Abstract
An 11-y-old spayed female German Shepherd was presented for a second opinion of ventral cervical swelling of 3-mo duration. On examination, the dog had significant dependent ventral cervical swelling. Enlarged lymph nodes with cystic changes and severe edematous facial swelling were noted on computed tomography. Fine-needle aspiration of the ventral cervical swelling revealed yellow-tinged fluid, with a predominance of lymphoid cells noted on cytologic examination. On cervical exploratory surgery, the left mandibular lymph node was surrounded by a large fluid pocket; biopsies of the lymph node were obtained. Impression smear cytology, flow cytometry, PCR for antigen receptor gene rearrangements, and histopathology were performed on samples from the left mandibular lymph node. Impression smear cytology revealed a population of atypical discrete cells. Flow cytometry identified a population of CD34+/CD45– large cells. A tumor of endothelial origin within the medulla of the lymph node was identified by histopathology, and lymphangiosarcoma was confirmed based on prospero-related homeobox gene 1 (PROX1) immunoreactivity. Our study describes the challenges in the diagnosis of a rarely reported entity and highlights that neoplastic endothelial cells should be considered as a differential when high proportions of CD34+/CD45– cells are present in flow cytometry.
Keywords: canine, flow cytometry, immunohistochemistry, lymphangiosarcoma, neoplasia, pathology
An 11-y-old, 25.6 kg (56.4 lb), spayed female German Shepherd dog was presented to a tertiary facility surgery service as a referral because of persistent ventral neck swelling. Previous work-up included multiple biopsy samples of the cervical and mandibular skin, and culture and cytology of fluid from the swollen areas. Prior treatments included dental extractions, antibiotic trials with clindamycin and amoxicillin–clavulanic acid (Clavamox; Zoetis, Parsippany, NJ), prednisone therapy, and parotid sialoadenectomy. The face and cervical region continued to swell despite all treatments.
On presentation, there was dependent edema on the face and ventral cervical region, with slight oozing of yellow-tinged fluid from the ventral aspect of the neck. The remainder of the physical exam was unremarkable. A complete blood count and serum biochemistry performed by the primary care veterinarian were unremarkable. A point-of-care analysis (Stat Profile pHOx Ultra; Nova Biomedical, Waltham, MA) was performed prior to anesthesia, with no significant findings. Computed tomography (CT) of the head and neck revealed absence of the left parotid salivary gland with a pocket of fluid around its expected location. Additional findings included: bilateral medial retropharyngeal lymphadenomegaly, severe facial swelling of indeterminate etiology, and absence of multiple teeth consistent with previous dental extractions. Fluid, containing predominantly lymphoid cells, was aspirated from the region. Surgical exploration of the cervical region was performed to obtain lymph node biopsies. The left mandibular salivary gland was identified and removed to facilitate visualization of the left mandibular lymph node. The left medial retropharyngeal lymph node was not found. The left mandibular lymph node was excised, and impression smears were made before fixation in formalin.
Cytologic examination of the impression smears revealed predominantly large, atypical discrete cells with fewer lymphoid cells (Fig. 1). The atypical cells were observed singly and within variably sized loose aggregates. These cells were round-to-oval with variably distinct cell borders and abundant, pale blue-gray cytoplasm, which occasionally contained a few small, discrete vacuoles. Rare cells displayed a spindled morphology (Fig. 1, inset). The cells typically contained a single oval-to-indented nucleus, although occasional bi-, tri- and multinucleate cells were noted. Nuclei were eccentrically placed and had lacy-to-coarse chromatin with 1–6 nucleoli that varied in size, to include occasional macronucleoli. Overall, cells displayed moderate anisocytosis and anisokaryosis and had moderate nuclear-to-cytoplasmic ratios. Nuclear molding was occasionally noted, and rare mitotic figures were seen. Lymphoid cells were predominantly lymphocytes, with a moderate increase in plasma cells that displayed mild anisocytosis and anisokaryosis. Occasional binucleation and plasma cells with Mott cell morphology were observed. Lymphocytes were ~ 70% small lymphocytes and 30% intermediate and large lymphocytes. These findings were consistent with mild-to-moderate reactive lymphoid hyperplasia. The identity of the discrete atypical cells was unknown, but a malignant neoplastic population was suspected.
Figure 1.

