Abstract
Objective:
We aimed to test for an association between the amount of circulating fetal cell-free DNA and trisomy, and whether NIPS failure due to low fetal fraction indicates trisomy risk.
Method:
Maternal BMI, maternal age, fetal sex, gestational age, fetal cfDNA fraction, and NIPS results was collected on 2,374 pregnancies. Additional clinical information was available for 1,180 research consented patients. We investigated associations between fetal fraction and available variables and determined the success rate of repeat NIPS testing.
Results:
Fetal trisomy was marginally associated with decreased fetal fraction (p = 0.067). However, the proportions of trisomy events were not significantly increased in women who had failed NIPS due to low fetal fraction (< 4%) (OR = 1.37 [0.3 – 7.4]; p = 0.714). 66% of repeated NIPS after a second blood draw were successful.
Conclusion:
Failure to meet the clinical cutoff of 4% fetal fraction established for NIPS accuracy did not suggest increased risk for trisomy in our cohort. Because repeat testing was successful in the majority of cases and most failures were explained by high BMI and low gestational age, a redraw may be an appropriate next step before invasive screening due to concerns for trisomic pregnancies.
Keywords: Non Invasive Prenatal Screening, NIPS, cffDNA, trisomy, fetal fraction, aneuploidy
Introduction
Aneuploidy—the presence of an abnormal number of chromosomes—is the leading cause of pregnancy loss, responsible for over 25% of all miscarriages1. The few types of autosomal aneuploidies that are compatible with life lead to intellectual and developmental disabilities. The consequence of a sex chromosome aneuploidy can range from embryonic lethality (e.g. monosomy X) to very minor phenotypes (e.g. XXX and XXY)2. Detecting aneuploidy prenatally is useful to informed pregnancy management and family planning. Traditional cytogenetic analysis of a fetus entails assaying nucleated fetal cells collected by amniocentesis or chorionic villus sampling. However, these procedures are invasive and may pose a small risk of miscarriage3. Recent advances in technologies allow for non-invasive prenatal screening (NIPS) of fetal aneuploidies, via a maternal blood draw. This technique is possible because the placenta, which is fetal in origin, sheds cell-free DNA (cfDNA) into the maternal circulation4, with cell-free fetal DNA (cffDNA) comprising approximately 10–12% of the maternal plasma sample during pregnancy5, though this value can vary greatly by individual pregnancy. NIPS can be performed as early as 10 weeks of gestation.
A common approach to NIPS employs Next Generation Sequencing (NGS) methodologies to obtain depth-based counts of each chromosome present in the maternal plasma sample. Aneuploidy is apparent by a marked increase (trisomy) or decrease (monosomy) in coverage relative to that expected for any particular chromosome6. Genetic testing laboratories that offer NIPS typically screen for trisomies 13, 18, and 21, in addition to sex chromosome aneuploidies. Currently, the use of NIPS for microdeletions, microduplications, or autosomal aneuploidies other than 13, 18 and 21 are not recommended7. In a general obstetric population, a meta-analysis found a pooled sensitivity of 95.9% for trisomy 21 , 86.5% for trisomy 18, and 77.5% for trisomy 13, all with a specificity approaching 100%8. The positive predictive values (PPV) varies by chromosome and risk category (high versus low) but is substantially greater for commonly tested autosomes compared to sex chromosomes (trisomy 21 = 82% ,monosomy X = 26%)9. Therefore, NIPS is not considered a diagnostic test requires the confirmation by amniocentesis or chorionic villus sampling. Due to success of this screening technique, current guidelines recommend that all pregnant women be offered the option of cffDNA screening tests10.
Since both fetal and maternal DNA are present in the maternal plasma sample, accurate prenatal assessment depends largely on the fraction of fetal cfDNA present. The cutoff requirement for fetal fraction is dependent on the test provider. For most testing laboratories, the cutoff fraction for confidence in analysis is 4% fetal cfDNA. Samples containing less than 4% fetal cfDNA are typically considered not reportable due to lowered specificity and sensitivity6,11. Several factors contribute to a decreased fetal fraction, including a high maternal Body Mass Index (BMI) and earlier gestational age12. Of significance, some studies have indicated that the presence of trisomy may result in decreased fetal fraction12,13. The observation of decreased fetal fraction in aneuploid pregnancies has prompted guidelines suggesting pregnant women with failed NIPS screening due to low fetal fraction be directly offered invasive testing rather than a second cfDNA screening7. However, because NIPS is becoming more widely available, and invasive tests are costly, may not be readily available to all patients, and may pose a risk to the fetus3, we aimed to evaluate the clinical utility of NIPS retesting after initial failure due to low fetal fraction. Specifically, we investigated the percentage of NIPS failures that are successful after a second blood draw and whether a NIPS failure due to low fetal fraction is indicative of increased trisomy risk.
