Abstract

In the present study, we report the design and synthesis of new derivatives of the β-keto-enol grafted on pyridine and furan moieties (L1–L11). Structures of compounds were fully confirmed by Fourier transform infrared spectroscopy (FT-IR), 1H NMR, 13C NMR, electrospray ionization/liquid chromatography-mass spectrometry (ESI/LC-MS), and elemental analysis. The compounds were screened for antifungal and antibacterial activities (Escherichia coli, Bacillus subtilis, and Micrococcus luteus). In vitro evaluation showed significant fungicidal activity for L1, L4, and L5 against fungal strains (Fusarium oxysporum f.sp albedinis) compared to the reference standard. Especially, the exceptional activity has been demonstrated for L1 with IC50 = 12.83 μg/mL. This compound and the reference benomyl molecule also showed a correlation between experimental antifungal activity and theoretical predictions by Petra/Osiris/Molinspiration (POM) calculations and molecular coupling against the Fgb1 protein. The highest inhibition of bacterial growth for L1 is due to its strongest binding to the target protein. This report may stimulate the further synthesis of examples of this substance class for the development of new drugs.
1. Introduction
Fungal infection is a common disease that causes a serious growing threat to mankind including high morbidity and mortality every year around the world,1−3 especially among immune-compromised patients.4,5 Therefore, it is urgent to discover novel antifungal inhibitors, particularly those with new action modes, with low toxicity and bioavailability. Furthermore, it should be safer and more effective against sensitive and drug-resistant fungi. In recent years, the fused heterocyclic compounds containing bridgehead nitrogen- or oxygen-donor atoms have attracted great interest due to their biological activities and chemical medicinal properties. Indeed, pyridine and furan derivatives have been recently reported to possess pharmacological potential as fungicidal,6,7 anti-inflammatory,8,9 antiviral,10,11 antibacterial,12,13 and even anticancer agents.14,15
On the other hand, heterocyclic compound bearing β-keto-enol functionality has been a fruitful source of inspiration for pharmaceutical and medicinal industries due to their widespread potential biological activities and their versatile utility in the world of medicinal chemistry,16,17 such as antioxidant,18 anti-inflammatory,19 anticancer,20 and antifungal21 activities. In this context, β-keto-enol functionality was found in numerous natural products such as the curcumin and its derivatives (Figure 1), which have spurred numerous studies in medicinal chemistry.22−24 Some compounds have even been approved for treating patients with human immunodeficiency virus (HIV).25,26
Figure 1.
Molecular structures of some drugs containing keto-enol functionality.
Recently, several analogic compounds containing the β-keto-enol phamacophore have been designed and synthesized such as the keto-enol tetrazoles and triazoles as anti-HCV agents,27 the triazolyl-keto-enol calix[4]arene as a potent integrase strand transfer inhibitor,28 coumarinyl chalcones as highly selective agents for breast cancer cell lines,29 etc. On the other hand, virtual screening combined with other computational methods, such as ligand–target Docking, can be very useful to confirm biological activities.30,31 While the classic drug development process is very expensive and tedious, computational methods are highly efficient and economical.32 Modern drug designing extensively relies on computer-assisted techniques like ligand-based drug design (LBDD) and structure-based drug design (SBDD) approaches. Molecular docking is a contemporary approach under the umbrella of SBDD. Molecular docking is highly useful to identify the structural/pharmacophoric features that govern the bioactivity profile of a molecule. The identification of a pharmacophoric pattern is then used for future optimizations. This approach is highly economical, time saving, and result oriented. Considering all these observations, and in continuation of our previous work on biologically important β-keto-enol heterocyclic compound, we report herein a new series of β-keto-enol group embedded with heterocyclic moieties such as pyridine and furan derivatives. Their antifungal activities were in vitro evaluated and correlated by Petra/Osiris/Molinspiration (POM) calculations and molecular coupling against the Fgb1 protein.
2. Results and Discussion
2.1. Synthesis
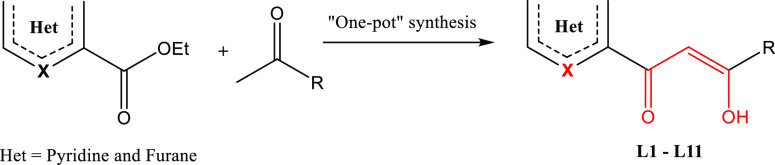
The synthetic route of the target compounds (L1–L11) was carried out following a Claisen condensation under mild conditions (Scheme 1).21,33−41 Indeed, a solution of ethyl heterocycle-2-carboxylate was added to a suspension of sodium in toluene, and then ketone derivatives were added at 0 °C. After neutralization of the generated keto-enolate salts to pH = 5.5 using acetic acid, the obtained residue was filtered through silica using CH2Cl2/MeOH as an eluent to afford the title products in acceptable yields. These products are presumably in anticonformation isomeric forms as already demonstrated for similar products analyzed too by single-crystal X-ray diffraction.21,33−41
Scheme 1. Synthetic Route for the Compounds L1–L11.
Intramolecular hydrogen bonding is the main factor that governs the structure of keto-enol tautomers in solution.37 In fact, the stable β-keto-enol form is generally present in large amount due to the conjugation effect of the enol with the carbonyl group and the presence of an intramolecular hydrogen bond (Scheme 1).381H NMR spectra of (L1–L11) exhibited peaks at δ = 6.20–8.10 ppm assigned to the vinylic proton (−(−OH)C=CH−) of the enolic tautomer. In addition, a very small peak at δ = 3.14–4.87 ppm for the methylene protons (−CO–CH2–CO−) of the β-diketones tautomer form was observed. Indeed, according to the 1H NMR integration of signals, the parent β-diketones exist almost exclusively in the enol form (>90%), and only a trace of the keto form is detected. 13C NMR signals assigned with the aid of distortionless enhancement by polarization transfer (DEPT) experiments were also performed to confirm the previous result. Indeed, one signal at around δ = 92–95 ppm was observed for the vinylic proton (−CH=C–(OH−)−) of the enolic forms. Also, a very small negative signal of ketone form (−CO–CH2–CO−) was observed on the DEPT-135 spectra at around δ = 176–184 ppm. Representative NMR and mass spectra are given in Supporting Information (SI) Figures S1–S20. The target products were obtained in majority in the β-keto-enol tautomer form (90%), whereas the remaining 10% represents the β-diketone tautomer form. This trend is confirmed by single-crystal X-ray diffraction, which systematically showed the β-keto-enol in this substance class, along with a mesomeric effect.21,33−41 We recall that there are two driving forces responsible for the formation of keto-enol isomers in solution:42 (i) the electronic force, in which the formation of the enol isomer is controlled by the electronegativity of the R and R′ substituents fixed on the β-diketone (R′COCH2COR) and (ii) the resonance force (mesomeric effect), which has priority over the inductive effect when one or two substituents R and R′ are aromatic.
2.2. Pharmacology
The compounds (L1–L11) were evaluated for their antifungal activity against Fusarium oxysporum and two Gram-positive (strains, viz., Bacillus subtilis and Micrococcus luteus). A complementary screening was performed too against two Gram-negative bacterial strains (bacterium, viz., Escherichia coli) but very weak or absence of antibacterial activity was noticed. Only small inhibition areas were observed compared to the gentamicin standard. These results can be interpreted by the lack of pharmacophore sites that can serve as potential and specific features for inhibiting bacterial growth.43 In contrast, most of these molecules have strong antifungal activity against FOA, depending on their dose (Table 1). Their activity largely depends on the activity relationships of the structure (SAR) and shows an interesting influence of the substitution pattern by observing the influence of the R substituent (Figure 2).
Table 1. Structures and Anti-Fusarium Activities of L1–L11.
In the presence of the tested compounds (cm).
NS = not significant.
