Abstract
Background
The risk of vertical and perinatal transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, which causes COVID-19), the most appropriate management, and the neonate's risk of developing COVID-19 during the perinatal period are unknown. Therefore, we aimed to elucidate best practices regarding infection control in mother–newborn dyads, and identify potential risk factors associated with transmission.
Methods
In this observational cohort study, we identified all neonates born between March 22 and May 17, 2020, at three New York Presbyterian Hospitals in New York City (NY, USA) to mothers positive for SARS-CoV-2 at delivery. Mothers could practice skin-to-skin care and breastfeed in the delivery room, but had to wear a surgical mask when near their neonate and practice proper hand hygiene before skin-to-skin contact, breastfeeding, and routine care. Unless medically required, neonates were kept in a closed Giraffe isolette in the same room as their mothers, and were held by mothers for feeding after appropriate hand hygiene, breast cleansing, and placement of a surgical mask. Neonates were tested for SARS-CoV-2 by use of real-time PCR on nasopharyngeal swabs taken at 24 h, 5–7 days, and 14 days of life, and were clinically evaluated by telemedicine at 1 month of age. We recorded demographics, neonatal, and maternal clinical presentation, as well as infection control practices in the hospital and at home.
Findings
Of 1481 deliveries, 116 (8%) mothers tested positive for SARS-CoV-2; 120 neonates were identified. All neonates were tested at 24 h of life and none were positive for SARS-CoV-2. 82 (68%) neonates completed follow-up at day 5–7 of life. Of the 82 neonates, 68 (83%) roomed in with the mothers. All mothers were allowed to breastfeed; at 5–7 days of life, 64 (78%) were still breastfeeding. 79 (96%) of 82 neonates had a repeat PCR at 5–7 days of life, which was negative in all; 72 (88%) neonates were also tested at 14 days of life and none were positive. None of the neonates had symptoms of COVID-19.
Interpretation
Our data suggest that perinatal transmission of COVID-19 is unlikely to occur if correct hygiene precautions are undertaken, and that allowing neonates to room in with their mothers and direct breastfeeding are safe procedures when paired with effective parental education of infant protective strategies.
Funding
None.
Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide with substantial consequences for public health.1 New York City (NY, USA) has been particularly affected, with around 200 000 confirmed cases as of May 17, 2020. Adults with comorbidities are at greatest risk for severe disease and death; however, little is known about the consequences of SARS-CoV-2 infection in pregnant women and fetuses.2, 3, 4 Information regarding neonatal outcomes is scarce, and optimal management of the mother and neonate is unknown.
Respiratory viruses uncommonly result in intrauterine transmission of infection to fetuses; therefore, intrauterine transmission of SARS-CoV-2 is anticipated to be low. Two case reports describing isolation of SARS-CoV-2 from amniotic fluid5 and placental tissue6 and isolation of SARS-CoV-2 from the nasopharynx of the two neonates within 48 h of life suggested probable congenital infection; however, the rate of congenital infections of neonates born to SARS-CoV-2-positive mothers remains unknown. The risk of perinatal transmission, especially when breastfeeding, and the neonate's risk of developing COVID-19 during the perinatal period are also unknown.2, 7 Guidelines have been provided by various medical societies, but because of a scarcity of clinical data, they differ in recommended management strategies for mother–infant dyads.8, 9, 10, 11, 12, 13, 14
We aimed to follow up neonates born to mothers positive for SARS-CoV-2 at time of delivery, to elucidate best practices regarding infection control and identify potential risk factors associated with transmission.
Methods
Study design and participants
For this observational cohort study, we identified all neonates born between March 22 and May 17, 2020, at New York Presbyterian—Komansky Children's Hospital, Weill Cornell Medicine, New York Presbyterian—Lower Manhattan Hospital, and New York Presbyterian—Queens in New York City to mothers who tested positive for SARS-CoV-2 from a nasopharyngeal swab sample at the time of delivery. As a result of inadvertent exposure of health-care professionals to SARS-CoV-2 from asymptomatic women in labour, and concern that symptoms of labour can mimic those of COVID-19, universal screening of all pregnant women presenting in labour was implemented in our Labour and Delivery units on March 25, 2020.
