Abstract
Aims
To explore whether variability in dietary cholesterol and phytosterol absorption impacts the risk of coronary artery disease (CAD) using as instruments sequence variants in the ABCG5/8 genes, key regulators of intestinal absorption of dietary sterols.
Methods and results
We examined the effects of ABCG5/8 variants on non-high-density lipoprotein (non-HDL) cholesterol (N up to 610 532) and phytosterol levels (N = 3039) and the risk of CAD in Iceland, Denmark, and the UK Biobank (105 490 cases and 844 025 controls). We used genetic scores for non-HDL cholesterol to determine whether ABCG5/8 variants confer greater risk of CAD than predicted by their effect on non-HDL cholesterol. We identified nine rare ABCG5/8 coding variants with substantial impact on non-HDL cholesterol. Carriers have elevated phytosterol levels and are at increased risk of CAD. Consistent with impact on ABCG5/8 transporter function in hepatocytes, eight rare ABCG5/8 variants associate with gallstones. A genetic score of ABCG5/8 variants predicting 1 mmol/L increase in non-HDL cholesterol associates with two-fold increase in CAD risk [odds ratio (OR) = 2.01, 95% confidence interval (CI) 1.75–2.31, P = 9.8 × 10−23] compared with a 54% increase in CAD risk (OR = 1.54, 95% CI 1.49–1.59, P = 1.1 × 10−154) associated with a score of other non-HDL cholesterol variants predicting the same increase in non-HDL cholesterol (P for difference in effects = 2.4 × 10−4).
Conclusions
Genetic variation in cholesterol absorption affects levels of circulating non-HDL cholesterol and risk of CAD. Our results indicate that both dietary cholesterol and phytosterols contribute directly to atherogenesis.
Keywords: Dietary cholesterol, Phytosterols, Absorption, Genetics, ABCG5/8
See page 2629 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa445)
Introduction
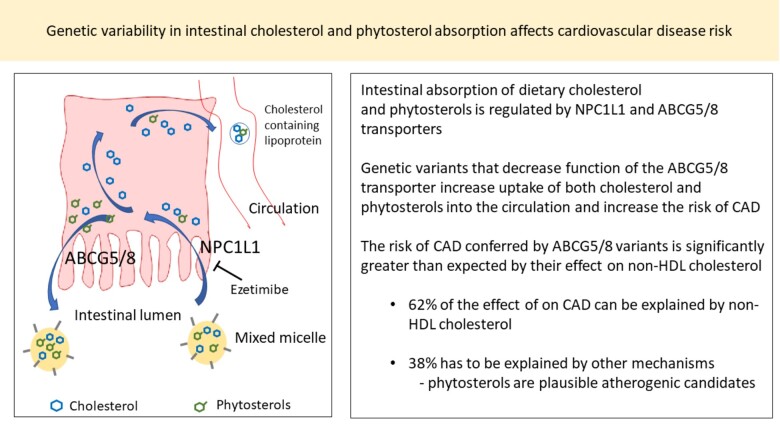
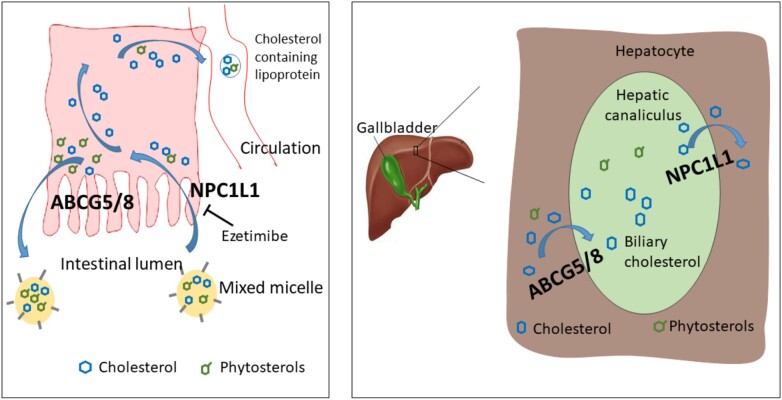
The ABCG5 and ABCG8 genes encode the obligate heterodimers of the ATP-binding cassette (ABC) transporters G5 and G8 (ABCG5/8) that have a major role in preventing accumulation of dietary sterols, including cholesterol and sterols derived from plants (phytosterols), in the body.1 The ABCG5/8 transporter is mainly expressed in the small intestine on the absorptive surface of enterocytes and in the liver on hepatocytes facing the bile canaliculi (Figure 1).
Figure 1.
A schematic representation of sterol transport in the intestine and liver. Dietary cholesterol and non-cholesterol sterols are transported from the intestinal lumen into enterocytes via NPC1L1.While approximately 50–60% of the cholesterol that has entered the enterocytes is taken up into the circulation, the majority of phytosterols are pumped back into the intestinal lumen by ABCG5/8. In addition to excreting phytosterols, the hepatic ABCG5/8 transporters have a major role in removing cholesterol from the body. Decreased ABCG5/8 function in the liver reduces gallstone risk through diminished cholesterol saturation in bile. Phytosterols in the diet are believed to attenuate the pool of absorbable cholesterol by displacing cholesterol from intestinal micelles.
Translational perspective
The importance of dietary cholesterol absorption in the regulation of cholesterol levels in blood and the risk of coronary artery disease (CAD) has been the subject of controversy. We find that sequence variants that decrease the function of the ABCG5/8 transporter increase absorption of both cholesterol and phytosterols and increase the risk of CAD. The findings provide mechanistic insights indicating harmful effects of dietary cholesterol on cardiovascular disease. We also find that the impact of ABCG5/8 variants on the risk of CAD is not fully explained by non-HDL cholesterol. Thus, in addition to dietary cholesterol other dietary sterols such as phytosterols may contribute directly to atherogenesis, raising questions about the safety of supplementing food with phytosterols for the purpose of cardiovascular risk reduction.
While the NPC1L1 transporter, the target of the cholesterol-lowering drug ezetimibe,2 is responsible for the non-selective uptake of sterols into enterocytes and hepatocytes from the intestinal lumen and bile, respectively, the ABCG5/8 excretes them back into the intestinal lumen and bile1 (Figure 1).
