Abstract
Aims
The association of body weight and weight change with mortality and cardiovascular (CV) outcome in patients with diabetes mellitus (DM) is not clearly established. We assessed the relationship between weight, weight change, and outcomes in patients with established CV risk factors and type 2 DM or pre-diabetes.
Methods and results
A total of 12 521 participants from the ORIGIN trial were grouped in BMI categories of low body weight [body mass index (BMI) < 22 kg/m2] normal (22–24.9), overweight (25–29.9), obesity Grades 1–3 (30–34.9, 35–39.9, ≥40 kg/m2, respectively). Outcome variables included total and CV mortality and composite outcomes of CV death, non-fatal stroke, or myocardial infarction plus revascularization or heart failure hospitalization. Follow-up was 6.2 years (interquartile range 5.8–6.7 years). After multivariable adjustment, lowest risks were seen in patients with overweight and mild obesity for total mortality [overweight: hazard ratio (HR) 0.80 (95% confidence interval (CI) 0.69–0.91); obesity Grade 1: HR 0.82 (0.71–0.95), both P < 0.01)] and CV mortality [overweight: HR 0.79 (0.66–0.94); obesity Grade 1: 0.79 (0.65–0.95), all compared to patients with normal BMI, P < 0.05]. Obesity of any severity was not associated with higher mortality. Low body weight was related to higher mortality [HR 1.28 (1.02–1.61); CV mortality: HR 1.34 (1.01–1.79), P < 0.05]. A continued 2-year weight loss was associated with higher risk of mortality [HR 1.32 (1.18–1.46), P < 0.0001] and CV mortality [HR 1.18 (1.02–1.35), compared to patients without weight loss, P < 0.05]. In turn, weight gain was not related to any adverse outcome.
Conclusion
Obesity in patients with DM or pre-diabetes and CV risk profile was not associated with higher mortality or adverse CV outcome. The lowest mortality risk was seen in patients with overweight and moderate obesity (BMI 25–35 kg/m2). Weight loss was an independent risk factor for higher mortality compared to no weight loss.
Keywords: Diabetes mellitus, Body weight, Weight change, Outcome, Risk factor, Obesity paradigm
See page 2678 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa398)
Introduction
Overweight and obesity are established risk factors for cardiovascular (CV) disease1–3 which is the leading cause of death in modern society.4 Excessive body weight as a key component of the metabolic syndrome predisposes to insulin resistance and promotes development of type 2 diabetes mellitus (DM) which, too, is a risk factor for CV disease. Weight control is therefore widely promoted as a major preventive measure to reduce the burden of diabetes and subsequent CV disease. In accord with this concept, current guidelines for prevention and treatment of DM5 and of CV disease6 recommend weight reduction irrespective of CV comorbidity to improve disease status and outcome.
The impact of obesity on mortality in patients with established type 2 DM or at high risk of DM and with a prevalent CV risk profile is, however, less clear. It appears that with increasing age the optimum (or ideal) body weight is shifting towards a higher body mass index (BMI) range compared to the WHO-defined ‘ideal BMI’ of 18.5–25 kg/m2.7 , 8 Increasing evidence demonstrates a survival benefit with overweight and mild obesity in patients at risk or with established CV disease.9 Previous registry data suggest a survival benefit in overweight patients with DM without CV disease compared to normal weight.10 While most clinical data on body weight and outcome report on a single-time assessment of body weight, prospective evidence on weight change affecting outcome is limited and not supportive of a beneficial effect of weight reduction. The LOOK-AHEAD trial applied a comprehensive lifestyle intervention program to overweight patients with type 2DM.11 A reduction of body weight and of subsequent surrogate markers of the metabolic syndrome was indeed achieved. Yet, the study showed that this weight reduction did not improve any of the outcome variables on mortality or morbidity. A 19-year follow-up study of patients with intentional weight loss of the randomized clinical trial ‘Diabetes Care in General Practice’ (DCGP) showed that weight loss in obese patients regardless of intention did not improve outcome but was an independent risk factor for increased all-cause mortality.12
The LOOK-AHEAD trial included 5145 relatively healthy patients with DM (14% with CV comorbidity), and the DCGP study was a population based cohort study in 1381 patients with newly diagnosed type 2 DM and a moderate CV risk profile (26–30% macrovascular comorbidity). In contrast, the ORIGIN trial investigated 12 537 patients with type 2 DM or pre-diabetes with 100% prevalent CV risk factors.13 The aim of this analysis from the ORIGIN trial data is to assess the relationship between body weight, weight change, and CV outcome in this cohort at high risk of CV events.
Methods
Previous publications describe the design and the results of the ORIGIN trial (ClinicalTrials.gov number, NCT00069784, funded by Sanofi).13 , 14 In this randomized, controlled trial, patients with CV risk factors and either pre-diabetes or type 2 DM were allocated to receive insulin therapy or standard care and to receive n-3 fatty acids or placebo in a 2 × 2 factorial design. A total of 12 537 participants were enrolled in 573 clinical sites in 40 countries. Mean follow-up was 6.2 years (interquartile range 5.8–6.7 years). Body weight and height were assessed at baseline and weight was assessed at 2-month and at yearly follow-up throughout the trial. The primary outcome was a composite endpoint of CV death, non-fatal myocardial infarction (MI), or non-fatal stroke. Further outcome measures were individual components of the primary endpoint, all-cause mortality, total stroke, and total MI. The ORIGIN trial complies with the Declaration of Helsinki and was approved by the Ethical Committee at each site and each participant provided written informed consent.
Body weight and weight change assessment
Body mass index was assessed as ratio of body weight and height squared and was categorized according to the WHO criteria as underweight (BMI < 18.5 kg/m2), normal weight (18.5–<25 kg/m2), overweight (25–<30 kg/m2), obesity Grade 1 (30–<35 kg/m2), Grade 2 (35–<40 kg/m2) and Grade 3 (≥40 kg/m2). Due to the low number of participants with a BMI below 18.5 kg/m2, we expanded in this analyses the category of low body weight to all participants with a BMI below 22 kg/m2. Accordingly and in line with accumulating epidemiologic evidence, we used as reference group (normal weight) patients with a BMI 22–<25 kg/m2.3
Weight change was assessed as % weight change from baseline. Sustained weight gain was defined as continued weight gain of ≥1.0 kg/year over 2 years with no intervening weight loss of ≥0.5 kg. Sustained weight loss was assessed as continued weight loss of ≥1 kg/year over 2 years with no intervening weight gain of ≥0.5 kg.
