Abstract
Increasingly being recognized is the role of the complex microenvironment to regulate cell phenotype; however, the cell culture systems used to study these effects in vitro are lagging. The complex microenvironment is host to a combination of biological interactions, chemical factors, and mechanical stimuli. Many devices have been designed to probe the effects of one mechanical stimulus, but few are capable of systematically interrogating all combinations of mechanical stimuli with independent control. To address this gap, we have developed the MechanoBioTester platform, a decoupled, multi-stimulus cell culture model for studying the cellular response to complex microenvironments in vitro. The system uses an engineered elastomeric chamber with a specially defined region for incorporating different target materials to act as the cell culture substrate. We have tested the system with several target materials including: polydimethylsiloxane elastomer, polyacrylamide gel, poly(1,8-octanediol citrate) elastomer, and type I collagen gel for both 2D and 3D co-culture. Additionally, when the chamber is connected to a flow circuit and our stretching device, stimuli in the form of fluid flow, cyclic stretch, and hydrostatic pressure are able to be imparted with independent control. We validated the device using experimental and computational methods to define a range of capabilities relevant to physiological microenvironments. The MechanoBioTester platform promises to function as a model system for mechanobiology, biomaterial design, and drug discovery applications that focus on probing the impact of a complex microenvironment in an in vitro setting. The protocol described within provides the details characterizing the MechanoBioTester system, the steps for fabricating the MechanoBioTester chamber, and the procedure for operating the MechanoBioTester system to stimulate cells.
Keywords: mechanical stimulation, 3D mechanobiology, complex microenvironment, cell culture model, multi stimulus
1. INTRODUCTION
The human body experiences numerous interacting mechanical stimuli from the mechanical loading of the musculoskeletal system to the rhythmic loading of the cardiovascular system. In turn, tissue level stimuli are transferred to the cellular level such that the cells forming these tissue detect and respond to these mechanical stimuli by mechanotransduction pathways.1 More specifically, cells exist in a 3D viscoelastic extracellular matrix through which they can be stimulated by external fluid flow, stretching, pressure, chemical factors, neighboring cells, and the properties of the matrix they inhabit.2–4 The complex microenvironment is the combination of these biological interactions, chemical factors, and mechanical stimuli. In recent years, the relative importance of mechanical stimulation to regulate cell phenotype has been revised to be on par with that of chemical factors.
To reach this point, many in vitro cell culture models have been developed to understand the mechanobiological response of cells to each individual mechanical stimulus. These devices have been designed to expose adherent cells to either fluid flow, cyclic stretch, or applied pressure.5–7 And by using these systems, deeper understandings of cellular regulation, differentiation, and pathology have been gained.2,5,8 Another factor increasingly being recognized has been the effect of material properties in regulating cellular function.2,9–13 However, these historic devices for fluid flow, cyclic stretch, and applied pressure have relied on supraphysiologically stiff glass, silicone, or tissue culture polystyrene as the cell culture substrate. Moreover, these methods do not provide a means for stimulation by multiple factors, which would better emulate the cellular microenvironment in vitro. Only recently, has there been an effort to better reproduce the in vivo microenvironment by developing systems to apply pairwise combinations of mechanical stimuli to cultured cells.2,7,14,15 We have previously reviewed these devices and their results as they related to the vascular microenvironment to draw attention to the synergistic and antagonistic effects in cellular response when cells are stimulated by pairs of mechanical stimuli.2,14 Despite their significant interaction, few have further investigated combinations of more than two mechanical stimuli.15–19 One explanation for this is that these systems are traditionally complicated, confounded, and material-specific. Additionally, many of these devices are built in-house and are lab-specific. Because no standard device is used for mechanical stimulation studies, it is difficult to compare results as each device and stimulation mechanism has its own benefits and limitations. A cell culture model capable of applying all combinations of mechanical stimulation with independent control of each stimuli would reduce this experimental variability.
Furthermore, cells interact with other cell types in vivo and the need to capture this effect is growing. Using an organ-on-a-chip model has revealed unique interactions between endothelial and lung epithelial cells exposed to cyclic strain.20 To date, endothelial cell and vascular smooth muscle cell co-culture has been studied under static conditions or under limited mechanical stimulation (principally fluid shear stress) in 2D or 3D settings; however, these cell types are additionally exposed to cyclic stretch and hydrostatic pressure in the body.21 A system capable of stimulating cells in co-culture while imitating the tissue microenvironment would enable a greater understanding of biological and mechanical interactions in regulating cellular phenotype.
Thus, to further probe the complex microenvironment in vitro, it is necessary to have a system for independently controlling mechanical stimulation. Herein, we describe the characterization and fabrication of the MechanoBioTester, a custom-built system engineered to decouple the effects of fluid flow, cyclic stretch, hydrostatic pressure, and material properties for their independent control and application in combination. This system relies on a chamber specific for this purpose and a complementary series of equipment to support its functionality. We validated the device using experimental and computational methods to define a range of tested capabilities relevant to physiological microenvironments. The methods for fabricating the chamber, seeding the chamber with cells, and using the chamber to stimulate cells are described. We propose this platform has the ability to function as a cell culture model for mechanobiology, biomaterial design, and drug discovery applications that focus on probing the impact of complex microenvironments in an in vitro setting.
OVERVIEW: THE MECHANOBIOTESTER IS A DECOUPLED, MULTI-STIMULUS CELL CULTURE MODEL
This newly developed cell culture model is capable of independently varying flow regime, fluid shear stress, unidirectional cyclic stretch, hydrostatic pressure, and substrate properties for the systematic testing of all combinations of these stimuli in vitro (Table 1). Independent variation of stimuli means; for example, that the flow rate can be adjusted without changing the other stimuli conditions or the stretching conditions can be changed without effecting the flow rate and is not dependent on the material system used. Moreover, the system features the ability to test these conditions in both 3D and co-culture settings. We have tested the capabilities presented herein; however, the system is not limited to these values.
Table 1.
Capabilities of the MechanoBioTester Cell Culture Model
| Stimulus | Capability | In vivo Property |
|---|---|---|
| Substrate Material§ | Polydimethylsiloxane elastomer (PDMS) Polyacrylamide gel (PA) Poly(1,8-octanediol citrate) elastomer (POC) Type I collagen gel (Col) |
Tissue-specific ECM |
| Substrate Modulus | 5 – 1500 kPa (PDMS)22 0.1 – 100 kPa (PA)23,24 1850 – 6440 kPA (POC)25,26 0.1 – 20 kPa (Col)27,28 |
Brain: ∼ 1 kPa9,29 Muscle: ∼ 10 kPa9,29 Collagenous bone: ∼ 100 kPa9,29 Blood vessel: 8 – 100 kPa30 |
| Substrate Topography | Isotropic or Anisotropic | Tissue-specific ECM architecture31,32 |
| Flow Regime | Laminar or Disturbed Unidirectional or Reciprocating |
Straight blood vessel: Laminar/Unidirectional33 Curved/bifurcated blood vessel: Disturbed/Reciprocating33 |
| Fluid Shear Stress | 0 – 2* Pa | Blood vessel average: 1.5 Pa33 |
| Cyclic Stretch | 0 – 15% strain 0 – 1† Hz |
Blood vessel: 2 – 10%, 1 Hz2,8 Lung alveoli: 5 – 15%, 0.2 Hz34,35 Articular cartilage: 5 – 10%, 0.1 – 1 Hz36 |
| Hydrostatic Pressure | 0 – 4⊥ kPa 0 – 1‡ Hz |
Blood pressure: 0 – 16.5 kPa2 Lung pressure: 0.2 – 2.5 kPa35 |
Included are only the materials we have tested; it is expected that more materials are also possible.
Upper limit dependent on peristaltic pump and fluid properties.
Upper limit dependent on greatest height of any flow circuit component.
Upper limit dependent on stepper motor and peristaltic pump properties.
Upper limit dependent on stepper motor properties.
The device centers around a novel, deformable, polydimethylsiloxane elastomer (PDMS) chamber, which is approximately 100 mm x 100 mm x 5 mm (Figure 1). The chamber is fabricated in PDMS because of the material’s biocompatibility, elasticity, low-cost, optical transparency, and formability. Through the center of the chamber is a 10 mm wide, 2 mm high, and 100 mm long rectangular flow channel. The length of the channel was optimized to ensure steady, laminar flow developed without effects from the inlet and outlet. The chamber geometry was engineered to include a pair of struts perpendicular to the flow channel direction. These are for clamping and displacing to elicit a predictable strain field at the center of the flow channel. Additionally, the struts include interlocks to ensure alignment of chamber halves during fabrication to reduce chamber-to-chamber variability. Located at this central position is a 10 mm wide, 1 mm deep, and 20 mm long inset in both the top and bottom walls of the flow channel, termed the cell culture region (CCR) (Figure 1). This inset provides a region for introducing materials of interest different from that of the PDMS chamber. The CCR is fillable with any material capable of physically or chemically bonding to PDMS. By separating construction material of the chamber from that of the cell culture material, the system gained material-independence meaning this device is not exclusive to a single material. We successfully filled and bonded a suite of diverse biomaterials to the CCR: polydimethylsiloxane elastomer, polyacrylamide gel, poly(1,8-octanediol citrate), and type I collagen gel. Collectively, these materials cover a broad range of applications and mechanical properties relevant to physiological and pathological conditions. Moreover, any material with a stiffness less than that of the chamber (~1.5 MPa) and capable of sufficient bonding to PDMS is able to be used for stretching. A complete chamber is formed by sealing two chamber halves together and affixing our custom, biocompatible, 3D printed luer lock fittings (Figure S1). We tested two different silicone formulations for sealing the chamber halves together: Sylgard 184 and ReproRubber® Thin Pour. Each had their specific advantages and disadvantages. Sylgard 184 is useful because it is optically transparent; however, it takes approximately 24 – 48 hours to fully cure at 37 °C. Whereas, ReproRubber® Thin Pour cures in ~10 minutes at room-temperature drastically reducing fabrication time; however, it cures green in color, which can introduce minor background fluorescence near the walls of the chamber during fluorescence microscopy.
Figure 1.
The bioreactor chamber. A rectangular flow channel (dotted line) with a central region termed the “cell culture region” (CCR) (shaded blue) runs through the chamber. Perpendicular to the flow channel are struts used for clamping and stretching the inner channel. The struts of each chamber half have inverse mechanical interlocks to facilitate alignment of the two halves during chamber fabrication. Arrows at the inlet and outlet fittings show the direction of fluid flow. Arrows at the struts show the direction of chamber displacement to create the region of well-defined strain.
