Abstract
Most cancers arise in individuals over the age of 60. As the world population is living longer and reaching older ages, cancer is becoming a substantial public health problem. It is estimated that, by 2050, more than 20% of the world’s population will be over the age of 60 — the economic, healthcare and financial burdens this may place on society are far from trivial. In this Review, we address the role of the ageing microenvironment in the promotion of tumour progression. Specifically, we discuss the cellular and molecular changes in non-cancerous cells during ageing, and how these may contribute towards a tumour permissive microenvironment; these changes encompass biophysical alterations in the extracellular matrix, changes in secreted factors and changes in the immune system. We also discuss the contribution of these changes to responses to cancer therapy as ageing predicts outcomes of therapy, including survival. Yet, in preclinical studies, the contribution of the aged microenvironment to therapy response is largely ignored, with most studies designed in 8-week-old mice rather than older mice that reflect an age appropriate to the disease being modelled. This may explain, in part, the failure of many successful preclinical therapies upon their translation to the clinic. Overall, the intention of this Review is to provide an overview of the interplay that occurs between ageing cell types in the microenvironment and cancer cells and how this is likely to impact tumour metastasis and therapy response.
Cancer is often defined as a disease of ageing. The incidence of most cancers increases dramatically as we age and cancer has been shown to be the number one cause of death in both males and females aged 60–79 years1. The probability of developing invasive cancer in patients over 60 is more than double that of younger patients, with a median age of diagnosis at 65 and a median age of death at 74 (REF1). These statistics place a huge socioeconomic burden on society as improvements in healthcare and technology are resulting in much longer life expectancies. The World Health Organization estimates that the proportion of the world’s population over 60 years old will shift from 12% to 22% by 2050, with a total of over 2 billion people.
The mechanisms of both cancer and ageing underlie a time-dependent accumulation of cellular damage. Despite the preconceived notion that the processes of cancer (hyperproliferation and increased cellular survival) and ageing (decreased function and fitness) in the context of a cell are opposing, studies highlight that many of the hallmarks of ageing are shared with cancer2. These include epigenetic changes, altered intracellular communication, changes in proteostasis, mitochondrial dysfunction and cellular senescence. Some of these shared features may be attributed to the fact that the majority of cancers arise in aged individuals3, and therefore the hallmarks of ageing are already a part of the phenotype of cancer cells. However, an important distinguishing feature is that many studies now show that ageing can dramatically affect the normal cells of the tumour microenvironment (TME), which can act to promote tumour progression and metastasis. Fibroblasts and immune cells appear particularly susceptible to this age-related impact.
Tumour progression most often requires genetic mutations in growth pathways to drive a hyperproliferative phenotype as well as mutations that enable the bypass of senescence; many of the key factors associated with the ageing of cells, including an increased accumulation of genomic damage (point mutations, deletions and translocations), telomere attrition, epigenetic alteration, impaired proteostasis and deregulation of nutrient sensing2·4·5, can often promote this. Environmental factors to which we are exposed as we age, such as ultraviolet (UV) radiation exposure, alcohol, smoking and pollution, further contribute to the chronic accumulation of DNA damage and other events associated with cellular ageing. Further exemplifying the importance of ageing in cancer, recent studies have highlighted that the multistage model of carcinogenesis (involving tumour initiation, promotion and progression) requires incorporation of ageing-dependent somatic selection to ensure this model is capable of generalizing cancer incidence across tissues and species6. Previous studies have also shown that this process of somatic selection is non-cell-autonomous, and is in fact defined by microenvironment-imposed increases in positive selection for prior accumulated genetic and/or phenotypic diversity in aged tissues7.
Paradoxically, while many of these factors involved in aged tissue evolution can lead to eventual transformation to malignant and hyperplastic growth in self-renewing tissues, these processes also contribute to growth arrest (senescence), apoptosis and degradation of other cells and structural tissue components. It has been well documented that cancer risk and many of these degradative features within tissues and cells exponentially increase as we age5. Studies are now finally beginning to mechanistically link the complex interrelationship between an aged local and systemic microenvironment and its contribution towards tumour initiation and progression. Furthermore, age-induced reprogramming of these stromal populations in an established TME also appears to play a major role in driving efficient metastatic progression. Interestingly, conflicting statistics regarding age and disease outcome have been reported across different cancer types (BOX 1); this phenomenon likely suggests that different stromal tissue environments across the body may be reprogrammed differently during ageing, which consequently impacts tumour growth and progression with respect to the tissue of origin.
Box 1 |. Tumour aggressiveness does not correlate with age in all tumours.
In many tumour types, age predicates a more aggressive tumour phenotype. However, for some cancers, such as breast and colon cancer, whilst the overall incidence remains higher in aged patients, the cancers diagnosed in younger patients are more aggressive, leading to poorer outcomes. The reasons for this disparity are unclear, but may involve the following:
-
Lack of screening
In younger individuals, routine screenings, such as mammograms and colonoscopies, are not mandated. Therefore, it is likely that cancers in the young are diagnosed much later than those diagnosed through routine and regular screening.
-
Driver mutations that promote more aggressive disease
Driver mutations in genes such as BRCA1 or BRCA2 and APCin breast cancer and colorectal cancer, respectively, may drive earlier manifestations of tumours and, depending on the subtype, tumours in younger patients with breast and colon cancer can be more aggressive212,213. However, in melanoma, driver mutations of the BRAF gene are similarly present in 69% of patients under the age of 45; however, these tumours are less aggressive in the young than in older patients, most of whom are BRAF wild-type214,215.
-
Hormonal influences
Breast cancer may be more susceptible to hormonal influences than other cancers such as melanoma; therefore, fluctuations during menstruation may affect the aggressive nature of breast cancers and changes during menopause may somewhat mitigate the aggressiveness. Additionally, it has been shown that melanomas diagnosed during pregnancy can be quite aggressive and recent data indicate this to be the result of aberrant expression of oestrogen receptors216 and the subsequent signalling downstream thereof.
-
Obesity
Obesity is emerging as a risk factor for multiple types of cancer. While conventional wisdom associates obesity with increasing age, the rates of obesity are rapidly rising in younger patients, which may explain the increasing rates of cancer in this population. For diseases such as breast and colon cancer, which are increasing in younger adults, obesity-associated inflammation may increase the aggressiveness of these cancers217.
This Review will focus on the role of the aged microenvironment in driving tumour progression. We will discuss the interactions between cancer cells and the aged TME, focusing on how ageing can reprogramme stromal fibroblast populations, the extracellular matrix (ECM) and immune infiltration to drive cancer initiation and progression. Finally, we will examine how the ageing TME can govern responses of tumour cells to chemotherapy, targeted therapies and immunotherapies.
The ageing stroma
Stromal homeostasis
The stromal microenvironment within tissue is made up of various components, including fibroblasts, endothelial cells, pericytes, adipocytes, ECM and immune cells, and plays a major role in tissue homeostasis. Fibroblasts are the most common stromal component within tissues across the body; these cells are required for synthesis of the ECM, including collagen, and for the structural integrity of connective tissue, and also play a key role in wound healing and inflammation8. Their predominant mode of action in regulating many of these processes is through the secretion of soluble factors, including cytokines, chemokines, growth factors, enzymes and structural components of the ECM5,9, into the microenvironment. Given the structural and functional complexity of different tissues, the microenvironment plays an important context-specific role in the regulation of the soluble factors secreted by fibroblasts along with their migratory and proliferative characteristics. The fibroblast renewal rate (which is the sum of the total number and proliferative capacity of fibroblasts) is highly diverse between different tissues, with factors such as local temperature, vascularization, mechanical stress and hormonal response contributing to this rate10. Changes that occur in fibroblasts during ageing are also likely to differ between organ sites and often involve senescence.
Antagonistic pleiotropy in ageing stromal environments
Antagonistic pleiotropy in an ageing context is defined as a singular gene trait that elicits a phenotype that is beneficial and increases fitness early in life but later, in an aged organism, becomes detrimental. Senescence is a classic example of antagonistic pleiotropy, and accumulation of senescent cells is one of the key pathological features associated with ageing5,11–13. As senescent fibroblast populations accumulate throughout the body as we age, senescence was originally used as a model to study ageing of fibroblasts both in vitro and in vivo14. However, senescence is a somewhat artificial model of ageing, and data indicate that normal ageing and senescence have few markers in common. Despite this, cellular senescence is linked to many of the cellular processes of ageing and can also occur in direct response to intrinsic or extrinsic oncogenic stimuli; it is important to note that there are many forms of senescence that occur irrespective of ageing (oncogene induced, replication induced, stress induced and therapy induced)15–17. While in humans it is impossible to truly distinguish whether senescence induction is due directly to age or these other factors, the key distinguishing feature is the accumulation of these senescent populations within aged stromal microenvironments and tissue. There is still much debate as to how the accumulation of senescent cells occurs in the elderly. It has been hypothesized that, as we age, a reduction in immune function (discussed later) decreases recognition and clearance of these growth arrested cells, which eventually results in their accumulation18; however, other studies argue that this hypothesis lacks evidence19.
One of the key features of senescence in cells is a widespread change in epigenetic gene expression, whereby cells dramatically increase the secretion of proinflammatory cytokines, chemokines, growth factors and proteases; this secretome is defined as the senescence-associated secretory phenotype (SASP)9. Typically, the SASP is thought to be made up of about 75 secreted factors, including granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-6 (IL-6), IL-8 and IL-10 (REFS5,9,14); however, many of these secreted factors were identified in studies using oncogene-induced senescent models and may not necessarily reflect true age-induced senescence. The SASP can be beneficial, alerting the immune system to tissue damage or that clearance of senescent cells is required, but can also be detrimental as it plays a role in driving tumour cell invasion and progression (discussed in detail below). Whilst the mechanisms underlying age-related SASP transformation are still being uncovered, many genetically engineered mouse models (GEMMs) have been key in determining its pathological and homeostatic role (BOX 2). p16INK4A activation appears to be one of the major contributors towards senescence induction in cells20, yet its contribution towards age-related accumulation of senescent cells needs further clarification. In line with this, when modelled in vivo, its contribution towards senescent cell accumulation is described as more of a ‘molecular’ form of ageing as opposed to a ‘chronological’ one20. A recent study reported the development of a GEMM using a Cdkn2aINK4A reporter allele that is sensitive enough to allow enumeration, isolation and characterization of individual p16INK4A-expressing cells21. This GEMM demonstrated that a dramatic accumulation of p16INK4A-expressing cells occurs across various tissues throughout the ageing process, and characterized the pathological changes associated with the age-induced SASP in peritoneal macrophages, highlighting the potential for other stromal components, besides fibroblasts, to contribute towards SASP-related pathologies. Much of the literature suggests that age-induced activation of these senescent programmes occurs via DNA damage; however, studies have shown that tumour-associated fibroblasts undergo chromatin remodelling via histone deacetylase (HDAC) modulation to achieve a SASP irrespective of DNA damage22. Recently, it was also shown that L1 (also known as LINE-1) retrotrans- posable elements are derepressed at the transcriptional level to elicit a type I interferon (IFN) response, which in turn contributes to maintenance of a SASP23. These findings further support the hypothesis that dynamic changes within an aged microenvironment, particularly within the TME, play a key role in reprogramming cells towards a SASP.
Box 2 |. Mouse models of senescence and ageing.
Several genetically engineered mouse models (GEMMs) have been created and have enabled investigation of the pathology of the senescence-associated secretory phenotype (SASP) as well as characterization of its secretome. These seminal studies were critical for the subsequent understanding of SASP involvement in the progression of many different cancer types, as discussed throughout the Review. A few models are described in more detail here.
The innovative INK-ATTAC model employed an apoptosis-inducible FKBP-CAS8 fusion protein driven by the Cdkn2aINK4A promoter that allowed visualization (through a GFP tag) of p16INK4A-positive cell populations and their selective removal via treatment with the drug AP20187 (REF.218). In a mouse model of accelerated ageing, life-long p16INK4A-expressing cell elimination decreased the incidence of several age-related pathologies, while their removal in late life attenuated progression of already established pathologies.
-
The first studies to elucidate an involvement of the SASP in homeostasis highlighted the importance of programmed senescence in embryonic development219,220.
The generation of the p16–3MR mouse model years later enabled another method of detection, isolation and elimination of senescent cells221. These studies found that senescent fibroblasts and endothelial cells appeared early during wound healing and secreted platelet-derived growth factor AA (PDGF-AA) to accelerate wound closure221; however, accumulation of senescent cells during ageing severely impacted tissue and cartilage regeneration and was a contributor towards osteoarthritis222.
p53 null and p16INK4A null animal models were employed to investigate reprogramming of cells in vivo towards induced pluripotent stem cells (iPSCs) using OCT4, KLF4, SOX2 and MYC (OKSM) factors, and highlighted the context-specificity involved in senescence induction and homeostasis or pathology223,224. oKSM expression led to iPSC reprogramming in many tissues but also induced senescence via p16INK4A in control animals in a similar manner to both tissue damage and ageing. These SASP cells secreted high amounts of interleukin-6 (IL-6) that invoked OKSM-like reprogramming of neighbouring cells, suggesting that senescence induction contributed to reprogramming-like cellular plasticity upon tissue damage, which is hypothesized to be important in wound healing and tissue repair. However, they also found that p53 null cells within tissues underwent greater cellular damage during senescence induction and induced exacerbated SASP-mediated secretion even in the absence of p16INK4A. This finding is indicative of how age-induced evolution of stromal tissues may lead to the accumulation of pathological SASP cells, and that this may drive reprogramming of the microenvironment towards a pathological state224.