Photomicrographs of a touch impression of the left mandibular lymph node. Impression smear cytology revealed reactive lymphoid hyperplasia and a population of discrete, large, atypical cells (asterisks). The atypical cells were round-to-oval with variably distinct cell borders and abundant, pale blue-gray cytoplasm, which occasionally contained a few small, discrete vacuoles. Inset: rare cells had a spindled morphology. Giemsa stain.
Flow cytometry of the left mandibular lymph node was subsequently performed. The small cells were normally distributed lymphocytes; the large cells consisted predominantly of lymphocytes and inflammatory cells. However, ~ 30% of the large cell population did not express pan-leukocyte antigen CD45, but had positive expression of CD34 (Fig. 2). Based on the case history, these CD34+/CD45– cells were suspected to be endothelial in origin (lymphatic or vascular endothelium), although there is one case report describing a plasma cell tumor as being CD34+/CD45–.15 Therefore, PCR for antigen receptor rearrangements (PARR) was performed as described previously,15 with polyclonal rearranged immunoglobulin genes detected.
Figure 2.

Flow cytometry dot plots of the fine-needle aspirate sample obtained from the left mandibular lymph node. CD45-negative cells (purple) are large, as indicated by high degree of forward scatter. CD34-positive cells (blue) are in the same location as the large, CD45-negative cells. CD21 = B-cell marker; CD34 = hematopoietic precursor and endothelial cell marker; CD45 = pan-leukocyte marker; CLII = MHC class II.
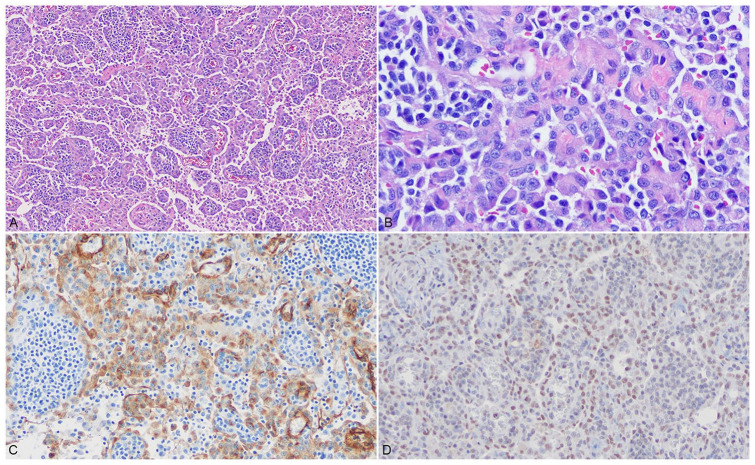
Histologically, the mandibular salivary gland exhibited diffuse interlobular edema with no signs of a mucocele. The mandibular lymph node was multifocally infiltrated by a poorly demarcated, unencapsulated population of neoplastic spindle cells forming and projecting into clefts resembling vascular channels supported by thin fibrovascular cores and containing only scattered erythrocytes (Fig. 3A). Neoplastic cells had indistinct cell borders and scant eosinophilic, fibrillar cytoplasm. Nuclei were round-to-ovoid, with finely stippled chromatin, and a single, small but distinct, basophilic nucleolus (Fig. 3B). Anisocytosis and anisokaryosis were moderate; the mitotic count was 2 per 2.37 mm2. All neoplastic cells demonstrated strong, intracytoplasmic immunopositivity for vimentin and von Willebrand factor (Fig. 3C) and were diffusely immunonegative for cytokeratin AE1/AE3, confirming an endothelial cell origin. In order to further differentiate the endothelial neoplasm, prospero-related homeobox gene 1 (PROX1) immunohistochemistry was performed at the Indiana Animal Disease Diagnostic Laboratory (West Lafayette, IN; Suppl. Data). Neoplastic cells also exhibited diffuse, intranuclear immunopositivity for PROX1, indicating that the neoplastic cells were derived from lymphatic endothelium specifically (Fig. 3D). Cross-reactivity of blood vessel endothelium to PROX1 was not noted within the sample specimen or positive canine lymph node control tissue (Suppl. Fig. 1). Considering the histopathologic and immunohistochemical findings, lymphangiosarcoma was diagnosed.
Figure 3.
