Abstract
Campylobacter infection is a leading cause of ovine abortion worldwide. Campylobacter fetus and C. jejuni are the major species involved. We report herein on abortion storms in 4 Danish sheep flocks. Initially, no pathogenic bacteria were isolated from placental and fetal tissues on aerobic and selective media despite the presence of severe suppurative and necrotizing placentitis with numerous bacteria located intracellularly in trophoblasts. Fluorescence in situ hybridization (FISH) was then applied on abortion material from 13 cases; species-specific oligonucleotide probes directed against either C. fetus or C. jejuni were used in combination with a general bacterial probe. C. fetus was detected as the only lesion-associated bacterial species in 4 cases from 2 flocks, and C. jejuni in 6 cases from the other 2 flocks, thereby establishing the likely etiology of the abortion storms in all 4 flocks. FISH is a useful detection tool in culture-negative cases with tissue lesions suggestive of bacterial infection. Furthermore, FISH is a fast and economical method to detect and identify the zoonotic agent Campylobacter within ovine abortion material.
Keywords: campylobacter, FISH, fluorescence in situ hybridization, sheep abortion, zoonosis
In situ demonstration of bacteria or bacterial antigens is a valuable and widely used principle in diagnostic pathology.11,13 In situ techniques that work on formalin-fixed specimens are preferred given that formalin fixation is the most commonly used method for tissue preservation in diagnostic pathology.3,13 Fluorescence in situ hybridization (FISH) is a rapid method for simple identification of microbial pathogens and works well on formalin-fixed tissue samples.4 FISH allows identification of bacteria at different phylogenetic levels based on specific hybridization of short fluorescence-labeled oligonucleotide probes to bacterial ribosomal RNA (rRNA).7 As a microscopic technique, FISH has the unique potential to provide information about spatial resolution, morphology, and identification of key pathogens in mixed species samples.4,7,8
Campylobacter spp. are motile, comma- or s-shaped, gram-negative bacteria that require microaerophilic conditions for culture. The organisms are remarkably sensitive to environmental conditions (e.g., dehydration, atmospheric oxygen, sunlight, and elevated temperature).18 Campylobacter infection is a leading cause of ovine abortion worldwide, with flock abortion rates of 5–50%.18 Infection of pregnant ewes with Campylobacter spp. often leads to late-term abortions, stillbirths, or the birth of weak lambs.12 When Campylobacter infection occurs initially within a flock, naive ewes are exposed to, and get infected by, large numbers of Campylobacter spp. from abortion material, resulting in an abortion storm.12 Histologically, the typical finding is suppurative necrotizing placentitis with intracellular bacteria distending the trophoblasts and invading the chorionic villus stroma.12 Once the agent has crossed the placental barrier, suppurative bronchopneumonia and multifocal necrotizing hepatitis are further common lesions seen on histologic examination.12
In 2007, and still today, the majority of Danish sheep are kept in small flocks and under non-commercial conditions: 90% and 87% of flocks consisted of <50 ewes in 2007 and 2017, respectively (Statistics Denmark, https://statistikbanken.dk/HDYR2). This implies close physical contact between lambing ewes and their owners and caretakers. According to the diagnostic record archive of the Danish National Veterinary Institute, C. fetus is seldom identified as an abortifacient agent in sheep in Denmark; C. jejuni has not been identified as an abortifacient agent prior to our study (National Veterinary Institute. [Results of diagnostic investigations in sheep and goats 1995–2000]. Frederiksberg C, Denmark: Technical University of Denmark. Danish). From 1998 to 2018, 3 Campylobacter-related abortion storms have been investigated, and C. fetus ssp. fetus was diagnosed as the abortifacient agent in all of them.
Campylobacter spp. are a major global cause of gastroenteritis in humans, with C. jejuni being the most frequently isolated species.6 Campylobacteriosis is the most common bacterial gastrointestinal infection in humans in Denmark,10 and was the most commonly reported zoonosis in the European Union in 2017.6 C. fetus infection in humans appears to be primarily zoonotic, with sheep and cattle as major reservoirs.20 Direct contact with animals is an important route of exposure, especially for some professions, such as farming or veterinary work.20 We applied the culture-independent FISH method in 2 species-specific assays to identify the intralesional bacteria observed in abortion material from abortion storms in 4 sheep flocks in Denmark.