Outcomes
The primary outcome for this analysis was all-cause mortality. We further investigated the two composite outcomes of the ORIGIN protocol comprising: (a) primary composite endpoint of first occurrence of CV death, non-fatal MI, or non-fatal stroke; and (b) expanded composite endpoint of first occurrence of these three outcomes or revascularization or hospitalization for heart failure (HF). Further analyses included individual components of these composites and further outcomes such as total MI and total stroke.
Statistical analyses
Data analysis followed a prospectively designed analysis plan and used SAS software (version 9.4 for Solaris). Clinical characteristics are presented as means (± standard deviation) for continuous variables and by frequency (percentage) for categorical variables.
For comparison of baseline characteristics between BMI groups, analysis of variance (ANOVA) for continuous variables and χ2 test for categorical variables were used. Event rates between groups were analysed by adjusted Cox regression model. Time to event curves for BMI subgroups were constructed for outcome variables with the use of adjusted survival curves. Hazard ratios (HR) and 95% confidence intervals (95% CI) for each outcome according to BMI group were calculated using the BMI category 22–<25 kg/m2 as reference in Cox regression models adjusted (a) for age and sex (minimum adjustment) and (b) for age, sex, smoking status, previous CV events, duration of DM, waist circumference, systolic blood pressure, medication with ACE or ARB, beta-blocker medication, statin, LDL level, HbA1c level, eGFR, allocation to treatment arm glargine, and allocation to treatment arm n-3 fatty acid (full adjustment model). Previous CV events were defined according to the ORIGIN protocol as a history of MI, stroke, or revascularization.14 Heart failure was an exclusion criterion for the trial. Weight change analyses were performed using a multivariate adjusted Cox models that included weight gain and weight loss as time varying variables. A nominal P-value of 0.05 was used to indicate statistical significance.
Results
A total of 12 521 participants with baseline BMI measurements (mean age 63.5 years, 35% female) were included in this analysis (99.9% of the total study population). During a median follow-up of 6.2 years (interquartile range 5.8–6.7 years), these individuals suffered 1910 deaths (15.3%) of which 1153 (9.2%) were CV deaths. Clinical characteristics and medications in BMI subgroups are shown in Table 1. Overweight and obese participants were younger, had a higher prevalence of hypertension, and a higher blood pressure and LDL cholesterol than the normal weight participants. Obese participants also had a lower prevalence of prior CV events than normal weight participants at the time of randomization. No linear trends across BMI categories were seen for smoking status, previously known type 2 DM, or newly diagnosed DM at baseline.
Table 1.
Baseline characteristics of study participants according to body mass index categories
| BMI <22 (n = 478) |
22–24.9 (n = 1561) |
25–29.9 (n = 5044) |
30–34.9 (n = 3600) |
35–39.9 (n = 1300) |
≥40 (n = 538) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N* | N* | N* | N* | N* | N* | P-value | ||||||
| BMI (kg/m2) | 20.5 (1.4) | 23.8 (0.8) | 27.6 (1.40) | 32.1 (1.4) | 37.0 (1.40) | 43.8 (3.8) | <0.0001 | ||||||
| Age (years) | 65.0 (8.9) | 64.7 (8.1) | 64.4 (7.8) | 62.9 (7.5) | 61.6 (7.4) | 60.2 (6.7) | <0.0001 | ||||||
| Males n (%) | 272 (56.9) | 1052 (67.4) | 5043 | 3594 (71.3) | 2312 (64.2) | 703 (54.1) | 203 (37.7) | <0.0001 | |||||
| Waist males (cm) | 272 | 82.2 (10.1) | 1049 | 89.6 (8.2) | 3587 | 97.8 (8.8) | 2311 | 108.2 (8.4) | 699 | 117.7 (10.2) | 199 | 128.1 ( 13.4) | <0.0001 |
| Hip males (cm) | 272 | 88.9 (9.1) | 1049 | 94.5 (8.7) | 3587 | 100.6 (8.6) | 2311 | 107.8 (9.0) | 699 | 115.6 ( 11.2) | 192 | 125.1 (14.2) | <0.0001 |
| Waist females (cm) | 206 | 77.2 (10.4) | 509 | 83.9 (10.6) | 1447 | 91.2 (9.3) | 1287 | 100.7 (9.1) | 596 | 108.4 (9.8) | 332 | 117.9 (11.0) | <0.0001 |
| Hip females (cm) | 206 | 88.3 (9.1) | 509 | 94.6 (9.2) | 1442 | 101.8 (9.3) | 1287 | 111.3 (9.1) | 595 | 120.0 (9.5) | 312 | 131.1 ( 10.8) | <0.0001 |
| Ever smoked n (%) | 230 (48.1) | 842 (53.9) | 3017 (59.8) | 2227 (61.9) | 745 (57.3) | 274 (50.9) | <0.0001 | ||||||
| Previous diabetes n (%) | 405 (84.7) | 1314 (84.2) | 5043 | 4145 (82.2) | 2892 (80.3) | 1095 (84.2) | 456 (84.8) | 0.0009 | |||||
| New diabetesa n (%) | 15 (3.1) | 85 (5.4) | 316 (6.3) | 241 (6.7) | 73 (5.6) | 28 (5.2) | 0.0312 | ||||||