Research in context.
Evidence before this study
Little is known about the possible consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnant women and fetuses. There is scant information regarding neonatal outcomes, and optimal management of the mothers and neonates is unknown. We searched PubMed and LitCovid for all manuscripts published in English from Feb 1 to May 20, 2020, with the key terms “newborns and COVID-19”, “perinatal outcome COVID-19”. We found a few small case series advising separation from mothers and formula feeding for at least 14 days for all neonates born to a mother testing positive for SARS-CoV-2 at time of delivery. However, these interventions are mainly expert opinions and there is no prospective and actual data showing that these procedures are effective and needed.
Added value of this study
To the best of our knowledge, this is the largest cohort of neonates born to mothers positive for SARS-CoV-2 at the time of delivery, with prospective follow-up up to 1 month of life. In our cohort, 68 (83%) of 82 neonates with complete follow-up data roomed in with the mothers and all were allowed to breastfeed. Prospective real-time PCR testing for SARS-CoV-2 was negative in all neonates tested at 1 week and 2 weeks of life. None of the neonates had symptoms of COVID-19 as of 1 month of age.
Implications of all the available evidence
Our findings support the published literature and confirm that perinatal transmission of COVID-19 is unlikely to occur if correct hygiene precautions are undertaken. In view of the benefits of early mother–neonate bonding and breastfeeding, rooming in with the mother and direct breastfeeding are safe and should be promoted, but these procedures need to be paired with effective parental education of infant protective strategies, such as use of surgical masks when near the neonate and frequent hand hygiene.
Neonates were referred to newly created neonatal COVID-19 outpatient clinics from the three hospitals from March 22, 2020.
This study was approved by the Weill Cornell Medicine institutional review board, protocol number 20-04021816. It was approved as exempt from consent as our testing and clinical follow-up was created as part of standard medical care for this population. Institutional Review Board approval was obtained to retrospectively collect the data obtained as part of clinical care.
Procedures
Testing for SARS-CoV-2 was done by use of real-time PCR (rtPCR; RealStar SARS-CoV-2 RT-PCR Kit, [Altona Diagnostics USA, Plain City, OH], cobas SARS-CoV-2 Test [Roche Molecular Systems, Branchburg, NJ], and Xpert Xpress SARS-CoV-2 [Cepheid, Sunnyvale, CA]). Turnaround time from specimen collection to result reporting was 24 h for the Altona and Roche tests and 1–2 h for Cepheid Xpert. Neonates were tested for SARS-CoV-2 by rtPCR on a nasopharyngeal swab sample at 12–24 h, 5–7 days, and 14 days of life and as indicated at subsequent visits. These timepoints were chosen to provide an opportunity for repeat testing and routine neonatal care. The 5–7-day timepoint was used to allow for routine neonatal follow-up, because many paediatricians' offices were not seeing patients in person; thus, a visit to our clinic at that time allowed for repeat testing along with routine neonate evaluation. The 14-day timepoint was decided on the basis of what was known about viral shedding and antibody responses at that time,15 and we purported that mothers were unlikely, if asymptomatic, to remain infectious at that time. Thus, if the neonate remained SARS-CoV-2 rtPCR-negative at 14 days, the likelihood of continued risk of transmission from the mother was unlikely.
The data used in this study were collected from inpatient medical records at time of birth and the outpatient medical record at the neonatal COVID-19 clinic visits on days 5–7 and 14 and by telemedicine visits at 1 month of life. Data collected included demographics, neonatal and maternal clinical presentation at time of delivery, during hospitalisation, and once discharged, microbiology results (SARS-CoV-2 rtPCR testing), and infection control practices in the hospital and at home. Neonates were assessed at all timepoints for fever, hypothermia, respiratory distress, lethargy, cough, rhinorrhoea, irritability, rash, diarrhoea, and feeding intolerance. Mothers were assessed at each outpatient clinic visit for self-reported symptoms of fever, cough, anosmia, ageusia, shortness of breath, sore throat, rhinorrhoea, myalgias, vomiting, and diarrhoea. Infection control practices included in the data collection were disposition of the neonate at delivery (allowed to room in with mother or admitted to a dedicated isolation room), feeding method (direct breastfeeding or expressed breast milk or formula), and self-reported frequency of hand hygiene, breast cleansing, and use of surgical masks at home.