Methods
Information including maternal BMI, maternal age, fetal sex, gestational age, fetal cfDNA fraction, and NIPS test results was collected at the time of NIPS testing on a cohort of 2,374 pregnancies referred to the Mayo Clinic Genomics Laboratory between January 2016 and September 2018. Additional clinical information was available for 1,181 research consented patients who delivered at Mayo Clinic during this period. IRB approval was waived for this retrospective data collection that did not include patient identifiers. The Mayo Clinic Investigational Review Board approved all methods related to the acquisition of medical records for these subjects (IRB #: 18–003290) and all subjects were at least 18 years of age. Abstracted data was collected and managed using a REDCap database14. Clinical variables recorded included the presence of fetal trisomy, maternal smoking status, the occurrence of intrauterine growth restriction (IUGR) or intrauterine fetal demise (IUFD), preeclampsia, gestational hypertension, and pregnancies achieved via advanced reproductive technologies (ART). Additionally, we obtained information regarding follow up prenatal testing strategies.
For each maternal plasma sample, circulating cffDNA was purified from the plasma component of anticoagulated maternal whole blood and converted into a genomic DNA library for sequencing. Fetal fraction was determined by a proprietary read count based on multivariate regression model (SeqFF) developed by Sequenom 15. Women who had a non-reportable test due to low fetal fraction (< 4%) were offered retesting after a second blood draw and outcomes were recorded. For samples ≥ 4% fetal fraction, sequencing reads were aligned to the human genome, and a Z-score generated for chromosomes 13, 18, 21 and X. Each sample was also evaluated for the presence or absence of Y chromosome to determine sex.
For the statistical analysis, we excluded two participants whose BMI were considered abnormally high (i.e. > 60) and six participants with NIPS testing failed due to a negative fetal fraction value, likely due to an algorithmic issue. We first conducted linear regression analyses to investigate the effect of well described confounders BMI and gestational age on fetal fraction12. Additional clinical data allowed for the investigation of various putative confounders, including maternal age, fetal sex, smoking status, use of ART to achieve pregnancy, intrauterine fetal demise (IUFD), intrauterine growth restriction (IUGR), preeclampsia, and hypertension. We conducted linear regression analyses to test for associations between these aforementioned clinical data and fetal fraction. Next, we conducted Firth’s logistic regression analyses to determine whether the proportions of trisomy events differed between women who failed versus passed NIPS test (< 4% vs ≥ 4%), controlling for BMI and gestational age. We used Firth’s penalized likelihood method16 to adjust for separation bias due to low prevalence of patients with fetal fraction below 4% who also had a trisomy event.
Results
From the full cohort (n = 2,374), 95% of patients (n = 2,264) had a fetal fraction above the established cutoff of 4%. Of the 5% of women (n = 110) with fetal fraction < 4%, 59% (n = 65) opted for a second blood draw for retesting. The average time between first draw and second draw was 18.3 days (SD = 8.2). Of those retested, 66% (n = 43) had a fetal fraction above 4% and received a result. All 43 successfully retested pregnancies were not indicated to have aneuploidy according to medical chart review. Figure 1 depicts outcomes of those who failed their second NIPS test or opted out of a second NIPS test (n = 67). Fetal outcome information was available for an additional 30 research consented pregnancies; 28 of which were negative for aneuploidy, one was positive for trisomy 18 and another was triploid. Information regarding the decision to undergo invasive screening was available for 48 pregnancies that initially had NIPS fail due to low fetal fraction. Two of 48 (4%) opted for invasive testing. Tables 1 and 2 summarize baseline patient characteristics for all patients (n = 2,366) and research-consented patients (n = 1,180) included in the statistical analysis, respectively, using counts, percentages, mean, standard deviation, median, and quartiles by NIPS results (i.e. pass or fail).
Figure 1. Study flow diagram.