Rare inactivating mutations in the ABCG5/8 genes cause autosomal recessive phytosterolaemia (also termed sitosterolaemia). This rare disorder is characterized by impaired sterol elimination from enterocytes and hepatocytes leading to excessive intestinal absorption of cholesterol and phytosterols, as well as reduced secretion to bile.3 Although autosomal recessive phytosterolaemia frequently involves hypercholesterolaemia, sometimes to the extreme,3 this is not always the case and significant premature atherosclerosis has been documented in the absence of substantial hypercholesterolaemia.3 , 4
Common variants at the ABCG5/8 locus associate with low-density lipoprotein (LDL) cholesterol,5 , 6 phytosterol levels, and the risk of coronary artery disease (CAD).7 , 8 Alleles that associate with decreased levels of LDL cholesterol also associate with increased risk of gallstones,7 , 9 , 10 likely mediated through an effect on cholesterol saturation in bile. Furthermore, NPC1L1 variants associate with LDL cholesterol and CAD,5 , 11 and rare NPC1L1 inactivating variants associate with reduced levels of LDL cholesterol and phytosterols.12
While evidence from genetic studies and randomized clinical trials of cholesterol-lowering drugs demonstrates that the relationship between non-HDL/LDL cholesterol and CAD is causal,13 , 14 the contribution of dietary cholesterol to cardiovascular diseases (CVDs) and mortality has been debated for decades.15 Over the last few years, the importance of dietary cholesterol has been deemphasized in dietary recommendations in many countries.16 , 17
The role of phytosterols in atherosclerotic disease is also a matter of controversy.18–20 The ESC/EAS Guidelines for the management of dyslipidaemias17 recommend food enriched with phytosterols as a lifestyle intervention to reduce cholesterol levels by interfering with intestinal cholesterol absorption.21
Here, we explore whether variability in dietary cholesterol and phytosterol absorption impacts the risk of CAD, using sequence variants of the ABCG5/8 genes as instruments.
Methods
Detailed description of the studies included and methods used is provided in Supplementary material online, Methods. Briefly, we analysed data from three studies of individuals of European origin from Iceland, Denmark, and UK Biobank. We examined association of sequence variants in ABCG5/8 with non-HDL cholesterol22 in up to 610 532 individuals, phytosterol levels in 3039 individuals, and the risk of CAD in 105 490 cases and 844 025 controls. Variant associations were also assessed in public data from the Global Lipids Genetics Consortium23 (N up to 333 359) and CARDIoGRAM Exome24 (42 355 cases and 78 240 controls).
Logistic or linear regression, assuming additive models, was used to test for the association of variants with binary or quantitative traits, respectively. Variant association results from the different study groups were combined into meta-analyses assuming fixed effects. All P-values reported in this study are two-sided.
We constructed individual-level genetic risk scores (GRS) for levels of non-HDL cholesterol22 and calculated into the study subjects. The GRSs were generated for each individual by summing the product of the allele count and the corresponding non-HDL cholesterol effect size.
Results
Coding variants in ABCG5/8 and association with non-high density lipoprotein cholesterol and coronary artery disease
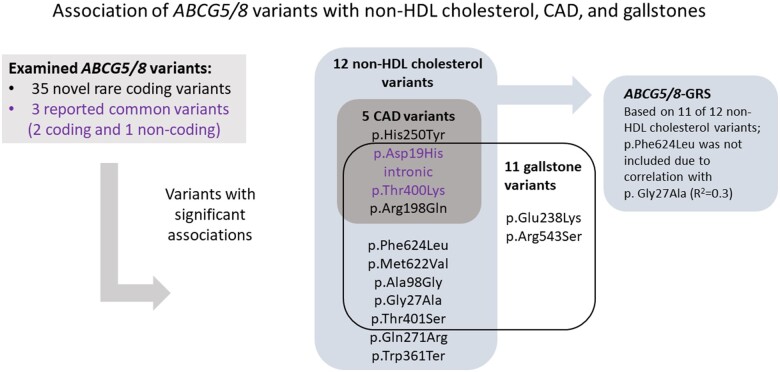
We identified 35 rare [minor allele frequency (MAF) ≥0.01% and <1%] coding variants in 28 075 whole-genome sequenced Icelanders that we subsequently imputed into chip-typed Icelanders and their close relatives (Supplementary material online, Methods). Six common (MAF > 5%) variants (five coding and one intronic) reported5–10 to associate with LDL cholesterol, CAD, and gallstones were also examined. We tested these ABCG5/8 variants for association with non-HDL and LDL cholesterol in datasets from Iceland, Denmark, the UK Biobank, and the Global Lipids Genetics Consortium (GLGC),23 and in a meta-analysis (N up to 943 891; Supplementary material online, Tables S1 and S2).
Of the 35 rare coding variants, nine associate with non-HDL cholesterol (P < 0.05/41 = 1.2 × 10−3) (Table 1, Figure 2, and Supplementary material online, Table S1). We note that two or more of these nine rare variants never occur on the same haplotype (D´= −1, pairwise R 2 < 3.0 × 10−6), with the exception of p. Phe624Leu in ABCG5 that is always observed on the background of p. Gly27Ala in the same gene (D´ = 1 and R 2 = 0.32).
Table 1.
The association of ABCG5/8 variants with non-high density lipoprotein cholesterol, coronary artery disease, and gallstones
| EA frequency (%) | Non-HDL cholesterol (N up to 943 891)a
|
CAD (N up to 147 825 cases/922 265 controls)b
|
Gallstones (N up to 27 441 cases/738 791 controls)c
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCG-[5/8] | Coding change | rsName | EA/non-EA | Iceland/Denmark/UK Biobank/GLGC | Β | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| 5 | p.Phe624Leu | rs150401285 | G/A | 0.031/0.138/0.098/0.065 | 0.19 | (0.14, 0.25) | 1.9 × 10−11 | 1.12 | (0.96, 1.31) | 0.16 | 0.37 | (0.24, 0.57) | 6.3 × 10−6 |
| 5 | p.Met622Val | rs140374206 | C/T | 0.104/0.541/0.649/0.503 | 0.05 | (0.02, 0.07) | 8.2 × 10−5 | 1.02 | (0.96, 1.09) | 0.5 | 0.70 | (0.61, 0.82) | 6.1 × 10−6 |