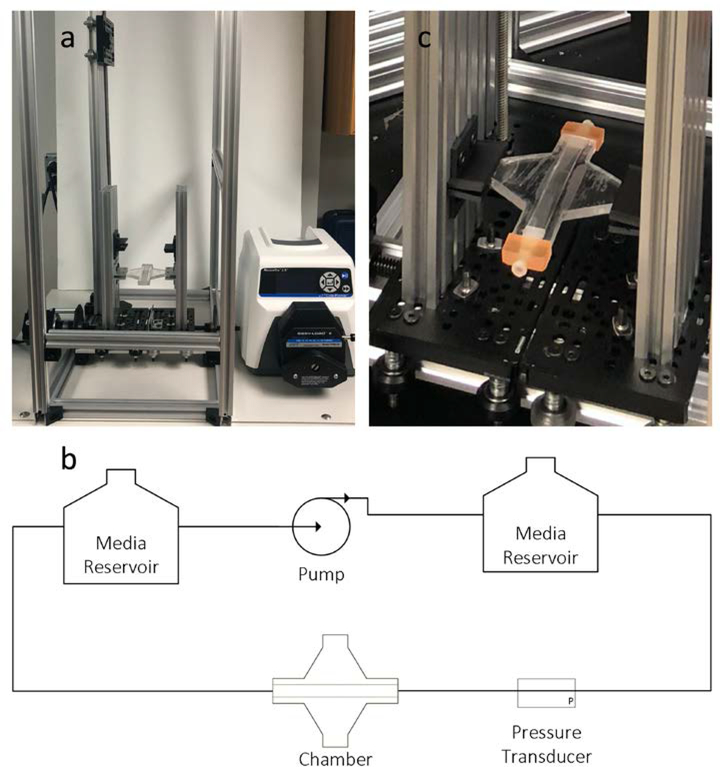
After seeding cells and sealing the chamber, it is then transferred to the system frame, which houses the flow circuit and chamber stretcher (Figure 2a). The flow circuit consists of a peristaltic pump, two media reservoirs, and an in-line pressure transducer (Figure 2b). One media reservoir is placed in a 37 °C water bath to maintain cell culture media temperature. The cell culture media is supplemented with HEPES to maintain cell culture media pH. The second media reservoir is attached to a meter long vertically oriented linear actuator to control the hydrostatic pressure in the chamber. The chamber stretcher is a bidirectional lead screw actuator with clamps for securing to the chamber struts (Figure 2c). The chamber stretcher works such that, as the lead screw rotates, the clamps mechanically move together or apart at the same rate to stretch the chamber. Thus, the deformation of the chamber is symmetric about the chamber centerline, which facilitates live-cell imaging because the image does not move out of the field of view.
Figure 2.
The MechanoBioTester system. The system setup (a) is a simple flow circuit consisting of media reservoirs, peristaltic pump, in-line pressure transducer, and the bioreactor chamber (b). The peristaltic pump is used to generate flow. The media reservoir connected to the outflow of the pump serves a dual purpose 1) it is to minimize the intrinsic pulsatility in the flow rate from the pump and 2) to keep proper temperature and pH of the flowing cell culture media. While the other media reservoir is used for adjusting the hydrostatic pressure in the chamber by changing its relative height with respect to the chamber. The in-line pressure transducer is used to monitor the pressure in the chamber. The chamber stretcher is a bidirectional lead screw linear actuator, which clamps onto the struts of the chamber (c).
A DinoLite USB microscope with 480/510 nm and 570/610 nm excitation/emission capabilities can be incorporated to image the CCR in real-time using fluorescent protein expressing or fluorescently-labeled cells (Figure S2). The peristaltic pump, linear actuators, and sensors are all connected to our custom, open-source control box (Figure 3a). A series of three Arduino microcontrollers are used to coordinate the different electronic components and record sensor data (Figure 3b). The code for the control box is available on GitHub. The MechanoBioTester system was designed to be a single device for mechanically stimulating cells in culture by combinations of material properties, fluid flow, cyclic stretch, and hydrostatic pressure.
Figure 3.
The MechanoBioTester control box. The control box is an external peripheral constructed using open-source electronics (a). The system uses a series of 3 Arduino microcontrollers to coordinate the peristaltic pump, linear actuators, and sensors (b).
Flow Characterization: The Flow Channel Mimics a Parallel-Plate Flow Chamber
To determine the flow characteristics of the chamber, we performed computational fluid dynamics (CFD) simulations (Supplementary Methods 1). The flow in the chamber assumed a steady, well-developed laminar flow field over the CCR (Figure S4a). The fluid shear stress or wall shear stress (WSS) over the CCR was shown to vary linearly with the fluid flow rate. As expected, these results matched the theoretical solution of the Navier-Stokes equation for a parallel-plate flow chamber because the chamber geometry is a simple, rectangular channel (Figure S4b). Using colored dye, we confirmed laminar flow through the chamber by observing continuous, non-mixing streamlines of dye (Video S1). Additionally, by partially filling the CCR to form a step feature, recirculating flow was shown to develop (Figure S5). The simple flow channel geometry enabled the development of controllable flow characteristics.
Strain Characterization: The Bonding of PDMS, PA, POC, and Collagen to the CCR Was Sufficient to Transfer Strain to These Materials
To confirm the strain field in the CCR for the different bonded materials, we employed digital image correlation (DIC) particle tracking methods (Supplementary Methods 2).37 From these measurements we calculated the average equivalent strain using the equation,
in which εeq is the equivalent strain, ν is the material’s Poisson’s ratio assumed to be 0.5, εx is the strain in the x-direction, εy is the strain in the y-direction, and γxy is the shear strain. As expected, the strain varied linearly with strut displacement (Figure 4). Furthermore, the variability of the strain in the CCR was minimal and consistent for a sinusoidal displacement profile (Figure 4). We also performed ANSYS mechanical simulations of the stretched chamber, which agreed well with the experimental results (Supplementary Methods 1). The simulation results showed more clearly the strain field that developed over the entire chamber geometry. The simulations revealed a near-uniform strain field in the CCR (Figure S6a) and both the simulated and measured CCR average equivalent strain matched (Figure S6b) with deviations only at larger strut displacements (>4 mm). Using the chamber stretcher, the stretching mechanism was made independent of both flow and pressure.
Figure 4.
Strain transfer to the CCR. The first two columns on the left show images of the tested material substrates: PDMS, PA, POC and Col impregnated with charcoal powder, before (relaxed) and after being stretched. The third column shows a cyclic strain profile achieved using the microcontroller directed chamber stretcher. The profiles for each material were consistent over multiple periods (T). The fourth panel shows the linear relationship between strut displacement and CCR average equivalent strain for each material. The average equivalent strain varied slightly with each CCR filler material at larger displacements.
Pressure Characterization: To Modify the Hydrostatic Pressure in the Chamber the Media Reservoir Was Mounted to a Separate Linear Actuator
To measure the hydrostatic pressure in the chamber, a PendoTech in-line pressure transducer was connected to the inlet of the chamber and kept level with the chamber. By raising or lowering the media reservoir of the closed loop flow circuit, the hydrostatic pressure in the chamber was able to be increased or decreased independent of the flow and stretching mechanisms. As expected, the hydrostatic pressure varied linearly with changes in the media reservoir height according to the relationship P = ρgh, where P is the hydrostatic pressure in the chamber, ρ is the density of the fluid taken as 1000 kg/m3 for water, g is the acceleration due to gravity taken as 9.799 m/s2 in Gainesville, FL, and h is the relative height difference between the chamber and the media reservoir (Figure S7a). Moreover, transient pressure profiles were produced by dynamically moving the media reservoir with the linear actuator (Figure S7b). The in-line pressure transducer had an additional benefit by being able to monitor changes induced by each of the mechanical stimulation conditions (Figure S8). For instance, along with observing hydrostatic pressure changes in the system, increases or decreases in flow rate or changes in stretching frequency could be observed as changes in the measured pressure by the transducer. By using controlled height differences in the flow circuit, the hydrostatic pressure in the chamber is made independent of the flow and stretching mechanisms.
Fluid-Structure Characterization: Simulations Revealed Optimized Conditions for Decoupling the Fluid Flow and Stretch Interactions in the Chamber
To understand the interactions occurring in the chamber, extensive fluid-structure interaction (FSI) simulations were performed (Supplementary Methods 1). When the chamber is stretched this changes the flow channel dimensions, which consequently influences the flow through the channel. As the chamber struts are pulled, the flow channel widens causing a vacuum effect pulling in more fluid and thus negatively spiking the flow rate through the channel. Then as the struts are returned to their initial position, the channel narrows to its original dimensions causing a pumping effect pushing out more fluid and thus positively spiking the flow rate through the channel. For that reason, it appeared the flow and stretch remained coupled. This was overcome by dynamically changing the flow rate to compensate and negate this fluid-structure interaction. We tested both steady and unsteady inlet flow rates to determine an inlet flow rate function that minimized the variability of the WSS over the CCR. From the simulations, a steady inlet flow rate and a stretching profile imparted a sinusoidal-esque waveform on the WSS at the center of the CCR (Figure 5a). This same sinusoidal-esque waveform was observed by the PendoTech in-line pressure transducer (Figure 5b). Upon recognizing this, we tested the effect of using an inlet flow rate with a sinusoidal waveform of the type, Qin = Qave − A sin(ωt − ϕ), where Qave is the average inlet flow rate over the stretching period, A is the flow rate amplitude, ω is the frequency, and ϕ is the phase angle between the cyclic stretch waveform and the flow rate waveform. It was found for a given displacement that the variability of the WSS at the center of the CCR was minimized by a phase angle of ~90º and a ratio of of ~4% for a waveform of this type and a cyclic stretching amplitude of 3.5 mm of strut displacement (Figure 6). The ratio of to minimize the WSS variability decreased with decreasing strut displacement. Simulation videos further illustrate the reduced variability in WSS across the channel wall by using these optimized parameters (Video S2 & S3). Using colored dye, we confirmed laminar flow was maintained through the chamber during stretching by observing continuous, non-mixing streamlines of dye (Video S4).
Figure 5.
FSI simulation results. The simulation used a squared sinusoidal function to displace the struts 3.5 mm at a frequency of 1 Hz and a constant inlet flow rate of 600 mL/min (a). Cyclically stretching the chamber resulted in a sinusoidal-esque response in the WSS at the center of the CCR. The effect of stretching the chamber was observed by the in-line PendoTech pressure transducer due to the pumping action from stretching the chamber, which mirrors the simulated variation in the wall shear stress (b). A change in the dynamic pressure results in a change in the local flow rate through the chamber, which would lead to a variation in the WSS in the chamber.
Figure 6.
Optimized flow parameters from FSI simulations. The standard deviation of the wall shear stress (σWSS) was minimized using a inlet flow rate with a generalized sine wave of the form, Qin = Qave − A sin(ωt − ϕ). A transformed full 3rd order regression model was used to fit the simulation data with an R2 = 96% (a). The impact of the optimized parameters is clear from the plot of the WSS over the center of the CCR during one period (b). It was found for a given displacement that the variability of the WSS at the center of the CCR was minimized by a phase angle of ~90º and a ratio of of ~4% for a waveform of this type and a cyclic stretching amplitude of 3.5 mm of strut displacement.