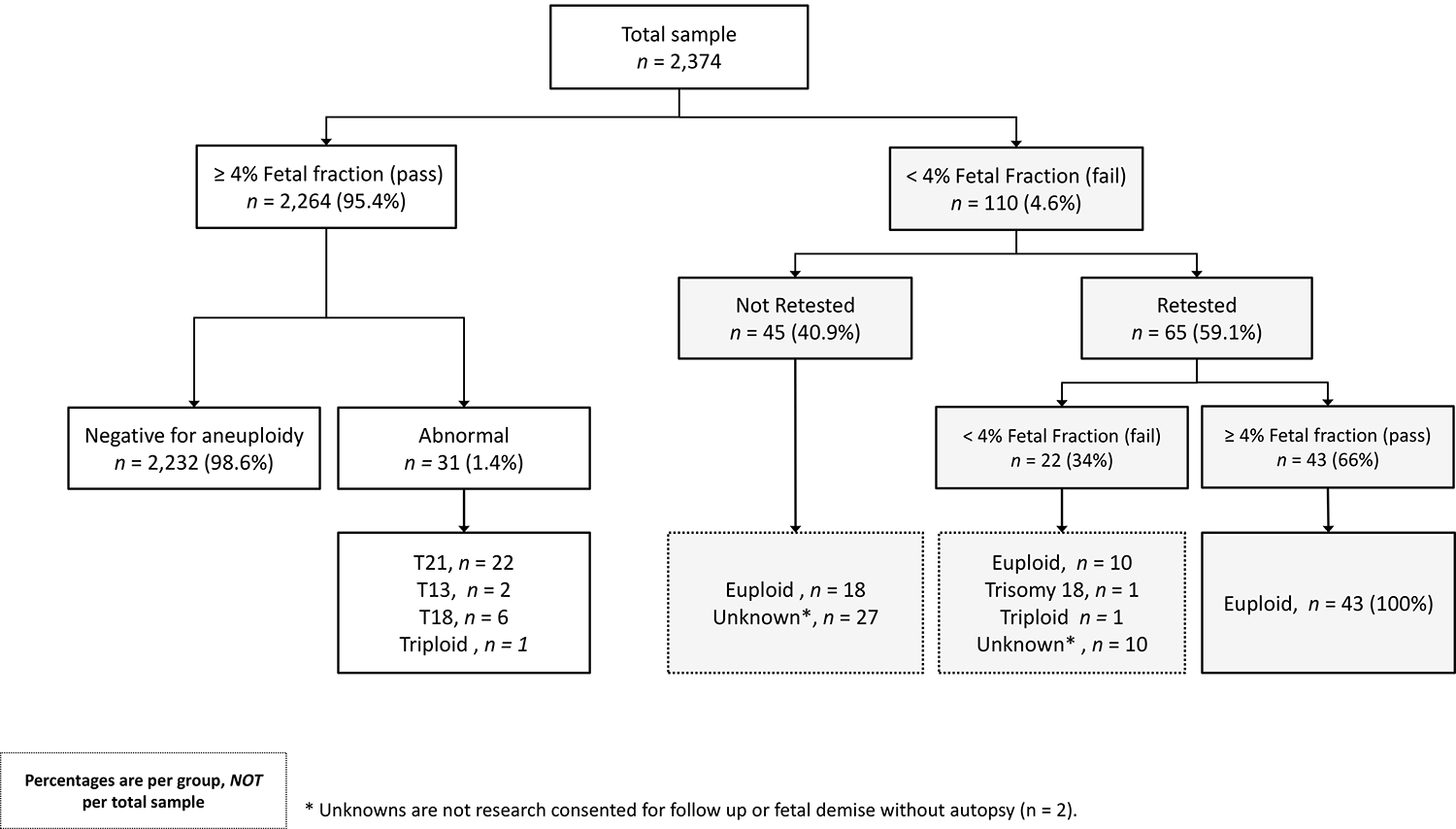
Percentages are per group, not per total sample. Unknowns were not research consented for follow up or fetal demise without autopsy (n = 2).
Table 1.
Baseline characteristics of patients included in statistical analysis (n = 2,366). Two triploid events were included in the trisomy category, with one triploid event the NIPS pass and one in the NIPS fail sub cohorts. Percentage of trisomy was calculated from a total of 71 as unknowns were excluded.
| NIPS results | |||
|---|---|---|---|
| Total | Fail | Pass | |
| (n = 2,366) | (n = 103) | (n = 2,263) | |
| Fetal fraction | |||
| Mean | 9.4 | 2.8 | 9.7 |
| SD | 4.3 | 0.8 | 4.2 |
| Median | 8.8 | 2.9 | 9.0 |
| Q1 – Q3 | 6.6 – 11.6 | 2.3 – 3.3 | 6.9 – 11.8 |
| BMI | |||
| Mean | 27.4 | 34.3 | 27.1 |
| SD | 6.6 | 7.6 | 6.3 |
| Median | 25.9 | 34.3 | 25.7 |
| Q1 – Q3 | 22.5 – 31 | 30.1 – 39.4 | 22.3 – 30.5 |
| Gestational age (weeks) | |||
| Mean | 13.7 | 13.1 | 13.7 |
| SD | 4.34 | 3.11 | 4.4 |
| Median | 12.1 | 12.9 | 12.1 |
| Q1 – Q3 | 11.0 – 14.4 | 11.8 – 14.1 | 11 – 14.4 |
| Presence of trisomy | |||
| Yes (%) | 33 | 2 (2.8) | 31 (1.4) |
| No (%) | 2,296 | 69 (97.2) | 2,227 (98.6) |
| Unknown (%) | 37 | 32 | 5 |
| Fetal sex | |||
| Male (%) | 1,207 | 53 (51.5) | 1,154 (51.7) |
| Female (%) | 1,129 | 50 (48.5) | 1,079 (48.3) |
| Unknown (%) | 30 | 0 | 30 |
| Maternal age (years) | |||
| Mean | 32.3 | 32.9 | 32.3 |
| SD | 5.2 | 5.2 | 5.2 |
| Median | 33 | 34 | 33 |
| Q1 – Q3 | 29 – 36 | 30 – 37 | 29 – 36 |
Table 2.