SASP in cancer progression
Senescence plays an important role in the regulation of cancer cells, where oncogenic transformation of a normal cell can result in it becoming senescent, initially preventing its growth. However, malignant cells often bypass this process through genetic mutation or epigenetic down regulation of tumour suppressor-associated pathways such as p53–p21 and p16INK4A–RB pathways24. For example, in melanocytes, the BRAF oncogene induces senescence, but these cells are able to undergo malignant transformation and bypass senescence via activation of the canonical WNT signalling pathway25. Non-malignant senescent cells that are able to persist have also been shown to dramatically contribute towards tumour initiation and progression in many mouse models of cancer9,13,24, and this may involve non-canonical WNT signalling26.
In addition to cancer cells, this process is particularly prevalent in fibroblasts and age-related decreases in the number and proliferation of healthy stromal cells27,28 (discussed in detail later) can potentially dictate a local microenvironment that primarily contains senescent populations. Despite the homeostatic importance of programmed senescence in stromal cells, age-related accumulation of SASP cells can contribute to cancer progression by reprogramming both primary and metastatic microenvironments (including premetastatic niches) over time to a state that is more permissive for the growth of malignant cells (FIG. 1). Senescent fibroblasts and many SASP factors have been shown to induce cancer cell proliferation and invasion in culture29–31. Furthermore, co-injection of senescent fibroblasts, but not non-senescent fibroblasts, stimulates the growth and progression of various mouse and human tumours in syngeneic and immunocompromised mice, respectively31,32.
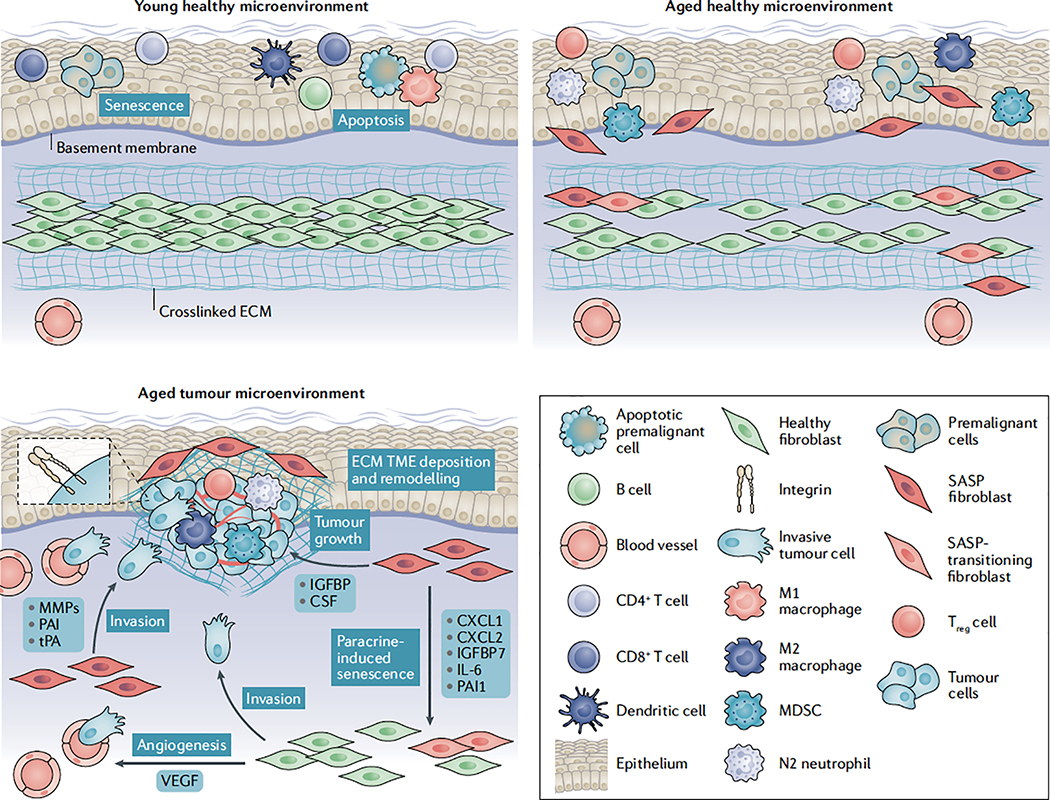
Fig. 1 |. Stromal deregulation in the aged microenvironment drives tumorigenesis and progression.
Fibroblasts are the most common stromal component within tissues. They are responsible for regulating tissue structure via extracellular matrix (ECM) deposition and for supporting cellular and microenvironmental homeostasis via the tightly regulated secretion of soluble factors such as cytokines, chemokines, growth factors and other key signalling proteins. Within younger, healthier tissues, the fibroblast secretome provides a very growth-restrictive microenvironment for premalignant cells and helps in the prevention of other pathological conditions. Furthermore, studies highlight that, under healthy conditions, fibroblasts can undergo a short-lived senescence-associated secretory phenotype (SASP) that, through secretion of approximately 75 defined soluble factors (such as granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-6 (IL-6), IL-8 and IL-10), can increase immune infiltrates and provide other factors important in the clearance of apoptotic, senescent and malignant cells while also aiding in the wound healing response5,9,14,20. Following injury and damage, these senescent fibroblasts are quickly cleared by the body221. As the body ages, the healthy fibroblast renewal rate decreases dramatically27,28 and immunosenescence results in a decrease in effector immune cell function126. As a result, aged tissue microenvironments have an accumulation of SASP-associated fibroblasts5,9,14 and a switch towards more immunosuppressive immune infiltrates, such as myeloid-derived suppressor cells (MDSCs)149–151 and regulatory T (Treg) cells141–144, when compared with a younger tissue microenvironment. It can be hypothesized that this establishes a tumour- permissive, chronic inflammatory microenvironment that enables cancer cells to eventually expand in number and progress unabated by the immune system. Systemic increases in immunosuppressive M2 macrophages133 and N2 neutrophils138 in the elderly may also further contribute to increased immunosuppression, whereas immunosenescence of effector T cells, natural killer cells, macrophages and dendritic cells, all dramatically decrease their cytotoxic activities and infiltration within an aged tumour- promoting microenvironment127–130. Extensive accumulation of senescent cells has been shown to be responsible for many age-related pathologies218 and is a major driver of cancer progression. Many of the soluble factors within the SASP have been shown to promote tumour invasion (matrix metalloproteinases (MMPs), plasminogen activator inhibitors (PAIs) and tissue-type plasminogen activator (tPA))9,33–35, growth (insulin-like growth factor binding protein (IGFBP)39–42 and colony-stimulating factor (CSF)41) and angiogenesis (vascular endothelial growth factor (VEGF)45) as well as to sustain a protumour milieu by supporting tumour evasion of immune surveillance (CXC-chemokine ligand 1 (CXCL1) and CXCL2 (REFS37,38), IL-6 (REF.4), IL-10 (REF.9) and GM-CSF9). Many of these factors also appear to have powerful paracrine effects that can induce SASP in surrounding stromal cells46–49. SASP fibroblasts also promote extensive ECM remodelling to increase key signalling components involved in tumorigenesis and to promote invasion of tumour cells and immune cell trafficking74,98. Recent evidence now also suggests that ageing reprogrammes the secretome of healthy human fibroblasts within the skin, whereby aged skin fibroblasts secrete factors such as secreted frizzled-related protein 2 (SFRP2), which significantly increases tumour cell invasion and dissemination, tumour angiogenesis, and resistance to targeted therapy71.
The great diversity of SASP-related secretory factors involved in these processes emphasizes their ability to regulate many facets of tumour development. Studies have highlighted increased secretion of matrix metalloproteinases (MMPs), such as MMP1, MMP3 and MMP10 (REFS33–35), and other proteases, such as plasminogen activator inhibitor 1 (PAI1) and PAI2 and tissue-type plasminogen activator (tPA), as drivers of tumorigenesis9. These act to reinforce the invasive phenotype in many malignancies via remodelling of the ECM, which is important given the age-related breakdown of ECM structure and function (discussed in detail later). MMPs can also regulate the activity of other soluble factors via cleavage and induce their activation and/or degradation9,36. SASP fibroblasts secrete large amounts of chemokines, cytokines and growth factors such as CXC-chemokine ligand 1 (CXCL1), CXCL2 (REFS37,38), IL-6 (REF.4), insulin-like growth factor binding proteins (IGFBPs)39–42 and colony stimulating factors (CSFs)41. These are all linked with tumorigenesis, modulation of the TME towards a protumour immune microenvironment and resistance to certain types of therapies9,13,43. Systemic angiogenesis also decreases in the elderly44; SASP fibroblasts can overcome this within local microenvironments and the TME through the secretion of angiogenic factors such as vascular endothelial growth factors (VEGFs)45. The accumulation of SASP cells also appears to induce a positive feedback loop within their microenvironment as secreted factors such as CXCL1 and CXCL2 (REF.46), IGFBP7 (REF.47), IL-6 (REF.48) and PAI1 (REF.49) appear to have powerful paracrine effects in the maintenance and induction of other senescent cells.
Other SASP factors, such as IL-1α, are also drivers of both tumour initiation and progression50,51. Genetic knockout of the senescence-inducing factor SIN3B in a KrasG12D-driven mouse model of spontaneous pancreatic intraepithelial neoplasia (PanIN; the non-invasive precursor lesions of pancreatic ductal adenocarcinoma (PDAC)) revealed that the initiation and progression of pancreatic lesions was significantly reduced compared with control mice50. Furthermore, SIN3B deletion significantly reduced SASP factor IL-1α secretion in the pancreata of knockout mice and several primary pancreatic duct epithelial cell (PDEC) lines. A follow-up study to this employed senescent uncoupling of IL-1α via doxycycline-inducible IL-1 receptor (IL-1R) knockdown in lung fibroblasts, which significantly decreased the secretion of IL-1α and other SASP factors (IL-1β, IL-6 and IL-8) compared with control cells51. The authors further confirmed that not only did the IL-1 pathway control the majority of the SASP via autocrine and paracrine signalling through IL-1α secretion, but that the uncoupling of IL-1α via conditional knockout in the spontaneous PanIN model described above significantly reduced the number of neoplastic lesions.
Finally, SASP-associated fibroblasts also undergo dramatic metabolic changes such as mitochondrial dysfunction, hydrogen peroxide production and a switch towards aerobic glycolysis52. These changes lead to increased production and secretion of high energy metabolites, such as lactate, ketones and glutamine, into the microenvironment, along with secretion of molecules such as nitric oxide and reactive oxygen species (ROS)53–56. Together, these factors enhance cancer cell aggressiveness and accelerate age-related cellular damage, which can further promote a permissive metabolic microenvironment for cancer development. However, it is clear that more studies investigating direct age-related changes in the metabolic reprogramming of stromal components may help in uncovering further mechanisms that link the aged microenvironment with tumour progression.
Impact of ageing on other TME components
The bone microenvironment is another important niche shown to have age-related changes. Osteoblasts are responsible for bone formation and are almost indistinguishable from fibroblasts in terms of gene expression, with only two osteoblast-specific transcripts so far identified (core-binding factor subunit α1 (CBFA1, also known as RUNX2)57 and BGLAP, the gene encoding osteocalcin58); however, microenvironmental regulation of these cells enables a dramatic change in their function, resulting in an ECM that is mineralized59. Importantly, many studies highlight age-related changes in this stromal component, leading to an accumulation of senescent populations that contribute to both primary and metastatic tumour progression60. The Fibroblasts Accelerate Stromal-Supported Tumorigenesis (FASST) mouse model uses a stromal-specific, oestrogen-responsive Cre recombinase to create senescent osteoblasts in mice by inducing expression of the cell cycle inhibitor p27Kip1 (REF.60). To investigate metastatic tumour burden in the bone, a study used intracardiac injection of NT2.5 mouse breast cancer cells and found that FASST mice had significantly increased metastasis compared with control mice. Senescent osteoblasts in the FASST mice significantly increased bone remodelling via secretion of IL-6, which functioned to increase osteoclastogenesis and overall tumour cell growth. Importantly, treatment with a neutralizing IL-6 antibody inhibited metastatic outgrowth, primarily by inhibiting osteoclast-driven remodelling in the FASST mice. Given that other cancers, such as multiple myeloma, are also dependent on a supportive bone microenvironment for their progression61, it will be critical to further understand how this stromal environment ages and supports tumour progression.
As fibroblasts are the most common stromal component within tissues, much of the literature on ageing focuses on SASP-related effects and the underlying cancer pathologies driven by these populations. However, there is a clear role for other senescent populations, such as endothelial cells, epithelial cells, immune cells, stem cells and even certain tumour cells, in modulating the TME through acquiring a SASP (this has been extensively reviewed in REF62). Interestingly, there are many examples where senescence in these populations can contextually produce protumorigenic or antitumorigenic effects; nevertheless, direct age-related studies within these cell types are still limited. Triple negative breast cancer is an example of a cancer subtype where older women have a better outcome compared with younger women63. A recent study used a xenograft model with human BPLER triple negative breast cancer cells in nude mice and found that tumours showed delayed onset, slower growth kinetics and less metastasis in aged mice (>10 months old) compared with young mice (8–10 weeks old). The study further showed that a subset of tumour-infiltrating haematopoietic cells in young mice upregulated the CSF1 receptor (CSF1R) and secreted the growth factor granulin to induce robust tumour growth and metastasis. Importantly, bone marrow-derived cells from young mice were sufficient to activate a tumour-supportive microenvironment and induce tumour progression when transplanted into the aged mice.