| 5 | p.His250Tyr | rs776502883 | A/G | 0.091/NA/NA/NA | 0.58 | (0.42, 0.75) | 6.0 × 10−12 | 1.96 | (1.35, 2.83) | 3.9 × 10−4 | 0.80 | (0.40, 1.61) | 0.52 |
| 5 | p.Arg198Gln | rs141828689 | T/C | 0.145/0.157/0.132/0.138 | 0.16 | (0.11, 0.21) | 2.8 × 10−11 | 1.29 | (1.11, 1.49) | 6.2 × 10−4 | 0.42 | (0.31, 0.59) | 3.3 × 10−7 |
| 5 | p.Phe125Leu | NA | G/A | 0.027/NA/NA/NA | 0.41 | (0.11, 0.70) | 6.5 × 10−3 | 2.48 | (1.30, 4.71) | 5.7 × 10−3 | 0.46 | (0.12, 1.73) | 0.25 |
| 5 | p.Ala98Gly | rs145164937 | C/G | 0.021/0.231/0.176/NA | 0.11 | (0.06, 0.17) | 1.6 × 10−4 | 1.11 | (0.96, 1.29) | 0.16 | 0.42 | (0.29, 0.60) | 1.8 × 10−6 |
| 5 | p.Gly27Ala | rs56204478 | G/C | 0.072/0.358/0.354/NA | 0.14 | (0.10, 0.18) | 2.7 × 10−13 | 1.08 | (0.98, 1.19) | 0.13 | 0.50 | (0.42, 0.61) | 4.5 × 10−12 |
| 8 | p.Asp19His | rs11887534 | C/G | 5.461/6.104/6.49/5.8 | −0.11 | (−0.11, −0.10) | 3.5 × 10−203 | 0.93 | (0.91, 0.95) | 9.4 × 10−13 | 1.94 | (1.87, 2.00) | <1 × 10−300 |
| 8 | intron | rs4299376 | G/T | 27.958/29.541/31.667/25.17 | 0.07 | (0.06, 0.07) | 6.6 × 10−266 | 1.05 | (1.04, 1.06) | 1.1 × 10−22 | 0.80 | (0.79, 0.82) | 1.1 × 10−119 |
| 8 | p.Glu238Lys | rs34754243 | A/G | 0.019/0.292/0.162/NA | −0.01 | (−0.06, 0.05) | 0.83 | 1.11 | (0.97, 1.26) | 0.13 | 1.51 | (1.21, 1.89) | 3.1 × 10−4 |
| 8 | p.Arg263Gln | rs137852990 | A/G | 0.117/NA/NA/0.013 | 0.17 | (0.05, 0.29) | 5.5 × 10−3 | 1.14 | (0.81, 1.60) | 0.5 | 0.41 | (0.22, 0.75) | 4.0 × 10−3 |
| 8 | p.Gln271Arg | rs770309304 | G/A | 0.106/NA/NA/NA | 0.40 | (0.25, 0.55) | 2.9 × 10−7 | 1.36 | (0.95, 1.96) | 0.091 | 0.39 | (0.20, 0.79) | 8.2 × 10−3 |
| 8 | p.Trp361Ter | rs137852987 | A/G | 0.147/0.159/0.076/0.111 | 0.13 | (0.08, 0.18) | 9.9 × 10−8 | 1.14 | (1.00, 1.31) | 0.059 | 0.66 | (0.48, 0.90) | 8.5 × 10−3 |
| 8 | p.Thr400Lys | rs4148217 | A/C | 19.076/18.405/18.502/NA | −0.04 | (−0.05, −0.04) | 2.8 × 10−48 | 0.97 | (0.95, 0.98) | 3.2 × 10−6 | 1.11 | (1.09, 1.14) | 6.8 × 10−19 |
| 8 | p.Thr401Ser | rs144200355 | T/A | 0.006/0.174/0.233/0.157 | −0.09 | (−0.14, −0.05) | 5.7 × 10−6 | 0.91 | (0.80, 1.03) | 0.12 | 1.55 | (1.27, 1.88) | 1.3 × 10−5 |
| 8 | p.Arg543Ser | rs201690654 | T/G | 0.032/0.036/0.058/0.029 | 0.08 | (−0.01, 0.16) | 0.069 | 1.13 | (0.86, 1.48) | 0.39 | 0.35 | (0.20, 0.62) | 3.1 × 10−4 |
The effect (β) on non-HDL cholesterol is given in standard deviation units. p.Arg263Gln causes phytosterolemia in 2 Icelandic sisters. p. Phe125Leu has borderline significant association with non-HDL cholesterol and CAD.
CAD, coronary artery disease; CI, confidence interval; EA, effect allele; HDL, high-density lipoprotein; OR, odds ratio.
Combined Iceland (N = 139 033), Denmark (N = 113 038), UK Biobank (N = 358 461), and/or Global Lipids Genetics Consortium (GLGC) (N up to 333 359).
Combined Iceland (39 020 cases/319 620 controls), Denmark (33 603 cases/148 707 controls), UK Biobank (32 867 cases/375 698 controls), and CARDIoGRAM exome (42 335 cases/78 240 controls).
Combined Iceland (9024 cases/348 643 controls) and UK Biobank (18 417 cases/348 643 controls).
Figure 2.
A schematic representation of the ABCG5/8 variant associations.
All six reported common variants associate with non-HDL cholesterol in our dataset (Supplementary material online, Table S1). However, these associations are fully captured by three variants with low pairwise correlation (R 2 ≤ 0.1; Supplementary material online, Table S3), p. Asp19His (rs11887534), the intronic rs4299376, and p. Thr400Lys (rs4148217) (Figure 2 and Supplementary material online, Table S3).
Next, we examined the association of the 35 rare coding and the 3 common variants with CAD in Iceland (39 020 cases and 319 620 controls) and in a meta-analysis of data from Iceland, Denmark, UK Biobank, and the public CARDIoGRAM Exome24 (combined up to 147 825 cases and 922 265 controls) (Table 1 and Supplementary material online, Table S4). Two rare variants associate with CAD (P < 0.05/35 = 1.4 × 10−3), p. His250Tyr (OR = 1.96, P = 3.9 × 10−4), and p. Arg198Gln (OR = 1.29, P = 6.2 × 10−4), and both are predicted to have deleterious impact on the protein (Supplementary material online, Table S5). Furthermore, His250 is located in a highly conserved motive (GERP score =5.56; top 0.3% genome wide), the histidine loop (H-loop) in the nucleotide-binding domain of ABC transporters (Supplementary material online, Methods). We also replicate the CAD association of the common variants7 , 8 (Table 1). The alleles of the five variants that associate with higher risk of CAD all associate with higher levels of non-HDL cholesterol.
The association of the 12 non-HDL cholesterol ABCG5/8 variants with other atherosclerosis-related phenotypes is shown in Supplementary material online, Table S6. We find several nominally significant associations between rare ABCG5/8 coding variants and other CVD. For example, p. His250Tyr that has the largest effect on non-HDL cholesterol and phytosterols and associates with CAD, also associates with aortic valve stenosis (P = 0.0056), heart failure (P = 0.0018), and sudden cardiac death (P = 3.1 × 10−5).
None of the variants associates (at P < 8.3 × 10−4, corrected for the number of variants and traits tested) with the atherosclerotic risk factors, hypertension, type 2 diabetes, body mass index, triglyceride, or HDL cholesterol, except the common intronic variant rs4299376 that has small effect on triglyceride levels (β = 0.0096, P = 6.5 × 10−6) (Supplementary material online, Table S6).