Photomicrographs of a section of the left mandibular lymph node. A. The sinuses are diffusely infiltrated by a population of neoplastic spindle cells forming vascular channels largely devoid of erythrocytes. Neoplastic cells surround and separate preexisting lymphoid aggregates. H&E. B. Neoplastic cells project into the vascular channels and exhibit moderate anisocytosis and anisokaryosis. H&E. C. Neoplastic cells exhibit strong, membranous-to-cytoplasmic immunolabeling for von Willebrand factor. D. Neoplastic cells exhibit strong, nuclear immunolabeling for PROX1.
The dog recovered uneventfully from anesthesia and was maintained overnight on hydromorphone (0.1 mg/kg [0.22 mg/lb], IV, q6h), gabapentin (10 mg/kg [22 mg/lb], PO, q8h), and lactated Ringer solution (5 mL/kg/h [11 mL/lb/h], IV). The dog was discharged from the hospital 48 h after surgery. The swelling was static, with yellow-tinged fluid dripping from the previous fine-needle aspiration site. However, following discharge, the swelling of the cervical region progressed according to client communication, and another trial of inflammatory dose prednisone was started. The owners declined further tests or treatments. Cervical swelling remained minimal under prednisone maintenance therapy (1 mg/kg/day [2.2 mg/lb/day]). Unfortunately, tapering trials of the prednisone led to immediate worsening of the swelling, and the patient was subsequently returned to the higher doses of prednisone. Given the perceived poor quality of life, the patient was euthanized 4.5 mo after onset of clinical signs (2.5 mo after definitive diagnosis), and an autopsy was performed at the same tertiary facility. Autopsy findings included marked facial subcutaneous edema and a 15 × 15 × 15 cm, hairless, pale purple, soft, fluctuant mass spanning the ventral neck from the caudal mandible to the thoracic inlet (Fig. 4A). On cut section, the mass was diffusely pale tan-to-gray, and exuded abundant clear, watery-to-gelatinous fluid (Fig. 4B). There was no gross evidence of metastatic disease. Histologic assessment of the ventral cervical mass was consistent with lymphangiosarcoma. Given that the patient’s lymph nodes appeared grossly normal, further histologic assessment was not performed.
Figure 4.

A. A 15 × 15 × 15 cm, hairless, pale-purple, soft, fluctuant mass spanned the ventral neck from the caudal mandible to the thoracic inlet. B. On cut surface, the mass is diffusely pale tan-to-gray and exudes abundant clear, watery-to-gelatinous fluid.
Lymphangiosarcoma is an uncommon neoplasm that arises from the lymphatic endothelium. It is considered rare in domestic animals, with cases most commonly reported in dogs and cats, and occasional equine cases reported.1,2,4,5,7-10,12-14,16,17,19,20 In dogs, large breeds are overrepresented, with ages ranging from 8 wk to 13 y.1,10,13,14,17,20 Lymphangiosarcoma is most often reported in the subcutis as a poorly demarcated mass or focal swelling along the ventral midline or on the limbs.1,4,10,12,13,16,17,20 The most commonly reported locations include the inguinal region, ventral cervical area, and the hindlimb and forelimb.1,7,10,13,16,17 Masses are frequently associated with subcutaneous edema, and a lymphorrheic effusion from the affected area may also be observed.1,6,10,12,16,17,20 The biologic behavior of lymphangiosarcoma is considered aggressive, with extensive local infiltration and rapid metastatic spread from the primary site.1,6,9,20 Diagnostic modalities for lymphangiosarcoma may involve cytology, radiography, abdominal ultrasonography, lymphoscintigraphy, and/or advanced imaging such as magnetic resonance imaging, CT, or CT lymphography.1,2,9,10,20 Definitive diagnosis is typically made with histopathology of affected tissues and concurrent immunohistochemical stains.1,6,10,13,14,16,17,20
Given the paucity of reported lymphangiosarcoma cases, optimal treatment is not well established. However, a combination of surgery and chemotherapy is often recommended.1,9,10,13,16,17 Surgery generally requires wide surgical margins, which is often difficult given the poorly defined borders and highly infiltrative nature of these tumors.1 In our case, aggressive surgical resection was not an option given the location and extent of the affected area. Previously reported chemotherapy of lymphangiosarcoma has included cytotoxic chemotherapy with doxorubicin, carboplatin, vinorelbine, or lomustine; or metronomic chemotherapy with chlorambucil, meloxicam, cyclophosphamide, or tyrosine kinase inhibitors.1,9,10,13,16,17 In the human literature, prolonged survival has been reported with the use of surgery and radiation therapy; however, there are few reports regarding the utility of radiation therapy for treatment of lymphangiosarcoma in dogs. In a case series, one dog that received a combination of surgery, full-course radiation therapy, and cytotoxic chemotherapy had an overall survival time of 574 d.1 Based on the limited veterinary literature, the reported survival time for dogs who do not receive any treatment is 60–876 d, with median survival time of 168 d.1 With surgical treatment alone, the median reported survival time is 487 d.1 The use of an anti-inflammatory dose of prednisone as a sole therapy has been reported in one case with a survival time of 90 d.1 The German Shepherd in our report was treated with an anti-inflammatory dose of prednisone with marginal improvement in clinical signs. This dog’s overall survival time was 135 d, which is longer than that previously reported for prednisone use alone.