Baseline characteristics of research-consented patients at the time of NIPS testing (n = 1,180). IUFD includes spontaneous abortions.
| NIPS results | |||
|---|---|---|---|
| Total | Fail | Pass | |
| (n = 1,180) | (n = 48) | (n = 1,132) | |
| IUFD | |||
| Yes (%) | 19 | 2 (4.2) | 17 (1.5) |
| No (%) | 1,148 | 46 (95.8) | 1,102 (98.5) |
| Unknown (%) | 14 | 0 | 13 |
| IUGR | |||
| Yes (%) | 35 | 1 (2.1) | 34 (3.0) |
| No (%) | 1,132 | 47 (97.9) | 1,085 (97.0) |
| Unknown (%) | 13 | 0 | 13 |
| Pregnancy achieved via ART | |||
| Yes (%) | 134 | 7 (14.6) | 127 (11.4) |
| No (%) | 1030 | 41 (85.4) | 989 (88.6) |
| Unknown (%) | 16 | 0 | 16 |
| Smoking status | |||
| Yes (%) | 90 | 4 (8.3) | 86 (7.7) |
| No (%) | 1,073 | 44 (91.7) | 1,029 (92.3) |
| Unknown (%) | 17 | 0 | 17 |
Consistent with previous findings, fetal fraction was negatively associated with BMI (p < 0.001; Figure 2A) and positively associated with gestational age (p < 0.001; Figure 2B). Fetal fraction was not significantly associated with maternal age, fetal sex, IUFD, IUGR, preeclampsia, hypertension, use of advanced reproductive technologies to achieve pregnancy, or smoking status in our research consented cohort of 1,180. We then investigated the associations between fetal fraction and presence of trisomy, controlling for BMI and gestational age (Table 2). There was a total of 33 trisomy events in our cohort including trisomy 21 (n = 21), trisomy 18 (n = 7), trisomy 13 (n = 2) and triploidy (n = 2). The presence of trisomy was associated with decreased fetal fraction at a marginal level (p = 0.067). Women carrying a fetus with trisomy had a mean fetal fraction of 8.26% (SD = 4.25) versus 9.55% (SD = 4.28) in those with euploid pregnancies (Figure 3).
Figure 2. Associations between fetal fraction and BMI (A) and gestational age (B).
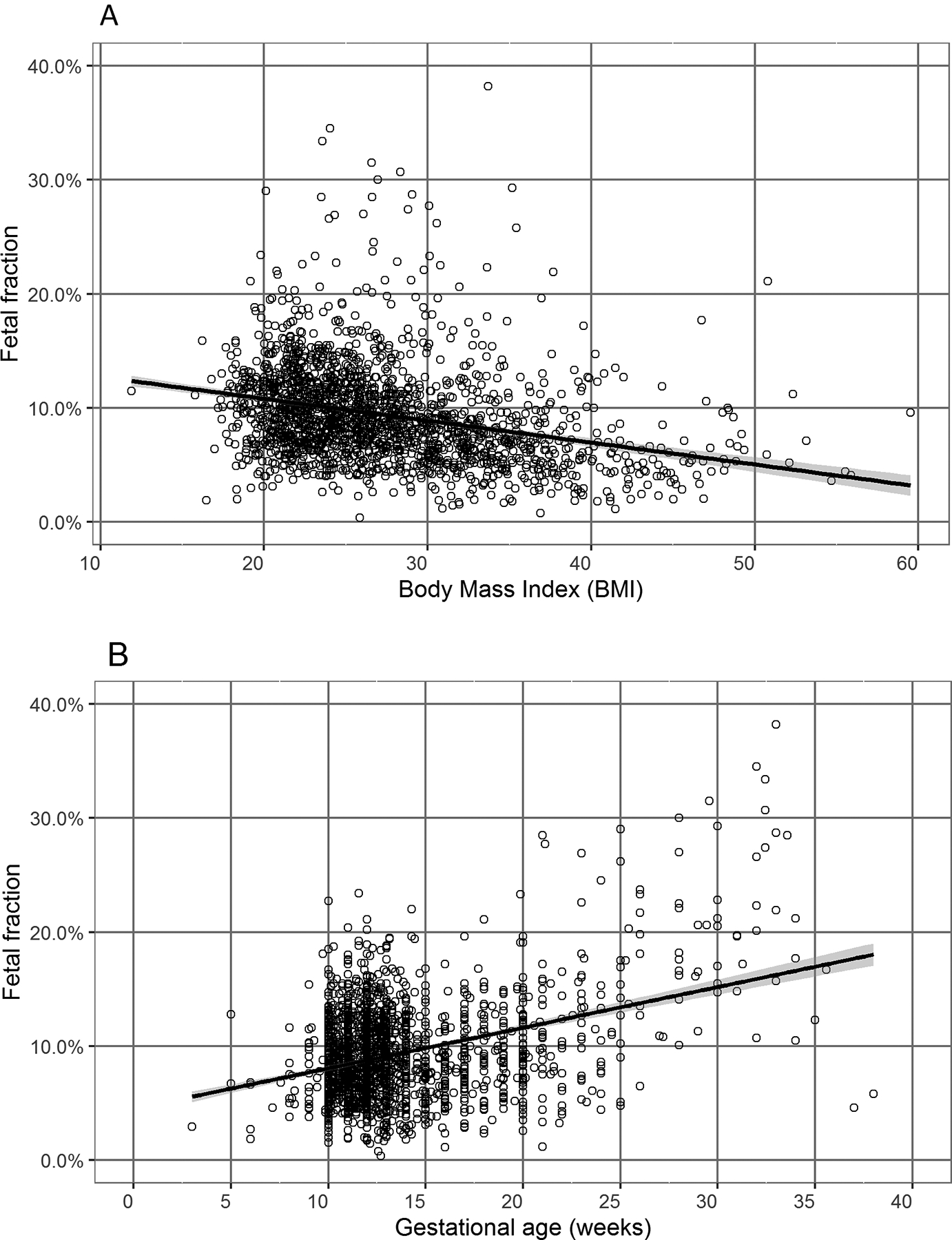
Fetal fraction was negatively associated with BMI (p < 0.001; Figure 2A) and positively associated with gestational age (p < 0.001; Figure 2B).