Standard of care at all participating institutions is to initiate newborn skin-to-skin contact with mothers in the first hour of life if medically appropriate. This practice was not altered during the pandemic. Mothers who were positive for SARS-CoV-2 could practice skin-to-skin care and breastfeed in the delivery room with some modifications to usual processes. Among the precautions, mothers donned a surgical mask when near their neonate and practiced proper hand hygiene before skin-to-skin contact, breastfeeding, and routine care. All neonates who roomed in with their mothers were kept in a closed Giraffe isolette (General Electric Healthcare, Chicago, IL) and were held by mothers for feeding after appropriate hand hygiene, breast cleansing, and placement of a surgical mask. Only mothers were allowed to be present on the postpartum unit for the duration of the neonate's stay. All mothers were allowed to breastfeed their neonate while in the hospital and after being discharged home. Mothers of neonates admitted to the NICU were allowed to visit once 14 days had elapsed after they tested positive and if they were afebrile for at least 72 h.
Statistical analysis
We did a descriptive analysis with results presented as proportions for categorical variables and median and simple ranges for continuous variables. Stata 13 software was used for all analyses.
Role of the funding source
There was no funding source for this study.
Results
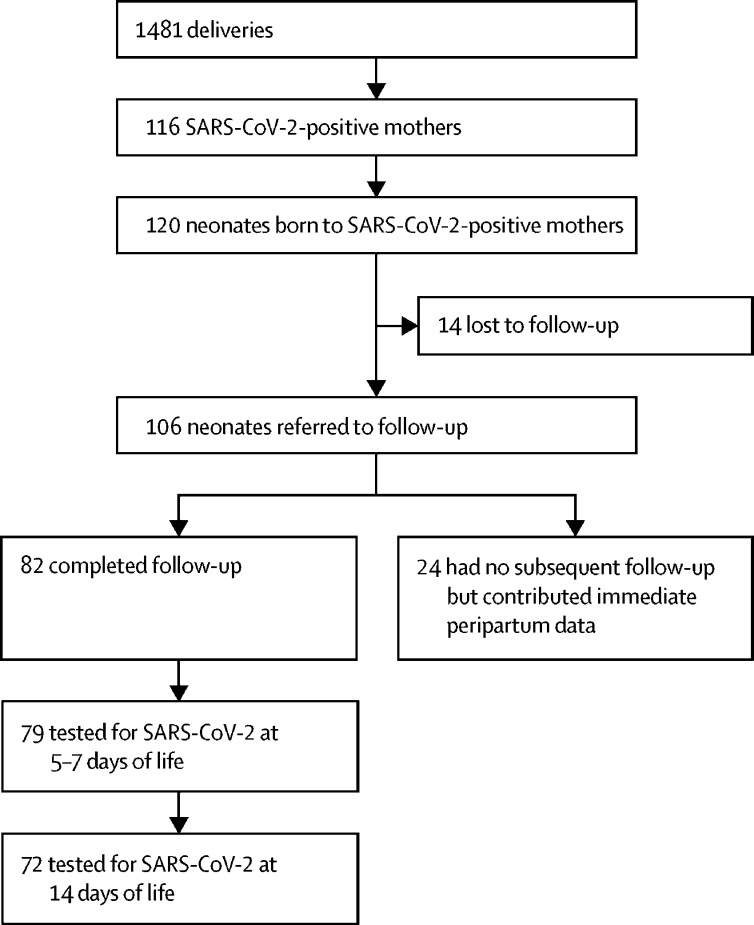
Between March 22 and May 17, 2020, there were 1481 deliveries, with 116 (8%) mothers testing positive for SARS-CoV-2. Of the 120 neonates identified, 106 (88%) were referred to our outpatient clinic (figure ). 14 (12%) of 120 neonates were lost to follow-up, because some providers were not aware of the follow up clinic and did not make referrals. Only the 82 (69%) neonates with completed follow-up data at day 5–7 of life were included in the final analysis of this study; however, the immediate peripartum data is provided for the 24 neonates who were referred but had no subsequent follow-up (table 1 ).