| Diabetes duration years (SD) | 7.16 (7.57) | 6.14 (7.04) | 4.87 (5.97) | 4.28 (5.33) | 3.78 (4.57) | 3.72 (4.71) | <0.0001 | ||||||
| Previous CV event n (%) | 296 (61.9) | 960 (61.5) | 3178 (63.0) | 2084 (57.9) | 648 (49.8) | 204 (37.9) | <0.0001 | ||||||
| Hypertension n (%) | 309 (64.6) | 1124 (72.0) | 3906 (77.4) | 2980 (82.8) | 1152 (88.6) | 483 (89.8) | <0.0001 | ||||||
| SBP (mmHg) | 141.4 (24.4) | 144.1 (23.0) | 5043 | 145.3 (21.6) | 3598 | 146.9 (21.1) | 1298 | 147.7 (21.4) | 537 | 147.1 (22.1) | <0.0001 | ||
| DBP (mmHg) | 79.0 (11.7) | 81.2 (11.9) | 5041 | 83.3 ( 11.6) | 3596 | 85.4 (11.9) | 87.6 ( 12.1) | 88.3 ( 13.0) | <0.0001 | ||||
| LDL cholesterol (mmol/L) | 472 | 2.92 (1.05) | 1551 | 2.87 (1.03) | 4961 | 2.87 (1.03) | 3533 | 2.90 (1.04) | 1265 | 2.97 (1.1) | 532 | 3.02 (0.98) | 0.0028 |
| HbA1c (%) | 471 | 6.5 (0.96) | 1537 | 6.5 (1.0) | 4963 | 6.5 (1.0) | 3570 | 6.5 (0.9) | 1284 | 6.6 (0.93) | 534 | 6.6 (0.9) | 0.0158 |
| eCRF (mL/min/1.73 m2) | 79.5 (26.2) | 1560 | 77.2 (21.2) | 5037 | 76.9 (21.4) | 3595 | 77.1 (20.6) | 1299 | 78.5 (20.8) | 78.5 (21.8) | 0.0232 | ||
| Glargine allocation n (%) | 241 (50.4) | 756 (48.4) | 2587 (51.3) | 1766 (49.1) | 639 (49.2) | 262 (48.7) | 0.2271 | ||||||
| Omega 3 allocation n (%) | 248 (51.9) | 773 (49.5) | 2537 (50.3) | 3599 | 1787 (49.7) | 656 (50.5) | 271 (50.4) | 0.9381 | |||||
| Thiazide diuretic n (%) | 48 (10.0) | 220 (14.1) | 832 (16.5) | 764 (21.2) | 342 (26.3) | 163 (30.3) | <0.0001 | ||||||
| ACE-I or ARB n (%) | 279 (58.4) | 964 (61.8) | 3412 (67.6) | 2612 (72.6) | 989 (76.1) | 420 (78.1) | <0.0001 | ||||||
| Beta blocker n (%) | 178 (37.2) | 725 (46.4) | 2671 (53.0) | 2035 (56.5) | 718 (55.2) | 267 (49.6) | <0.0001 | ||||||
| Other BP drug n (%) | 117 (37.0) | 566 (36.3) | 1981 (39.3) | 1541 (42.8) | 601 (46.2) | 273 (50.7) | <0.0001 | ||||||
| Statin n (%) | 206 (43.1) | 815 (52.2) | 2827 (56.0) | 2012 (55.9) | 651 (50.1) | 225 (41.8) | <0.0001 | ||||||
| Antiplatelet drug n (%) | 318 (66.5) | 1085 (69.5) | 3583 (71.0) | 2522 (70.1) | 841 (64.7) | 310 (57.6) | <0.0001 | ||||||
Values are n (%) or mean (SD), N* number of patients if missing data, P-values for Log-rank test between categories. aNewly diagnosed diabetes at baseline. Previous CV event is defined according to the ORIGIN protocol as previous myocardial infarction, stroke or revascularization [15].
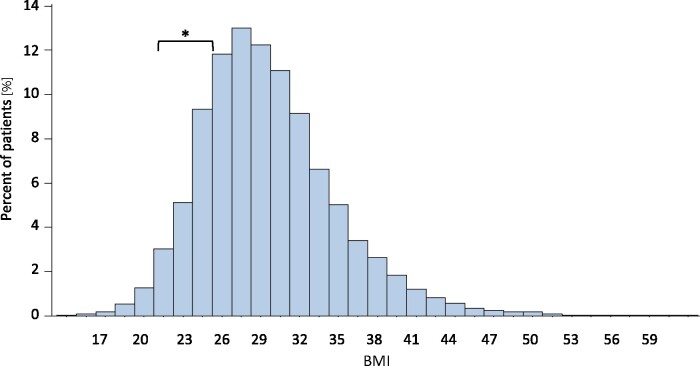
The distribution of BMI within the study population is shown in Figure 1. The majority of participants were overweight (40.3%) and mildly obese (28.8%) while only 12.5% had normal weight. Absolute event numbers for all outcomes and composite endpoints are shown in Table 2. For total mortality, CV mortality, and for the primary composite endpoint a consistent inverse relation of event rates and BMI categories was observed with lowest event rates in severely obese or obese Grade 2 (CV mortality) patients (all P < 0.01).
Figure 1.
Frequency distribution of body mass index in the ORIGIN study population. The body mass index range 22–24.9 kg/m2 is considered as normal body mass index (square bracket*).
Table 2.
Incidence of events event for all outcomes and composite endpoints in BMI categories in absolute numbers and percent per BMI subgroup
|
BMI <22 (
n = 478)
|
22–24.9 (
n = 1561)
|
25–29.9 (n = 5044) |
30–34.9 (n = 3600) |
35–39.9 (n = 1300) |
≥40 (n = 538) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | n | % | n | % | n | % | n | % | n | % | n | % | P-value |
| All-cause mortality | 107 | 22.4 | 289 | 18.5 | 763 | 15.1 | 507 | 14.1 | 175 | 13.5 | 69 | 12.8 | <0.0001 |
| CV mortality | 69 | 14.4 | 181 | 11.6 | 467 | 9.3 | 303 | 8.4 | 93 | 7.2 | 40 | 7.4 | 0.0004 |
| Primary composite endpointa | 100 | 20.9 | 304 | 19.5 | 867 | 17.2 | 528 | 14.7 | 184 | 14.2 | 67 | 12.5 | 0.0024 |
| Expanded composite endpointb | 129 | 27.0 | 463 | 29.7 | 1468 | 29.1 | 1007 | 28.0 | 334 | 25.7 | 113 | 21.0 | 0.7444 |
| Non-fatal MI | 27 | 5.6 | 77 | 4.9 | 253 | 5.0 | 164 | 4.6 | 64 | 4.9 | 16 | 3.0 | 0.5437 |
| Non-fatal Stroke | 20 | 4.2 | 86 | 5.5 | 243 | 4.8 | 129 | 3.6 | 42 | 3.2 | 16 | 3.0 | 0.0608 |
| Total MI | 30 | 6.3 | 84 | 5.4 | 283 | 5.6 | 176 | 4.9 | 71 | 5.5 | 17 | 3.2 | 0.3661 |
| Total stroke | 27 | 5.6 | 101 | 6.5 | 292 | 5.8 | 157 | 4.4 | 54 | 4.2 | 18 | 3.3 | 0.0618 |
| Revascularization | 43 | 9.0 | 198 | 12.7 | 777 | 15.4 | 555 | 15.4 | 153 | 11.8 | 40 | 7.4 | 0.0005 |
| HF hospitalization | 20 | 4.2 | 75 | 4.8 | 232 | 4.6 | 215 | 6.0 | 74 | 5.7 | 36 | 6.7 | <0.0001 |
P-values for differences between BMI groups, adjusted Cox regression model (type 3).