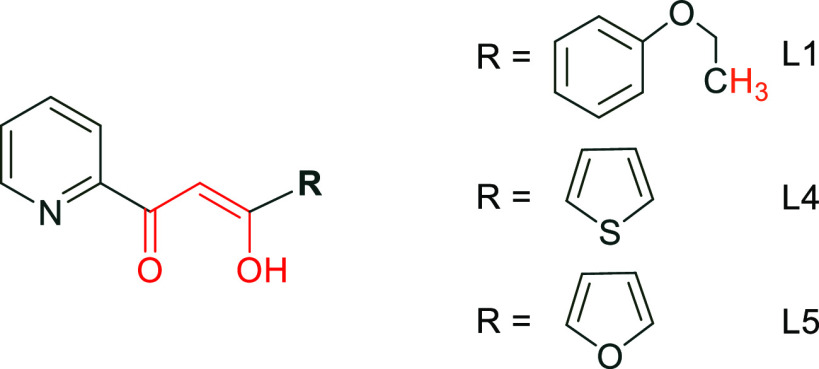
Figure 2.
R substituent for L1, L4, and L5.
Indeed, L4 with the thiophene group has an IC50 = 17 μg/mL, whereas L1, which contains the ethoxyphenyl, has a decreased IC50 = 12.83 μg/mL. Replacement of thiophene by a furan group (L5) results however in an increased IC50 = 34 μg/mL.
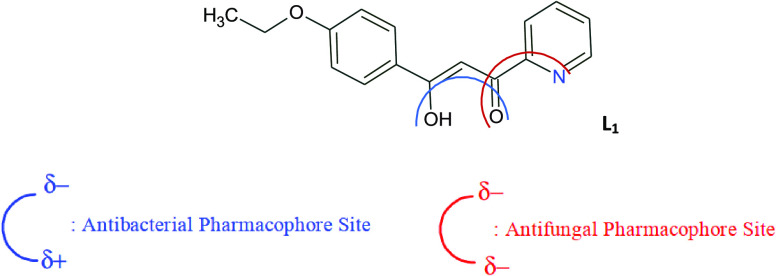
It is known that the negative charges of the keto and pyridine groups contribute positively to the antifungal activity, rather the antibacterial activity.43 This is consistent with the antifungal mode of action of compounds with pharmacophore sites (Xδ−···Yδ+) (Figure 3). Indeed, the fungal activity of L1 is very substantial and decreases slightly in the case of L4 because of ethoxyphenyl groups. The greater activity of the pharmacophore site is due to its physicochemical properties and its ability to penetrate the envelope of fungal cells and reach its cellular site of action.
Figure 3.
Antibacterial and antifungal pharmacophore sites for L1.
It is worth noting that the present results are the best among previously tested analogues against the FOA fungus.21,33 In connection with a pronounced biological interest in heterocyclic compounds, this study suggests that the nature of the R substituent (Figure 2) should be considered seriously to be able to tune properties and determine the structural activity ratio for this new class of antifungal agent.
2.3. Computational Studies
2.3.1. POM Analyses of Compounds
The theoretical toxicity risks of L1–L11, which were calculated using the POM program,44 show that some of our compounds are nontoxic and can be used as therapeutic agents (Table 2). Besides, the assessment of the mutagenicity of the free drug assumes that the structures of the compounds examined are not mutagenic and have a low risk compared to synthetic drugs. The standards for irritant and reproductive effects are related.
Table 2. Osiris Calculations of Toxicity Risks of L1–L11.

Highly toxic (red), slightly toxic (orange), and not toxic (green). MW, molecular weight; MUT, mutagenic; TUM, tumorigenic; IRRIT, irritant; and REP, reproductive effective.
Sol: solubility, DL: druglikness, DS: Drug-Score.
The hydrophilicity of each compound was evaluated by the cLog P value because the absorption is known to be strongly influenced by this parameter. Our results show that all compounds have cLog P values that are within acceptable standards, with the highest value of 2.98 for L7, and may be effective against multiple biological targets as shown in Table 3. Therefore, the good absorption of test compounds L1–L11 may be attributed to their good solubility.45 In addition, Table 3 shows the similarity of the drug of compounds L1–L11 in the area of incompatibility with known standard medicinal products (benomyl for fungi). The Drug-Score (DS) combines the risks of toxicity, cLog P, Log S (S = solubility), molecular weight (MW), and toxicity into a useful value that can be used to assess the overall potential of a compound to qualify as a drug. Overall, L1–L11 are considered as good candidates, in terms of the bioactivity score prediction, against regular human receptors such as GPCR ligand, ion-channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor, and enzyme inhibitor. Compounds L1–L11 are subject to important tautomerism/mesomerism chemical processes leading to the regeneration of combined pharmacophore sites, as shown in Figure 3 for L1. In addition, Figure 3 shows that L1 is more likely to inhibit fungal activity because its pharmacodynamic sites are more specific than multiple substitution sites. POM analyses show the crucial role and influence of substituents on bioactivity, which indicate unfavorable structural parameters in the actual design of the drug: a stronger substitution does no longer guarantee efficiency in bioactivity.
Table 3. Molinspiration Calculations of (L1–L11) and Benomyl.
| molinspiration
calculationsa |
drug-likenessb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| molecule | TPSA | NONH | NV | VOL | GPCRL | ICM | KI | NRL | PI | EI |
| L1 | 59.42 | 2 | 0 | 248.06 | –0.52 | –0.31 | –0.67 | –0.46 | –0.45 | 0.12 |
| L2 | 50.19 | 1 | 0 | 223.60 | –0.69 | –0.32 | –0.78 | –0.79 | –0.65 | 0.11 |
| L3 | 63.08 | 1 | 0 | 201.56 | –0.49 | –0.20 | –0.60 | –0.66 | –0.41 | 0.31 |
| L4 | 50.19 | 1 | 0 | 196.43 | –0.92 | –0.65 | –1.00 | –1.14 | –0.84 | –0.00 |
| L5 | 63.33 | 1 | 0 | 187.28 | –0.84 | –0.64 | –1.10 | –1.12 | –0.95 | –0.02 |
| L6 | 50.19 | 1 | 0 | 196.43 | –0.69 | –0.28 | –0.83 | –0.82 | –0.65 | 0.17 |
| L7 | 50.44 | 1 | 0 | 235.43 | –0.67 | –0.44 | –0.80 | –0.59 | –0.66 | –0.07 |
| L8 | 63.58 | 1 | 0 | 173.01 | –1.27 | –0.86 | –1.49 | –1.40 | –1.33 | –0.35 |
| L9 | 59.67 | 1 | 0 | 216.98 | –1.03 | –0.67 | –1.20 | –0.93 | –1.06 | –0.29 |
| L10 | 50.44 | 1 | 0 | 182.15 | –1.54 | –1.15 | –1.66 | –1.64 | –1.55 | –0.57 |
| L11 | 50.44 | 1 | 0 | 208.00 | –1.14 | –0.72 | –1.34 | –1.08 | –1.18 | –0.35 |
| benomyl | 85.26 | 2 | 0 | 264.53 | 0.36 | 0.21 | 0.33 | –0.68 | –0.04 | 0.28 |
TPSA, total molecular polar surface area; NONH, number of OH···N and O···NH interactions; NV, number of violation of five Lipinsky rules; and VOL, volume.
GPCRL, GPCR ligand; ICM, ion-channel modulator; KI, kinase inhibitor; NRL, nuclear receptor ligand, PI, protease inhibitor; and EI, enzyme inhibitor.