Figure.
Study profile
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Table 1.
Demographics and neonatal characteristics
| Neonates referred who completed follow-up (N=82) | Neonates referred with no follow-up (N=24) | |||
|---|---|---|---|---|
| Sex | ||||
| Male | 41 (50%) | 14 (58%) | ||
| Female | 41 (50%) | 10 (42%) | ||
| Race | ||||
| White | 30 (37%) | 12 (50%) | ||
| Black | 10 (12%) | 1 (4%) | ||
| Asian | 14 (17%) | 1 (4%) | ||
| Other or declined to answer | 28 (34%) | 10 (42%) | ||
| Ethnicity | ||||
| Hispanic, Latino, or Spanish | 23 (28%) | 6 (25%) | ||
| Not Hispanic, Latino, or Spanish | 32 (39%) | 14 (58%) | ||
| Other or declined to answer | 27 (33%) | 4 (17%) | ||
| Insurance | ||||
| Private | 45 (55%) | 11 (46%) | ||
| Public | 37 (45%) | 11 (46%) | ||
| NA | 0 | 2 (8%) | ||
| Born by | ||||
| Caesarean section | 36 (44%) | 7 (29%) | ||
| Vaginal delivery | 46 (56%) | 17 (71%) | ||
| Gestational age, weeks | 38 (27–41) | 39 (37–41) | ||
| Preterm (<37 weeks) | 14 (17%) | 0 | ||
| 34–36 weeks | 11 (13%) | .. | ||
| 32–33 weeks | 2 (2%) | .. | ||
| 28–31 weeks | 0 | .. | ||
| <28 weeks | 1 (1%) | .. | ||
| Term | 68 (83%) | .. | ||
| Birthweight, grams | 3110 (840–4115) | 3410 (2020–4250) | ||
| ≥2500 | 71 (87%) | 23 (96%) | ||
| 1500–2499 | 10 (12%) | 1 (4%) | ||
| 1000–1499 | 0 | 0 | ||
| <1000 | 1 (1%) | 0 | ||
| Admitted to | ||||
| Newborn nursery | 70 (85%) | 24 (100%) | ||
| Neonatal intensive care unit | 12 (15%) | 0 | ||
| Rooming In with mother | ||||
| Yes | 68 (83%)* | 21 (88%) | ||
| No | 14 (17%) | 3 (13%)† | ||
| Rupture of membrane | ||||
| <18 h | 67 (82%) | 20 (83%) | ||
| ≥18 h | 9 (11%) | 0 | ||
| NA | 6 (7%) | 4 (17%) | ||
| Breastfeeding | ||||
| At 5–7 days of life | ||||
| Yes | 64 (78%) | NA | ||
| No | 18 (22%) | NA | ||
| At 1 month of life‡ | ||||
| Yes | 45/53 (85%) | NA | ||
| No | 8/53 (15%) | NA | ||
Data are n (%), n/N (%), or median (range). NA=not available.
Three were separated from their mothers after 24 h per clinical indications; phototherapy in one, prolonged QT syndrome in one, and significant feeding intolerance and possible short bowel syndrome in one.
Reasons for isolation from mother were transient tachypnoea of neonate in two and antibiotic administration for 48 h owing to maternal fever in one.
Proportions calculated from the 53 neonates who reached 1 month of age as of May 17, 2020 (follow-up ongoing).
Of the 82 neonates, 41 (50%) were female, 36 (44%) were born by caesarean section, 68 (83%) were born at term, 14 (17%) were preterm, and the median gestational age was 38 weeks (range 27–41). The most common indications for caesarean sections were arrest of labour (12 [33%] of 36) and non-reassuring fetal tracing (6 [17%] of 36). Mode of delivery was not affected by SARS-CoV-2 test results. 12 (15%) neonates were admitted to the neonatal intensive care unit (NICU) and 70 (85%) received routine neonatal care.
