Abstract
Research question
Can the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus induce testis damage and dysfunction?
Design
This is the description of the case of a young man presenting with heavy testicular pain as the first symptom of COVID-19 infection. A review of the literature is also presented.
Results
SARS-CoV-2 may enter into the host cell by binding to angiotensin-converting enzyme 2. This receptor seems to be widely expressed in different testicular cell types, making possible the occurrence of orchitis in male patients with COVID-19 infection. From a review of the literature, it seems that there is currently no evidence of sexual transmission of SARS-CoV-2; however, the possibility of virus-induced testis damage and dysfunction cannot be excluded.
Conclusions
Further studies are necessary on the pathological effect of SARS-CoV-2 in the male reproductive system and to ensure a proper andrological follow-up for male patients.
Introduction
From the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic up to 30 May 2020 there were over 5.8 million cases worldwide and over 230,000 cases in Italy (www.salute.gov.it). At the beginning of March 2020, before the national lockdown in Italy, some regions in Northern Italy were declared as a ‘red zone’; among these was the authors’ region, Emilia-Romagna, in the middle of which Modena is located. Here, almost 200 cases per day were registered during the epidemic's peak.
Case report
In mid-April, a 43-year-old man with a medical history of type 1 diabetes presented to the emergency department of the authors’ University Hospital with low-grade fever and severe bilateral testicular pain that had started 3 days earlier. When asked in the emergency department, he reported not having had unsafe sex for years and not previously having suffered from venereal disease. On emergency department admission, no evidence of inguinal hernia or urinary tract infection was instrumentally or clinically detected. Thus, given the stability of the clinical outlook, the patient was discharged with paracetamol therapy.
Twelve hours later, due to the onset of dyspnoea, the patient returned to the emergency department complaining again of uncontrollable testicular pain, prevalent on the left side. A chest X-ray showed multiple thickening concomitant with bilateral consolidations. An oropharyngeal swab tested positive for SARS-CoV-2, so the patient was transferred to the infectious disease department with a diagnosis of coronavirus disease (COVID-19) pneumonia associated with testicular pain. On admission to the infectious disease department, testicular examination performed by the attending urologist showed absence of redness and palpable swelling, but presence of pain in the scrotum and inguinal lymph nodes. Urine microbiological investigations were performed and did not show any kind of infection. However, antibiotic therapy was started, with amoxicillin/clavulanic acid and azithromycin.
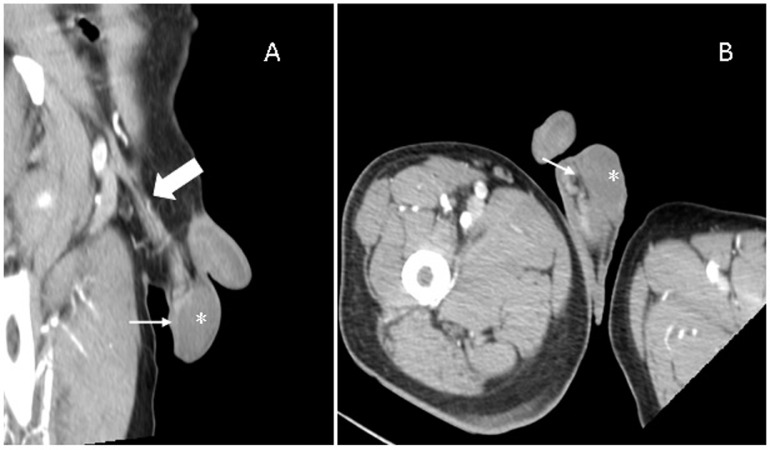
The following day, due to a progressive worsening of oxygenation, with a need to upgrade oxygen therapy up to 15 l/min, the interleukin-6 blocking agent tocilizumab 8 mg/kg was prescribed. Shortly before tocilizumab administration, blood biochemistry tests showed an increase in lactate dehydrogenase to 1213 U/l, D-dimer to 1150 ng/ml and C-reactive protein to 23.80 mg/dl. In the meanwhile, a computed tomography (CT) scan of the lung and abdomen was performed along with some scans of the testicles that demonstrated regular and symmetrical enhancement of the testis, epididymis, testicular artery and pampiniform plexus (Figure 1 ). To complete the examination, an ultrasound was performed due to the persistence of severe pain. The ultrasound report showed a slightly inhomogeneous aspect of the left epididymis, mild accentuation of the vascularization pattern and slight swelling of the left epididymis. Thus, the ultrasound picture was compatible with epididymitis.
Figure 1.
Contrast-enhanced computed tomography scan demonstrating regular and symmetrical enhancement of the testis (*), epididymis (thin arrows), testicular artery and pampiniform plexus (thick arrow). There is also no evidence of testicular enlargement or scrotal fluid collection. (A) Multiplanar reformat on the long axis of the right epididymis. (B) Multiplanar reformat on the long axis of the left epididymis.