2.3.2. Molecular Docking and Molecular Dynamics Simulations
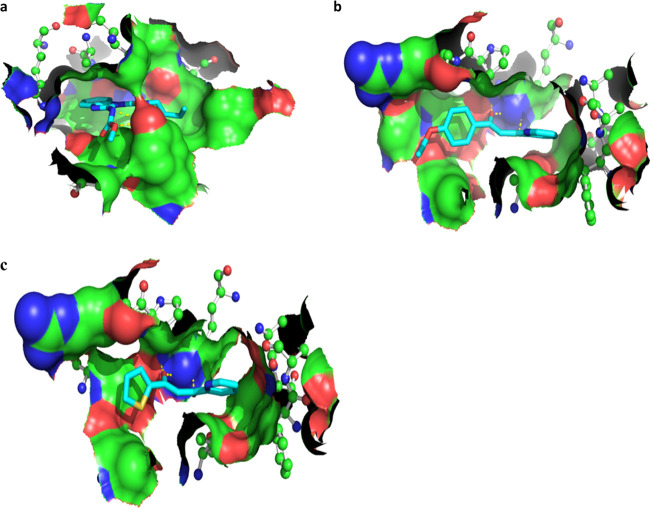
Two best-performing molecules (L1 and L4) as well as benomyl as a positive control, presenting minimum IC50 values, were further evaluated in molecular docking experiments to predict their binding affinity to β-tubulin of F. oxysporum (Figure 4 and Table 4). This in silico experimental design takes into consideration tubulin-binding properties of benomyl as an antimitotic antifungal agent and the information about the receptor-binding site, which was previously validated with the co-crystallized ligand taxol.46,47 As an outcome, the Gibbs free energy of binding (ΔGbind) and Kd values were determined to accurately predict the minimum inhibitory concentration (MIC) values obtained from the MIC assay, which shows a good correlation between the experimental and theoretical data (Table 4). In particular, the L1 compound represented the stronger binding (ΔGbind = −7.0 kcal/mol and Kd = 7.08 μM) to β-tubulin compared to L4 (ΔGbind = −6.5 kcal/mol and Kd = 16.23 μM), which correlates with their IC50 values. On the other hand, the 50 ns MD simulations of L1 and benomyl compounds bound to β-tubulin confirmed the previous results of molecular docking and antifungal assay, where the reference substance achieved the highest inhibition of bacterial growth due to its strongest binding to the target protein (Table 4).
Figure 4.
Binding conformations predicted from the ADVina runs for benomyl (a), L1 (b), and L4 (c) bound to β-tubulin of F. oxysporum. The protein (Fgb1) binding site is shown by the molecular surface and colored according to the protein atomic composition. All protein residues are drawn as ball-and-stick models. Hydrogen bonds are visualized as dashed lines. The ligand molecules are depicted in sticks, and hydrogen atoms were removed to enhance clarity.
Table 4. Binding Free Energies (ΔGbind, ΔGGBSA, ΔGPBSA), Dissociation Constants (Kd), and Minimum Inhibitory Concentrations IC50 Capable of Inhibiting F. oxysporum Growth at 50% for the Analyzed Substances.
| molecule | ΔGbind (kcal/mol) | Kd (μM) | IC50 (μg/mL) | ΔGGBSA (kcal/mol) | ΔGPBSA (kcal/mol) |
|---|---|---|---|---|---|
| L1 | –7.0 | 7.08 | 12.83 | –31.01 | –2.76 |
| L4 | –6.5 | 16.23 | 17.0 | ||
| benomyl | –7.2 | 5.05 | 5.0 | –41.38 | –11.45 |
3. Conclusions
We have designed and synthesized a different new set of new β-keto-enol pyridine and furan derivatives. They were characterized by elemental analysis, 1H NMR, 13C NMR, Fourier transform infrared spectroscopy (FT-IR), and electrospray ionization/liquid chromatography-mass spectrometry (ESI/LC-MS). Most of the newly synthesized compounds demonstrated appropriate antifungal activities that were evaluated in vitro. In this context, we noticed that L1 exhibited excellent antifungal activity at IC50 = 12.83 μg/mL concentration against tested fungicide strains; which is even equal to benomyl fungicide reference. These results exhibited by such β-keto-enol group pyridine and furan derivatives are encouraging and may stimulate further studies for the development of new drugs.
4. Materials and Methods
4.1. General Information
All chemical reagents used in this study were of analytical grade (Aldrich, purity >99%). Melting points were measured with a BUCHI 510 mp apparatus. 1H and 13C NMR spectra were recorded on a Bruker AC 300 spectrometer operating at 300 MHz for proton and 75.47 for carbon nuclei. Molecular weights were determined using a JEOL JMS DX-300 mass spectrometer. Elemental analysis was performed by CNRST Rabat. Infrared (IR) spectra were acquired on a Shimadzu infrared spectrophotometer using KBr disks. X-ray diffraction data collection was carried out on the four-circle Oxford Xcalibur diffractometer (Mo Kα radiation, λ = 0.71073 Å). The in vitro antifungal and antibacterial activities were tested by the agar diffusion technique.
4.2. General Procedure for the Synthesis of β-Keto-enol Heterocycles
The β-keto-enol pyridine and furan derivatives were prepared using our previously reported procedure.21,33−35 Briefly, aryl methyl ketones (17.39 mmol) were added at 0 °C to a mixture of sodium (0.4 g, 17.39 mmol) and ethyl heterocycle-2-carboxylate (13.23 mmol) in toluene (50 mL). The mixture was kept under stirring at room temperature for 2 days. The resulting solid was treated thoroughly with toluene and neutralized with acetic acid to pH of 5.5. The organic layer obtained after extraction with CH2Cl2 was dried over anhydrous sodium sulfate and concentrated in vacuo. The obtained residue was filtered through silica using CH2Cl2/MeOH as an eluant followed by recrystallization from methanol (95%) to give the desired products L1–L11 as solids in acceptable yields.
4.2.1. 3-(4-Ethoxyphenyl)-3-hydroxy-1-(pyridin-2-yl)prop-2-en-1-one (L1)
Brown powder; yield: 45% (1.3 g); mp = 198–199 °C; Rf = 0.5 (CH2Cl2/MeOH 7/3)/silica. IR (KBr, cm–1): ν(OH) = 3444; ν(C=O) = 1599; ν(enolic C=C) = 1549; 1H NMR (DMSO-d6): δ 1.31 (t, 3H, CH2–CH3); 3.14 (s, 0.1H, keto, CH2); 4.06 (q, 2H, O-CH2); 6.96 (d, 2H, Ar-H3,5); 7.49 (s, 0.9H, enol, CH); 7.73 (t, 1H, Py-Hβ); 7.78 (t, 1H, Py-Hδ); 7.84 (m, 2H, Ar-H2,6); 8.03 (t, 1H, Py-Hγ); 8.75 (d, 1H, Py-Hα); 13C NMR (DMSO-d6): δ 14.94 (1C, CH2–CH3); 48.24 (1C, keto, CH2); 63.90 (1C, OCH2); 92.87 (1C, enol, CH); 114.64 (2C, Ar-C3,5); 123.25 (1C, Py-Cδ); 130.12 (2C, Ar-C2,6); 138.14 (1C, Py-Cγ); 150.11 (1C, Py-Cα); 182.16 (1C, C-OH); 186.77 (1C, C=O); Anal. Calcd. for C16H15NO3: C 71.36, H 5.61, N 5.20. Found C 71.32, H 5.58, N 5.18; M.S: m/z, 270.01 (M + H)+.
4.2.2. 3-(4-Bromophenyl)-3-hydroxy-1-(pyridin-2-yl)prop-2-en-1-one (L2)
Brown powder; yield: 31% (0.9 g); mp = 236–238 °C; Rf = 0.73 (CH2Cl2/MeOH 8/2)/silica. IR (KBr, cm–1): ν(OH) = 3442; ν(C=O) = 1596; ν(enolic C=C) = 1554; 1H NMR (DMSO-d6): δ 3.54 (s, 0.1H, keto, CH2); 5.71 (s, 0.9H, enol, CH); 7.68 (m, 2H, Ar-H2,6); 7.74 (m, 1H, Py-Hβ); 7.77 (m, 1H, Py-Hγ); 7.84 (m, 2H, Ar-H3,5); 8.08 (t, 1H, Py-Hδ); 8.79 (d, 1H, Py-Hα); 13C NMR (DMSO-d6): δ 48.24 (1C, keto, CH2); 95.93 (1C, enol, CH); 122.25 (1C, Py-Cδ); 125.35 (1C, Py-Cβ); 125.64 (1C, Ar-C4); 128.56 (2C, Ar-C3,5); 131.44 (1C, Ar-C1); 132.10 (2C, Ar-C2,6); 137.14 (1C, Py-Cγ); 149.61 (1C, Py-Cα); 167.13 (2C, Py-Cε); 182.24 (1C, C-OH); 184.46 (1C, C=O); Anal. Calcd. for C14H10BrNO2: C 55.29, H 3.31, Br 26.27, N 4.61; Found C 55.30, H 3.32, Br 26.24, N 4.64; M.S: m/z, 305 (M + H)+.