20 (26%) of 78 mothers reported never being symptomatic, and 58 (74%) were symptomatic; 27 (46%) of these 58 mothers had symptoms onset more than 2 weeks before delivery and were asymptomatic at delivery, and 31 (54%) had symptoms onset within 2 weeks before delivery or during labour (table 2 ). Of the 22 mothers who reported symptoms within 7 days of delivery, 18 (82%) had cough, nine of whom also reported a fever. Mothers with symptom onset more than 2 weeks before delivery were more prevalent during the last weeks of the study period.
Table 2.
Maternal and household characteristics
| All mothers (N=78) | ||
|---|---|---|
| Maternal symptoms | ||
| Yes | 58 (74%) | |
| No | 20 (26%) | |
| Type of maternal symptoms* | ||
| Cough | 29/58 (50%) | |
| Anosmia or ageusia | 27/58 (47%) | |
| Fever | 24/58 (41%) | |
| Rhinorrhea | 11/58 (19%) | |
| Myalgia | 11/58 (19%) | |
| Shortness of breath or respiratory distress | 8/58 (14%) | |
| Headaches | 7/58 (12%) | |
| Gastrointestinal | 5/58 (9%) | |
| Other | 9/58 (15%) | |
| Onset of maternal symptoms | ||
| 0–7 days before labour | 22/58 (38%) | |
| 8–14 days before labour | 9/58 (16%) | |
| ≥15 days before labour | 27/58 (46%) | |
| Symptomatic household member† | ||
| Yes | 44/73 (60%) | |
| Father | 27/73 (61%) | |
| Other adult | 13/73 (30%) | |
| Sibling | 8/73 (18%) | |
| No | 22/73 (54%) | |
| Use of mask†‡ | ||
| Always | 62/73 (85%) | |
| Frequently or sometimes | 6/73 (8%) | |
| Never | 3/73 (4%) | |
| Use of hand hygiene†‡ | ||
| Always | 70/73 (96%) | |
| Frequently or sometimes | 1/73 (1%) | |
| Never | 0/73 | |
Data are n (%) or n/N (%).
In multiple cases more than one symptom was reported by 58 mothers.
Proportions calculated from the 73 neonates discharged home by day 5–7 of life; data not available for two families; in multiple cases more than one household member was symptomatic.
Self-reported data.
Of the 82 neonates, 68 (83%) roomed in with the mothers (table 1). Of these 68 neonates who roomed in, three (4%) were separated from their mothers after 24 h per clinical indications; one required 3 days of phototherapy, one remained under brief observation in the NICU for prolonged QT syndrome, and one had clinically significant feeding intolerance and short bowel syndrome requiring prolonged NICU admission. 14 (17%) neonates were separated from their mothers immediately after birth; four were kept in a dedicated isolation room in the newborn nursery owing to maternal medical condition or maternal preference, and ten were directly admitted to the NICU. Of the 12 neonates admitted to the NICU, five had a length of stay of 2–4 days and reasons for admission were prolonged QT syndrome, mild respiratory distress, and tachycardia. Median length of hospital stay for all 82 neonates was 2 days (range 1–21).
By 5–7 days of life, 73 (89%) of 82 neonates were discharged home to parents, and the remaining nine (11%) remained hospitalised. 44 (60%) of these 73 neonates were discharged home to an environment where a household member, other than mother, had reported symptoms consistent with COVID-19. In most cases, the symptomatic household member was an adult, and in multiple cases, more than one household member was ill (table 2).
Self-reported use of masks and hand hygiene practices were done always by 62 (85%) of 73 parents, frequently or sometimes by six (8%), and never by three (4%), despite recommendations given at time of hospital discharge; data were not available for two families (table 2).
At 5–7 days of life, 18 (22%) of 82 neonates were exclusively formula fed, whereas the remaining 64 (78%) were receiving breastmilk, through direct latching or bottle administration, with or without addition of formula (table 1).
rtPCR results from a nasopharyngeal swab obtained at birth were available for all 120 neonates initially identified. 119 (99%) neonates had a negative rtPCR and one result was reported as invalid (table 3 ). 79 (96%) of the 82 neonates included in the final analysis had a repeat rtPCR at 5–7 days of life and all were negative, including the infant whose initial rtPCR result was reported as invalid. 72 (88%) neonates had repeat rtPCR testing at day 14 of life, of whom 70 (97%) had a negative test result. Two (3%) results were reported as invalid and testing was not repeated, because both neonates were clinically well.
