Abstract
COVID-19, the acute respiratory tract infection (RTI) caused by the Coronavirus, Sars-CoV-2, has swept around the world. No country has been spared from its onslaught. Treatments that can reduce the risk of infection and mortality from the disease are desperately needed. Though high quality randomized controlled trials are lacking, some observational and interventional studies that explore the link between vitamin D and RTIs exist. Vitamin D modulates both innate as well as adaptive immunity and may potentially prevent or mitigate the complications associated with RTIs. Evidence linking vitamin D to COVID-19 include that the outbreak occurred in winter in the northern hemisphere at a time when vitamin D levels are lowest in resident populations, that blacks and minority ethnic individuals who are known to have lower levels of vitamin D appear to be disproportionately affected and have more severe complications from the disease, that vitamin D deficiency has been shown to contribute to acute respiratory distress syndrome and that case fatality rates increase with age and in populations with comorbid conditions such as diabetes, hypertension, and cardiovascular disease, all of which are associated with lower vitamin D levels. This narrative review summarizes the current knowledge about the epidemiology and pathophysiology of COVID-19, the evidence linking vitamin D and RTIs, especially COVID-19, the mechanistic reasons behind the possible protective effect of vitamin D in COVID-19, and the evidence with regard to vitamin D supplementation in RTIs. It concludes with some recommendations regarding supplementation of vitamin D in patients with COVID-19.
Keywords: COVID-19, Coronavirus, Vitamin D, Vitamin D supplementation, SARS-CoV-2
1. Introduction
Many infectious diseases have contributed to shaping human evolution, and multiple pandemics have swept through the world over the last several centuries. Related to Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), COVID-19 is caused by the coronavirus, SARS-CoV-2. It affects the respiratory tract and manifests as pneumonia in humans. The causative agent was identified in January 2020 from throat swab samples conducted on infected patients by the Chinese Center for Disease Control and Prevention (CCDC). The disease was named COVID-19 by the World Health Organization (WHO) (https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020) and was subsequently declared as a pandemic in March 2020. Since the first cases were reported in Wuhan, China, at the end of 2019, the disease has raged across the world with more than 11.2 million cases and 529,601 deaths recorded to date worldwide (as of July 18, 2020). Whilst the highest incidence has been recorded in the United States, Brazil, and India in descending order (www.worldometers.info – accessed July 18, 2020), countries in the most densely populated region of the world, namely the Asia-Pacific have also been severely affected, with no country being spared from its ravages (Table 1).
Table 1.
COVID-19 in Asia Pacific region (as of July 18, 2020).
| Region | Country | Population (million) | Total cases | Cases/1 million | Total deaths | Deaths/1 million |
|---|---|---|---|---|---|---|
| East Asia | ||||||
| 1 | China | 1439 | 83,644 | 58 | 4634 | 3 |
| 2 | Hong Kong | 7.4 | 1778 | 237 | 12 | 2 |
| 3 | Japan | 126 | 23,473 | 186 | 985 | 8 |
| 4 | South Korea | 51 | 13,711 | 267 | 294 | 6 |
| South Asia | ||||||
| 1 | Bangladesh | 164 | 202,066 | 1226 | 2581 | 16 |
| 2 | India | 1380 | 1,055,932 | 765 | 26,508 | 19 |
| 3 | Pakistan | 220 | 261,916 | 1185 | 5582 | 25 |
| 4 | Sri Lanka | 21 | 2703 | 126 | 11 | 0.5 |
| Southeast Asia | ||||||
| 1 | Brunei | 0.4 | 141 | 322 | 3 | 7 |
| 2 | Cambodia | 16 | 171 | 10 | 0 | 0 |
| 3 | Indonesia | 273 | 84,882 | 310 | 4016 | 15 |
| 4 | Laos | 19 | 19 | 3 | 0 | 0 |
| 5 | Malaysia | 32 | 8764 | 271 | 122 | 4 |
| 6 | Myanmar | 54 | 339 | 6 | 6 | 0.1 |
| 7 | Philippines | 109 | 65,304 | 596 | 1773 | 16 |
| 8 | Singapore | 5.8 | 47,655 | 8143 | 27 | 5 |
| 9 | Thailand | 69 | 3246 | 46 | 58 | 0.8 |
| 10 | Vietnam | 97 | 382 | 4 | 0 | 0 |
| Oceania | ||||||
| 1 | Australia | 25 | 11,441 | 448 | 118 | 5 |
| 2 | New Zealand | 5 | 1550 | 310 | 22 | 4 |
A possible protective role for vitamin D; the so called “Sunshine vitamin” in COVID-19 has been brought up in discussions that pertain to prevention and management of severe outcomes from the disease, and the mainstream media has been deluged with reports speculating on the matter. This article reviews the current knowledge about COVID-19, the epidemiological evidence that links vitamin D and respiratory infections such as COVID-19, discusses the mechanisms behind a possible protective effect of vitamin D on the disease, and provides a perspective on whether and how vitamin D supplementation can be employed to mitigate the risks associated with this highly infectious disease.
2. Pathophysiology of COVID-19 respiratory infection
The pathophysiologic basis of the clinical manifestations of COVID-19 is believed to be secondary to an uncontrolled and complex immune response of the human body to the virus. It is believed that angiotensin-converting enzyme 2 (ACE2) is the host cell receptor responsible for mediating infection by SARS-CoV-2. ACE2 is predominantly expressed by epithelial cells of the lung, intestine, kidney, heart, and blood vessels. ACE2 was identified as a key receptor for SARS (severe acute respiratory syndrome) coronavirus infections [1]. SARS-CoV-2 binds to ACE2 receptors to gain access into host cells. Once the virus attaches to ACE2 receptors, it downregulates activity and expression of ACE2 [2]. Both ACE and ACE2 belong to the ACE family of dipeptidyl carboxydipeptidases. However, they exert distinct physiological functions. ACE cleaves angiotensin I to angiotensin II. Angiotensin II activates angiotensin II receptor type 1. This activation leads to vasoconstrictive, proinflammatory, and pro-oxidative effects [3]. ACE2 on the other hand, degrades angiotensin II to angiotensin 1-7 and angiotensin I to angiotensin 1-9. Binding of angiotensin 1-9 to the Mas receptor leads to anti-inflammatory, antioxidative, and vasodilatory effects. It is postulated that SARS-CoV-2 binding to ACE2 may attenuate ACE2 activity, skewing the ACE/ACE2 balance to a state of heightened angiotensin II activity leading to acute lung injury. Enhanced production of pro-inflammatory cytokines and chemokines including granulocyte colony stimulating factor (GCSF), interferon gamma inducible protein 10 (IP10), macrophage chemotactic protein-1, macrophage chemotactic protein −1 (MCP1), macrophage inflammatory protein (MIP)1A, and tumour necrosis factor (TNF) alpha, by immune effector cells, leading to a cytokine storm is thought to be the cause of multiple systemic and respiratory symptoms in COVID-19 as it is in SARS-CoV [4] and MERS CoV infections [5]. The binding affinity of the SARS-CoV-2 spike protein and ACE2 determines viral multiplication and severity of COVID-19.
