Abstract
Patient: Male, 72-year-old
Final Diagnosis: SARS-CoV-2
Symptoms: Dyspnea
Medication: Standard
Clinical Procedure: C-reactive protein apheresis
Specialty: Immunology
Objective:
Unusual clinical course
Background:
C-reactive protein (CRP) plasma levels in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel viral disease, are surprisingly high. Pulmonary inflammation with subsequent fibrosis in SARS-CoV-2 infection is strongly accelerated. Recently, we have developed CRP apheresis to selectively remove CRP from human plasma. CRP may contribute to organ failure and pulmonary fibrosis in SARS-CoV-2 infection by CRP-mediated complement and macrophage activation.
Case Report:
A 72-year-old male patient at high risk was referred with dyspnea and fever. Polymerase chain reaction analysis of throat smear revealed SARS-CoV-2 infection. CRP levels were ∼200 mg/L. Two days after admission, CRP apheresis using the selective CRP adsorber (PentraSorb® CRP) was started. CRP apheresis was performed via peripheral venous access on days 2, 3, 4, and 5. Following a 2-day interruption, it was done via central venous access on days 7 and 8. Three days after admission the patient was transferred to the intensive care unit and intubated due to respiratory failure. Plasma CRP levels decreased by ∼50% with peripheral (processed blood plasma ≤6000 mL) and by ∼75% with central venous access (processed blood plasma ≤8000 mL), respectively. No apheresis-associated side effects were observed. After the 2-day interruption in apheresis, CRP levels rapidly re-increased (>400 mg/L) and the patient developed laboratory signs of multi-organ failure. When CRP apheresis was restarted, CRP levels and creatinine kinases (CK/CK-MB) declined again. Serum creatinine remained constant. Unfortunately, the patient died of respiratory failure on day 9 after admission.
Conclusions:
This is the first report on CRP apheresis in a SARS-CoV-2 patient. SARS-CoV-2 may cause multi-organ failure in part by inducing an excessive CRP-mediated autoimmune response of the ancient innate immune system.
MeSH Keywords: Blood Component Removal, C-Reactive Protein, COVID-19, Multiple Organ Failure, Pulmonary Fibrosis, SARS Virus
Background
C-reactive protein (CRP), the prototype human acute phase protein, remains scientifically enigmatic. Whereas CRP, as part of the ancient humoral immune response, is generally considered to be immunoprotective [1], increasing evidence suggests that it may also be harmful under certain circumstances [2–5]. The major inductors of CRP synthesis are interleukin-1 (IL-1) and interleukin-6 (IL-6) [1–3]. Two well-described immunological CRP functions are (1) activation of the classical complement pathway via C1q binding [6] and (2) binding to human immunoglobulin Fcγ receptors (mainly FcγRIIa) after opsonization of biological particles for macrophages [7–9]. The latter may also significantly contribute to the removal of affected cells in the context of organ damage [10]. Notably, complement activation and binding to Fcγ receptors are both antibody functions. For this reason, it is likely that CRP was the first antibody-like molecule in the evolution of the mammalian immune system. As CRP, in binding to “antigens”, has functions different from antibodies, its effectiveness may depend on the unique and manifold increase in plasma concentration as observed during an acute phase response. Because CRP functions have been taken over by antibodies with time, CRP may well be an atavism in the human immune system [5].
A new public health crisis exists with the emergence and spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [11,12]. The virus originated in bats and, in December 2019, was obviously transmitted to human beings through yet unknown intermediary animals in Wuhan, Hubei province, China. SARS-CoV-2 is transmitted by contact with infected droplets or by inhalation [11,12]. The incubation period ranges from 2 to 14 days [13]. Many people are asymptomatic. Diagnosis is made through detection of the virus in respiratory secretions by PCR analysis. Symptoms include sore throat, cough, breathlessness, fever, fatigue, and malaise among others. The course of the disease is mild in most people; however, in some individuals (usually those with comorbidities and the elderly), the disease may progress to viral pneumonia and, finally, acute respiratory distress syndrome (ARDS) and multi-organ failure. The fatality rate is excessively high in intubated patients for several reasons [12]. Common laboratory findings, especially in fatal cases, include low lymphocyte counts and, given a viral disease, surprisingly high plasma levels of CRP [11–13]. As yet, there is no known vaccine or proven specific antiviral treatment [11–14]. Primary treatment is symptomatic and supportive.