Variant effects on phytosterol levels
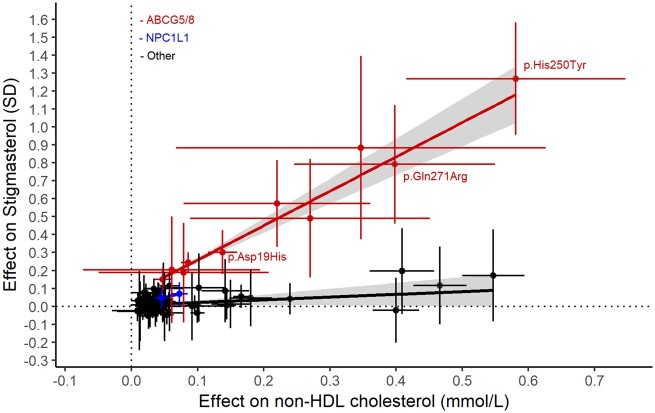
We measured three of the most common phytosterols (sitosterol, campesterol, and stigmasterol) in serum from 3039 Icelanders, enriched for carriers of the rare coding variants in ABCG5/8 that associate with non-HDL cholesterol. Sufficiently many serum samples were available from carriers of seven rare variants and of those six associate with phytosterol levels. The variant p. His250Tyr with greatest effect on non-HDL cholesterol also has the greatest effect on all three phytosterols (β for stigmasterol =1.27 SD, P = 2.2 × 10−15) (Table 2).
Table 2.
Association of ABCG5/8 variants with phytosterol levels
| Stigmasterol |
Sitosterol |
Campesterol |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 3039) |
(N = 3039) |
(N = 3039) |
|||||||||
| ABCG-[5/8] | Coding change | rsName | EA/non-EA | N carriers measured | EA frequency in measured samples (%) | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| 5 | p.Phe624Leu | rs150401285 | G/A | 19 | 0.31 | 0.88 (0.37, 1.39) | 6.9 × 10−4 | 0.85 (0.33, 1.37) | 1.2 × 10−3 | 0.81 (0.30, 1.32) | 1.7 ×10−3 |
| 5 | p.His250Tyr | rs776502883 | A/G | 50 | 0.82 | 1.27 (0.96, 1.58) | 2.2 × 10−15 | 1.17 (0.86, 1.49) | 3.9 × 10−13 | 1.10 (0.79, 1.41) | 4.4 × 10−12 |
| 5 | p.Arg198Gln | rs141828689 | T/C | 54 | 0.89 | 0.20 (−0.09, 0.50) | 0.18 | 0.29 (−0.01, 0.59) | 0.056 | 0.30 (0.01, 0.59) | 0.044 |
| 5 | p.Gly27Ala | rs56204478 | G/C | 51 | 0.84 | 0.49 (0.16, 0.82) | 3.7 × 10−3 | 0.43 (0.09, 0.76) | 0.012 | 0.40 (0.08, 0.73) | 0.016 |
| 8 | p.Asp19His | rs11887534 | C/G | 266 | 4.38 | −0.30 (−0.42, −0.18) | 1.1 × 10−6 | −0.32 (−0.45, −0.20) | 1.6 × 10−7 | −0.26 (−0.38, −0.13) | 3.5 × 10−5 |
| 8 | intronic | rs4299376 | G/T | 1529 | 25.16 | 0.24 (0.19, 0.30) | 2.6 × 10−17 | 0.27 (0.22, 0.33) | 1.6 × 10−21 | 0.23 (0.18, 0.29) | 3.1 × 10−16 |
| 8 | p.Gln271Arg | rs770309304 | G/A | 40 | 0.66 | 0.79 (0.46, 1.12) | 2.6 × 10−6 | 0.90 (0.56, 1.23) | 1.2 × 10−7 | 0.73 (0.40, 1.06) | 1.3 × 10−5 |
| 8 | p.Arg263Gln | rs137852990 | A/G | 72 | 1.22 | 0.57 (0.33, 0.82) | 3.6 × 10−6 | 0.45 (0.21, 0.69) | 3.0 × 10−4 | 0.40 (0.15, 0.64) | 1.3 × 10−3 |
| 8 | p.Trp361Ter | rs137852987 | A/G | 59 | 0.97 | 0.19 (−0.09, 0.47) | 0.18 | 0.17 (−0.11, 0.45) | 0.23 | 0.16 (-0.11,0.44) | 0.25 |
| 8 | p.Thr400Lys | rs4148217 | A/C | 1049 | 17.26 | −0.15 (−0.22, −0.09) | 3.5 × 10−6 | −0.16 (−0.23, −0.10) | 1.6 × 10−7 | −0.13 (−0.20, −0.07) | 3.6 × 10−5 |
The effect (β) is given in standard deviation units. N carriers measured refers to the number of carriers with phytosterol measurements. EA frequency is for the phytosterol measured dataset, enriched with rare variant carriers.
CI, confidence interval; EA, effect allele.
In the Icelandic dataset, we identified seven homozygous or compound heterozygous carriers of rare ABCG5/8 coding variants. Two homozygous carriers of p. Arg263Gln in ABCG8 have extremely high phytosterol levels consistent with autosomal recessive phytosterolaemia (see Supplementary material online, Note).
In agreement with the role of the ABCG5/8 transporter in regulating intestinal absorption of both cholesterol and phytosterols, the ABCG5/8 variant effects on non-HDL cholesterol and phytosterol levels are highly correlated (R 2 = 0.97, Figure 3 and Supplementary material online, Table S7C and G). In the Icelandic data, 1 mmol/L increase in non-HDL cholesterol driven by the ABCG5/8 variants associates with 2.56 SD increase in stigmasterol levels (P = 1.1 × 10−8). Two common NPC1L1 variants measured in our dataset associate with phytosterol levels (Supplementary material online, Table S7), but the phytosterol effect per unit change in non-HDL cholesterol is smaller than that observed for the ABCG5/8 variants (Figure 3). The apparent difference in the effects of NPC1L1 and ABCG5/8 variants on phytosterol levels is consistent with the non-selective uptake of sterols into enterocytes mediated by NPC1L1,1 , 25 as opposed to the preferential excretion of phytosterols from enterocytes into the intestinal lumen mediated by ABCG5/8.1
Figure 3.
The relationship between variant effects on non-high-density lipoprotein cholesterol and stigmasterol. The crosses show 95% confidence intervals. SD, standard deviation units. The red (for ABCG5/8 variants) and black (for variants outside ABCG5/8 locus) lines are the best lines fitting the stigmasterol effects for non-high-density lipoprotein cholesterol variants using weighted regression with one over standard error squared as weights. The grey-shaded area around the line is the 95% confidence interval. NPC1L1 variants are plotted in blue. See data in Supplementary material online, Table S7C and G.
Consistent correlation between effects on non-HDL cholesterol and phytosterol levels is not observed for non-HDL cholesterol associating variants outside the ABCG5/8 and NPC1L1 loci (R 2 = 0.13, P = 0.0012, Figure 3 and Supplementary material online, Table S7).