We included aborted fetuses and/or placentas from 13 late-term abortion cases originating from abortion storms in 4 Danish sheep flocks (A = 900; B = 1,150; C = 750; D = 70 ewes). Placental tissue and/or aborted fetuses were submitted to the National Veterinary Institute, Denmark, for routine diagnostic investigation during the lambing seasons in 2007 and 2009. In 6 cases, placental tissue and 1 fetus were submitted; only the fetus or only placenta were submitted in 4 and 3 cases, respectively. The animals were considered free of Brucella melitensis, B. abortus, and Chlamydia abortus because those agents have never been reported in sheep and goats in Denmark (Danish Animal Health Report 2017, https://www.foedevarestyrelsen.dk/Publikationer/Alle%20publikationer/Animal%20Health%202017.pdf).
As part of the initial routine examinations, tissue specimens of fetal lung, liver, placenta (mostly cotyledons), and abomasal contents were cultured aerobically on blood agar at 37°C overnight. Specific examination for C. fetus was carried out on abomasal contents using thioglycolate agar plates at 37°C under microaerophilic conditions for 48–96 h. In 3 cases, fetal intestinal contents (cases A1 and A2) or placental tissue (case C1) were also cultured for C. fetus. Placenta samples from cases D1, D2, and D3 were additionally cultured under anaerobic conditions. The fetuses and fetal placentas were examined macroscopically, and tissue samples were taken from the placenta, liver, lung, heart, and brain for histologic examination. Tissue samples were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 7 µm (brain) or 3 µm (other tissues), and stained with hematoxylin and eosin.
Immunohistochemistry for toxoplasmosis was carried out on sections of brain and placenta using a polyclonal rabbit anti–Toxoplasma gondii antibody (BioGenex, Fremont, CA) diluted 1:1,000. The tissue sections were incubated with the primary antibody overnight at room temperature. Signal development was carried out using a polymer system with PermaRed as chromogen (SuperVision RED 2 AP-polymer-kit; DCS Diagnostics, Hamburg, Germany). In case of an inconclusive immunohistochemistry result, total nucleic acids were extracted from scrolls of paraffin-embedded placenta and tested for T. gondii DNA (ID Gene Toxoplasma gondii duplex kit; IDvet, Grabels, France) according to the manufacturer’s instructions.
Control samples for FISH were prepared by injecting pure cultures of the following bacterial species suspended in a 0.9% sterile saline solution into sterile porcine lung samples: C. fetus ssp. fetus CCUG 6823T, C. fetus ssp. venerealis NCTC 10354T, C. jejuni ssp. jejuni ATCC 33560T, C. coli ATCC 33559T, C. lari ssp. lari CCUG 23947T, C. upsaliensis CCUG 23626, Arcobacter cibarius CCUG 48482T, and Listeria monocytogenes LO28 and CIP 103575. Sections of the following specimens were used as further FISH controls: bovine placenta from a case of natural Coxiella burnetii infection, bovine lung from a case of experimental Chlamydia psittaci strain DC15 infection, bovine intestine from a case of natural Chlamydia pecorum infection, and ovine placenta from a case of natural Chlamydia abortus infection. The Chlamydia-infected specimens were confirmed positive by real-time PCR assays described previously.5,14 The Coxiella control was confirmed positive by FISH using the probe Cburn8 (Table 1).
Table 1.