Based on the assumption that CRP may contribute to organ failure and accelerated pulmonary fibrosis (by CRP-mediated complement and macrophage activation) [15] as well as abnormalities of surfactant function [16], we have used recently established selective CRP apheresis [4,10,17,18] as a method to efficiently and selectively remove CRP from the plasma of a SARS-CoV-2 patient at high risk.
Case Report
A 72-year-old man with symptoms of cough, breathlessness, and fever lasting for 7 days was referred to Kempten Hospital, Germany, by his general practitioner. Concomitant diseases included type 2 diabetes mellitus, arterial hypertension, prostate cancer (under active surveillance), peripheral arterial disease, ankylosing spondylitis (under treatment with sulfasalazine), chronic obstructive lung disease (on anti-obstructive medication), diffuse goiter, and chronic kidney disease (stage 3, creatinine 1.63 mg/dL, reference range 0.70–1.20 mg/dL; GFR 41.9 mL/min/1.73 m2). Thus, he was considered a high-risk SARS-CoV-2 patient. Interestingly, the patient did not report on any stay in a SARS-CoV-2 risk area or any contact with SARS-CoV-2-infected subjects. At admission, oxygen saturation was 85%. Laboratory values revealed excessive elevation of CRP plasma levels (206.3 mg/L, reference range 0.00–5.00 mg/L) without leukocytosis though lymphopenia of 10% (reference range 17–47%). Chest x-ray in bed revealed streaky infiltrates on both sides.
Two blood cultures were taken, SARS-CoV-2 and influenza PCR analysis of throat smear was initiated, and empiric antibiotic therapy with ampicillin/sulbactam (2/1) and clarithromycin was started. The patient was transferred to the infection ward. PCR results in the evening of admission day revealed SARS-CoV-2 positivity. Laboratory results 1 day after admission indicated previous infection with Chlamydia pneumoniae (AK IgM [EIA] <10 U/mL, standard value <10; reference range 10–15 U/mL; AK IgA [EIA]: 75 U/mL, standard value <10, reference range 10–13 U/mL; AK IgG [EIA] 74 U/mL, standard value <10, reference range 10–12 U/mL) as well as sero-negativity for Legionella pneumoniae and Mycoplasma pneumoniae. No bacterial or fungal infection was detected. Influenza PCR was negative.
Continuous CRP elevation on day 2 after admission (and on day 2 of antibiotic therapy) prompted us to begin with CRP apheresis under the premise of continuous standard therapy of SARSCoV-2 infection. After correspondence with the Ethical Review Committee of Ulm University, Germany, written informed consent for CRP apheresis (declared as an individual healing attempt) was obtained from the patient.