Primary composite endpoint: composite of first occurrence of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.
Expanded composite endpoint: composite of first occurrence of these three outcomes or revascularization or heart failure hospitalization.
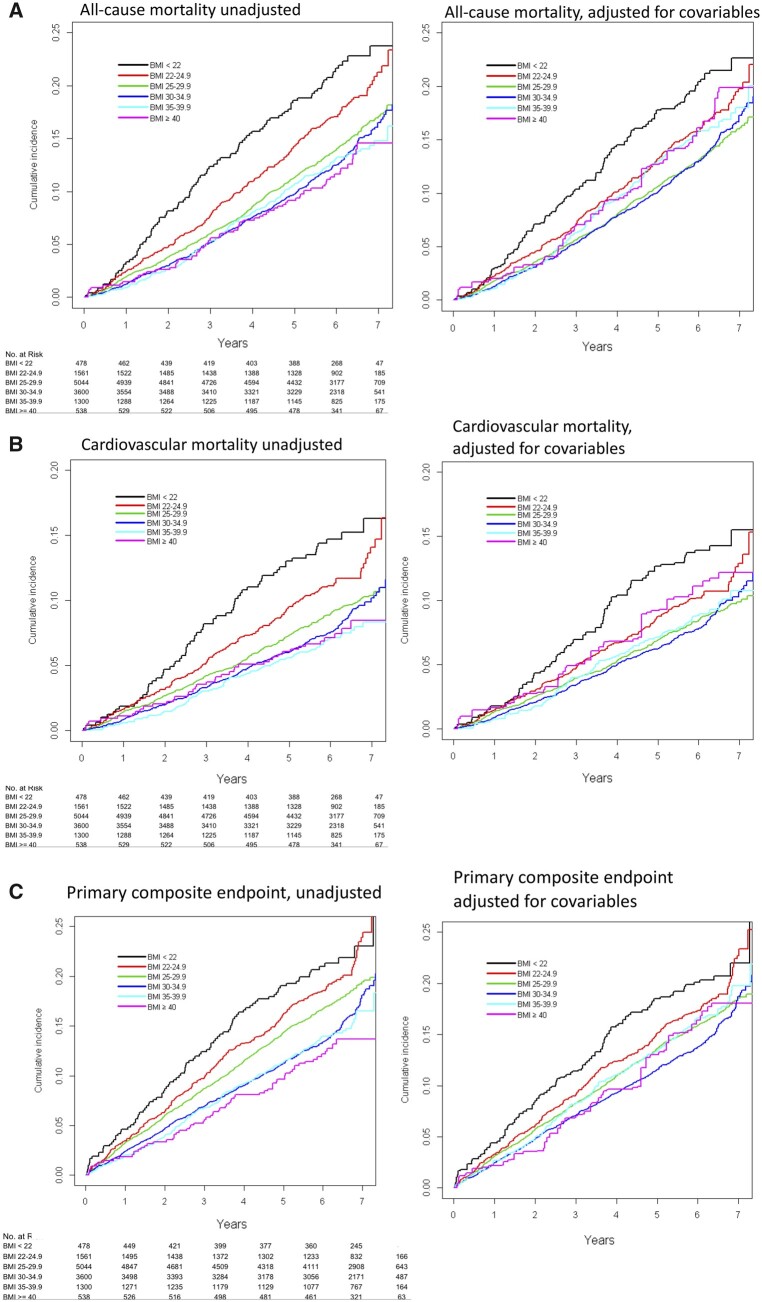
Time to event curves for total mortality and for CV mortality as well as for primary composite endpoint are shown in Figure 2A–C.
Figure 2.
Time to event curves for outcomes in body mass index categories of the ORIGIN study population. Unadjusted (left) and multivariate adjusted (right) models. (A) All-cause mortality, (B) cardiovascular mortality, (C) primary composite endpoint.
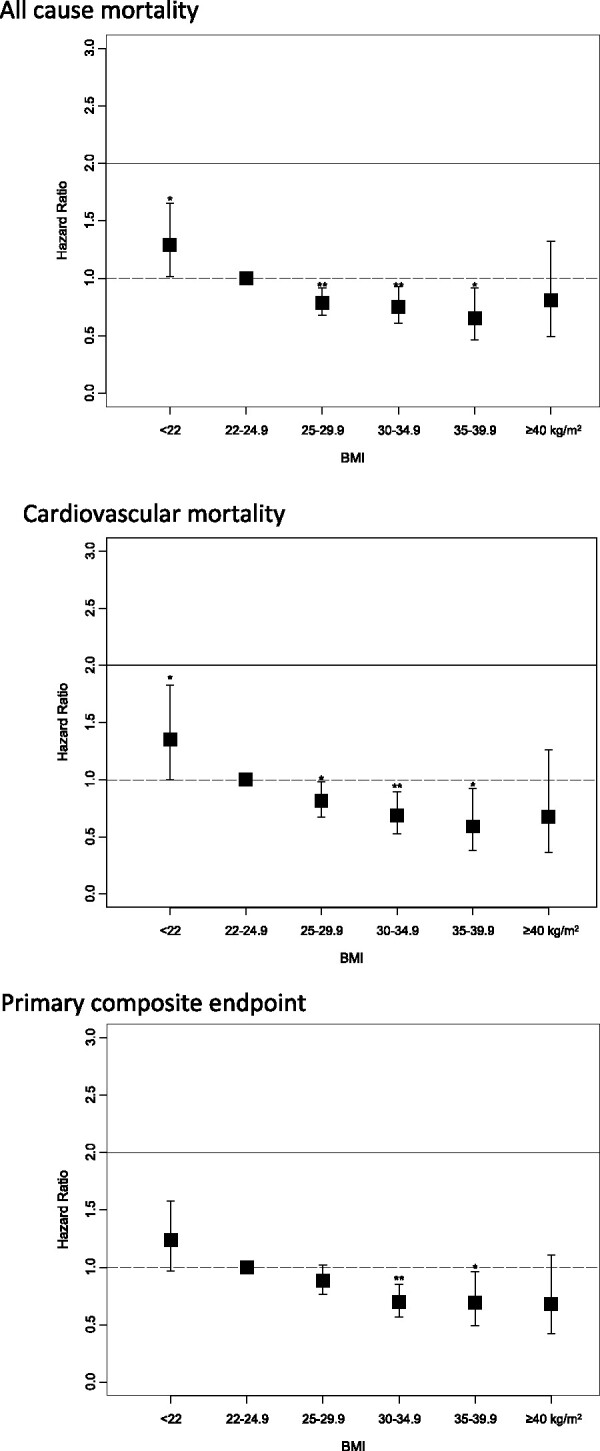
Hazard ratios for outcomes in BMI subgroups after adjustment for age and sex and after full adjustment for all available covariables are shown in Table 3. Adjusted risk models show that patients with overweight and with mild to moderate obesity have a significant lower risk of all-cause mortality, CV mortality, and for the primary composite endpoint compared to the reference group (Table 3 and Take home figure). Outcomes for non-fatal endpoints are shown in Supplementary material online, Table S1. None of the mortality endpoints or the composite outcomes showed a higher risk in CV outcomes with higher BMI. In contrast, patients with low body weight had a higher risk of all-cause mortality and of CV mortality (Table 3). A sensitivity analysis further subdivided the low BMI subgroup into tertiles and showed a heterogeneous distribution of outcome across tertiles. This suggests that the adverse outcomes in this subgroup were not driven exclusively by a subset of patients with extremely low BMI (Supplementary material online, Table S2).