Higher levels of ACE2 are associated with better outcomes for coronavirus disease and it has been shown that in the lung, ACE2 protects against acute lung injury [1]. ACE2 expression in lungs has been shown to decrease by 67% in older female rats and even more, by 78% in older male rats, as compared to younger groups in an animal model study [6]. Though difficult to draw conclusions given that this was a rodent study, and though it is likely there are other reasons such as co-morbid illnesses and other gender specific factors, this decrease of ACE2 with age and gender appears to parallel the increase in COVID-19 mortality noted in older males in worldwide case fatality rate estimates.
COVID -19 has differentially impacted populations around the world. Analyses of confirmed, severely affected, and deceased cases have revealed certain environmental and demographic patterns. It is now becoming increasingly evident that the mortality rates of COVID-19 vary significantly among countries and even amongst populations indigenous to or residing within a particular country. The causes of these disparities are not clearly understood. Postulations include differences in COVID-19 testing strategies and policies, quality of health care services, demographic characteristics including the prevalence of elderly within a given population [7], and even the possible emergence of mutated strains of the virus in different parts of the world [8].
Another postulate explaining differences in mortality and severity in COVID-19, that is gaining increasing traction is the role of Vitamin D in the infectious process.
3. Vitamin D - the sunshine vitamin
Vitamin D, a fat-soluble vitamin, plays a major role in calcium homeostasis. The human body obtains it either through exogenous intake of food rich in vitamin D or from endogenous synthesis from a thermal reaction that converts 7-dehydrocholesterol in the skin to vitamin D3 (cholecalciferol) following exposure to Ultraviolet B (UVB)-radiation. Vitamin D3 is also found in animal food sources eg, fatty fish such as salmon, mackerel and tuna, cod liver oil, milk, etc. Vitamin D2 (ergocalciferol) is found in vegetable sources such as sun-exposed yeast and mushrooms. Enzymatic 25-hydroxylation of D3 and D2 occurs in the liver with formation of 25-hydroxyvitamin D (25(OH)D). 25(OH)D is the major circulating vitamin D with a half-life of approximately 2–3 weeks and defines vitamin D status of an individual. Subsequent 1-α hydroxylation occurs in the kidneys and other organs, with generation of biologically active 1,25-dihydroxyvitamin D (1–25(OH)D). 1,25(OH)D, the activated form of vitamin D, interacts with the nuclear vitamin D receptor (VDR) in various tissues to exert the biological effects of vitamin D [9].
A growing body of mostly observational evidence comparing outcomes from various countries suggest inverse links between vitamin D levels and outcomes including severity of, as well as mortality from COVID-19. COVID-19 made its appearance and started spreading across the northern hemisphere in the winter of 2019. Levels of 25(OH)D in populations residing in these northern climes have been found to be at their lowest during winter [10,11]. Also, a correlation between geographical location, mean temperature, low humidity, and the distribution of COVID-19 infections has been found [12]. A link between mortality rates and northerly latitudes that persists after adjustment for age has been noted [13]. Initial observations about countries in southern hemispheres such as Australia having lower incidences and mortality rates, appeared to substantiate this point. An exception to this has been the high incidences and case fatality rates noted subsequently in an equatorial country in the southern hemisphere such as Brazil. It must be noted that the seasonality of many respiratory viral infections in equatorial countries such as Brazil is related to rainy periods [14,15] (rather than to winter as it is in northern latitudes). There also appears to be an association between vitamin D status and mortality from COVID-19, with mortality rates found to be higher in countries with populations with lower prevalent vitamin D levels, for example in India and in middle eastern countries such as Iran [16,17]. 25(OH)D levels has been shown to have little seasonal variation in the Middle East with low vitamin D levels found in the population year round [18].
The lower infection and mortality rates in Nordic countries such as Norway, Finland, and Sweden are an exception to the trend of more negative outcomes in northern latitudes. However, this is likely explained by populations in these countries having relatively sufficient vitamin D levels even in winter due to widespread fortification of food. Countries such as Italy and Spain that have relatively sunny climates had very high rates of infection and mortality rates, but, it is well documented that these regions have surprisingly high prevalence of vitamin D deficiency [19].
Though convincing observational studies linking vitamin D levels to severity of COVID-19 infections in the Asia Pacific have not yet been performed, it is plausible that a potential link might exist, as has been shown in 2 studies, the publications of which are available only in pre-print versions and therefore cannot be authenticated completely. In one of these, a retrospective study from a single centre in Indonesia that included 780 cases with laboratory-confirmed infection of SARS-CoV-2, univariate analysis showed that older and male cases with pre-existing conditions and below normal vitamin D levels had increased odds of death. After controlling for age, sex, and comorbidity, vitamin D status was found to be strongly associated with COVID-19 mortality outcome of cases (https://emerginnova.com/patterns-of-covid19-mortality-and-vitamin-d-an-indonesian-study/). In another retrospective study conducted in a hospital in the Philippines, multinomial logistic regression analysis reported that for each standard deviation increase in serum 25(OH)D, the odds of having a mild clinical outcome rather than a severe outcome were approximately 7.94 times (OR = 0.126, P < 0.001), while the odds of having a mild clinical outcome rather than a critical outcome were approximately 19.61 times (OR = 0.051, P < 0.001) (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3571484).