CHAMBER FABRICATION AND CELL CULTURE REGION PREPARATION
Chamber
Materials
Reagents
Dow Sylgard 184 (e.g. Ellsworth Adhesives, Germantown, WI, cat no: 4019862)
Ethanol, 200 proof (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: 22–032-601)
Deionized water
Equipment
Disposable plastic cups
Disposable plastic stir rods
Disposable plastic bags
Chamber molds (CNC milled from polycarbonate sheet)
Analytical balance
Vortex
Vacuum oven
Sonicator
Procedure
The chamber is composed of two separate halves that are fused together. The top and bottom halves of the chamber are fabricated by casting Sylgard 184, a polydimethylsiloxane elastomer (PDMS), into custom designed molds (Supplementary Methods 4). Molds were CNC milled from polycarbonate. Sylgard 184 is prepared by pouring the base and curing agent in a 10:1 mass ratio into a plastic disposable cup followed by mixing with a disposable stir rod and vortexing for ~2 minutes. Disposable cups and stir rods are suggested because before curing Sylgard 184 is a sticky, viscous polymer. ~8 g of Sylgard 184 is needed to fill each chamber position in the mold. ~2 g should be prepared in excess for each chamber position in the mold to account for losses. Then Sylgard 184 in the molds is degassed for 1 hour in the vacuum oven at room-temperature and at ~5 inHg and subsequently cured for 2 hours in the vacuum oven at 70 °C and at ambient pressure. A vacuum chamber at room-temperature can be used for degassing as well. Following cooling and demolding, the chamber halves are cleaned by placing them in a disposable plastic bag filled with 70% (v/v) ethanol-water solution and sonicating for 10 minutes. After drying, the inset cell culture region (CCR) is prepared for bonding the substrate material of interest (Figure 7). In specific scenarios, a chamber may be made without the CCR inset using a different mold (Supplementary Methods 4). Chamber assembly was designed to be a straightforward process: 1) cast the chamber, 2) fill the CCR, 3) seal the chamber.
Figure 7:
Chamber fabrication. First, the Sylgard 184 polydimethylsiloxane elastomer chamber halves are cast in molds (1). Then the CCR region is treated for bonding and the substrate filler material of interest is added (2). Two chamber halves are then glued together and sealed with our custom luer lock fittings to make a complete chamber (3).
CCR Substrate: Polydimethylsiloxane elastomer (PDMS)
Materials
Reagents
Dow Sylgard 184 (e.g. Ellsworth Adhesives, Germantown, WI, cat no: 4019862)
Dow Sylgard 527 (e.g. Ellsworth Adhesives, Germantown, WI, cat no: 1696742)
Equipment
Chamber half without CCR inset
Disposable plastic cup
Disposable plastic stir rod
Polycarbonate strip (100 mm X 10 mm X 5 mm) (cut from a sheet of polycarbonate)
Polycarbonate coverslip (30 mm X 10 mm X 5 mm) (cut from a sheet of polycarbonate)
Vortex
Oven
Autoclave
Procedure
To achieve the experimental working range of elastic moduli, Dow Sylgard 184 and Dow Sylgard 527 are mixed separately according to the manufacturer’s instructions and then mixed together according to the ratios described by Palchesko et al.22 The PDMS mixture can be prepared in disposable plastic cups using disposable plastic stir rods for mixing. In this situation, a CCR is unnecessary and a chamber without a CCR inset is used. A small drop of PDMS mixture prepared according to Palchesko et al is placed in the channel.22 Using the polycarbonate strip, the drop is spread across the channel surface to make a very thin, even surface layer of the desired PDMS formulation. The chamber half then is baked for 1 hour in an oven at 70°C. Before curing to impart a surface roughness to the PDMS, an abraded polycarbonate coverslip can be placed in contact with the uncured PDMS. Next, all chamber parts are sterilized by steam autoclaving at 121 °C for 20 minutes. Chambers can be stored at room-temperature until needed.
CCR Substrate: Polyacrylamide gel (PA)
Materials
Reagents
Deionized water
Benzophenone (e.g. Millipore Sigma, Burlington, MA, cat no: B9300)
Acetone (e.g. Millipore Sigma, Burlington MA, cat no: 179124)
Methanol (e.g. Millipore Sigma, Burlington MA, cat no: 179337)
Acrylamide 40% solution (e.g. Bio-Rad, Hercules, CA, cat no: 1610140)
Bis-acrylamide 2% solution (e.g. Bio-Rad, Hercules, CA, cat no: 1610142)
Ammonium persulfate (e.g. Bio-Rad, Hercules, CA, cat no: 1610700)
Tetramethylethylenediamine (TEMED) (e.g. Bio-Rad, Hercules, CA, cat no: 1610800)
Sterile phosphate-buffered saline (PBS) (e.g. Corning, Corning, NY, cat no: 21–040-CV)
Equipment
Chamber half with CCR inset
Rectangular glass coverslip (22 mm X 10.5 mm) (e.g. Electron Microscopy Sciences, Hatfield, PA, cat no: 72191–22)
Sterile 150 mm diameter disposable plastic petri dish (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: FB0875714)
UV lamp
Biosafety cabinet
Procedure
The CCR of the chamber is prepared for bonding PA to PDMS by impregnating the PDMS surface with benzophenone, a UV photoinitiator, following the methods of Simmons et al.38 First, prepare a 10% (w/v) benzophenone solution by dissolving benzophenone in a water/acetone mixture (35:65 w/w). Transfer the chamber to a 150 mm diameter disposable petri dish. In a fume hood, add 200 μL of the benzophenone solution to the CCR of a chamber half and incubate it for 60 seconds. Pipette off the benzophenone solution, rinse with methanol three times, and let the chamber air dry. During this time prepare a PA precursor solution by mixing acrylamide solution, bis-acrylamide solution, TEMED, and 10% (w/v) ammonium persulfate solution following the protocol of Fischer et al39. Mixtures of acrylamide and bis-acrylamide can be prepared in advance. 10% (w/v) solutions of ammonium persulfate in water should be prepared fresh. The mechanical properties of the polyacrylamide are controlled by varying the relative concentrations of acrylamide and bis-acrylamide in the precursor solution. After drying, fill the benzophenone treated CCR with 250 μL of PA precursor solution. Place a rectangular glass coverslip over the CCR. Make sure there are no bubbles. Allow the PA to gel polymerize under a UV-lamp emitting at a wavelength of 365 nm for 45 minutes in a biosafety cabinet. This process will both bond the two materials together and UV-sterilize the chamber halves by conducting the step in the biosafety cabinet.40 Transfer the chamber to a fresh sterile 150 mm diameter disposable petri dish using sterile tweezers. In a similar manner as for PDMS, a surface topography can be transferred to the PA by using an abraded polycarbonate coverslip instead of the glass coverslip. All remaining steps are conducted aseptically in a biosafety cabinet. Following gel polymerization, the coverslip is gently removed, and the part is equilibrated in sterile PBS overnight. Chambers can be stored hydrated in sterile PBS at 4 °C until needed for approximately 2 weeks.
CCR Substrate: Poly(1,8-octanediol citrate) elastomer (POC)
Materials
Reagents
Ethanol, 200 proof (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: 22–032-601)
Deionized water
1, 8 octanediol (e.g. Millipore Sigma, Burlington MA, cat no: O3303)
Citric acid (e.g. Millipore Sigma, Burlington MA, cat no: 251275)
Equipment
Chamber half with CCR inset
250 mL round bottom flask
Magnetic stir bar
Hotplate with magnetic stirring
Silicone oil bath
Analytical balance
Ethylene oxide gas sterilizer
Procedure
POC elastomer is synthesized following the protocols of Yang et al.26 Briefly, 1,8-octanediol and citric acid are combined in a round bottom flask with a magnetic stir bar at an equimolar ratio and are melted in a 165 °C silicone oil bath. Upon complete melting, the temperature is reduced to 140 °C for the polycondensation reaction to proceed. The resulting viscous pre-polymer melt is purified by recrystallization in water and the purified pre-polymer is dissolved in ethanol to make a 50 % (w/v) solution. This solution is then added to the CCR region dropwise and the ethanol is allowed to evaporate. This process is repeated until the CCR is filled with POC pre-polymer. Subsequently, the filled CCR is baked at 80 °C for 2 days to thermally crosslink the POC pre-polymer to form the POC elastomer. Chambers are gas sterilized using ethylene oxide. Chambers can be stored at room-temperature until needed.
CCR Substrate: Type I collagen gel (Col)
Materials
Reagents
Sterile deionized water
sulfo-SANPAH (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: A35395)
PureCol® EZ Gel type I collagen (Advanced Biomatrix, San Diego, CA, cat no: 5074)
Equipment
Chamber half with CCR inset
Sterile 150 mm diameter disposable plastic petri dish (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: FB0875714)
Sterile tweezers
UV lamp
Biosafety cabinet
Autoclave
Procedure
For embedding cells in the 3D gel proceed to section Seeding Cells in the Chamber. Begin by sterilizing the chamber half by steam autoclaving at 121 °C for 20 minutes. All remaining steps are conducted aseptically in a biosafety cabinet. Prepare a sterile 2 mM solution of sulfo-SANPAH dissolved in sterile deionized water. Transfer the chamber to a sterile 150 mm diameter disposable petri dish using sterile tweezers. Fill the CCR with 200 μL of sulfo-SANPAH solution and UV irradiate for 15 minutes. Remove the sulfo-SANPAH solution and rinse twice with sterile deionized water. Then fill the CCR with 400 μL of PureCol® EZ Gel type I collagen gel and incubate for 2 hours at 37 °C to allow the collagen solution to gel. Use the chamber immediately for cell culture following gelation.