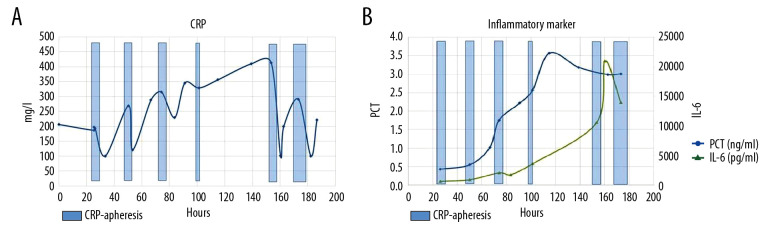
CRP apheresis using the selective CRP adsorber (PentraSorb® CRP, Pentracor GmbH, Germany) was started via peripheral venous access on days 2, 3, 4, and 5 after admission. Due to ARDS with consecutive respiratory exhaustion (Figure 1), the patient was transmitted to the intensive care unit (ICU) on day 3 after admission and intubated in the evening. ARDS category [19] during the ICU stay changed from moderate (PaO2/FIO2 ≤200 mmHg) to severe (PaO2/FIO2 ≤100 mmHg). Antibiosis was escalated to piperacillin/tazobactam. Ampicillin/sulbactam (2/1) and clarithromycin (no atypical bacteria) were stopped. Following a 48 h interruption of CRP apheresis due to uncertainty about continuation (treatment of acute myocardial infarction with maximum 3 consecutive CRP apheresis sessions [17,18]) and organizational difficulties caused by a necessary alternating change from ventral to dorsal patient position, we decided to continue CRP apheresis via central venous Shaldon catheter on day 7. Central venous access markedly improved efficiency of CRP elimination (Figure 2). The major reason to continue was the fact that laboratory parameters and clinical presentation indicating multi-organ failure increased after discontinuation of CRP apheresis (Figure 3).
Figure 1.
Supine chest x-ray on days 1 (A), 3 (B), and 7 (C) showing progressive bilateral infiltrates, predominant basal distribution, and development of fibrosing alveolitis.
Figure 2.
(A) CRP levels (reference range 0.00–5.00 mg/L) during the course of the patient’s hospital stay. CRP levels were highly elevated at admission and decreased with each CRP apheresis session. CRP apheresis sessions 1, 2, 3, and 4 (blue columns) used peripheral venous access (processed blood plasma ≤6000 mL) and sessions 5 and 6 (blue columns) used central venous access (processed blood plasma ≤8000 mL). (B) IL-6 and PCT levels during the course of the hospital stay. Both IL-6 and PCT increased with time, although the PCT increase was only moderate.
Figure 3.
(A) CRP apheresis sessions (blue columns) and course of CK/CK-MB and LDH plasma levels. Interpretation see text. (B) CRP apheresis sessions (blue columns) and course of bilirubin and creatinine levels as well as international normalized ratio (INR). Marked CK/CK-MB increase occurred during the interruption of CRP apheresis and marked CK/CK-MB decrease occurred following CRP apheresis restart.
In each apheresis session (with the exception of day 5 after admission), ≥6000 mL of plasma was treated. The course of CRP plasma levels and the course of other laboratory results are depicted in Figures 2–4. CRP apheresis efficiently counteracted CRP elevation and reduced CRP plasma levels (Figure 2). CRP apheresis obviously also influenced creatinine and bilirubin plasma levels, as well as creatinine-kinases (CK/CK-MB) and l-lactate-dehydrogenase (LDH) (Figure 3). Thus, 3 parameters determining the Sequential Organ Failure Assessment (SOFA) score [20] – bilirubin (1.58 mg/dL, reference range <1.2 mg/dL), creatinine (2.1 mg/dL, reference range 0.7–1.2 mg/dL), and platelet count (220 000/µL, reference range 146,000–328,000) remained relatively low until time of death. Respiratory parameters, however, did not improve (Figure 4). Extracorporeal membrane oxygenation, in this rapidly progressive disease with severely predamaged lungs and very poor prognosis, was not considered an option.
Figure 4.
(A) CRP apheresis sessions (blue columns) and course of Horovitz quotient [17]. Respiratory failure with time. (B) CRP apheresis sessions (blue columns) and course of lactate. Respiratory failure with time.
Unfortunately, the patient died of respiratory failure on day 9 after admission.
Discussion
Although CRP has been known since 1930 [21], the molecule’s role in the human immune system is only partly known. Paradoxically, in spite of its widespread clinical use, relatively little is known about CRP’s biological functions. Based on the assumption that CRP may indeed contribute to organ failure and accelerated pulmonary fibrosis by CRP-mediated complement and macrophage activation [15–19], we attempted to treat a high-risk SARS-CoV-2 patient with markedly elevated CRP plasma levels by efficient and selective CRP removal via CRP apheresis. To date, CRP apheresis is the only available method to selectively eliminate CRP from the human body.