Table 3.
Outcome in BMI subgroups, Cox regression models (HR, 95% confidence intervals) adjusted for age and sex (upper line) and full adjustment (lower line); BMI category 22–24.9 kg/m2 was used as reference category
| BMI <22 |
25–29.9
|
30–34.9
|
35–39.9
|
≥40 kg/m2
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| All-cause mortality | |||||||||||||||
| Age and sex adjusted | 1.28 | 1.03–1.60 | 0.029 | 0.80 | 0.70–0.92 | 0.001 | 0.83 | 0.72–0.96 | 0.011 | 0.94 | 0.77–1.14 | 0.518 | 1.02 | 0.77–1.35 | 0.876 |
| Full adjustment | 1.29 | 1.01–1.65 | 0.038 | 0.79 | 0.68–0.91 | 0.002 | 0.75 | 0.61–0.93 | 0.008 | 0.65 | 0.46–0.92 | 0.014 | 0.81 | 0.50–1.32 | 0.395 |
| CV mortality | |||||||||||||||
| Age and sex adjusted | 1.31 | 0.99–1.74 | 0.056 | 0.78 | 0.66–0.93 | 0.006 | 0.79 | 0.66–0.96 | 0.015 | 0.80 | 0.62–1.04 | 0.099 | 0.96 | 0.67–1.38 | 0.833 |
| Full adjustment | 1.35 | 1.00–1.83 | 0.048 | 0.81 | 0.67–0.98 | 0.033 | 0.69 | 0.53–0.89 | 0.005 | 0.59 | 0.38–0.92 | 0.020 | 0.68 | 0.36–1.26 | 0.217 |
| Primary composite endpointa | |||||||||||||||
| Age and sex adjusted | 1.13 | 0.90–1.42 | 0.278 | 0.86 | 0.75–0.98 | 0.023 | 0.79 | 0.68–0.91 | 0.001 | 0.87 | 0.72–1.06 | 0.163 | 0.83 | 0.63–1.10 | 0.189 |
| Full adjustment | 1.24 | 0.97–1.58 | 0.090 | 0.88 | 0.76–1.02 | 0.089 | 0.70 | 0.57–0.85 | 0.001 | 0.69 | 0.49–0.96 | 0.029 | 0.68 | 0.42–1.11 | 0.122 |
| Expanded composite endpointb | |||||||||||||||
| Age and sex adjusted | 0.95 | 0.78–1.16 | 0.637 | 0.96 | 0.87–1.07 | 0.449 | 0.98 | 0.88–1.10 | 0.763 | 1.03 | 0.89–1.19 | 0.718 | 0.86 | 0.70–1.07 | 0.190 |
| Full adjustment | 1.03 | 0.83–1.27 | 0.798 | 0.95 | 0.85–1.070 | 0.421 | 0.86 | 0.74–1.01 | 0.072 | 0.86 | 0.66–1.12 | 0.254 | 0.78 | 0.53–1.12 | 0.217 |
Full adjustment for age, sex, ever smoker, previous cardiovascular event, LDL, HbA1c, eGFR, allocation to glargine, allocation to omega 3 fatty acid, duration of DM, waist circumference, systolic blood pressure, ACE or ARB medication, beta-blocker medication, statin medication. Hip circumference, hypertension and diastolic blood pressure were not included in the model because of collinearity.
Primary composite endpoint: composite of first occurrence of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.
Expanded composite endpoint: composite of first occurrence of these three outcomes or revascularization or heart failure hospitalization.
Take home figure.

Outcome in body mass index categories, multivariable adjusted Cox regression models (hazard ratio, 95% confidence intervals), body mass index category 22–24.9 kg/m2 as reference category. *P < 0.05; **P < 0.01.
Weight change analyses
Weight gain and weight loss (% of body weight) during the first year of follow-up was assessed in multivariable adjusted models (Table 4). Weight gain was related to lower mortality (all-cause and CV mortality) and to better outcome (composite outcomes, stroke, revascularization, or HF hospitalization) in comparison to patients with no weight gain. In contrast, weight loss was related to higher risk of death, and to worse outcome compared to patients with no weight loss.
Table 4.
Outcome analyses for subgroups with weight gain and weight loss (per 5% of body weight change) during the first year of follow (Cox proportional hazard analyses (HR, 95% confidence intervals)
| Weight gain (% body weight) |
Weight loss (% body weight) |
|||||
|---|---|---|---|---|---|---|
| Outcome variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| All-cause mortality | 0.857 | 0.827–0.887 | <0.0001 | 1.167 | 1.127–1.208 | <0.0001 |
| CV mortality | 0.917 | 0.874–0.962 | 0.0004 | 1.091 | 1.039–1.144 | 0.0004 |
| Primary composite endpointa | 0.910 | 0.878–0.944 | <0.0001 | 1.099 | 1.060–1.140 | <0.0001 |
| Secondary composite endpointb | 0.898 | 0.873–0.924 | <0.0001 | 1.113 | 1.083–1.145 | <0.0001 |
| Total stroke | 0.897 | 0.841–0.958 | 0.0012 | 1.114 | 1.044–1.190 | 0.0012 |
| Non-fatal stroke | 0.914 | 0.850–0.983 | 0.0160 | 1.094 | 1.017–1.176 | 0.0160 |
| Total MI | 0.956 | 0.897–1.018 | 0.1588 | 1.047 | 0.982–1.115 | 0.1588 |
| Non-fatal MI | 0.935 | 0.875–0.999 | 0.0460 | 1.070 | 1.001–1.143 | 0.0460 |
| Revascularization | 0.901 | 0.868–0.936 | <0.0001 | 1.110 | 1.068–1.152 | <0.0001 |
| HF hospitalization | 0.859 | 0.809–0.912 | <0.0001 | 1.164 | 1.097–1.236 | <0.0001 |
Weight gain and weight loss were treated as time varying covariables in multivariable adjusted models (Cox regression model adjusted for age, sex, ever smoker, previous cardiovascular event, LDL, HbA1c, eGFR, allocation to glargine, allocation to omega 3 fatty acid, duration of DM, waist circumference, systolic blood pressure, ACE or ARB medication, beta blocker medication, statin medication. Hip circumference, hypertension, and diastolic blood pressure were not included in the model because of collinearity.). HR’s compare the category vs. all subjects outside the category.
