Abstract
In January 2020, a cluster of pneumonia cases was reported in Wuhan, China. A global pandemic followed. The infection, called novel coronavirus disease 2019 (COVID-19), is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Common symptoms of COVID-19 illness included fever, cough, and abnormal findings on chest computed tomography. Nucleic acid testing, in the form of real-time reverse transcriptase polymerase chain reaction, is essential for diagnosing COVID-19 from respiratory samples from infected patients. Still, many questions remain surrounding the optimization of pre-analytical factors, such as specimen selection, collection, and transport. This review summarizes the current publications that describe viral density and specimen suitability for molecular detection methods. Of note, many of the reports represent studies with small sample sizes, and information may change as more is learned about specimen types as the pandemic continues.
Background
On 31 December 2019, a cluster of zoonotic pneumonia cases in Wuhan, China, was reported and included 41 hospitalized patients [1]. The disease, now known as novel coronavirus disease 2019 (COVID-19), is caused by the 2019 novel coronavirus (2019-nCoV), a betacoronavirus [1]. Since the first reports, the number of cases rapidly expanded, and the 2019 novel coronavirus was categorized as a public health outbreak of international concern by the World Health Organization on 30 January 2020. The virus was renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on 11 February 2020 because the virus is genetically related to the coronavirus responsible for the SARS outbreak of 2003. The global pandemic was declared on 11 March 2020, and the disease was named COVID-19 [2].
Many patients presented to health care providers with mild symptoms that progressed to pneumonia, followed by severe respiratory illness requiring admission to the intensive care unit and an elevated incidence of mortality. Common symptoms of COVID-19 include fever and cough, shortness of breath, myalgia, fatigue, and abnormal findings on chest computed tomography, as well as various other symptoms. Complications included a syndrome that resembled acute respiratory distress syndrome [1]. The first documented case of infection in the United States presented in the state of Washington on 19 January 2020 [3], and the number of infections quickly progressed, and the United States leads the world in confirmed COVID-19 cases.
Nucleic acid testing, most commonly reverse transcriptase polymerase chain reaction (RT-PCR), plays a significant role in the diagnosis of COVID-19 from respiratory samples from infected patients. However, despite the launch of commercially available diagnostic assays for the detection of SARS-CoV-2 nucleic acids, with emergency use authorization claims granted by the Food and Drug Administration (FDA) [4], diagnostic uncertainty remains. Pre-analytical factors, such as specimen selection, collection, and transport, are critical to optimal assay accuracy [5]. Furthermore, there is a current need for information describing the viral loads in different body sites.
The COVID-19 pandemic brought diagnostic challenges to clinical laboratories across the globe, particularly in the ability to reliably provide accurate and rapid test results. Detection of SARS-CoV-2 RNA is a critical diagnostic factor for the diagnosis of COVID-19 from clinical specimens. Early in the pandemic, reports of false-negative real-time RT-PCR (rRT-PCR) results were documented, and the sensitivity of rRT-PCR for the detection of SARS-CoV-2 came into question [6, 7, 8]. These reports did not compare the assay’s limit of detection with those of new commercial assays, but they did control for optimized specimen collection processes.
It is now understood that the disease usually begins in the upper respiratory tract (URT) and can progress to the lower respiratory tract (LRT) in more severe cases [5]. Therefore, when the initial nasopharyngeal (NP) sample tested negative in patients with a high pre-test probability of disease, it was recommended that repeat testing (also referred to as serial testing) be performed with an LRT specimen [7]. It is important to note that sub-optimal specimen quality can also impact test results; therefore, re-collection and testing of the URT (or LRT) specimens in patients with negative RT-PCR results and high suspicion or probability of infection are recommended [5].
Suboptimal pre-analytical practices may limit the accuracy of RT-PCR. Lack of attention to rapidly emerging literature may limit test performance by impacting the following testing parameters: specimen collection devices (including swab material and transport media), sample selection, sample collection (i.e., poor-quality collection), specimen transport and storage, the presence of interfering substances, and testing outside the diagnostic window. Guidance documents for providers with concise communication and wide distribution of clear instructions for specimen collection, management, and storage are crucial to clinical operations to ensure high-quality specimens are submitted for testing. Information about specimen types is changing quickly, and it is likely that what we now know about sample types used for SARS-CoV-2 testing will be a small subset of what we will learn in the future.