Non-senescent aged stroma and tumour growth
While the accumulation of senescent fibroblasts and other cells is well documented with age, there is much debate as to whether these SASP-related effects on tumour development can truly be attributed to the ageing process11,64. Interestingly, studies of slower ageing animal species with great longevity, such as lobsters and rainbow trout, have been shown to retain telomerase activity, allowing long term cellular proliferation capacity65,66. It has been hypothesized that these species have significantly slower rates of senescent cell accumulation4,67; however, no direct studies have been performed to confirm this. Yet, it is important to note that many studies have now shown that the mode of senescence initiation (induced by oncogenes, replication, stress or therapy) dramatically alters the SASP factors secreted by these cells and, thus, not all senescence is equal nor may it truly be indicative of ageing15–17.
Aged tissues have been found to have a dramatic decrease in fibroblast numbers, proliferative capacity and density27,28,68. Recent studies are investigating the mechanisms underlying these changes as well as how aged, non-senescent fibroblasts are genetically reprogrammed and appear to change their identity compared with younger populations. Studies in the ageing mouse skin (from mice aged 10 and 16 months) have shown that, rather than a uniform loss in fibroblast density, there appear to be highly localized clusters of cell loss69. The study determined that the loss of fibroblast density that occurs with ageing is not a uniform loss of cells, but an accumulation of localized cell losses that are not recovered by proliferation. Interestingly, rather than filling these gaps by increasing cell numbers, fibroblasts instead maintain positional stability within the tissue and extend their protrusions to ensure membrane occupancy of the volume. Along with the decrease in fibroblast number, a recent study has also highlighted that dermal aged fibroblasts appear to evolve phenotypically in non-pathological conditions in the aged skin70. Specifically, the study shows that the genomic identity of aged fibroblasts (from 18-month-old mice) changes dramatically compared with the clearly demarcated populations of fibroblasts in the young skin. Interestingly, aged fibroblasts dramatically decrease the expression of genes involved in ECM production but gain adipogenic traits, which is highly influenced by systemic metabolism as caloric restriction inhibits this aged phenotype but a high-fat diet accelerates it.
In line with these findings, recent studies from our group have now begun to show that non-senescent aged fibroblasts from healthy human donors appear to promote tumour progression71 and have a dramatically different secretome compared with senescent fibroblasts72. Using a syngeneic mouse model of skin melanoma, subcutaneous injection of YUMM1.7 cells resulted in faster growing primary tumours in younger animals (8 weeks old); however, aged mice (52 weeks old) had a significantly increased vessel density and number of lung micrometastases71. Using organotypic 3D human skin reconstructions, aged fibroblasts taken from healthy human donors (>55 years old) induced significantly more invasion but less proliferation of several human melanoma cell lines when compared with fibroblasts taken from younger healthy donors (<35 years old)71. Proteomic analysis of conditioned media (CM) from the culture of these young versus aged dermal fibroblasts confirmed that secreted frizzled-related protein 2 (SFRP2, a canonical-WNT antagonist) secretion was significantly higher in aged CM and was responsible for the increases in melanoma cell invasiveness. Furthermore, recombinant SFRP2 treatment in young mice significantly increased tumour angiogenesis and lung metastasis71.
Collectively, these studies highlight the importance of age-related changes in the fibroblast secretome of both senescent and non-senescent populations and the contribution thereof to tumour progression (FIG. 1). Given the large variation in organ structure and fibroblast (and other stromal cell) function within different tissues, an important future research avenue will be to examine other fibroblast populations with respect to their secretome in young and aged healthy patients, as many of the studies of aged-fibroblast evolution have only been performed with dermal cell populations. This may help better our understanding of how age may contribute to other cancer types originating in various tissues and the potential involvement of aged stromal cell populations in the establishment of a premetastatic niche. It may also reveal information related to metastatic site specificity (organotropism) of different primary cancer types.
The matrix and ageing
The ECM
In addition to secreted factors, fibroblasts also play a role in laying down an ECM. The ECM is primarily responsible for the architectural integrity of most tissues and governs protein and cell-specific trafficking across the body. It is defined as the non-cellular component of tissue, made up of fibrillar proteins, scaffold proteins and other molecules that provide structural and biochemical support for cells. Components of the ECM serve as ligands for cell surface receptors such as integrins and control central homeostatic processes, including adhesion, apoptosis, proliferation, migration, survival and differentiation. The ECM is highly dynamic and constantly undergoing remodelling controlled by a delicate balance between degradation and deposition. Often described as a master regulator of cell phenotype, the loss of tissue ECM integrity encompasses one of the hallmarks of cancer and its dysregulation is heavily associated with tumour progression and metastasis73–76.
ECM composition diversity across organs
The ECM microenvironment across organs differs greatly owing to the complex variation required for specific organ structure and function. While there is diversity in many of the biochemical cues in an ECM microenvironment, heterogeneity in stiffness and elasticity are also observed throughout the body and these factors change dramatically as we age. In healthy individuals, softer tissues such as the brain and breast require a more elastic, ‘looser’ connective tissue environment, whereas harder tissues such as the skin and bone require stiffer structures to provide a protective barrier. This is achieved via regulation of crosslinking of ECM components and overall collagen density. For example, in the skin, collagen and elastin are tightly bound to each other by hyaluronic acid (HA) in a basket-weave pattern. HA alterations have been shown to increase the ability of fibroblasts to contract elastin and collagen matrices77. Moreover, the role of HA in cancer is believed to be tissue specific; reduced HA is associated with increased tumorigenesis in normally HA-rich tissues such as skin78. Furthermore, elegant studies in naked mole rats have demonstrated that their fibroblasts secrete a high molecular mass HA, which accumulates in tissue and is hypothesized to contribute to the cancer resistance and exceptional longevity observed in these animals79. Changes in ECM stiffness also provide cells with biophysical cues that can dramatically alter the cell phenotype80.
Pathologically, this diversity in ligand expression and stiffness plays a major role in cancer progression (FIG. 2). It was initially hypothesized that cancer metastasis followed a linear model whereby increasing ECM stiffness favoured progression; however, recent mathematical modelling of cancer cells suggests that stiffness fits a biphasic model, whereby too much stiffening inhibits progression as cell nuclei are unable to efficiently fit through the smaller pores created in very stiffly crosslinked matrices81. This can ultimately affect tumour cell invasion and influence the immune cell infiltrate72. Importantly, discrete morphological alterations in the ECM, such as the realignment of fibres, strain-stiffening and increased crosslinking, also play a key role in these processes82,83. As such, there is a clear need for understanding the diverse ECM remodelling occurring in different environments, particularly in aged patients, that enables efficient cancer progression.
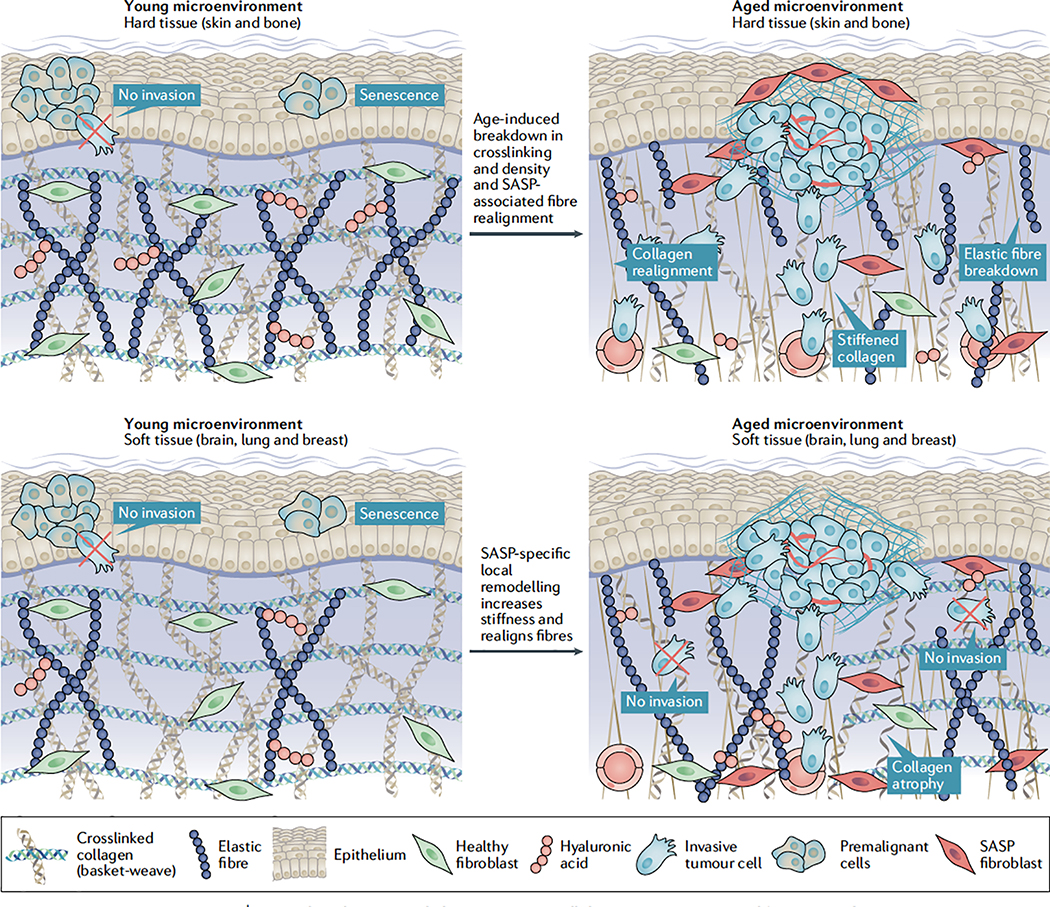
Fig. 2 |. Age-induced contextual changes in extracellular matrix structure and function in the tumour microenvironment.
The extracellular matrix (ECM) provides a scaffold responsible for protein and cell-specific trafficking across the body, while also providing structural integrity for tissue microenvironments. Components of the ECM serve as ligands for cell surface receptors known as integrins and these, along with the biophysical properties of the ECM including stiffness, crosslinking and fibre alignment, provide extrinsic cues that dramatically alter cellular phenotype. The loss of tissue ECM integrity encompasses one of the hallmarks of cancer, and ECM dysregulation is heavily associated with tumour progression and metastasis73–76. There is dramatic diversity in both the biochemical and biophysical nature of the ECM environment across different tissues. For example, the ‘basket-weave’ pattern seen in the ECM of skin has not been observed in breast tissues (not shown in this figure for simplicity). In healthy, younger individuals, softer tissues, such as the brain, lung and breast, require a more elastic, ‘looser’ connective tissue environment (bottom, left), whereas harder tissues, such as the skin and bone, require stiffer structures to provide a protective barrier (top, left). It has been clearly established that ECM integrity decreases substantially across the body as we age, which includes a decrease in collagen density84–86 and ECM fibre area and thickness87–89 as well as changes in the mechanical properties of the ECM such as stiffness85. Furthermore, natural age-related turnover of collagen crosslinking proteins, such as fibulin, fibrillin and elastin90,91, decreases crosslinking within the ECM, which further impairs integrity92 (top and bottom, right). Studies reveal that age-related decreases in ECM integrity in the skin, which is known to be a stiff tissue microenvironment, can promote cancer progression72. Specifically, these studies show that age-related decreases in the secretion of the ECM remodelling protein hyaluronan and proteoglycan link protein 1 (HAPLN1) from fibroblasts (and consequent breakdown of hyaluronic acid (HA)) result in significantly decreased collagen crosslinking and density while also increasing fibre alignment, resulting in increased melanoma cell invasion and progression (top, right). By contrast, the different context of softer tissue microenvironments means that they often require increased crosslinking and stiffening to promote efficient tumour progression100,105. Age-induced accumulation of senescence-associated secretory phenotype (SASP) stromal cells appears to be a key mechanism in inducing ECM stiffness in site-specific niches within these environments, such as the lung99, which may ultimately allow increased tumorigenesis and dissemination; however, further studies are required to directly link these processes (bottom, right).
The ECM in ageing and cancer
It has been clearly established that ECM integrity decreases substantially as we age. The most well-known and visually recognizable example of this is the wrinkling process, which occurs in the skin. Age-related changes in the physical properties of the ECM include decreases in collagen density84–86, ECM fibre area and thickness87–89, and changes in the mechanical properties of the ECM, for example stiffness85. Furthermore, physiological age-related turnover of collagen crosslinking proteins such as fibulin, fibrillin and elastin90·91 decreases crosslinking within the ECM, which further impairs integrity92.