Material Validation
Biocompatible synthetic polymers and natural matrices can be bonded to the chamber. To demonstrate the system’s material functionality, we bonded four different established chemistries to the CCR. First, we demonstrated tunability of the chamber channel elasticity with different PDMS elastomer formulations to show that the simplest material was possible. We then confirmed polyacrylamide gel (PA), a tunable material used for mechanobiology experiments, could bond to the CCR, as well. This was done by grafting the PA during its polymerization process to the PDMS by forming radicals at the surface layer of the PDMS. Radicals were formed using the photoinitiator benzophenone, which was first embedded in the surface of the PDMS and activated by UV irradiation during the PA polymerization process. To demonstrate the systems applicability to biomaterial development, we demonstrated poly(1,8-octanediol citrate) elastomer (POC), a hemocompatible biomaterial, could bond to PDMS.41–44 POC is a sticky, tacky polymer. Without additional treatment to either material, it was capable of forming a sufficiently strong bond with PDMS through intermolecular interactions. The final material we bonded to the CCR was a type I collagen gel (Col) to show that the MechanoBioTester system was not limited to synthetic, 2D material systems, but that natural, 3D matrices are also supported. To do this, the heterobifunctional crosslinker sulfo-SANPAH was used to chemically link the two materials together. The crosslinker’s nitrophenyl azide group was first reacted with the PDMS surface by UV irradiation to non-specifically insert itself into PDMS methyl groups. Then the exposed, surface bound sulfo-NHS esters from the sulfo-SANPAH were used to non-specifically react with primary amines on the collagen as it gelled. We believe other biomaterials such as alginate and hyaluronic acid hydrogels can be sufficiently bonded to the CCR using the appropriate chemistries. For these materials we propose first treating the PDMS with (3-aminopropyl)triethoxysilane (APTES) to put a primary amine group at the surface of the PDMS after which then using a carbodiimide crosslinker to link to the carboxylic acid groups on the alginate and hyaluronic acid hydrogels. In a similar manner, silk fibroin and poly(ethylene glycol) (PEG) hydrogels could be bonded to the CCR by first using APTES to put primary amines at their hydroxyl groups and then using sulfo-SANPAH to conjugate them to the PDMS surface as we have shown for other primary amine containing species. Successful bonding of these various materials broadens the cell culture model’s applicability for investigating mechanobiology and cell-material interactions.
Moreover, topographies could be transferred to the material within the CCR during the material’s polymerization process. We showed this functionality by transferring a uniform surface roughness to a PA filled CCR using an abraded polycarbonate coverslip during gel polymerization (Figure S3). Because the chamber is flexible, it is possible to peel it away from a rigid mold, enabling more complex topographies to be transferred by micro-molding using etched silicon wafers. The CCR allows the system to be used for testing a variety of materials independent of the PDMS chamber.
CELL CULTURE
CCR Substrate Treatment for Cell Adhesion
Materials
Reagents
Sterile deionized water
sulfo-SANPAH (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: A35395)
Primary amine-containing peptide, protein, DNA, or other species
Sterile phosphate-buffered saline (PBS) (e.g. Corning, Corning, NY, cat no: 21–040-CV)
Equipment
Chamber with prepared CCR substrate
Sterile 150 mm diameter disposable plastic petri dish (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: FB0875714)
Biosafety cabinet
Procedure
This procedure is applicable for either PDMS treated or PA filled CCR. POC and collagen gels support cell adhesion on their own and thus surface functionalization for cell adhesion is not necessary for those substrates.
All further work is conducted aseptically in a biosafety cabinet. Adapting the methods of Li et al., the prepared CCR of the chamber is functionalized with 0.4 to 2 mM sulfo-SANPAH diluted in sterile deionized water.45 400 μL of sulfo-SANPAH is added to the CCR of the chamber and then UV irradiated for 15 minutes. The reacted sulfo-SANPAH is removed and the CCR of the chamber is rinsed three times with sterile deionized water. Afterwards, 400 μL of a primary amine-containing species is added to the CCR of the chamber and is incubated overnight at room-temperature. Following incubation, remove the primary amine-containing species solution and rinse three times with PBS to remove any excess material. Add 400 μL of PBS to the chamber to maintain hydration of the conjugated species to the CCR. Chambers can be stored in sterile 150 mm diameter disposable petri dishes at 4 °C until they are needed for cell culture. We have functionalized the CCR using 0.1 mM RGD peptide (abcam, Cambridge, UK, cat no: ab142698) diluted in sterile deionized water, 0.4 μM vascular endothelial growth factor (VEGF) receptor-2 binding DNA aptamer with a 5’ amine functionality (Integrated DNA Technologies, Coralville, IA) diluted in sterile deionized water, and 10 mg/mL rat tail type I collagen in 0.03N acetic acid (Corning, Corning, NY, cat no: 354249) diluted with sterile deionized water to 0.3 mg/mL.46 Stability of the amine-containing species and CCR material will be different for each treatment and requires testing by the investigator.
Seeding Cells in the Chamber
Materials
Reagents
Sterile deionized water
sulfo-SANPAH (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: A35395)
PureCol® EZ Gel type I collagen (Advanced Biomatrix, San Diego, CA, cat no: 5074)
Sterile phosphate-buffered saline (PBS) (e.g. Corning, Corning, NY, cat no: 21–040-CV)
For 2D culture, at most 60,000 cells per chamber being seeded (gives a seeding density of 30,000 cells/cm2)
For 3D culture, CCR inset volume is 200 mm3 (200 μL) prepare the number of cells accordingly
Cell-specific cell culture media
Equipment
Chamber with prepared CCR substrate for cell adhesion (for 2D culture) or chamber half with CCR inset (for 3D culture)
Standard cell culture supplies
Microcentrifuge
Sterile 150 mm diameter disposable plastic petri dish (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: FB0875714)
Sterile tweezers
UV lamp
Biosafety cabinet
Autoclave
Procedure for 2D culture
All steps are conducted aseptically in a biosafety cabinet. Prepare a cell suspension that gives the desired seeding density for a 2 cm2 area in 400 μL of cell culture media. Remove any liquid from the CCR of the chamber and rinse once with cell culture media. Dropwise add 400 μL of the prepared cell suspension across the CCR of the chamber. Place the chamber in a cell culture incubator for 3 to 4 hours to allow cells to attach to the CCR surface. Cell attachment time may vary depending on cell type and CCR treatment. Monitor cell culture media volume during the cell attachment period to ensure it does not evaporate. If longer times are necessary for attachment, after 3 to 4 hours replenish with additional cell culture media.
Procedure for 3D culture
Sterilize all chamber parts by steam autoclaving at 121 °C for 20 minutes. All steps should be conducted aseptically in a biosafety cabinet. Prepare a sterile 2 mM solution of sulfo-SANPAH dissolved in sterile deionized water. Transfer the chamber to a sterile 150 mm diameter disposable petri dish using sterile tweezers. Fill the CCR with 200 μL of sulfo-SANPAH solution and UV irradiate for 15 minutes. During this time, in a sterile microcentrifuge tube prepare a cell suspension with the desired cell number for seeding the gel. Centrifuge the cell suspension to pellet the cells according to the specific cell’s centrifugation conditions. Following UV-irradiation, remove the sulfo-SANPAH solution and rinse twice with sterile deionized water. Aspirate the supernatant from the microcentrifuge tube and gently resuspend the pellet in 400 μL of the PureCol® EZ Gel type I collagen gel. Prior to seeding, confirm cell compatibility with PureCol® EZ Gel. Dropwise pipette the cell solution into the CCR and incubate it for 2 hours in a cell culture incubator to allow the collagen solution to gel. After gelation, add 200 μL of cell culture media to hydrate the gel. For subsequently seeding cells on top of the gel, follow the Procedure for 2D culture.
Chamber Sealing
Materials
Reagents
Cell-specific cell culture media
Dow Sylgard 184 (e.g. Ellsworth Adhesives, Germantown, WI, cat no: 4019862)
ReproRubber Thin Pour (ReproRubber, Islandia, NY, cat no: 16135)
Sterile phosphate-buffered saline (PBS) (e.g. Corning, Corning, NY, cat no: 21–040-CV)
Equipment
2 cell seeded chamber halves or 1 cell seeded chamber half and 1 autoclaved chamber half without CCR inset
Disposable plastic cup
Disposable plastic stir rod
Sterile microcentrifuge tube
Sterile 1000 μL micropipette tip
Sterile 30 mm diameter disposable plastic petri dish
Sterile 150 mm diameter disposable plastic petri dish (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: FB0875714)
2 sets of sterile tweezers
2 sterile one-way stopcocks (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: NC9889364)
2 sterile 3D printed, custom luer lock fittings (printed using Dental SG resin with a Form 2, sterilized by autoclaving at 121 °C for 20 minutes)
2 sterile polypropylene male luer caps (e.g. Cole-Parmer, Vernon Hills, IL, cat no: EW-30800–30)
2 sterile 10 mL disposable plastic luer syringes (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: 14–955-459)
Biosafety cabinet
Procedure
A complete chamber is formed by gluing the top and bottom parts together. Chamber sealing can be completed using either PDMS (Dow Sylgard 184, 10:1 base to curing agent) or ReproRubber® Thin Pour, a two-part platinum catalyzed, addition cured silicone. Sylgard 184 is prepared by pouring the base and curing agent at a 10:1 mass ratio into a plastic disposable cup followed by mixing with a disposable stir rod while vortexing for ~2 minutes. The mixed Sylgard 184 is then poured into a sterile microcentrifuge tube outside of the biosafety cabinet. For ReproRubber® Thin Pour, each component is poured into a separate sterile microcentrifuge tube outside of the biosafety cabinet. All remaining steps are conducted aseptically in a biosafety cabinet. 400 μL of cell culture media is left on the chamber during gluing to keep the cells hydrated and also to exclude the silicones from contacting the cells. If using ReproRubber, a small amount of the two parts from their respective microcentrifuge tubes are poured into a sterile 30 mm diameter disposable plastic petri dish and mixed together using a 1000 μL micropipette tip. For gluing, either of the uncured silicones are applied as a thin layer to the contact area of one chamber half using a micropipette tip. Then using sterile tweezers, another chamber half is positioned and tapped down onto the bottom chamber half. This other chamber half can be one with seeded cells or a chamber half without a CCR inset. A PDMS glued chamber is then transferred to a cell culture incubator to cure overnight. A ReproRubber glued chamber is ready for the next step in ~10 minutes and may be left in the biosafety cabinet until then. Next, the seam formed by the joined chamber parts is coated in a thin layer of PDMS (Dow Sylgard 184, 10:1 base to curing agent) and again cured at 37 °C overnight. It is important to ensure there is sufficient cell culture media in the chamber during overnight curing. The same sealing process is used for ReproRubber® Thin Pour, but only requires ~10 minutes for curing. Then custom 3D printed luer lock fittings (Supplementary Methods 4) are inserted into the chamber openings, sealed to the chamber using PDMS (Dow Sylgard 184, 10:1 base to curing agent), and cured at 37 °C overnight. The same sealing process is used for ReproRubber® Thin Pour, but only requires ~10 minutes for curing. This sealing procedure is necessary to ensure a water-tight chamber. ReproRubber is the preferred method for chamber sealing. Affix a sterile one-way stopcock to each fitting of the chamber in the closed position. With the chamber horizontal, attach a sterile syringe filled with ~3 mL of PBS. Then hold the chamber vertical with the syringe end on the bottom and the open end at the top. Turn the two stopcocks to the open position and slowly fill the chamber with PBS by depressing the syringe plug. Fill the chamber until the PBS just enters the upper stopcock and then slowly retract the syringe plug to empty the chamber of all liquid. Then return the chamber to the horizontal position and attach another syringe filled will ~3 mL of cell culture media to the other stopcock. Remove the PBS filled syringe and then hold the chamber vertically with the cell culture media syringe at the bottom and the open stopcock at the top. Slowly fill the chamber with cell culture media by depressing the syringe plug until the cell culture media just passes the opening of the top stopcock. Close the two stopcocks, return the chamber to the horizontal position, and remove the cell culture media syringe. Affix a male luer cap to each stopcock and place the chamber in a cell culture incubator until needed for experimentation. As necessary for the specific cell type in the chamber, cell culture media should be changed following the described method above.