Association with coronary artery disease is not fully explained by non-high density lipoprotein cholesterol
We then explored whether ABCG5/8 impacts the risk of CAD beyond what is expected by their effect on non-HDL cholesterol. We constructed 2 GRS for non-HDL cholesterol, one using ABCG5/8 variants (GRS-ABCG5/8) and another using reported variants outside the ABCG5/8 locus (GRS-other) (Supplementary material online, Methods, Table S7 and S8) and compared their effects on CAD in 85 544 cases and 648 442 controls/non-CAD cases from Iceland, Denmark, and UK Biobank (Table 3). NPC1L1 variants were not included in these GRSs. We scaled the units of the GRSs to mmol/L of non-HDL cholesterol and the odds ratios (OR) for CAD are calculated per 1 mmol/L of the genetically predicted increase in non-HDL cholesterol. The ABCG5/8 GRS associates with double the risk of CAD for a 1 mmol increase in non-HDL cholesterol (OR 2.01, 95% CI 1.75–2.31; P = 9.8 × 10−23, Table 3) compared with a 54% increase in CAD risk for GRS-other (OR = 1.54, 95% CI 1.49–1.59; P = 1.1 × 10−154, P for difference in effects = 2.4 × 10−4). This greater effect of the GRS-ABCG5/8 on CAD indicates that there are atherogenic effects of ABCG5/8 variants that are not mediated through non-HDL cholesterol.
Table 3.
Disparate effects of genetic risk scores for non-high density lipoprotein cholesterol on the risk of coronary artery disease
| GRS-other |
GRS-ABCG5/8
|
GRS-NPC1L1
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-HDL cholesterol variants, outside ABCG5/8 and NPC1L1 loci |
Non-HDL cholesterol variants at ABCG5/8 locus |
Non-HDL cholesterol variants at NPC1L1 locus |
||||||||
| Cases/controls | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Iceland | 19 074/124 037 | 1.47 | (1.37, 1.59) | 1.3 × 10−23 | 1.96 | (1.48, 2.58) | 2.0 × 10−6 | 1.89 | (1.18, 3.01) | 0.0079 |
| Denmark | 33 603/148 707 | 1.64 | (1.54, 1.75) | 7.3 × 10−55 | 2.30 | (1.63, 3.26) | 2.5 × 10−6 | 2.94 | (1.73, 5.00) | 7.2 × 10−5 |
| UK Biobank | 32 867/375 698 | 1.51 | (1.45, 1.58) | 3.3 × 10−81 | 1.96 | (1.63, 2.35) | 4.9 × 10−13 | 1.64 | (1.13, 2.37) | 0.0087 |
| Combined | 85 544/648 442 | 1.54 | (1.49, 1.59) | 1.1 × 10−154 | 2.01 | (1.75, 2.31) | 9.8 × 10−23 | 1.95 | (1.51, 2.52) | 2.6 × 10−7 |
| Phet (for difference in effects on CAD) | ||||||||||
| GRS-ABCG5/8 vs. GRS-other | 2.4 × 10−4 | |||||||||
| GRS-NPC1L1 vs. GRS-other | 0.067 | |||||||||
The effects on CAD are given per 1 mmol/L of genetically elevated non-HDL cholesterol levels.
CAD, coronary artery disease; CI, confidence interval; GRS, genetic risk score; HDL, high-density lipoprotein; OR, odds ratio.
P het: P-value for heterogeneity in effects.
For comparison, we examined the association of a GRS based on four NPC1L1 variants with CAD. Although the results for GRS-NPC1L1 were similar to GRS-ABCG5/8, there were fewer variants behind this risk score than for the GRS-ABCG5/8 resulting in less accurate CAD risk estimate. The CAD risk conferred by NPC1L1 variants was not significantly different from that expected by non-HDL cholesterol variants at other loci (P = 0.067) (Table 3).
Association with gallstones and haematologic traits
Since the ABCG5/8 transporter is known to affect biliary cholesterol secretion and gallstone formation, we tested the 35 rare coding and 3 common variants for association with gallstone risk in a meta-analysis including data from Iceland and the UK Biobank (27 441 cases and 738 791 controls). We identified associations between eight rare coding variants and gallstones (P < 1.4 × 10−3 = 0.05/35) and replicated the association of the common variants9 , 10 (Table 1 and Supplementary material online, Table S9). We note that among eight rare variants that associate with gallstone risk, six also associate with non-HDL cholesterol, with the non-HDL cholesterol increasing alleles consistently associating with lower risk of gallstones (Table 1). However, we do not observe a clear dose–response relationship between variant effects on non-HDL cholesterol and on gallstones (Table 1 and Supplementary material online, Table S9).
Because of the reported macrothrombocytopenia and haemolytic anaemia in some phytosterolaemia patients,3 we tested ABCG5/8 variants for association with platelet traits and haemoglobin (Supplementary material online, Table S10). The three common variants associate with mean platelet volume (rs4299376: P = 2.5 × 10−15, p. Asp19His: P = 1.7 × 10−4, p. Thr400Lys: P = 1.9 × 10−4) and with haemoglobin levels (rs4299376: P = 0.030, p. Asp19His: P = 3.2 × 10−8, p. Thr400Lys: P = 3.3 × 10−4). Furthermore, the rare variant p. His250Tyr that has the largest effect on phytosterol levels, associates with greater mean platelet volume (P = 5.7 × 10−3). The directions of the effects on platelet size and haemoglobin levels are consistent with those reported in phytosterolaemia (Supplementary material online, Table S10).
Discussion
We identified several rare ABCG5/8 coding variants with substantial impact on circulating levels of non-HDL cholesterol and phytosterols, and demonstrate that heterozygous carriers are at increased risk of CAD and other CVD (Take home figure).
Take home figure.
Genetic analysis using ABCG5/8 variants as instruments indicates that both dietary cholesterol and phytosterols contribute directly to atherogenesis.
The role of dietary cholesterol absorption in the regulation of circulating cholesterol and subsequent CVD is debated.15 We show that for variants at the ABCG5/8 locus, the effect on non-HDL cholesterol is highly correlated (R 2 = 0.97) with the effect on phytosterols that are only derived from the diet. This is consistent with the common mechanism of intestinal absorption of cholesterol and phytosterols, regulated by NPC1L1 and ABCG5/8 sterol transporters. Indeed, phytosterol levels are frequently used as surrogate markers of intestinal cholesterol absorption.26 Thus, the results indicate that increased intestinal absorption has a major contribution to the levels of cholesterol, although cholesterol removal through the liver may also play a role. However, less consistent relationship between variant effects on non-HDL cholesterol and on the formation of gallstones, a marker of cholesterol efflux to bile,27 suggests a smaller role for this mechanism. Furthermore, in carriers of the ABCG5/8 variants that associate with increased non-HDL cholesterol less cholesterol from the enterohepatic circulation is expected to be within the gut than in non-carriers since these variants associate with less secretion of cholesterol to bile. This suggests that the ABCG5/8 variants affect cholesterol levels in blood, mainly through regulation of dietary cholesterol absorption. Our findings thus provide mechanistic insights into how dietary cholesterol may affect CVD. A cautious view towards dietary cholesterol is also proposed by a recent large observational study, finding that higher consumption of dietary cholesterol associates with incident CVD and all-cause mortality in a dose-dependent manner.28 In line with what other studies have suggested (reviewed in Ref.29), our results support the opinion that ‘high cholesterol absorbers’ might benefit in particular from moderation in cholesterol intake and ezetimibe treatment.