4.2.3. 3-Hydroxy-1,3-di(pyridin-2-yl)prop-2-en-one (L3)
Brown powder; yield: 59% (2.5 g); mp = 108–110 °C; Rf = 0.82 (CH2Cl2/MeOH 6/4)/silica. IR (KBr, cm–1): ν(OH) = 3451; ν(C=O) = 1617; ν(enolic C=C) = 1568; 1H NMR (CDCl3-d): δ 4.87 (s, 0.1H, keto, CH2); 7.37 (dd, 2H, Py-Hβ); 8.79 (td, 2H, Py-Hγ); 8.07 (t, 2H, Py-Hδ), 8.10 (s, 1H, enol, C-H); 8.68 (dd, 2H, Py-Hα); 13C NMR (CDCl3-d): δ 48.32 (1C, keto, CH2); 94.56 (1C, enol, C-H); 121.97–122.14 (2C, Py-Cδ); 126.39–127.20 (2C, Py-Cβ); 136.90 (2C, Py-Cγ); 148.90–149.52, (2C, Py-Cα); 152.43 (2C, Py-Cε); 184.44 (1C, C=O); 196.97 (1C; C-OH); Anal. Calcd. for C13H10N2O2: C 69.02, H 4.46, N 12.38; Found C 69.04, H 4.43, N 12.36; M.S: m/z, 227.16 (M + H)+.
4.2.4. 3-Hydroxy-1-(pyridin-2-yl)-3-(thiophen-2-yl)prop-2-en-1-one (L4)
Colorless crystals; yield: 38% (1.5 g); mp = 94–96 °C; Rf = 0.13 (CH2Cl2/MeOH, 9/1)/silica. IR (KBr, cm–1): ν(OH) = 3426; ν(C=O) = 1622; ν(enolic C=C) = 1514. 1H NMR (CDCl3-d): δ 4.80 (s, 0.1H, keto, CH2); 7.18 (dd, 1H, Th-Hβ); 7.39 (s, 1H, enol, C-H); 7.43 (dd, 1H, Py-Hγ); 7.67 (dd, 1H, Py-Hβ); 7.86 (td, 1H, Py-Hδ); 7.92 (dd, 1H, Th-Hγ); 8.11 (dt, 1H, Th-Hα); 8.71 (dd, 1H, Py-Hα); 13C NMR (CDCl3-d): δ 49.10 (1C, keto, CH2); 94.12 (1C, enol, C-H); 122.06 (1C, Py-Cδ); 126.28 (1C, Th-Cβ); 128.53 (1C, Py-Cβ); 131.33 (1C, Th-Hγ); 133.36 (1C, Th-Cα); 137.20 (1C, Th-Cε); 142.59 (1C, Py-Cγ); 149.54 (1C, Py-Cα); 151.89 (1C, Py-Cε); 177.99 (1C, C=O); 184.16 (1C; C-OH); Anal. Calcd. for C12H9NO2S: C 62.32; H 3.92; N 6.06; Found: C 61.79; H 3.96; N 6.09; M.S: m/z, 232.02 (M + H)+.
4.2.5. 3-(Furan-2-yl)-3-hydroxy-1-(pyridin-2-yl)prop-2-1-one (L5)
Yellow powder; yield: 30% (0. 98 g); mp = 102–104 °C; Rf = 0.6 (CH2Cl2/MeOH 9/1)/silica. IR (KBr, cm–1): ν(OH) = 3428; ν(C=O) = 1625; ν(enolic C=C) = 1515. 1H NMR (CDCl3-d) δ 4.69 (s, 0.1H, keto, CH2); 6.58 (dd, 1H, Fu-Hβ); 7.29 (dd, H, Fu-Hγ); 7.37 (s, 0.9H, enol, C-H); 7.41 (dd, 1H, Py-Hβ); 7.63 (dd, 1H, Fu-Hα); 7.84 (td, 1H, Py-Hγ); 8.09 (td, 1H, Py-Hδ); 8.69 (dd, 2H, Py-Hα). 13C NMR (CDCl3-d) δ 48.52 (1C, keto, CH2); 93.82 (1C, enol, C-H), 112.79 (1C, Fu-Cβ), 116.67 (1C, Fu-Hγ), 122.01 (1C, Py-Cδ), 126.33 (1C, Py-Cβ), 126.33 (1C, Py-Cγ), 137.15 (1C, Fu-Cα), 149.56 (1C, Py-Cα), 151.18 (1C, Py-Cε), 151.96 (1C, Fu-Cε), 178.73 (1C, C=O), 179.58 (1C; C-OH); Anal. Calcd. for C12H9NO3: C 66.97, H 4.22, N 6.51; Found: C 66.99, H 4.20, N 6.54; MS m/z: 216 [M + H]+.
4.2.6. 3-Hydroxy-1-(pyridin-2-yl)-3-(thiophen-3-yl)prop-2-en-1-one (L6)
Yellow powder; yield: 42% (1. 32 g); mp = 79–80 °C; Rf = 0.37 (CH2Cl2/MeOH 9/1)/silica. IR (KBr, cm–1): ν(OH) = 3424; ν(C=O) = 1625; ν(enolic C=C) = 1514. 1H NMR (CDCl3-d) δ 4.77 (s, 0.1H, keto, CH2); 7.38 (s, 0.9H, enol, C-H); 7.40 (m, 1H, Th-Hα) 7.44 (dd, 1H, Py-Hγ); 7.65 (dd, 1H, Py-Hε); 7.87 (td, 1H, Th-Hβ); 8.15 (dt, 1H, Py-Hδ); 8.20 (dd, 1H, Th-Hε); 8.71 (dd, 1H, Py-Hα); 13C NMR (DMSO-d6) δ 49.65 (1C, keto, CH2); 94.52 (1C, enol, C-H); 122.16 (1C, Py-Cδ); 126.43 (1C, Py-Cβ); 126.70 (2C, Th-Cα, Th-Cβ); 130.72 (1C, Th-Cε); 137.20 (1C, Py-Cγ); 139.68 (1C, Py-Cα); 149.41 (1C, Th-Cγ); 152.55 (1C, Py-Cε); 182.01 (1C; C-OH); 182.77 (1C, C=O); Anal. Calcd. for C12H9NO2S: C 62.32, H 3.92, N 6.06; Found: C 62.29, H 3.94, N 6.09; MS m/z: 232.06 [M + H]+.
4.2.7. 1-(Furan-2-yl)-3-hydroxy-3-(naphthalene-1-yl)prop-2-en-1-one (L7)
Yellow powder; yield: 28% (0. 79 g); mp = 162–164 °C; Rf = 0.24 (CH2Cl2)/silica. IR (KBr, cm–1): ν(OH) = 3434; ν(C=O) = 1629; ν(enolic C=C) = 1533. 1H NMR (CDCl3) δ 3.35 (s, 0.1H, keto CH2); 6.27 (s, 0.9H, enol, C-H); 7.56 (m, 1H, Fu-Hβ); 7.62 (d, 1H, Fu-Hα); 8.08 (d, 2H, Ar-H1,6); 8.20 (m, 1H, Ar-H4); 13C NMR (CDCl3) δ 49.50 (1C, keto CH2); 94.72 (1C, C-H, enol); 113.40 (1C, Fu-Cβ); 114.04 (2C, Ar-C3,5); 122.74 (1C, Fu-Cγ); 124.66 (1C, Ar-C1); 125.63 (1C, Ar-C4); 125.91 (1C, Ar-C9); 145.92 (1C, Fu-Cα); 178.52 (1C, C=O), 180.55 (1C, C-OH); Anal. Calcd. for C17H12O3: C 77.26, H 4.58; Found: C 77.26, H 4.58; MS m/z: 265 [M + H]+.