Oligonucleotide probes used to identify various bacteria in ovine abortion samples.
| Probe | Target | Systematic name* | Sequence 5’–3’† | Reference |
|---|---|---|---|---|
| Cburn | Coxiella burnetii | S-S-Cburn-0443-a-A-18 | CTTGAGAATTTCTTCCCC | 8 |
| EUB338 | Domain bacteria | S-D-Bact-0338-a-A-18 | GCTGCCTCCCGTAGGAGT | 2 |
| CAMP653 | Campylobacter genus | S-G-Camp-0653-a-A-18 | CTGCCTCTCCCTYACTCT | 19 |
| Cafet | Campylobacter fetus | S-S-Cafet-0986-a-A-18 | TAGTTGGATATCAAGCCC | Current study |
| Cajej | Campylobacter jejuni | L-S-Cajej-1693-a-A-21 | AGCTAACCACACCTTATACCG | 15 |
According to The Oligonucleotide Probe Database nomenclature.1
Y = C or T.
Sections of fetal placenta, lung, liver, and the control samples were mounted on slides (Superfrost Plus; Gerhard Menzel, Braunschweig, Germany) and hybridized as described previously.8 In brief, all hybridizations were carried out at 40–42°C for 16 h and at a final probe concentration of 5 ng/µL. After hybridization, the slides were washed in a washing buffer, rinsed in water, air dried, and mounted (Vectashield; Vector Laboratories, Burlingame, CA) for fluorescence microscopy. For evaluation of the amount and histologic localization of bacterial micro-colonies, the domain bacterial probe EUB338 was applied (Table 1).2 Screening for the presence of Campylobacter spp. was performed using the Campylobacter genus–specific probe CAMP653.19 Campylobacter species identification was carried out using the C. jejuni–specific probe Cajej15 and the newly designed C. fetus–specific probe Cafet. Examination for Coxiella burnetii was carried out on specimens of fetal placenta using probe Cburn.8 The oligonucleotide probes (Eurofins MWG Synthesis, Ebersberg, Germany) were 5’-labeled with either fluorescein isothiocyanate (FITC; probe EUB338), the isothiocyanate derivative Cy3 (probes CAMP653, Cajej, Cafet), or Alexa Fluor 488 (probe Cburn). Hybridized specimens were examined with an AxioImager M1 microscope, equipped for epifluorescence with a 100-W HBO lamp and filter sets 24 (excitation at 485/578 nm), 38 (excitation at 470 nm), and 43 (excitation at 550 nm) for detection of double-staining FITC/Cy3 and single-staining FITC and Cy3, respectively. Images were obtained (AxioCam MRm v.3 FireWire monochrome camera; AxioVision software v.4.5; Carl Zeiss, Oberkochen, Germany).
On postmortem examination, fetus A1 had hemoabdomen as a result of liver rupture. In the liver of A2, multifocal areas of presumptive necrosis measuring up to 1 cm diameter were observed. No macroscopic changes were found in the fetuses or placentas of the remaining cases.
The placenta was available in 9 cases and all had severe placentitis on histologic examination (Table 2). Placental lesions were dominated by severe necrosis of chorionic villi, exudation of neutrophils, and myriads of small rod-shaped bacteria organized as micro-colonies within the cytoplasm of distended trophoblasts. Large numbers of bacteria also distended subepithelial capillaries in the chorionic villi and invaded the villus stroma (Fig. 1). Furthermore, stromal mineralization of villi, focal hemorrhages, and fibrin deposition were found in most of the placental specimens. Vasculitis in the chorionic stroma was present in cases B2, C1, D1, D2, and D3. Fetal lung and liver were submitted and investigated in 10 cases. Suppurative bronchopneumonia was found in cases A1, A2, and C1. Multifocal necrotizing hepatitis with mild-to-moderate, mainly neutrophilic infiltrates was present in cases A1, A2, B1, B3, and C2.
Table 2.