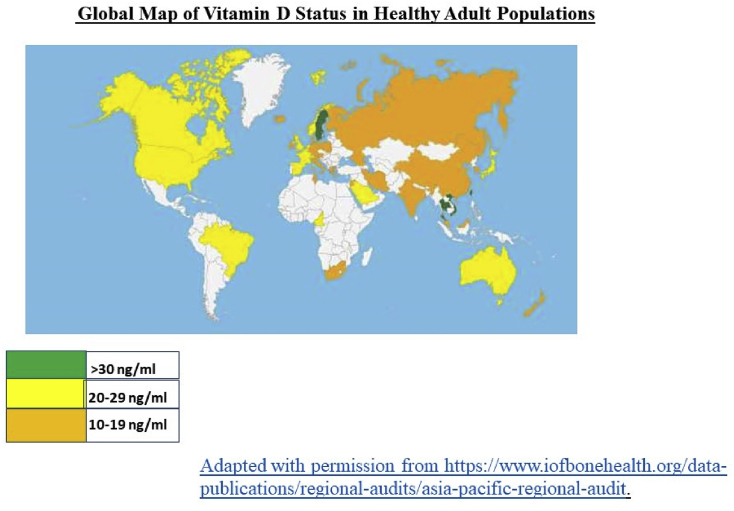
Low levels of vitamin D are observed region-wide in the Asia Pacific with very low levels reported in countries even with abundant sunshine year-round such as India, the Middle East etc (https://www.iofbonehealth.org/data-publications/regional-audits/asia-pacific-regional-audit) (Fig. 1). The low levels of vitamin D have been attributed to several causes including urbanization, low sun exposure, inadequate dietary vitamin D intake, lack of food fortification with vitamin D, pigmented skin, and traditional dress code. Another factor that may contribute to increased prevalence of vitamin D deficiency is environmental pollution. Regions that have been hit hard by COVID-19 in the Asia Pacific region include areas associated with high air pollution in China [20], and Delhi, India [21]. Interestingly, population density expressed per country was not significantly associated with mortality rates from COVID-19 in the study described earlier that had shown increased mortality rates with northerly latitudes [13].
Fig. 1.
Global map of Vitamin D status in healthy adult populations.
Though subject to debate, another possible indirect link between lower levels of vitamin D and increased severity and higher mortality from COVID-19 stems from the observation that black and minority ethnic populations that include Asians who have lower levels of vitamin D appear to be disproportionately affected with COVID-19 [22].
Fatality rates that may be as high as 20%, are seen in the elderly and in those with pre-existing co-morbidities such as diabetes, hypertension, obesity and cardiovascular disease [23,24].
Case fatality rates (CFR) from COVID-19 clearly increase in elderly individuals. Though several reasons including the incidence of increasing comorbidities with age may account for this, it also appears to parallel the decrease in serum 25(OH)D levels (and its ultimate hydroxylation product 1,25(OH)D) with age. One of the reasons for this decrease is the less efficient production of vitamin D in the skin due to lower levels of 7-dehydrocholesterol [25]. Medical conditions affecting endogenous vitamin D synthesis such as chronic kidney disease and other disorders associated with lower levels of vitamin D such as diabetes [26], hypertension [27], and cardiovascular diseases [28] are also more common in elderly individuals. Though it is unlikely that these observations alone are sufficient to completely explain why CFR from COVID-19 is higher in the elderly and in people with underlying comorbidities, it raises some interesting questions that deserve to be explored further.
4. Extra skeletal role of vitamin D
The actions of vitamin D in the human body are diverse [29]. Besides its classical role in the regulation of calcium and phosphorous homeostasis and bone metabolism, it has other important clinical functions including modulation of immune and cardiovascular function and cell proliferation. These extra-skeletal actions are made possible by ligation of the biologically active form of vitamin D −1,25(OH)D, with its ubiquitously distributed nuclear vitamin D receptor (VDR) and subsequent transcription of vitamin D-dependent genes. The presence of 1-alpha-hydroxylase enzyme in other extra renal sites such as brain, prostate, uterus, pancreas and immune cells provide additional sources of active vitamin D for extra skeletal physiologic functions [30].
5. Vitamin D as an immunomodulator
The body’s defence against any viral infection involves both innate (cellular) immunity and adaptive immunity. While innate immunity utilizes receptors that recognize broad structural motifs present in bacteria or viruses but are generally absent in host cells, adaptive immunity relies on antibodies and T-cells that can recognize viral antigens with high degrees of specificity.
Vitamin D has modulatory effects on both the innate and adaptive immune system and thus has an important role in fighting against invading pathogens. Vitamin D can simultaneously boost the innate immune system by suppressing cytokine production and thus reduce invading pathogen load and, at the same time decrease the overactivation of the adaptive immune system that occurs in infections and thereby help the body to respond adequately to the pathogen load. VDR are present in many immune reactive cells such as monocytes, macrophages, dendritic cells, and T- and B-lymphocytes [31]. The expression of the VDR on these cells is activated by contact with viral and bacterial ligands.
5.1. Innate immune system
Vitamin D supports host immunity by enhancing local synthesis of anti-microbial peptides including cathelicidins, IL-37 and defensins [32,33]. These host-derived peptides induce microbial killing by perturbing their cell membranes as well as by neutralizing endotoxin biological activity [34]. Critical to the innate immune response are the toll-like receptors (TLR) that recognize pathogenic molecules, and when activated, release cytokines and induce reactive oxygen species and antimicrobial peptides. Several of the toll-like receptors affect or are affected by vitamin D receptor induction [35]. Supplementation of vitamin D to levels 40 ng/ml or more has been shown to result in a marked reduction in the induced cytokine profile, specifically IL-6, TNF and IFN-alpha in peripheral blood mononuclear cells of vitamin D deficient subjects [36].
5.2. Adaptive immune system
Vitamin D has shown to be a key modulator of the adaptive immune system. It suppresses the release of inflammatory cytokines and chemokines such as IL-2, tumor necrosis factor-α and interferon-γ, and thus suppresses responses mediated by T helper cell type 1 (Th1) [37,38]. This may help to reduce the cytokine storm that is noted in Covid-19 patients [39] and which is the pathophysiologic driver behind systemic inflammatory response syndrome and acute respiratory distress syndrome [40,41]. TLRs are also instrumental in activating adaptive immunity. TLR2 senses lipopeptides from bacteria and leads to activation of NF-κB and induction of cytokine production and release [42]. Optimal vitamin D levels after supplementation has been shown to improve the expression of TLR2 and hence the body’s ability to fight infections [36].