Figure 3. Fetal fraction of pregnancies with vs. without trisomy grouped by pass or failed NIPS.
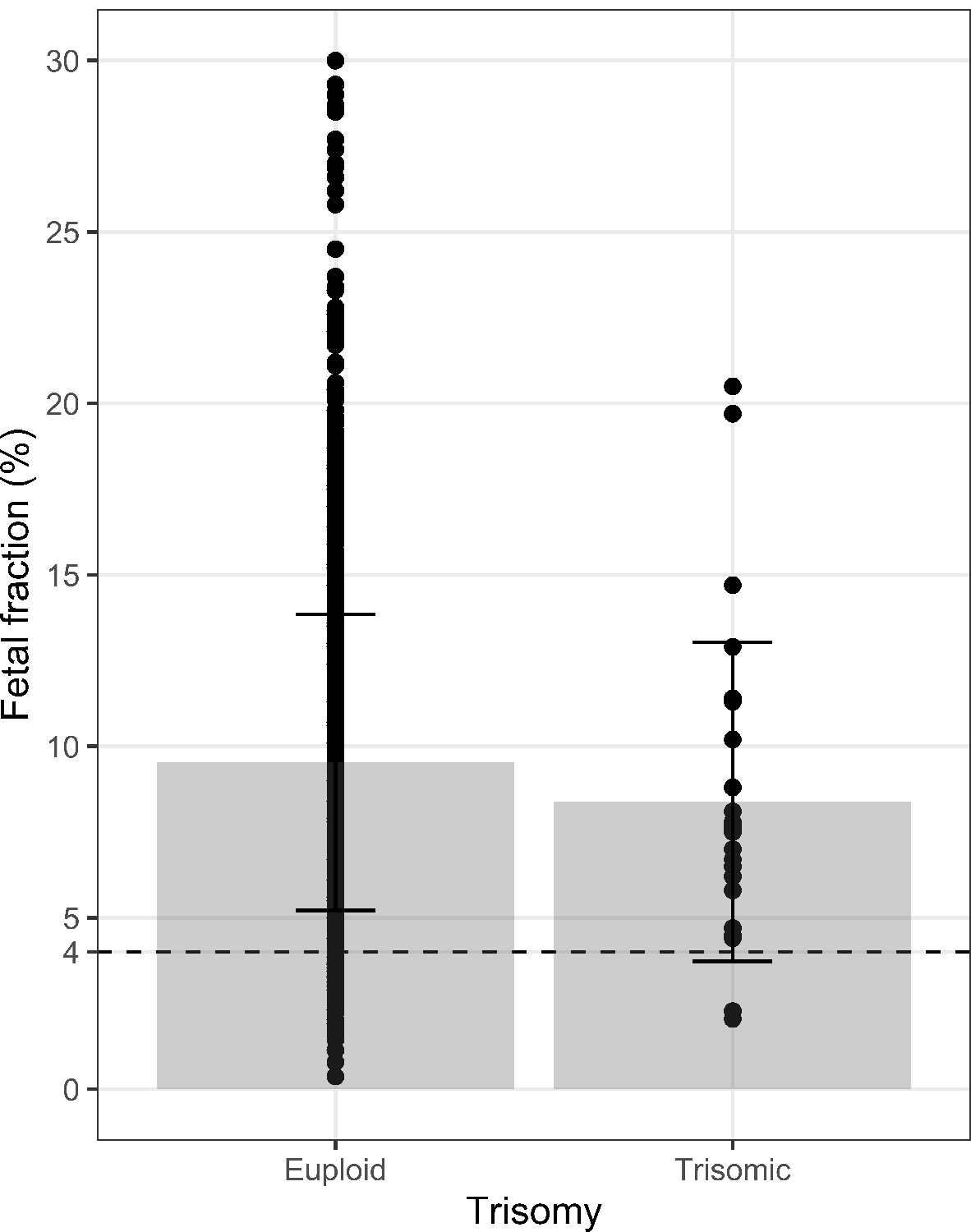
Trisomy pregnancies M = 8.26%; SD = 4.25 versus euploid pregnancies M = 9.55%; SD = 4.28, 4% fetal fraction cut off indicated by dotted line).