Summary of the histopathologic, bacteriologic, fluorescence in situ hybridization (FISH), and immunohistochemical results for ovine abortion cases.
| Case† | Histopathology | FISH |
C. fetus isolated‡
(site) |
C. jejuni isolated (site) |
Aerobe bacterial culture (tissues) |
Toxoplasmosis§
(tissues) |
Placenta assessed | Cause of abortion | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Camp. (tissues) |
C. fetus (tissues) |
C. jejuni (tissues) |
C. burnetii placenta |
||||||||
| A1 | Pneumonia, hepatitis | + (li, lu) |
+ (li, lu) |
− (li, lu) |
NA | − (aoc, int) |
NA | NG (li, lu, aoc) | − (br, he, li, lu) |
− | C. fetus |
| A2 | Pneumonia, hepatitis | + (li, lu) |
+ (li, lu) |
− (li, lu) |
NA | − (aoc, int) |
NA | NG (li, lu, aoc) | − (br, he, li, lu) |
− | C. fetus |
| B1 | Hepatitis | − (li, lu) |
− (li, lu) |
− (li, lu) |
NA | NA | NA | NA | − (br, he, li, lu) |
− | Unknown |
| B2 | Placentitis | + (pl) |
+ (pl) |
− (pl, li, lu) |
− | NA | NA | NA | − (br, he, li, lu, pl) |
+ | C. fetus |
| B3 | Placentitis, hepatitis | + (pl) |
+ (pl, li, lu) |
− (pl, li, lu) |
− | NA | NA | NA | − (br, he, li, lu, pl) |
+ | C. fetus |
| C1 | Placentitis, pneumonia | + (pl) |
− (pl, li, lu) |
+ (pl) |
− | − (aoc, pl) |
NA | NG (li, lu, aoc); mixed flora (pl) | − (br, he, li, lu, pl) |
+ | C. jejuni |
| C2 | Hepatitis | − (li, lu) |
− (li, lu) |
− (li, lu) |
NA | − (aoc) |
NA | NG (li, lu, aoc) | − (br, he, li, lu) |
− | Unknown |
| C3 | Placentitis | − (pl) |
− (pl) |
− (pl) |
− | NA | NA | NA | − (pl) |
+ | Unknown |
| C4 | Placentitis | + (pl) |
− (pl) |
+ (pl) |
− | NA | NA | NA | − (pl) |
+ | C. jejuni |
| C5 | Placentitis | + (pl) |
− (pl) |
+ (pl) |
− | NA | NA | NA | − (pl) |
+ | C. jejuni |
| D1 | Placentitis | NA | − (pl, li, lu) |
+ (pl) |
− | − (aoc) |
+¦
(aoc) |
NG (li, lu); mixed flora (pl, aoc) | − (br, he, li, lu, pl) |
+ | C. jejuni |
| D2 | Placentitis | NA | − (pl, li, lu) |
+ (pl, li#) |
− | − (aoc) |
+¦
(aoc) |
NG (li); mixed flora (pl, lu, aoc) | − (br, he, li, lu, pl) |
+ | C. jejuni |
| D3 | Placentitis | NA | − (pl, li, lu) |
+ (pl) |
− | − (aoc) |
+¦
(aoc) |
NG (li); mixed flora (pl, lu aoc) | − (br, pl) |
+ | C. jejuni |
aoc = abomasal contents; br = brain; Camp. = Campylobacter; he = heart; int = intestinal contents; li = liver; lu = lung; NA = data not available; NG = no growth; pl = placenta; − = no/negative; + = yes/positive.
Grouped by flock of origin A–D, one abortion case per number.
Culture on thioglycolate agar plates at 37°C under microaerophilic conditions for 48–96 h.
Based on histologic and immunohistochemical findings, in cases B2 and B3 combined with real-time PCR.
Isolated in pure culture under anaerobic conditions.
Single C. jejuni organisms were detected within the lumen of large hepatic veins.
Figure 1.