On day 2, a further worsening of oxygenation (PaO2/FiO2 ratio less than 70 mmHg) developed concomitantly with severe tachypnoea and dyspnoea. The patient was discharged from the infectious disease department and admitted to the intensive care unit (ICU) for non-invasive ventilation strategy. In ICU, a new testicular ultrasound was performed confirming the previous findings and conserved vascular flows.
On day 3, due to the persistence of poor oxygenation unresponsive to non-invasive strategies, the patient was intubated and mechanically ventilated, and rescue therapy with a prone position was implemented.
On day 4, severe cardiogenic shock developed, necessitating inotrope and vasopressor infusion. Unfortunately, the patient died 3 days later because of irreversible cardiogenic shock following myocarditis of unknown origin.
On autopsy examination, the pathologists’ attention focused on the myocardium, which revealed highly sclerotic and stenotic coronary vessels with marked thickening of the left ventricular wall. No positive immunoreactivity to SARS-CoV-2 antibody was detected in the myocytes. Unfortunately, the testicles were only macroscopically examined and no noteworthy morphological alterations were described.
Ethical committee approval was not required for publication of this case. The authors asked and received from the patient's relatives permission to publish this case report since it suggests that, even in the absence of histopathological confirmation, the testicular pain could be related to epididymitis following SARS-CoV-2 infection, given the fulminant evolution of the COVID-19 disease.
Discussion
Similar to severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome, SARS-CoV-2 infection can cause a respiratory syndrome with a variable spectrum of symptoms such as fever, cough and dyspnoea; this highlights that SARS-CoV-2 has the respiratory tract as its main target. In addition, several studies have provided evidence of potential infection with SARS-CoV-2 of the cardiovascular, digestive and urinary systems, as indicated in the review by Behzad and colleagues (Behzad et al., 2020). However, the relationship between SARS-CoV-2 and the reproductive system has not yet been clarified.
It is known that viruses such as human immunodeficiency virus, hepatitis B virus and mumps virus can enter the testis and cause viral orchitis that may result in male infertility and testicular tumour (Dejucq et al., 2001). During the past SARS epidemic, several studies demonstrated the relationship between members of the coronavirus family (SARS-CoV) and orchitis. Even if SARS-CoV virus has not been detected in testicular tissue (Ding et al., 2004), testicular damage and germ cell destruction was clearly observed in these cases (Xu et al., 2006).
Like previous coronaviruses, with which it shares 78% genetic homology, SARS-CoV-2, seems to have a strong interaction capacity for human angiotensinconverting enzyme 2 (ACE2). ACE2 belongs to the angiotensin-converting enzyme family of dipeptidyl carboxydipeptidases and is homologous to angiotensin 1 converting enzyme. This receptor, which is mainly found in pulmonary epithelium but also in other tissues such as the intestines, kidneys and testes (Li et al., 2020), mediates the entrance into human cells of SARS-CoV-2, which then completes intracellular replication and virus release, and induces cytotoxicity. This explains why the route of viral infection depends on the expression and distribution of the corresponding receptor (Jayawardena et al., 2019).
Recent studies have demonstrated a high expression of ACE2 in kidney and testicular tissue, in particular in spermatogonia, Sertoli cells and Leydig cells, suggesting possible effects on spermatogenesis and the possible occurrence of orchitis in male SARS-CoV-2 patients (Cardona Maya et al., 2020; Fan et al., 2020; Liu et al., 2020; Wang et al., 2020).
In order to clarify this possibility, Song and co-workers (Song et al., 2020) employed real-time PCR (real-time-PCR) testing to detect SARS-CoV-2 in semen or testicular biopsy specimens from 13 hospitalized male patients, but no positive RT-PCR result was found. In line with this, Paoli and colleagues (Paoli et al., 2020) investigated the presence of SARS-CoV-2 in semen and urine samples of a volunteer with a positive nasopharyngeal swab, but both these samples were found to be negative for viral mRNA. A very recent study included 18 semen samples from recovered male patients obtained 8–54 days after symptoms had ended, and two samples from patients with active COVID-19 infection. No RNA was detected by RT-PCR in the semen, including semen samples from the two patients with acute COVID-19 infection. Of note, participants with a moderate infection showed an impairment of sperm quality (Holtmann et al., 2020). These results show that there is no current evidence of sexual transmission of SARS-CoV-2, but the possibility of virus-induced testis damage and dysfunction cannot be excluded (Chen et al., 2020). As with previous coronaviruses, viral binding to the ACE2 receptor in the testis may lead to tissue inflammation and the development of orchi-epididymitis with testicular pain, allowing SARS-CoV-2, which is believed to induce a systemic vasculitis – in particular of small vessels – to be identified as another possible cause of testicular damage and orchitis (Corona et al., 2020).
