| Pvalb-IRES-Cre | B6.Cg-Pvalbtrn1.1(cre)Aibs/J | Khdrbs3trn1.1Schei/J | D | ND | 0 (0/23) | - | - | Elisabetta Furlanis, LisaTraunmuller, Peter Scheiffele |
| B6.129P2-Pvalbtrn1(cre)Arbr/J | Rpl22trn1.1Psam/J | A | ND | 0(0/19) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6.129P2-Pvalbtrn1(cre)Arbr/J | Rpl22trn1.1Psam/J | B | 0 (0/24) | ND | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6.129P2-Pvalbtrn1(cre)Arbr/J | Rpl22trn1.1Psam/J | F | 0(0/11) | ND | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6.129P2-Pvalbtrn1(cre)Arbr/J | Rpl22trn1.1Psam/J | E | 0(0/16) | 0(0/16) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6.129P2-Pvalbtrn1(cre)Arbr/J | Rpl22trn1.1Psam/J | G | 0 (0/3) | 0 (0/3) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6.129P2-Pvalbtrn1(cre)Arbr/J | Trpm7trn1Clph | C | 0 (0/57) | ND | - | - | Cui Chen, Wei Li, Nashat Abumaria | |
| Rbp4-Cre | Tg(Rbp4-cre) KL100Gsat/Mmucd | Ai93e | Not specified | - | - | observed | Steinmetz etal., 2017 | - |
| B6.Cg-Tg(Rbp4-cre) KL100Gsat/Mmucd | Rpl22trn1.1Psam/J | E | 0(0/14) | 0(0/14) | - | - | Susanne Falkner, Peter Scheiffele | |
| B6.Cg-Tg(Rbp4-cre) KL100Gsat/Mmucd | Rpl22trn1.1Psam/J | B | ND | 0(0/16) | - | - | Susanne Falkner, Peter Scheiffele | |
| B6.FVB(Cg)-Tg(Rbp4-cre)KL100Gsat/Mmucd/GENSAT | Gt(ROSA) 26Sortrn2(CAG-tdTomato)Fawa | F | 26.7% (4/15) | ND | - | - | Naosuke Hoshina, Hisashi Umemori | |
| Rgs9-Cre | Rgs9trn1(cre)Yql | Cnr1trn1.2Ltz | Not specified | - | - | observed | Davis et al., 2018 | - |
| Rgs9trn1(cre)Yql | B6.Cg-Rem2trn3551(T2A-mkate2)Arte | A | 7.6% | 55.1% | - | Liput, 2018 | - | |
| Rorb-Cre | B6.129S-Rorbtrn1.1(cre)Hze/J | Ai93e | Not specified | - | - | observed | Steinmetz etal., 2017 | - |
| B6.129S-Rorbtrn1.1(cre)Hze/J | Rpl22trn1.1Psam/J | E | 0(0/17) | 0(0/17) | - | - | Susanne Falkner, Peter Scheiffele | |
| B6.129S-Rorbtrn1.1(cre)Hze/J | Rpl22trn1.1Psam/J | G | 0 (0/22) | 0 (0/22) | - | - | Susanne Falkner, Peter Scheiffele | |
| B6.129S-Rorbtrn1.1(cre)Hze/J | Rpl22trn1.1Psam/J | C | ND | 0 (0/20) | - | - | Susanne Falkner, Peter Scheiffele | |
| Scnn1a-Cre | B6;C3-Tg(Scnn1a-cre)2Aibs/J | Rpl22trn1.1Psam/J | A | 0(0/17) | ND | - | - | Elisabetta Furlanis, Peter Scheiffele |
| B6;C3-Tg(Scnn1a-cre)2Aibs/J | Rpl22trn1.1Psam/J | C | 0(0/17) | ND | - | - | Elisabetta Furlanis, Peter Scheiffele | |
| Scx-Cre | Tg(Scx-GFP/cre)1Stzr | Ai9e | B | 0 (from >10 litters) | 0 (from >10 litters) | - | - | Wenjia You, Constance L. Cepko |
| SERT-Cre | B6.129(Cg)-Slc6a4trn1(cre)Xz/J | Lepftrn1.1Chua | C | - | - | ~100% | Lam et al., 2011 | - |
| B6.129(Cg)-Slc6a4trn1(cre)Xz/J | Stxbp1trn1Mver | A | observed (from >20 litters) | observed (from >20 litters) | - | Dudok et al., 2011 | Matthijs Verhage | |
| Sim1-Cre | Tg(Sim1-cre)1Lowl/J | Rai1trn2.1Luo/J | A | 0 (0/95) | ND | - | - | Wei-Hsiang Huang, Liqun Luo |
| Six3-Cre | Tg(Six3-cre)69Frty/GcoJ | Isl1trn1.1Whk | A | 1/1 | 2/3 | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay |
| Tg(Six3-cre)69Frty/GcoJ | Syktrn1.2Tara | A | 1/1 | 1/2 | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay | |
| Tg(Six3-cre)69Frty/GcoJ | Tgfb3trn1Moaz | A | 1/2 | 3/4 | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay | |
| Tg(Six3-cre)69Frty/GcoJ | Flrt2trn1c(EUCOMM)Wtsi | A | 3/3 | 4/4 | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay | |
| Tg(Six3-cre)69Frty/GcoJ | Ptf1atrn3Cvw | A | 1/1 | 1/1 | - | Ray et al., 2018 | Ariane Pereira, Jeremy N. Kay | |
| Tg(Six3-cre)69Frty/GcoJ | Pcdhgtrn2Xzw | multiple | observed | observed | - | Ing-Esteves etal., 2018 | Joshua R. Sanes | |
| Tg(Six3-cre)69Frty/GcoJ | Pcdhaem1Jrs | multiple | observed | observed | - | Ing-Esteves etal., 2018 | Joshua R. Sanes | |
| Tg(Six3-cre)69Frty/GcoJ | Chat/Slc18a3trn1.2Vpra | A or C | 51.9.% (177/341 100% (12/12 including 58 Cre including 4 Cre negative offspring) negative offspring) | - | Martyn et al., 2012 | Marco A.M. Prado, Vania F. Prado | ||
| Sox10-Cre | Tg(Sox10-cre)1Wdr | Gria2trn3Rsp | F | observed | 0 | - | Kougioumtzidou etal., 2017 | - |
| Tg(Sox10-cre)1Wdr | Gjb2trn1Ugds | Not specified | observed | 0 or less than male | Crispino et al., 2011; Takada etal., 2014 | - | ||
| B6;CBA-Tg(Sox10-cre) 1Wdr/J | Slc16a1lox/lox | C | ND | 0 (from >35 litters) | - | - | Thomas Phillips, Jeffrey Rothstein | |
| Sst-IRES-Cre | B6-Ssttrn2.1(cre)Zjh | Ai9e | multiple | - | - | <5% | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele |
| B6-Ssttrn2.1(cre)Zjh | Khdrbs3trn1.1Schei/J | E | 0 (0/5) | 0 (0/5) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6-Ssttrn2.1(cre)Zjh | Khdrbs3trn1.1Schei/J | C | 0(0/16) | 0(0/10) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6-Ssttrn2.1(cre)Zjh | Rpl22trn1.1Psam/J | E | 0 (0/26) | 0 (0/26) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6-Ssttrn2.1(cre)Zjh | Rpl22trn1.1Psam/J | C | 0(0/12) | 0(0/2) | - | - | Elisabetta Furlanis, Lisa Traunmuller, Peter Scheiffele | |
| B6; 129S4;CD1-Ssttrn2.1(cre)Zjh | Mafbtrn1.1Good | H | 0 (from >60 litters) | 0 (from >60 litters) | - | Pai et al., 2019 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| B6; 129S4;CD1-Ssttrn2.1(cre)Zjh | Maftrn2.1Cbm | H | 0 (from >60 litters) | 0 (from >60 litters) | - | Pai et al., 2019 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| B6; 129S4;CD1-Ssttrn2.1(cre)Zjh | Ai14e | H | 0 (from >60 litters) | 0 (from >60 litters) | - | Pai et al., 2019 | Emily Ling-Lin Pai, John L.R. Rubenstein | |
| Synapsin1-Cre | B6.Cg-Tg(Syn1-cre)671Jxm/J | Prkar2btrn3Gsm | F | observed | 0 or less than male | - | Zheng et al., 2013 | - |
| B6.Cg-Tg(Syn1-cre)671Jxm/J | Hif1atrn1Rsjo | C | 63% | 0 | - | Zheng et al., 2013 | - | |
| B6.Cg-Tg(Syn1-cre)671Jxm/J | Erc2trn1.1Sud/J | A | ND | 0 (0/39) | - | - | Jiexin Wang, Pascal S. Kaeser | |
| Thy1-CreER | Tg(Thy1-cre/ERT2,-EYFP) HGfng/PyngJ | Fgf22trn1a(EUCOMM)Hmgu | A, B, C, D, E, F | - | - | 0 (0/27) | - | Hisashi Umemori |
| VAChT.Cre.Fast | B6;129− Tg(SLC18A3-cre)KMisa/0 | Chat/Slc18a3trn1.2Vpra | A or C | 6.1% (7/115) | 1.3% (1/76) | - | - | Marco A.M. Prado, Vania F. Prado |
| VGAT/VIAAT-Cre | Slc32a1trn2(cre)Lowl/J | Rai1trn2.1Luo/J | A | 0(0/103) | ND | - | - | Wei-Hsiang Huang, Liqun Luo |
| VGAT/VIAAT-Cre | B6.FVB-Tg(Slc32a1-cre) 2.1Hzo/FrkJ/ | Dnmt3atrn3.1Enl | AorF | 60.9% (14/23) | 0.7% (from >100 mice) | - | - | Laura Lavery, Huda Y. Zoghbi |
| VGluT1-IRES2-Cre-D | Slc17a7trn1.1(cre)Hze/J | Ai34e | A, F, H | 33.3% (10/30) | 38.5% (5/13) | - | - | Jiexin Wang, Pascal S. Kaeser |
| B6;129S-Slc17a7trn1.1(cre)Hze/J | Rai1trn2.1Luo/J | A | 0 (0/20) | ND | - | - | Wei-Hsiang Huang, Liqun Luo | |
| VGluT2-IRES-cre | Slc17a6trn2(cre)Lowl/J | Rai1trn2.1Luo/J | A | 0 (0/142) | ND | - | - | Wei-Hsiang Huang, Liqun Luo |
| Slc17a6trn2(cre)Lowl/J | Ai14e | E or G | 0 (from >3 years breeding) | 0 (from >3 years breeding) | - | - | Kevin T. Beier | |
| VGluT3-Cre | Tg(Slc17a8-icre) 1Edw/SealJ | Chat/Slc18a3trn1.2Vpra | C | 1.9% (5/265 including 2 Cre negative offspring) | 1.9% (1/52) | - | - | Marco A.M. Prado, Vania F. Prado |
| Tg(Slc17a8-icre) 1Edw/SealJ | Ai14e | multiple | - | - | ~30% (from >60 litters) | - | Kenneth Pelkey, Chris J. McBain | |
| VIP-Cre | B6-Viptrn1(cre)Zh/J | Khdrbs3trn1.1Schei/J | C | 0 (0/22) | ND | - | - | Lisa Traunmuller, Peter Scheiffele |
| B6-Viptrn1(cre)Zh/J | Khdrbs3trn1.1Schei/J | E | 0 (0/7) | 0(0/7) | - | - | Lisa Traunmuller, Peter Scheiffele | |
| B6-Viptrn1(cre)Zh/J | Rpl22trn1.1Psam/J | E | 0(0/31) | 0(0/31) | - | - | Lisa Traunmuller, Peter Scheiffele | |
| B6-Viptrn1(cre)Zh/J | Ai9e | multiple | - | - | <10% | - | Lisa Traunmuller, Peter Scheiffele | |
| Wnt1-Cre | B6.Cg-H2afvTg(Wnt1-cre)11Rth | Gt(ROSA)26Sortrn1Sor | B | 0 (0/8) | 0 (0/6) | - | Weng et al., 2008 | - |
| B6.Cg-H2afvTg(Wnt1-cre)11Rth | Neo1trn1.1Jfcl | F | 0 (0/37) | ND | Emilie Dumontier, Jean-Francois Cloutier |
Breeding strategy: A: Targetf/+; Cre driver X Targetf/f; B: Targetf/+; Cre driver X Target+/+; C: Targetf/f; Cre driver X Targetf/f; D: Targetf/f; Cre driver X Target+/+; E: Targetf/+; Cre driver 5; F: Targetf/+; Cre driver X Targetf/+; G: Targetf/f; Cre driver 5; H: Targetf/f; Cre driver X Targetf/+.
The numbers (x/y) indicate that x offspring with germline recombination were found from y offspring with the target locus in cumulative data from multiple litters. ND indicates not determined.
Where no contributors are listed, the information is from the reference. Where contributors are listed, the associated publication reported findings from the crosses, but not information about germline recombination.
Contributors providing information on germline recombination. Please contact the Lead Contact for the electronic addresses of principal investigators.
Ai9: B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J; Ai14: B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; Ai32: B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J; Ai93: B6;129S6-Igs7tm93.1(tetO-GCaMP6f)Hze/J; ROSAmT/mG: Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; RCE:loxp: Gt(ROSA)26Sortm1.1(CAG-EGFP)Fsh/Mmjax; Ai34: 129S-Gt(ROSA)26Sortm34.1(CAG-Syp/tdTomato)Hze/J.
A comprehensive resource of relevant information on Cre driver lines could be invaluable to mitigate undesired germline recombination by serving as a guide for choosing among similar Cre lines and for designing optimal breeding schemes. We thus pooled information to present new combined data on germline recombination rates and parental sex effects for Cre driver lines for neuroscience research. The collective data from all previously published and unpublished sources are reported in Table 1.
Of the 64 Cre driver lines analyzed, over half (64.1%) exhibited some germline recombination. The mosaic nature of germline deletion for most of the Cre driver lines renders the genotype of individual offspring to be unpredictable. Furthermore, of the 29 Cre driver lines for which sufficient information is available on parental sex effects, the majority (82.8%) showed a sex bias, with 62.1% demonstrating germline recombination solely or selectively through the male parent and 20.1% solely or selectively through the female parent. Only 17.2% showed nearly equal rates of germline recombination in male and female parents. These findings highlight the importance of assessing potential germline recombination for every mouse and the value of tracking parental sex bias toward optimizing breeding schemes to minimize unwanted germline recombination.