The role of phytosterols in atherosclerotic disease is a matter of an ongoing dispute.18–20 We demonstrate that the degree of CAD risk conferred by ABCG5/8 variants is greater than predicted by their effect on non-HDL cholesterol levels. Based on the effect of non-HDL cholesterol variants in other genes than ABCG5/8 and NPC1L1 as reflected in GRS-other, non-HDL cholesterol can only explain around 62% of the CAD risk inferred from effect of variants in GRS-ABCG5/8 on CAD, the remaining 38% must be due to other mechanisms. The excess risk is unlikely driven through other traditional risk factors for CAD since the ABCG5/8 variants do not associate with them. In contrast, the rare and common ABCG5/8 variants have a consistent close relationship with phytosterol levels, making elevated phytosterol levels a plausible explanation for the excess CAD risk. The chemical relatedness to cholesterol also makes phytosterols credible atherogenic candidates. Evidence from humans with phytosterolaemia, from animal studies, and in vitro experiments further support atherogenic effect of phytosterols.3 , 30 , 31
While our results indicate that genetic susceptibility to high absorption of cholesterol and phytosterols increases the risk of CAD, the total and relative amount of these dietary components in the gut may also play a role in the net absorption. Thus, high intakes may increase absorption because of more availability. However, phytosterols in the diet may also reduce intraluminal availability of cholesterol, through physicochemical interferance.21
While our findings raise concerns about the safety of phytosterol-supplemented food, given their propensity to raise phytosterol levels in blood,21 harmful effects of phytosterol supplementation cannot be concluded based on our data. Ultimately, it needs to be established in clinical trials whether the non-HDL/LDL cholesterol-lowering effects of phytosterol-supplemented food products truly lower cardiovascular risk, or whether swapping the cholesterol with another atherogenic lipid might override this effect, or possibly increase risk.
The main limitation to our study is that we cannot demonstrate directly the dietary origin of the non-HDL cholesterol in blood. Neither was our study equipped to address the effects of various proportions of cholesterol and phytosterols in diet on the amount absorbed, or on the effect on CVD.
In conclusion, we used genetics to demonstrate a role of dietary cholesterol in the regulation of non-HDL cholesterol levels and the risk of CVD. Furthermore, other dietary sterols such as phytosterols may contribute directly to atherogenesis.
Supplementary Material
Acknowledgements
We thank all the individuals who participated in the study and whose contribution made this work possible as well as our colleagues who contributed to data collection, sample handling, and genotyping. This research has been conducted using the UK Biobank Resource under Application Number ‘24711’. Data on coronary artery disease have been contributed by the Myocardial Infarction Genetics and CARDIoGRAM Exome investigators and have been downloaded from www.CARDIOGRAMPLUSC4D.ORG. Data on blood lipids were contributed by the Global Lipids Genetics Consortium and was downloaded from http://csg.sph.umich.edu/willer/public/lipids2017EastAsian/.
Funding
This work was supported by deCODE genetics/Amgen. S.B. is supported by the Novo Nordisk Foundation (NNF14CC0001 and NNF17OC0027594). F.W.A. is supported by University College London Hospital National Institute for Health Research Biomedical Research Centre (BRC288A).
Conflict of interest: The authors affiliated with deCODE genetics/Amgen, Inc. are employed by the company.
Contributor Information
Anna Helgadottir, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Gudmar Thorleifsson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Kristjan F Alexandersson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Vinicius Tragante, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; Department of Cardiology, Division Heart & Lungs, University Medical Center Utrecht, Utrecht University, 3584 CX Utrecht, the Netherlands.
Margret Thorsteinsdottir, ArcticMass, Sturlugata 8, 102 Reykjavik, Iceland; Faculty of Pharmaceutical Sciences, University of Iceland, Hofsvallagata 53, 107 Reykjavik, Iceland.
Finnur F Eiriksson, ArcticMass, Sturlugata 8, 102 Reykjavik, Iceland.
Solveig Gretarsdottir, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Eythór Björnsson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; Department of Internal Medicine, Landspitali – National University Hospital of Iceland, 101 Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland.
Olafur Magnusson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Gardar Sveinbjornsson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Ingileif Jonsdottir, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland.
Valgerdur Steinthorsdottir, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Egil Ferkingstad, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Brynjar Ö Jensson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Hreinn Stefansson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Isleifur Olafsson, Department of Clinical Biochemistry, Landspitali –National University Hospital of Iceland, Hringbraut, 101 Reykjavik, Iceland.
Alex H Christensen, Herlev-Gentofte Hospital, Copenhagen University Hospital, Gentofte Hospitalsvej 1, 2900 Hellerup, Denmark.
Christian Torp-Pedersen, Department of Cardiology and Clinical Research, Nordsjaellands Hospital, Dyrehavevej 29, 3400 Hillerød, Denmark; Department of Cardiology, Aalborg University Hospital, Hobrovej 18-22, 9000 Aalborg, Denmark.
Lars Køber, The Capital Region’s Unit of Inherited Cardiac Diseases, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Ole B Pedersen, Department of Clinical Immunology, Næstved Hospital, Ringstedgade 61, 4700 Næstved, Denmark; Department of Clinical Medicine, Copenhagen University, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Christian Erikstrup, Department of Clinical Immunology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus, Denmark.
Erik Sørensen, Department of Clinical Immunology, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Søren Brunak, Translational Disease Systems Biology, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Karina Banasik, Translational Disease Systems Biology, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Thomas F Hansen, Translational Disease Systems Biology, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark; Department of Neurology, Danish Headache Center, Glostrup, Rigshospitalet, Valdemar Hansens vej 1-23, 2600 Glostrup, Denmark; Institute for Biological Psychiatry, Mental Health Center Sct. Hans, Boserupvej 2, 4000 Roskilde, Denmark.
Mette Nyegaard, Department of Biomedicine, Aarhus University, Høegh-Guldbergs Gade 10, 8000 Aarhus, Denmark.
Gudmundur I Eyjolfssson, The Laboratory in Mjodd, Thonglabakki 6, 109 Reykjavik, Iceland.
Olof Sigurdardottir, Department of Clinical Biochemistry, Akureyri Hospital, Eyrarlandsvegur, 600 Akureyri, Iceland.
Bjorn L Thorarinsson, Department of Neurology, Landspitali – National University Hospital of Iceland, Hringbraut, 101 Reykjavik, Iceland.
Stefan E Matthiasson, Laekning, Medical Clinics, Lágmúli 5, 108 Reykjavik, Iceland.
Thora Steingrimsdottir, Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland; Department of Obstetrics and Gynecology, Landspitali – National University Hospital, Hringbraut, 101 Reykjavik, Iceland.
Einar S Bjornsson, Department of Internal Medicine, Landspitali – National University Hospital of Iceland, 101 Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland.