Primary composite endpoint: composite of first occurrence of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.
Expanded composite endpoint: composite of first occurrence of these three outcomes or revascularization or heart failure hospitalization.
Sustained weight gain over 2 years with no weight loss during these 2 years was observed in 2863 patients and sustained weight loss over 2 years was observed in 1755 patients. Sustained weight gain did not relate to a higher risk for any of the assessed outcome variables in comparison to patients without weight gain. In turn, sustained weight loss was related to worse outcome for all-cause mortality and for CV mortality (Table 5) compared to no weight loss. Patients who remained at stable weight had a better mortality outcome compared to patients with sustained loss or gain of body weight.
Table 5.
Outcomes for subgroups with 2-year sustained weight gain, weight loss, or stable weight during the first 2 years of the follow-up (HR, 95% confidence intervals)
| Sustained weight gain |
Sustained weight loss |
Stable weight |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| All-cause mortality | 1.000 | 0.896–1.115 | 0.996 | 1.315 | 1.182–1.463 | <0.0001 | 0.824 | 0.751–0.904 | <0.0001 |
| CV mortality | 1.109 | 0.968–1.272 | 0.136 | 1.175 | 1.020–1.353 | 0.025 | 0.828 | 0.734–0.933 | 0.0019 |
| Primary composite endpointa | 1.040 | 0.939–1.152 | 0.453 | 1.049 | 0.942–1.168 | 0.385 | 0.940 | 0.859–1.028 | 0.172 |
| Secondary composite endpointb | 0.983 | 0.909–1.064 | 0.676 | 1.122 | 1.036–1.216 | 0.0046 | 0.933 | 0.871–0.999 | 0.0460 |
| Total stroke | 1.036 | 0.864–1.243 | 0.699 | 0.918 | 0.754–1.119 | 0.397 | 1.030 | 0.878–1.208 | 0.717 |
| Non-fatal stroke | 0.983 | 0.804–1.202 | 0.868 | 0.876 | 0.704–1.090 | 0.235 | 1.106 | 0.928–1.318 | 0.259 |
| Total MI | 0.945 | 0.787–1.134 | 0.543 | 1.005 | 0.832–1.214 | 0.958 | 1.040 | 0.888–1.217 | 0.626 |
| Non-fatal MI | 0.904 | 0.745–1.096 | 0.304 | 1.014 | 0.832–1.236 | 0.892 | 1.068 | 0.906–1.260 | 0.432 |
| Revascularization | 0.944 | 0.845–1.054 | 0.304 | 1.195 | 1.070–1.335 | 0.0017 | 0.919 | 0.834–1.012 | 0.0858 |
| HF hospitalization | 1.024 | 0.853–1.228 | 0.801 | 1.196 | 0.995–1.437 | 0.0563 | 0.865 | 0.748–1.013 | 0.0725 |
Weight gain, weight loss, and stable weight were treated as time varying covariables in multivariable adjusted models (Cox regression model adjusted for age, sex, ever smoker, previous cardiovascular event, previous diabetes, LDL, HbA1c, eGFR, allocation to glargine, and allocation to omega 3 fatty acid.). HR’s compare the category vs. all subjects outside the category.
Primary composite endpoint: composite of first occurrence of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.
Expanded composite endpoint: composite of first occurrence of these three outcomes or revascularization or heart failure hospitalization.
Discussion
The analysis showed that in patients with CV risk factors and with either type 2 DM or prediabetes, people who were overweight and mildly obese had a lower all-cause and CV mortality, as well as a fewer composite CV outcomes (non-fatal MI, non-fatal stroke, or CV death) than people whose BMI was 22–25 kg/m2. It also showed higher mortality and CV outcomes in those patients with BMI below this threshold.
The relation of body weight and outcome is reported in most observational studies based on single-time assessment of body weight. Our study extends the present knowledge as we report on weight change in relation to outcome in these patients with DM or prediabetes. Weight loss but not weight gain was associated with worse outcomes compared to those with stable body weight. This is in line with recent data in patients with coronary artery disease, where fluctuation in body weight was associated with higher mortality and higher rate of CV events.15
Our findings are in line with previous reports on an inverse relation of body weight and outcome across a wide range of CV disease settings9 and in multiple population-based cohort studies.7 This finding was initially termed ‘obesity paradox’ as it is counterintuitive to the setting of primary prevention where excessive body weight is clearly established as a risk factor for CV disease. The reproducibility of these findings and recent pathophysiologic insights that suggest a benefit of excessive energy stores in the context of disease related catabolic conditions led to challenge the terminology of a paradox (i.e. unexpected and unexplained).16
In patients with DM two meta-analyses including 18 longitudinal clinical trials and registries17 and 16 cohort studies18 report the lowest mortality in overweight and mildly obese patients. Registry data from 10 568 patients with DM and no CV disease10 and data from the DIAMOND prospective cohort registry in 1192 patients with DM after acute MI19 showed a lower all-cause mortality in overweight patients (BMI > 25 kg/m2) compared to non-obese patients. A prospective cohort study including 11 449 Chinese adults with DM showed lowest mortality in obese patients and significantly lower risk of death in overweight patients compared to patients with normal weight.20
Cardiovascular death is the most common cause of death in diabetic patients and the prevalent CV risk profile in the cohort of the ORIGIN study may contribute to explain the observed association of body weight and outcome in these patients. This is supported by a recent report on over 170 000 patients from 14 prospective studies.21 All participants in the ORIGIN trial had a CV comorbidity. In contrast, in a recent study in patients with incident type 2 DM who were free of CV disease, overweight, and obesity were not associated with improved outcome.22 In the latter study, self-reported measures were used to calculate BMI. Notably, a systematic bias in self-reported weight data has repeatedly been observed leading to overestimation of the association of obesity with worse outcome.23 The present study used measured values instead of self-reported date for the calculation of BMI thus avoiding the potential bias from self-reported BMI values.