4.2.8. 1,3-Di(Furan-2-yl)-3-hydroxyprop-2-en-1-one (L8)
Yellow powder; yield: 31% (0. 82 g); mp = 68–70 °C; Rf = 0.42 (CH2Cl2)/silica. IR (KBr, cm–1): ν(OH) = 3448; ν(C=O) = 1640; ν(enolic C=C) = 1532. 1H NMR (DMSO-d6) δ 4.43 (s, 0.1H, keto, CH2); 6.69 (s, 0.9H, enol, C-H); 6.73 (dd, 1H, Fue-Hβ); 6.76 (dd, 1HFuc-Hβ); 7.46 (dd, 1H, Fue-Hγ); 7.54 (dd, 1H, Fuc-Hγ); 8.00 (dd 1H, Fue-Hα); 8.02 (dd, 1H, Fue-Hα); 13C NMR (DMSO-d6) δ 49.23 (1C, keto, CH2); 92.21 (1C, C-H, enol); 113.33 (1C, Fue-Cγ); 113.62 (1C, Fuc-Cγ); 117.39 (1C, Fue-Cβ); 120.52 (1C, Fuc-Cβ); 148.42 (1C, Fue-Cα); 148.99 (1C, Fuc-Cα); 149.51 (1C, Fue-Cδ); 152.01 (1C, Fuc-Cδ); 174.44 (1C, C=O), 182.62 (1C, C-OH); Anal. Calcd. for C11H8O4: C 77.26, H 4.58; Found: C 77.23, H 4.56; MS m/z: 205.08 [M + H]+.
4.2.9. 1-(Furan-2-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one (L9)
Yellow powder; yield: 43% (0. 82 g); mp = 174–176 °C; Rf = 0.22 (CH2Cl2/MeOH 9/1)/silica. IR (KBr, cm–1): ν(OH) = 3430; ν(C=O) = 1626; ν(enolic C=C) = 1533. 1H NMR (CDCl3) δ 3.36 (s, 0.1H, keto CH2); 3.86 (s, 3H, Ar-OCH3); 6.73 (s, 0.9H, enol, C-H); 6.98 (m, 1H, Fu-Hβ); 7.46 (d, 2H, Ar-H3,5); 7.58 (d, 1H, Fu-Hγ): 7.97 (m, 1H, Ar-H2,6); 8.02 (d, 1H, Fu-Hα); 13C NMR (CDCl3) δ 54.69 (1C, Ar-OCH3); 49.50 (1C, keto CH2); 94.43 (1C, C-H, enol); 113.40 (1C, Fu-Cβ); 114.04 (2C, Ar-C3,5); 122.74 (1C, Fu-Cγ); 129.43 (3C, Ar-C1,2,6); 131.54 (1C, Fu-Cα); 178.52 (1C, C=O), 181.84 (1C, C-OH); Anal. Calcd. for C14H12O4: C 68.85, H 4.95; Found: C 68.84, H 4.93; MS m/z: 245 [M + H]+.
4.2.10. 1-(Furan -2-yl)-3-hydroxy-3-(thiophen-2-yl)prop-2-en-1-one (L10)
Yellow powder; yield: 28% (0. 84 g); mp = 72–74 °C; Rf = 0.69 (CH2Cl2)/silica. IR (KBr, cm–1): ν(OH) = 3445; ν(C=O) = 1639; ν(enolic C=C) = 1546. 1H NMR (MeOD-d4) δ 4.52 (s, 0.1H, keto, CH2); 6.66 (dd, 1H, Fu-Hβ); 6.70 (s, 0.9H, enol, C-H); 7.20 (dd, 1H, Th-Hβ); 7.26 (dd, 1H, Fu-Hγ); 7.78 (dd, 1H, Th-Hγ); 7.79 (dd, 1H, Fu-Hα); 7.89 (dd, 1H, Th-Hα); 13C NMR (MeOD-d4) δ 46.91 (1C, keto, CH2); 92.65 (1C, C-H, enol); 112.51 (1C, Fu-Cγ); 115.44 (1C, Fu-Cβ); 128.36 (1C, Th-Cγ); 130.76 (1C, Th-Cβ); 132.89 (1C, Th-Cα); 141.06 (1C, Fu-Cα); 146.78 (1C, Th-Cδ); 149.75 (1C, Fu-Cδ); 172.37 (1C, C=O), 180.70 (1C, C-OH); Anal. Calcd. for C11H8O3S: C 59.99, H 3.66; Found: C 59.96, H 3.63; MS m/z: 221 [M + H]+.
4.2.11. 1-(Furan -2-yl)-3-hydroxy-3-p-tolylprop-2-en-1-one (L11)
Brown powder; yield: 32% (0. 72 g); mp = 102–103 °C; Rf = 0.73 (CH2Cl2/MeOH 5/5)/silica. IR (KBr, cm–1): ν(OH) = 3431; ν(C=O) = 1621; ν(enolic C=C) = 1531. 1H NMR (CDCl3) δ 2.34 (s, 3H, Ar-CH3); 3.37 (s, 0.1H, keto CH2); 7.29 (s, 0.9H, enol, C-H); 7.26 (t, 1H, Fu-Hβ); 7.52 (d, 1H, Fu-Hγ): 7.57 (d, 2H, Ar-H3,5); 7.64 (d, 1H, Fu-Hα); 7.81 (t, 1H, Ar-H4); 13C NMR (CDCl3) δ 21.58 (1C, Ar-CH3); 49.50 (1C, keto CH2); 94.56 (1C, C-H, enol); 112.55 (1C, Fu-Cγ); 117.62 (1C, Fu-Cβ); 128.48 (2C, Ar-C3,5); 129.59 (3C, Ar-C2,4,6); 143.50 (1C, Fu-Cα); 178.52 (1C, C=O), 180.84 (1C, C-OH); Anal. Calcd. for C14H12O3: C 73.67, H 5.30; Found: C 73.64, H 5.33; MS m/z: 229 [M + H]+.
4.3. Biological Evaluation
4.3.1. Antifungal Assay
We determined the in vitro antifungal potential of the 11 compounds against the pathogenic fungus F. oxysporum f.sp albedinis (FAO) using the agar diffusion technique.48 Different volumes (50, 200, and 500 μL) of a DMSO solution of the compounds were tested to prepare Petri dishes with different concentrations with papa dextrose agar medium (PDA). Subsequently, 6 mm diameter disks of the microorganism (FAO) were placed in the middle of these Petri dishes. After incubation at 28 °C for 7 days, the inhibition percentages were calculated and the semi-maximal inhibitory concentration (IC50) was determined using a nonlinear regression algorithm of the percentage dose inhibition graph. Benomyl was used as a standard drug (positive control).
4.3.2. Antibacterial Test
The antibiotic effect of L1–L11 was evaluated against a Gram-negative bacterium, viz., E. coli, and two Gram-positive strains, viz., B. subtilis and Micrococcus luteus, according to the recommendations of the National Clinical Laboratory Standards Committee (NCCLS). For the tests, compounds were dissolved in dimethylsulfoxide (DMSO) and sterile WHATMAN paper disks (6 mm in diameter) were impregnated with different volumes of each compound and then placed in the middle of the Petri dishes containing the culture media (Muller–Hinton agar) previously inoculated with cultures during the night of the target strains. After 24 h of incubation at 37 °C, the diameter of the zones of inhibition around each disk was measured. Gentamicin was used as a standard drug.