Our studies of young and aged healthy human fibroblast samples revealed that young dermal fibroblasts secrete higher levels of ECM constituents, including proteoglycans, glycoproteins and cartilage-linking proteins, when compared with aged dermal fibroblasts72. The most abundant secreted ECM component from young fibroblasts was hyaluronan and proteoglycan link protein 1 (HAPLN1). HAPLN1 is a crosslinking protein that stabilizes proteoglycan monomer aggregates with HA; it was originally thought to be secreted exclusively by tumour cells and to contribute to tumour cell invasion93. However, we find that HAPLN1 is secreted by young fibroblasts and that this secretion is lost from healthy aged human skin fibroblasts — an observation that may reflect the complex interplay that occurs between tumour cells and fibroblasts, which changes with age. Furthermore, there are data showing that LOXL1 is critical for ECM-mediated migration of cancer cells94, yet our proteomics data show that LOXL1 is no longer secreted by aged fibroblasts72. Collectively, these changes in the ECM components secreted by aged dermal fibroblasts result in a decrease in collagen density and fibre crosslinking as well as a dramatic increase in fibre alignment, which can promote melanoma cell invasion in vitro.
A follow-up study from our group investigated the importance of ECM breakdown on the formation of visceral metastases95. Paradoxically, older patients with melanoma have lower rates of sentinel lymph node metastases but have inferior survival and increased visceral metastases96. Given the importance of HAPLN1 in age-dependent ECM breakdown in the dermis, our study found that lymphatic expression of HAPLN1 was prognostic of long-term survival in patients with melanoma. HAPLN1 secreted from fibroblasts reduced lymphatic endothelial cell permeability via modulation of vascular endothelial–cadherin (VE-cadherin) junctions in vitro, whereas reconstitution of HAPLN1 in aged mice increased lymph node metastases while reducing visceral metastases95. These findings may have considerable clinical implications for other cancers that follow a sequential model of progression, where cancer cells spread from the primary tumour, to the lymph node, and then finally to visceral sites97.
Impact of ECM stiffness on the aged TME
Age-related increases in senescent stromal components with a SASP can lead to secretion of soluble factors that contribute to ECM remodelling and matrix stiffening in the local microenvironment98. This appears to be a key factor in softer tissue-associated TMEs, which require increased crosslinking and stiffening to allow tumour dissemination and progression. Such a phenomenon can account for the fact that, while body-wide breakdown of the ECM architecture occurs rapidly with ageing, local age- related stromal accumulation of SASP cells may foster a tumour-specific niche required for progression (FIG. 2). Studies using samples from young and aged lungs of both humans and mice have shown that age-related accumulation of senescent cells with a SASP can increase collagen density and crosslinking99. While no studies have directly linked these aged changes in ECM structure and lung cancer specifically, malignancies of the lung are directly correlated with age and increases in ECM stiffness, crosslinking and collagen density100 and, as such, direct studies investigating SASP cell accumulation and tumorigenesis in an aged context are warranted in these soft-tissue environments. In vitro 3D and in vivo systems have led to the hypothesis that ECM remodelling within breast tissue can act in both an oncogenic and tumour-suppressive role dependent upon the context101,102. For example, age-related changes in stromal ECM density correlate with higher likelihood of breast cancer, while compromised integrity of the basement membrane or the myoepithelial layer allows premalignant or malignant luminal cells to contact ECM components such as collagen I, leading to MMP secretion and aggressive tumorigenesis101. This has been underscored by the fact that ageing of human breast fibroblasts via passaging in culture has been shown to significantly increase the secretion of MMPs, proteases, growth factors and other ECM-modifying components103.
Furthermore, immune cells are significantly impacted by ECM remodelling during tumour progression104. This is evident in breast cancer, as ECM stiffening has a significant correlation with immune infiltration105. Interestingly, in our study, HAPLN1 also contributed to altered immune cell motility in melanoma in 3D in vitro assays and in allogeneic mouse models. Specifically, HAPLN1 significantly increased migration and infiltration of effector CD8+ T cells into the tumour, while reducing immunosuppressive regulatory T (Treg) cell infiltration72. Overall, these findings highlight the importance of physical changes in the ECM as a mediator of both immune and tumour cell trafficking. As discussed below, there is a direct influence of ageing on immune cell type specificity and function within systemic and local microenvironments; yet, no studies have been performed to date investigating the potential consequential effects on ECM structure and functionality. Understanding these influences may allow a therapeutic avenue to alter the ECM and potentially inhibit metastasis.
The immune microenvironment and ageing
Inflammaging: an age-related driver of cancer progression
The immune system plays a vital role in recognizing and inhibiting malignant tissue growth. Thus, the evasion of immune surveillance is a critical step necessary for tumour initiation, growth and progression towards metastasis73. One of the hallmarks of ageing is an increase in systemic low-grade chronic inflammation, a process termed ‘inflammaging’106. This persistent inflammatory response can lead to tissue degeneration and disrupted acute inflammation and is heavily associated with cancer induction and progression107 (FIG. 3). Cellular senescence has been proposed as a key contributor linking inflammaging with many age-related malignancies107,108. SASP induction in stromal populations results in the persistently increased secretion of multiple inflammatory cytokines that maintains a low- grade adaptive immune response. Other age-related changes to the gut microbiota· obesity and tissue degradation also appear to drive the inflammaging response4. Overall, these age-related processes appear to drive chronic inflammation by increasing systemic levels of IL-1, IL-6· IL-lα, IL-1β, IL-33· GM-CSF, IFNγ, tumour necrosis factor (TNF) and C-reactive protein (CRP), all of which have been shown to contribute to multiple morbidities and mortalities in the elderly4,109.
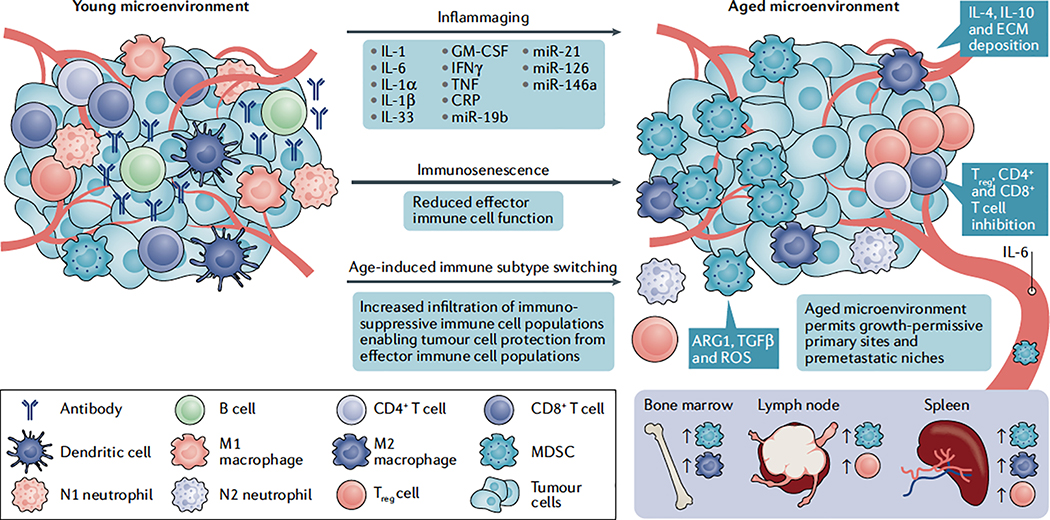
Fig. 3 |. Immune cell switching, inflammaging and immunosenescence as drivers of age-induced tumour progression.
Many of the hallmarks of cancer require immune modulation as an important step in enabling efficient progression12,73. One of the critical factors involved in age-related pathologies is immunosenescence, a process defined as a dramatic decline in overall immune function126,198. Subpopulations of effector immune cells, including T cells, natural killer cells, macrophages and dendritic cells, exhibit a dramatic decrease in cytotoxic activity during the ageing process127–130. In younger and healthier tissues, these effector cells are responsible for acute responses to malignant tissue growth and clearance of senescent cells; thus, age-related immunosenescence plays a key role in promoting tumour formation and accumulation of senescence-associated secretory phenotype (SASP)-secreting cells. These age-related decreases in effector immune cell function also appear to induce tissue-specific switching towards more immunosuppressive cell populations. Many studies show that immunosuppressive myeloid-derived suppressor cells (MDSCs)149–151 and regulatory T (Treg) cells141–144 are significantly increased in aged tissues and blood, and depending on the context, contribute towards the progression of tumours in aged mouse models144,152. These immune components are also critical for the establishment of premetastatic niches across many cancer types148,153; however, a direct relationship between these immune cell populations and metastatic niche formation with ageing has yet to be investigated. Furthermore, in the elderly, effector cells, such as neutrophils and macrophages, appear to switch phenotypically towards immunosuppressive N2 (REF138) and M2 (REF133) states, respectively, both of which have been shown to promote tumorigenesis of various cancer types135,139; yet, more direct studies showing their involvement in age-related tumorigenesis are warranted. The accumulation of SASP stromal components also results in ‘inflammaging’, a process defined by persistent low-grade inflammation107,108,126. This process has been shown to disrupt acute inflammatory responses towards malignant tissue, induce infiltration of immunosuppressive MDSCs111 and Treg cells112, and promote secretion of anti-inflammatory components such as cytokines, chemokines and ‘inflamma-miRs’116–119. As such, ‘inflammaging’ appears to play a key role in impairing antitumour immunity in aged individuals. ARG1, arginase 1; CRP, C-reactive protein; ECM, extracellular matrix; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFNγ, interferon-γ; IL, interleukin; ROS, reactive oxygen species; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor.
One of the key elements that appear to link inflam- maging to many types of cancer involves the recruitment of myeloid-derived suppressor cells (MDSCs). MDSCs are a heterogeneous population of cells defined by their myeloid origin and are potent repressors of T cells through secretion of arginase 1 (ARG1), transforming growth factor-β (TGFβ) and ROS110. A study using the Ret transgenic spontaneous melanoma mouse model, which over time develops malignant skin melanoma that progresses towards lymph, liver and lung metastases, showed that persistent upregulation of inflammaging factors such as IL-1β, GM-CSF and IFNγ correlates with progression111. These factors induced MDSC infiltration in primary and metastatic tumour sites, and isolated MDSCs from these melanoma-bearing mice significantly reduced T cell proliferation ex vivo. Treatment with the phosphodiesterase type 5 inhibitor sildenafil, which has been shown to reduce MDSC immunosuppressive activities, decreased tumour growth, systemically decreased inflammaging mediators, restored T cell function and increased mice survival.
Similar links have been found with increased infiltration of immunosuppressive Treg populations in chronic inflammatory mouse models, which mimic the chronic inflammation that often precedes and may lead to certain malignancies such as skin and colorectal can- cer112. Treg cells play a key role in maintaining tolerance to self-antigens and suppress the induction and proliferation of effector T cells (such as CD4+ and CD8+ T cells) via the secretion of cytokines and enzymes113. One study induced chronic, tumour-promoting allergic contact dermatitis (ACD) in mice 6–8 weeks old by treating them with 1-fluoro-2,4-dinitrobenzene (DNFB) and found that IL-33 expression was key in inducing the transition from acute, tumour suppressing inflammation to chronic inflammation112. The authors then employed a genetic IL-33 knockout mouse model and found that treatment with the carcinogen 7,12-dimethyl- benz(a)anthracene (DMBA), which promoted significant tumour formation on the skin of control animals, protected against tumour formation and progression. Treg cells were significantly reduced in DNFB-treated IL-33 knockout mice, and importantly knockout of the IL-33R from Treg cells significantly reduced ACD- induced skin carcinogenesis. Interestingly, extension of this study to look at colitis-induced colorectal cancer also showed the IL-33-Treg cell axis to be a key driver of carcinogenesis. Despite the potential involvement of IL-33 as a mediator of various pathologies and inflammaging in aged models109,114,115, more direct studies are required to confirm any age-related effects of the IL-33-Treg cell axis in tumorigenesis.
Studies in breast cancer show that another key inflammaging component, IL-6 was crucial in driving tumour progression· with high serum levels correlating with both age and a poor patient prognosis116,117. Several microRNAs termed ‘inflamma-miRs’ have been linked with many different human malignancies118. Specifically, age-related increases in miR-19b, miR-21, miR-126 and miR-146a appear to drive progression in many cancer types, at least in part, through inflammaging. miR-21, a biomarker for inflammaging119, has been shown to be overexpressed in many malignancies and reduces expression of potent anti-inflammatory IL-10 and TGFβ120, while binding to Toll-like receptor 8 (TLR8) and inducing secretion of inflammaging cytokines IL-6 and TNF121.
Many of the models performed under conditions of chronic inflammation show a direct link to disease progression and inflammaging-associated signalling. However, few models have confirmed a causative association between many of these components and age-related cancer progression. Further studies are warranted to access the mechanistic intricacies associated with inflammaging and tumorigenesis.
Age-induced immunosenescence and cancer
Immunosenescence is another contributing factor to many age-related pathologies, including cancer. It is defined as an age-related dysregulation ofthe immune system, whereby subpopulations of effector immune cells and overall immune function decline. This process is the result of multiple factors, including thymic atrophy122, decreases in naive T cells123, a reduction in memory T cell function124 and decreased antigen recognition diversity by T cells125. Inflammaging appears to play a key role in accelerating many of these processes, as chronic inflammatory signals and responses associated with this process are often inhibitory126. Along with T cell loss of function, natural killer cells, macrophages and dendritic cells, which play an early role in tumour recognition and suppression, appear to also undergo phenotypic decreases in cytotoxic activity as we age127–130. A key example highlighting the involvement of these processes in cancer progression has been shown in a squamous cell carcinoma (SCC) model of ageing131. In a GEMM conditionally expressing mutant HRAS in keratinocytes, it was found that aged mice (18–22 months old) developed SCC at a much higher rate than young mice (2–4 months old). Analysis of the immune system of these aged mice demonstrated a shift towards a protumorigenic T helper 2 cell anti-inflammatory response as well as increased expression of the inhibitory programmed cell death 1 ligand 1 (PDL1) and senescence-associated β-galactosidase on effector immune cells in the dermis.