The moderate association between decreased fetal fraction and risk for a trisomy event uncovered in our cohort is consistent with other published findings12,13. Observations such as these have raised concern for women who received a failed NIPS result due to low fetal fraction, as they may be at increased risk for aneuploidy. We therefore conducted a Firth’s logistic regression analysis to investigate associations between presence of trisomy and NIPS results, i.e., pass (> 4% cffDNA) versus failed (> 4% cffDNA). NIPS failure was associated with increased BMI (p = 0.002) but not with gestational age (p = 0.448). Nonetheless, we retained gestational age in subsequent regression models because gestational age was positively associated with fetal fraction (p < 0.001). We then investigated whether women with failed NIPS due to low fetal fraction (< 4%) were at increased risk of fetal trisomy, controlling for BMI and gestational age. The results indicated that the proportion of trisomy events among women who failed NIPS test due to fetal fraction below 4% (2.8%, or 2 out of 71) were not significantly higher (OR = 1.37 [0.3 – 7.4]; p = 0.714) than those above the clinical cut off of 4% (1.4%, or 31 out of 2,258). Although the presence of trisomy may be moderately associated with a reduction in fetal fraction, the NIPS assay fetal fraction cutoff of 4% (employed for sensitivity reasons) is not helpful in distinguishing pregnancies with trisomies from those without (Figure 3). Our results also suggest that BMI is the single most important predictor of a failed NIPS test due to low fetal fraction, with 74% of samples with inadequate cffDNA (vs. 27% of samples with adequate cffDNA) being from clinically obese patients (Figure 4). Patients who benefited to retesting after had a lower mean BMI and later calculated gestational age compared to those with two consecutive NIPS failures (BMI = 32.9 (SD 7.5) GA = 14.9 (SD 3.21) and BMI = 36.5 (SD 8.5) GA = 13.9 (SD 2.05), respectively).
Figure 4. The majority of NIPS failures could be explained by high BMI.
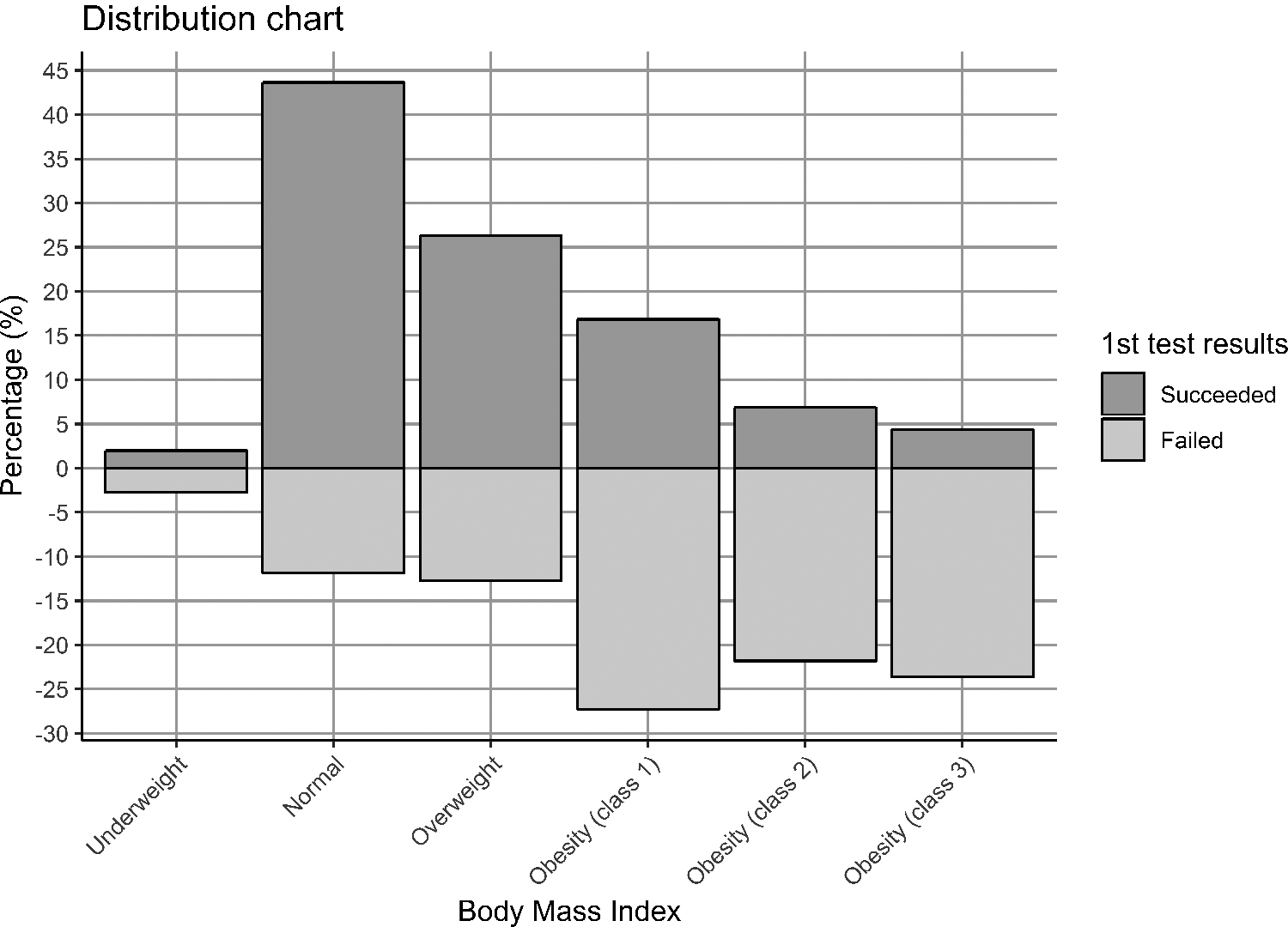
Obesity class as defined by CDC; Underweight: < 18.5, Normal: 18.5–24.9, Overweight: 25.0–29.9, Obesity class 1: 30.0–34.9, Obesity class 2: 35.0–39.9, Obesity class 3: 40.0 +.