Notably, in patients with DM a range of body weight at BMI near 30 kg/m2 is the commonly observed average weight distribution. Hence, lower body weight may per se indicate some underlying problem associated with illness or metabolic imbalance which may contribute to explain the observed findings. Cancer related death is often discussed to account for higher mortality in underweight subject. In the ORIGIN cohort, however, the proportion of CV death was higher (64%) in the subgroup of low body weight as in all other BMI subgroups (Table 2). It was shown previously in the ORIGIN population that mean BMI was not different between patients with vs. without cancer (BMI 29.9 ± 4.8 vs. 29.8 ± 5.3 kg/m2, P = 0.68) and that body weight was not associated with cancer mortality in multivariate analysis.24 Cancer mortality is therefore unlikely to account for the higher mortality rate in patients with low body weight in this cohort study. We cannot exclude that diseases which were not recorded in the ORIGIN dataset were more prevalent among patients with low body weight. However, a range of comorbidities and clinical variables with known impact on CV outcome were recorded and were included in our analyses (Table 1). Smoking is a commonly discussed factor to explain higher mortality among subjects with lower body weight. Smoking status was assessed in the ORIGIN study, and it was shown that smoking history was lowest in subjects with low body weight and was highest in patients with overweight and mild obesity. The multivariable outcome analyses included adjustment for smoking status. However, smoking status was self-reported and a reporting bias cannot be full excluded.
Limitations
Our study is a post hoc analysis of a large clinical trial database and limitations of such analysis apply. Despite multivariate adjustments, some baseline differences may still be unaccounted for, thus definite conclusions may only be possible from a prospective randomized trial on weight management. From the observed associations, no causal interaction can be concluded. Our study cannot answer the question of potential benefits from intentional weight reduction as the ORIGIN study protocol did not include any weight change advice or any intervention towards weight modification. The randomized controlled LOOK-AHEAD trial investigated prospectively a comprehensive weight reduction program in patients with type 2 DM and a significant weight reduction was achieved. This intentional weight reduction, however, did not translate into improved total or CV survival of the patients. A post hoc analysis of the LOOK AHEAD Study has shown that if intentional weight reduction of more than 10% within 1 year can be achieved than a 20% lower CV disease risk can be observed (for composite of non-fatal acute MI or stroke, hospitalized angina, or CV death HR 0.8, 95% CI 0.65–0.99).25 A smaller weight reduction was, however, not effective. A lack of survival benefit from intentional weight loss was also reported in a long-term cohort study of 19 years follow-up in overweight patients with DM.12 The current study may not allow conclusions on more advanced obesity. A recent report from a retrospective observations study showed that in more advanced obesity (mean BMI 45.1 kg/m2) weight reduction by gastrointestinal surgery resulted in improved mortality and CV event rates.26 This retrospective reports warrants confirmation in randomized clinical trials.
In the ORIGIN study, no intervention on body weight and no weight change advice were given within the study protocol. Therefore, the observed weight change in the study is either unintended or may result from individual weight loss attempts of patients at their own initiative. Other observational studies on weight change in DM without intentional weight management strategies could not show a benefit of weight reduction for improved prognosis. An analysis from the Veterans Health Administration (VHA) including 145 198 patients treated with oral antidiabetic therapy showed that weight loss of >5% body weight but not weight gain was consistently associated with higher risk of 5-year mortality.27 We have previously reported on weight change in patients with DM and CV comorbidity of the PROactive study.28 In this patient cohort, weight loss was associated with higher mortality compared to patients with no weight loss while weight gain had no negative effect. A nationwide longitudinal study in more than 11 million South Korean subjects showed that weight loss across all BMI categories was associated with increased mortality.29
Taken together, interventional and observational studies in patients with DM and CV comorbidity did repeatedly not show a benefit for survival from intended weight reduction and suggest a higher risk associated with unintended weight loss. The widely held weight management recommendation is to promote weight reduction in any patient with BMI >25 kg/m2 and irrespective of any CV comorbidity. Further research is needed to clarify if recommendations on weight management should differentiate more clearly between primary prevention and patients with established disease and advanced CV risk profiles.
The discussion is ongoing that other anthropometric measures such as waist circumference may yield better signals than BMI to assess CV risk. Combined models have shown that within BMI groups waist circumference can further subdivide patients for CV risk.30 However, BMI is the most widely used measure with well-defined categories and is strongly implemented in body weight management in both the medical and public domain. Therefore, the data on BMI in this representative patient population may be helpful to inform on individualized weight management.
Conclusions
Our investigation showed that in patients with type 2 DM and prevalent CV risk profile overweight and mild obesity are associated with lower all-cause mortality and CV mortality compared to patients with normal body weight (BMI 22–<25 kg/m2). In turn, patients with low body weight have a higher mortality. Weight loss was related to higher all-cause and CV mortality compared to no weight loss while weight gain was not. These findings are in contrast to conventional considerations on body weight in primary prevention.
Conflict of interest: W.D. reports grants and personal fees from Vifor, Pfizer, Boehringer Ingelheim, Sphingotec, ZS Pharma, Bayer, Medtronic outside the submitted work. H.C.G. holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He reports research grants from Eli Lilly, AstraZeneca, Merck, Novo Nordisk, and Sanofi; honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi; and consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Janssen, Sanofi, Kowa, and Cirius outside the submitted work; J.R., C.A., and S.H. report being an employee of Sanofi during the conduct of the study. H.J. has nothing to disclose. S.D.A. reports grants and personal fees from Vifor Int, Bayer, Boehringer Ingelheim, Servier, V-Wave, Novartis, outside the submitted work.
Supplementary Material
Contributor Information
Wolfram Doehner, Berlin Institute of Health Center for Regenerative Therapies (BCRT), Charité Universitätsmedizin Berlin, 13353 Berlin, Germany; Division of Cardiology and Metabolism, Department of Cardiology (Virchow Hospital), German Centre for Cardiovascular Research (DZHK), Partner Site Berlin and Center for Stroke Research Berlin, Charité Universitätsmedizin Berlin, 13353 Berlin, Germany.
Hertzel C Gerstein, Population Health Research Institute, McMaster University, Hamilton Health Sciences, L8S 4K1 Hamilton, ON, Canada.