Diagnosis of lymphangiosarcoma can be challenging because lymphatic endothelium closely resembles blood vascular endothelium.1,2,6,20 Histopathology is considered the gold standard for diagnosis of lymphangiosarcoma. Histologically, these tumors are infiltrative, non-encapsulated masses composed of elongate-to-plump spindle cells forming irregular channels. In comparison to hemangiosarcoma, the irregular neoplastic vascular channels generally do not contain erythrocytes.1,2,4-10,12-14,16,17,19,20 Given the morphologic similarity between hemangiosarcoma and lymphangiosarcoma, immunohistochemistry is recommended for definitive diagnosis. Although both hemangiosarcoma and lymphangiosarcoma are immunopositive to vimentin, CD31, and von Willebrand factor, positive immunoreactivity for lymphatic endothelial receptor 1 (LYVE1) or PROX1 is considered specific for lymphatic endothelium.1,4-6,19 Other reported special stains include the use of lectin histochemical stains: Ricinus communis agglutinin 1 (RCA1) has been found to be widely distributed in lymphangiosarcomas; Phaseolus vulgaris erythroagglutinin (PHA-E) is found predominantly in hemangiosarcomas.2
In addition to histologic analysis, transmission electron microscopy has been used to differentiate the 2 types of angiosarcomas. Lymphangiosarcomas are reported to have a discontinuous or absent basement membrane, fewer micropinocytic vesicles and intercellular junctions, and lack supporting pericytes.4,8,14,16 In comparison, hemangiosarcomas have a continuous basement membrane, many micropinocytic vesicles and intercellular junctions, with the presence of surrounding pericytes.
Unfortunately, cytology is often not helpful in the diagnosis of lymphangiosarcoma given that cytologic examination of fine-needle aspirates of masses typically reveals a high percentage of small lymphocytes with possible concurrent mild neutrophilic inflammation.1,10 However, one case report in a dog describes pleomorphic spindle cells observed on fine-needle aspirate cytology.9 In addition, an equine case report described similar findings to those in our case on examination of impression smears, with a predominance of large atypical rounded cells.8
To our knowledge, flow cytometry has not been reported previously as an aid in the diagnosis of lymphangiosarcoma. Although CD34 is commonly used in flow cytometry to identify hematopoietic precursor cells and other stem cells,18 endothelial cells are also known to express CD34.3 Given that endothelial cells are not leukocytes, the large lymphatic endothelial cells in the sample did not display the pan-leukocyte antigen CD45. Flow cytometry has also historically been used in the evaluation of canine hemangiosarcoma.11 Our case highlights that, although CD34 is commonly used in veterinary medicine as a marker of hematopoietic lineage, other differentials should be considered when increased proportions of CD34+ cells are noted on flow cytometry.
Supplemental Material
Supplemental material, Supplemental_material for Pathologic and flow cytometric features of a case of canine ventral cervical lymphangiosarcoma by Carolina N. Azevedo, Allyson A. Sterman, Lauren W. Stranahan, Brianne M. Taylor, Dominique J. Wiener, Jacqueline R. Davidson and Karen E. Russell in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Drs. Anne Avery and Kari Frankhouse of the CSU Clinical Immunology Laboratory for providing the flow cytometry images and for their expertise in the interpretation of the flow cytometry in this case. We also thank Dr. Jose Ramos-Vara with the Indiana Animal Disease Diagnostic Laboratory for performing the PROX1 immunohistochemistry.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Carolina N. Azevedo  https://orcid.org/0000-0002-0725-4930
https://orcid.org/0000-0002-0725-4930
Supplementary material: Supplementary material for this article is available online.