Upper Respiratory Tract Samples
Nasopharyngeal, mid-turbinate, and oropharyngeal swabs
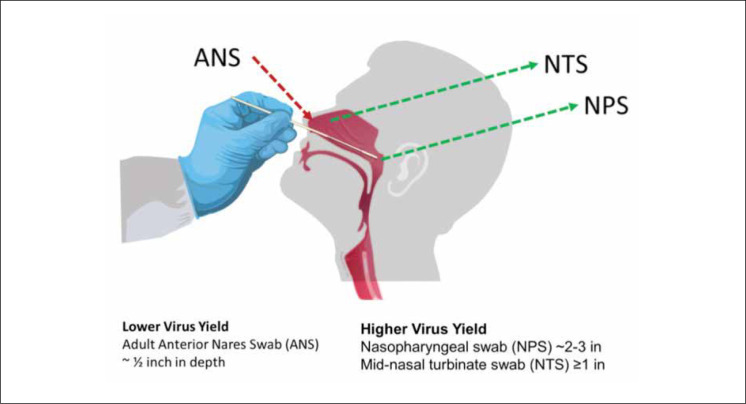
Patients with viral pneumonia do not typically produce purulent sputum; therefore, the most common collection method used to obtain a specimen for respiratory pathogen testing is the use of NP swabs (NPS). NPS and oropharyngeal swabs (OPS) (also called throat swabs) were among the first specimens suggested for COVD-19 testing. Mid-turbinate swabs (MTS) and anterior-nares (i.e., nasal) swabs (ANS) are now also accepted for testing (Fig. 1 ).
Figure 1.
Diagram illustrating the differences among clinical samples collected from different areas of the nasal cavity. Adapted by Geisinger Laboratory Medicine from the Centers for Disease Control and Prevention (https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html).
Despite its popularity, an NPS sample is challenging to collect from some patients, and suboptimal sampling may result in false-negative results [9, 10]. For this reason, MTS may be considered, as they are less invasive for the patient and are considered safer for the specimen collector due to little or no induced patient gagging and coughing.
An LRT specimen may be required if the URT specimen is negative and pre-test probability is high or if the severity of disease dictates (i.e., the patient is intubated and/or unconscious). In these cases, LRT specimens may need to be obtained by bronchoscopy or tracheal aspiration.
To assess the differences between URT and LRT samples, one study used SARS-CoV-2 quantitative RT-PCR assays to test different specimen types from 82 infected individuals. Additionally, serial samples (OPS, sputum, stool, and urine) were collected from two patients daily over time to assess viral loads after symptom onset. Estimates of the viral density of SARS-CoV-2 ranged from 104 to 107 copies per ml, and sputum samples generally showed higher viral loads than OPS samples. Overall, viral loads were best in sputum, followed by OPS and stool samples. No viral RNA was detected in urine samples. In this study, the viral loads in OPS and sputum samples appeared to be highest early after symptom onset and peaked at approximately 5 to 6 days after symptom onset, as opposed to peaks in viral density observed in SARS-CoV-2 patients, which typically occur around 10 days after onset. Lastly, viral RNA was detected from infected patients a day before symptom onset, suggesting that individuals may be infectious while asymptomatic [11].
Another study monitored the viral loads in the URT over time, specifically the days since symptom onset. This study tested NPS, MTS, and OPS samples from 18 patients, 17 of whom were symptomatic and 1 asymptomatic with a positive test result, using polyester flock swabs for all sampling. This study’s finding agreed with those mentioned above, in which high viral loads were detected early in the disease, where NPS and MTS showed higher viral loads than OPS [12]. These findings were supported by another study in which the viral loads of respiratory samples from 76 confirmed COVID-19 patients were analyzed quantitatively, and the average viral load in sputum was found to be significantly higher than those in OPS and nasal swabs (P < 0.001) [13].
A higher positive rate was observed in NPS than in OPS in a study of 353 patients who had both specimens tested simultaneously. NPS from inpatients showed a higher positive rate than those from outpatients. Virus detection was better when both swabs were tested than with NPS alone; however, the results were unreliable between sample types (kappa = 0.308) [6].