6. Vitamin D and respiratory tract infections
As early as in the 19th century, cod liver oil (a rich source of vitamin D) was used for treating tuberculosis (TB). In 1903, Finsen received the Nobel Prize for demonstrating that Lupus vulgaris, the epidermal form of TB, could be cured using light from an electric arc lamp. In the early 1900s, increasing awareness of the benefits of “heliotherapy” or sun exposure, in the treatment of infectious diseases led to the development of sanatoriums in “sun-rich areas”. These sanatoriums enabled regimented sun exposure, diet, and exercise. These sanatoriums primarily hosted TB patients [43].
Studies that have been conducted exploring the correlation between vitamin D levels and the incidence of respiratory tract infections (RTI) have produced heterogenous and inconsistent results. Most have been observational, and most have reported statistically significant associations between low vitamin D levels and increased risk of both upper and lower RTIs. A negative correlation between vitamin D levels and upper respiratory infections was demonstrated in a large population-based nutrition survey [44]. The effect was stronger in patients with underlying obstructive airway disease such as chronic obstructive pulmonary disease or asthma. Patients with severe vitamin D deficiency have been found to have not only increased incidence of pneumonia but also higher rates of admission to intensive care units and mortalities [[45], [46], [47]].
On the other hand, sufficient vitamin D levels, have shown to be positively associated with better outcomes in certain respiratory infection such as mycobacterium tuberculosis and influenza virus infections [48,49]. A serum 25(OH)D concentration above 38 ng/ml was found to be inversely associated with risk of viral respiratory tract infections in a prospective study of nearly 200 adults [50]. The risk of nosocomial infections decreased by a third with each 10 ng/dl rise in serum vitamin D levels in an observational study conducted in Spain [51].
7. Mechanism behind vitamin D’s possible protective role against and in RTI specifically COVID-19
As mentioned previously, COVID-19 infection is thought to be associated with an inflammatory cytokine storm that can result in life threatening acute lung injury and ARDS [24,52]. Vitamin D deficiency appears to contribute to the pathogenesis of ARDS in animal and human experimental models of the disorder, and pharmacological repletion of vitamin D has been shown to reduce alveolar capillary damage in deficient patients [53]. Detectable serum SARS-COV-2 RNA (RNAemia) in patients with COVID-19 has been found to be associated with elevated IL-6 concentrations and poor prognosis. IL-6 elevations are possibly part of the cytokine storm that can worsen outcomes in COVID-19 infected patients [54].
As mentioned earlier, SARS-CoV-2 utilizes Angiotensin converting enzyme (ACE2) as an entry receptor into the lung cells and downregulates its expression [1,2]. Calcitriol (1,25-dihydroxyvitamin D3), an activated analogue of vitamin D3 has been shown to exhibit anti-inflammatory effects in acute lung injury and ARDS through modulating expression of ACE enzymes in lungs [55]. Pre-clinical studies suggest that administration of calcitriol has a pronounced impact on ACE2/Ang(1–7)/MasR axis with enhanced expression of ACE2, MasR and Ang(1–7) generation [56]. Another salient virulence factor in COVID-19 infection could be the ability of the S1 domain of the COVID-19 spike glycoprotein to bind to human CD26/DPP-4 (57). CD26/DPP-4 is a key immunoregulatory factor for host hijacking and pathogen virulence. Some of the unique residues of S1 domain of COVID-19 spike glycoprotein are also presumed to interact with the ACE2 protein [58]. The expression of the DPP-4/CD26 receptor has been shown to be reduced significantly in vivo upon the correctness of vitamin D insufficiency [59]. However, there is insufficient evidence to elucidate the effect, if any, of sustained DPP-4 inhibition (as can be achieved through the correction of vitamin D insufficiency or through the use of DPP-4 inhibitors used in the treatment of diabetes) on coronavirus infections.
Vitamin D can modulate the body’s reaction to an invading organism through other mechanisms also. Mice studies have shown that vitamin D deficiency can impair the ability of macrophages to mature, to produce macrophage-specific surface antigens, to produce the lysosomal enzyme acid phosphatase, and to secrete hydrogen peroxide (H2O2), a function integral to their antimicrobial function [57], [60], [61].
It can be thus surmised that there are mechanistic explanations for the observed consequence of vitamin D deficiency on respiratory infectious processes in that it does modulate expression of various cytokines and chemokines involved in the immune reactive process, and, in the setting of COVID-19, vitamin D may help to blunt or dampen the immune system reaction to the virus. However, it has to be noted that neither vitamin D nor its metabolites have been consistently shown to influence replication or clearance of respiratory viruses from human respiratory epithelial cell cultures in pre-clinical studies [58] and more studies are needed to clarify the effects of the vitamin on viral entry and adhesion to the respiratory epithelium. The most promising avenue of research appears to be further exploration of the ACE2/Ang(1–7)/MasR axis and its modulation by vitamin D.
8. Clinical studies on vitamin D supplementation in RTIs
There is no consensus as to what the optimal 25(OH)D level needed for skeletal and extraskeletal benefits is. Baseline vitamin D status determines whether a meaningful impact from vitamin D supplementation will be obtained. The potential benefit of vitamin D supplementation for RTI was found to be the greatest when the baseline 25(OH)D level was below 10 ng/ml in a systematic review and meta-analysis of 25 well conducted high-quality randomized controlled trials [62]. Vitamin D deficiency was present in 69% of intensive care unit (ICU) patients and it was associated with higher mortality compared to patients with 25(OH)D levels of more than 20 ng/ml in an observational study at a university hospital in Turkey [63]. The risk of ARDS following esophagectomy (which is considered as a surgery with high risk for development of ARDS) was 3.5 times higher (after adjustment for age, gender, smoking status, and tumor staging) when the pre-operative 25(OH)D was less than 10 ng/ml [53].
Clinical trials of vitamin D supplementation for the prevention and treatment of acute RTIs, however, have reported heterogenous results. A meta-analysis of 11 randomized controlled trials revealed a significant 64% reduction in the risk of RTIs (95% CI 0.49–0.84; P = 0.0014) after vitamin D supplementation [64]. Another systematic review and meta-analysis of 25 RCTs that included 11,321 participants showed that vitamin D supplementation helped protect against RTIs. Subgroup analysis further revealed that the protection was most beneficial in groups with vitamin D deficiency (<10 ng/ml) and individuals taking daily or weekly vitamin D3 or D2 supplementation without receiving additional bolus doses [62].
