Editor—Coronavirus disease 2019 (COVID-19) is a novel viral respiratory disease that was declared a global pandemic by the WHO on March 11, 2020. The pathophysiology of the disease remains under investigation; however, a new perspective has emerged that neutrophils may play a central role in the organ damage and mortality associated with COVID-19.1
The most abundant leucocyte in peripheral blood, neutrophils play a crucial role in immune response to infection by killing pathogens (bacteria, fungi, viruses) by means of phagocytosis and oxidative burst. A third mechanism by which neutrophils kill invading organisms was discovered in 2004: formation of neutrophil extracellular traps (NETs).2 NETs are web-like structures of DNA studded with proteins that are extruded from the nucleus of neutrophils and function to trap and kill circulating pathogens. Like much of the immune response, netosis (the process of forming NETs) functions well as long as it is closely regulated. When dysregulation of netosis occurs, collateral damage ensues. Excessive production of NETS has been associated with disease progression in a range of pathological conditions including pre-eclampsia, lupus erythematosis, myocardial infarction, and sepsis.3, 4, 5, 6 Interestingly, the organ systems most commonly damaged by NETS, the pulmonary, cardiovascular, and renal systems, are the same organ systems that are most affected in severe COVID-19.1
NETs and COVID-19
Elevated levels of citrullinated histone H3 (Cit-H3) have been observed in hospitalised patients with COVID-19.7 Cit H3 is a specific biomarker of the presence of NETs. Whether the presence of markers of netosis bears clinical relevance is unknown as no longitudinal cohort studies have been published. However, in numerous disease models elevated Cit H3 is associated with poor outcomes.8, 9, 10 Interestingly, serum from COVID-19 patients triggered NET release from control neutrophils in vitro, suggesting COVID-19 creates a cellular environment in which netosis is more likely to occur.
NETs and acute respiratory distress syndrome
A subgroup of COVID-19 patients develops an acute respiratory distress syndrome (ARDS)-like state that frequently requires ICU-level support. Although there is some disagreement as to whether these patients fit the Berlin definition of ARDS, such discussions are to some degree academic.11 What is clear is that COVID-19 can cause a severe viral pneumonia associated with profound hypoxaemia and need for mechanical ventilation. NETs have been shown to contribute to disease progression in pulmonary infections,12, 13, 14 and animal models suggest that therapies that reduce formation of or lyse NETs reduce lung injury and mortality.15 , 16 Levels of NETs in the plasma and broncho-alveolar lavage fluid correlate with disease severity in patients with pneumonia-induced ARDS.17
NETs and thrombosis
A hypercoagulable state has been described in COVID-19 patients resulting in a high incidence of venous thromboembolic phenomena that contribute to the disease burden.18 NETs activate the contact pathway of the coagulation system while at the same time neutrophil elastase (a component of NETs) degrades natural antithrombotic agents such as antithrombin III and tissue factor pathway inhibitor.19 NETs are a prognostic indicator of venous thromboembolism in cancer patients and partly explain the hypercoagulable state associated with cancer.20 An animal model has shown that aberrant production of NETs causes microvascular thrombi particularly in the lungs.21
NETs and the COVID-19 cytokine storm
A proportion of COVID-19 patients develop a dysregulated release of pro-inflammatory cytokines that is termed a cytokine storm. Onset of this disease state in COVID-19 patients is associated with high mortality, and suppression of these overactivated cytokines is a therapeutic target of current interest. NETs have been shown to induce macrophages to secrete interleukin-1 (IL-1), which in turn induces IL-6.22 , 23 Both these ILs are seen as key players of the cytokine response, and antagonists to these cytokines (tocilizumab and anakinira) are currently being investigated in COVID-19 patients. Decreasing NET formation may help to dampen the upstream signal stimulating the release of these cytokines.
NETs as a therapeutic target
A recombinant DNAase (Dornase Alfa) is currently licensed for use in cystic fibrosis patients where it functions to dissolve NETs present in sputum and hence reduce the associated viscosity.24 Its use has been suggested in COVID-19 patients who may also have thick gelatinous airway secretions.1 Colchicine is currently being trialled in COVID-19 patients with a hypothesis that it may reduce neutrophil recruitment and hence NET formation (ClinicalTrials.gov identifiers: NCT04326790, NCT04328480, NCT04322565, NCT04322682).