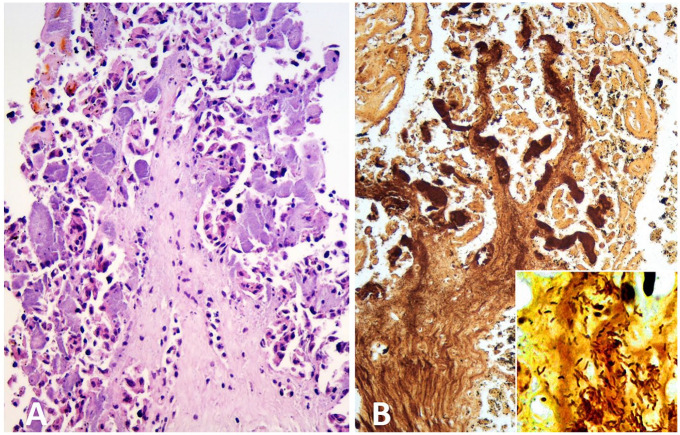
Aborted ovine placenta from case D1. A. Chorionic villus with severe trophoblast necrosis, mild stromal infiltration with neutrophils, and numerous severely distended subepithelial capillaries filled with finely granular basophilic material (bacterial micro-colonies). H&E. B. Silver-stained material within severely distended subepithelial capillaries and the stroma of a necrotic chorionic villus. Inset: comma-shaped, silver-stained bacteria invading the villus stroma. Warthin–Starry silver stain.
None of the 10 submitted fetuses had histologic lesions typical of toxoplasmosis in the brain, heart, or liver. In cases C3, C4, and C5, only placenta was submitted. All cases were immunohistochemically negative for T. gondii, except that cases B2 and B3 were inconclusive. Toxoplasmosis was ruled out in these cases by PCR. Based on the histopathologic, immunohistochemical, and PCR findings, toxoplasmosis was excluded as the cause of abortion in all 13 cases.
There was either no growth or growth of mixed flora without signs of abortifacient species on aerobic cultures of the lung, liver, and abomasal contents of 7 fetuses, and of placenta in 4 of these 7 cases (Table 2). In cases D1, D2, and D3 (the only cases subjected to anaerobic culture), C. jejuni was isolated from abomasal contents in pure culture. All samples examined specifically for C. fetus were negative (7 samples of abomasal contents, 2 samples of fetal intestinal contents, and 1 placenta sample).
Based on the histopathologic and bacteriologic findings (Table 2), Campylobacter infection was suspected. Sections of fetal placenta, lung, and liver from flocks A–C were then screened for the presence of Campylobacter spp. and other bacteria by simultaneous hybridization with the Campylobacter genus–specific probe CAMP653 and the general bacterial probe EUB338. Campylobacter spp. were detected in 7 cases (Table 2); Campylobacter was found to be the predominant bacterial population present and the only one associated with lesions.
A species-specific oligonucleotide probe, Cafet, targeting 16S rRNA in C. fetus including the subspecies C. fetus ssp. fetus and C. fetus ssp. venerealis, was designed using ARB software.21 The specificity of the probe was further evaluated for published 16S rRNA gene sequences in the NCBI database (http://www.ncbi.nlm.nih.gov/) using a BLASTn search for homology (Table 3). The probe has at least 2 mismatches to non-target 16S rRNA gene sequences in the NCBI database, except for the species Campylobacter avium, a species that has only been isolated from birds.16 The Cafet probe was further evaluated in combination with the general bacterial probe EUB338, targeting 99% of all known bacteria,2 using the C. fetus ssp. fetus and ssp. venerealis containing control samples. Cafet and EUB338 targeted cells of similar type and distribution and in equal numbers. The bacterial cells were found permeable for the probes and with a ribosomal content sufficient to result in an evident fluorescence signal. Furthermore, the hybridization specificity of the Cafet probe was tested on control tissue sections containing the following non-target species that are known abortifacients in sheep: Chlamydia abortus, Chlamydia pecorum, Chlamydia psittaci, Coxiella burnetii, L. monocytogenes, C. jejuni ssp. jejuni, and Escherichia coli, as well as the following closely related non-target species: C. coli, C. lari ssp. lari, C. upsaliensis, and A. cibarius. No Cafet hybridization signal was detected with any of these species.
Table 3.