Example Cell Culture Experiment
The CCRs of PDMS chambers prepared with Dow Sylgard 184 substrates were functionalized with 0.1 mM RGD peptide (abcam, Cambridge, UK, cat no: ab142698) diluted in sterile deionized water or 0.4 μM VEGF receptor-2 binding DNA aptamer with a 5’ amine functionality (Integrated DNA Technologies, Coralville, IA) diluted in sterile deionized water. 0.4 mM sulfo-SANPAH in sterile deionized water was used to conjugate the RGD peptide and 5’ amine-containing DNA aptamer. Human umbilical vein endothelial cells (HUVECs) (LifeLine Cell Technologies) were cultured at passage number 6 to 9 in complete VascuLife® VEGF Endothelial Medium (LifeLine Cell Technologies). Cells were passaged using cell dissociation buffer (gibco) and seeded at 10,000 cells/cm2 onto the functionalized PDMS substrates. Cell dissociation buffer was used to maintain the cell surface proteins necessary for adhesion. Representative images were taken and processed using the NIH ImageJ software. Green fluorescent protein expressing human umbilical vein endothelial cells (GFP-HUVECs) (Angio-Proteomie) were cultured at passage number 8 in Endothelial Growth Medium (Angio-Proteomie). Cell suspensions were seeded at a density of 10,000 cells/cm2 into a completed chamber. The GFP-HUVECs were allowed to attach for 4 hours before further chamber manipulation. The chamber was then flushed and rinsed with PBS (Corning) to remove unattached cells. Subsequently, the chamber was filled with Endothelial Growth Medium. For co-culture experiments, human aortic smooth muscle cells (AoSMCs) (Cell Applications) were cultured at passage number 8 in complete Human Smooth Muscle Cell Growth Media (Cell Applications). During passaging, the AoSMCs were labeled with Cell Proliferation Staining Reagent - Orange Fluorescence - Cytopainter (abcam) to make the cells visible in the gel while imaging. AoSMCs were then mixed into the PureCol® EZ Gel type I collagen gel (Advanced Biomatrix) solution at 500,000 cells/mL. The AoSMC cell suspension in collagen gel was used to fill the CCR. After gelation, 200 μL of Human Smooth Muscle Cell Growth Media was pipetted onto the gel to saturate it. The AoSMCs were allowed to attach within the gel overnight. Following, GFP-HUVECs were seeded on top of the gel at a density of 30,000 cell/cm2 and allowed to attach for 4 hours prior to completing chamber fabrication. A 1:2 ratio of AoSMC to GFP-HUVEC media was used for the rest of co-culture after initial seeding. Prior to imaging, live cell nuclei were labeled with Hoechst 33342 solution (ThermoFisher Scientific) following the manufacturer’s instructions. Fluorescence images of chamber cross-sections were taken using a Nikon TE-2000 inverted epifluorescence microscope and processed using the NIH ImageJ software.
Anticipated Results and Discussion
The CCR was functionalized with diverse chemistries to support cell attachment and filled with a cell-laden gel enabling 3D, co-culture studies. We successfully conjugated the adhesion peptide sequence arginine-glycine-aspartate (RGD) and DNA aptamer. Their conjugation to the surface was confirmed by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) (Supplementary Methods 3). This was observed in the absorbance spectra by the presence of a broad peak centered at 3500 cm−1 corresponding to the stretching of the N-H bond of the amine groups found in all three species and not the substrate material, PDMS (Figure S9). Human umbilical vein endothelial cells (HUVECs) were shown to readily attach to and spread on the functionalized PDMS surface after conjugation of the two chemistries (Figure S9). We showed conjugation to PDMS using sulfo-SANPAH, though this chemistry and others can be used to conjugate these same species to PA and POC. By using established conjugation chemistries, our cell culture model system can be a platform for studying the interactive effects of surface chemistry and ECM composition with mechanical stimulation on cellular behavior. Using sulfo-SANPAH, we bonded a Type I collagen gel to the CCR inset to form a 3D collagen matrix to demonstrate the system is not limited to only 2D substrates. Green fluorescent protein expressing human umbilical vein endothelial cells (GFP-HUVEC) were seeded onto the surface of the collagen gel filled CCR. The cells readily spread and grew in the chamber showing little to no cytotoxicity. Moreover, the cells remained segregated to the CCR demonstrating the spatial control in conjugating various chemistries to the CCR (Figure 8).
Figure 8.
GFP-HUVECs remained segregated to the cell culture region (CCR). GFP-HUVECs were seeded onto a type I collagen gel filled CCR. Cells remained confined to the CCR after seeding observed by the sharp interface between the corner of the CCR, the flow channel, and the chamber wall (dotted line). The image was taken using a DinoLite USB microscope with 480/510 nm and 570/610 nm excitation/emission capabilities.
Equipped with this functionality, we aimed to demonstrate a 3D vascular co-culture model could be achieved in the CCR inset. Human aortic smooth muscle cells (AoSMC) were embedded in a collagen gel matrix formed in the CCR and following gelation GFP-HUVECs were seeded on top of the gel (Figure 9). Because the chamber is PDMS, the CCR region can be excised from the chamber and sectioned for histological analysis with little difficulty enabling cross-sectional analyses of cellular invasion or development in 3D matrices after mechanical stimulation. The MechanoBioTester can support numerous surface functionalities, matrix architectures, and cell types for their study under applied decoupled combinations of mechanical stimulation.
Figure 9.
Demonstration of a full thickness cross-section of the 3D co-culture model contained in the CCR of the chamber (a). A higher magnification view of GFP-HUVEC-AoSMC interface in the collagen gel (b). A full thickness image of the GFP-HUVEC-AoSMC 3D vascular co-culture model after 3 days of culture (c).
MECHANICAL STIMULATION
Flow Circuit Assembly
Materials
Reagents
Cell-specific cell culture media
Polyvinylpyrrolidone (PVP) (Millipore Sigma, Burlington MA, cat no: P0930)
HEPES (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: 11344041)
Equipment
Cell seeded sealed chamber
4 sterile one-way stopcocks (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: NC9889364)
2 sterile two-way stopcocks (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: NC9218896)
1 sterile 10 mL disposable plastic luer syringes (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: 14–955-459)
200 μm pore size sterile syringe filters (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: SLGPR33RS)
2 autoclaved 100 mL glass bottles (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: 06–414-1A)
2 autoclaved Thermo Scientific™ Nalgene™ Top Works™ Flexible Systems (Thermo Fisher Scientific, Waltham, MA, cat no: 14–831-342B)
Masterflex L/S® Precision Pump Tubing, PharMed® BPT, L/S 17 (Cole-Parmer, Vernon Hills, IL, cat no: EW-06508–17)
Masterflex L/S® Precision Pump Tubing, PharMed® BPT, L/S 16 (Cole-Parmer, Vernon Hills, IL, cat no: EW-06508–16)
PVDF 1/8” barb to male luer fittings (e.g. Nordson Medical, Loveland, CO, cat no: MTLL230-J1A)
PVDF 1/4” barb to female luer fittings (e.g. Nordson Medical, Loveland, CO, cat no: FTLL230-J1A)
PVDF 1/4” barb to male luer fittings (e.g. Nordson Medical, Loveland, CO, cat no: MTLL230-J1A)
2 sterile 250 mL glass bottles (e.g. Thermo Fisher Scientific, Waltham, MA, cat no: 06–414-1B)
PendoTech PRESS-S-000 Single-Use Pressure Sensor, Sterile, Polycarbonate, Luer Fitting (e.g. Cole-Parmer, Vernon Hills, IL, cat no: UX-19406–32)
Water bath at 37 °C
Biosafety cabinet
Autoclave
Viscometer (e.g. Brookfield)
Procedure
All flow circuit tubing and media bottles should be prepared and autoclaved ahead of time. Three lengths of L/S 17 tubing should be sized and cut for the flow circuit. Each length of tubing should be approximately 2 to 3 feet. A separate piece of L/S 16 tubing should be sized and cut to a length of approximately 1 foot. Two of the L/S 17 lengths should be affixed with male to barb fittings on one end and female to barb fittings on the other. The remaining length of L/S 17 tubing should be affixed with female to barb fittings on both ends. The L/S 16 length should be affixed with male to barb fittings on both ends. The 1/4” internal diameter tubing of the two Thermo Scientific™ Nalgene™ Top Works™ should be affixed with male to barb fittings. Each of these tops should then be screwed onto one of the 100 mL glass bottles. Autoclave all of these components at 121 °C for 20 minutes. Store the autoclaved components until needed.
For flow experiments, cell culture media needs to be thickened to reach physiological fluid shear stresses in the chamber. Media is thickened by adding PVP to make a 10% (w/v) PVP in cell culture media solution. ~200 mL of cell culture media is necessary to fill the flow circuit. Mass 20 g of PVP and add it to one of the 250 mL glass bottles. In the biosafety cabinet, transfer 200 mL of cell culture media to the bottle with PVP. Add HEPES to the bottle to yield a 10 to 25 mM concentration. The specific concentration of HEPES should be determined for the specific cell type being cultured. Seal the bottle, agitate the media to facilitate dissolution of the PVP, and place the bottle in the water bath to further expedite dissolution. Once the PVP is completely dissolved, return to the biosafety cabinet and sterile filter the PVP-media solution into the other sterile 250 mL glass bottle. The sterile-filtered 10% (w/v) PVP-media solution should be stored according to the cell culture media’s requirements. Measure the viscosity of the PVP-media solution at 37 °C using a viscometer. This measurement will be required for calculating the exact flow rate necessary for the desired fluid shear stress by applying the equation in Figure S4b.