Despite the inferiority of the OPS in multiple small studies [6, 11, 12, 13, 14], another small study, which followed the clinical courses of 9 hospitalized patients admitted for COVID-19, found similar viral densities in OPS and NPS [15].
Pooling of NPS samples
Pooling of specimens has long been utilized as a method to screen large numbers of samples at lower cost. Individual samples are combined into a pool and tested. The pooling of specimens for the detection of SARS-CoV-2 has been suggested to save reagents and personnel time. In one small study, a Web-based tool was utilized to determine the optimal number of specimens per pool. When a pool size of 5 samples was assessed, the authors observed an overall increase in the testing capability of at least 69% [16]. As the demand for testing more of the general population increases, others suggest pooling of specimens when screening asymptomatic individuals. The rate of positivity is generally low (≤10%) in this group of individuals, which would save on testing.
Moreover, due to the limited availability of testing supplies, screening asymptomatic individuals by pooling specimens would lessen the number of test kits required. Another study utilized larger sample pool sizes of 9 or 10 as a strategy to detect community transmission of SARS-CoV-2 [17]. The number of pools tested was 292, consisting of 2,740 NPS and bronchoalveolar lavage (BAL) samples, with a positivity rate of 0.07%. It is important to note that pooling strategies are not standardized; therefore, a careful assessment of local data (i.e., positivity rate) is necessary before adopting a pooling protocol, and results must be interpreted with caution.
Saliva
Saliva is an offshoot of OPS; however, it can be collected in larger volumes than that collected for an OPS. Saliva was evaluated as another option for non-invasive sample collection. In one study, 200 prospectively collected saliva samples were compared to paired NPS and OPS (combined into one sample for testing [NPS/OPS]) in persons seeking care at an acute respiratory infection clinic. Compared to NPS/OPS as the reference standard, the sensitivity and specificity of the saliva samples were lower than those of NPS/OPS at 84.2% (95% confidence interval [CI], 60.4% to 96.6%) and 98.9% (95% CI, 96.1% to 99.9%), respectively. Overall result agreement between the two specimens in a population with 9.5% prevalence was 97.5% (kappa coefficient, 0.851; 95% CI, 0.723 to 0.979; P < 0.001). Because saliva collection is non-invasive, it has been adopted as a specimen in some settings; however, caution in result interpretation is warranted, as sensitivity using saliva samples is lower than for NPS and OPS [18].
All specimens tested positive in a case study that evaluated a variety of specimen types (NPS, OPS, saliva, sputum, and urine) from 2 COVID-19 patients. Specimens were collected every 2 days during hospital days 1 to 9, and RT-PCR assay results showed that the viral load was highest in the nasopharynx. Saliva samples showed that viral density was lower but easily detectable. Interestingly, the samples of saliva were collected at 1 hour, 2 hours, and 4 hours after using a chlorhexidine mouthwash on hospital days 3 and 6. Although the viral load in saliva was temporarily decreased for 2 hours after using the chlorhexidine mouthwash, SARS-CoV-2 was detected by 4 hours after the mouthwash and up to hospital day 6 for both patients (day 7 for one patient). Chlorhexidine mouthwash effectively reduced the SARS-CoV-2 load in saliva for a short time but did not eliminate viral detection [19].
Another study of 23 confirmed SARS-CoV-2-infected individuals showed that viral density in saliva was highest during the first week after symptom onset. Although the viral load in saliva declined with time, one patient had detectable viral RNA 25 days after symptom onset [20]. Other studies documented detectable viral RNA for as long as 6 weeks after symptom onset [21]. In one report, it appeared that more severe cases had prolonged shedding of viral DNA [22].
Lower Respiratory Tract Samples
Sputum
Because of the false-negative OPS and in some cases NPS documented early in the pandemic, the diagnostic value of analyzing sputum samples to improve accuracy was assessed. Not all individuals can produce sputum; however, one study was able to pair OPS and sputum samples obtained from 52 cases. Using an RT-PCR assay, the positive rates from sputum specimens and OPS were 76.9% and 44.2%, respectively, with sputum having a significantly higher positive rate than OPS (P = 0.001) [23]. The superiority of sputum samples over OPS was documented in a small study in which sputum samples generally showed higher loads of SARS-CoV-2 than OPS [11].