Sequence comparison within the targeting site for the probe Cafet targeting Campylobacter fetus.
| Probe name or reference strain | Sequence | GenBank accession |
|---|---|---|
| Cafet | 3′-CCCGAACTATAGGTTGAT-5′٭ | |
| C. fetus ssp. fetus ATCC 27374T | 5′-******************-3′ | NR118514 |
| C. fetus ssp. venerealis ATCC 19438T | 5′-******************-3′ | NR118515 |
| C. fetus ssp. testudinum 03-427T | 5′-******************-3′ | NR133993 |
| C. avium CCUG 56292T | 5′-******************-3′ | NR118510 |
| C. jejuni ssp. jejuni ATCC 33560T | 5′-*************T*AG*-3′ | KF541294 |
| C. jejuni ssp. doylei LMG 8843T | 5′-*************T*AG*-3′ | NR043599 |
| C. coli ATCC 33559T | 5′-*************T*AG*-3′ | NR118511 |
| C. lari ATCC 35221T | 5′-*************T*AG*-3′ | NR118523 |
| C. upsaliensis NCTC 11541T | 5′-A***************A*-3′ | LR134372 |
Pairing is represented by asterisks; mismatch pairings within the probe target site are indicated.
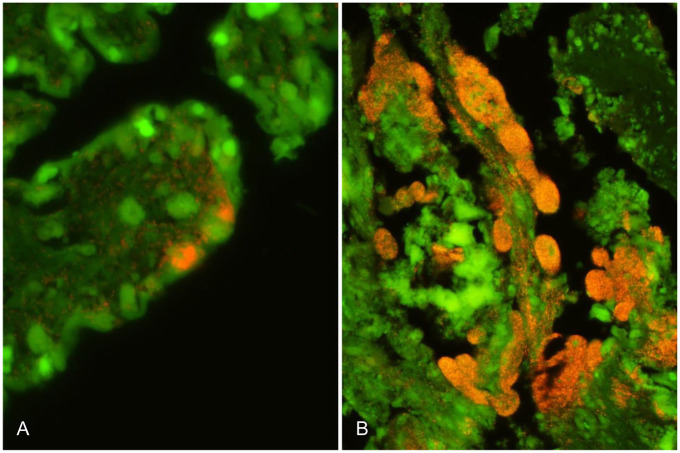
FISH using the specific probes Cafet and Cajej was then applied on sections of fetal placenta, lung, and liver from all 13 cases (Table 2). C. fetus was detected in all tissue lesions in cases from flocks A and B except for in the liver of B1 (Table 2). C. fetus was found in large numbers in the cytoplasm of distended trophoblasts covering the chorionic villi as well as invading the underlying villus stroma (Fig. 2A). Furthermore, C. fetus was found within alveolar macrophages and pneumocytes in the cases with pneumonia (A1, A2) as well as associated with the necrotic foci in the livers of cases A1, A2, and B3. All tissue specimens from flocks A and B were negative for C. jejuni. C. jejuni was detected in close association with tissue lesions in all cases from flocks C and D, except for cases C2 and C3. In tissue sections from the latter 2 cases, only a few bacteria were detected by the EUB338 probe; however, those bacteria were neither associated with the necrotic foci in the liver (C2) nor the placental lesions (C3). In case C1, C. jejuni was present in the placental lesions only, but not in the pneumonic lesions. C. jejuni was found in large numbers in the cytoplasm of distended trophoblasts covering the chorionic villi as well as invading the underlying villus stroma (Fig. 2B). All tissue specimens from flocks C and D were negative for C. fetus. All placental samples were negative for Coxiella burnetii when hybridized with the specific probe Cburn.
Figure 2.
Aborted ovine placenta. A. Detection of Campylobacter fetus by fluorescence in situ hybridization (FISH) in the placenta of case B3. Numerous C. fetus organisms (specific orange fluorescence signal from probe Cafet) are distending the cytoplasm of infected trophoblasts as well as invading the underlying chorionic villus stroma. B. Detection of Campylobacter jejuni by FISH in the placenta from case D1. Trophoblasts and subepithelial capillaries are severely distended as a result of accumulation of numerous C. jejuni organisms (orange). C. jejuni organisms also invade the villus stroma.