References
- 1. Curran KM, et al. Lymphangiosarcoma in 12 dogs: a case series (1998–2013). Vet Comp Oncol 2016;14:181–190. [DOI] [PubMed] [Google Scholar]
- 2. Diessler ME, et al. Cutaneous lymphangiosarcoma in a young dog: clinical, anatomopathological and lectin histochemical description. J Vet Med A Physiol Pathol Clin Med 2003;50:452–456. [DOI] [PubMed] [Google Scholar]
- 3. Fina L, et al. Expression of the CD34 gene in vascular endothelial cells. Blood 1990;75:2417–2426. [PubMed] [Google Scholar]
- 4. Galeotti F, et al. Feline lymphangiosarcoma—definitive identification using a lymphatic vascular marker. Vet Dermatol 2004;15:13–18. [DOI] [PubMed] [Google Scholar]
- 5. Gerding JC, et al. Presumed primary ocular lymphangiosarcoma with metastasis in a miniature horse. Vet Ophthalmol 2015;18:502–509. [DOI] [PubMed] [Google Scholar]
- 6. Halsey CH, et al. The use of novel lymphatic endothelial cell-specific immunohistochemical markers to differentiate cutaneous angiosarcomas in dogs. Vet Comp Oncol 2016;14:236–244. [DOI] [PubMed] [Google Scholar]
- 7. Hinrichs U, et al. Lymphangiosarcomas in cats: a retrospective study of 12 cases. Vet Pathol 1999;36:164–167. [DOI] [PubMed] [Google Scholar]
- 8. IJzer J, et al. Lymphangiosarcoma in a horse. J Comp Pathol 2000;122:312–316. [DOI] [PubMed] [Google Scholar]
- 9. Itoh T, et al. Lymphangiosarcoma in a dog treated with surgery and chemotherapy. J Vet Med Sci 2004;66:197–199. [DOI] [PubMed] [Google Scholar]
- 10. Kim JH, et al. Successful management of lymphangiosarcoma in a puppy using a tyrosine kinase inhibitor. Can Vet J 2018;59:367–372. [PMC free article] [PubMed] [Google Scholar]
- 11. Lamerato-Kozicki AR, et al. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol 2006;34:870–878. [DOI] [PubMed] [Google Scholar]
- 12. Lin D, et al. Pathology in practice. Ventral abdominal lymphangiosarcoma. J Am Vet Med Assoc 2017;250:623–626. [DOI] [PubMed] [Google Scholar]
- 13. Marcinowska A, et al. A novel approach to treatment of lymphangiosarcoma in a boxer dog. J Small Anim Pract 2013;54:334–337. [DOI] [PubMed] [Google Scholar]
- 14. Mineshige T, et al. Lymphangiosarcoma with bone formation of the auricle in a dog. J Vet Med Sci 2015;77:739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rout ED, et al. Progression of cutaneous plasmacytoma to plasma cell leukemia in a dog. Vet Clin Pathol 2017;46:77–84. [DOI] [PubMed] [Google Scholar]
- 16. Sagartz JE, et al. Lymphangiosarcoma in a young dog. Vet Pathol 1996;33:353–356. [DOI] [PubMed] [Google Scholar]
- 17. Sicotte V, et al. Use of surgery and mitoxantrone chemotherapy in a dog with disseminated lymphangiosarcoma. J Am Vet Med Assoc 2012;241:1639–1644. [DOI] [PubMed] [Google Scholar]
- 18. Sidney LE, et al. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014;32:1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugiyama A, et al. Lymphangiosarcoma in a cat. J Comp Pathol 2007;137:174–178. [DOI] [PubMed] [Google Scholar]
- 20. Williams JH. Lymphangiosarcoma of dogs: a review. J S Afr Vet Assoc 2005;76:127–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for Pathologic and flow cytometric features of a case of canine ventral cervical lymphangiosarcoma by Carolina N. Azevedo, Allyson A. Sterman, Lauren W. Stranahan, Brianne M. Taylor, Dominique J. Wiener, Jacqueline R. Davidson and Karen E. Russell in Journal of Veterinary Diagnostic Investigation