Assembly of the flow circuit should be conducted aseptically in the biosafety cabinet. Transfer the autoclaved components, sterile stopcocks, sterile syringe filters, sterile PendoTech pressure sensor, and cell seeded complete chamber to the biosafety cabinet. Warm the PVP-media solution in the water bath. Affix a three-way stopcock to both ends of the chamber so that the ports perpendicular to the chamber flow axis are facing the same direction. Connect the L/S 16 tubing to the ports perpendicular to the chamber flow axis on the three-way stopcocks. This tubing is a bypass line. Attach the PendoTech pressure sensor to one end of the chamber at one of the available ports on the three-way stopcock. Attach one of the two L/S 17 tubing lengths with male and female ports to one of the available ports on the three-way stopcock on the chamber. Repeat for the open end of the PendoTech pressure sensor on the chamber with the other length of L/S 17 tubing with male and female ports. Affix a one-way stopcock to each port of the Nalgene™ Top Works™ bottles with fittings. Connect each of the L/S 17 tubing lengths attached to the chamber to separate Nalgene™ Top Works™ bottles. Use the remaining L/S 17 length to connect the available ports of the two Nalgene™ Top Works™ bottles together. For two sterile syringe filters, attach a one-way stopcock to their luer connection. Insert each syringe filter with stopcock into the 1/8” internal diameter tubing on each of the Nalgene™ Top Works™ bottles. Equally distribute the warmed PVP-media to each of the Nalgene™ Top Works™ bottles. Ensure all of the one-way stopcocks are in the closed position and that the three-way stopcocks are closed to the chamber direction and are open to the bypass line. The flow circuit is now ready to be transferred to the frame, peristaltic pump, and chamber stretcher.
Transfer the flow circuit assembly to the benchtop. Bring the chamber over and down into the frame. Position the chamber in the clamps of the chamber stretcher and secure it in place. Attach the media bottle downstream of the PendoTech pressure sensor to the vertical linear actuator using zip ties. Place the media bottle upstream of the chamber into the water bath. Load the tubing downstream of the media bottle attached to the vertical linear actuator into the peristaltic pump. Open the one-way stopcocks attached to the 1/4” tubing of the Nalgene™ Top Works™ media bottles. Turn on the peristaltic pump and flow media at 50 mL/min through the system to fill the tubing. At this time the media should be flowing through the bypass line. Flow media until there are no bubbles in the tubing. Turn off the pump and set it to a flow rate of 5 mL/min. Close the three-way stopcocks to the bypass line and open them to the chamber. Open the one-way stopcocks attached to the chamber. Turn on the pump and let media flow through the chamber. Turn on the control box and connect the laptop via the USB A to USB B cable to the Arduino Mega in the control box. On the laptop in the Arduino Serial Monitor, type the desired stimulation conditions following the instructions described in the ReadMe file available on GitHub. Stimulation conditions can be changed at any time and the set conditions will run indefinitely until the control box is turned off. The laptop can be disconnected from the control box without interrupting the MechanoBioTester operation. At the end of the desired stimulation time, turn off the control box. Close the three-way stopcocks to the chamber and close the one-way stopcocks attached to the chamber. Unclamp the chamber and disconnect it from the flow circuit. The cells in the chamber can be used for further analyses such as phase microscopy or immunofluorescence microscopy, protein expression, gene expression, or another technique that is accessible to cells cultured in vitro. Cell culture media can be collected from the flow circuit as well. All reusable components such as tubing, bottles, and 3D printed fittings should be washed with light detergent, rinsed with deionized water, and autoclaved as previously described. All wash water and rinsate should be disposed of as liquid biohazardous waste. Disposable components such as stopcocks, syringe filters, and chambers should be discarded as solid biohazardouse waste.
Example Mechanical Stimulation Experiment
HUVECs were mechanically stimulated by fluid flow or cyclic stretch for 24 hours. In all experiments, chambers were fabricated using Sylgard 184 at 1:10 mixing ratio and treated for cell adhesion using type I collagen. In all experiments, HUVECs were seeded at 30,000 cells/cm2 and allowed to attach for ~4 hours prior to chamber sealing. After complete chamber sealing, the chamber was flushed with PBS and then refilled with fresh media. Before stimulation experiments, the cells were cultured overnight in the chamber kept in a cell culture incubator. For fluid flow experiments, the cell culture media viscosity was increased with polyvinylpyrrolidone to make a 10% (w/v) PVP-media solution. The viscosity of the PVP-media solution was measured at 37 °C using a Brookfield viscometer and ranged from 4 – 5 cP. Accounting for the measured PVP-media viscosity and chamber dimensions, the flow rate was adjusted to achieve the desired wall shear stress for any given flow experiment. The flow rate was incrementally increased by 10 mL/min every 30 minutes up to the flow rate that gave the desired wall shear stress. For cyclic stretch experiments, media was flowed through the chamber at 10 mL/min to provide media renewal and mitigate air bubble formation in the chamber. Media was not modified with PVP for cyclic stretch experiments. The seeded chambers were connected to the MechanoBioTester system and stimulated for 24 hours at the desired experimental conditions. Following stimulation, chambers were disconnected, rinsed with PBS, and opened. The cells were fixed with 10% formalin (Sigma Aldrich) for 10 minutes and rinsed with PBS three times. Cells were then permeabilized with 0.1% Triton X-100 (Fisher Scientific) in PBS and rinsed with PBS three times. Cell cytoskeletal F-actin was then stained with Texas Red labeled phalloidin (Invitrogen) diluted in 1% bovine serum albumin (Fisher Scientific) in 0.1% Tween 20 (Fisher Scientific) in PBS following the manufacturer’s instructions. The cells were then mounted using ProLong Diamond Antifade Mountant with DAPI (Invitrogen) and imaged using a Nikon TE-2000 inverted epifluorescence microscope. Cell alignment was quantified from 7–10 images of cell F-actin imaged randomly across the CCR using the Directionality plugin available with the NIH ImageJ software.
Anticipated Results and Discussion
After 24 hours of mechanical stimulation by either fluid flow or cyclic stretch, HUVECs were observed to align in the direction of the flow channel. Alignment was qualitatively visualized by fluorescent imaging of HUVEC cytoskeletal F-actin. Stress fibers organized in the direction of flow or perpendicular to the direction of stretch (Figure 10c, d). Alignment was not observed for HUVECs exposed to lower levels of fluid flow (WSS = 0.5 Pa) (Figure 10b) compared to higher levels of fluid flow (WSS = 1.5 Pa) (Figure 10c). Quantitatively, the distribution of cytoskeletal F-actin fiber directionality was shown to narrow and center around the direction of the flow channel for both fluid flow with a WSS = 1.5 Pa or cyclic stretch with a magnitude of 10% strain and frequency of 1 Hz compared to the unstimulated static control (Figure 10d, g, h). At the same time, the degree of alignment increased with increasing fluid flow (Figure 10f, g). From these simple, proof-of-concept experiments the MechanoBioTester was shown to stimulate HUVECs in a recognizable manner. Stimulation by either fluid flow or cyclic stretch recapitulated the well-document response of F-actin organization in the direction of fluid flow or perpendicular to the direction of cyclic stretch by endothelial cells after 24 hours of stimulation.2,5,33 These experiments support that the MechanoBioTester is able to confer the characterized mechanical stimulation to the cultured cells at the CCR.
Figure 10:
Mechanical stimulation of HUVECs. Representative images from staining of HUVEC F-actin cytoskeletal stress fibers following no stimulation (static condition) (a), following stimulation by fluid flow with WSS = 0.5 Pa (b), following stimulation by fluid flow with WSS = 1.5 Pa (c), and following stimulation by cyclic stretching with a magnitude of 10% strain and frequency of 1 Hz (d). White arrows indicate the direction of stimulation. Quantification of F-actin cytoskeletal stress fiber alignment with respect to the flow channel axis (left to right) following no stimulation (static condition) (e), following stimulation by fluid flow with WSS = 0.5 Pa (f), following stimulation by fluid flow with WSS = 1.5 Pa (g), and following stimulation by cyclic stretching with a magnitude of 10% strain and frequency of 1 Hz (h). 7–10 images were analyzed. Error bars represent one standard deviation.
4. BENEFITS AND LIMITATIONS
Benefits
The MechanoBioTester is a cell culture model for the systematic analysis of complex microenvironments. The system decouples mechanical stimuli to enable the independent control of flow regime, fluid shear stress, unidirectional cyclic stretch, hydrostatic pressure, and material properties. Many models use different principles to achieve the same applied mechanical stimulus (cone-and-plate viscometer versus parallel-plate flow chamber), which introduces variability between experiments and these devices in general are designed for one mechanical stimulus.2,7 The MechanoBioTester was designed to reduce this variability and serve as a complete system for many mechanobiology studies.
The chamber geometry was designed to decouple fluid flow from cyclic stretch. By recognizing the fluid flow negligibly affected the cyclic stretch mechanism while at the same time the cyclic stretch significantly affected the fluid flow mechanism, the system was designed to be closed for stretch and open for flow. In this way, the stretching parameters (magnitude and frequency) are first set and the flow rate then is modulated to compensate for the variability in flow rate induced by the chamber stretching. By using the volume of fluid in the closed loop flow circuit, the hydrostatic pressure could be varied independent of the fluid flow and stretching by simply raising or lowering a media reservoir in relation to the chamber. Together this achieved, a defined region in the chamber, the CCR, in which the effects of fluid flow, cyclic stretch, and hydrostatic pressure could be well-defined and independently controlled.
The benefit of the MechanoBioTester is its versatility. For instance, if stretching is not of interest then the stiffness and PDMS bonding requirements are unnecessary– the device is still available for investigations concerned with fluid flow, hydrostatic pressure, and material. All combinations are able to be systematically varied giving it the ability to tease out the specific effects of different mechanical stimuli with a single device.
The system is straight forward to fabricate and use– cast it, fill it, seal it, test it. The PDMS chamber halves are first cast. The CCR is then treated with the appropriate conjugation chemistry to bond the CCR filler material of interest. Next, the CCR is filled with the desired experimental material, treated for cell adhesion if necessary, seeded with cells, and both halves are then joined and sealed together to form the complete chamber. The chamber then is ready to be connected to the system to enable flow, stretch, and/or pressure stimulation. The equipment for such stimulation are modular, open components and are controlled using open, inexpensive electronics (Arduino microcontrollers). This reduces the overall device cost and enables the investigator to easily modify system components for their specific studies. Even though the MechanoBioTester is a simple system, it was designed to enable complex analyses. Because the chamber is optically transparent it enables in situ live-cell imaging capabilities by using mobile fluorescent imaging technologies such as USB fluorescence microscopes or by transferring the chamber to a conventional inverted fluorescence microscope without additional equipment. Additionally, the optically transparent chamber enables photoactive materials to be investigated during mechanical stimulation empowering the study of dynamic material systems.10,47,48 Moreover, the system enables multiple analyses from a single stimulation condition. Each chamber has two CCR regions (top and bottom), which can be used to test different material conditions or act as experimental replicates. Because the chamber is compact and self-enclosed, multiple chambers can be connected to a single system by using a multichannel pump head and by stacking the chambers in the chamber stretcher. Furthermore, the chambers are robust because of the multistep sealing process, which enables the chambers to be transported with ease to conduct analyses using equipment in other lab spaces, buildings, or institutions. The bioreactor system supports multifaceted experimentation in a simple package.