4.4. Computational Studies
4.4.1. POM Analyses of Compounds
The POM program is an efficient bioinformatics platform that can process virtually all organic compounds and most organometallic ones, leading to the identification of their pharmacophore sites. The POM program also leads to its optimization based on geometric, physicochemical parameters of each site and electronic load distribution of the corresponding heteroatoms X, Y, and Z.49,50
4.4.1.1. Osiris Calculations
The prediction results are valued and color-coded. Properties with a high risk of undesirable effects such as mutagenicity or poor intestinal absorption are shown in red, while a green color indicates a drug conformation behavior. The hydrophilicity of the series (L1–L11) has been expressed in terms of the cLog P value, where P is a partition coefficient of the molecule between n-octanol and water. It has been established that absorption is greatly affected by hydrophilicity. In particular, when cLog P is greater than 5, absorption decreases. This intratemplate effect is in favor of the constitution of an important antifungal/antiviral pharmacophore site N···O as hypothesized by the POM theory.51,52
4.4.1.2. Molinspiration Calculations
The bioactivity scores (BS) of L1–L11 were calculated for different parameters such as TPSA, NH···O or N-HO interaction and molecular volume and compared with some standard medications. All of the mentioned parameters were calculated with the Molinspiration online software (www.molinspiration.com), which predicted a moderate biological activity for the synthesized compounds (L1–L11).
4.4.2. Molecular Docking and Molecular Dynamics Simulations
The ADVina algorithm was implemented to perform the molecular docking of the hit and reference molecules (benomyl) to the binding site of F. oxysporum β-tubulin.53 The protein three-dimensional (3D) structure, namely, Fusarium oxysporum Guanine nucleotide-binding protein β (Fgb1), was generated using the SWISS-MODEL server,54 as a fully automated protein homology modeling workflow. The docking grids with a 3D size of 22.5 Å were used in the study. The binding site was determined according to the information obtained from the α-tubulin-taxol crystallized complex (PDB ID: 1JFF) with Cartesian coordinates of 332.24 Å (x-axis), 424.26 Å (y-axis), and 323.41 Å (z-axis). The PyMOL molecular imaging software and the in-house Python data processing scripts were utilized to calculate the dissociation constants (Kd) from the ΔGbind (Gibbs free energy of binding) values, generate high-quality graphics, and analyze the results (PyMOL Molecular Graphics System, Version 1.7.2.1, Schrödinger, LLC). All molecular dynamics (MD) simulations were performed using the AMBER 16 package,55 with the FF99SB and GAFF force fields for the protein and its ligands. The systems were solvated with the TIP3P water models and neutralized by adding the Na+ ions using the tLEap input script available from the AmberTools package. Long-range electrostatic interactions were modeled via the particle–mesh Ewald method.56 The SHAKE algorithm,57 was applied to constrain the length of covalent bonds, including the hydrogen atoms. Langevin thermostat was implemented to equilibrate the temperature of the system at 300 K. A 2.0 fs time step was used in all of the MD setups. For the minimization and equilibration phases, 50 000 steps and 1 ns period were used, respectively. Finally, 50 ns classical MD simulations, with no constraints were performed for each of the protein–ligand complexes using the molecular mechanics combined with the Poisson–Boltzmann (MM-PBSA) or generalized Born (MM-GBSA) surface area term.58,59 The MM-PBSA/GBSA solvation models were applied as a postprocessing end-state method to calculate the free energies of molecules in the solution employing the optimized python script (MM-PBSA.py).
Acknowledgments
This work was supported by a bilateral WBI action with Morocco (COP 22 Program 2018-2022), the Fonds De La Recherche Scientifique—FNRS (CDR 33694457), and PPR-P10 CNRST project (Morocco).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02365.
Mass, 1H NMR, and 13C spectra of ligands used in this work (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fisher M. C.; Henk D. A.; Briggs C. J.; Brownstein J. S.; Madoff L. C.; McCraw S. L.; Gurr S. L. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola A. M.; Albuquerque P.; Paes H. C.; Fernandes L.; Costa F. F.; Kioshima E. S.; Abadio A. K. R.; Bocca A. L.; Felipe M. S. Antifungal drugs: new insights in research & development. Pharmacol. Ther. 2019, 195, 21–38. 10.1016/j.pharmthera.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Brown G. D.; Denning D. W.; Gow N. A.; Levitz S. M.; Netea M. G.; White T. C. Hidden killers: human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Xie G. H.; Fang X. M.; Fang Q.; Wu X. M.; Jin Y. H.; Wang J. L.; Guo Q. L.; Gu M. N.; Xu Q. P.; Wang D. X.; Yao S. L.; Yuan S. Y.; Du Z. H.; Sun Y. B.; Wang H. H.; Wu S. J.; Cheng B. L. Impact of invasive fungal infection on outcomes of severe sepsis: a multicenter matched cohort study in critically ill surgical patients. Crit. Care 2008, 12, R5. 10.1186/cc6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana K. V.; Kandi S.; Bharatkumar V.; Sharada C. V.; Rao R.; Mani R.; Rao S. D. Invasive fungal infections: a comprehensive review. Am. J. Infect. Dis. Microbiol. 2013, 1, 64–69. 10.12691/ajidm-1-4-2. [DOI] [Google Scholar]
- Ahme W.; Yan X.; Hu D.; Adnan M.; Tang R. Y.; Cui Z. N. Synthesis and fungicidal activity of novel pyrazole derivatives containing 5-Phenyl-2-Furan. Bioorg. Med. Chem. 2019, 27, 115048 10.1016/j.bmc.2019.115048. [DOI] [PubMed] [Google Scholar]
- Sun B.; Dong Y.; Lei K.; Wang J.; Zhao L.; Liu M. Design, synthesis and biological evaluation of amide-pyridine derivatives as novel dual-target (SE, CYP51) antifungal inhibitors. Bioorg. Med. Chem. 2019, 27, 2427–2437. 10.1016/j.bmc.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Dennis Bilavendran J. D.; Manikandan A.; Thangarasu P.; Sivakumar K. Synthesis and discovery of pyrazolo-pyridine analogs as inflammation medications through pro- and anti-inflammatory cytokine and COX-2 inhibition assessments. Bioorg. Chem. 2020, 94, 103484 10.1016/j.bioorg.2019.103484. [DOI] [PubMed] [Google Scholar]
- Boukharsa Y.; Lakhlili W.; El harti J.; Meddah B.; Tiendrebeogo R. Y.; Taoufik J.; Faouzi M.; Ibrahimi A.; Ansar A. Synthesis, anti-inflammatory evaluation in vivo and docking studies of some new 5-(benzo[b]furan-2-ylmethyl)-6-methyl-pyridazin-3(2H)-one derivatives. J. Mol. Struct. 2018, 1153, 119–127. 10.1016/j.molstruc.2017.09.092. [DOI] [Google Scholar]