Bronchoalveolar lavage
One study assessed the detection of SARS-CoV-2 in a variety of sample types, including BAL, sputum, and bronchoscope brush biopsy samples; blood; feces; urine; and nasal and pharyngeal swabs. Of 1,070 specimens collected from 205 patients infected with SARS-CoV-2, BAL specimens showed the highest positive rates (14 of 15; 93%), followed by sputum (72 of 104; 72%) and nasal swabs (5 of 8; 63%). Bronchoscope brush biopsy samples showed lower positivity (6 of 13; 46%), followed by pharyngeal swabs (126 of 398; 32%), feces (44 of 153; 29%), and blood (3 of 307; 1%). None of the 72 urine specimens tested positive [14]. In another study, LRT specimens, sputum, or endotracheal aspirates had significantly higher SARS-CoV-2 RNA density than NPS or OPS specimens [24].
Because early studies indicated sputum and BAL samples, if available, can be superior to OPS, especially late in the course of illness [23, 24, 25], and because reports show that viral RNA may become undetectable when the patient is tested later in the course of an illness [26], our laboratory immediately deployed a risk-based method to assess negative NPS results. LRT specimens, including sputum, tracheal aspirate, and BAL, were sought when the pre-test probability was high and the URT sample yielded a negative result. This approach was found to improve the detection of SARS-CoV-2 by increasing the accuracy of diagnosis in cases when clinical suspicion for COVID-19 was high.
Ocular Samples
For one case series, the authors retrospectively performed a chart review of their hospitalized patients with confirmed COVID-19 and sought those with ocular manifestations of the disease. NP and conjunctival swabs from 38 patients were assessed by RT-PCR for the presence of SARS-CoV-2. Positive findings from NPS were observed for 28 patients (73.7%). Only 2 of the patients (5.2%) also tested positive for SARS-CoV-2 in their conjunctival swabs. A total of 12 patients (31.6%; 95% CI, 17.5 to 48.7) had ocular abnormalities consistent with conjunctivitis, including conjunctival swelling, redness, or increased tear secretions. Of these, 11 had NPS with positive findings (91.7%; 95% CI, 61.5 to 99.8). Positive results were also noted for 2 (16.7%) of the conjunctival swabs. Of note, patients with ocular findings appeared to have more severe disease than those without an ocular finding [27]. In contrast, a study of 15 critically ill patients showed that only 1 (6.67%) conjunctival swab specimen was positive for the detection of SARS-CoV-2 [24].
Gastrointestinal and Anal Samples
Stool
Because of the challenge of collecting NPS in pediatric patients, other specimen types, such as OPS, stool, and rectal or anal swabs were examined. One early study in adults showed that stool samples tested positive for a prolonged period compared to OPS, called throat swabs in this study [28]. Similarly, a study of three mild pediatric cases demonstrated that stool samples tested positive for weeks after the OPS samples became negative [29]. Moreover, detection of viral shedding in the stool (5/14; 35.7%) was found to be equally accurate as OPS testing in a small case series in which a positive stool test was not correlated with gastrointestinal symptoms [30]. When COVID-19 patients with mild disease and one or more gastrointestinal symptoms (diarrhea, nausea, and/or vomiting), with or without respiratory symptoms, were compared with patients presenting with solely respiratory symptoms, the following patterns were observed.
Patients with gastrointestinal symptoms had a longer duration between symptom onset and viral clearance (P < 0.001), which also correlated with a delayed diagnosis compared to those with respiratory symptoms alone. Moreover, stool from patients with gastrointestinal symptoms was more likely to test positive than stool from those with respiratory symptoms alone (73.3% versus 14.3%; P = 0.033) [31].
In contrast, another study showed that the presence of SARS-CoV-2 RNA in stool was not linked to the presence of gastrointestinal symptoms or disease severity. Of 42 laboratory-confirmed cases, 8 (19.05%) had gastrointestinal symptoms, whereas 28 (66.67%) patients tested positive for SARS-CoV-2 RNA in stool specimens. The presence of viral RNA in the stool continued to be detected in 18 (64.29%) patients, for 7 (range, 6 to 10) days after the OPS turned negative. In this study, the presence of the virus in the stool did not appear to vary based on illness severity [32]. A systematic review and meta-analysis of published gastrointestinal symptoms and detection of the virus in stool were also reported [33]. Although several studies demonstrated that viral RNA in the stool could be detected for prolonged periods after OPS were negative and symptoms resolved [33], none were able to establish whether the virus was infectious. One study attempted to isolate infectious virus from various sample types, including sputum, OPS, and stool. Active virus replication was observed for sputum and OPS, but not stool, despite high viral loads [34].