An immune-cell switch in TMEs and premetastatic niches
Age-induced immunosenescence occurs largely in effector T cells and other immune cell types crucial for tumour immunity. It has been hypothesized that these changes may induce a shift towards the activation and infiltration of more immunosuppressive cell populations in the elderly, which may be key to their increased predisposition to cancer and metastasis132 (FIG. 3). Studies have shown that M2 tumour associated macrophages (TAMs), which have an immunosuppressive phenotype, are significantly higher in the spleen and bone marrow of aged (> 24–28 months old) mice133. Furthermore, stimulation of macrophages isolated and cultured from aged mice with mesothelioma or lung carcinoma cell-derived supernatants increased the levels of the M2-derived immunosuppressive cytokine IL-4, a phenotype not observed in macrophages isolated from young mice. Stimulation of M2 TAMs towards an M1 proinflammatory phenotype by treating aged mice with a combination IL-2 agonist and anti-CD40 therapy reduced immunosuppressive IL-4 and IL-10 expression and resulted in a decrease in tumour growth133,134. While the binary M1–M2 classification of macrophages is heavily debated, there is a large amount of evidence that these M2-like immunosuppressive macrophages are key drivers of tumour progression in an ageing context135. TAMs have also been shown to play a role in setting up a premetastatic niche in the liver by secreting CXCL1 and inducing the recruitment of MDSCs, which is necessary for efficient formation of colorectal cancer liver metastases136. More direct studies on the local and systemic effects of this M2 TAM switch in aged microenvironments and tumorigenesis are clearly warranted.
While neutrophils appear to undergo immunosenescence throughout ageing, they have still been shown to infiltrate injured tissue in the elderly137. Interestingly, studies have shown that neutrophils in aged patients and mice produce more anti-inflammatory cytokines than their younger counterparts138. Neutrophils are well- established mediators of tumour progression through their proinflammatory effects in tumour models of young mice; however, a subpopulation of anti-inflammatory ‘N2’ tumour-associated neutrophils (TANs) are now implicated in many cancers139. Their role in the context of ageing and cancer has not been well defined, yet studies in young mice suggest that these immunosuppressive N2 TANs, which are increased systemically in aged patients, exhibit a similar function to MDSCs140. Nevertheless, direct age-related studies of N2 TAN involvement in the TME are lacking.
Studies on the contribution of Treg cells to an age- related decline in immune response are contradictory132. Many studies show a dramatic increase in Treg cell numbers and function in age-related pathologies, and in organs such as the lymph nodes and spleen141–144; however, our studies and others show no change in Treg cell numbers or function or a reduced contribution in other aged tissues and cancers145–147, suggesting that they have a context-specific role in different TMEs and tissue environments. An early study using a model of pre-B cell acute lymphoblastic leukaemia (pre-B-ALL), whereby BM-185 cell lines were implanted into young (2–3 months old) and aged (18–22 months old) mice, found that young mice rejected these tumours whereas they grew efficiently in aged mice144. Analysis of the young and aged animals confirmed that Treg cells were significantly increased in the spleen and lymph nodes of older animals. The study employed a neutralizing antibody against CD25, a marker largely expressed on Treg cells (and at lower levels on activated T cells, B cells, thymocyte subsets and pre-B cells) and showed that neutralization of CD25+ Treg cells induced the rejection of tumours in aged mice and restored the activity of cytotoxic T cells. Furthermore, Treg cell recruitment also appears to be a crucial process in the establishment of the premetastatic niche in many cancer types148. Nonetheless, it remains to be established if the age-related increases in Treg cells seen systemically in certain mouse models is directly linked with increases in age-related metastasis.
MDSCs show a consistent increase during ageing in human blood149, as well as in the bone marrow and lymphoid organs150 of mice (17–19 months old)151. In a syngeneic mouse model of breast cancer, young mice (2 months old) rejected implantation of TS/A mammary adenocarcinoma cells, whilst aged mice (12 months old) showed efficient tumour growth152. It was found that regression within these young mice correlated with significant effector T cell infiltration, whereas aged mice had significantly increased numbers of MDSCs in the TME. These tumour-specific MDSCs were highly immunosuppressive in vitro and depletion of MDSCs significantly reduced tumour growth in aged mice, while adoptive transfer of aged-mouse MDSCs into young mice increased tumorigenesis. Importantly, MDSCs are also one of the immune cell types most highly associated with the formation of the premetastatic niche in cancer153.
While there are a great many signalling molecules associated with MDSC infiltration and activation, it is still unclear how they are recruited towards aged primary TMEs or in the formation of the premetastatic niche. However, as described in earlier sections, there is extensive cross-talk between senescence-associated stromal populations that accumulate during ageing and an immunosuppressive phenotype. A study employing the FASST mouse model described earlier showed that senescence induction within this model154 mimicked the mosaic pattern of senescent stromal cell accumulation observed in aged human tissues such as the skin, lung, breast and bone155. Stromal senescence significantly increased the numbers of immunosuppressive MDSCs and Treg cells in healthy mice adjacent to senescent populations, primarily via secretion of IL-6 (REF154). Furthermore, these immunosuppressive populations inhibited CD8+ T cell functionality and promoted tumour growth of implanted PDSC5 mouse squamous cell carcinoma cells in FASST mice. Finally, the authors of this study confirmed that senescent stromal cells were increased in the aged human skin (63–73 years old) compared with skin from younger individuals (29–33 years old), and this increase correlated with increased IL-6 expression and MDSC infiltration. These studies additionally suggest that accumulation of senescent stromal cells is sufficient to establish a tumour-permissive, chronic inflammatory microenvironment that allows cancer to grow and progress unabated by the immune system. Given the fact that age-related increases in systemic MDSCs and other age-related processes, such as inflammaging and ECM modulation, may directly link MDSCs, Treg cells and other immunosuppressive cell populations with age-related cancer predisposition and premetastatic niche formation, further investigations into the role of immunosuppressive immune cell types in these processes are highly warranted.
Interesting contradictory observations in prostate cancer show that age-induced increases in effector T cells and proinflammatory cytokines appear to contribute to increases in tumour growth. Prostate fibroblasts cultured from young (<55 years old) and aged (>65 years old) healthy individuals showed that aged fibroblasts secrete increased amounts of cytokines and interleukins, which increase the growth of epithelial cells156 and may affect the function of immune cells. Importantly, another study in prostate cancer highlighted that CD3+, CD4+ and CD8+ T cell infiltration was protumorigenic and correlated with tumour growth157. This further highlights the context dependence of effector versus immunosuppressive immune cell infiltration in microenvironments with age, which in turn establishes tumour growth and premetastatic niche formation.
Ageing and response to therapy
There are many challenging aspects regarding the treatment of cancer in elderly patients. Often, age-related health conditions put clinicians in a dilemma as to whether potentially beneficial therapies can be safely administered at standard dosages and improve survival or whether the potential side effects will likely affect the patient’s quality of life. Approximately 50% of all cancers occur in patients over 65 years old, with an estimate of that number rising to 70% as life expectancy continues to increase158; however, there is very limited data from clinical trials in patients above this age. Only 40% of patients enrolled in cancer clinical trials are over 65 and less than 10% are over 75 years of age159. Importantly, most preclinical studies are designed in mice 6–8 weeks old, which vastly misrepresents the true clinical age when most cancers arise in patients. It is thought that the age of 18 months more closely represents the true geriatric age in mice, equivalent to 60 years old in humans; however, this may differ for different organs. Recent advances in the understanding of the mechanisms underlying the aged TME and response to therapy may reveal crucial knowledge that will allow more efficient and less intensive targeting of cancer types in elderly individuals.
Chemotherapeutic response in aged patients
Chemotherapy is a non-specific, aggressive treatment avenue that results in targeting of fast-growing malignant cells. It can be used alone or in combination with other targeted therapies. Given its limited specificity, there are many side effects associated with treatment that can often be life threatening in more elderly individuals. There are many examples of how the aged microenvironment can contribute to chemotherapeutic resistance. Senescent fibroblasts, other stromal components and cancer cells with a SASP are already highly resistant to chemotherapy due to their non-proliferative nature. Additionally, these cells can secrete factors that promote intrinsic resistance of cancer cells to chemotherapy in a paracrine fashion160–162, can induce remodelling of the ECM to upregulate ligands and support structures that promote resistance (referred to as cell adhesion-mediated drug resistance)163–166, and alter immune cell types within the microenvironment to support a protumorigenic niche9,12,13,162,167. Furthermore, studies also support the fact that chemotherapeutic treatment can cause further SASP induction in tumour, immune and stromal cells via therapy-induced senescence. A recent study using the p16–3MR mouse model described in BOX 2 highlighted that therapy-induced senescent fibroblasts within mice lead to a persistent inflammaging response, and that elimination of these cells in vivo significantly decreased the short-term and long-term side effects associated with chemotherapeutic cytotoxicity, decreased cancer recurrence and reduced metastasis post therapy168. Given that many chemotherapeutic regimens are performed cyclically to minimize collateral damage to the patient, this accentuated build-up of senescent populations over time, which is already higher in aged patients, may lead to more aggressive tumours and resistant tumour niches being established throughout the body.
Chemotherapy has also been shown to further accelerate immunosenescence in aged patients. Increased immunosenescence of CD8+ T cells was observed in patients with breast169 and lung cancer170 receiving chemotherapy. A similar phenomena was seen with CD3+ effector T cells in patients with breast cancer171 and testicular cancer172 receiving chemotherapy. In all cases, CD3+ and CD8+ T cell populations did not increase again post therapy and patients had a worst outcome. Many of these studies suggest that, while chemotherapy may be initially beneficial, in many cases, it may later contribute to accelerated ageing of microenvironments and increased residual disease in patients. Interestingly, studies in therapy-naive and pretreated patients with incurable malignancies showed that treatment with recombinant IL-7 caused a marked increase in CD3+, CD4+ and CD8+ T cells and tumour regression173, suggesting that some therapeutic avenues might have potential to overcome the age-related acceleration associated with chemotherapeutic treatments.
Chemotherapy can also have off-target effects that include stem-cell decline and bone marrow exhaustion. Studies have shown that mesenchymal stem cells (MSCs) in 16-month-old aged mice were much more sensitive to doxorubicin treatment compared with mice aged 1 and 8 months174. Patients can have stem cell transplants whilst undergoing high-dose chemotherapy regimens to avoid this; however, this therapy may be too toxic for many elderly patients and there appear to be limitations associated with its use175. For example, a cohort of patients with cancers, including myeloma, lymphoma and leukaemia, that were receiving haematopoietic stem cell transplants during treatment had significantly increased p16INK4A expression in effector T cells. Further analysis of gene expression in effector T cells from these patients showed clear signs of immunosenescence and T cell ageing176. The study further acknowledges the possibility that off-target effects associated with transplantation and chemotherapy may also occur, including damage to the thymus, which may lead to accelerated thymic ageing.
Overall, there may be potential clinical benefit in targeting chemotherapeutic-induced acceleration of age-related tumorigenic events resulting in decreased cytotoxicity and increased survival. There are many cases where chemotherapy in elderly patients is well tolerated and prolongs survival irrespective of the location of the cancer177. Given that a large clinical factor in determining chemotherapeutic treatment regimens in the elderly is whether the benefits of treatment outweigh the side effects, further research is necessary to better understand the cytotoxic effects at the molecular level, along with organ function decline in older patients.
Targeted therapy response in aged patients
Targeted therapy represents the standard of personalized care that aims to target cancer cells or the TME for specific drivers of growth and progression individual to that tumour type. While there are many patients whose tumours initially respond to targeted therapy, a large subset become resistant178. There are numerous studies showing direct changes in intrinsic signalling pathways associated with resistance to targeted therapy179; however, the direct relationship between these changes and the microenvironment is not well defined, particularly with regards to age. Targeted therapy often has less off-target effects compared with chemotherapy and radiotherapy and, as such, cells appear to undergo more intrinsic changes based on genetic mutations, epigenetic alterations and genomic instability to induce a resistant TME. Our group has recently performed one of the first age-related mechanistic studies showing that healthy aged dermal fibroblasts significantly increase resistance to targeted BRAF therapy in allogeneic mouse models of melanoma via secretion of SFRP2 into the TME71. Furthermore, treatment of young mice with recombinant SFRP2 was able to increase resistance within this previously sensitive mouse model.