Based on FISH assays, we diagnosed C. fetus as the cause of abortion in 4 of 5 abortion cases from flocks A and B, and thereby established C. fetus as the likely etiology of the abortion storms in both flocks. C. jejuni was diagnosed as the cause of abortion in 6 of 8 cases from flocks C and D, and was thereby established as the likely etiology of the abortion storms in these 2 flocks. The cause of abortion remained unknown in cases B1, C2, and C3. In Denmark, T. gondii is the most often diagnosed infectious abortifacient in sheep (National Veterinary Institute. [Results of diagnostic investigations in sheep and goats 1995–2000]. Frederiksberg C, Denmark: Technical University of Denmark. Danish). Lesions similar to those observed in the placenta and fetal organs analyzed in our study can be caused by infection with Brucella melitensis, B. abortus, and Chlamydia abortus.12 However, infection of sheep with those agents has never been recorded in Denmark (Danish Animal Health Report 2017).
Our cases are the most recently recorded ovine Campylobacter abortions in Denmark. The establishment of C. jejuni as the cause of abortion in cases C1, C4, and C5 emphasizes the importance of considering C. jejuni as an ovine abortifacient in Denmark, where C. fetus ssp. fetus has been the only Campylobacter species detected in ovine abortions prior to our study, according to the diagnostic record archive of the Danish National Veterinary Institute. C. fetus ssp. fetus has been the cause of the majority of ovine Campylobacter abortions worldwide. In the United States, C. jejuni replaced C. fetus as the predominant Campylobacter species causing ovine abortion during the late 1980s and early 1990s.17
The distribution of lesions in fetuses A1, A2, and B3 and the occurrence of C. fetus in the lung (A1, A2) and liver (A1, A2, B3), as shown by FISH, strongly suggest oral (via amniotic fluid) and hematogenous routes of infection. Infection through the amniotic fluid caused bronchopneumonia, whereas hematogenous spread through the umbilical vein caused necrotizing hepatitis. In the C. jejuni FISH-positive cases, C. jejuni was only detected in association with placental lesions, but not within lung or liver lesions. C. jejuni and, to a lesser extent, C. fetus commonly exist as commensals in the gastrointestinal tract of sheep.18 The direct detection of Campylobacter intracellularly in chorionic trophoblasts in areas of placental necrosis and inflammation rules out accidental fecal contamination and thus allows the establishment of Campylobacter as the cause of abortion.
Isolation of C. fetus was unsuccessful in our study, even though a C. fetus–specific culture method was applied, and numerous C. fetus were demonstrated within lung and liver lesions using FISH. These findings together with the assumption that abomasal content mainly consists of amniotic fluid, which, if infested with bacteria, is the most likely source of fetal lung infection, probably reflect decreased viability of the bacteria by the time culture was attempted. Furthermore, our findings suggest that FISH has higher sensitivity than the traditional culture method. FISH is a fast, economical, and simple method when compared to most amplification-based molecular techniques and leads to immediate differentiation of infectious agents without delay inherent in microbial culture.9
In our study, FISH proved to be a useful tool to detect and identify the zoonotic agent Campylobacter within ovine abortion material, a potential source of human infection. Cafet, the C. fetus–specific oligonucleotide probe developed in our study, targets almost the same area of the 16S ribosomal RNA of C. fetus as the recently published oligonucleotide probe C. fetus 1.9 Both probes were designed using ARB software. C. fetus 1 was shown to be able to identify C. fetus, including all subspecies, by hybridizing whole-cell bacterial cultures.9 In our study, probe Cafet was used successfully to identify C. fetus in situ in tissue specimens from clinical cases potentially containing complex bacterial communities. We conclude that the targeted part of the 16S rRNA sequence is well suited to identify C. fetus in different sample types.
Acknowledgments
We thank Annie Ravn Pedersen, Helle Ruby, and Susanne Primdahl for their technical assistance in preparing the histologic and FISH specimens.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The study was funded by the National Veterinary Institute, Denmark.