Interventional studies with high dose vitamin D in critically ill patients have failed to show marked positive ICU related end points. Supplementation with high doses of vitamin D3 (25,000 IU or 50,000 IU) daily for five days reduced hospital length of stay in mechanically ventilated patients, but did not improve other ICU outcomes such as mortality, time on ventilator, and nosocomial infection [65]. A randomized, double-blind, placebo-controlled, phase 3 trial of vitamin D3 (single enteral dose of 540,000 international units orally or through a nasogastric tube) administered within 12 h of ICU admission to vitamin D deficient patients (mean vitamin D level of 11 ng/ml) did not show any difference in 90 -day mortality or in other clinically important secondary end points [66].
The disparate doses and differences in frequency of administration might explain why the therapeutic outcomes differed considerably in the intervention studies with vitamin D that have been conducted so far. Quarterly bolus supplementation of oral cholecalciferol 100,000 IU was not found to reduce the incidence of pneumonia in infants in a RCT done in Kabul, Afghanistan [67]. Large bolus doses of vitamin D, especially given within a short period of time, can potentially lead to an immune suppressive effect with negative impact on clinical outcomes. This was evidenced in a trial where cholecalciferol intake of 10,000 IU/day that resulted in achieving a mean value of 25(OH)D of 72 ng/ml showed reduced proliferative responses of peripheral blood monocytes in patients with multiple sclerosis [68]. Administration of supraphysiologic doses of cholecalciferol can result in elevated phosphate and FGF23, both of which are known to inhibit activation of vitamin D at renal as well as extrarenal sites [9]. This may explain, in part, why many of these studies with bolus cholecalciferol supplementation lead to poorer or nil outcomes compared to daily dosing schedules.
In view of conflicting reviews and the heterogenous evidence, it is difficult to draw firm conclusions and to generalize the results of studies exploring the role of vitamin D supplementation for RTIs at a population level. Some plausible explanations for inconsistent results include variation in doses and intervals of vitamin D supplementation, vitamin D status of the population studied, hereditary variation in VDR and vitamin D binding protein, possible pathogen specific beneficial effect, and perhaps interindividual variability in conversion to active vitamin D due to genetic polymorphisms in hydroxylase enzymes.
9. To supplement or not to supplement?
Do we adhere to the tenet of Primum Non Nocere (First do no harm) or, whether as physicians grappling with a new and virulent enemy, do we follow the contrary principle of Melius Anceps Remedium Quam Nullum (It is better to do something than do nothing)?
The US Institute of Medicine (IOM) issued vitamin D and calcium supplementation guidelines in 2011 [69]. The institute recognized that no study has reported adverse effects including hypercalciuria or nephrocalcinosis with supplementation at doses of vitamin D less than 10,000 IU/day. However, the IOM still set the upper intake level at 4000 IU/day. This was partly out of concerns originating from observational studies that suggested deleterious reverse J-shaped relationships between serum 25(OH)D concentrations and health outcomes including mortality and cardiovascular events [70]. Intermittent high bolus dosing of vitamin D has also been associated with an increased risk of falls and fractures [71].
To date, no published data examining the effectiveness of vitamin D supplementation in patients infected with COVID-19 exist. However, several randomized open-label and blinded trials of supplementation are currently in progress (Table 2) and limited published data regarding the correlation of vitamin D levels with COVID-19 severity and mortality rates associated with it exist. A recent National Institute for Health and Care Excellence (NICE) Rapid Evidence Review [72] that included 5 studies; a study with an observational retrospective cohort design [73], a study that was a case-control survey [74], an observational prognostic study using univariable and multivariable regression [75], and 2 studies [16], [76] that were observational prognostic ones using correlation, concluded that all 5 of the studies (Table 3) had high risk of bias with very low quality of evidence, and that there was no evidence at the current time to support taking vitamin D supplements to specifically prevent or treat COVID-19 [72]. None of the studies included in the rapid evidence review were intervention studies of vitamin D supplementation, so no information on appropriate doses or adverse events was provided. It was acknowledged, however, in the NICE review that a systematic review and randomized controlled trials on vitamin D and COVID-19 were underway and that new evidence would be considered once it became available.
Table 2.
Ongoing randomized open-label and blinded trials of vitamin D supplementation in COVID-19 (NIH Trial Net database).
| Name of Trial | Design | Start Date | End Date | Sample Size | Intervention | Outcome |
|---|---|---|---|---|---|---|
| Vitamin D on Prevention and Treatment of COVID-19 (COVITD-19) (NCT04334005) |
Randomized, double-blind | April 10 2020 | June 30 2020 | 200 | Single dose of 25,000 IU of oral colecalciferol | Composite of cumulative death for all causes and for specific causes |
| Low-risk, Early Aspirin and Vitamin D to Reduce COVID-19 Hospitalizations (LEAD COVID-19) (NCT04363840) |
Randomized open label parallel assignment | May 2020 | December 2020 | 1080 | Aspirin 81 mg orally daily for 14 days plus a dietary supplement of 50,000 IU of vitamin D orally once weekly for 2 weeks | Hospitalization for COVID-19 symptoms |
| Open Label Phase II Pilot Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection (HELP-COVID-19) (NCT04335084) |
Randomized Double-Blind, Placebo-Controlled | April 2020 | July 2020 | 600 | Hydroxychloroquine for 1 day and a dietary supplement of vitamin C, vitamin D and Zinc for 12 weeks | Prevention of COVID-19 symptoms in medical workers who are at elevated risk of COVID-19 due to exposure to positive patients in the Emergency department or Intensive Care Unit |
| Impact of Zinc and Vitamin D3 Supplementation on the Survival of Aged Patients Infected with COVID-19 (ZnD3-CoVici) (NCT04351490) | Randomized open label parallel assignment | April 2020 | July 2020 | 3140 | Zinc Gluconate orally 15 mg X 2 per day, 25 -OH-colecalciferol drinkable solution 10 drops (2000 IU) per day for 2 months | Survival rate in subjects asymptomatic at inclusion time |