Studies in melanoma have found that tumour B cell infiltration results in the secretion of insulin-like growth factor 1 (IGF1), which can induce resistance to BRAF and MEK inhibitors180. While these studies were only performed in young mice (6–8 weeks old), recent studies have shown that immunosuppressive age-associated B cell (ABC) numbers are considerably increased in aged (> 24 months old) mice at the expense of other B cell subtypes181, which may contribute to the previously described resistance to targeted therapy in elderly individuals71. MDSCs, which, as described earlier, increase systemically with age151, also appear to induce resistance to anti-angiogenic therapies182,183 and other targeted therapies used in the treatment of multiple myeloma184, prostate cancer185, hepatocellular carcinoma186 and melanoma187; however, no direct link with age has been investigated in these models. It has been proposed that combination therapies with drugs that target resistance-promoting components of the tumour stroma and ECM may dramatically increase the durability of targeted therapy188–190. Given that many facets of these components are altered to induce protumorigenic effects with age, studies into aged TMEs and potential premetastatic niches and their contribution to targeted therapy resistance are clearly warranted.
Immunotherapy response in aged patients
Immunotherapies that modulate the immune microenvironment to target and eliminate tumour cells have revolutionized cancer treatment over the past decade; however, resistance to these treatments occurs in a large subset of patients191. This resistance highlights the complexity and adaptability of cancer cells responding to immune responses in different tissues. Given the dramatic changes in immune profiles and function as we age, treatments targeting the immune system within the elderly have considerable clinical implications. Despite this, there are still few direct studies assessing the efficacy of immunotherapies in aged cancer models.
The most common treatments that have proven clinical efficacy across a broad range of cancers are immune checkpoint inhibitors against programmed cell death 1 (PD1), PDL1 and cytotoxic T lymphocyte-associated antigen 4 (CTLA4). The PD1 and CTLA4 receptors, when activated, act as inhibitory signals on effector T cells with, for example, PDL1 activating PD1 to induce inhibitory T cell effects. Many cancer cells and other stromal components upregulate signalling through immune checkpoint pathways to evade antitumour immunity. Interestingly, PD1 surface expression has been suggested to contribute to the age-dependent functional decline in effector memory T cells192. PD1 expression increases on T cells with age193 and, consequently, anti-PD1 improves T cell function in aged mice194. The mTOR inhibitor rapamycin has been shown to reduce age-related increases in PD1 expression, suggesting a role for these inhibitors in increasing tumour immunity in aged tissues194. There is also a dramatic increase in PDL1 expression in CD8+ effector T cells in aged mice compared with young mice, and anti-PDLl improves their proliferation in vitro and their antitumour immunity in aged hosts when compared with young hosts in a mouse lymphoma model195. A recent study also showed that PDL1 and indoleamine 2,3-dioxygenase 1 (IDO1) are increased during ageing in the brain of healthy human adults, and that this correlates with increased circulating Treg cells and decreased CD8+ T cells196. These observations suggest that, for diseases like lymphoma, glioblastoma and leukaemia, older patients may be less responsive to immunotherapy. A recent study in aged mice (>12 months old) using 4T1 or Met1-derived transplantable orthotopic mouse models of triple negative breast cancer found little difference in tumour growth and progression when compared with younger mice (6–8 weeks old)197. However, neither of the aged mouse models responded efficiently to anti- PDL1 or anti-CTLA4 treatment, whereas both young mouse models showed significant (but not complete) regression on these treatments. Furthermore, within aged mouse TMEs, CD8+ T cells displayed markers of exhaustion and IFNγ levels were significantly decreased; similarly, in aged patients with triple negative breast cancer, IFNγ gene expression was found to be reduced. Stimulation of the TME with IFNγ in aged tumour-bearing mice significantly increased response to immune checkpoint blockade.
Intense review of published data sets has revealed that currently available immune checkpoint inhibitors are highly effective in older adults198,199; however, there are many conflicting reports, coupled with a lack of enrolled aged patients in trials, to properly determine whether toxicity is increased in the elderly200–204. A recent study from our group investigated whether age-mediated differences in intratumoural immune populations played a role in response to immunotherapy in patients with melanoma147. Patients over the age of 60 responded more efficiently to anti-PD1 and the likelihood of response to anti-PD1 increased with age; this could be recapitulated in young and aged melanoma mouse models. Additionally, analysis of tumour immune cell subtypes revealed that aged mice had significantly increased CD8+ T cell to Treg cell ratios, indicating more immunogenic tumours147. Furthermore, depletion of Treg cells using an anti-CD25 therapy significantly increased the anti-PD1 response in younger mice147.
Our study corroborates previous findings that Treg cell depletion in aged melanoma mouse models was ineffective in inducing antitumour immunity but significantly decreased tumour growth in young mice150. However, other studies in melanoma have shown contradictory results. For example, anti-PDL1 treatment of B16 melanomas only showed efficacy in young mice, but treatment efficacy could be partially restored in aged mice by combining anti-PDL1 with anti-CTLA4 (REF205). Further retrospective studies in patients with melanoma201 and non-small-cell lung cancer206 treated with either anti- PD1 or anti-PDL1 showed little difference in overall survival, progression free survival and toxicity between aged groups. Based on the massive diversity in immune cell profiles, infiltrates and activity across various tumour models and in differing tissue microenvironments, the immunotherapeutic response likely differs dramatically in young versus aged patients. Genetic and environmental diversity between patient populations also limits collective analyses across various racial and ethnic backgrounds. Unfortunately, few studies have directly investigated these response rates. In addition, given the discrepancy seen when targeting one immune checkpoint compared with another in aged models, tailoring specific immunotherapeutic treatments to age may be warranted. Importantly, many groups such as the Alliance for Clinical Trials are now focused on implementing strategies for the accrual of older patients with cancer into clinical trials207. These strategies, along with a focus on furthering the understanding of age-related changes at the molecular level in TMEs and premetastatic niches, may be crucial in efficiently predicting patient response across a wide variety of cancers. It may also reveal other avenues for effective co-targeting of tumour-promoting immune cell subtypes.
Conclusions and perspectives
Overall, recent data suggest that the ageing microenvironment may have dramatic effects on tumour progression. Normal age-related changes in stromal and immune populations may work together to drive the progression of tumour cells from an initial or slow- growing state to highly aggressive and metastatic disease. The outcome of these changes involve variations in secreted factors, changes in the biophysical architecture of the TME and even changes on a more macroscopic level such as a breakdown in vasculature integrity208.
The impact of the ageing microenvironment on tumour progression reveals the need for a better understanding of the role that age plays in response to therapy and in driving metastasis. Furthermore, these data suggest that we need a paradigm shift in the way we approach preclinical studies. For example, there is often a debate as to when preclinical mouse models reach a so-called ‘aged phenotype’. Many studies use an age of > 10 months, whereas others have provided evidence to suggest that mice do not reach a geriatric phenotype until 18 months of age209. Interestingly, studies have highlighted that mice appear to reach an immunological age starting at 10 months210 and a longitudinal study in humans was recently undertaken showing that immunological age is not equivalent to chronological age211. Collectively, this highlights the complexity of modelling ageing in vivo given that distinct tissues and the various biological systems age differently. It is also suggestive that certain biological systems, such as the immune system, may not necessarily need to be studied in 18-month-old geriatric mice to show an ageing phenotype; however, the potential complex interplay between the immune system and the aged ECM and stroma clearly warrants further investigation between younger (6–8 weeks old) mice versus 10-month-old (immunological age) and 18-month-old (geriatric age) models to properly contextualize chronological and molecular ageing with tumour progression.
Moreover, in vitro studies of cancer cell interactions with human primary stromal populations are often performed with stromal cells isolated from neonatal or non-age-controlled tissues. In vivo preclinical studies of drug development employ testing in mice aged 6–8 weeks, yet mice do not reach the human equivalent of 60 years old until 18–24 months of age. These emerging data highlight the need for age-specific models across all types of cancer. In conclusion, it is becoming clear that targeting age-related changes in cancer may provide novel ways in which to alleviate this growing public health burden.
Acknowledgements
This review is dedicated to the memory of Dr Aarti Hurria, a prominent geriatric oncologist at the City of Hope, Los Angeles, CA. Dr Hurria was a loving mentor to so many, a pioneer in the field of geriatric oncology, and a positive force in the field of ageing and cancer. This review was supported by research grants from the National Cancer Institute R01CA1746046 (ATW) and R01CA207935 (ATW, MEF).
Footnotes
Competing interests
The authors declare no competing interests.
Proteostasis
The homeostatic maintenance of protein folding for proper structure and function.
Cellular senescence
Refers to the potentially irreversible arrest of cell proliferation (growth) that occurs when cells experience certain stressors.
Thymic atrophy
The loss in cellularity of the thymus.
Strain-stiffening
The property of increasing stiffness with increasing deformation.
Osteoclastogenesis
The process by which stem cells or stromal cells commit towards an osteoclast lineage.
References
- 1.Siegel RL, Miller KD & Jemal A Cancer statistics. CA Cancer J. Clin. 68, 7–30 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Aunan JR, Cho WC & Soreide K The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. 8, 628–642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu T Educational initiatives in geriatric oncology — who, why, and how? J. Geriatr. Oncol. 7, 390–396 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Zinger A, Cho WC & Ben-Yehuda A Cancer and aging — the inflammatory connection. Aging Dis. 8, 611–627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campisi J Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozhok A & DeGregori J A generalized theory of age-dependent carcinogenesis. eLife 8, e39950 (2019).This paper shows that the multistage model of carcinogenesis (tumour initiation, promotion and progression) requires incorporation of ageing- dependent somatic selection to ensure this model is capable of generalizing cancer incidence across tissues and species.
- 7.Rozhok AI, Salstrom JL & DeGregori J Stochastic modeling indicates that aging and somatic evolution in the hematopoietic system are driven by non-cell- autonomous processes. Aging 6, 1033–1048(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R & Zeisberg M Fibroblasts in cancer. Nat. Rev. Cancer 6, 392 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Coppe JP, Desprez PY, Krtolica A & Campisi J The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruchti C, Haller D, Nuber M & Cottier H Regional differences in renewal rates of fibroblasts in young adult female mice. Cell Tissue Res. 232, 625–636 (1983). [DOI] [PubMed] [Google Scholar]
- 11.Faragher RG, McArdle A, Willows A & Ostler EL Senescence in the aging process. F1000Res. 6, 1219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHugh D & Gil J Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 217, 65–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calcinotto A et al. Cellular senescence: aging, cancer, and injury. Physiol. Rev. 99, 1047–1078 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Campisi J & Robert L Cell senescence: role in aging and age-related diseases. Interdiscip. Top Gerontol. 39, 45–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan S et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 8, 1316–1329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson DM, McBryan T, Jeyapalan JC, Sedivy JM & Adams PD A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age 36, 9637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salama R, Sadaie M, Hoare M & Narita M Cellular senescence and its effector programs. Genes Dev. 28, 99–114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton DGA & Stolzing A Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res. Rev. 43, 17–25 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Waaijer MEC et al. Are skin senescence and immunosenescence linked within individuals? Aging Cell 18, e12956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S & Sharpless NE Senescence in health and disease. Cell 169, 1000–1011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JY et al. Cells exhibiting strong p16INK4a promoter activation in vivo display features of senescence. Proc. Natl Acad. Sci. USA 116, 2603–2611 (2019).This study highlights p16INK4A as a key driver of senescence while showing that p16INK4A-expressing cells accumulate with age and exhibit clinically targetable features.
- 22.Pazolli E et al. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 72, 2251–2261 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Cecco M et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019).This paper shows that L1 retrotransposable elements become transcriptionally derepressed during ageing, which promotes type 1 IFN activation and age-associated inflammaging.
- 24.Lee S & Schmitt CA The dynamic nature of senescence in cancer. Nat. Cell Biol. 21, 94–101 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Kaur A, Webster MR & Weeraratna AT In the Wnt-er of life: Wnt signalling in melanoma and ageing. Br. J. Cancer 115, 1273–1279 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webster MR et al. Wnt5A promotes an adaptive, senescent-like stress response, while continuing to drive invasion in melanoma cells. Pigment Cell Melanoma Res. 28, 184–195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varani J et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 168, 1861–1868 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunin AG, Kornilova NK, Petrov VV & Vasil’eva OV Age-related changes in the number and proliferation of fibroblasts in the human skin [Russian]. Adv. Gerontol. 24, 43–47 (2011). [PubMed] [Google Scholar]
- 29.Kim E et al. Senescent fibroblasts in melanoma initiation and progression: an integrated theoretical, experimental, and clinical approach. Cancer Res. 73, 6874–6885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrenson K et al. Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia 12, 317–325 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krtolica A, Parrinello S, Lockett S, Desprez PY & Campisi J Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D & Hornsby PJ Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 67, 3117–3126 (2007). [DOI] [PubMed] [Google Scholar]
- 33.West MD, Pereira-Smith OM & Smith JR Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp. Cell Res. 184, 138–147 (1989). [DOI] [PubMed] [Google Scholar]
- 34.Millis AJ, Hoyle M, McCue HM & Martini H Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp. Cell Res. 201, 373–379 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Zeng G & Millis AJ Differential regulation of collagenase and stromelysin mRNA in late passage cultures of human fibroblasts. Exp. Cell Res. 222, 150–156 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Gialeli C, Theocharis AD & Karamanos NK Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 278, 16–27 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Lesina M et al. RelA regulates CXCL1/CXCR2- dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J. Clin. Invest. 126, 2919–2932 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coppe JP et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLOS ONE 5, 245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanada F et al. IGF binding protein-5 induces cell senescence. Front. Endocrinol. 9, 53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Severino V et al. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 4, e911 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppe JP et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLOS Biol. 6, 2853–2868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elzi DJ et al. Plasminogen activator inhibitor 1- insulin-like growth factor binding protein 3 cascade regulates stress-induced senescence. Proc. Natl Acad. Sci. USA 109, 12052–12057 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodier F & Campisi J Four faces of cellular senescence. J. Cell Biol. 192, 547–556 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moriya J & Minamino T Angiogenesis, cancer, and vascular aging. Front. Cardiovasc. Med. 4, 65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coppe JP, Kauser K, Campisi J & Beausejour CM Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 281,29568–29574 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Acosta JC et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Wajapeyee N, Serra RW, Zhu X, Mahalingam M & Green MR Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132, 363–374 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuilman T et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Kortlever RM, Higgins PJ & Bernards R Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8, 877–884 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rielland M et al. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. J. Clin. Invest. 124, 2125–2135 (2014).The study shows that the histone deacetylase- associated protein SIN3B drives oncogene-induced senescence and promotes PDAC progression by increasing inflammation.