Recombination in Germline Cells
As discussed in the introduction, Cre activity in the germline cells of the ovary or testes mediates germline recombination. Relevant to the Cre driver lines chosen as examples here, native Gpr26 is expressed in the testes (Jones et al., 2007), consistent with the selective paternal germline recombination of Gpr26-Cre. However, native Dlx5 and Dlx6 are expressed in both the ovary and testes (Bouhali et al., 2011; Nishida et al., 2008); yet, only maternal germline recombination was observed for Dlx5/6-Cre. Moreover, expression in the ovary or testes may not reflect expression by germline cells, and the Cre driver lines may not reproduce native expression patterns. Recent scRNA-seq data from male germline cells (Lukassen et al., 2018a, 2018b) circumvents the former limitation. Yet, even restricting analyses to KI Cre driver lines, expression levels of the native driver genes at these KI loci in male germline cells showed no apparent relation to whether the Cre driver line mediated paternal germline recombination (Figure S1). Circumventing the second limitation, that Cre expression may not be adequately reflected by the native driver gene expression, the Cre portal of the MGI database (Bult et al., 2019; Heffner et al., 2012) reports Cre recombinase activity patterns for many lines. For the majority (75%) of the 16 Cre driver lines with information about reproductive system germline cell activity in the MGI database, the data were consistent with our findings on germline recombination in Table 1. For 3 cases, germline cell Cre activity was reported but recombination was not observed; for example, ChAT-Cre was positive for Cre recombinase activity in oocytes (MGI) yet did not exhibit maternal germline recombination at any of several target loci (Table 1). Tg(Grik4-cre)G32–4Stl and Sst-IRES-Cre were listed as negative for Cre recombinase activity in germline cells yet showed some germline recombination, although for Sst-IRES-Cre only at one of six target loci, thus consistent with the MGI data for most target loci. Among the 30 other lines that showed germline recombination in Table 1, the MGI database did not list any as negative for Cre recombinase activity in germline cells, although several were listed as negative either in the testes or ovary. Thus, a conservative interpretation of the MGI database Cre recombinase activity may be helpful to predict the occurrence of germline recombination. Basing predictions for germline recombination at the majority of target loci on Cre activity in germ cells yields good measures with precision 0.700, recall0.875, accuracy 0.750, diagnostic odds ratio 11.67, and F1 score0.778 (from 16 lines: 7 true positive, 5 true negative, 3 false positive, and 1 false negative).
In principle, one would expect mice expressing Cre recombinase fused with the estrogen receptor (CreER) to lack germline recombination unless they are exposed to tamoxifen to activate the CreER. Indeed, of the 7 driver lines studied here that express CreER or the improved version CreERT2 (Feil et al., 1997), 85.7% did not show germline recombination. However, Tg(hs799-cre/ERT2,-GFP)405Jlr showed maternal germline recombination in the absence of tamoxifen administration (Table 1). This mouse line exhibits some tamoxifen-independent activity of CreERT2, possibly associated with high expression of the CreERT2 allele (Silberberg et al., 2016). Such tamoxifen-independent activity can also lead to an age-dependent increase in cell-specific recombination; for example, untreated Tg(Plp1-cre/ERT)3Pop mice showed increasing recombination in oligodendrocytes with age (Traka et al., 2016). Thus, it is not safe to assume that CreER driver lines lack germline recombination.
Recombination in Zygotes
Our data in Table 1 focus on germline recombination occurring when the Cre driver and the target locus are together in the male or female germline cells of F1 mice, resulting in the transmission of a recombined allele to F2 mice. It is also possible for recombination to occur directly in the F1 zygote when the Cre driver is inherited from one parent and the target locus from the other parent, resulting in global recombination and also germline transmission of the recombined allele. Indeed, this occurs with deleter (Tg(CMV-cre)1Cgn) and EIIa-Cre (Tg(EIIa-cre) C5379Lmgd) mouse lines commonly used in crosses with floxed mice to generate lines with global recombination (Lakso et al., 1996; Schwenk et al., 1995). In F1 mice crossing EIIa-Cre with a floxed target, half of the mice showed global recombination resulting from Cre activity in the one-cell zygote and half showed mosaic recombination (Lakso et al., 1996). Furthermore, virtually all floxed loci undergo global recombination in F1 offspring of females carrying Vasa-Cre (Tg(Ddx4-cre)1Dcas), even in offspring lacking Cre, due to apparent perdurance of Cre protein in the zygote (Gallardo et al., 2007). However, global recombination was not commonly observed in our F1 mice combining Cre drivers and target loci. For example, we observed no recombination in tail tissue of F1 mice from Dlx5/6-Cre X Ai32 crosses (0/9 with maternal Cre) or Gpr26-Cre X Ai32 crosses (0/19 with paternal Cre) despite global recombination in some F2 mice (Figures 1 and 2). Furthermore, in the crosses described in Figures 1A and 2A, recombination in the F2 zygote would likely affect both Clstn3f/f alleles, yet recombination was only observed for one of the two alleles, suggesting that recombination occurred in germline cells of F1 mice but not in F2 zygotes. Similarly, in the publications discussed here reporting global recombination in F2 mice resulting from germline recombination in F1 mice, global recombination was not observed in F1 mice where analyzed (Simmons et al., 2016; Weng et al., 2008). Thus, recombination can occur in zygotes combining a Cre driver and a target locus but the prevalence appears to be considerably lower than in germline cells carrying both the Cre driver and the target locus.
Comparisons among Related Cre Driver Lines
Different Cre driver lines with some common transcriptional regulatory elements frequently behaved differently regarding germline recombination. Perhaps the most interesting comparison is for the pairs of Cre driver lines targeting common transcriptional regulatory elements by both random transgenic insertion and KI approaches. For one such pair, Grik4-Cre, germline recombination was observed with both approaches. For the other two such pairs, Pcp2/L7-Cre and VGAT/VIAAT-Cre, germline recombination was observed for the transgenic line but not the KI line. While we cannot rule out differences related to target locus selectivity (see below), intrinsic differences between these related Cre driver lines seems likely. Potential factors contributing to germline recombination in the transgenic lines include ectopic expression due to a limited regulatory region, genetic and epigenetic effects of the transgene insertion site, and high expression due to multi-copy integration (although the latter would not apply to the Pcp2/L7-Cre transgenic line, which was generated through embryonic stem cells to avoid multi-copy integration (Barski et al., 2000).
Differences in germline recombination were observed even among Cre driver lines generated using similar strategies. Both Nestin-Cre transgenic lines showed germline recombination but with some differences in frequencies. The four CaMKII-Cre transgenic lines were generated with similar targeting strategies (Dragatsis and Zeitlin, 2000; Minichiello et al., 1999; Rios et al., 2001; Tsien et al., 1996a). While all four CaMKII-Cre lines showed paternal germline recombination, two lacked maternal germline recombination, one had a low rate, and the last was not tested maternally. Comparing the two lines generated with exactly the same strategy (Minichiello et al., 1999; Rios et al., 2001), Tg(Camk2a-cre)93Kln showed stronger overall Cre expression than Tg(Camk2a-cre)159Kln (Tolson et al., 2010) and a higher rate of paternal germline recombination. Differences were also observed between the two Emx1-Cre KI lines and between the two GFAP-Cre lines. In both cases, one line showed roughly equal paternal and maternal germline recombination and the other only paternal germline recombination. In comparing the two Pvalb-Cre KI lines, Pvalb-IRES-Cre did not exhibit germline recombination in multiple crosses from different labs, while Pvalb-2A-Cre showed germline recombination through both parents. These findings are consistent with the stronger overall Cre activity in Pvalb-2A-Cre mice than in Pvalb-IRES-Cre mice (Madisen et al., 2010) and detection of Cre activity in spermatids of Pvalb-2A-Cre but not Pvalb-IRES-Cre mice (Kobayashi and Hensch, 2013). Using tools such as an IRES to attenuate Cre expression may be beneficial to reduce germline recombination for KI driver lines where Cre expression in germ cells is lower than that in the nervous system. Song and Palmiter (2018) reported success in reducing germline recombination by generating a new Cre driver line with attenuated Cre expression by altering the codons, removing a nuclear localization signal, or adding destabilizing signals.
Target Locus Selectivity
The germline recombination prevalence could also depend on the specific target locus. Among the Cre driver lines crossed with multiple target loci, 81.6% (31/38) showed consistent results for all target loci in terms of occurrence of germline recombination and parental sex bias where known. Quantitative data for multiple targets were available for nine of these lines, of which the majority (six) showed target-specific differences. In addition to Dlx5/6-Cre as mentioned above, Tg(Camk2a-cre)93Kln, Tg(Nes-cre)1Kln, Tg(Pcp2-cre)2Mpin, Tg(Six3-cre)69Frty, and Tg(Slc17a8-icre)1Edw showed substantial locus-dependent differences in germline recombination rates. For example, Tg(Pcp2-cre)2Mpin generated Cre-negative germline-recombined offspring at rates of 14.3%, 69.0%, or 84.0% at different floxed loci. Furthermore, 15.8% (6/38) of the Cre driver lines showed germline recombination at some target loci but not others. In most (5/6) cases, recombination occurred for reporter genes at the Rosa26 locus but not for other floxed target genes. Another line showed target-consistent germline recombination with paternal Cre but recombined only at the Rosa26 locus with maternal Cre. Thus, overall, the majority of Cre driver lines behaved consistently in terms of the presence of germline recombination events and parental sex effects at different target loci, but the target loci influenced recombination rates over a wide range. Target locus-specific differences in recombination could be due to differences in the length of loxP-flanked sequences, chromosomal location, epigenetic modification, and accessibility reflected by transcriptional activity in germ cells (Liu et al., 2013; Long and Rossi, 2009; Zheng et al., 2000). Indeed, the Rosa26 locus, which we found particularly prone to germline recombination, is widely used for gene targeting because it supports strong ubiquitous expression and appears to lack gene silencing effects (Soriano, 1999).
These findings dispel the common belief that a reporter can be used as a readout of recombination at another target locus. For example, using the Ai32 RosaLSL-tg locus as a reporter for Dlx5/6-Cre-mediated recombination would have missed nearly half the instances of germline recombination seen at the Clstn3f locus. Conversely, the Ai9 RosaLSL-tg reporter locus showed germline recombination by Sst-IRES-Cre and VIP-Cre not seen at multiple other target loci. While we focus here on undesirable germline recombination, this caveat likely applies more generally, that cell-type-specific recombination at one locus cannot be inferred from recombination at a different locus. Indeed, in the example discussed above crossing female Emx1-Cre;Wwp1f/f;Wwp2f/f with male Wwp1f/f;Wwp2f/f mice, mosaic recombination in tail tissue occurred frequently at the Wwp2 locus with little or none at the Wwp1 locus. Strategies to amplify Cre expression may help to achieve recombination at all floxed target loci in Cre positive cells. For example, in mice intercrossed with the Tg(iSuRe-Cre) line, which both amplifies Cre expression and uses MbTomato as a reporter of recombination, MbTomato-positive cells were recombined at other floxed loci with high confidence (Fer-nández-Chacón et al., 2019).
To demonstrate directly the differential sensitivity of target loci to Cre-mediated germline recombination, we assayed offspring from female Dlx5/6-Cre; Ai32 RosaLSL-tg/+; Clstn3f/+ mice crossed with WT. In this small sample, germline recombination occurred more frequently at the Clstn3f/+ locus than at the Ai32 RosaLSL-tg/+ locus, as expected from the difference in frequencies reported in Table 1. Importantly, one mouse showed germline recombination at the Clstn3f locus but not at the Ai32 RosaLSL-tg locus (Figure 3, lane 2), implying differential recombination in the maternal germ cells. In this case, using Ai32 as a reporter to assess whether recombination occurred at the locus of interest (Clstn3f) would be misleading.
Figure 3. Differential Recombination at Two Target Loci.
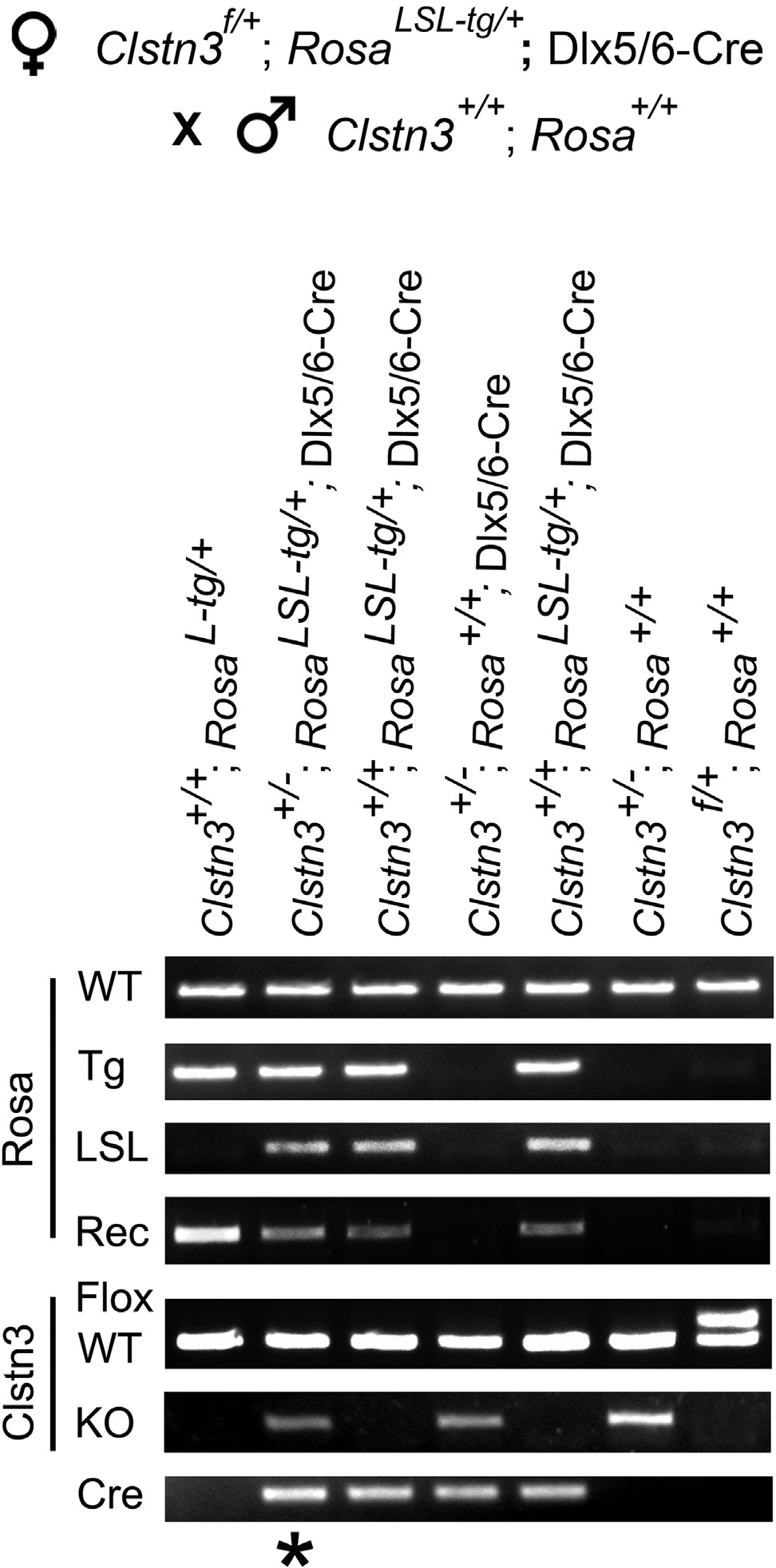
Breeding scheme and genotyping result from tail tissue for a litter from crossing female Clstn3f/+;RosaLSL-tg/+; Dlx5/6-Cre with male WT mice. In one offspring (*), ubiquitous recombination happened at the Clstn3f/+ locus but not at the Ai32 RosaLSL-tg/+ locus implying differential activity of Cre at these target loci in the female germ cells. This mouse exhibited mosaic deletion in tail tissue at the Rosa locus as indicated by the presence of WT, LSL, and Rec PCR bands (see Figure 1B for a diagram).