Discussion
CfDNA-based NIPS for trisomies 13, 18 and 21 is currently recognized as the most sensitive prenatal screening tool for trisomy events and has a high success rate. On average, fetal cfDNA comprises of 10–12% of the maternal plasma sample during pregnancy, and increases with gestational age5. A widely accepted cffDNA fraction required for a sensitive NIPS result is 4%6,11. However, the decision to use 4% as a cutoff for fetal fraction is dependent on the test provider. In our cohort, 95% of patients had a fetal fraction above this established cutoff. Although the majority of NIPS tests are successful, a small percentage fail due to low fetal fraction. Previous studies have observed the association between decreased fetal fraction and trisomy12,13 while others observe non-significant trends17. Therefore, valid concerns for increased aneuploidy risk have been proposed in the case of failure due to low fetal fraction. Various factors contribute to a low fetal fraction, most notably high BMI and early gestational age12. Consistent with previous findings, fetal fraction was negatively associated with BMI and positively associated with gestational age among our study samples. Because these variables heavily influence the proportion of circulating cffDNA in the maternal bloodstream, taking maternal BMI and gestational age into consideration would likely lead to a more accurate risk assessment for women with failed NIPS due to low fetal fraction18.
The biological explanation for the decrease in fetal fraction observed in trisomy pregnancies is poorly understood. There is speculation that placental health, which may be compromised in these pregnancies, could affect the levels of cffDNA in maternal blood19. For this reason, we investigated whether the association between fetal fraction and presence of trisomy could be potentially confounded by other clinical events that may be indicative of poor placental health, such as IUGR, eventual IUFD, preeclampsia, and gestational hypertension. We also investigated whether fetal fraction was influenced by pregnancies achieved through ART, smoking status, maternal age, and/or fetal sex. Our results indicated no such associations after controlling for maternal BMI and gestational age. Future research may be warranted to further investigate the role of these variables with circulating cffDNA in maternal blood.
The possible increased risk of trisomic pregnancies in women with low fetal fraction have prompted guidelines for women with failed NIPS testing due to inadequate cffDNA to be recommended invasive procedures for diagnostic testing while rescreening by NIPS is discouraged7. Because invasive testing is costly, may not be easily accessible to all patients, and may pose a risk to the fetus, research investigating cffDNA screening test outcomes is vital to the development of an accurate standard procedure for identifying women who may benefit from a second attempt at NIPS. It is important to note that there are various circumstances when a second screen following a failed NIPS result would not be recommended. If the assay was performed at an appropriate gestational age and in a woman of average BMI the provider may choose to first offer diagnostic testing, especially for cases in which timing may be critical. However, we found that many patients declined diagnostic testing following a failed NIPS result, despite current recommendations. Patient choice and provider discretion will vary based on individual circumstances.
The results of our study indicate that decreased fetal fraction is only marginally associated with the presence of trisomy. The strength of the association uncovered in our cohort may be understated as limited sample sizes of affected trisomies precluded us from investigating specific trisomies individually. Some studies have shown that decreased fetal fraction is only is associated only with trisomies 18 and 13, while it may even be slightly increased in trisomy 21 18,20,21. However, the majority of affected samples in our cohort were still well above the 4% fetal fraction that most laboratories currently require for a successful NIPS interpretation. Unknown outcomes were not research consented for follow up. We therefore could not assess pregnancy outcomes of those cases. Although some unknowns may be aneuploidy, they are most likely to be associated with the mother’s high BMI as the average BMI of women who had two consecutive failures was 36.5. Of the women with failed NIPS test who decided to have further testing, many opted for a second NIPS versus an invasive option. Additionally, the majority of failed NIPS cases could be explained by high BMI or early gestational age and 66% of NIPS re-draws were successful with results available in approximately two weeks after testing. In conclusion, although decreased fetal fraction may be associated with fetal trisomy, failure to meet the recommended clinical cutoff of 4% fetal fraction established for NIPS accuracy is not a reliable predictor of increased risk. Therefore, for many cases of failed NIPS due to inadequate cffDNA, counseling patients on the option of a redraw may be an appropriate next step prior to invasive screening.