- 51.Lau L, Porciuncula A, Yu A, Iwakura Y & David G Uncoupling the senescence-associated secretory phenotype from cell cycle exit via IL-1 inactivation unveils its pro-tumorigenic role. Mol. Cell Biol. 39, e00586–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elkhattouti A, Hassan M & Gomez CR Stromal fibroblast in age-related cancer: role in tumorigenesis and potential as novel therapeutic target. Front. Oncol. 5, 158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavlides S et al. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle 9, 3485–3505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Outschoorn UE et al. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int. J. Biochem. Cell Biol. 43, 1045–1051 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balliet RM et al. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle 10, 4065–4073 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Outschoorn UE et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle 9, 3515–3533 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ducy P, Zhang R, Geoffroy V, Ridall AL & Karsenty G Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Ducy P et al. Increased bone formation in osteocalcin- deficient mice. Nature 382, 448–452 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Ducy P, Schinke T & Karsenty G The osteoblast: a sophisticated fibroblast under central surveillance. Science 289, 1501–1504 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Luo X et al. Stromal-initiated changes in the bone promote metastatic niche development. Cell Rep. 14, 82–92 (2016).This paper demonstrates that senescent osteoblasts increase osteoclastogenesis via IL-6, which promotes a permissive microenvironment for the outgrowth of metastatic breast cancer.
- 61.Hameed A, Brady JJ, Dowling P, Clynes M & O’Gorman P Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis 7, 33–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faget DV, Ren Q & Stewart SA Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 19, 439–453 (2019).An in-depth review highlighting the context specificity of SASP induction in cancer progession.
- 63.Liedtke C et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res. Treat. 152, 667–673 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Burton DG & Faragher RG Cellular senescence: from growth arrest to immunogenic conversion. Age 37, 27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klapper W et al. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Lett. 439, 143–146 (1998). [DOI] [PubMed] [Google Scholar]
- 66.Klapper W, Heidorn K, Kuhne K, Parwaresch R & Krupp G Telomerase activity in ‘immortal’ fish. FEBS Lett. 434, 409–412 (1998). [DOI] [PubMed] [Google Scholar]
- 67.Lasry A & Ben-Neriah Y Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 36, 217–228 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Andrew W, Behnke RH & Sato T Changes with advancing age in the cell population of human dermis. Gerontologia 10, 1–19 (1964). [DOI] [PubMed] [Google Scholar]
- 69.Marsh E, Gonzalez DG, Lathrop EA, Boucher J & Greco V Positional stability and membrane occupancy define skin fibroblast homeostasis in vivo. Cell 175, 1620–1633.e13 (2018).This paper shows that aged skin results in a decrease in fibroblast number and proliferation; however, these fibroblasts extend their protrusions to ensure membrane occupancy of skin volume.
- 70.Salzer MC et al. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175, 1575–1590.e22 (2018).This study reveals that healthy aged fibroblasts in the skin undergo a genetic switch and decrease expression of genes involved in ECM production but gain adipogenic traits, which is highly influenced by systemic metabolism.
- 71.Kaur A et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532, 250–254 (2016).This study shows that healthy human aged fibroblasts undergo dramatic reprogramming of their secretome and secrete SFRP2 to drive increased metastasis and resistance to targeted therapy.
- 72.Kaur A et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. CancerDiscov. 9, 64–81 (2019).This study shows that healthy human aged fibroblasts lose expression of HAPLN1, which leads to remodelling of the ECM within the skin to drive invasion, metastasis and immune cell motility.
- 73.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).An in-depth review of the many hallmarks associated with cancer progression; it discusses stromal, ECM, immune and other components implicated in cancer progression with age.
- 74.Walker C, Mojares E & Del Rio Hernandez A Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 19, 3028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poltavets V, Kochetkova M, Pitson SM & Samuel MS The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front. Oncol. 8, 431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pickup MW, Mouw JK & Weaver VM The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang-Lee LL & Nimni ME Crosslinked CNBr-activated hyaluronan-collagen matrices: effects on fibroblast contraction. Matrix Biol. 14, 147–157 (1994). [DOI] [PubMed] [Google Scholar]
- 78.Karjalainen JM et al. Reduced level of CD44 and hyaluronan associated with unfavorable prognosis in clinical stage I cutaneous melanoma. Am. J. Pathol. 157, 957–965 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian X et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cox TR & Erler JT Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 4, 165–178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmadzadeh H et al. Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proc. Natl Acad. Sci. USA 114, E1617–E1626 (2017).This paper shows that ECM realignment and contractility fit a biphasic model for cancer cell invasion.
- 82.Levental KR et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, Abhilash AS, Chen CS, Wells RG & Shenoy VB Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys. J. 107, 2592–2603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diridollou S et al. Skin ageing: changes of physical properties of human skin in vivo. Int. J. Cosmet. Sci. 23, 353–362 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Panwar P et al. Changes in structural-mechanical properties and degradability of collagen during aging- associated modifications. J. Biol. Chem. 290, 23291–23306 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fisher GJ et al. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 138, 1462–1470 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Lee HO et al. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 11, 245 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oh JH et al. Intrinsic aging- and photoaging- dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 62, 192–201 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Marcos-Garces V et al. Age-related dermal collagen changes during development, maturation and ageing — a morphometric and comparative study. J. Anat. 225, 98–108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roark EF et al. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J. Histochem. Cytochem. 43, 401–411 (1995). [DOI] [PubMed] [Google Scholar]
- 91.Sephel GC & Davidson JM Elastin production in human skin fibroblast cultures and its decline with age. J. Invest. Dermatol. 86, 279–285 (1986). [DOI] [PubMed] [Google Scholar]
- 92.Martin H & Dean M An N-terminal peptide from link protein is rapidly degraded by chondrocytes, monocytes and B cells. Eur. J. Biochem. 212, 87–94 (1993). [DOI] [PubMed] [Google Scholar]
- 93.Naba A et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. 11, M111.014647 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Northey JJ, Przybyla L & Weaver VM Tissue force programs cell fate and tumor aggression. Cancer Discov. 7, 1224–1237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ecker BL et al. Age-related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov. 8, 82–95 (2019).This study demonstrates that HAPLN1 loss with age increases lymphatic permeability and allows efficient metastatic spread of melanoma cells.
- 96.Page AJ et al. Increasing age is associated with worse prognostic factors and increased distant recurrences despite fewer sentinel lymph node positives in melanoma. Int. J. Surg. Oncol. 2012, 456987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leong SP et al. Clinical patterns of metastasis. Cancer Metastasis Rev. 25, 221–232 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Mavrogonatou E, Pratsinis H, Papadopoulou A, Karamanos NK & Kletsas D Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol. 75–76, 27–42 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Calhoun C et al. Senescent cells contribute to the physiological remodeling of aged lungs. J. Gerontol. A Biol. Sci. Med. Sci. 71, 153–160 (2016).This paper shows that aged accumulation of senescent stroma induces dramatic changes in ECM structure and function within the lung.
- 100.Burgstaller G et al. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur. Respir. J. 50, 1601805 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Bissell MJ & Hines WC Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17, 320–329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bissell MJ, Radisky DC, Rizki A, Weaver VM & Petersen OW The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation 70, 537–546 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martens JW et al. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb. Haemost. 89, 393–404 (2003). [PubMed] [Google Scholar]
- 104.Jiang D & Lim SY Influence of immune myeloid cells on the extracellular matrix during cancer metastasis. Cancer Microenviron. 9, 45–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Acerbi I et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 7, 1120–1134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lopez-Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leonardi GC, Accardi G, Monastero R, Nicoletti F & Libra M Ageing: from inflammation to cancer. Immun. Ageing 15, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olivieri F, Prattichizzo F, Grillari J & Balistreri CR Cellular senescence and inflammaging in age-related diseases. Mediators Inflamm. 2018, 9076485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greene MA & Loeser RF Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 23, 1966–1971 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Veglia F, Perego M & Gabrilovich D Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyer C et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc. Natl Acad. Sci. USA 108, 17111–17116 (2011).This study shows that inflammaging induces MDSC infiltration and activation and drives tumour progression in the Ret transgenic melanoma model.
- 112.Ameri AH et al. IL-33/regulatory T cell axis triggers the development of a tumor-promoting immune environment in chronic inflammation. Proc. Natl Acad. Sci. USA 116, 2646–2651 (2019).This paper demonstrates that chronic inflammatory factor IL-33 drives an increased Treg cell response and progression of melanoma and colorectal cancer.
- 113.Shevach EM Foxp3+ T regulatory cells: still many unanswered questions-a perspective after 20 years of study. Front. Immunol. 9, 1048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garbern JC et al. Dysregulation of IL-33/ST2 signaling and myocardial periarteriolar fibrosis. J. Mol. Cell Cardiol. 128, 179–186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim DW et al. Age-related increase of IL-33 in non-eosinophilic nasal polyps. J Allergy Clin. Immunol. 141, AB67 (2018). [Google Scholar]
- 116.Knupfer H & Preiss R Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res. Treat 102, 129–135 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Maggio M, Guralnik JM, Longo DL & Ferrucci L Interleukin-6 in aging and chronic disease: a magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci. 61, 575–584 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Olivieri F et al. MicroRNAs linking inflamm-aging, cellular senescence and cancer. Ageing Res. Rev. 12, 1056–1068 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Olivieri F et al. Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech. Ageing Dev. 133, 675–685 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Merline R et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci. Signal. 4, ra75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fabbri M et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl Acad. Sci. USA 109, E2110–E2116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palmer DB The effect of age on thymic function. Front. Immunol. 4, 316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haynes L, Eaton SM, Burns EM, Randall TD & Swain SL CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc. Natl Acad. Sci. USA 100, 15053–15058 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saule P et al. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4+ versus effector memory and terminally differentiated memory cells in CD8+ compartment. Mech. Ageing Dev. 127, 274–281 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Yager EJ et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205, 711–723 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fulop T et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8, 1960 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gayoso I et al. Immunosenescence of human natural killer cells. J. Innate Immun. 3, 337–343 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Colonna-Romano G et al. Impairment of gamma/delta T lymphocytes in elderly: implications for immunosenescence. Exp. Gerontol. 39, 1439–1446 (2004). [DOI] [PubMed] [Google Scholar]
- 129.Linton PJ & Thoman ML Immunosenescence in monocytes, macrophages, and dendritic cells: lessons learned from the lung and heart. Immunol. Lett. 162, 290–297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Linehan E & Fitzgerald DC Ageing and the immune system: focus on macrophages. Eur. J. Microbiol. Immunol. 5, 14–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Golomb L et al. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ. 22, 1764–1774 (2015).This study shows that age induces immunosenescence of effector immune cells and drives a T helper 2-type anti-inflammatory immune response, promoting SCC progression.
- 132.Hurez V, Padron A, Svatek RS & Curiel TJ Considerations for successful cancer immunotherapy in aged hosts. Exp. Gerontol. 107, 27–36 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Jackaman C et al. Targeting macrophages rescues age-related immune deficiencies in C57BL76J geriatric mice. Aging Cell 12, 345–357 (2013).This paper shows that aged mice have substantially more M2 macrophages and targeting these with an IL-2 agonist–CD40 antibody combination immunotherapy promotes a switch towards an antitumour macrophage phenotype.
- 134.Jackaman C & Nelson DJ Intratumoral interleukin-2/agonist CD40 antibody drives CD4+-independent resolution of treated-tumors and CD4+-dependent systemic and memory responses. Cancer Immunol. Immunother. 61, 549–560 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aras S & Zaidi MR TAMeless traitors: macrophages in cancer progression and metastasis. Br. J. Cancer 117, 1583–1591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang DZ, Sun HY & DuBois RN CXCL1 is critical for pre-metastatic niche formation and metastasis in colorectal cancer. Cancer Res. 77, 3655–3665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gomez CR, Nomellini V, Faunce DE & Kovacs EJ Innate immunity and aging. Exp. Gerontol. 43, 718–728 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schroder AK & Rink L Neutrophil immunity of the elderly. Mech. Ageing Dev. 124, 419–425 (2003). [DOI] [PubMed] [Google Scholar]
- 139.Grecian R, Whyte MKB & Walmsley SR The role of neutrophils in cancer. Br. Med. Bull 128, 5–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fridlender ZG & Albelda SM Tumor-associated neutrophils: friend or foe? Carcinogenesis 33, 949–955 (2012). [DOI] [PubMed] [Google Scholar]
- 141.Zhao L et al. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged BALB/c mice. J. Leukoc. Biol. 81, 1386–1394 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Kryczek I et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 69, 3995–4000 (2009). [DOI] [PubMed] [Google Scholar]
- 143.Rosenkranz D et al. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J. Neuroimmunol. 188, 117–127 (2007). [DOI] [PubMed] [Google Scholar]
- 144.Sharma S, Dominguez AL & Lustgarten J High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J. Immunol. 177, 8348–8355 (2006).This study shows that aged animals have increased numbers of Treg cells in the spleen and lymph nodes, which increase progression of pre-B-ALL tumours.