Broader Implications—Other Recombinase Systemsand Organisms
The principles discussed here apply to all genetically targeted recombinase systems, including lox variants, Flp-frt, and Drerox systems. For example, the En1-Dre KI mouse line shows variable paternal germline recombination (Nouri and Awatramani, 2017). Moreover, the problem of unwanted recombination may be compounded by intersectional strategies involving multiple recombinases. For example, if the desired gene expression requires the cell-specific expression of both Cre and Flp introduced from different driver lines, then germline recombination by Cre will result in gene expression regulated only by Flp, and vice versa. Such intersectional strategies constitute a powerful tool for achieving exquisite cellular specificity in gene targeting (Huang and Zeng, 2013) but require vigilant monitoring to ensure the desired cell-specific expression.
Furthermore, while we focus here on mouse models, the same issues apply to all genetically targeted organisms using site-specific recombinase systems. Similar unwanted germline recombination with a parental sex bias was observed in the rat tyrosine hydroxylase-Cre line (Liu et al., 2016). In this case, recombination occurred in F2 offspring when the Cre driver and the target locus were together in the female germline cells (18/18, including Cre-negative offspring) but not in the male germline cells (0/19) and not in F1 zygotes. Similar unwanted germline recombination was also observed in zebrafish Cre enhancer trap driver lines with differential expression patterns in the brain (Table 2; Tabor et al., 2019). Among the 6 lines surveyed here, 2 showed only paternal germline deletion, 1 only maternal, and 2 with a strong paternal bias. Thus, zebrafish Cre driver lines show varied rates of germline recombination with a parental sex bias, similar to mouse Cre driver lines.
Table 2.
Prevalence of Germline Recombination in Zebrafish Cre Driver Lines that Show Nervous System Recombination
| Cre Line Common Name | Full Cre Line Name/Source | Target Gene/Reporter | Breeding Strategya | Germline Recombination Efficiency, Cre from Fatherb | Germline Recombination Efficiency, Cre from Motherb | Reference/Associated Publicationc | Contributorsd |
|---|---|---|---|---|---|---|---|
| y492-Cre | Et(REX2-SCP1-Ocu.Hbb2:Cre-2A-Cerulean)y492 | Tg(actb2:LOXP-EGFP-LOXP-LY-TagRFPT)y272 | A | 4.8% (3/63) | 0% (0/134) | Tabor et al., 2019 | Jennifer Sinclair, Harold Burgess |
| y547-Cre | Et(REX2-SCP1-Ocu.Hbb2:Cre)y547 | Tg(actb2:LOXP-EGFP-LOXP-LY-TagRFPT)y272 | B | 82.3% (51/62) | 38.1% (8/21) | Tabor et al., 2019 | Jennifer Sinclair, Harold Burgess |
| y549-Cre | Et(REX2-SCP1-Ocu.Hbb2:Cre)y549 | Tg(actb2:LOXP-EGFP-LOXP-LY-TagRFPT)y272 | B | 0% (0/58) | 6.7% (2/30) | Tabor et al., 2019 | Jennifer Sinclair, Harold Burgess |
| y559-Cre | Et(REX2-SCP1-Ocu.Hbb2:Cre)y559 | Tg(actb2:LOXP-EGFP-LOXP-LY-TagRFPT)y272 | B | 3.4% (3/89) | 2.4% (1/42) | Tabor et al., 2019 | Jennifer Sinclair, Harold Burgess |
| y546-Cre | Et(REX2-SCP1-Ocu.Hbb2:Cre-2A-Cerulean)y546 | Tg(actb2:LOXP-EGFP-LOXP-LY-TagRFPT)y272 | B | 51.1% (23/45) | 6.2% (4/64) | Tabor et al., 2019 | Jennifer Sinclair, Harold Burgess |
| y555-Cre | Et(REX2-SCP1-Ocu.Hbb2:Cre)y555 | Tg(actb2:LOXP-EGFP-LOXP-LY-TagRFPT)y272 | A | 3.2% (3/95) | 0% (0/64) | Tabor et al., 2019 | Jennifer Sinclair, Harold Burgess |
Breeding strategy: A: Targetf/f; Cre driver X Target+/+; B: Targetf/+; Cre driver X Target+/+.
The numbers (x/y) indicate that x offspring with germline recombination were found from y offspring with the target locus in cumulative data from multiple clutches.
The associated publication reported the generation and characterization of the Cre driver lines but not detailed information about germline recombination.
Contributors providing information on germline recombination. Please contact the Lead Contact for electronic addresses of principal investigators.
Alternatives to complement genetic strategies to achieve spatially and temporally controlled recombination exist, notably viral vectors to deliver recombinase-dependent expression cassettes to Cre driver lines or to deliver recombinases to floxed target lines. This is a powerful and commonly used approach that circumvents any potential for germline recombination but has other limitations. Perhaps the most serious limitation is animal to animal variability in recombination efficiency and targeted brain regions due to differences in viral vector injection sites. In addition, the small capacity of adeno-associated viral vectors, which are mostly commonly used in the nervous system, limits the potential for cell-type specificity. Despite ongoing improvements through engineering transcriptional control elements and capsids (Bedbrook et al., 2018), viral vectors are unlikely to achieve the highest specificity and reproducibility possible with genetic methods.
Guidelines
We recommend researchers to consider the following suggestions when using Cre driver lines:
Always genotype every animal for the WT, floxed, and recombined alleles at the target locus of interest. This is the only way to ensure all the animals have their expected genotypes. If recombined alleles are observed, concerns of leaky Cre expression in tail or ear tissue could be addressed by testing Cre-negative animals. Beyond the focus here on reducing unwanted germline recombination, the differential sensitivity of distinct target loci to Cre recombinase implies that cell-type-specific recombination patterns must also be confirmed at the locus of interest and not just at a separate reporter locus. Thus, validation by in situ hybridization and/or immunostaining for the target of interest in the region of interest is important to confirm cell-type specificity and efficiency of local recombination.
If there are multiple Cre driver lines that could give the desired Cre expression pattern, check for information on germline recombination rates. Check the MGI database for recombinase activity in male or female germline cells, as such activity is a good predictor of germline recombination. If such information is lacking, typically KI driver lines tend to have lower undesirable germline recombination than random insertion transgenic driver lines, and tools that attenuate Cre expression such as an IRES can reduce germline recombination.
Choose an optimal breeding strategy to reduce or avoid germline recombination. If information on germline recombination frequencies is not available, test breeding strategies with Cre recombinase transmitted exclusively through the male parent or the female parent. Given the parental sex effects observed here for the majority of Cre driver lines, there is often one better way to mitigate or even avoid undesired germline recombination altogether. Detailed strategies for breeding and genotyping are suggested in Figures 4 and 5.
In publishing a paper using Cre driver lines, clearly indicate that all WT, floxed, and recombined alleles were assessed by genotyping, report the frequencies of germline recombination and parental sex bias, and indicate how cell-type-specific recombination at the target locus was assessed. Deposit new information on frequencies of germline recombination and parental sex bias in the MGI database.
Figure 4. Breeding and Genotyping Strategies for Conditional KO/KI Mice.
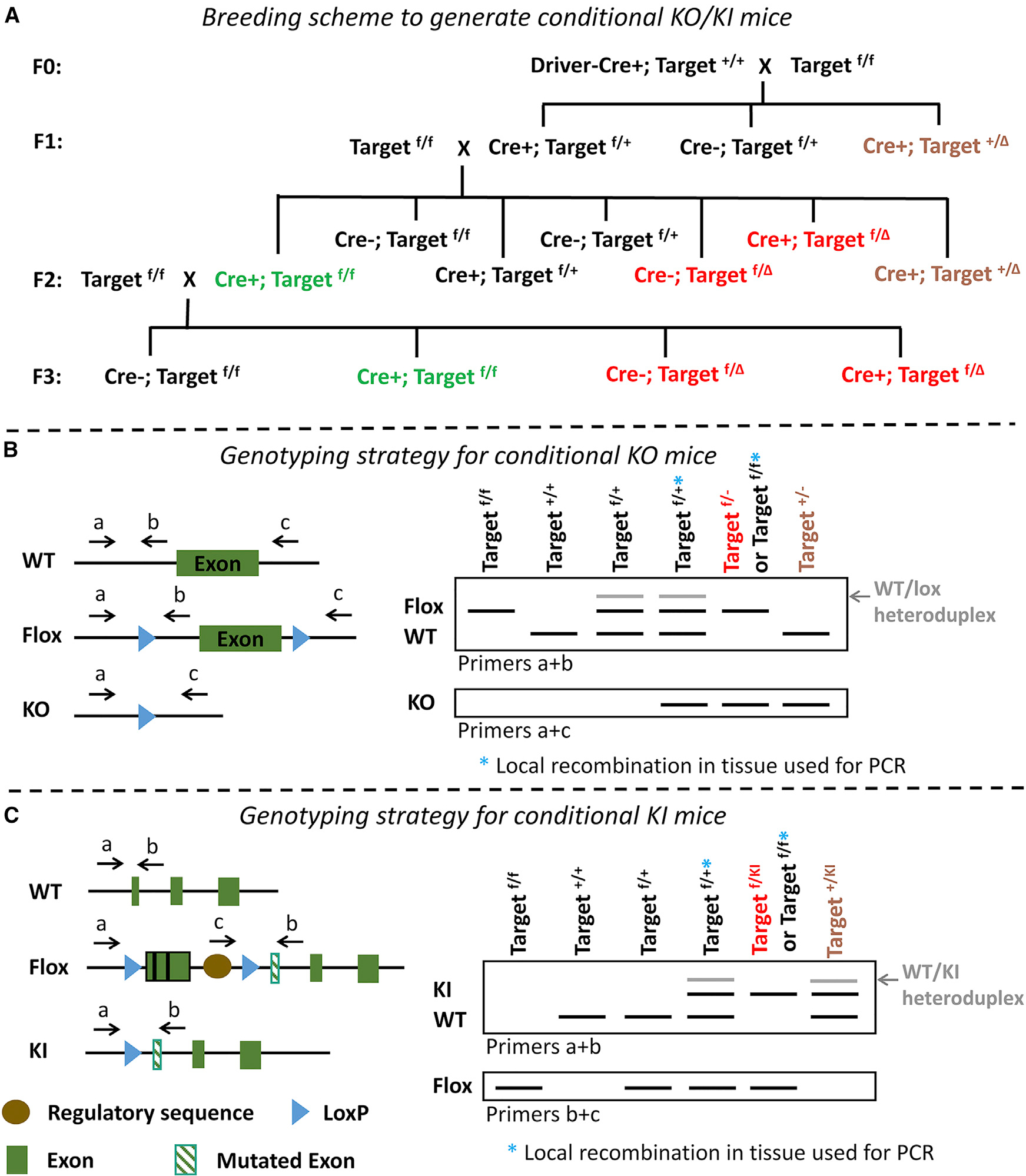
(A) A recommended breeding scheme is outlined. TargetΔ indicates a target allele that has undergone recombination in male or female germline cells (red) or more rarely in zygotes (brown); thus, Target– or TargetKI. Targetf/+ instead of Targetf/f mice can be used for the F0 cross, reducing the frequency of generating Cre+; Targetf/+ mice for the F1 cross. Routine use of F2 crosses to generate experimental mice is recommended to minimize required animal numbers, but F1 crosses can also be used. It is recommended that F1 crosses using both male and female Cre+; Targetf/+ mice be established and the resultant germline recombination rates be tracked in offspring. Then male or female Cre+; Targetf/f mice can be used for the F2 crosses, depending on which sex gave the lowest germline recombination rate in the F1 crosses. It is important that Cre+; Targetf/f mice (green experimental mice) be validated by immunostaining or in situ hybridization for the target protein/RNA in the region of interest to confirm consistent recombination in the expected cell type. Cre–; Targetf/f mice can be used as controls; separate breeding of congenic Cre+; Target+/+ and WT controls is also recommended. “Cre+” refers to mice with one allele of the Cre transgene. In these recommended breeding schemes, Cre+ mice are not bred to Cre+ mice as this would result in a subset of offspring have 2 alleles of the Cre driver gene. This scenario can be problematic. For random insertion transgenic Cre drivers, it is typically not possible to differentiate among mice with one or two Cre driver alleles by PCR genotyping, leading to unknown variation in Cre expression levels upon subsequent breeding of these mice (which could result in further variability in germline recombination rates). For KI Cre driver lines, it is generally possible to differentiate among mice with one or two Cre driver alleles. However, homozygous insertion of the Cre driver may result in deleterious effects not seen with heterozygous Cre drivers, due to possible disruption of the native gene at the random or targeted insertion site. An exception may apply to targeted insertion Cre driver lines shown to have normal native gene expression; then, if one wanted to maximize Cre expression level, one might breed Cre+ with Cre+ mice and select those with 2 Cre alleles for further breeding.(B and C) Recommended genotyping strategies are diagrammed for conditional KO and KI mice, assuming a mini-gene strategy was used for conditional KI. Genotyping should also be done for the presence of the Cre driver gene (as in Figures 1 and 2, not shown here). The black PCR bands are diagnostic, and the gray bands are additional heteroduplexes that may appear. Potential PCR products that are too large to be generated under typical conditions are not diagrammed here, but these may be generated under some conditions (B with a+c primers for WT and Flox alleles, and C with a+b primers for Flox allele). For mice with one target allele, the presence of Flox, WT, and KO/KI bands indicates the occurrence of local recombination in the tissue used for genotyping rather than ubiquitous germline recombination (Targetf/+*). For mice with two target alleles, the presence of Flox and KO/KI bands indicates either germline recombination (Targetf/– or Targetf/KI) or local recombination in the tissue used for genotyping (Targetf/f*). The additional absence of a Cre driver identifies such mice to be Targetf/– or Targetf/KI, but such Cre+ mice would have to be bred further, or local recombination in genotyping tissue ruled out, to determine whether the recombination is germline.