To the authors’ knowledge, apart from this case, testicular pain with COVID-19 infection has been reported only by Kim and colleagues (Kim et al., 2020), who described an atypical clinical presentation of SARS-CoV-2 infection with abdominal and testicular pain in a 42-year-old man. However, the clinical course of the patient described by Kim and colleagues was benign, while unfortunately the patient described in the current case report died due to a fulminant evolution of his clinical course. In the SARS-CoV-2 pandemic era, paying the utmost attention to the clinical manifestation of testicular pain, in addition to protecting healthcare workers from possible contagion, will allow the proper early identification of patients who could rapidly deteriorate.
The frequency of such an atypical presentation of SARS-CoV-2 infection is unknown, but it should be considered for every patient who comes to the emergency department. Further studies are necessary to investigate the possible short- and long-term effects of SARS-CoV-2 on the male reproductive system and to ensure proper andrological follow-up for swab-positive male patients even in the absence of pulmonary symptoms.
Biography

Antonio La Marca is Professor of Obstetrics and Gynecology at the University of Modena and Reggio Emilia, Italy. His clinical activity covers all fields of reproductive medicine and surgery. He has published extensively and his current h-index is 44, with more than 8000 citations.
Key message.
Testicular pain as the first symptom of COVID-19 may suggest orchitis as possible in men patients with SARS-CoV- infection. Further studies are necessary on the pathological effect of SARS-CoV-2 in the male reproductive system and to ensure a proper andrological follow-up for male patients.
Alt-text: Unlabelled box
Declaration: The authors report no financial or commercial conflicts of interest.
References
- Behzad S., Aghaghazvini L., Radmard A.R., Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin. Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona Maya W.D., Du Plessis S.S., Velilla P.A. SARS-CoV-2 and the testis: similarity with other viruses and routes of infection [published online ahead of print, 2020 Apr 17] Reprod. Biomed. Online. 2020;40(6):763–764. doi: 10.1016/j.rbmo.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Lou D. Rising Concern on Damaged Testis of COVID-19 Patients [published online ahead of print, 2020 Apr 25] Urology. 2020 doi: 10.1016/j.urology.2020.04.069. S0090-4295(20)30459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G., Baldi E., Isidori A.M., Paoli D., Pallotti F., De Santis L., Francavilla F., La Vignera S., Selice R., Caponecchia L., Pivonello R., Ferlin A., Foresta C., Jannini E.A., Lenzi A., Maggi M., Lombardo F. SARS-CoV-2 infection, male fertility and sperm cryopreservation: a position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (Società Italiana di Andrologia e Medicina della Sessualità) J. Endocrinol. Invest. 2020:1–5. doi: 10.1007/s40618-020-01290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejucq N., Jégou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol. Mol. Biol. Rev. 2001;65(2):208–231. doi: 10.1128/MMBR.65.2.208-231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Li K., Ding Y., Lu L.W., Wang J. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. medRxiv. 2020 doi: 10.3389/fmed.2020.563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann N., Edimiris P., Andree M., Edimiris P., Andree M., Doehmen C., Baston-Buest D., Adams O., Kruessel J., Bielfeld A.P. Assessment of SARS-CoV-2 in human semen-a cohort study [published online ahead of print. Fertil. Steril. 2020 doi: 10.1016/j.fertnstert.2020.05.028. S0015-0282(20)30519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena N., Burga L.N., Poirier J.T., Bostina M. Virus-Receptor Interactions: Structural Insights For Oncolytic Virus Development. Oncolytic Virother. 2019;8:39–56. doi: 10.2147/OV.S218494. Published 2019 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Thomsen T., Sell N., Goldsmith A.J. Abdominal and testicular pain: An atypical presentation of COVID-19 [published online ahead of print, 2020 Mar 31] Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.03.052. S0735-6757(20)30194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. Published 2020 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen Y., Tang W., Zhang L., Chen W., Yan Z., Yuan P., Yang M., Kong S., Yan L., Qiao J. Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes [published online ahead of print, 2020 Apr 30] Sci. China Life Sci. 2020:1–10. doi: 10.1007/s11427-020-1705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D., Pallotti F., Colangelo S., Basilico F., Mazzuti L., Turriziani O., Antonelli G., Lenzi A., Lombardo F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab [published online ahead of print, 2020 Apr 23] J. Endocrinol. Invest. 2020:1–4. doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Wang Y., Li W., Hu B., Chen G., Xia P., Wang W., Li C., Hu Z., Yang X., Yao B., Liu Y. Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of COVID-19 patients. medRxiv. 2020 [Google Scholar]
- Wang Z., Xu X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells. 2020;9(4):920. Published 2020 Apr 9 [DOI] [PMC free article] [PubMed]
- Xu J., Qi L., Chi X., Yang J., Wei X., Gong E., Peh S., Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol. Reprod. 2006;74(2):410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]