Our patient received optimized standard therapy according to medical guidelines, best practice, and latest recommendations.
This therapy included lung protective ventilation with low tidal volumes, best positive end-expiratory pressure (after PEEP-trial), intermittent prone positioning of 16 to 20 h, empiric antibiotic treatment of a potential bacterial superinfection, and so forth. Compassionate use of remdesivir was considered but not feasible. As the patient’s clinical condition and clinical chemistry parameters worsened, we interdisciplinarily discussed selective CRP apheresis as an ultimate bail-out therapy with the Ethical Review Committee’s advice, the patient himself, and with his family. Due to our broad experience with apheresis in the setting of clinical routine and clinical trials as well as the ad hoc availability of this technique and the lack of alternative therapies, we finally came to the decision to start selective CRP apheresis in this patient.
Up to the present day, SARS-CoV-2 represents an unknown virus and thus, there is scant expertise on the resulting pulmonary sequelae (i.e., SARS-CoV-2-induced ARDS). In general, the basic science on the underlying mechanisms, molecular key players, and therapeutic approaches is virtually absent. In particular, whether CRP or complement deposits may be found in affected tissues in autopsy specimens of SARS-CoV-2 patients is as yet unknown. Indeed, most information comes from clinicians treating SARS-CoV-2 in their daily routine. They often report the following remarkable observations related to SARSCoV-2 pneumonia: (1) exceptionally high prognosis-associated CRP and IL-6 plasma levels [22] in general, and especially in the setting of a viral infection; (2) in many cases, only moderately elevated procalcitonin levels [11–14], as was the case in our patient; (3) frequently no bacterial or fungal superinfection detectable [11–14], as was the case in our patient; and (4) in comparison with other viral pulmonary infections, early and aggressive lung injury with consecutive ARDS and fibrosis [11–14].
Based on these clinical observations, the question arises whether the virus itself or an inadequate, massive, and excessive immune response is responsible for the fatal clinical course in some subjects. As CRP plasma levels are extraordinarily elevated in many patients, this molecule attracts special attention as a mediator and key player for destructive processes triggered by SARS-CoV-2. As a result, lowering CRP plasma levels may cause beneficial effects and slow the immunological self-destruction of the lung and other organs. Selective CRP apheresis may thus represent an adequate tool to reach this target. Certainly, CRP obviously holds an important role in immune defense and thus, it would be imprudent to completely eliminate this molecule in humans, especially during an ongoing infection. Given that CRP levels in our patient never declined to a plasma concentration of <100 mg/L, immunosuppression due to a lack of CRP is unlikely. In fact, a controlled decrease of CRP plasma levels in SARS-CoV-2 patients may represent an approach for achieving better survival of SARS-CoV-2 pneumonia.
This is a hypothesis-generating case report demonstrating that, in a high-risk SARS-CoV-2 patient, (1) CRP apheresis effectively reduced CRP plasma levels; (2) CRP apheresis in an ICU was more practical and efficient via central venous compared with peripheral venous access; and (3) CRP apheresis may have influenced other laboratory parameters (and related clinical course).
Seen from the provisional end, we were not successful because we were not able to save this high-risk SARS-CoV-2 patient’s life. However, 2 further potentially important observations have to be taken into account. First, effective CRP apheresis may have positively influenced laboratory parameters indicating failure of heart (creatinine kinases CK/CK-MB), kidney (creatinine), and liver (bilirubin). Whether this potential organ-protective effect is clinically useful in combination with further therapeutic approaches (such as drugs targeting the virus directly or, alternatively, nonspecific cytokine lowering by, for example, CytoSorb therapy) remains to be determined. Second, the start of CRP apheresis may have been too late to be successful in this patient. CRP apheresis in high-risk patients probably needs to be started at lower CRP levels to avoid a deleterious course of 2019-nCoV-2 infection. Also, lower risk patients with fewer comorbidities may benefit from a continuous CRP apheresis with well-defined target CRP plasma levels.