Rectal/anal swabs
RT-PCR results from anal swab samples continued to test positive well after paired OPS tested negative [28]. However, in a study of 212 pediatric patients, the diagnostic accuracy of anal swabs was directly compared to that of OPS. The detection rates of SARS-CoV-2 from the two different specimen types were significantly different, with positive rates of 78.2% from OPS and 52.6% from anal swabs (McNemar test, P = 0.0091; kappa = 0.311, P < 0.0001) [35].
In a small case series, 39% (11 of 28) of anal swab specimens had detectable concentrations of viral RNA, and 73% (8 of 11) of those patients had severe cases of COVID-19 [36]. Another study corroborated that critically ill patients have detectable amounts of viral RNA in anal swabs (27%; 4/15) with increased positivity in the stool (69%; 11/16) [24].
Blood Samples
In some reports, viral RNA was not detected in blood samples; however, SARS-CoV-2 nucleic acid was detected in sera collected from critically ill COVID-19 patients [37]. These patients also exhibited extremely high interleukin 6 levels, which suggested a close correlation with the detection of viral RNA in the serum samples (R = 0.90) [37].
In another assessment of critically ill patients, viral RNA was detected in 6 of 57 (10.5%) serum samples. Patients with serum viral RNA progressed to more severe disease stages, which suggested a correlation between disease severity and serum RNA (P = 0.0001) [36]. Lastly, 6.3% (1 of 16) of serum samples from critically ill patients demonstrated positive RT-PCR results for SARS-CoV-2 [24].
Urine Samples
In several small case series, each with patients for whom positive SARS-CoV-2 RNA signals were detected in NPS, viral RNA was not detectable in urine specimens [15, 32, 38]. In another study of 206 patients, 72 of whom had urine collected, none of the 72 urine specimens tested positive [14]. In contrast, two studies reported infrequent detection of viral RNA in urine samples [24, 39], but those results have yet to be corroborated by more extensive studies.
Correlation of Positive Results from Samples Collected during Asymptomatic, Mild, and Severe Disease Stages
Not only did variations in viral loads exist between sample types, they also differed depending on the severity of the disease. The viral loads in nasal swabs and OPS obtained from patients with severe disease appeared to be higher than those in swabs collected from patients with mild to moderate disease; however, the sample size was small, and the 95% CI was wide [12]. In sputum, the viral loads in acute and early stage of illness were higher than those found later, during the recovery stage [13].
In one study, the viral load that was detected in one asymptomatic patient was similar to that found in the symptomatic patients, which suggested viral transmission could possibly occur from asymptomatic or minimally symptomatic patients [12]. In other cases, the viral RNA shedding patterns have been documented to vary with disease severity [40]. In one such study, 76 patients with laboratory-confirmed COVID-19 were tested; the mean estimated viral density of severe cases was higher than that of the density found in mild cases. Detectable virus remained for the first 12 days after illness onset, whereas in mild cases, 90% of patients tested negative on RT-PCR after 10 days [41].
Duration and Symptomatic Status in RT-PCR-Positive Patients
A patient is determined to be infected by the presence of the virus, which can be detected in different body fluids, secretions, and excreta. Results indicated that samples from the LRT had slower resolution of viral shedding than URT samples [24]. Reports of prolonged viral shedding (>55 days) are documented [24]. Older age was correlated with higher viral loads (P = 0.020) [20]. While asymptomatic patients can transmit the virus [42] in some cases, mild infection with documented positive test results occurred without evidence of transmission to close contacts [43]. Finally, in 50% of patients, seroconversion occurred after 7 days (14 days in all) but was not associated with declining viral loads [15].
Long-term-care facilities, especially nursing homes, appear to have high rates of documented case transmission by asymptomatic individuals. In one skilled nursing facility, a point prevalence study was performed 23 days after the first resident tested positive. Of the 48 residents with laboratory-confirmed infection, 27 (56%) were asymptomatic at the time of testing; 24 developed symptoms, with a median time to onset of 4 days. In samples from 24 pre-symptomatic residents, viable virus was recovered from 17 residents (71%). Based on the results from this study, over half of residents with positive test results were asymptomatic at the time of testing and most likely contributed to transmission [44].