- 145.Thomas DC, Mellanby RJ, Phillips JM & Cooke A An early age-related increase in the frequency of CD4+ Foxp3+ cells in BDC2.5NOD mice. Immunology 121,565–576 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kozlowska E, Biernacka M, Ciechomska M & Drela N Age-related changes in the occurrence and characteristics of thymic CD4+ CD25+ T cells in mice. Immunology 122, 445–453 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kugel CH 3rd et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin. Cancer Res. 24, 5347–5356 (2018).This study demonstrates that aged patients and preclinical animal models of melanoma respond better to anti-PD1 compared with younger counterparts owing to a decrease in Treg to CD8+ T cell ratio.
- 148.Aguado BA, Bushnell GG, Rao SS, Jeruss JS & Shea LD Engineering the pre-metastatic niche. Nat. BiomedEng. 1,0077 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Verschoor CP et al. Blood CD33+HLA-DR− myeloid- derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 93, 633–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hurez V et al. Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res. 72, 2089–2099 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Enioutina EY, Bareyan D & Daynes RA A role for immature myeloid cells in immune senescence. J. Immunol. 186, 697–707 (2011). [DOI] [PubMed] [Google Scholar]
- 152.Grizzle WE et al. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech. Ageing Dev. 128, 672–680 (2007).This paper shows that increases in systemic MDSCs in aged mice increase progression of mammary adenocarcinoma tumours.
- 153.Wang Y, Ding Y, Guo N & Wang S MDSCs: key criminals of tumor pre-metastatic niche formation. Front. Immunol. 10, 172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruhland MK et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 7, 11762 (2016).This paper shows that induced stromal senescence in the FASST mouse model significantly increases numbers of immunosuppressive MDSCs and Treg cells in healthy mice and enables a permissive niche for tumour growth.
- 155.Dimri GP et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Begley LA, Kasina S, MacDonald J & Macoska JA The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 43, 194–199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Strasner A & Karin M Immune infiltration and prostate cancer. Front. Oncol. 5, 128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Daste A et al. Targeted therapy and elderly people: a review. Eur. J. Cancer 69, 199–215 (2016). [DOI] [PubMed] [Google Scholar]
- 159.Singh H et al. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: a 10-year experience by the US Food and Drug Administration. J. Clin. Oncol. 35 (Suppl. 15), 10009 (2017). [Google Scholar]
- 160.Bavik C et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 66, 794–802 (2006). [DOI] [PubMed] [Google Scholar]
- 161.Schosserer M, Grillari J & Breitenbach M The dual role of cellular senescence in developing tumors and their response to cancer therapy. Front. Oncol. 7, 278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Canino C et al. SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene 31,3148–3163 (2012). [DOI] [PubMed] [Google Scholar]
- 163.Hazlehurst LA & Dalton WS Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 20, 43–50 (2001). [DOI] [PubMed] [Google Scholar]
- 164.Meads MB, Gatenby RA & Dalton WS Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat. Rev. Cancer 9, 665–674 (2009). [DOI] [PubMed] [Google Scholar]
- 165.Sethi T et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 5, 662–668 (1999). [DOI] [PubMed] [Google Scholar]
- 166.Sherman-Baust CA et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell 3, 377–386 (2003). [DOI] [PubMed] [Google Scholar]
- 167.Sidi R et al. Induction of senescence markers after neo-adjuvant chemotherapy of malignant pleural mesothelioma and association with clinical outcome: an exploratory analysis. Eur. J. Cancer 47, 326–332 (2011). [DOI] [PubMed] [Google Scholar]
- 168.Demaria M et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. CancerDiscov. 7, 165–176 (2017).This study shows that targeting senescent fibroblasts using the p16–3MR mouse model decreases the short-term and long-term side effects associated with chemotherapeutic cytotoxicity, decreases cancer recurrence and reduces metastasis post therapy.
- 169.Onyema OO et al. Chemotherapy-induced changes and immunosenescence of CD8+ T-cells in patients with breast cancer. Anticancer Res. 35, 1481–1489 (2015). [PubMed] [Google Scholar]
- 170.Onyema OO et al. Shifts in subsets of CD8+ T-cells as evidence of immunosenescence in patients with cancers affecting the lungs: an observational case-control study. BMC Cancer 15, 1016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sanoff HK et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl Cancer Inst. 106, dju057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bourlon MT et al. Immunosenescence in testicular cancer survivors treated with chemotherapy. J. Clin. Oncol. 35 (Suppl. 15), e16037 (2017). [Google Scholar]
- 173.Morre M & Beq S Interleukin-7 and immune reconstitution in cancer patients: a new paradigm for dramatically increasing overall survival. Target Oncol. 7, 55–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bashiri Dezfouli A et al. Evaluation of age effects on doxorubicin-induced toxicity in mesenchymal stem cells. Med. J. Islam. Repub. Iran 31, 98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang CL, Huang T, Wu BL, He WX & Liu D Stem cells in cancer therapy: opportunities and challenges. Oncotarget 8, 75756–75766 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wood WA et al. Chemotherapy and stem cell transplantation increase p16INK4a expression, a biomarker of T-cell aging. EBioMedicine 11, 227–238 (2016).This study shows that chemotherapy and stem cell transplantation accelerate immunosenescence of T cells.
- 177.Grigorescu AC Chemotherapy for elderly patients with advanced cancer: a pilot study in institute of oncology bucharest. J. Transl. Intern. Med. 3, 24–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sabnis AJ & Bivona TG Principles of resistance to targeted cancer therapy: lessons from basic and translational cancer biology. Trends Mol. Med. 25, 185–197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Holohan C, Van Schaeybroeck S, Longley DB & Johnston PG Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013). [DOI] [PubMed] [Google Scholar]
- 180.Somasundaram R et al. Tumor-associated B-cells induce tumor heterogeneity and therapy resistance. Nat. Commun. 8, 607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Riley RL, Khomtchouk K & Blomberg BB Age-associated B cells (ABC) inhibit B lymphopoiesis and alter antibody repertoires in old age. Cell Immunol. 321,61–67 (2017). [DOI] [PubMed] [Google Scholar]
- 182.Shojaei F et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 25, 911–920 (2007). [DOI] [PubMed] [Google Scholar]
- 183.Shojaei F et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc. Natl Acad. Sci. USA 106, 6742–6747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Romano A et al. PMN-MDSC and arginase are increased in myeloma and may contribute to resistance to therapy. Expert Rev. Mol. Diagn. 18, 675–683 (2018). [DOI] [PubMed] [Google Scholar]
- 185.Calcinotto A et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559, 363–369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen Y et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology 59, 1435–1447 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Steinberg SM et al. Myeloid cells that impair immunotherapy are restored in melanomas with acquired resistance to BRAF inhibitors. Cancer Res. 77, 1599–1610 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Roma-Rodrigues C, Mendes R, Baptista PV & Fernandes AR Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 20, 840 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Almeida FV, Douglass SM, Fane ME & Weeraratna AT Bad company: microenvironmentally mediated resistance to targeted therapy in melanoma. Pigment Cell Melanoma Res. 32, 237–247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fang H & Declerck YA Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 73, 4965–4977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sharma P, Hu-Lieskovan S, Wargo JA & Ribas A Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y & Inomata M Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp. Gerontol. 44, 517–522 (2009). [DOI] [PubMed] [Google Scholar]
- 193.Lages CS, Lewkowich I, Sproles A, Wills-Karp M & Chougnet C Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging Cell 9, 785–798 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hurez V et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune- deficient mice. Aging Cell 14, 945–956 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mirza N et al. B7-H1 expression on old CD8+ T cells negatively regulates the activation of immune responses in aged animals. J. Immunol. 184, 5466–5474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ladomersky E et al. The coincidence between increasing age, immunosuppression, and the incidence of patients with glioblastoma. Front. Pharmacol. 10, 200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sceneay J et al. Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. CancerDiscov. 9, 1208–1227 (2019).This paper demonstrates that aged 4T1 or Metl-derived transplantable orthotopic mouse models do not respond to immunotherapy owing to increased CD8+ T cell exhaustion and decreased IFNγ production.
- 198.Elias R, Hartshorn K, Rahma O, Lin N & Snyder-Cappione JE Aging, immune senescence, and immunotherapy: a comprehensive review. Semin. Oncol. 45, 187–200 (2018). [DOI] [PubMed] [Google Scholar]
- 199.Elias R, Karantanos T, Sira E & Hartshorn KL Immunotherapy comes of age: immune aging & checkpoint inhibitors. J. Geriatr. Oncol. 8, 229–235 (2017). [DOI] [PubMed] [Google Scholar]
- 200.Singh H et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers. J. Clin. Oncol. 34 (Suppl. 15), 10010 (2016). [Google Scholar]
- 201.Betof AS et al. Impact of age on outcomes with immunotherapy for patients with melanoma. Oncologist 22, 963–971 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gettinger SN et al. Nivolumab (NIVO) safety profile: summary of findings from trials in patients (pts) with advanced squamous (SQ) non-small cell lung cancer (NSCLC). Eur. J. Cancer 51, S631 (2015). [Google Scholar]
- 203.Hodi FS et al. Clinical response, progression-free survival (PFS), and safety in patients (pts) with advanced melanoma (MEL) receiving nivolumab (NIVO) combined with ipilimumab (IPI) vs IPI monotherapy in CheckMate 069 study. J. Clin. Oncol. 33 (Suppl. 15), 9004 (2015). [Google Scholar]
- 204.Friedman CF et al. Efficacy and safety of checkpoint blockade for treatment of advanced melanoma (mel) in patients (pts) age 80 and older (80+). J. Clin. Oncol. 34 (Suppl. 15), 10009 (2016). [Google Scholar]
- 205.Figueiredo ASP, Hurez V, Liu A & Curiel TJ Age and sex affect αCTLA-4 efficacy alone and combined with αB7-H1 or regulatory T cell depletion in a melanoma model. J. Immunol. 196, 213.4 (2016). [Google Scholar]
- 206.Lichtenstein MRL et al. Impact of age on outcomes with immunotherapy in patients with non-small cell lung cancer. J. Thorac. Oncol. 14, 547–552 (2019). [DOI] [PubMed] [Google Scholar]
- 207.Freedman RA et al. Promoting accrual of older patients with cancer to clinical trials: an alliance for clinical trials in oncology member survey (A171602). Oncologist 23, 1016–1023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu X et al. Age-related impairment of vascular structure and functions. Aging Dis. 8, 590–610 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dutta S & Sengupta P Men and mice: relating their ages. Life Sci. 152, 244–248 (2016). [DOI] [PubMed] [Google Scholar]
- 210.Hale JS, Boursalian TE, Turk GL & Fink PJ Thymic output in aged mice. Proc. Natl Acad. Sci. USA 103, 8447–8452 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Alpert A et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25, 487–495 (2019).This longitudinal study performed in humans shows that immunological age is not equivalent to chronological age and is better modelled using an immune ageing ‘IMM-AGE’ score.
- 212.Partridge AH et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J. Clin. Oncol. 34, 3308–3314 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Berian JR, Benson AB 3rd & Nelson H Young age and aggressive treatment in colon cancer. JAMA 314, 613–614 (2015). [DOI] [PubMed] [Google Scholar]
- 214.Bauer J et al. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res. 24, 345–351 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tas F & Erturk K Patient age and cutaneous malignant melanoma: elderly patients are likely to have more aggressive histological features and poorer survival. Mol. Clin. Oncol. 7, 1083–1088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Natale CA et al. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. eLife 7, e31770 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu PH et al. Association of obesity with risk of early- onset colorectal cancer among women. JAMA Oncol. 5, 37–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Baker DJ et al. Clearance of p16lnk4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).The INK-ATTAC GEMM used in this study highlights the occurrence of p16INK4A-induced senescence in multiple pathologies while also showing that clearance of p16INK4A-expressing senescent cells may be effective in delaying these age-associated disorders.
- 219.Storer M et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).This study underlines the homeostatic importance of senescence induction in embryonic growth and development.
- 220.Munoz-Espin D et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).This paper demonstrates the importance of programmed senescence in embryonic development.
- 221.Demaria M et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31,722–733 (2014).This paper shows the homeostatic importance of senescent cell induction for wound healing via secretion of the SASP factor PDGF-AA.
- 222.Jeon OH et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mosteiro L, Pantoja C, de Martino A & Serrano M Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell 17, e12711 (2018).This study highlights p16INK4a and IL-6 as key drivers of senescence-induced in vivo reprogramming and the implications thereof for tissue damage and pathology.
- 224.Mosteiro L et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, aaf4445 (2016). [DOI] [PubMed] [Google Scholar]