Table 3.
Serial rtPCR testing results
| 24 h of life (N=120) | 5–7 days of life (N=82) | 14 days of life (N=82) | |
|---|---|---|---|
| rtPCR done | |||
| Yes | 120 (100%) | 79 (96%) | 72 (88%) |
| No | 0 | 3 (4%) | 10 (12%) |
| Result | |||
| Positive | 0 | 0 | 0 |
| Negative | 119/120 (99%) | 79/79 (100%) | 70/72 (97%) |
| Invalid* | 1/120 (<1%) | 0 | 2/72 (3%) |
Data are n (%) or n/N (%). rtPCR=real-time PCR.
No reaction to any of the targets, including the internal control.
Three (4%) of 82 neonates had a telemedicine visit at days 7 and 14 of life and were asymptomatic during the entire observation period, but they were not retested.
Aside from seven (8%) preterm neonates admitted to NICU for standard care for prematurity, all other neonates were asymptomatic at birth, except four who had transient tachycardia or transient tachypnoea. Of the 82 neonates who had a follow up visit at 5–7 days of life, 81 (99%) were asymptomatic; one (1%) was readmitted because of hypothermia, for whom repeat rtPCR upon admission was negative. The 75 (91%) neonates who were followed up at 14 days of life continued to remain asymptomatic and were growing appropriately.
As of May 17, 2020, 53 (65%) of 82 neonates have been followed up by telemedicine at 1 month of life; five (6%) missed the scheduled appointment and all the remaining neonates were not yet 1 month of age (follow-up is ongoing). 77 (94%) of 82 infant's parents were symptom-free at the day 14 of life visit and were instructed to discontinue mask precautions at home when around the neonate. Five (6%) of 82 infant's parents were still symptomatic and were instructed to continue wearing a mask for an extra week.
At the telemedicine visit at 1 month of life, three sets of parents were still using masks when next to the neonate despite our recommendation, mainly because of parental fear of still being contagious.
Of the 53 neonates, 45 (85%) were still breastfeeding and eight (15%) were exclusively formula fed.
Parents reported new-onset congestion without fever for three neonates. These three infants were retested for SARS-CoV-2 by nasopharyngeal PCR, all of which were negative. One neonate had 1 day of fever and was evaluated in the emergency department, where blood and urine cultures and repeat SARS-CoV-2 PCR were negative; he was discharged home without admission. All neonates continued to be clinically well and are growing appropriately.
Discussion
To our knowledge, this is the largest US cohort of neonates born to mothers who tested positive for SARS-CoV-2 at time of delivery and who were subsequently followed with serial testing and clinically up to 1 month of life. In our case series, no infant had SARS-CoV-2 virus detected by a nasopharyngeal swab in the immediate postnatal period (24 h), nor at 5–7 or 14 days of life. Additionally, all infants remained asymptomatic during the study period. This finding supports the previous reports of a low risk of perinatal transmission with strict infection control practices.
To date, there are several case reports of neonates who have tested positive for SARS-CoV-2 within 48 h. Zeng and colleagues16 reported on three of 33 neonates, born to SARS-CoV-2-positive mothers, with positive nasopharyngeal and anal swabs on days 2 and 4 of life. Two individual case reports suggestive of probable congenital infection have been published. Zamaniyan and colleagues5 described a preterm neonate born to a mother with severe COVID-19; the neonate tested positive by nasopharyngeal swab at 48 h and amniotic fluid was also positive for SARS-CoV-2 by rtPCR. Kirtsman and colleagues6 reported a term neonate born to a mother with 1 day of fever and cough at time of delivery. The mother's placental tissue was rtPCR-positive for SARS-CoV-2 on both the parenchymal and chorionic sides. The infant tested positive for SARS-CoV-2 at birth by nasopharyngeal swab. In a study from the UK,17 six neonates were positive within 12 h after birth; however, whether this represented a false positive from maternal contamination has not been confirmed, because testing was obtained very soon after birth and was not repeated at a later time to confirm. In two reports from China,18, 19 three neonates born to mothers with COVID-19 pneumonia were positive for IgG and IgM; however, presence of IgM alone is not a reliable marker for vertical transmission and its significance is unclear.20 Several other case series have been published to date; however, none of these case series followed neonates with serial rtPCR testing past the first week of life.7, 21, 22, 23, 24, 25, 26 The overall risk of COVID-19 infection due to in-utero or perinatal exposure for infants remains to be determined.