Figure 5. Breeding and Genotyping Strategy for Conditional Reporter Mice.
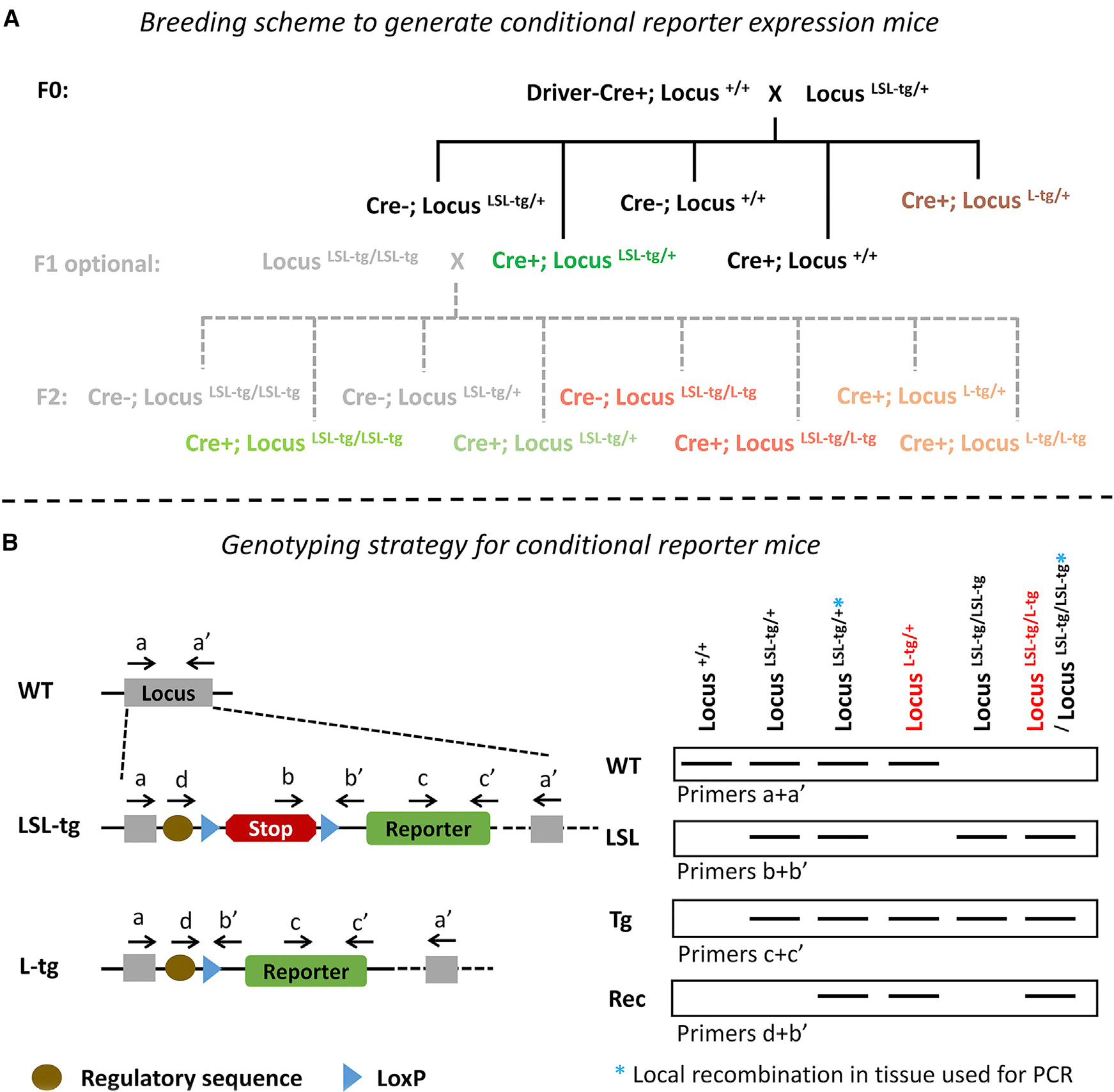
(A) For conditional reporter mice, it is simplest to breed F0 mice and study F1 Cre+;LocusLSL-tg/+ mice. Thus, the Cre driver gene and target locus are not together in the germline so unwanted global recombination could only occur by recombination in the zygote, which is not as common as in germline cells. It is important that Cre+;LocusLSL-tg/+ mice (green, experimental mice) be validated by immunostaining or in situ hybridization for the transgene protein/RNA in the region of interest to confirm consistent recombination in the expected cell type. The optional F1 breeding scheme could be used to increase reporter expression level in Cre+; Locus LSL-tg/LSL-tg mice, but this also results in possible germline recombination. If F1 crosses are performed, both male and female Cre+; LocusLSL-tg/+ mice should be used initially to track resultant germline recombination rates so that the sex resulting in the lowest germline recombination rate can be used in further F1 crosses. LocusLSL-tg+ indicates a lox-stop-lox-transgene cassette that expresses the transgene upon Cre-mediated recombination, but our recommendation applies to other Cre-dependent loci such as those using a flip excision or double-inverted orientation mechanism. LocusL-tg+ indicates a globally recombined locus resulting from recombination in male or female germline cells (red) or more rarely in the zygote (brown). “Cre+” refers to mice with one allele of the Cre transgene (see Figure 4 legend).
(B) A recommended genotyping strategy is diagrammed for conditional reporter mice. Only the first four lanes depicting PCR bands are relevant to F1 mice in the above breeding scheme. Genotyping should also be done for the presence of the Cre driver gene (as in Figures 1 and 2, not shown here). Potential PCR products that are too large to be generated under typical conditions are not diagrammed here, but these may be generated under some conditions (with a+a’ primers for LSL-tg and L-tg alleles, and d+b’ primers for the LSL-tg allele). For mice with one target allele, the presence of WT, LSL, Tg, and Rec bands indicates the occurrence of local recombination in the tissue used for genotyping rather than ubiquitous germline recombination (LocusLSL-tg/+*). For mice with two target alleles, the presence of LSL, Tg, and Rec bands indicates either germline recombination (LocusLSL-tg/L-tg) or local recombination in the tissue used for genotyping (LocusLSL-tg/LSL-tg*). The additional absence of a Cre driver identifies such mice to be LocusLSL-tg/L-tg, but such Cre+ mice would have to be bred further or local recombination in genotyping tissue ruled out to determine whether the recombination is germline.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Ann Marie Craig (acraig@mail.ubc.ca). Inquiries concerning the e-mail addresses of the investigators who contributed information regarding specified Cre driver lines in Table 1 should be directed to the Lead Contact.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All mice used and agencies approving procedures are as follows: the Canadian Council for Animal Care and the University of British Columbia Animal Care Committee: Clstn3tm1Amcr/J (Pettem et al., 2013), Ai32 (Madisen et al., 2012), B6-Tg(dlx5a-cre)1Mekk/J (Zerucha et al., 2000), B6-Tg(Gpr26-cre)KO250Gsat/Mmucd (GENSAT Project); Association for the Assessment and Accreditation of Laboratory Animal Care and Stanford University’s Administrative Panel on Laboratory Animal Care: Tg(Sim1-cre)1Lowl/J (Balthasar et al., 2005), B6.129S1(Cg)-Rai1tm2.1Luo/J (Huang et al., 2016), B6.129S2-Emx1tm1(cre)Krj/J (Gorski et al., 2002), B6N.Cg-Gad2tm2(cre)Zjh/J (Taniguchi et al., 2011), B6.Cg-Tg(Nes-cre)1Kln/J (Tronche et al., 1999), B6J.129S6(FVB)-Slc32a1tm2(cre)Lowl/MwarJ (Vong et al., 2011), B6;129S-Slc17a7tm1.1(cre)Hze/J (Harris et al., 2014), Slc17a6tm2(cre)Lowl/J (Vong et al., 2011); University of California San Francisco Laboratory Animal Research Center: Tg(Nkx2–1-cre)2Sand (Xu et al., 2008), Tg(hs799-cre/ERT2,-GFP)405Jlr (Silberberg et al., 2016), Tg(I12b–cre)1Jlr (Potter et al., 2009), Mafbtm1.1Good (Yu et al., 2013), Maftm2.1Cbm (Wende et al., 2012), Gt(ROSA) 26Sortm32(CAG-tdTomato)Hze, B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Madisen et al., 2010), Ssttm2.1(cre)Zjh (Taniguchi et al., 2011); The Canadian Council for Animal Care and the University Western Ontario Animal Care Committee: Tg(Drd2-cre)ER44Gsat/Mmucd (Gong et al., 2007), En1tm2(cre)Wrst/J (Kimmel et al., 2000), 129(Cg)-Foxg1tm1(cre)Skm/J (Hébert and McConnell, 2000), C57BL/6J-Tg(Nkx2–1-cre)2Sand/J (Xu et al., 2008), Tg(Six3-cre)69Frty/GcoJ (Furuta et al., 2000), B6;129- Tg(SLC18A3-cre)KMisa/0 (Misawa et al., 2003), Tg(Slc17a8-icre)1Edw/SealJ (Divito et al., 2015), B6,129-Chat/Slc18a3tm1.2Vpra (Martins-Silva et al., 2011); Fudan University and Tsinghua University Committees on Animal Care and Use: Trpm7tm1Clph/J (Jin et al., 2008), B6-Tg(Camk2a-cre)T29–1Stl (Tsien et al., 1996b), B6.129P2-Pvalbtm1(cre)Arbr/J (Hippenmeyer et al., 2005); The NINDS Animal Care and Use Committee: Gria1tm2Rsp (Engblom et al., 2008), Gria2tm3Rsp (Shimshek et al., 2006), Gria3tm1Rsp (Sanchis-Segura et al., 2006), Grin1tm2Stl (Tsien et al., 1996b), B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Madisen et al., 2010), Slc6a3tm1.1(cre)Bkmn/J (Bäckman et al., 2006); Nie-dersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit: Wwp1tm1.1Hkb/N (Ambrozkiewicz et al., 2018), Wwp2tm1.1Hkb/N (Ambrozkiewicz et al., 2018), B6.129S2-Emx1tm1(cre)Krj/J (Gorski et al., 2002), Neurod6tm1(cre)Kan (Goebbels et al., 2006), Tg(Camk2a-cre)159Kln (Minichiello et al., 1999), Fdft1tm1Kan (Saher et al., 2005); The Kobe University Animal Care and Use Committee: Grik4tm1(cre)Ksak (Akashi et al., 2009), B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (Madisen et al., 2010); Institutional Animal Care and Use Committee of Harvard Medical School: Tg(Six3-cre)69Frty/GcoJ (Furuta et al., 2000), B6.SJL-Slc6a3tm1.1(cre)Bkmn/J (Bäckman et al., 2006), Rims1tm3Sud/J (Kaeser et al., 2008), Rims2tm1.1Sud/J (Kaeser et al., 2011), Ai34 (MGI Direct Data Submission, J:170755), Tg(Nes-cre)1Kln/J (Tronche et al., 1999), B6.Cg-Tg(Syn1-cre)671Jxm/J (Zhu et al., 2001), Erc2tm1.1Sud/J (Kaeser et al., 2009), Slc17a7tm1.1(cre)Hze/J (Harris et al., 2014), B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (Madisen et al., 2010), B6; Bhlhe22tm3.1(cre)Meg (Ross et al., 2010), B6;129P-Klf3tm1(cre/ERT2)Pzg/J (GenitoUrinary Development Molecular Anatomy Project (GUDMAP)), Neurog2tm1(cre/Esr1)And (Zirlinger et al., 2002), Tg(Scx-GFP/cre)1Stzr (Blitz et al., 2009), B6;129S4-Foxd1tm1(GFP/cre)Amc/J (Humphreys et al., 2010); The Canadian Council for Animal Care and the Montreal Neurological Institute Animal Care Committee: Neo1tm1.1Jfcl (Kam et al., 2016), B6.Cg-H2afvTg(Wnt1-cre)11Rth (Danielian et al., 1998; Rowitch et al., 1999); Stanford University’s Administrative Panel on Laboratory Animal Care: B6.SJL-Slc6a3tm1.1(cre)Bkmn/J (Bäckman et al., 2006), B6-Tg(Drd1-Cre) EY262GSat (Gong et al., 2003), B6-Gad2tm2(cre)Zjh/J (Taniguchi et al., 2011), Slc17a6tm2(cre)Lowl/J (Vong et al., 2011), B6-Tg(Adora2a-Cre)KG139GSat (Gong et al., 2007), B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; the National Institutes of Health: B6.Cg-Gt(ROSA) 26Sortm14(CAG-tdTomato)Hze/J (Madisen et al., 2010), Gt(ROSA)26Sortm1.1(CAG-EGFP)Fsh/Mmjax (Sousa et al., 2009), C57BL/6J-Tg(Nkx2–1-cre)2Sand/J (Xu et al., 2008), Tg(Slc17a8-icre)1Edw/SealJ (Grimes et al., 2011), Tg(Htr3a-cre)NO152Gsat/Mmucd (Chittajallu et al., 2013); Boston Children’s Hospital: C57BL/6-Tg(Grik4-cre)G32–4Stl/J (Nakazawa et al., 2002), B6.FVB(Cg)-Tg(Adora2a-cre) KG139Gsat/Mmucd/GENSAT, B6.FVB(Cg)-Tg(Drd1-cre)EY262Gsat/Mmucd/GENSAT, B6.FVB(Cg)-Tg(Rbp4-cre)KL100Gsat/Mmucd/GENSAT (Gong et al., 2007), B6.SJL-Slc6a3tm1.1(cre)Bkmn/J/JAX (Bäckman et al., 2006), Tg(Thy1-cre/ERT2,-EYFP)HGfng/PyngJ (Young et al., 2008), Gt(ROSA)26Sortm2(CAG-tdTomato)Fawa (Rock et al., 2011), Fgf22tm1a(EUCOMM)Hmgu (Terauchi et al., 2016); Cantonal Veterinary Office of Basel-Stadt, Switzerland: B6-Tg(Camk2a-cre)T29–1Stl, B6.Cg-Cux2tm3.1(cre/ERT2)Mull/Mmmh (Franco et al., 2012), B6-Tg(Grik4-cre)G32–4Stl/J (Nakazawa et al., 2002), B6.Cg-Tg(Ntsr1-cre)GN220Gsat/Mmucd (Gong et al., 2003), B6.129-Pcp2tm1(cre)Nobs (Saito et al., 2005), B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (Madisen et al., 2010), B6.129P2-Pvalbtm1(cre)Arbr/J, B6.Cg-Tg(Rbp4-cre)KL100Gsat/Mmucd (Gong et al., 2003), B6.129S-Rorbtm1.1(cre)Hze/J (Harris et al., 2014), B6;C3-Tg(Scnn1a-cre)2Aibs/J (Madisen et al., 2010), B6-Ssttm2.1(cre)Zjh, B6-Viptm1(cre)Zjh/J (Taniguchi et al., 2011), Khdrbs3tm1.1Schei/J (Traunmüller et al., 2014), Rpl22tm1.1Psam/J (Sanz et al., 2009); Institutional Animal Care and Use Committee at Duke University: B6;129S6-Chattm2(cre)Lowl/J (Rossi et al., 2011), B6.Cg-Csf1rtm1.2Jwp/J (Li et al., 2006), B6.129P2 (Cg)-Cx3cr1tm2.1(cre/ERT2)Litt/WganJ (MGI Direct Data Submission MGI: J:190965), Epas1tm1Mcs/J (Gruber et al., 2007), B6;129-Flrt2tm1c(EUCOMM)Wtsi/RobH (Del Toro et al., 2017), FVB-Tg(GFAP-cre)25Mes/J (Zhuo et al., 2001), Isl1tm1(cre)Sev/J (Yang et al., 2006), Megf10tm1c(KOMP)Jrs (Kay et al., 2012), Tgfb3tm1Moaz (Doetschman et al., 2012), Ptf1atm3Cvw (Krah et al., 2015), Isl1tm1.1Whk (Mu et al., 2008), Tg(Six3-cre)69Frty/GcoJ, B6.129P2-Syktm1.2Tara/J (Saijo et al., 2003), CBy.B6-Gt(ROSA)26Sortm1(HBEGF)Awai/J (Buch et al., 2005), B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (Muzumdar et al., 2007), B6.Cg-Gt(ROSA) 26Sortm14(CAG-tdTomato)Hze/J (Madisen et al., 2010); the Animal Resource Committee of Keio University: Grin2ctm2(icre)Mwa (Miyazaki et al., 2012), Cacna1atm1Kano (Hashimoto et al., 2011), Grik4tm1(cre)Ksak (Akashi et al., 2009), Grik2tm1.1 Ksak (Matsuda et al., 2016), Adgrb3tm1Ksak (Kakegawa et al., 2015), Atg5tm1Myok (Hara et al., 2006), B6.129-Tg(Pcp2-cre)2Mpin/J (Barski et al., 2000), PhotonSABER-LSL; The Institutional Animal Care and Use Committee and the Duke Division of Laboratory Animal Resources oversight: Tg(Slc1a3-cre/ERT)1Nat/J (Wang et al., 2012), Nlgn2tm1.1Sud/J (Liang et al., 2015), Gt(ROSA) 26Sortm14(CAG-tdTomato)Hze/J (Madisen et al., 2010); the nstitutional and Dutch governmental guidelines for animal welfare: B6.129(Cg)-Slc6a4tm1(cre)Xz/J (Zhuang et al., 2005), Stxbp1tm1Mver (Heeroma et al., 2004), B6-Gad2tm2(cre)Zjh/J (Taniguchi et al., 2011), Baylor College of Medicine Institutional Animal Care and Use Committee: Dnmt3atm3.1Enl (Kaneda et al., 2004), B6.FVB-Tg(Slc32a1-cre)2.1Hzo/FrkJ/ (Chao et al., 2010), the Johns Hopkins University Institutional Animal Care and Use Committee: B6.Cg-Tg(Gfap-cre)77.6Mvs/2J (Gregorian et al., 2009), Slc16a1lox/lox (Jha et al., 2019), B6;CBA-Tg(Sox10-cre)1Wdr/J (Matsuoka et al., 2005). Genotyping was performed using primers and tissues described in Table S1.
Procedures involving zebrafish were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee and are described in Tabor et al. (2019).
METHOD DETAILS
Brain slice imaging
Offspring from Ai32 crosses were euthanized at P14–16, brains extracted and sectioned in ice cold artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO47H2O, 2 CaCl2, 26 NaHCO3 and 15 D-glucose which was bubbled continuously with carbogen (95% O2/5% CO2) to adjust the pH to 7.3. Slices (300mm) were then recovered in 31°C ACSF for 20 min and fixed in 4% paraformaldehyde + 4% sucrose in PBS (pH 7.4) for 12 min. They were then washed in PBS containing the nuclear counterstain DAPI (4’,6 diamidino-2-phenylindole), and mounted in elvanol (Tris-HCl, glycerol, and polyvinyl alcohol with 2% 1,4-diazabicyclo[2,2,2]octane). Tiled and individual images were captured on a Zeiss LSM 700 confocal microscope.
DATA AND CODE AVAILABILITY
Information from Table 1 will be incorporated in the Mutation details section for each Cre line in the MGI webpage, tagged in bold as “Germline Recombination.” This particular Mutation details field is already exported to various resources, including mutant mouse repositories for display on strain data pages.
Supplementary Material