Ragnar Danielsen, Division of Cardiology, Department of Internal Medicine, Landspitali – National University Hospital of Iceland, Hringbraut, 101 Reykjavik, Iceland.
Folkert W Asselbergs, Department of Cardiology, Division Heart & Lungs, University Medical Center Utrecht, Utrecht University, 3584 CX Utrecht, the Netherlands; Institute of Cardiovascular Science, Faculty of Population Health Sciences, University College London, Gower Street, London WC1E 6BT, UK; Health Data Research UK and Institute of Health Informatics, University College London, Gower Street, London WC1E 6BT, UK.
David O Arnar, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland; Division of Cardiology, Department of Internal Medicine, Landspitali – National University Hospital of Iceland, Hringbraut, 101 Reykjavik, Iceland.
Henrik Ullum, Department of Clinical Immunology, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Henning Bundgaard, The Capital Region’s Unit of Inherited Cardiac Diseases, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Patrick Sulem, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Unnur Thorsteinsdottir, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland.
Gudmundur Thorgeirsson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland; Division of Cardiology, Department of Internal Medicine, Landspitali – National University Hospital of Iceland, Hringbraut, 101 Reykjavik, Iceland.
Hilma Holm, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland.
Daniel F Gudbjartsson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; School of Engineering and Natural Sciences, University of Iceland, Dunhagi, 101 Reykjavik, Iceland.
Kari Stefansson, deCODE genetics/Amgen, Inc., Sturlugata 8, 102 Reykjavik, Iceland; Faculty of Medicine, University of Iceland, Vatnsmýrarvegur, 101 Reykjavik, Iceland.
References
- 1. Lammert F, Wang D-H. New insights into the genetic regulation of intestinal cholesterol absorption. Gastroenterology 2005;129:718–734. [DOI] [PubMed] [Google Scholar]
- 2. Sudhop T, LüTjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 2002;106:1943–1948. [DOI] [PubMed] [Google Scholar]
- 3. Ajagbe BO, Othman RA, Myrie SB. Plant sterols, stanols, and sitosterolemia. J AOAC Int 2015;98:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolovou G, Voudris V, Drogari E, Palatianos G, Cokkinos DV. Coronary bypass grafts in a young girl with sitosterolemia. Eur Heart J 1996;17:965–966. [DOI] [PubMed] [Google Scholar]
- 5. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee J-Y, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RYL, Wright AF, Witteman JCM, Wilson JF, Willemsen G, Wichmann H-E, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJG, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BWJH, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PKE, Lucas G, Luben R, Loos RJF, Lokki M-L, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw K-T, Kaprio J, Kaplan LM, Johansson Å, Jarvelin M-R, Cecile J. W., Janssens A, Ingelsson E, Igl W, Kees Hovingh G, Hottenga J-J, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJC, de Faire U, Crawford G, Collins FS, Chen Y-D. I, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai E-S, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJP, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010;466:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, Penninx B, Janssens A, Wilson JF, Spector T, Martin NG, Pedersen NL, Kyvik KO, Kaprio J, Hofman A, Freimer NB, Jarvelin M-R, Gyllensten U, Campbell H, Rudan I, Johansson A, Marroni F, Hayward C, Vitart V, Jonasson I, Pattaro C, Wright A, Hastie N, Pichler I, Hicks AA, Falchi M, Willemsen G, Hottenga JJ, de Geus EJ, Montgomery GW, Whitfield J, Magnusson P, Saharinen J, Perola M, Silander K, Isaacs A, Sijbrands EJ, Uitterlinden AG, Witteman JC, Oostra BA, Elliott P, Ruokonen A, Sabatti C, Gieger C, Meitinger T, Kronenberg F, Döring A, Wichmann HE, Smit JH, McCarthy MI, van Duijn CM, Peltonen L. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet 2009;41:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stender S, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. The ABCG5/8 cholesterol transporter and myocardial infarction versus gallstone disease. J Am Coll Cardiol 2014;63:2121–2128. [DOI] [PubMed] [Google Scholar]
- 8. Teupser D, Baber R, Ceglarek U, Scholz M, Illig T, Gieger C, Holdt LM, Leichtle A, Greiser KH, Huster D, Linsel-Nitschke P, Schäfer A, Braund PS, Tiret L, Stark K, Raaz-Schrauder D, Fiedler GM, Wilfert W, Beutner F, Gielen S, Großhennig A, König IR, Lichtner P, Heid IM, Kluttig A, El Mokhtari NE, Rubin D, Ekici AB, Reis A, Garlichs CD, Hall AS, Matthes G, Wittekind C, Hengstenberg C, Cambien F, Schreiber S, Werdan K, Meitinger T, Loeffler M, Samani NJ, Erdmann J, Wichmann H-E, Schunkert H, Thiery J. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ Cardiovasc Genet 2010;3:331–339. [DOI] [PubMed] [Google Scholar]
- 9. Buch S, Schafmayer C, Völzke H, Becker C, Franke A, Eller-Eberstein H V, Kluck C, Bässmann I, Brosch M, Lammert F, Miquel JF, Nervi F, Wittig M, Rosskopf D, Timm B, Höll C, Seeger M, ElSharawy A, Lu T, Egberts J, Fändrich F, Fölsch UR, Krawczak M, Schreiber S, Nürnberg P, Tepel J, Hampe J. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet 2007;39:995–999. [DOI] [PubMed] [Google Scholar]