Because of space limitations on the postpartum units, isolation of neonate from mothers was not always possible nor was it desirable to most mothers when the risks and benefits of isolation were discussed. In our cohort, all neonates were allowed to room in with mothers (neonate was kept in a closed isolette, 6 feet [1·83 m] apart from their mother unless feeding), unless not permitted by the mother's or neonate's condition. Moreover, all mothers were given the option to directly breastfeed following appropriate infection control precautions (ie, frequent hand hygiene, use of surgical masks at all times, and breast cleansing). These practices are controversial with regard to COVID-19 transmission in the published literature with divergent views.
Our findings suggest that alternative safe methods exist for caring for neonates in the immediate postnatal period besides the recommendations of the American Academy of Pediatrics,11 the US Centers for Disease Control and Prevention,14 and the Chinese expert consensus.12 As preferred management to decrease the risk of perinatal transmission, they advise isolation of the neonates immediately after delivery, formula or expressed breast milk feeding, and no contact, if possible, with the mother for 14 days or at least 7 days from symptoms onset. This recommendation contrasts with the WHO,13 UK Royal College of Obstetricians & Gynecologists,9 and the Italian Society of Neonatology recommendations on management of neonates born to SARS-CoV-2-positive mothers, endorsed by the Union of European Neonatal and Perinatal Societies,8 which advocate for the promotion of breastfeeding and the initial mother–infant relationship after childbirth. Some factors favour our approach. The well known benefits of early mother–neonate bonding and breastfeeding should be prioritised during the perinatal period if the risks are deemed low. Additionally, most neonates were discharged within 24–48 h to home, where in most cases the mother was the primary provider and continued breastfeeding. We have shown that rooming in with the mother and breastfeeding are safe if associated with adequate parental education of safe infection control practices, such as use of surgical masks at all times and frequent hand hygiene.
Findings from our cohort support the published literature, which consists mainly of small case series that suggest that perinatal transmission of SARS-CoV-2 to neonates from infected mothers or family members are rare events provided close attention to infection precautions are maintained. Such precautions are crucial, because SARS-CoV-2 is an easily transmittable virus. However, our cohort differs from the other reports by confirming through serial rtPCR testing and clinical follow-up that none of the neonates developed asymptomatic infection. Given that 25–50% of SARS-CoV-2-positive mothers are asymptomatic, neonates might also show a similar prevalence of asymptomatic infection. There are case reports of neonates who became clinically symptomatic after hospital discharge, such as a report from Coronado Munoz and colleagues27 of a neonate who presented with respiratory symptoms at 5 days of life and who eventually tested positive for SARS-CoV-2. Contact source analysis showed that family members had overt clinical COVID-19 signs.27 This finding further supports the need for scrupulous precautions to prevent horizontal spread of infection. There is evidence that asymptomatic carriers are common, as reported by Wang and colleagues28 and confirmed in our cohort of mothers, with 26% of cases being entirely asymptomatic. Because about half of the mothers were symptomatic shortly before or during delivery, acquisition of protective maternal antibodies in all infants is unlikely. Moreover, our data show no difference in neonatal outcome on the basis of whether mothers were symptomatic or not. Whether infants in our cohort were less likely to acquire infection due to inherent physiological differences or by early infection control measures remains to be clarified.