| COVID-19 and Vitamin D Supplementation in a Multi-center Randomized Controlled Trial of High Dose Versus Standard Dose Vitamin D3 in High-Risk COVID-19 Patients (CoVit Trial) (NCT04344041) | Randomized open label parallel assignment | April 2020 | July 2020 | 260 | Either a single dose of colecalciferol 400,000 IU compared to single dose of 50,000 IU | Number of deaths from any cause during the 14 days following inclusion and intervention |
| Prevention and treatment with Calcifediol of Coronavirus induced acute respiratory syndrome (SARS) COVID-19 (COVIDIOL) (NCT04366908) |
Multi-center, randomized, open label parallel assignment | April 29 2020 | August 28 2020 | 1008 | Best available therapy plus Calcefediol 532 mcg orally on the day of admission and 266 mcg orally on day 3, 7, 14, 21 and 28 or Best available therapy only | Proportion of patients admitted to Intensive Care Unit or died at day 28 |
| Preventive and Therapeutic Effects of Oral 25-hydroxyvitamin D3 on Coronavirus (COVID-19) in Adults (Oral 25-hydroxyvitamin D3 and COVID-19) (NCT04386850) | Multi-center, randomized, double-blinded, placebo-controlled clinical trial with parallel groups and allocation 1:1 | April 14 2020 | November 15 2020 | 1500 | 25 mcg of 25 OHD3 orally daily to case group and placebo to control group for 2 months. One arm of the study is for patients testing positive for COVID-19. Another arm is to evaluate the preventive potential of 25 mcg of 25OHD3 in health care providers and hospital workers with a negative test for COVID-19 | Therapeutic efficacy of rapidly correcting vitamin D deficiency in adults with the use of 25-hydroxyvitamin D3 [25(OH)D3] for reducing the risk of acquiring the SARS-CoV-2 (COVID-19) viral infection and mitigating morbidity and mortality associated with COVID-19. |
| Improving Vitamin D status in the Management of COVID-19 (NCT04385940) | Randomized parallel assignment with quadruple masking | June 2020 | December 2020 | 64 | Subjects randomized to high-dose therapy will take 50,000 IU two times in the first week and once weekly over 2nd and 3rd weeks. Subjects in low -dose arm will take vitamin D 1000 IU daily for 3 weeks | To determine the relationship between baseline vitamin D deficiency and clinical characteristic and to assess patient response to vitamin D supplementation in week 3 and determine its association with disease progression and recovery |
Table 3.
Results of studies included in National Institute for Health and Care Excellence (NICE) Evidence Review exploring correlations between vitamin D levels and COVID-19.
| Study (Reference Number) | Country/Region | Sample size | Study design | Study population | Outcome studied | Results |
|---|---|---|---|---|---|---|
| Ilie et al. 2020 [16] | 20 countries in Europe | n ≥ 45,000 (as of 8 April 2020) | Cross sectional-Observational prognostic | General Population | The correlation between mean serum 25(OH)D level and COVID-19 cases and mortalities by country | A negative correlation observed between 25(OH)D levels and the number of COVID-19 cases (p = 0.050) and mortalities per million population (p = 0.05) respectively |
| D’Avolio et al. 2020 [73] | Switzerland | n = 1484 (107 COVID-19 positive/negative cohort from 1 March-14 April 2020 AND n = 1377 control patients from 1March-14 April 2019 | Retrospective Cohort | General Population | Serum 25(OH)D concentrations in SARS-CoV-2 PCR positive patients compared with PCR-negative patients from 2020 cohort and historic controls from 2019. The association between serum 25(OH)D status and COVID-19 infection | Significantly lower 25(OH)D levels seen in 2020 SARS-CoV-2 PCR positive cohort [11.1 ng/ml] compared with 2020 PCR negative cohort [24.6 ng/ml] (p = 0.004) A similar association seen when 2020 SARS-CoV-2 PCR positive cohort [11.1 ng/ml] compared to 2019 historic controls [24.6 ng/ml] (p < 0.001) |
| Fasano et al. 2020 [74] | Italy | n = 2693 (1486 patients with Parkinson’s disease [PD] and 1207 controls) | Case control Survey | Patients with Parkinson’s Disease | Primary Outcome: Risk of Developing COVID-19 in PD patients Secondary Outcome: The risk factors for COVID-19 infection in PD patients |
22.4% of PD patients on vitamin D supplementation did not develop COVID-19 while 12.4% of PD patients who did not take vitamin D supplementation developed COVID-19 Age adjusted OR: 0.56 (95% CI:0.32–0.99); (p = 0.048) |
| Hastie et al. 2020 [75] | UK | n = 348,598 (UK Biobank participants aged 37–73 years between 16 March 2020 to 14 April 2020) | Observational Prognostic using univariate and multivariate regression | General Population- UK Biobank participants across England, Scotland, and Wales | The prediction between baseline 25(OH)D status and subsequent COVID-19 infection | 25(OH)D levels showed a significant association with COVID-19 infection in univariate analysis (p = 0.013) but not after adjustment for confounders (p = 0.208) |
| Laird et al. 2020 [76] | 12 countries in Europe | n = 21,769 | Cross sectional -Observational Prognostic using correlation | Older adults | The correlation between serum 25(OH)D status by country and COVID-19 mortalities | A statistically significant correlation between low mean serum 25(OH)D levels and higher rate of COVID-19 mortalities per million population (p = 0.046) |
Given the possible detrimental effects of very high bolus doses of vitamin D, the absence of clear positive effects on mortality or prevention of nosocomial infections in critically ill patients with it, and, with no evidence to show that it reduces the incidence of RTI, we do not advocate the use of bolus doses of vitamin D in the setting of COVID-19. However, with circumstantial evidence suggesting that populations at risk for vitamin D deficiency have higher mortality rates from COVID-19 appear to be more susceptible to severe infections, and that doses less than 4000 IU/day are unlikely to be of harm, it seems prudent to recommend optimizing vitamin D status in accordance with recommendations made by national and international public health agencies. On extrapolating the studies that have been conducted in patients hospitalized for other reasons, it appears that measuring serum 25(OH)D levels in patients admitted with Covid-19 infections would be useful to determine baseline sufficiency status. Supplementing those whose levels are clearly below 10 ng/ml, to reach a concentration of 30 ng/ml or above, with daily doses not exceeding 4000 IU should be a reasonably safe option. In countries where resource constraints make testing for baseline levels of 25(OH)D in all patients difficult, and, it is known that vitamin D deficiency is prevalent in the population, supplementing with 1000–2000 IU daily is a pragmatic compromise and can be considered. At present, there is no evidence to support recommending additional intake of vitamin D to prevent COVID-19. General measures such as adequate exposure to sunlight and ensuring that at-risk individuals such as the elderly are getting adequate vitamin D either via diet or supplements (400–800 IU/day) also are recommended.