Lidocaine as a potential therapy
The local anaesthetic drug lidocaine has been shown to reduce markers of netosis.25 This prospective RCT looked at the influence of anaesthetic interventions on netosis expression in patients undergoing breast cancer surgery. Subjects who received a lidocaine infusion, commencing at induction and continuing for 24 h postoperatively, had lower levels of Cit-H3 detected in plasma compared with well-matched control subjects who did not receive lidocaine. This is the first trial to show that lidocaine can positively influence the development of NETs in patients undergoing surgery. A mechanism for how lidocaine could suppress the formation of NETs has not been described but could be partly explained by its known anti-inflammatory properties.26
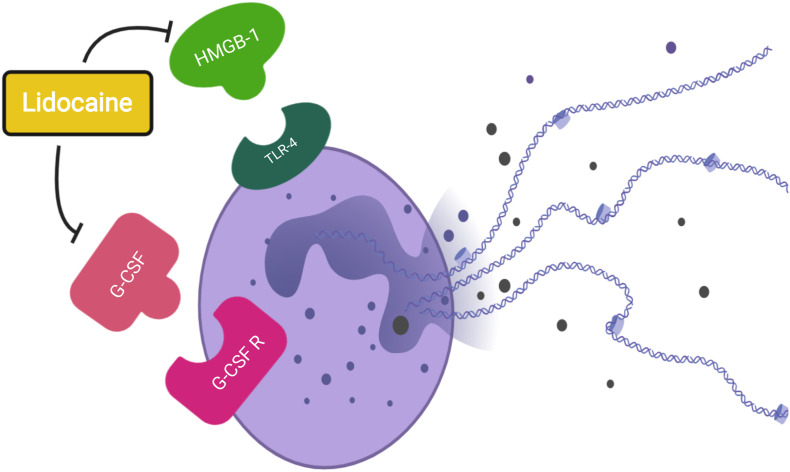
The evidence that lidocaine can suppress development of netosis in perioperative patients raises the possibility of repurposing it for use in COVID-19 patients. As evidence mounts that NETs play an important role in the pathological process of COVID-19, an agent that suppresses this could bring potential therapeutic benefits. Although lidocaine would not have a direct effect on the SARS CoV-2 virus, it may help to temper the immunological storm that is triggered in patients with severe disease (Fig. 1 ).
Fig 1.
Proposed mechanism of lidocaine suppression of netosis. Purple cell represents a neutrophil undergoing netosis. G-CSF, granulocyte colony stimulating factor; G-CSF R, granulocyte colony stimulating factor receptor; HMGB-1, high mobility group box-1; TLR-4, Toll-like receptor-4.
Lidocaine infusions have a strong record of safety in clinical medicine. They are frequently used in chronic pain conditions and in gastrointestinal surgery where they have been shown to reduce postoperative opioid requirements and enhance bowel recovery.27 , 28 We hypothesise that lidocaine infusion in COVID-19 patients may decrease the formation of NETs and modulate the severity of disease.
Apart from its primary role as a local anaesthetic agent, lidocaine exhibits cytoprotective properties. Its ability to delay the onset of ischaemia-related potassium efflux may explain its benefit in animal models of brain injury.29 In addition, lidocaine has been shown to exhibit a number of anti-inflammatory properties. The ability of lidocaine to inhibit high mobility group box-1 (HMGB-1),30 and granulocyte colony stimulating factor (G-CSF) merits further research as both HMGB-1 via Toll-like receptor 4 (TLR-4)31 and G-CSF32 are key mediators in the initiation of netosis.33
Triggering of netosis is a complex process that can occur through a variety of mechanisms. The generation of reactive oxygen species (ROS) is a well-described pathway with some evidence suggesting that commonly used anaesthetic drugs such as propofol may suppress ROS and subsequent NET formation in healthy volunteers.34 However, netosis can also occur through ROS-independent pathways such as the HMGB-1 and G-CSF pathways. The primary pathway of netosis in COVID-19 patients is not currently known.
Important limitations should be highlighted with this proposal. The current evidence for lidocaine in suppressing netosis is in the perioperative setting. Here it is the surgical stress response that triggers formation of NETs. The mechanisms by which netosis occurs are not fully understood and possibly differ between patients undergoing surgery and those with viral pneumonia.
The optimum timing and duration of administration of lidocaine with a view to suppressing netosis is unknown. Furthermore, it is not known if this results in longer-term clinical benefits once the infusion is stopped. The work by Galoș and colleagues25 showed that lidocaine can suppress a biomarker of netosis at 24 h after surgery, but whether this translates into meaningful clinical benefits for patients was not answered. However, we do know that higher levels of NETs in the postoperative setting correlate with disease progression in cancer surgery and a higher incidence of venous thromboembolism.35 , 36
Declarations of interest
The authors declare that they have no conflicts of interest.