Our study has several limitations. First, the study is limited by the sample size and a follow-up period of 1 month. A larger cohort and longer follow-up with repeat testing and serology might be needed to confirm that perinatal transmission is unlikely to occur if correct protective strategies are used. Second, a large proportion of neonates was lost to follow-up, which is, in part, justifiable owing to parents' fear of leaving their homes and using public transportation in the middle of a pandemic to attend the clinic for follow-up appointments. Third, we were unable to screen for presence of the virus in blood, urine, or stool due to absence of approved testing for these samples during the study period. Thus, it remains possible that the virus might be detectable only in the blood and urine rather than in the respiratory tract in congenitally infected neonates. However, neonates positive for SARS-CoV-2 at birth reported in the published literature so far had a positive nasopharyngeal swab. Finally, we relied solely on parental report regarding hand hygiene and mask usage at home, so there is the potential for recall bias.
In conclusion, our data suggest that perinatal transmission is unlikely to occur if correct hygiene precautions are undertaken and that rooming in and breastfeeding are safe procedures when paired with effective parental education of infant protective strategies.
Data sharing
The de-identified dataset supporting the conclusions of this Article can be made available from the corresponding author upon reasonable request.
Acknowledgments
Acknowledgments
We thank Jeffrey Perlman (Weill Cornell Medicine, New York Presbyterian—Komansky Children's Hospital) who provided helpful suggestions and comments during the revision of the manuscript.
Contributors
CMS and PD, examined the infants and contributed to the design, data organisation, analysis, discussion, main interpretation of results, and writing of the article. J-YH, KPA, and JD examined the children and contributed to the discussion of results and writing of the article. PT, JJ, MB, CC, LG, and AP contributed with epidemiological information and infant referrals and to writing of the article.
Declaration of interests
We declare no competing interests.
References
- 1.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Guo J, Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. 2020;395:760–762. doi: 10.1016/S0140-6736(20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favre G, Pomar L, Musso D, Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. 2020;395:E40. doi: 10.1016/S0140-6736(20)30311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamaniyan M, Ebadi A, Aghajanpoor Mir S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat Diagn. 2020 doi: 10.1002/pd.5713. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirtsman M, Diambomba Y, Poutanen SM. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020 doi: 10.1503/cmaj.200821. publised online May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Wang L, Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davanzo R, Moro G, Sandri F, Agosti M, Moretti C, Mosca F. Breastfeeding and coronavirus disease-2019: ad interim indications of the Italian Society of Neonatology endorsed by the Union of European Neonatal & Perinatal Societies. Matern Child Nutr. 2020;16:e13010. doi: 10.1111/mcn.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royal College of Obstetricians & Gynaecologists National guidance on managing coronavirus infection in pregnancy published. Royal College of Obstetricians & Gynaecologists. March 8, 2020. https://www.rcog.org.uk/en/news/national-guidance-on-managing-coronavirus-infection-in-pregnancy-published/
- 10.Li F, Feng ZC, Shi Y. Proposal for prevention and control of the 2019 novel coronavirus disease in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2020 doi: 10.1136/archdischild-2020-318996. published online March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics FAQs: Management of infants born to mothers with COVID-19. American Academy of Pediatrics, 2020. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/
- 12.Wang L, Shi Y, Xiao T. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition) Ann Transl Med. 2020;8:47. doi: 10.21037/atm.2020.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Clinical management of COVID-19. Interim guidance. World Health Organization. May 27, 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 14.CDC Evaluation and management considerations for neonates at risk for COVID-19. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-newborns.html
- 15.To KK, Tsang OT, Leung WS. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng L, Xia S, Yuan W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. published online March 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight M, Bunch K, Vousden N. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong L, Tian J, He S. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng H, Xu C, Fan J. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimberlin DW, Stagno S. Can SARS-CoV-2 infection be acquired in utero? More definitive evidence is needed. JAMA. 2020;323:1788–1789. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Peng H, Wang L. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Li L, Wu X. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215:127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1759541. published online April 2. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0901-SA. published online March 17. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020 doi: 10.1007/s11684-020-0772-y. published online April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J, Guo J, Fan C. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.04.014. P111.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coronado Munoz A. Nawaratne U, McMann D, Ellsworth M, Meliones J, Boukas K. Late-onset neonatal sepsis in a patient with Covid-19. N Engl J Med. 2020;382:e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Liu Y, Liu L, Wang X, Luo N, Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-Coronavirus-2 in Shenzhen, China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The de-identified dataset supporting the conclusions of this Article can be made available from the corresponding author upon reasonable request.