Janina Ried, Sanofi-Aventis Deutschland GmbH, Research & Development, 65926 Frankfurt, Germany.
Hyejung Jung, Population Health Research Institute, McMaster University, Hamilton Health Sciences, L8S 4K1 Hamilton, ON, Canada.
Christian Asbrand, Sanofi-Aventis Deutschland GmbH, Research & Development, 65926 Frankfurt, Germany.
Sibylle Hess, Sanofi-Aventis Deutschland GmbH, Research & Development, 65926 Frankfurt, Germany.
Stefan D Anker, Berlin Institute of Health Center for Regenerative Therapies (BCRT), Charité Universitätsmedizin Berlin, 13353 Berlin, Germany; Division of Cardiology and Metabolism, Department of Cardiology (Virchow Hospital), German Centre for Cardiovascular Research (DZHK), Partner Site Berlin and Center for Stroke Research Berlin, Charité Universitätsmedizin Berlin, 13353 Berlin, Germany.
References
- 1. Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med 2007;357:2371–2379. [DOI] [PubMed] [Google Scholar]
- 2.Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. [DOI] [PubMed] [Google Scholar]
- 5. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2019;doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 6. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afzal S, Tybjærg-Hansen A, Jensen GB, Nordestgaard BG. Change in body mass index associated with lowest mortality in Denmark, 1976-2013. JAMA 2016;315:1989–1996. [DOI] [PubMed] [Google Scholar]
- 9. Doehner W. Critical appraisal of the obesity paradox in cardiovascular disease: how to manage patients with overweight in heart failure? Heart Fail Rev 2014;19:637–644. [DOI] [PubMed] [Google Scholar]
- 10. Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, Perrone-Filardi P, Zhang J, Atkin SL. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med 2015;162:610–618. [DOI] [PubMed] [Google Scholar]
- 11. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Køster-Rasmussen R, Simonsen MK, Siersma V, Henriksen JE, Heitmann BL, de Fine Olivarius N. Intentional weight loss and longevity in overweight patients with type 2 diabetes: a population-based cohort study. PLoS One 2016;11:e0146889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ORIGIN Trial Investigators, Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328. [DOI] [PubMed] [Google Scholar]
- 14.Origin Trial Investigators, Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 2008;155:26–32. [DOI] [PubMed] [Google Scholar]
- 15. Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body-weight fluctuations and outcomes in coronary disease. N Engl J Med 2017;376:1332–1340. [DOI] [PubMed] [Google Scholar]
- 16. Doehner W, von Haehling S, Anker SD. Protective overweight in cardiovascular disease: moving from ‘paradox’ to ‘paradigm’. Eur Heart J 2015;36:2729–2732. [DOI] [PubMed] [Google Scholar]
- 17. Carnethon MR, Rasmussen-Torvik LJ, Palaniappan L. The obesity paradox in diabetes. Curr Cardiol Rep 2014;16:446. [DOI] [PubMed] [Google Scholar]
- 18. Kwon Y, Kim HJ, Park S, Park YG, Cho KH. Body mass index-related mortality in patients with type 2 diabetes and heterogeneity in obesity paradox studies: a dose-response meta-analysis. PLoS One 2017;12:e0168247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Won KB, Hur SH, Cho YK, Yoon HJ, Nam CW, Kim KB, Bae JH, Choi DJ, Ahn YK, Park JS, Kim HS, Choi RK, Choi D, Kim JH, Han KR, Park HS, Choi SY, Yoon JH, Kwon HC, Rha SU, Hwang KK, Lim DS, Jung KT, Oh SK, Lee JH, Shin ES, Kim KS. Comparison of 2-year mortality according to obesity in stabilized patients with type 2 diabetes mellitus after acute myocardial infarction: results from the DIAMOND prospective cohort registry. Cardiovasc Diabetol 2015;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu H, Wu S, Li Y, Sun L, Huang Z, Lin L, Liu Y, Ji C, Zhao H, Li C, Song L, Cong H. Body mass index and mortality in patients with type 2 diabetes mellitus: a prospective cohort study of 11,449 participants. J Diabetes Complications 2017;31:328–333. [DOI] [PubMed] [Google Scholar]
- 21. Lamelas P, Schwalm JD, Leong D, Jolly S, Mehta S, Bangdiwala S, Yusuf S. Varying effects of body mass index and mortality in different risk groups. Am J Cardiol 2018;122:1155–1160. [DOI] [PubMed] [Google Scholar]
- 22. Tobias DK, Pan A, Jackson CL, O'Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014;370:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiolero A, Peytremann-Bridevaux I, Paccaud F. Associations between obesity and health conditions may be overestimated if self-reported body mass index is used. Obes Rev 2007;8:373–374. [DOI] [PubMed] [Google Scholar]
- 24. Bordeleau L, Yakubovich N, Dagenais GR, Rosenstock J, Probstfield J, Chang Yu P, Ryden LE, Pirags V, Spinas GA, Birkeland KI, Ratner RE, Marin-Neto JA, Keltai M, Riddle MC, Bosch J, Yusuf S, Gerstein HC; ORIGIN Trial Investigators. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 2014;37:1360–1366. [DOI] [PubMed] [Google Scholar]
- 25.Look AHEAD Research Group, Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, Evans M, Foreyt J, Foster G, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jeffery RW, Johnson KC, Kitabchi AE, Knowler WC, Kriska A, Lang W, Lewis CE, Montez MG, Nathan DM, Neiberg RH, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Redmon B, Regensteiner J, Rejeski J, Ribisl PM, Safford M, Stewart K, Trence D, Wadden TA, Wing RR, Yanovski SZ. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW, Nissen SE. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019;322:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kocarnik BM, Moore KP, Smith NL, Boyko EJ. Weight change after initiation of oral hypoglycemic monotherapy for diabetes predicts 5-year mortality: an observational study. Diabetes Res Clin Pract 2017;123:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, Anker SD. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol 2012;162:20–26. [DOI] [PubMed] [Google Scholar]
- 29. Kim YH, Kim SM, Han KD, Son JW, Lee SS, Oh SW, Lee WY, Yoo SJ; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Change in weight and body mass index associated with all-cause mortality in Korea: a nationwide longitudinal study. J Clin Endocrinol Metab 2017;102:4041–4050. [DOI] [PubMed] [Google Scholar]
- 30. Coutinho T, Goel K, Corrêa de Sá D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease. J Am Coll Cardiol 2013;61:553–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.