- 10. Joshi AD, Andersson C, Buch S, Stender S, Noordam R, Weng L-C, Weeke PE, Auer PL, Boehm B, Chen C, Choi H, Curhan G, Denny JC, De Vivo I, Eicher JD, Ellinghaus D, Folsom AR, Fuchs C, Gala M, Haessler J, Hofman A, Hu F, Hunter DJ, Janssen HLA, Kang JH, Kooperberg C, Kraft P, Kratzer W, Lieb W, Lutsey PL, Darwish Murad S, Nordestgaard BG, Pasquale LR, Reiner AP, Ridker PM, Rimm E, Rose LM, Shaffer CM, Schafmayer C, Tamimi RM, Uitterlinden AG, Völker U, Völzke H, Wakabayashi Y, Wiggs JL, Zhu J, Roden DM, Stricker BH, Tang W, Teumer A, Hampe J, Tybjærg-Hansen A, Chasman DI, Chan AT, Johnson AD. Four susceptibility loci for gallstone disease identified in a meta-analysis of genome-wide association studies. Gastroenterology 2016;151:351–363.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stitziel NO, Won HH, Morrison AC, Peloso GM, Do R, Lange LA, Fontanillas P, Gupta N, Duga S, Goel A, Farrall M, Saleheen D, Ferrario P, König I, Asselta R, Merlini PA, Marziliano N, Notarangelo MF, Schick U, Auer P, Assimes TL, Reilly M, Wilensky R, Rader DJ, Hovingh GK, Meitinger T, Kessler T, Kastrati A, Laugwitz KL, Siscovick D, Rotter JI, Hazen SL, Tracy R, Cresci S, Spertus J, Jackson R, Schwartz SM, Natarajan P, Crosby J, Muzny D, Ballantyne C, Rich SS, O'Donnell CJ, Abecasis G, Sunaev S, Nickerson DA, Buring JE, Ridker PM, Chasman DI, Austin E, Kullo IJ, Weeke PE, Shaffer CM, Bastarache LA, Denny JC, Roden DM, Palmer C, Deloukas P, Lin DY, Tang ZZ, Erdmann J, Schunkert H, Danesh J, Marrugat J, Elosua R, Ardissino D, McPherson R, Watkins H, Reiner AP, Wilson JG, Altshuler D, Gibbs RA, Lander ES, Boerwinkle E, Gabriel S, Kathiresan S. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med 2014;371:2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci U S A 2006;103:1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen M-R, Tokgözoğlu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helgadottir A, Gretarsdottir S, Thorleifsson G, Hjartarson E, Sigurdsson A, Magnusdottir A, Jonasdottir A, Kristjansson H, Sulem P, Oddsson A, Sveinbjornsson G, Steinthorsdottir V, Rafnar T, Masson G, Jonsdottir I, Olafsson I, Eyjolfsson GI, Sigurdardottir O, Daneshpour MS, Khalili D, Azizi F, Swinkels DW, Kiemeney L, Quyyumi AA, Levey AI, Patel RS, Hayek SS, Gudmundsdottir IJ, Thorgeirsson G, Thorsteinsdottir U, Gudbjartsson DF, Holm H, Stefansson K. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat Genet 2016;48:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grundy SM. Does dietary cholesterol matter? Curr Atheroscler Rep 2016;18:68. [DOI] [PubMed] [Google Scholar]
- 16. Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML, Perez-Escamilla R, Siega-Riz AM, Story M, Lichtenstein AH. The 2015 dietary guidelines advisory committee scientific report: development and major conclusions. Adv Nutr 2016;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman Mj Backer GGD, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 18. Weingärtner O, Böhm M, Laufs U. Controversial role of plant sterol esters in the management of hypercholesterolaemia. Eur Heart J 2008;30:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol 2004;24:2326–2332. [DOI] [PubMed] [Google Scholar]
- 20. Hansel B, Carrié A, Brun-Druc N, Leclert G, Chantepie S, Coiffard A-S, Kahn J-F, Chapman MJ, Bruckert E. Premature atherosclerosis is not systematic in phytosterolemic patients: severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis 2014;234:162–168. [DOI] [PubMed] [Google Scholar]
- 21. Gylling H, Plat J, Turley S, Ginsberg HN, Ellegård L, Jessup W, Jones PJ, Lütjohann D, Maerz W, Masana L, Silbernagel G, Staels B, Borén J, Catapano AL, Backer GD, Deanfield J, Descamps OS, Kovanen PT, Riccardi G, Tokgözoglu L, Chapman MJ; European Atherosclerosis Society Consensus Panel on Phytosterols. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014;232:346–360. [DOI] [PubMed] [Google Scholar]
- 22. Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, Thorand B, Giampaoli S, Brambilla P, Tunstall-Pedoe H, Moitry M, Iacoviello L, Veronesi G, Grassi G, Mathiesen EB, Söderberg S, Linneberg A, Brenner H, Amouyel P, Ferrières J, Tamosiunas A, Nikitin YP, Drygas W, Melander O, Jöckel K-H, Leistner DM, Shaw JE, Panagiotakos DB, Simons LA, Kavousi M, Vasan RS, Dullaart RPF, Wannamethee SG, Risérus U, Shea S, de Lemos JA, Omland T, Kuulasmaa K, Landmesser U, Blankenberg S; Multinational Cardiovascular Risk Consortium. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet 2019;394:2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu X, Peloso GM, Liu DJ, Wu Y, Zhang H, Zhou W, Li J, Tang C. S-M, Dorajoo R, Li H, Long J, Guo X, Xu M, Spracklen CN, Chen Y, Liu X, Zhang Y, Khor CC, Liu J, Sun L, Wang L, Gao Y-T, Hu Y, Yu K, Wang Y, Cheung CYY, Wang F, Huang J, Fan Q, Cai Q, Chen S, Shi J, Yang X, Zhao W, Sheu WH-H, Cherny SS, He M, Feranil AB, Adair LS, Gordon-Larsen P, Du S, Varma R, Chen Y-DI, Shu X-O, Lam KSL, Wong TY, Ganesh SK, Mo Z, Hveem K, Fritsche LG, Nielsen JB, Tse H-F, Huo Y, Cheng C-Y, Chen YE, Zheng W, Tai ES, Gao W, Lin X, Huang W, Abecasis G, Kathiresan S, Mohlke KL, Wu T, Sham PC, Gu D, Willer CJ; GLGC Consortium. Exome chip meta-analysis identifies novel loci and East Asian–specific coding variants that contribute to lipid levels and coronary artery disease. Nat Genet 2017;49:1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stitziel NO, Stirrups KE, Masca NGD, Erdmann J, Ferrario PG, König IR, Weeke PE, Webb TR, Auer PL, Schick UM, Lu Y, Zhang H, Dube M-P, Goel A, Farrall M, Peloso GM, Won H-H, Do R, Iperen E. V, Kanoni S, Kruppa J, Mahajan A, Scott RA, Willenberg C, Braund PS, Capelleveen JC, van Doney ASF, Donnelly LA; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigator. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med 2016;374:1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis HR, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SPN, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem 2004;279:33586–33592. [DOI] [PubMed] [Google Scholar]
- 26. Matthan NR, Lichtenstein AH. Approaches to measuring cholesterol absorption in humans. Atherosclerosis 2004;174:197–205. [DOI] [PubMed] [Google Scholar]
- 27. Marschall H-U, Einarsson C. Gallstone disease. J Intern Med 2007;261:529–542. [DOI] [PubMed] [Google Scholar]
- 28. Zhong VW, Horn LV, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM, Allen NB. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA 2019;321:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lütjohann D, Stellaard F, Mulder MT, Sijbrands EJG, Weingärtner O. The emerging concept of ‘individualized cholesterol-lowering therapy’: a change in paradigm. Pharmacol Ther 2019;199:111–116. [DOI] [PubMed] [Google Scholar]
- 30. Weingärtner O, Teupser D, Patel SB. The atherogenicity of plant sterols: the evidence from genetics to clinical trials. J AOAC Int 2015;98:742–749. [DOI] [PubMed] [Google Scholar]
- 31. Bao L, Li Y, Deng S-X, Landry D, Tabas I. Sitosterol-containing lipoproteins trigger free sterol-induced caspase-independent death in ACAT-competent macrophages. J Biol Chem 2006;281:33635–33649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.