10. Future research
Randomized controlled trials of vitamin D supplementation in patients with COVID-19 infections with regards to mitigating the risk and improving clinical outcomes for different degrees of disease severity in COVID-19 infections are currently lacking. Given the absence of current effective antiviral therapy and expected delays to develop an effective vaccine, such trials are warranted.
11. Conclusions
Though epidemiological and circumstantial data linking vitamin D to the novel coronavirus disease COVID-19 exist, conclusive evidence regarding the role of vitamin D in protecting against or mitigating the severe respiratory complications of COVID-19 is lacking. However, in the context of a global crisis that demands rapid solutions, supplementation with daily or weekly modest doses of vitamin D in patients infected with COVID-19 in whom vitamin D levels are deficient may afford some benefit without causing harm. At a population level, it may be advisable to have adequate intake of vitamin D to meet national and international recommendations. Well conducted randomized controlled trials of vitamin D supplementation in patients with varying degrees of severity of COVID-19 infection are urgently warranted.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
CRediT author statement
Manju Chandran: Conceptualization, Data curation, Writing – original draft, review and editing.
Aye Chan Maung: Data curation, Writing – original draft.
Ambrish Mithal: Writing - review and editing.
Rajeev Parameswaran: Conceptualization.
Acknowledgments
The authors would like to gratefully acknowledge Dr. Dominique Pierroz, Science Manager and Mr. David Oldani, Operational Communication Coordinator at International Osteoporosis Foundation (IOF), Nyon, Switzerland for their help in procuring the IOF Global Vitamin D Map. ORCID Manju Chandran: 0000-0001-9119-8443. Aye Chan Maung: 0000-0001-8004-4422. Ambrish Mithal: 0000-0002-0822-3212. Rajeev Parameswaran: 0000-0002-3318-0357.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 3.Dijkman R., Jebbink M.F., Deijs M., Milewska A., Pyrc K., Buelow E. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J Gen Virol. 2012;93(Pt 9):1924–1929. doi: 10.1099/vir.0.043919-0. [DOI] [PubMed] [Google Scholar]
- 4.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X., Chen J., Wang X., Zhang F., Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowd J.B., Andriano L., Brazel D.M., Rotondi V., Block P., Ding X. Demographic science aids in understanding the spread and fatality rates of COVID-19. Proc Natl Acad Sci U S A. 2020;117:9696–9698. doi: 10.1073/pnas.2004911117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pachetti M., Marini B., Benedetti F., Giudici F., Mauro E., Storici P. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18:179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry H.L. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metabol. 2011;25:531–541. doi: 10.1016/j.beem.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill C.M., Kazantzidis A., Ryan M.J., Barber N., Sempos C.T., Durazo-Arvizu R.A. Seasonal changes in Vitamin D-Effective UVB availability in Europe and associations with population serum 25-Hydroxyvitamin D. Nutrients. 2016;8:533. doi: 10.3390/nu8090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeum K.J., Song B.C., Joo N.S. Impact of geographic location on vitamin D status and bone mineral density. Int J Environ Res Publ Health. 2016;13:184. doi: 10.3390/ijerph13020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes J., Dunstan F., Laird E., Subramanian S., Kenny R.A. Perspective: vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2, and thrombosis (R1) J Intern Med. 2020 Jul 2 doi: 10.1111/joim.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirve S., Newman L.P., Paget J., Azziz-Baumgartner E., Fitzner J., Bhat N. Influenza seasonality in the tropics and subtropics - when to vaccinate? PLoS One. 2016;11 doi: 10.1371/journal.pone.0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moura F.E.A., Perdigão A.C.B., Siqueira M.M. Seasonality of influenza in the tropics: a distinct pattern in northeastern Brazil. Am J Trop Med Hyg. 2009;81:180–183. [PubMed] [Google Scholar]
- 16.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes J.M., Subramanian S., Laird E., Kenny R.A. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51:1434–1437. doi: 10.1111/apt.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant W.B., Fakhoury H.M.A., Karras S.N., Al Anouti F., Bhattoa H.P. Variations in 25-hydroxyvitamin D in countries from the Middle East and Europe: the roles of UVB exposure and diet. Nutrients. 2019;11:2065. doi: 10.3390/nu11092065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cashman K.D., Dowling K.G., Škrabáková Z., Gonzalez-Gross M., Valtueña J., De Henauw S. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Q., Gu Y., Zhang M. Spatiotemporal trends of PM2.5 concentrations in central China from 2003 to 2018 based on MAIAC-derived high-resolution data. Environ Int. 2020;137:105536. doi: 10.1016/j.envint.2020.105536. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal K.S., Mughal M.Z., Upadhyay P., Berry J.L., Mawer E.B., Puliyel J.M. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002;87:111–113. doi: 10.1136/adc.87.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pareek M., Bangash M.N., Pareek N., Pan D., Sze S., Minhas J.S. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020;395:1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucato P., Solmi M., Maggi S., Bertocco A., Bano G., Trevisan C. Low vitamin D levels increase the risk of type 2 diabetes in older adults: a systematic review and meta-analysis. Maturitas. 2017;100:8–15. doi: 10.1016/j.maturitas.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Qi D., Nie X.L., Wu S., Cai J. Vitamin D and hypertension: prospective study and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gholami F., Moradi G., Zareei B., Rasouli M.A., Nikkhoo B., Roshani D. The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. 2019;19:248. doi: 10.1186/s12872-019-1236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khammissa R.A.G., Fourie J., Motswaledi M.H., Ballyram R., Lemmer J., Feller L. The biological activities of vitamin D and its receptor in relation to calcium and bone homeostasis, cancer, immune and cardiovascular systems, skin biology, and oral health. BioMed Res Int. 2018;2018:9276380. doi: 10.1155/2018/9276380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bikle D.D., Patzek S., Wang Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: case report and review. Bone Rep. 2018;8:255–267. doi: 10.1016/j.bonr.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Rosa M., Malaguarnera M., Nicoletti F., Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian K., Bergman P., Henriques-Normark B. Vitamin D promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophils. J Innate Immun. 2017;9:375–386. doi: 10.1159/000455969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams J.S., Ren S., Liu P.T., Chun R.F., Lagishetty V., Gombart A.F. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009 Apr 1;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agier J., Efenberger M., Brzezińska-Błaszczyk E. Cathelicidin impact on inflammatory cells. Cent Eur J Immunol. 2015;40:225–235. doi: 10.5114/ceji.2015.51359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojaimi S., Skinner N.A., Strauss B.J., Sundararajan V., Woolley I., Visvanathan K. Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J Transl Med. 2013;11:176. doi: 10.1186/1479-5876-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharifi A., Vahedi H., Nedjat S., Rafiei H., Hosseinzadeh-Attar M.J. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS. 2019;127:681–687. doi: 10.1111/apm.12982. [DOI] [PubMed] [Google Scholar]