- Li P.; Jang J.; Hsia C.; Groomes P. V.; Lian W.; Wispelaere M.; Pitts J. D.; Wang J.; Kwiatkowski N.; Gray N. S.; Yang P. L. Small Molecules Targeting the Flavivirus E Protein with Broad-Spectrum Activity and Antiviral Efficacy in Vivo. ACS Infect. Dis. 2019, 5, 460–472. 10.1021/acsinfecdis.8b00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gualda B.; Pu S. Y.; Froeyen M.; Herdewijn P.; Einav S.; De Jonghe S. Structure-activity relationship study of the pyridine moiety of isothiazolo[4,3-b]pyridines as antiviral agents targeting cyclin G-associated kinase. Bioorg. Med. Chem. 2020, 28, 115188 10.1016/j.bmc.2019.115188. [DOI] [PubMed] [Google Scholar]
- Fan Z.; Shi J.; Luo N.; Ding M.; Bao X. Synthesis, Crystal Structure, and Agricultural Antimicrobial Evaluation of Novel Quinazoline Thioether Derivatives Incorporating the 1,2,4-Triazolo[4,3-a]pyridine Moiety. J. Agric. Food Chem. 2019, 67, 11598–11606. 10.1021/acs.jafc.9b04733. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Zhao S.; Lv Z.; Feng L.; Wang Y.; Zhang F.; Bai L.; Deng J. Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur. J. Med. Chem. 2019, 162, 266–276. 10.1016/j.ejmech.2018.11.025. [DOI] [PubMed] [Google Scholar]
- Suzuki Y.; Otake A.; Ueno S.; Hayashi K.; Ishii H.; Miyoshi N.; Kuroiwa K.; Tachikawa M.; Fujimaki Y.; Nishiyama K.; Manabe K.; Yamazaki R.; Asai A. Discovery of a Potent Anticancer Agent PVHD303 with in Vivo Activity. ACS Med. Chem. Lett. 2020, 11, 1287–1291. 10.1021/acsmedchemlett.0c00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola E.; Donzello M. P.; Testani S.; Luccisano G.; Astolfi M. L.; Rizzoli C.; Cong L.; Mannina L.; Ercolani C.; Kadish K. M. Tetra-2,3-pyrazinoporphyrazines with Peripherally Appended Pyridine Rings. 19. Pentanuclear Octa(2-pyridyl)tetrapyrazinoporphyrazines Carrying Externally Carboranthiolate Groups: Physicochemical Properties and Potentialities as Anticancer Drugs. Inorg. Chem. 2019, 58, 1120–1133. 10.1021/acs.inorgchem.8b02269. [DOI] [PubMed] [Google Scholar]
- Paixão D. A.; Oliveira B. C. A.; Almeida J. C.; Sousa L. M.; Lopes C. D.; Carneiro Z. A.; Tezuka D. Y.; Clavijo J. C. T.; Ellena J.; Polloni L.; Machado P. A.; Albuquerque S.; de Oliveira Júnior R. J.; Guilardi S.; Guerra W. Crystal structure, anti-Trypanosoma cruzi and cytotoxic activities of Cu(II) complexes bearing β-diketone and α-diimine ligands. Inorg. Chim. Acta 2020, 499, 119164 10.1016/j.ica.2019.119164. [DOI] [Google Scholar]
- Radulović N. S.; Genčić M. S.; Stojanović N. M.; Randjelović P. J.; Baldovini N.; Kurteva V. Prenylated β-diketones, two new additions to the family of biologically active Hypericum perforatum L. (Hypericaceae) secondary metabolites. Food Chem. Toxicol. 2018, 118, 505–513. 10.1016/j.fct.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Cheng D.; Li W.; Wang L.; Lin T.; Poiani G.; Wassef A.; Hudlikar R.; Ondar P.; Brunetti L.; Kong A. Pharmacokinetics, Pharmacodynamics, and PKPD Modeling of Curcumin in Regulating Antioxidant and Epigenetic Gene Expression in Healthy Human Volunteers. Mol. Pharmaceutics 2019, 16, 1881–1889. 10.1021/acs.molpharmaceut.8b01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porchezhiyan V.; Kalaivani D.; Shobana J.; Noorjahan S. E. Synthesis, docking and in vitro evaluation of l-proline derived 1,3-diketones possessing anti-cancer and anti-inflammatory activities. J. Mol. Struct. 2020, 1206, 127754 10.1016/j.molstruc.2020.127754. [DOI] [Google Scholar]
- Malekshah R. E.; Salehi M.; Kubicki M.; Khaleghian A. Biological studies and computational modeling of two new copper complexes derived from β-diketones and their nano-complexes. J. Coord. Chem. 2019, 72, 1697–1714. 10.1080/00958972.2019.1606422. [DOI] [Google Scholar]
- Tighadouini S.; Radi S.; Abrigach F.; Benabbes R.; Eddike D.; Tillard M. Novel β-keto-enol Pyrazolic Compounds as Potent Antifungal Agents. Design, Synthesis, Crystal Structure, DFT, Homology Modeling, and Docking Studies. J. Chem. Inf. Model. 2019, 59, 1398–1409. 10.1021/acs.jcim.8b00828. [DOI] [PubMed] [Google Scholar]
- Brglez Mojzer E.; Knez Hrnčič M.; Škerget M.; Knez Ž.; Bren U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama A.; Yamakoshi H.; Hongo S.; Kanoh N.; Shibata H.; Iwabuchi Y. Structure-Activity Relationships of the Antitumor C5-Curcuminoid GO-Y030. Molecules 2015, 20, 15374–15391. 10.3390/molecules200815374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. L.; Ali A.; Du Y.; Fu H.; Jin H. X.; Chin T. M.; Khan M.; Guo M. L. Synthesis and Evaluation of Bisbenzylidenedioxotetrahydrothiopranones as Activators of Endoplasmic Reticulum (ER) Stress Signaling Pathways and Apoptotic Cell Death in Acute Promyelocytic Leukemic Cells. J. Med. Chem. 2014, 57, 5904–5918. 10.1021/jm401352a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgur Y.; Craigir R.; Cohen G. H.; Fujiwara T.; Yoshinaga T.; Fujishita T.; Sugimoto H.; Endo T.; Murai H.; Davies D. R. Structure of the HIV-1 integrase catalytic domain complexed with an inhibitor: A platform for antiviral drug design. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 13040–13043. 10.1073/pnas.96.23.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa V.; Petrocchi A.; Bonelli F.; Crescenzi B.; Donghi M.; Ferrara M.; Fiore F.; Gardelli C.; Gonzalez Paz O.; Hazuda D. J.; Jones P.; Kinzel O.; Laufer R.; Monteagudo E.; Muraglia E.; Nizi E.; Orvieto F.; Pace P.; Pescatore G.; Scarpelli R.; Stillmock K.; Witmer M. V.; Rowley M. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 2008, 51, 5843–5855. 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- Song W. H.; Liu M. M.; Zhong D. W.; Zhu Y. L.; Bosscher M.; Zhou L.; Ye D. Y.; Yuan Z. H. Tetrazole and Triazole as Bioisosteres of Carboxylic Acid: Discovery of Diketo Tetrazoles and Diketo Triazoles as Anti-HCV Agents. Bioorg. Med. Chem. Lett. 2013, 23, 4528–4531. 10.1016/j.bmcl.2013.06.045. [DOI] [PubMed] [Google Scholar]
- Luo Z. G.; Zhao Y.; Ma C.; Xu X. M.; Zhang X. M.; Huang N. Y.; He H. Q. Synthesis and Anti-Integrase Evaluation of Novel Calix[4]arene Derivatives Containing the Triazolyl 1,3-diketo Moiety. Chin. Chem. Lett. 2014, 25, 737–740. 10.1016/j.cclet.2014.03.012. [DOI] [Google Scholar]
- Patel K.; Karthikeyan C.; Raja Solomon V.; Hari Narayana Moorthy N. S.; Lee H.; Sahu K.; Singh Deora G.; Trivedi P. Synthesis of Some Coumarinyl Chalcones and Their Antiproliferative Activity Against Breast Cancer Cell Lines. Lett. Drug Des. Discovery 2011, 8, 308–311. 10.2174/157018011794839475. [DOI] [Google Scholar]
- Pinzi L.; Rastelli G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331 10.3390/ijms20184331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B.; Suresh J.; Mathew G. E.; Sonia G.; Krishnan G. K. Design, Synthesis, Toxicity Estimation and Molecular Docking Studies of N-(furan-2-yl)-1-(5-substituted) phenyl-1,3,4-oxadiazol-2-yl) methanimine as Antitubercular Agents. Indian J. Pharm. Sci. 2014, 76, 401–406. [PMC free article] [PubMed] [Google Scholar]