References
- 1.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkmann V., Reichard U., Goosmann C., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 3.Anurag K.G., Hasler P., Holzgreve W., Hahn S. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol. 2007;29:163–167. doi: 10.1007/s00281-007-0073-4. [DOI] [PubMed] [Google Scholar]
- 4.Knight J.S., Kaplan M.J. Lupus neutrophils: ‘NET’gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24:441–450. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- 5.Vajen T., Koenen R.R., Werner I., et al. Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-29026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien X.M., Biron B.M., Reichner J.S. Consequences of extracellular trap formation in sepsis. Curr Opin Hematol. 2017;24:66. doi: 10.1097/MOH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo Y., Yalavarthi S., Shi H., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tohme S., Yazdani H.O., Al-Khafaji A.B., et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Liu B., Fukudome E.Y., et al. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery. 2011;150:442–451. doi: 10.1016/j.surg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahimi F., Giaglis S., Hahn S., et al. Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: secondary analysis of a randomised controlled trial. Eur Respir J. 2018;51:1701389. doi: 10.1183/13993003.01389-2017. [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L., Coppola S., Cressoni M., et al. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendib I., de Chaisemartin L., Granger V., et al. Neutrophil extracellular traps are elevated in patients with pneumonia-related acute respiratory distress syndrome. Anesthesiology. 2019;130:581–591. doi: 10.1097/ALN.0000000000002619. [DOI] [PubMed] [Google Scholar]
- 13.Mikacenic C., Moore R., Dmyterko V., et al. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit Care. 2018;22:358. doi: 10.1186/s13054-018-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefrançais E., Mallavia B., Zhuo H., et al. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narasaraju T., Yang E., Samy R.P., et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S., Su X., Pan P., et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep. 2016;6:37252. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv X., Wen T., Song J., et al. Extracellular histones are clinically relevant mediators in the pathogenesis of acute respiratory distress syndrome. Respir Res. 2017;18:165. doi: 10.1186/s12931-017-0651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klok F., Kruip M., Van der Meer N., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs T.A., Brill A., Wagner D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demers M., Krause D.S., Schatzberg D., et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cedervall J., Zhang Y., Huang H., et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015;75:2653–2662. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 22.Warnatsch A., Ioannou M., Wang Q., et al. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarello C.A. Targeting the pathogenic role of interleukin 1β in the progression of smoldering/indolent myeloma to active disease. Mayo Clinic Proc. 2009;84:105–107. doi: 10.4065/84.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papayannopoulos V., Staab D., Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galoș E.V., Tat T.-F., Popa R., et al. Neutrophil extracellular trapping and angiogenesis biomarkers after intravenous or inhalation anaesthesia with or without intravenous lidocaine for breast cancer surgery: a prospective, randomised trial. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Hollmann M.W., Durieux M.E. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93:858–875. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- 27.Bailey M., Corcoran T., Schug S., et al. Perioperative lidocaine infusions for the prevention of chronic postsurgical pain: a systematic review and meta-analysis of efficacy and safety. Pain. 2018;159:1696–1704. doi: 10.1097/j.pain.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson U., Scott M., Hubner M., et al. Guidelines for perioperative care in elective colorectal surgery: enhanced Recovery after Surgery (ERAS®) society recommendations: 2018. World J Surg. 2019;43:659–695. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y., Wang D., Du M., et al. Lidocaine prolongs the safe duration of circulatory arrest during deep hypothermia in dogs. Can J Anaesth. 1998;45:692–698. doi: 10.1007/BF03012102. [DOI] [PubMed] [Google Scholar]
- 30.Wang H.-L., Liu Y.-Y., Yan H.-D., et al. Intraoperative systemic lidocaine inhibits the expression of HMGB1 in patients undergoing radical hysterectomy. Int J Clin Exp Med. 2014;7:3398. [PMC free article] [PubMed] [Google Scholar]
- 31.Tadie J.-M., Bae H.-B., Jiang S., et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol. 2013;304:L342–L349. doi: 10.1152/ajplung.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson M., Crowley P., Foley A., et al. Effect of perioperative lidocaine on metastasis after sevoflurane or ketamine-xylazine anaesthesia for breast tumour resection in a murine model. Br J Anaesth. 2018;121:76–85. doi: 10.1016/j.bja.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 33.Snoderly H.T., Boone B.A., Bennewitz M.F. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019;21:1–13. doi: 10.1186/s13058-019-1237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen M.-S., Lin W.-C., Yeh H.-T., et al. Propofol specifically reduces PMA-induced neutrophil extracellular trap formation through inhibition of p-ERK and HOCl. Life Sci. 2019;221:178–186. doi: 10.1016/j.lfs.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Erpenbeck L., Schön M. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2017;36:2483–2490. doi: 10.1038/onc.2016.406. [DOI] [PubMed] [Google Scholar]
- 36.Demers M., Wagner D.D. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Semin Thromb Hemost. 2014;40:277–283. doi: 10.1055/s-0034-1370765. [DOI] [PMC free article] [PubMed] [Google Scholar]