- 38.Lemire J.M. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 39.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu Meduri, Annane D., Chrousos G.P., Marik P.E., Sinclair S.E. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136:1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 41.Olszowiec-Chlebna M., Koniarek-Maniecka A., Brzozowska A., Błauż A., Rychlik B., Stelmach I. Vitamin D inhibits pro-inflammatory cytokines in the airways of cystic fibrosis patients infected by Pseudomonas aeruginosa- pilot study. Ital J Pediatr. 2019;45:41. doi: 10.1186/s13052-019-0634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekeredjian-Ding I., Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bredin C.P. Sanatoria revisited: sunlight and health. J Roy Coll Phys Edinb. 2018;48:92–93. doi: 10.4997/JRCPE.2018.120. [DOI] [PubMed] [Google Scholar]
- 44.Ginde A.A., Mansbach J.M., Camargo C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brance M.L., Miljevic J.N., Tizziani R., Taberna M.E., Grossi G.P., Toni P. Serum 25-hydroxyvitamin D levels in hospitalized adults with community-acquired pneumonia. Clin Respir J. 2018;12:2220–2227. doi: 10.1111/crj.12792. [DOI] [PubMed] [Google Scholar]
- 46.Mamani M., Muceli N., Ghasemi Basir H.R., Vasheghani M., Poorolajal J. Association between serum concentration of 25-hydroxyvitamin D and community-acquired pneumonia: a case-control study. Int J Gen Med. 2017;10:423–429. doi: 10.2147/IJGM.S149049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remmelts H.H.F., van de Garde E.M.W., Meijvis S.C.A., Peelen E.L.G.C.A., Damoiseaux J.G.M.C., Grutters J.C. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis. 2012;55:1488–1494. doi: 10.1093/cid/cis751. [DOI] [PubMed] [Google Scholar]
- 48.Nursyam E.W., Amin Z., Rumende C.M. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 49.Urashima M., Segawa T., Okazaki M., Kurihara M., Wada Y., Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 50.Sabetta J.R., DePetrillo P., Cipriani R.J., Smardin J., Burns L.A., Landry M.L. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laviano E., Sanchez Rubio M., González-Nicolás M.T., Palacian M.P., López J., Gilaberte Y. Association between preoperative levels of 25-hydroxyvitamin D and hospital-acquired infections after hepatobiliary surgery: a prospective study in a third-level hospital. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dancer R.C.A., Parekh D., Lax S., D’Souza V., Zheng S., Bassford C.R. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020 Apr 17:ciaa449. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui C., Xu P., Li G., Qiao Y., Han W., Geng C. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26:101295. doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vankadari Naveen, Wilce Jacqueline A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microb Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komolmit P., Charoensuk K., Thanapirom K., Suksawatamnuay S., Thaimai P., Chirathaworn C. Correction of vitamin D deficiency facilitated suppression of IP-10 and DPP IV levels in patients with chronic hepatitis C: a randomised double-blinded, placebo-control trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Amer Y., Bar-Shavit Z. Impaired bone marrow-derived macrophage differentiation in vitamin D deficiency. Cell Immunol. 1993;151:356–368. doi: 10.1006/cimm.1993.1245. [DOI] [PubMed] [Google Scholar]
- 61.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aygencel G., Turkoglu M., Tuncel A.F., Candır B.A., Bildacı Y.D., Pasaoglu H. Is vitamin d insufficiency associated with mortality of critically ill patients? Crit Care Res Pract. 2013;2013:856747. doi: 10.1155/2013/856747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergman P., Lindh A.U., Björkhem-Bergman L., Lindh J.D. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han J.E., Jones J.L., Tangpricha V., Brown M.A., Brown L.A.S., Hao L. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. Version 2. J Clin Transl Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.National Heart Lung, Blood Institute PETAL Clinical Trials Network. Ginde A.A., Brower R.G., Caterino J.M., Finck L., Banner-Goodspeed V.M., Grissom C.K. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N Engl J Med. 2019;381:2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manaseki-Holland S., Maroof Z., Bruce J., Mughal M.Z., Masher M.I., Bhutta Z.A. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379:1419–1427. doi: 10.1016/S0140-6736(11)61650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimball S., Vieth R., Dosch H.M., Bar-Or A., Cheung R., Gagne D. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Metab. 2011;96:2826–2834. doi: 10.1210/jc.2011-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Durup D., Jørgensen H.L., Christensen J., Tjønneland A., Olsen A., Halkjær J. A Reverse J-shaped association between serum 25-hydroxyvitamin D and cardiovascular disease mortality: the CopD Study. J Clin Endocrinol Metab. 2015;100:2339–2346. doi: 10.1210/jc.2014-4551. [DOI] [PubMed] [Google Scholar]
- 71.Sanders K.M., Seibel M.J. Therapy: new findings on vitamin D3 supplementation and falls - when more is perhaps not better. Nat Rev Endocrinol. 2016;12:190–191. doi: 10.1038/nrendo.2016.29. [DOI] [PubMed] [Google Scholar]
- 72.NICE National Institute for Health and Care Excellence Evidence review. Vitamin D for COVID-19. https://www.nice.org.uk/advice/es28/evidence [PubMed]
- 73.D’Avolio A., Avataneo V., Manca A., Cusato J., De Nicolò A., Lucchini R. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fasano A., Cereda E., Barichella M., Cassani E., Ferri V., Zecchinelli A.L. COVID -19 in Parkinson’s disease patients living in lombardy, Italy. Mov Disord. 2020 doi: 10.1002/mds.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laird E., Rhodes J., Kenny R.A. Vitamin D and inflammation: potential implications for severity of COVID-19. Ir Med J. 2020;113:81. [PubMed] [Google Scholar]