Table S1. Genotyping Primers, Related to Table 1.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Mouse: Adgrb3trn1Ksak | A gift from Dr. Kenji Sakimura | MGI:5708584 |
| Mouse: B6-Tg(Adora2a-Cre)KG139GSat | Mutant Mouse Resource & Research Centers (MMRRC) | MMRRC_036158-UCD |
| Mouse: Atg5trn1Myok | Riken BioResource Center | MGI:3663625 |
| Mouse: B6; Bhlhe22trn31(cre)Meg | A gift from Dr. Sarah Ross (Michael E Greenberg Lab) | MGI:4440745 |
| Mouse: Cacna1atrn1Kano | A gift from Dr. Masanobu Kano | MGI:5140539 |
| Mouse: Tg(Camk2a-cre)159Kln | A gift from Klein lab (Minichiello et al., 1999) | MGI:2176753 |
| Mouse: B6-Tg(Camk2a-cre)T29–1Stl | The Jackson Laboratory | IMSR_JAX:005359 |
| Mouse: B6;129S6-Chattrn2(cre)Lowl/J | The Jackson Laboratory | IMSR_JAX:006410 |
| Mouse: B6,129-Chat/Slc18a3trn1.2Vpra | Prado lab (Martins-Silva et al., 2011) | MGI:1101061 |
| Mouse: Clstn3trn1Amcr/J | Craig lab (Pettem et al., 2013) | MGI:5521371 |
| Mouse: B6.Cg-Csf1rtrn1.2Jwp/J | The Jackson Laboratory | IMSR_JAX:021212 |
| Mouse: B6.Cg-Cux2trn3.1(cre/ERT2)Mull/Mmmh | MMRRC | MMRRC_032779-MU |
| Mouse: Cx3cr1trn2.1(cre/ERT2)Litt/WganJ | The Jackson Laboratory | IMSR_JAX:021160 |
| Mouse: B6-Tg(dlx5a-cre)1Mekk/J | The Jackson Laboratory | IMSR_JAX:008199 |
| Mouse: Dnmt3atrn3.1Enl | Dr. Margaret Goodell, Baylor College of Medicine (Can be purchased from Riken BRC) | IMSR_RBRC03731 |
| Mouse: B6-Tg(Drd1-Cre)EY262GSat | MMRRC | MMRRC_017264-UCD |
| Mouse: Tg(Drd2-cre)ER44Gsat/Mmucd | MMRC | MMRRC_017263-UCD |
| Mouse: B6.129S2-Emx1trn1(cre)Krj/J | The Jackson Laboratory | IMSR_JAX:005628 |
| Mouse: En1trn2(cre)Wrst/J | The Jackson Laboratory | IMSR_JAX: 007916 |
| Mouse: Epas1trn1Mcs/J | The Jackson Laboratory | IMSR_JAX: 008407 |
| Mouse: Erc2trn1.1Sud/J | Generated by P.S. Kaeser and T.C. Sudhof, available at the Jackson Laboratory | IMSR_JAX:015831 |
| Mouse: Fdft1trn1Kan | Saher lab (Saher et al., 2005) | MGI:3579504 |
| Mouse: Fgf22trn1a(EUCOMM)Hmgu | European Conditional Mouse Mutagenesis Program (EUCOMM) | IMSR_EM:06822 |
| Mouse: Flrt2trn1c(EUCOMM)Wtsi | EMMA repository | MGI:6119416 |
| Mouse: B6;129S4-Foxd1trn1(GFP/cre)Amc/J | The Jackson Laboratory | IMSR_JAX:012463 |
| Mouse:129(Cg)-Foxg1trn1(cre)Skm/J | The Jackson Laboratory | IMSR_JAX: 006084 |
| Mouse: B6N.Cg-Gad2trn2(cre)Zjh/J | The Jackson Laboratory | IMSR _JAX:019022 |
| Mouse: FVB-Tg(GFAP-cre)25Mes/J | The Jackson Laboratory | IMSR _JAX:004600 |
| Mouse: B6.Cg-Tg(Gfap-cre)77.6Mvs/2J | The Jackson Laboratory | IMSR _JAX:024098 |
| Mouse: B6-Tg(Gpr26-cre) KO250Gsat/Mmucd | MMRRC | MMRRC_036915-UCD |
| Mouse: Gria1trn2Rsp | A gift from Dr. Rolf Sprengel | IMRS_JAX: 019012 |
| Mouse: Gria2trn3Rsp | A gift from Dr. Rolf Sprengel | MGI:3611335 |
| Mouse: Gria3trn1Rsp | A gift from Dr. Rolf Sprengel | MGI:3611328 |
| Mouse: Grik2trn1.1Ksak | A gift from Dr. Kenji Sakimura | MGI:6117330 |
| Mouse: B6-Tg(Grik4-cre)G32–4Stl/J | The Jackson Laboratory | IMSR_JAX:006474 |
| Mouse: Grik4trn1(cre)Ksak | Sakimura lab (Akashi et al., 2009) | MGI: 4360478 |
| Mouse: Grin1trn2Stl | The Jackson Laboratory | IMRS_JAX:005246 |
| Mouse: Grin2ctrn2(icre)Mwa | A gift from Dr. Masahiko Matanabe | MGI:5306941 |
| Mouse: CBy.B6-Gt(ROSA)26Softrn1(HBEGF)Awai/J | The Jackson Laboratory | IMRS_JAX: 007900 |
| Mouse: Gt(ROSA) 26Soitr1.1(CAG-EGFP)Fsh/Mmjax | The Jackson Laboratory | MMRRC_ 32037-JAX |
| Mouse: Gt(ROSA)26Sortrn2(CAG-tdTomato)Fawa | Dr. Fan Wang (Duke) | MGI:5305341 |
| Mouse: Gt(ROSA) 26Sortrn4(ACTB-tdTomato,-EGFP)Luo/J | The Jackson Laboratory | IMRS_JAX:007676 |
| Mouse: B6.Cg-Gt(ROSA) 26Sortrn9(CAG-tdTomato)Hze/J | The Jackson Laboratory | IMSR_JAX: 007909 |
| Mouse: Ai14: B6.Cg-Gt(ROSA)26Sortrn14(CAG-tdTomato)Hze/J | The Jackson Laboratory | IMSR_JAX:007914 |
| Mouse: Ai32: B6;129S-Gt(ROSA)26Sortrn32(CAG-COP4*H134R/EYFP)Hze/J | A gift from Dr. Timothy Murphy, originally from The Jackson Laboratory | IMSR_JAX:012569 |
| Mouse: Gt(ROSA)26Sortrn32(CAG-tdTomato)Hze | The Jackson Laboratory | IMSR_JAX:007908 |
| Mouse: Ai34:129S-Gt(ROSA) 26Sortrn34.1(CAG-Syp/tdTomato)Hze/J | Gift from Dr. David Ginty, available at the Jackson Laboratory | IMSR_JAX:012570 |
| Mouse: B6.Cg-H2afvTg(Wnt1-cre)11Rth | The Jackson Laboratory | IMSR_JAX:009107 |
| Mouse: Tg(hs799-cre/ERT2,-GFP)405Jlr | N/A | MMRRC: 037574-UCD |
| Mouse: Tg(Htr3a-cre)NO152Gsat/Mmucd | MMRRC | MMRRC_036680 |
| Mouse: Tg(I12b-cre)1Jlr | N/A | MMRRC_031698-UCD |
| Mouse: B6;129S6-Igs7trn93.1(tetO-GCaMP6f)Hze/J | The Jackson Laboratory | IMSR_JAX:024103 |
| Mouse: Isl1trn1(cre)Sev/J | The Jackson Laboratory | IMSR_Jax:024242 |
| Mouse: Isl1trn1.1Whk | Xiuqian Mu | MGI:3837972 |
| Mouse: Khdrbs3trn1.1Schei/J | Generated in the Scheiffele Laboratory (deposited at Jackson) | IMSR_JAX:029273 |
| Mouse: B6;129P-Klf3trn1(cre/ERT2Pzg/J | The Jackson Laboratory | IMSR_JAX:010985 |
| Mouse: Maftrn2.1Cbm | Gift from Dr. Carmen Birchmeier | MGI:5316895 |
| Mouse: Mafbtrn1.1Good | Gift from Dr. Lisa Goodrich | MGI:5581684 |
| Mouse: Megf10trn1c(KOMP)Jrs | Josh Sanes lab | MGI:6194031 |
| Mouse: Neo1trn1.1Jfcl | Cloutier lab (Kam et al., 2016) | MGI:6285614 |
| Mouse: B6.Cg-Tg(Nes-cre)1Kln/J | The Jackson Laboratory | IMSR_JAX: 003771 |
| Mouse: Neurod6trn1(cre)Kan | Goebbels lab (Goebbels et al., 2006) | MGI:2668659 |
| Mouse: Neurog2trn1(cre/Esr1)And | MMRRC | MGI:2652037 |
| Mouse: Tg(Nkx2–1-cre)2Sand | Gift from Dr. Stewart Anderson | IMSR_JAX:008661 |
| Mouse: B6;SJL-Nlgn2trn1.1Sud/J | The Jackson Laboratory | IMSR_JAX:025544 |
| Mouse: B6.Cg-Tg(Ntsr1-cre) GN220Gsat/Mmucd | MMRRC | MMRRC_030648-UCD |
| Mouse: B6.129-Pcp2trn1(cre)Nobs | A gift from Dr. Noboru Suzuki | MGI:3578623 |
| Mouse: B6.129-Tg(Pcp2-cre)2Mpin/J | The Jackson Laboratory | IMSR_JAX:004146 |
| Mouse: PhotonSABER-LSL | Generated by S. Matsuda and M. Yuzaki | N/A |
| Mouse: Ptf1atrn3Cvw | Christopher Wright | MGI:5788429 |
| Mouse: B6.129P2-Pvalbtrn1(cre)Arbr/J | The Jackson Laboratory | IMSR_JAX:017320 |
| Mouse: B6.129S1(Cg)-Rai1trn2.1Luo/J | Luo lab (Huang et al., 2016) | IMSR_JAX:029103 |
| Mouse: B6.FVB(Cg)-Tg(Rbp4-cre) KL100Gsat/Mmucd/GENSAT | MMRRC | MMRRC_037128-UCD |
| Mouse: Rims1trn3Sud/J | Generated by P.S. Kaeser and T.C. Sudhof, available at the Jackson Laboratory | IMSR_JAX:015832 |
| Mouse: Rims2trn1.1Sud/J | Generated by P.S. Kaeser and T.C. Sudhof, available at the Jackson Laboratory | IMSR_JAX:015833 |
| Mouse: B6.129S-Rorbtrn1.1(cre)Hze/J | The Jackson Laboratory | IMSR_JAX_023526 |
| Mouse: Rpl22trn1.1Psam/J | The Jackson Laboratory | IMSR_JAX:011029 |
| Mouse: B6;C3-Tg(Scnn1 a-cre)2Aibs/J | The Jackson Laboratory | IMSR_JAX_009613 |
| Mouse: Tg(Scx-GFP/cre)1Stzr | A gift from Dr. Jenna Lauren Galloway (Clifford Tabin Lab) | MGI:5317938 |
| Mouse: Tg(Sim1-cre)1Lowl/J | A gift from Dr. Bradford Lowell | IMSR_JAX:006395 |
| Mouse: Tg(Six3-cre)69Frty/GcoJ | A gift from Dr. Guillermo Oliver. Available at The Jackson Laboratory | IMSR_JAX: 019755 |
| Mouse: Tg(Slc1a3-cre/ERT)1Nat/J | The Jackson Laboratory | IMSR_JAX: 012586 |
| Mouse: Slc6a3trn1.1(cre)Bkmn/J | A gift from Dr. Thomas Hnasko, originally from The Jackson Laboratory | IMRS_JAX: 006660 |
| Mouse: B6.129(Cg)-Slc6a4trn1(cre)Xz/J | Available at the Jackson Laboratory | IMRS_JAX# 014554 |
| Mouse: Slc16a1lox/lox | Rothstein lab (Jha et al., 2019) | N/A |
| Mouse: Slc17a6trn2(cre)Lowl/J | The Jackson Laboratory | IMSR _JAX: 016963 |
| Mouse: B6;129S-Slc17a7trn1.1(cre)Hze/J | The Jackson Laboratory | IMSR _JAX: 023527 |
| Mouse: Tg(Slc17a8-icre)1Edw/SealJ | A gift from Dr. Robert Edward. Available at The Jackson Laboratory | IMSR_JAX: 018147 |
| Mouse: B6;129-Tg(Slc18a3-cre)KMisa/0 | Riken BioResource Center | RBRC_No.RBRC01515 |
| Mouse: B6.FVB-Tg(Slc32a1-cre) 2.1Hzo/FrkJ/ | The Jackson Laboratory | IMSR_JAX:017535 |
| Mouse: B6J.129S6(FVB)-Slc32a1trn2(cre)Lowl/MwarJ | The Jackson Laboratory | IMSR _JAX: 028862 |
| Mouse: B6;CBA-Tg(Sox10-cre)1Wdr/J | The Jackson Laboratory | IMSR _JAX: 025807 |
| Mouse: Ssttrn2.1(cre)Zjh | The Jackson Laboratory | IMSR_JAX:013044 |
| Mouse: Stxbp1trn1Mver | Verhage lab (Heeroma et al., 2004) | MGI:5509149 |
| Mouse: Syktrn1.2Tara | The Jackson Laboratory | IMSR_JAX:017309 |
| Mouse: B6.Cg-Tg(Syn1-cre)671Jxm/J | Gift from Dr. Lisa Goodrich, available at the Jackson Laboratory | IMSR_JAX:00396 |
| Mouse: Tgfb3trn1Moaz | The Jackson Laboratory | IMSR_JAX:024931 |
| Mouse: Tg(Thy1-cre/ERT2,-EYFP) HGfng/PyngJ | The Jackson Laboratory | IMSR_JAX:012708 |
| Mouse: Trpm7trn1Clph/J | The Jackson Laboratory | IMRS_JAX:018784 |
| Mouse: B6-Viptrn1(cre)Zjh/J | The Jackson Laboratory | IMSR_JAX:031628 |
| Mouse: Wwp1trn1.1Hkb/N | Kawabe lab (Ambrozkiewicz et al., 2018) | MGI:6281946 |
| Mouse: Wwp2trn1.1Hkb/N | Kawabe lab (Ambrozkiewicz et al., 2018) | MGI:6281948 |
| Zebrafsh D. rerio: Et(REX2-SCP1-Ocu.Hbb2:Cre-2A-Cerulean)y492 | Burgess lab (Tabor et al., 2019) | ZFIN:ZDB-ALT-180717–56 |
| Zebrafsh D. rerio: Et(REX2-SCP1-Ocu.Hbb2:Cre)y547 | Burgess lab (Tabor et al., 2019) | ZFIN:ZDB-ALT-180717–80 |
| Zebrafsh D. rerio: Et(REX2-SCP1-Ocu.Hbb2:Cre)y549 | Burgess lab (Tabor et al., 2019) | ZFIN:ZDB-ALT-180717–82 |
| Zebrafish D. rerio: Et(REX2-SCP1-Ocu.Hbb2:Cre)y559 | Burgess lab (Tabor et al., 2019) | ZFIN:ZDB-ALT-180717–92 |
| Zebrafish D. rerio: Et(REX2-SCP1-Ocu.Hbb2:Cre-2A-Cerulean)y546 | Burgess lab (Tabor et al., 2019) | ZFIN:ZDB-ALT-180717–79 |
| Zebrafish D. rerio: Et(REX2-SCP1-Ocu.Hbb2:Cre)y555 | Burgess lab (Tabor et al., 2019) | ZFIN:ZDB-ALT-180717–88 |
| Oligonucleotides | ||
| Primers, see Table S1 | ||
Highlights.
Most mouse Cre driver lines tested exhibited variable rates of germline recombination
Germline recombination exhibits parental sex bias and target locus selectivity
Similar principles apply to multiple organisms and recombinase systems
Guidelines are provided for detecting and minimizing unwanted germline recombination
ACKNOWLEDGMENTS
We thank Dr. Cynthia Smith from the Jackson Laboratory for her open acceptance of our data on the MGI database. We thank Dr. Timothy Murphy for his kind gift of the Ai32 mouse line. We thank Drs. Elizabeth M. Simpson, Sören Lukassen, and the University of British Columbia Dynamic Brain Circuits Cluster for discussion. Work by Lin Luo and A.M.C. was supported by Canadian Institutes of Health Research (FDN-143206) and Simons Foundation Autism Research Initiative (SFARI 608066). Work by M.C.A. and H.K. was supported by German Research Foundation (SPP1365/KA3423/1-1 and KA3423/3-1) and Japan Society for the Promotion of Science (JSPS) KAKENHI grant number 15K21769.