- Thafar M.; Raies A. B.; Albaradei S.; Essack M.; Bajic V. B. Comparison Study of Computational Prediction Tools for Drug-Target Binding Affinities. Front. Chem. 2019, 7, 782 10.3389/fchem.2019.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi S.; Tighadouini S.; Feron O.; Riant O.; Bouakka M.; Benabbes R.; Mabkhot Y. N. Synthesis of Novel β-Keto-Enol Derivatives Tethered Pyrazole, Pyridine and Furan as New Potential Antifungal and Anti-Breast Cancer Agents. Molecules 2015, 20, 20186–20194. 10.3390/molecules201119684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighadouini S.; Radi S.; Garcia Y. Selective chemical adsorption of Cd(II) on silica covalently decorated with a β-ketoenol-thiophene-furan receptor. Mol. Syst. Des. Eng. 2020, 7, 60 10.1039/C9ME00140A. [DOI] [Google Scholar]
- Radi S.; Tighadouini S.; Bacquet M.; Degoutin S.; Dacquin J.-P.; Eddike D.; Tillard M.; Mabkhot Y. N. β-Keto-enol Tethered Pyridine and Thiophene: Synthesis, Crystal Structure Determination and Its Organic Immobilization on Silica for Efficient Solid-Liquid Extraction of Heavy Metals. Molecules 2016, 21, 888 10.3390/molecules21070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi S.; Tighadouini S.; Eddike D.; Tillard M.; Mabkhot Y. N. Crystal Structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one, C15H16N2O3. Z. Kristallogr. - New Cryst. Struct. 2017, 232, 199–200. 10.1515/ncrs-2016-0190. [DOI] [Google Scholar]
- Radi S.; Tighadouini S.; Eddike D.; Tillard M.; Mabkhot Y. N. Crystal Structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en-1-one, C14H14N2O2. Z. Kristallogr. - New Cryst. Struct. 2017, 232, 201–202. 10.1515/ncrs-2016-0194. [DOI] [Google Scholar]
- Radi S.; Tighadouini S.; Eddike D.; Tillard M.; Mabkhot Y. N. Crystal Structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one, C16H18N2O3. Z. Kristallogr. - New Cryst. Struct. 2017, 232, 207–208. 10.1515/ncrs-2016-0196. [DOI] [Google Scholar]
- Radi S.; Tighadouini S.; Eddike D.; Tillard M.; Mabkhot Y. N. Crystal Structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one, C15H16N2O2. Z. Kristallogr. - New Cryst. Struct. 2017, 232, 209–210. 10.1515/ncrs-2016-0199. [DOI] [Google Scholar]
- Radi S.; Tighadouini S.; Eddike D.; Tillard M.; Mabkhot Y. N. Crystal Structure of (Z)-3-hydroxy-3-(4-methoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-one, C15H13NO3. Z. Kristallogr. - New Cryst. Struct. 2017, 232, 235–236. 10.1515/ncrs-2016-0219. [DOI] [Google Scholar]
- Tighadouini S.; Radi S.; Ferbinteanu M.; Garcia Y. Highly Selective Removal of Pb(II) by a Pyridylpyrazole-β-ketoenol Receptor Covalently Bonded onto the Silica Surface. ACS Omega 2019, 4, 3954–3964. 10.1021/acsomega.8b03642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis W. C. Ina.; Erasmus J. J. C.; Lamprecht G. J.; Conradie J.; Cameron T. S.; Aquino M. A. S.; Swarts J. C. Cyclic Voltammetry of Ferrocene-containing B-diketones as a Tool to Obtain Group Electronegativities. The Structure of 3-ferrocenoyl-1,1,1-trifluoro-2- hydroxyprop-2-ene. Can. J. Chem. 1999, 77, 378–386. 10.1139/v99-015. [DOI] [Google Scholar]
- Chohan Z. H.; Youssoufi M. H.; Jarrahpour A.; Ben Hadda T. Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: Indolenyl sulfonamide derivatives. Eur. J. Med. Chem. 2010, 45, 1189–1199. 10.1016/j.ejmech.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Jarrahpour A.; Fathi J.; Mimouni M.; Ben Hadda T.; Sheikh J.; Chohan Z.; Parvez A. Petra, Osiris and Molinspiration (POM) together as a successful support in drug design: antibacterial activity and biopharmaceutical characterization of some azo Schiff bases. Med. Chem. Res. 2012, 21, 1984–1990. 10.1007/s00044-011-9723-0. [DOI] [Google Scholar]
- Savjani K. T.; Gajjar A. K.; Savjani J. K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cob-Calan N. N.; Chi-Uluac L. A.; Ortiz-Chi F.; Cerqueda-García D.; Navarrete-Vázquez G.; Ruiz-Sánchez E.; Hernández-Núñez E. Molecular Docking and Dynamics Simulation of Protein β-Tubulin and Antifungal Cyclic Lipopeptides. Molecules 2019, 24, 3387 10.3390/molecules24183387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément M.-J.; Rathinasamy K.; Adjadj E.; Toma F.; Curmi P. A.; Panda D. Benomyl and Colchicine Synergistically Inhibit Cell Proliferation and Mitosis: Evidence of Distinct Binding Sites for These Agents in Tubulin. Biochemistry 2008, 47, 13016–13025. 10.1021/bi801136q. [DOI] [PubMed] [Google Scholar]
- Abrigach F.; Rokni Y.; Takfaoui A.; Khoutoul M.; Doucet H.; Asehraou A.; Touzani R. In vitro screening, homology modeling and molecular docking studies of some pyrazole and imidazole derivatives. Biomed. Pharmacother. 2018, 103, 653–661. 10.1016/j.biopha.2018.04.061. [DOI] [PubMed] [Google Scholar]
- Jarrahpour A.; Heiran R.; Sinou V.; Labour C.; Djouhri Bouktab L.; Brunel J. M.; Sheikh J.; Ben Hadda T. Synthesis of New β-Lactams Bearing the Biologically Important Morpholine Ring and POM Analyses of Their Antimicrobial and Antimalarial Activities. Iran J. Pharm. Res. 2019, 18, 34–48. [PMC free article] [PubMed] [Google Scholar]
- Rbaa M.; Jabli S.; Lakhrissi Y.; Ouhssine M.; Almalki F.; Ben Hadda T.; Messgo-Moumene S.; Zarrouk A.; Lakhrissi B. Synthesis, antibacterial properties and bioinformatics computational analyses of novel 8-hydroxyquinoline derivatives. Heliyon 2019, 5, e02689 10.1016/j.heliyon.2019.e02689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan D. T.; Masand V. H.; Patil K. N.; Ben Hadda T.; Jawarka R. D.; Thakur S. D.; Rastija V. CoMSIA and POM analyses of anti-malarial activity of synthetic prodiginines. Bioorg. Med. Chem. Lett. 2012, 22, 4827–4835. 10.1016/j.bmcl.2012.05.115. [DOI] [PubMed] [Google Scholar]
- Mabkhot Y. N.; Aldawsari F. D.; Al-Showiman S. S.; Barakat A.; Ben Hadda T.; Mubarak M. S.; Naz S.; Ul-Haq Z.; Rauf A. Synthesis, Bioactivity, Molecular Docking and POM Analyses of Novel Substituted Thieno[2,3-b]thiophenes and Related Congeners. Molecules 2015, 20, 1824–1841. 10.3390/molecules20021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A.; Bertoni M.; Bienert S.; Studer G.; Tauriello G.; Gumienny R.; Heer F. T.; de Beer T. A. P.; Rempfer C.; Bordoli L.; Lepore R.; Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case D. A.; Cheatham T. E.; Darden T.; Gohlke H.; Luo R.; Merz K. M. Jr.; Onufriev A.; Simmerling C.; Wang B.; Woods R. J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essmann U.; Perera L.; Berkowitz M. L.; Darden T.; Lee H.; Pedersen L. G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. 10.1063/1.470117. [DOI] [Google Scholar]
- Miyamoto S.; Kollman P. A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. 10.1002/jcc.540130805. [DOI] [Google Scholar]
- Iglesais E. Application of Organized Microstructures to Study Keto-Enol Equilbrium of β-Dicarbonyl Compounds. Curr. Org. Chem. 2004, 8, 1–24. 10.2174/1385272043486124. [DOI] [Google Scholar]
- Forsén S.; Nilsson M.. The Chemistry of the Carbonyl Group; Zabicky J., Ed.; Interscience Publishers: London, 1970; pp 157–240. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.