Bulleted statements:
What’s already known about this topic?
Fetal trisomy is associated with decreased cell-free fetal DNA during pregnancy.
Women with NIPS failures due to low fetal fraction are recommended invasive testing over re-screening.
What does this study add?
Fetal fraction was decreased in trisomy pregnancies, however the effect was minimal.
NIPS failures were mostly explained by high BMI/early gestational age.
Women with failed NIPS due to low fetal fraction were not at increased risk of trisomy and many repeated screens were successful (66%).
Funding:
Funding support for this project was provided by the NIH HD065987 (EALE).
Footnotes
Conflict of interest statement:
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Data availability:
Data available on request from corresponding author.
References
- 1.Hassold T, Abruzzo M, Adkins K, et al. Human Aneuploidy : Incidence , Origin , and Etiology. 1996;175(1 996):167–175. [DOI] [PubMed] [Google Scholar]
- 2.Visootsak J, Jr JMG. Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J Rare Dis. 2006;1(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akolekar R, Beta J, Picciarelli G, et al. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling : a systematic review and meta-analysis. Ultrasound Obs Gynecol. 2015;45:16–26. [DOI] [PubMed] [Google Scholar]
- 4.Flori E, Doray B, Gautier E, et al. Circulating cell-free fetal DNA in maternal serum appears to originate from cyto- and syncytio-trophoblastic cells . Case report. Hum Reprod. 2004;19(3):723–724. [DOI] [PubMed] [Google Scholar]
- 5.Dar P, Curnow KJ, Gross SJ, et al. Clinical experience and follow-up with large scale single-nucleotide polymorphism based noninvasive prenatal aneuploidy testing. Am J Obstet Gynecol. 2014;211(5):527.e1–527.e17. [DOI] [PubMed] [Google Scholar]
- 6.Benn P Non-Invasive Prenatal Testing Using Cell Free DNA in Maternal Plasma : Recent Developments and Future Prospects. J Clin Med. 2014;3:537–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy , 2016 update : a position statement of the American College of Medical Genetics and Genomics. 2016;18(10). [DOI] [PubMed] [Google Scholar]
- 8.Taylor-phillips S, Freeman K, Geppert J, et al. Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down , Edwards and Patau syndromes : a systematic review and meta-analysis. BMJ Open. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi DW. Turner syndrome : New insights from prenatal genomics and transcriptomics. Am J Med Genet Part C. 2019;181C:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy , 2016 update : a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18(10). [DOI] [PubMed] [Google Scholar]
- 11.Norton ME, Brar H, Weiss J, et al. Non-Invasive Chromosomal Evaluation ( NICE ) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(2):137.e1–137.e8. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Zhu Z, Gao Y, et al. Effects of Maternal and Fetal Characteristics on Cell-Free Fetal DNA Fraction in Maternal Plasma. Reprod Sci. 2015;22(11):1429–1435. [DOI] [PubMed] [Google Scholar]
- 13.Suzumori N, Ebara T, Yamada T, et al. Fetal cell-free DNA fraction in maternal plasma is affected by fetal trisomy. J Hum Genet. 2016;61(7):1–6. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, et al. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SK, Hannum G, Geis J, et al. Determination of fetal DNA fraction from the plasma of pregnant women using sequence read counts †. Prenat Diagn. 2015;(35):810–815. [DOI] [PubMed] [Google Scholar]
- 16.Firth D Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. [Google Scholar]
- 17.Hestand MS, Bessem M, Rijn P Van, et al. Fetal fraction evaluation in non-invasive prenatal screening ( NIPS ). Eur J Hum Genet. 2019;27:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKanna T, Ryan A, Krinshpun S, et al. Fetal fraction-based risk algorithm for non-invasive prenatal testing: screening for trisomies 13 and 18 and triploidy in women with low cell-free fetal DNA. Ultrasound Obstet Gynecol. 2019;53(1):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerson KD, Truong S, Haviland MJ, et al. Low fetal fraction of cell-free DNA predicts placental dysfunction and hypertensive disease in pregnancy. Pregnancy Hypertens. 2019;16(April):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rava RP, Srinivasan A, Sehnert AJ, et al. Circulating fetal cell-free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin Chem. 2014;60(1):243–250. [DOI] [PubMed] [Google Scholar]
- 21.Ashoor G, Syngelaki A, Poon LCY, et al. Fetal fraction in maternal plasma cell-free DNA at 11–13 weeks’ gestation: Relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41(1):26–32. [DOI] [PubMed] [Google Scholar]