Work by N.B. and F.B. was supported by European Research Council (ERC) Advanced Grant SYNPRIME and the German Research Foundation SFB 1286/A9 awards to N.B. Work by C.C., W. Li, and N.A. was supported by the Natural Science Foundation of China grant (81573408), Fudan University-Shanghai Institute of Materia Medica Chinese Academy of Science joint grant (FU-SIMM20174015), and the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01). Work by J.L.S. and H.A.B. was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Work by K.T.B. was supported by National Institutes of Health (NIH) grant F32 DA038913 and NIH grant K99/R00 DA041445. Work by W.Y. and C.C. was supported by a Howard Hughes Medical Institute (HHMI) salary awarded to C.C. Work by E.D. and J.-F.C. was supported by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada (NSERC). Work by J.A.S. and C.E. was supported by NIH grants RO1DA031833 and F31NS092419. Work by J. Wang and P.S.K. was supported by NIH grants R01NS083898, R01NS103484, and R01MH113349. Work by A.P. and J.N.K. was supported by NIH grants EY024694 to J.N.K. and EY5722 to Duke University. Work by M.A.H. and W. Lu was supported by National Institute of Neurological Disorders and Stroke (NINDS) and NIH Intramural Research Program. Work by W.-H.H. and Liqun Luo was supported by HHMI, SFARI Research Award 345098, and NIH grant R01NS050580 to Liqun Luo and NIH grant 5K99HD092545-02 toW.-H.H. Work by T.M. and K.M. was supported by Japan’s Ministry of Education, Culture, Sports, Science and Technology KAKENHI grant 16H06463 and JPSP KAKENHI grant 18K06503. Work by K.A.P. and C.J.M. was supported by a Eunice Kennedy Shriver National Institute of Child Health and Human Development Intramural Award. Work by S.G., G.S., and K.-A.N. was supported by ERC Advanced Grants AxoGLIA and MyeliNANO, the German Research Foundation (SPP1757), and the Max Planck Society. Work by M.A.M.P. and V.F.P. was supported by Canadian Institute of Health Research grants MOP136930, MOP89919, PJT 162431, and PJT 159781 and NSERC grant 06577-2018 RGPIN. Work by E.L.-L.P. and J.L.R.R. was supported by NIH NINDS grant R01 NS099099, National Institute of Mental Health (NIMH) grant R01 MH081880, and NIMH grant R01 MH049428. Work by J.R.S. was supported by NIH R37NS029169. Work by S.F., E.F., A.M.G., L.T., and P.S. was supported by ERC Advanced Grant SPLICECODE; Swiss National Science Foundation to P.S. and European Molecular Biology Organization EMBO ALTF-70-2015 and aALTF-760-2016 to A.M.G. Work by Y.T. was supported by JPSP KAKENHI grant 26251013. Work by H.U. was supported by NIH R01DA042744, R01MH111647, R01NS092578, and SFARI grants. Work by J. Wortel and M.V. was supported by an ERC Advanced Grant (322966) of the European Union. Work by J.M. and M.Y. was supported by Japan Science and Technology Agency (JPMJCR1854) and KAKENHI (16H06461 and 15H05772). Work byL.A.L. and H.Y.Z. was supported by NIH/NINDS grant 5R01NS057819-13 as well as HHMI to H.Y.Z.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.neuron.2020.01.008.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Akashi K, Kakizaki T, Kamiya H, Fukaya M, Yamasaki M, Abe M, Natsume R, Watanabe M, and Sakimura K (2009). NMDA receptor GluN2B (GluR ε 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J. Neurosci 29, 10869–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrozkiewicz MC, Schwark M, Kishimoto-Suga M, Borisova E, Hori K, Salazar-Lázaro A, Rusanova A, Altas B, Piepkorn L, Bessa P, et al. (2018). Polarity Acquisition in Cortical Neurons Is Driven by Synergistic Action of Sox9-Regulated Wwp1 and Wwp2 E3 Ubiquitin Ligases and Intronic miR-140. Neuron 100, 1097–1115. [DOI] [PubMed] [Google Scholar]
- Bäck S, Necarsulmer J, Whitaker LR, Coke LM, Koivula P, Heathward EJ, Fortuno LV, Zhang Y, Yeh CG, Baldwin HA, et al. (2019). Neuron-Specific Genome Modification in the Adult Rat Brain Using CRISPR-Cas9 Transgenic Rats. Neuron 102, 105–119. [DOI] [PubMed] [Google Scholar]
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, and Tomac AC (2006). Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis 44, 383–390. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. (2005). Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505. [DOI] [PubMed] [Google Scholar]
- Barski JJ, Dethleffsen K, and Meyer M (2000). Cre recombinase expression in cerebellar Purkinje cells. Genesis 28, 93–98. [PubMed] [Google Scholar]
- Bates B, Rios M, Trumpp A, Chen C, Fan G, Bishop JM, and Jaenisch R (1999). Neurotrophin-3 is required for proper cerebellar development. Nat. Neurosci 2, 115–117. [DOI] [PubMed] [Google Scholar]
- Bedbrook CN, Deverman BE, and Gradinaru V (2018). Viral Strategies for Targeting the Central and Peripheral Nervous Systems. Annu. Rev. Neurosci 41, 323–348. [DOI] [PubMed] [Google Scholar]
- Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, Johnson RL, Tabin CJ, Schweitzer R, and Zelzer E (2009). Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhali K, Dipietromaria A, Fontaine A, Caburet S, Barbieri O, Bellessort B, Fellous M, Veitia RA, and Levi G (2011). Allelic reduction of Dlx5 and Dlx6 results in early follicular depletion: a new mouse model of primary ovarian insufficiency. Hum. Mol. Genet 20, 2642–2650. [DOI] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJAJ, Kremer M, Wunderlich FT, Jung S, and Waisman A (2005). A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426. [DOI] [PubMed] [Google Scholar]
- Buchholz F, Refaeli Y, Trumpp A, and Bishop JM (2000). Inducible chromosomal translocation of AML1 and ETO genes through Cre/loxP-mediated recombination in the mouse. EMBO Rep. 1, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult CJ, Blake JA, Smith CL, Kadin JA, and Richardson JE; Mouse Genome Database Group (2019). Mouse Genome Database (MGD) 2019. Nucleic Acids Res. 47 (D1), D801–D806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H-T, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu H-C, Heintz N, et al. (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Craig MT, McFarland A, Yuan X, Gerfen S, Tricoire L, Erkkila B, Barron SC, Lopez CM, Liang BJ, et al. (2013). Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT(3A)R expression. Nat. Neurosci 16, 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C-I, Yoon S-P, Choi J-M, Kim S-S, Lee Y-D, Birnbaumer L, and Suh-Kim H (2014). Simultaneous deletion of floxed genes mediated by CaMKIIa-Cre in the brain and in male germ cells: application to conditional and conventional disruption of Goa. Exp. Mol. Med 46, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino G, Di Pasquale G, Scimemi P, Rodriguez L, Galindo Ramirez F, De Siati RD, Santarelli RM, Arslan E, Bortolozzi M, Chiorini JA, and Mammano F (2011). BAAV mediated GJB2 gene transfer restores gap junction coupling in cochlear organotypic cultures from deaf Cx26Sox10Cre mice. PLoS ONE 6, e23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, et al. (2018). A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 174, 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, and McMahon AP (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol 8, 1323–1326. [DOI] [PubMed] [Google Scholar]
- Davis MI, Crittenden JR, Feng AY, Kupferschmidt DA, Naydenov A, Stella N, Graybiel AM, and Lovinger DM (2018). The cannabinoid-1 receptor is abundantly expressed in striatal striosomes and striosome-dendron bouquets of the substantia nigra. PLoS ONE 13, e0191436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro BM, De Jaeger X, Martins-Silva C, Lima RDF, Amaral E, Menezes C, Lima P, Neves CML, Pires RG, Gould TW, et al. (2009). The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol. Cell. Biol 29, 5238–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Toro D, Ruff T, Cederfjäll E, Villalba A, Seyit-Bremer G, Borrell V, and Klein R (2017). Regulation of Cerebral Cortex Folding by Controlling Neuronal Migration via FLRT Adhesion Molecules. Cell 169, 621–635. [DOI] [PubMed] [Google Scholar]
- Divito CB, Steece-Collier K, Case DT, Williams S-PG, Stancati JA, Zhi L, Rubio ME, Sortwell CE, Collier TJ, Sulzer D, et al. (2015). Loss of VGLUT3 Produces Circadian-Dependent Hyperdopaminergia and Ameliorates Motor Dysfunction and l-Dopa-Mediated Dyskinesias in a Model of Parkinson’s Disease. J. Neurosci 35, 14983–14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T, Georgieva T, Li H, Reed TD, Grisham C, Friel J, Estabrook MA, Gard C, Sanford LP, and Azhar M (2012). Generation of mice with a conditional allele for the transforming growth factor beta3 gene. Genesis 50, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragatsis I, and Zeitlin S (2000). CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis 26, 133–135. [DOI] [PubMed] [Google Scholar]
- Dubois NC, Hofmann D, Kaloulis K, Bishop JM, and Trumpp A (2006). Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis 44, 355–360. [DOI] [PubMed] [Google Scholar]
- Dudok JJ, Groffen AJA, Toonen RFT, and Verhage M (2011). Deletion of Munc18–1 in 5-HT neurons results in rapid degeneration of the 5-HT system and early postnatal lethality. PLoS ONE 6, e28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Luján R, Halbout B, Mameli M, et al. (2008). Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron 59, 497–508. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, and Chambon P (1997). Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun 237, 752–757. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón M, Casquero-García V, Luo W, Francesca Lunella F, Ferreira Rocha S, Del Olmo-Cabrera S, and Benedito R (2019). iSuRe-Cre is a genetic tool to reliably induce and report Cre-dependent genetic modifications. Nat. Commun 10, 2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, and Müller U (2012). Fate-restricted neural progenitors in the mammalian cerebral cortex. Science 337, 746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U, Jockusch WJ, Sivakumar N, Möbius W, Corthals K, Li S, Quintes S, Kim Y, Schaap IAT, Rhee J-S, et al. (2012). Critical time window of neuronal cholesterol synthesis during neurite outgrowth.J. Neurosci 32, 7632–7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BL, and Oliver GC (2000). Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis 26, 130–132. [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, and Castrillon DH (2007). Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 45, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, and Heintz N (2013). GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sanz C, Espinosa A, Fregoso SP, Bluske KK, Cunningham CL, Martinez-Garay I, Zeng H, Franco SJ, and Müller U (2015). Lineage Tracing Using Cux2-Cre and Cux2-CreERT2 Mice. Neuron 86, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, and Nave K-A (2006). Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44, 611–621. [DOI] [PubMed] [Google Scholar]
- Gondo Y (2008). Trends in large-scale mouse mutagenesis: from genetics to functional genomics. Nat. Rev. Genet 9, 803–810. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, and Heintz N (2003). A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, and Gerfen CR (2007). Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci 27, 9817–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JLR, and Jones KR (2002). Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci 22, 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, et al. (2009). Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis.J. Neurosci 29, 1874–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Seal RP, Oesch N, Edwards RH, and Diamond JS (2011). Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis. Neurosci 28, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M, Hu C-J, Johnson RS, Brown EJ, Keith B, and Simon MC (2007). Acute postnatal ablation of Hif-2a results in anemia. Proc. Natl. Acad. Sci. USA 104, 2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, and Rajewsky K (1994). Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science 265, 103–106. [DOI] [PubMed] [Google Scholar]
- Guzman MS, De Jaeger X, Raulic S, Souza IA, Li AX, Schmid S, Menon RS, Gainetdinov RR, Caron MG, Bartha R, et al. (2011). Elimination of the vesicular acetylcholine transporter in the striatum reveals regulation of behaviour by cholinergic-glutamatergic co-transmission. PLoS Biol 9, e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, and Mizushima N (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889. [DOI] [PubMed] [Google Scholar]
- Harno E, Cottrell EC, and White A (2013). Metabolic pitfalls of CNS Cre-based technology. Cell Metab. 18, 21–28. [DOI] [PubMed] [Google Scholar]
- Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M, Ouellette B, Kidney J, et al. (2014). Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front. Neural Circuits 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, Shin H-S, Watanabe M, Sakimura K, and Kano M (2011). Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc. Natl. Acad. Sci. USA 108, 9987–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert JM, and McConnell SK (2000). Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev. Biol 222, 296–306. [DOI] [PubMed] [Google Scholar]
- Heeroma JH, Roelandse M, Wierda K, van Aerde KI, Toonen RFG, Hensbroek RA, Brussaard A, Matus A, and Verhage M (2004). Trophic support delays but does not prevent cell-intrinsic degeneration of neurons deficient for munc18–1. Eur. J. Neurosci 20, 623–634. [DOI] [PubMed] [Google Scholar]
- Heffner CS, Herbert Pratt C, Babiuk RP, Sharma Y, Rockwood SF, Donahue LR, Eppig JT, and Murray SA (2012). Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat. Commun 3, 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, and Arber S (2005). A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, and Zeng H (2013). Genetic approaches to neural circuits in the mouse. Annu. Rev. Neurosci 36, 183–215. [DOI] [PubMed] [Google Scholar]