Limitations
Nonetheless, the MechanoBioTester system is not without areas for improvement. Despite being simple to fabricate, the entire fabrication process can be lengthy. The time to prepare a complete chamber can take anywhere from 2 to 3 days with our current methods depending on the complexity of the experiment. Because of this, we investigated different biocompatible silicone formulations for sealing the chamber halves. Using ReproRubber® Thin Pour silicone, we were able to reduce fabrication time significantly from days to hours for PDMS and collagen filled CCRs. PA and POC filled CCRs require additional fabrication time because of the additional steps needed during their polymerization process. In addition, the chambers are single-use requiring new chambers to be fabricated for every experiment. To reduce the environmental footprint and operating cost of the system, we are targeting methods for cleaning and sterilizing waste chambers to be reused in later experiments. For instance, PDMS chambers could potentially be used multiple times after sufficient rinsing and sterilization steps.45 It is necessary to note that PDMS has its own limitations being known to absorb small, hydrophobic molecules and leach uncured oligomer both of which can unduly influence cultured cells when left unaddressed.49,50 To overcome this process, we are evaluating potential coatings and other chamber construction materials. In terms of stimulation, the MechanoBioTester is confined to unidirectional cyclic stretch limiting the device’s ability to investigate the combined effects of multidirectional cyclic stretch with the other stimuli.51 Additionally, the fluid flow and cyclic stretch are only decoupled to support their constant value conditions i.e. a constant cyclic stretch value paired with a constant WSS value. Investigations requiring more dynamic stretching and flow conditions would require further simulation to determine their new fluid-structure interaction. Another challenge to mechanical stimulation devices is the loss of cells during the stimulation experiment. In flow experiments this can be due to a rapid increase in wall shear stress or by air bubbles flowing over the cultured cells.52,53 To mitigate these effects as much possible, we allowed the cells to attach for an extended period of time (overnight) prior to stimulation. To prevent loss due to rapid shearing, we incrementally ramped the flow rate to the target flow rate that gave the desired wall shear stress. To prevent loss due to bubbles, a bypass line was incorporated to fill the flow circuit without engaging the chamber until all the air in the tubing was removed. Future versions of the device aim to address these concerns to ever improve the functionality of the system.
5. CONCLUSION
Here we introduced the MechanoBioTester, a cell culture model system specially designed for decoupling mechanical stimuli for their independent control and systematic testing. The MechanoBioTester is poised to make inroads in furthering our understanding of complex microenvironments. By decoupling and supporting independent control of mechanical stimuli, the interrelatedness, and non-linear interactions between these stimuli to affect cellular behavior can continue to be understood. And it supports the study of this in 3D, co-culture settings giving a more complete model of the in vivo microenvironment using an in vitro platform. It also can be used as a material and chemical biocompatibility screening tool. By being material-independent, the CCR can be filled with newly synthesized biomaterials to quickly investigate the cellular response to the material in a more physiologically-relevant model– one with multiple forms of mechanical stimulation. Likewise, drug toxicity can be mechanically mediated and thus, the MechanoBioTester is apt to be a platform for studying chemo-mechanical microenvironmental interactions to aid in drug discovery.21,54,55 Our group intends to utilize this system in the context of vascular mechanobiology; specifically, targeting combinations of wall shear stress, cyclic stretch, hydrostatic pressure, and substrate stiffness to understand how each component affects the regulation of mechanoresponsive pathways in endothelial cells and vascular smooth muscle cells. This cell culture model will not only allow us to investigate the main effects of these stimuli, but also their interactive effects. Mechanical stimuli exist in every part of the body and other groups may be interested in using the MechanoBioTester for studying the mechanobiology of other tissue-specific cells (osseous, chondrous, vascular, cardiac, lymphatic, pulmonary, and cancerous) with decoupled mechanical stimuli. It is with this comprehensive protocol that we hope other investigators will be able to apply the MechnanoBioTester to their own research endeavors. The MechanoBioTester is a next generation system for studying mechanobiology and complex microenvironments in vitro.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Breanne Schenk and the staff of the University of Florida Infinity Fab Lab for their help and expertise in 3D printing and laser cutting the numerous parts used by the MechanoBioTester system.
Funding Sources
Research reported in this publication was supported in part by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. This work was also supported by the National Science Foundation-CBET CAREER award (1453098) provided to Josephine B. Allen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Footnotes
The author, Nicolas Montoya, declares no competing financial interest.
For details on chamber molds, chamber fittings, building the frame, chamber stretcher, and control box please contact us.
The authors, Bryan D. James and Josephine B. Allen, declare that they are listed as inventors on a patent filing for the device described herein.
ASSOCIATED CONTENT
Supporting Information. The following files are available.
Supplementary Method 1: Methods for FEM and CFD simulations. Supplementary Method 2: Method for DIC strain mapping. Supplementary Method 3: Method for ATR-FTIR. Figure S1: Photographs of a sealed chamber and of a custom luer lock fitting. Figure S2: Photograph demonstrating the live-cell imaging capabilities of the chamber. Figure S3: Representative images of surface roughness transferred to CCR filler material. Figure S4: ANSYS Fluent simulation of flow channel. Figure S5: ANSYS Fluent simulation results for a partially filled CCR. Figure S6: ANSYS mechanical simulation of chamber stretching. Figure S7: Measurement of hydrostatic pressure at different reservoir heights. Figure S8: Monitoring of different mechanical stimulation methods by hydrostatic pressure measurement. Figure S9: Cell growth on various surface chemistries. (PDF)
Video S1: Dye streamlines of flow in chamber. (MOV)
Video S2: ANSYS simulation results of the wall shear stress in the flow channel during stretching using the optimized inlet flow rate conditions. (MP4)
Video S3: ANSYS simulation results of the wall shear stress in the flow channel during stretching using constant inlet flow rate conditions. (MP4)
Video S4: Dye streamline of flow with stretching in chamber. (MOV)
REFERENCES
- (1).Paluch EK; Nelson CM; Biais N; Fabry B; Moeller J; Pruitt BL; Wollnik C; Kudryasheva G; Rehfeldt F; Federle W Mechanotransduction: Use the Force(S). BMC Biol. 2015, 13 (1), 1–14. DOI: 10.1186/s12915-015-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).James BD; Allen JB Vascular Endothelial Cell Behavior in Complex Mechanical Microenvironments. ACS Biomater. Sci. Eng. 2018, 4 (11), 3818–3842. DOI: 10.1021/acsbiomaterials.8b00628. [DOI] [PubMed] [Google Scholar]
- (3).Hung BP; Hutton DL; Grayson WL Mechanical Control of Tissue-Engineered Bone. Stem Cell Res. Ther. 2013, 4 (1), 10 DOI: 10.1186/scrt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Guimarães CF; Gasperini L; Marques AP; Reis RL The Stiffness of Living Tissues and Its Implications for Tissue Engineering . Nat. Rev. Mater. 2020. DOI: 10.1038/s41578-019-0169-1. [DOI] [Google Scholar]
- (5).Chiu J-J; Chien S Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol. Rev. 2011, 91 (1), 327–387. DOI: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Samet MM; Lelkes PI The Hemodynamic Environment of Endothelium In Vitro and Its Simulation In Vitro In Mechanical Forces and the Endothelium; Lelkes PI, Ed.; Taylor & Francis: The Netherlands, 1992; pp 1–32. [Google Scholar]
- (7).Davis CA; Zambrano S; Anumolu P; Allen ACB; Sonoqui L; Moreno MR Device-Based In Vitro Techniques for Mechanical Stimulation of Vascular Cells: A Review. J. Biomech. Eng. 2015, 137 (4), 040801 DOI: 10.1115/1.4029016. [DOI] [PubMed] [Google Scholar]
- (8).Jufri NF; Mohamedali A; Avolio A; Baker MS Mechanical Stretch: Physiological and Pathological Implications for Human Vascular Endothelial Cells. Vasc. Cell 2015, 7 (1), 8 DOI: 10.1186/s13221-015-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Engler AJ; Sen S; Sweeney HL; Discher DE Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126 (4), 677–689. DOI: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- (10).Yang C; Tibbitt MW; Basta L; Anseth KS Mechanical Memory and Dosing Influence Stem Cell Fate. Nat. Mater. 2014, 13 (6), 645–652. DOI: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chaudhuri O; Gu L; Klumpers D; Darnell M; Bencherif SA; Weaver JC; Huebsch N; Lee HP; Lippens E; Duda GN; et al. Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater. 2016, 15 (3), 326–334. DOI: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Levental I; Georges PC; Janmey PA Soft Biological Materials and Their Impact on Cell Function. Soft Matter 2007, 3 (3), 299–306. DOI: 10.1039/B610522J. [DOI] [PubMed] [Google Scholar]
- (13).Greiner AM; Sales A; Chen H; Biela SA; Kaufmann D; Kemkemer R Nano- and Microstructured Materials for in Vitro Studies of the Physiology of Vascular Cells. Beilstein J. Nanotechnol. 2016, 7, 1620–1641. DOI: 10.3762/bjnano.7.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sinha R; Verdonschot N; Koopman B; Rouwkema J Tuning Cell and Tissue Development by Combining Multiple Mechanical Signals. Tissue Eng. Part B Rev. 2017, 23 (5), 494–504. DOI: 10.1089/ten.teb.2016.0500. [DOI] [PubMed] [Google Scholar]