In conclusion, this hypothesis-generating case report offers a number of interpretations and therapeutic considerations that have to be tested in clinical trials reviewed by the scientific community. Currently, a randomized pilot study utilizing CRP apheresis in SARS-CoV-2 patients is being planned. Depending on the results, a randomized multicenter trial may need to be performed.
Consent
Consent for publication has been obtained, in line with the COPE best practice guidelines. The individual (his relatives, respectively), who is being reported on, is/are aware of the possible consequences of that reporting.
Conclusions
SARS-CoV-2 may cause multi-organ failure by inducing an excessive CRP-mediated autoimmune response of the ancient innate immune system.
Acknowledgments
We gratefully acknowledge Veronika Ackermann, Silvana Filleböck, Silvia Hosp, Regina Mühlegg, and Kerstin Rziha, Medical Care Center Kempten, Germany, for performing each apheresis session in close contact with the patient; our study nurse Andrea Zimmermann for composing the figures; and the doctors and nurses of ward B4 and ICU of Klinikum Kempten, Bavaria, Germany, for treating the patient with all their patience and empathy.
Footnotes
Conflicts of interest
Ahmed Sheriff is Founder and Shareholder of Pentracor GmbH. Christopher Bock and Stefan Kayser are employees of Pentracor GmbH.
References:
- 1.Du Clos TW. Pentraxins: Structure, function, and role in inflammation. ISRN Inflamm. 2013;2013:379040. doi: 10.1155/2013/379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–28. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 4.Sheriff A, Schindler R, Vogt B, et al. Selective apheresis of C-reactive protein: A new therapeutic option in myocardial infarction? J Clin Apher. 2015;30:15–21. doi: 10.1002/jca.21344. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann O, Li K, Zaczkiewicz M, et al. C-reactive protein in human atherogenesis: facts and fiction. Mediators Inflamm. 2014;2014:561428. doi: 10.1155/2014/561428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 7.Bharadwaj D, Stein MP, Volzer M, et al. The major receptor for C-reactive protein on leukocytes is Fcgamma receptor II. J Exp Med. 1999;190:585–90. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolov DE, Rocker C, Hombach V, et al. Ultrasensitive confocal fluorescence microscopy of C-reactive protein interacting with FcgammaRIIa. Arterioscler Thromb Vasc Biol. 2004;24:2372–77. doi: 10.1161/01.ATV.0000147407.17137.02. [DOI] [PubMed] [Google Scholar]
- 9.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: Implications for atherosclerosis. Circulation. 2001;103:1194–97. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 10.Slagman AC, Bock C, Abdel-Aty H, et al. Specific removal of C-reactive protein by apheresis in a porcine cardiac infarction model. Blood Purif. 2011;31:9–17. doi: 10.1159/000320763. [DOI] [PubMed] [Google Scholar]
- 11.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382(21):2012–22. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Litvinova M, Wang W, et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: A descriptive and modelling study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30230-9. [Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Zhang YY, Huang XR, et al. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease. Hypertension. 2010;55:953–60. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]
- 16.Li JJ, Sanders RL, McAdam KP, et al. Impact of C-reactive protein (CRP) on surfactant function. J Trauma. 1989;29:1690–97. doi: 10.1097/00005373-198912000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Ries W, Heigl F, Garlichs C, et al. Selective C-reactive protein-apheresis in patients. Ther Apher Dial. 2019;23:570–74. doi: 10.1111/1744-9987.12804. [DOI] [PubMed] [Google Scholar]
- 18.Ries W, Sheriff A, Heigl F, et al. “First in man”: Case report of selective C-reactive protein apheresis in a patient with acute ST segment elevation myocardial infarction. Case Rep Cardiol. 2018;2018:4767105. doi: 10.1155/2018/4767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of Pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–48. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]