- Huang W-H, Guenthner CJ, Xu J, Nguyen T, Schwarz LA, Wilkinson AW, Gozani O, Chang HY, Shamloo M, and Luo L (2016). Molecular and Neural Functions of Rai1, the Causal Gene for Smith-Magenis Syndrome. Neuron 92, 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W-H, Wang DC, Allen WE, Klope M, Hu H, Shamloo M, and Luo L (2018). Early adolescent Rai1 reactivation reverses transcriptional and social interaction deficits in a mouse model of Smith-Magenis syndrome. Proc. Natl. Acad. Sci. USA 115, 10744–10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, and Duffield JS (2010). Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol 176, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison MA, Gu X, Adrover MF, Lee MR, Hnasko TS, Alvarez VA, and Lu W (2018). Genetic inhibition of neurotransmission reveals role of glutamatergic input to dopamine neurons in high-effort behavior. Mol. Psychiatry 23, 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing-Esteves S, Kostadinov D, Marocha J, Sing AD, Joseph KS, Laboulaye MA, Sanes JR, and Lefebvre JL (2018). Combinatorial Effects of Alpha- and Gamma-Protocadherins on Neuronal Survival and Dendritic Self-Avoidance. J. Neurosci 38, 2713–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janickova H, Rosborough K, Al-Onaizi M, Kljakic O, Guzman MS, Gros R, Prado MAM, and Prado VF (2017). Deletion of the vesicular acetylcholine transporter from pedunculopontine/laterodorsal tegmental neurons modifies gait. J. Neurochem 140, 787–798. [DOI] [PubMed] [Google Scholar]
- Jha MK, Lee Y, Russell KA, Yang F, Dastgheyb RM, Deme P, Ament XH, Chen W, Liu Y, Guan Y, et al. (2019). Monocarboxylate transporter 1 in Schwann cells contributes to maintenance of sensory nerve myelination during aging. Glia 68, 161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, and Clapham DE (2008). Deletion of Trpm7 disrupts embryonic development and theymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PG, Nawoschik SP, Sreekumar K, Uveges AJ, Tseng E, Zhang L, Johnson J, He L, Paulsen JE, Bates B, and Pausch MH (2007). Tissue distribution and functional analyses of the constitutively active orphan G protein coupled receptors, GPR26 and GPR78. Biochim. Biophys. Acta 1770, 890–901. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Kwon H-B, Chiu CQ, Deng L, Castillo PE, and Südhof TC (2008). RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions.J. Neurosci 28, 13435–13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Chávez AE, Liu X, Castillo PE, and Südhof TC (2009). ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron 64, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, and Südhof TC (2011). RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144, 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Mitakidis N, Miura E, Abe M, Matsuda K, Takeo YH, Kohda K, Motohashi J, Takahashi A, Nagao S, et al. (2015). Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron 85, 316–329. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Katoh A, Narumi S, Miura E, Motohashi J, Takahashi A, Kohda K, Fukazawa Y, Yuzaki M, and Matsuda S (2018). Optogenetic Control of Synaptic AMPA Receptor Endocytosis Reveals Roles of LTD in Motor Learning. Neuron 99, 985–998. [DOI] [PubMed] [Google Scholar]
- Kam JWK, Dumontier E, Baim C, Brignall AC, Mendes da Silva D, Cowan M, Kennedy TE, and Cloutier J-F (2016). RGMB and neogenin control cell differentiation in the developing olfactory epithelium. Development 143, 1534–1546. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, and Sasaki H (2004). Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429, 900–903. [DOI] [PubMed] [Google Scholar]
- Kay JN, Chu MW, and Sanes JR (2012). MEGF10 and MEGF11 mediate homotypic interactions required for mosaic spacing of retinal neurons. Nature 483, 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel RA, Turnbull DH, Blanquet V, Wurst W, Loomis CA, and Joyner AL (2000). Two lineage boundaries coordinate vertebrate apical ecto-dermal ridge formation. Genes Dev 14, 1377–1389. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, and Hensch TK (2013). Germline recombination by conditional gene targeting with Parvalbumin-Cre lines. Front. Neural Circuits 7, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolisnyk B, Al-Onaizi M, Soreq L, Barbash S, Bekenstein U, Haberman N, Hanin G, Kish MT, Souza da Silva J, Fahnestock M, et al. (2017). Cholinergic Surveillance over Hippocampal RNA Metabolism and Alzheimer’s-Like Pathology. Cereb. Cortex 27, 3553–3567. [DOI] [PubMed] [Google Scholar]
- Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, Attwell D, and Richardson WD (2017). Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6, e28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic J, Maroteaux G, Schut D, Loos M, Dubey M, Pitsch J, Remmelink E, Koopmans B, Crowley J, Cornelisse LN, et al. (2018). Protein instability, haploinsufficiency, and cortical hyper-excitability underlie STXBP1 encephalopathy. Brain 141, 1350–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krah NM, De La OJP, Swift GH, Hoang CQ, Willet SG, Chen Pan F, Cash GM, Bronner MP, Wright CV, MacDonald RJ, and Murtaugh LC (2015). The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. eLife. Published online July 7, 2015 10.7554/eLife.07125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, and Westphal H (1996). Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 93, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DD, Leinninger GM, Louis GW, Garfield AS, Marston OJ, Leshan RL, Scheller EL, Christensen L, Donato J Jr., Xia J, et al. (2011). Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab 13, 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen K, Zhu L, and Pollard JW (2006). Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis 44, 328–335. [DOI] [PubMed] [Google Scholar]
- Liang J, Xu W, Hsu Y-T, Yee AX, Chen L, and Südhof TC (2015). Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol. Psychiatry 20, 850–859. [DOI] [PubMed] [Google Scholar]
- Liput DJ (2018). Cre-Recombinase Dependent Germline Deletion of a Conditional Allele in the Rgs9cre Mouse Line. Front. Neural Circuits 12, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Willet SG, Bankaitis ED, Xu Y, Wright CVE, and Gu G (2013). Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation. Genesis 51, 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Brown A, Fisher D, Wu Y, Warren J, and Cui X (2016). Tissue Specific Expression of Cre in Rat Tyrosine Hydroxylase and Dopamine Active Transporter-Positive Neurons. PLoS ONE 11, e0149379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kershberg L, Wang J, Schneeberger S, and Kaeser PS (2018a). Dopamine Secretion Is Mediated by Sparse Active Zone-like Release Sites. Cell 172, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen C, Liu Y, Li W, Wang Z, Sun Q, Zhou H, Chen X, Yu Y, Wang Y, and Abumaria N (2018b). TRPM7 Is Required for Normal Synapse Density, Learning, and Memory at Different Developmental Stages. Cell Rep. 23, 3480–3491. [DOI] [PubMed] [Google Scholar]
- Long MA, and Rossi FMV (2009). Silencing inhibits Cre-mediated recombination of the Z/AP and Z/EG reporters in adult cells. PLoS ONE 4, e5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S, Bosch E, Ekici AB, and Winterpacht A (2018a). Characterization of germ cell differentiation in the male mouse through single-cell RNA sequencing. Sci. Rep 8, 6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S, Bosch E, Ekici AB, and Winterpacht A (2018b). Single-cell RNA sequencing of adult mouse testes. Sci. Data 5, 180192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson D, Sage J, Crowley D, Trumpp A, Bronson RT, and Jacks T (2003). Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol. Cell. Biol 23, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu Y-WA, Garcia AJ 3rd, Gu X, Zanella S, et al. (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci 15, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Silva C, De Jaeger X, Guzman MS, Lima RDF, Santos MS, Kushmerick C, Gomez MV, Caron MG, Prado MAM, and Prado VF (2011). Novel strains of mice deficient for the vesicular acetylcholine transporter: insights on transcriptional regulation and control of locomotor behavior. PLoS ONE 6, e17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn AC, De Jaeger X, Magalhães AC, Kesarwani R, Gonçalves DF, Raulic S, Guzman MS, Jackson MF, Izquierdo I, Macdonald JF, et al. (2012). Elimination of the vesicular acetylcholine transporter in the forebrain causes hyperactivity and deficits in spatial memory and long-term potentiation. Proc. Natl. Acad. Sci. USA 109, 17651–17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Budisantoso T, Mitakidis N, Sugaya Y, Miura E, Kakegawa W, Yamasaki M, Konno K, Uchigashima M, Abe M, et al. (2016). Transsynaptic Modulation of Kainate Receptor Functions by C1q-like Proteins. Neuron 90, 752–767. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, and Koentges G (2005). Neural crest origins of the neck and shoulder. Nature 436, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn JE, Liu S-M, Liu H, Dragatsis I, Dietrich P, Ludwig T, Boozer CN, and Chua SC Jr. (2005). Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am. J. Physiol. Endocrinol. Metab 289, E403–E411. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp H-P, Bonhoeffer T, and Klein R (1999). Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24, 401–414. [DOI] [PubMed] [Google Scholar]
- Misawa H, Nakata K, Toda K, Matsuura J, Oda Y, Inoue H, Tateno M, and Takahashi R (2003). VAChT-Cre. Fast and VAChT-Cre.Slow: postnatal expression of Cre recombinase in somatomotor neurons with different onset. Genesis 37, 44–50. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Yamasaki M, Hashimoto K, Yamazaki M, Abe M, Usui H, Kano M, Sakimura K, and Watanabe M (2012). Cav2.1 in cerebellar Purkinje cells regulates competitive excitatory synaptic wiring, cell survival, and cerebellar biochemical compartmentalization. J. Neurosci 32, 1311–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Young A, Petros T, Karayannis T, McKenzie Chang M, Lavado A, Iwano T, Nakajima M, Taniguchi H, Huang ZJ, et al. (2015). Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons. J. Neurosci 35, 12869–12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Fu X, Beremand PD, Thomas TL, and Klein WH (2008). Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc. Natl. Acad. Sci. USA 105, 6942–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Eppig JT, Smedley D, Simpson EM, and Rosenthal N (2012). Beyond knockouts: cre resources for conditional mutagenesis. Mamm. Genome 23, 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, and Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, and Tonegawa S (2002). Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Miyagawa S, Vieux-Rochas M, Morini M, Ogino Y, Suzuki K, Nakagata N, Choi H-S, Levi G, and Yamada G (2008). Positive regulation of steroidogenic acute regulatory protein gene expression through the interaction between Dlx and GATA-4 for testicular steroidogenesis. Endocrinology 149, 2090–2097. [DOI] [PubMed] [Google Scholar]
- Nishiyama J, Miura E, Mizushima N, Watanabe M, and Yuzaki M (2007). Aberrant membranes and double-membrane structures accumulate in the axons of Atg5-null Purkinje cells before neuronal death. Autophagy 3, 591–596. [DOI] [PubMed] [Google Scholar]