- (15).Pradhan S; Banda OA; Farino CJ; Sperduto JL; Keller KA; Taitano R; Slater JH Biofabrication Strategies and Engineered In Vitro Systems for Vascular Mechanobiology. Adv. Healthc. Mater. 2020, 1901255 DOI: 10.1002/adhm.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Feinberg AW; Wilkerson WR; Seegert CA; Gibson AL; Hoipkemeier-Wilson L; Brennan AB Systematic Variation of Microtopography, Surface Chemistry and Elastic Modulus and the State Dependent Effect on Endothelial Cell Alignment. J. Biomed. Mater. Res. Part A 2008, 86A (2), 522–534. DOI: 10.1002/jbm.a.31626. [DOI] [PubMed] [Google Scholar]
- (17).Estrada R; Giridharan GA; Nguyen M-D; Roussel TJ; Shakeri M; Parichehreh V; Prabhu SD; Sethu P Endothelial Cell Culture Model for Replication of Physiological Profiles of Pressure, Flow, Stretch, and Shear Stress in Vitro. Anal. Chem. 2011, 83 (8), 3170–3177. DOI: 10.1021/ac2002998. [DOI] [PubMed] [Google Scholar]
- (18).Estrada R; Giridharan GA; Nguyen M-D; Prabhu SD; Sethu P Microfluidic Endothelial Cell Culture Model to Replicate Disturbed Flow Conditions Seen in Atherosclerosis Susceptible Regions. Biomicrofluidics 2011, 5 (3), 032006 DOI: 10.1063/1.3608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ding Y; Floren M; Tan W High-Throughput Screening of Vascular Endothelium-Destructive or Protective Microenvironments: Cooperative Actions of Extracellular Matrix Composition, Stiffness, and Structure. Adv. Healthc. Mater. 2017, 6 (11), 1601426 DOI: 10.1002/adhm.201601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Huh D; Matthews BD; Mammoto A; Montoya-Zavala M; Hsin HY; Ingber DE Reconstituting Organ-Level Lung Functions on a Chip. Science (80-. ). 2010, 328 (5986), 1662–1668. DOI: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Truskey GA Endothelial Vascular Smooth Muscle Cell Coculture Assay for High Throughput Screening Assays to Identify Antiangiogenic and Other Therapeutic Molecules. Int. J. High Throughput Screen. 2010, 171 DOI: 10.2147/IJHTS.S13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Palchesko RN; Zhang L; Sun Y; Feinberg AW Development of Polydimethylsiloxane Substrates with Tunable Elastic Modulus to Study Cell Mechanobiology in Muscle and Nerve. PLoS One 2012, 7 (12). DOI: 10.1371/journal.pone.0051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Denisin AK; Pruitt BL Tuning the Range of Polyacrylamide Gel Stiffness for Mechanobiology Applications. ACS Appl. Mater. Interfaces 2016, 8 (34), 21893–21902. DOI: 10.1021/acsami.5b09344. [DOI] [PubMed] [Google Scholar]
- (24).Syed S; Karadaghy A; Zustiak S Simple Polyacrylamide-Based Multiwell Stiffness Assay for the Study of Stiffness-Dependent Cell Responses. J. Vis. Exp. 2015, No. 97 DOI: 10.3791/52643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yang J; Webb AR; Pickerill SJ; Hageman G; Ameer GA Synthesis and Evaluation of Poly(Diol Citrate) Biodegradable Elastomers. Biomaterials 2006, 27 (9), 1889–1898. DOI: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- (26).Yang J; Webb AR; Ameer GA Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Adv. Mater. 2004, 16 (6), 511–516. DOI: 10.1002/adma.200306264. [DOI] [Google Scholar]
- (27).Mason BN; Starchenko A; Williams RM; Bonassar LJ; Reinhart-King CA Tuning Three-Dimensional Collagen Matrix Stiffness Independently of Collagen Concentration Modulates Endothelial Cell Behavior. Acta Biomater. 2013, 9 (1), 4635–4644. DOI: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Yamamura N; Sudo R; Ikeda M; Tanishita K Effects of the Mechanical Properties of Collagen Gel on the In Vitro Formation of Microvessel Networks by Endothelial Cells. Tissue Eng. 2007, 13 (7), 1443–1453. DOI: 10.1089/ten.2006.0333. [DOI] [PubMed] [Google Scholar]
- (29).Barnes JM; Przybyla L; Weaver VM Tissue Mechanics Regulate Brain Development, Homeostasis and Disease. J. Cell Sci. 2017, 130 (1), 71–82. DOI: 10.1242/jcs.191742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wood JA; Liliensiek SJ; Russell P; Nealey PF; Murphy CJ Biophysical Cueing and Vascular Endothelial Cell Behavior. Materials (Basel). 2010, 3 (3), 1620–1639. DOI: 10.3390/ma3031620. [DOI] [Google Scholar]
- (31).Liliensiek SJ; Nealey P; Murphy CJ Characterization of Endothelial Basement Membrane Nanotopography in Rhesus Macaque as a Guide for Vessel Tissue Engineering. Tissue Eng Part A 2009, 15 (9), 2643–2651. DOI: 10.1089/ten.TEA.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Gillies AR; Lieber RL Structure and Function of the Skeletal Muscle Extracellular Matrix. Muscle Nerve 2011, n/a–n/a. DOI: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chien S Mechanotransduction and Endothelial Cell Homeostasis: The Wisdom of the Cell. Am. J. Physiol. Circ. Physiol. 2007, 292 (3), H1209–H1224. DOI: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- (34).Birukov KG; Jacobson JR; Flores AA; Ye SQ; Birukova AA; Verin AD; Garcia JGN Magnitude-Dependent Regulation of Pulmonary Endothelial Cell Barrier Function by Cyclic Stretch. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285 (4), L785–L797. DOI: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- (35).Waters CM; Roan E; Navajas D Mechanobiology in Lung Epithelial Cells: Measurements, Perturbations, and Responses In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. DOI: 10.1002/cphy.c100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Stojkovska J; Bugarski B; Obradovic B Evaluation of Alginate Hydrogels under in Vivo–like Bioreactor Conditions for Cartilage Tissue Engineering. J. Mater. Sci. Mater. Med. 2010, 21 (10), 2869–2879. DOI: 10.1007/s10856-010-4135-0. [DOI] [PubMed] [Google Scholar]
- (37).Eberl C; Thompson R; Gianola D; Bundschuh S Digital Image Correlation and Tracking with Matlab Version 1.2.0.0. MathWorks File Exchange 2010. [Google Scholar]
- (38).Simmons CS; Ribeiro AJS; Pruitt BL Formation of Composite Polyacrylamide and Silicone Substrates for Independent Control of Stiffness and Strain. Lab Chip 2013, 13 (4), 646 DOI: 10.1039/c2lc41110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Fischer RS; Myers KA; Gardel ML; Waterman CM Stiffness-Controlled Three-Dimensional Extracellular Matrices for High-Resolution Imaging of Cell Behavior. Nat. Protoc. 2012, 7 (11), 2056–2066. DOI: 10.1038/nprot.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Tse JR; Engler AJ Preparation of Hydrogel Substrates with Tunable Mechanical Properties. Curr. Protoc. Cell Biol. 2010, 47 (1), 10.16.1–10.16.16. DOI: 10.1002/0471143030.cb1016s47. [DOI] [PubMed] [Google Scholar]
- (41).Goins A; Ramaswamy V; Lichlyter D; Webb A; Allen JB Fabrication of a Bilayer Scaffold for Small Diameter Vascular Applications. J. Biomed. Mater. Res. Part A 2018, 106 (11), 2850–2862. DOI: 10.1002/jbm.a.36473. [DOI] [PubMed] [Google Scholar]
- (42).Dulany K; Goins A; Kelley A; Allen JB Fabrication of a Free Radical Scavenging Nanocomposite Scaffold for Bone Tissue Regeneration. Regen. Eng. Transl. Med. 2018, 4 (4), 257–267. DOI: 10.1007/s40883-018-0067-x. [DOI] [Google Scholar]
- (43).Motlagh D; Allen J; Hoshi R; Yang J; Lui K; Ameer G Hemocompatibility Evaluation of Poly(Diol Citrate)in Vitro for Vascular Tissue Engineering. J. Biomed. Mater. Res. Part A 2007, 82A (4), 907–916. DOI: 10.1002/jbm.a.31211. [DOI] [PubMed] [Google Scholar]
- (44).Baler K; Ball JP; Cankova Z; Hoshi RA; Ameer GA; Allen JB Advanced Nanocomposites for Bone Regeneration. Biomater. Sci. 2014, 2 (10), 1355 DOI: 10.1039/C4BM00133H. [DOI] [PubMed] [Google Scholar]
- (45).Li B; Chen J; Wang JH-C RGD Peptide-Conjugated Poly(Dimethylsiloxane) Promotes Adhesion, Proliferation, and Collagen Secretion of Human Fibroblasts. J. Biomed. Mater. Res. Part A 2006, 79A (4), 989–998. DOI: 10.1002/jbm.a.30847. [DOI] [PubMed] [Google Scholar]
- (46).Ramaswamy V; Monsalve A; Sautina L; Segal MS; Dobson J; Allen JB DNA Aptamer Assembly as a Vascular Endothelial Growth Factor Receptor Agonist. Nucleic Acid Ther. 2015, 25 (5), 227–234. DOI: 10.1089/nat.2014.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Günay KA; Ceccato TL; Silver JS; Bannister KL; Bednarski OJ; Leinwand LA; Anseth KS PEG–Anthracene Hydrogels as an On-Demand Stiffening Matrix To Study Mechanobiology. Angew. Chemie 2019, ange.201901989. DOI: 10.1002/ange.201901989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Rosales AM; Vega SL; DelRio FW; Burdick JA; Anseth KS Hydrogels with Reversible Mechanics to Probe Dynamic Cell Microenvironments. Angew. Chemie Int. Ed. 2017, 56 (40), 12132–12136. DOI: 10.1002/anie.201705684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Toepke MW; Beebe DJ PDMS Absorption of Small Molecules and Consequences in Microfluidic Applications. Lab Chip 2006, 6 (12), 1484 DOI: 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- (50).Regehr KJ; Domenech M; Koepsel JT; Carver KC; Ellison-Zelski SJ; Murphy WL; Schuler LA; Alarid ET; Beebe DJ Biological Implications of Polydimethylsiloxane-Based Microfluidic Cell Culture. Lab Chip 2009, 9 (15), 2132 DOI: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sinha R; Le Gac S; Verdonschot N; van den Berg A; Koopman B; Rouwkema J Endothelial Cell Alignment as a Result of Anisotropic Strain and Flow Induced Shear Stress Combinations. Sci. Rep. 2016, 6 (1), 29510 DOI: 10.1038/srep29510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lane WO; Jantzen AE; Carlon TA; Jamiolkowski RM; Grenet JE; Ley MM; Haseltine JM; Galinat LJ; Lin F-H; Allen JD; et al. Parallel-Plate Flow Chamber and Continuous Flow Circuit to Evaluate Endothelial Progenitor Cells under Laminar Flow Shear Stress. J. Vis. Exp. 2012, No. 59 DOI: 10.3791/3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Wipff P-J; Majd H; Acharya C; Buscemi L; Meister J-J; Hinz B The Covalent Attachment of Adhesion Molecules to Silicone Membranes for Cell Stretching Applications. Biomaterials 2009, 30 (9), 1781–1789. DOI: 10.1016/j.biomaterials.2008.12.022. [DOI] [PubMed] [Google Scholar]
- (54).Feng S; Mao S; Zhang Q; Li W; Lin J-M Online Analysis of Drug Toxicity to Cells with Shear Stress on an Integrated Microfluidic Chip. ACS Sensors 2019, 4 (2), 521–527. DOI: 10.1021/acssensors.8b01696. [DOI] [PubMed] [Google Scholar]
- (55).Savoji H; Mohammadi MH; Rafatian N; Toroghi MK; Wang EY; Zhao Y; Korolj A; Ahadian S; Radisic M Cardiovascular Disease Models: A Game Changing Paradigm in Drug Discovery and Screening. Biomaterials 2019, 198, 3–26. DOI: 10.1016/j.biomaterials.2018.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.