ORCID iD: Godelind A. Wolf-Jäckel  https://orcid.org/0000-0002-5719-3443
https://orcid.org/0000-0002-5719-3443
References
- 1. Alm EW, et al. The oligonucleotide probe database. Appl Environ Microbiol 1996;62:3557–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amann RI, et al. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 1990;56:1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arreaza G, et al. Pre-analytical considerations for successful next-generation sequencing (NGS): challenges and opportunities for formalin-fixed and paraffin-embedded tumor tissue (FFPE) samples. Int J Mol Sci 2016;17:1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boye M, et al. Specific detection of Lawsonia intracellularis in porcine proliferative enteropathy inferred from fluorescent rRNA in situ hybridization. Vet Pathol 1998;35:153–156. [DOI] [PubMed] [Google Scholar]
- 5. Ehricht R, et al. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes 2006;20:60–63. [DOI] [PubMed] [Google Scholar]
- 6. European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J 2018;16:5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frickmann H, et al. Fluorescence in situ hybridization (FISH) in the microbiological diagnostic routine laboratory: a review. Crit Rev Microbiol 2017;43:263–293. [DOI] [PubMed] [Google Scholar]
- 8. Jensen TK, et al. Application of fluorescent in situ hybridisation for demonstration of Coxiella burnetii in placentas from ruminant abortions. APMIS 2007;115:347–353. [DOI] [PubMed] [Google Scholar]
- 9. Karg M, et al. Identification of Campylobacter fetus by fluorescence in situ hybridization (FISH). J Microbiol Methods 2018;151:44–47. [DOI] [PubMed] [Google Scholar]
- 10. Kuhn KG, Mølbak K. Determinants of sporadic Campylobacter infections in Denmark: a nationwide case-control study among children and young adults. Clin Epidemiol 2018;10:1695–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makki JS. Diagnostic implication and clinical relevance of ancillary techniques in clinical pathology practice. Clin Med Insights Pathol 2016;9:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moeller RB., Jr Disorders of sheep and goats. In: Njaa BL, ed. Kirkbride’s Diagnosis of Abortion and Neonatal Loss in Animals. 4th ed. Oxford, UK: Wiley-Blackwell, 2012:49–88. [Google Scholar]
- 13. Mostegl MM, et al. Influence of prolonged formalin fixation of tissue samples on the sensitivity of chromogenic in situ hybridization. J Vet Diagn Invest 2011;23:1212–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pantchev A, et al. New real-time PCR tests for species-specific detection of Chlamydophila psittaci and Chlamydophila abortus from tissue samples. Vet J 2009;181:145–150. [DOI] [PubMed] [Google Scholar]
- 15. Poppert S, et al. Identification of thermotolerant Campylobacter species by fluorescence in situ hybridization. J Clin Microbiol 2008;46:2133–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rossi M, et al. Campylobacter avium sp. nov., a hippurate-positive species isolated from poultry. Int J Syst Evol Microbiol 2009;59: 2364–2369. [DOI] [PubMed] [Google Scholar]
- 17. Sahin O, et al. Emergence of a tetracycline-resistant Campylobacter jejuni clone associated with outbreaks of ovine abortion in the United States. J Clin Microbiol 2008;46:1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sahin O, et al. Campylobacter-associated diseases in animals. Annu Rev Anim Biosci 2017;5:21–42. [DOI] [PubMed] [Google Scholar]
- 19. Schmid MW, et al. Development and application of oligonucleotide probes for in situ detection of thermotolerant Campylobacter in chicken faecal and liver samples. Int J Food Microbiol 2005;105:245–255. [DOI] [PubMed] [Google Scholar]
- 20. Wagenaar JA, et al. Campylobacter fetus infections in humans: exposure and disease. Clin Infect Dis 2014;58:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Westram R, et al. ARB: a software environment for sequence data. In: de Bruijn FJ, ed. Handbook of Molecular Microbial Ecology I: Metagenomics and Complementary Approaches. Hoboken, NJ: Wiley-Blackwell, 2011:399–406. [Google Scholar]