- Nouri N, and Awatramani R (2017). A novel floor plate boundary defined by adjacent En1 and Dbx1 microdomains distinguishes midbrain dopamine and hypothalamic neurons. Development 144, 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan ED, Creson TK, Kramár EA, Rojas C, Seese RR, Babyan AH, Shi Y, Lucero R, Xu X, Noebels JL, et al. (2014). Reduced cognition in Syngap1 mutants is caused by isolated damage within developing forebrain excitatory neurons. Neuron 82, 1317–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai EL-L, Vogt D, Clemente-Perez A, McKinsey GL, Cho FS, Hu JS, Wimer M, Paul A, Fazel Darbandi S, Pla R, et al. (2019). Mafb and c-Maf Have Prenatal Compensatory and Postnatal Antagonistic Roles in Cortical Interneuron Fate and Function. Cell Rep. 26, 1157–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, Kawabe H, Chen F, Zhang L, et al. (2013). The specific a-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron 80, 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, and Rubenstein JLR (2009). Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol. Cell. Neurosci 40, 167–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puñal VM, Paisley CE, Brecha FS, Lee MA, Perelli RM, Wang J, O’Koren EG, Ackley CR, Saban DR, Reese BE, and Kay JN (2019). Large-scale death of retinal astrocytes during normal development is non-apoptotic and implemented by microglia. PLoS Biol 17, e3000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray TA, Roy S, Kozlowski C, Wang J, Cafaro J, Hulbert SW, Wright CV, Field GD, and Kay JN (2018). Formation of retinal direction-selective circuitry initiated by starburst amacrine cell homotypic contact. eLife 7 Published online April 3, 2018 10.7554/eLife.34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempe D, Vangeison G, Hamilton J, Li Y, Jepson M, and Federoff HJ (2006). Synapsin I Cre transgene expression in male mice produces germline recombination in progeny. Genesis 44, 44–49. [DOI] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, and Jaenisch R (2001). Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol 15, 1748–1757. [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, and Hogan BLM (2011). Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesen-chymal transition. Proc. Natl. Acad. Sci. USA 108, E1475–E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. (2010). Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, and Elmquist JK (2011). Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH, S-Jacques B, Lee SM, Flax JD, Snyder EY, and McMahon AP (1999). Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J. Neurosci 19, 8954–8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp AC, Allison MB, Jones JC, Patterson CM, Faber CL, Bozadjieva N, Heisler LK, Seeley RJ, Olson DP, and Myers MG Jr. (2018). Specific subpopulations of hypothalamic leptin receptor-expressing neurons mediate the effects of early developmental leptin receptor deletion on energy balance. Mol. Metab 14, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, and Nave K-A (2005). High cholesterol level is essential for myelin membrane growth. Nat. Neurosci 8, 468–475. [DOI] [PubMed] [Google Scholar]
- Saijo K, Schmedt C, Su I-H, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, and Tarakhovsky A (2003). Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat. Immunol 4, 274–279. [DOI] [PubMed] [Google Scholar]
- Saito H, Tsumura H, Otake S, Nishida A, Furukawa T, and Suzuki N (2005). L7/Pcp-2-specific expression of Cre recombinase using knock-in approach. Biochem. Biophys. Res. Commun 331, 1216–1221. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, and Spanagel R (2006). Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J. Neurosci 26, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, and Amieux PS (2009). Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 106, 13939–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B, and Henderson N (1988). Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA 85, 5166–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EE, Taylor DS, Prigge JR, Barnett S, and Capecchi MR (2000). Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc. Natl. Acad. Sci. USA 97, 13702–13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk F, Baron U, and Rajewsky K (1995). A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshek DR, Jensen V, Celikel T, Geng Y, Schupp B, Bus T, Mack V, Marx V, Hvalby Ø, Seeburg PH, and Sprengel R (2006). Forebrain-specific glutamate receptor B deletion impairs spatial memory but not hippocampal field long-term potentiation. J. Neurosci 26, 8428–8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg SN, Taher L, Lindtner S, Sandberg M, Nord AS, Vogt D, Mckinsey GL, Hoch R, Pattabiraman K, Zhang D, et al. (2016). Subpallial Enhancer Transgenic Lines: a Data and Tool Resource to Study Transcriptional Regulation of GABAergic Cell Fate. Neuron 92, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AB, Bloomsburg SJ, Billingslea SA, Merrill MM, Li S, Thomas MW, and Fuerst PG (2016). Pou4f2 knock-in Cre mouse: A multi-faceted genetic tool for vision researchers. Mol. Vis 22, 705–717. [PMC free article] [PubMed] [Google Scholar]
- Song AJ, and Palmiter RD (2018). Detecting and Avoiding Problems When Using the Cre-lox System. Trends Genet 34, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet 21, 70–71. [DOI] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, and Fishell G (2009). Characterization of Nkx6–2-derived neocortical interneuron lineages. Cereb. Cortex 19 (19, Suppl 1), i1–i10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Buetfering C, Lecoq J, Lee CR, Peters AJ, Jacobs EAK, Coen P, Ollerenshaw DR, Valley MT, de Vries SEJ, et al. (2017). Aberrant Cortical Activity in Multiple GCaMP6-Expressing Transgenic Mouse Lines. eNeuro. Published online September 6, 2017 10.1523/ENEURO.0207-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N, and Hamilton D (1981). Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol 150, 467–486. [DOI] [PubMed] [Google Scholar]
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji R-R, and Eroglu C (2017). Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor KM, Marquart GD, Hurt C, Smith TS, Geoca AK, Bhandiwad AA, Subedi A, Sinclair JL, Rose HM, Polys NF, and Burgess HA (2019). Brain-wide cellular resolution imaging of Cre transgenic zebrafish lines for functional circuit-mapping. eLife 8 Published online February 8, 2019. 10.7554/eLife.42687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana N, Touahri Y, Dixit R, David LA, Adnani L, Cantrup R, Aavani T, Wong RO, Logan C, Kurek KC, and Schuurmans C (2018). Hamartoma-like lesions in the mouse retina: an animal model of Pten hamartoma tumour syndrome. Dis. Model. Mech 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Beyer LA, Swiderski DL, O’Neal AL, Prieskorn DM, Shivatzki S, Avraham KB, and Raphael Y (2014). Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy. Hear. Res 309, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Bullock B, Lehtinen MK, and Umemori H (2016). Retrograde fibroblast growth factor 22 (FGF22) signaling regulates insulin-like growth factor 2 (IGF2) expression for activity-dependent synapse stabilization in the mammalian brain. eLife 5, e12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi A, Gavin E, Wilson J, and Umemori H (2017). Selective Inactivation of Fibroblast Growth Factor 22 (FGF22) in CA3 Pyramidal Neurons Impairs Local Synaptogenesis and Affective Behavior Without Affecting Dentate Neurogenesis. Front. Synaptic Neurosci 9, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, and Kublaoui BM (2010). Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J. Neurosci 30, 3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M, Podojil JR, McCarthy DP, Miller SD, and Popko B (2016). Oligodendrocyte death results in immune-mediated CNS demyelination. Nat. Neurosci 19, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunmüller L, Bornmann C, and Scheiffele P (2014). Alternative splicing coupled nonsense-mediated decay generates neuronal cell type-specific expression of SLM proteins. J. Neurosci 34, 16755–16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, and Schütz G (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet 23, 99–103. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, and Martin GR (1999). Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 13, 3136–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, and Sahin M (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, and Tonegawa S (1996a). Subregion-and cell type-restricted gene knockout in mouse brain. Cell 87, 1317–1326. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, and Tonegawa S (1996b). The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S Jr., and Lowell BB (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, and Nathans J (2012). Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende H, Lechner SG, Cheret C, Bourane S, Kolanczyk ME, Pattyn A, Reuter K, Munier FL, Carroll P, Lewin GR, and Birchmeier C (2012). The transcription factor c-Maf controls touch receptor development and function. Science 335, 1373–1376. [DOI] [PubMed] [Google Scholar]
- Weng DY, Zhang Y, Hayashi Y, Kuan C-Y, Liu C-Y, Babcock G, Weng W-L, Schwemberger S, and Kao WW-Y (2008). Promiscuous recombination of LoxP alleles during gametogenesis in cornea Cre driver mice. Mol. Vis 14, 562–571. [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. (2011). Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcinski A, Morabito M, Lawton AK, Stephen DN, and Joyner AL (2019). Genetic deletion of genes in the cerebellar rhombic lip lineage can stimulate compensation through adaptive reprogramming of ventricular zone-derived progenitors. Neural Dev. 14, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, and Anderson SA (2008). Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol 506, 16–29. [DOI] [PubMed] [Google Scholar]
- Yang L, Cai C-L, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, and Evans S (2006). Isl1Cre reveals a common Bmp pathway in heart and limb development. Development 133, 1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Qiu L, Wang D, Zhao S, Gross J, and Feng G (2008). Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice. Nat. Neurosci 11, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W-M, Appler JM, Kim Y-H, Nishitani AM, Holt JR, and Goodrich LV (2013). A Gata3-Mafb transcriptional network directs post-synaptic differentiation in synapses specialized for hearing. eLife 2, e01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller A, Crestani F, Camenisch I, Iwasato T, Itohara S, Fritschy JM, and Rudolph U (2008). Cortical glutamatergic neurons mediate the motor sedative action of diazepam. Mol. Pharmacol 73, 282–291. [DOI] [PubMed] [Google Scholar]
- Zerucha T, Stühmer T, Hatch G, Park BK, Long Q, Yu G, Gambarotta A, Schultz JR, Rubenstein JLR, and Ekker M (2000). A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J. Neurosci 20, 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dublin P, Griemsmann S, Klein A, Brehm R, Bedner P, Fleischmann BK, Steinhäuser C, and Theis M (2013). Germ-line recombination activity of the widely used hGFAP-Cre and nestin-Cre transgenes. PLoS ONE 8, e82818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Sage M, Sheppeard EA, Jurecic V, and Bradley A (2000). Engineering mouse chromosomes with Cre-loxP: range, efficiency, and somatic applications. Mol. Cell. Biol 20, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Yang L, Sikorski MA, Enns LC, Czyzyk TA, Ladiges WC, and McKnight GS (2013). Deficiency of the RIIb subunit of PKA affects locomotor activity and energy homeostasis in distinct neuronal populations. Proc. Natl. Acad. Sci. USA 110, E1631–E1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y-X, Zhao M, Li D, Shimazu K, Sakata K, Deng C-X, and Lu B (2003). Cerebellar deficits and hyperactivity in mice lacking Smad4. J. Biol. Chem 278, 42313–42320. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, and Parada LF (2001). Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 15, 859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, and Hen R (2005). Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J. Neurosci. Methods 143, 27–32. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, and Messing A (2001). hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31, 85–94. [DOI] [PubMed] [Google Scholar]
- Zirlinger M, Lo L, McMahon J, McMahon AP, and Anderson DJ (2002). Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc. Natl. Acad. Sci. USA 99, 8084–8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Genotyping Primers, Related to Table 1.
Data Availability Statement
Information from Table 1 will be incorporated in the Mutation details section for each Cre line in the MGI webpage, tagged in bold as “Germline Recombination.” This particular Mutation details field is already exported to various resources, including mutant mouse repositories for display on strain data pages.