Summary
LRT samples appear to have higher positivity rates than URT and other samples, especially later in the illness course. Optimal sample collection and testing of NPS during the initial presentation of illness remains the recommended testing standard. Testing of specimens from multiple sites may improve sensitivity and reduce false-negative test results, especially if clinical suspicion is high. Urine has not been found to contain detectable viral RNA, and the presence of RNA in serum is low. Stool or anal swabs may be an option for testing early or late in the illness, but it does not correlate with severity. No standard methods are available for stool, and the sample type does not always yield positive results. Saliva, ANS, and OPS are convenient but may not be optimal samples, according to existing publications. Viral dynamics in infected patients are yet to be fully determined. Persistence and clearance of viral RNA from different patient specimens remain unclear. Epidemiology, known symptoms, and newly documented symptoms will be essential as laboratories monitor and compare illness with the presence of the virus in clinical specimens. The ability to distinguish live virus from detectable virus remains a challenge. This review represents publications from the early phases of the pandemic, but scores of new publications appear on a weekly basis. Therefore, it will be important for clinical laboratories to continually monitor the literature as the pandemic progresses and new discoveries are published.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cionatta DVM. WHO declares a COVID-19 pandemic. Acta Biomen. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell SL, St George K, Rhoads DD, Butler-Wu SM, Dharmarha V, McNult P. Understanding, verifying and implementing emergency use authorization molecular diagnostics for the detection of SARS-CoV-2 RNA. J Clin Microbiol. 2020 doi: 10.1128/JCM.00796-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Tan L, Wang X, Liu W, Lu Y, Cheng L. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients who received tests with both specimens simultaneously. Int J Infect Dis. 2020;94:107–109. doi: 10.1016/j.ijid.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullis SSM, Crothers JW, Wayne S, Hale AJ. A Cautionary tale of false-negative nasopharyngeal COVID-19 testing. IDCases. 2020 doi: 10.1016/j.idcr.2020.e00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao AT, Tong YX, Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020 doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piras A, Rizzo D, Longoni E, Turra N, Urru S, Saba PP. Nasopharyngeal swab collection in the suspicion of Covid-19. Am J Otolaryngol. 2020 doi: 10.1016/j.amjoto.2020.102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piras A, Rizzo D, Uzzau S, De Riu G, Rubino S, Bussu F. Inappropriate nasopharyngeal sampling for SARS-CoV-2 detection is a relevant cause of false-negative reports. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820931793. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 16.Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153:715–718. doi: 10.1093/ajcp/aqaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan CA, Sahoo MK, Pinsky BA. Sample pooling as a strategy to detect community transmission of SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasomsub E, Watcharananan SP, Boonyawat K, Janchompoo P, Wongtabtim G, Suksuwan W. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35:e195. doi: 10.3346/jkms.2020.35.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang JT, Ran RX, Lv ZH, Feng LN, Ran CY, Tong YQ. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C, Xiang J, Yan M, Li H, Huang S, Shen C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19) Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0187. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201:1435–1438. doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gualano G, Musso M, Mosti S, Mencarini P, Mastrobattista A, Pareo C. Usefulness of bronchoalveolar lavage in the management of patients presenting with lung infiltrates and suspect COVID-19-associated pneumonia: a case report. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang Province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T, Cui X, Zhao X, Wang J, Zheng J, Zheng G. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 33.Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 35.Yuan C, Zhu H, Yang Y, Cai X, Xiang F, Wu H. Viral loads in throat and anal swabs in children infected with SARS-CoV-2. Emerg Microbes Infect. 2020:1–17. doi: 10.1080/22221751.2020.1771219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo IL, Lio CF, Cheong HH, Lei CI, Cheong TH, Zhong X. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16:1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott SE, Zabel K, Collins J, Hobbs KC, Kretschmer MJ, Lach M. First mildly ill, non-hospitalized case of coronavirus disease 2019 (COVID-19) without viral transmission in the United States – Maricopa County, Arizona, 2020. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]