Highlights
-
•
Patients in the experimental group received intravenous ribavirin versus supportive care only in the control group.
-
•
Ribavirin was not associated with reduced negative conversion time of SARS-CoV-2 PCR test compared with the control group.
-
•
Ribavirin did not reduce the mortality rate compared with the control group.
Keywords: 2019 novel coronavirus, SARS-CoV-2, COVID-19, Respiratory infection, Treatment, Ribavirin
Abstract
The aim of this study was to compare ribavirin therapy versus supportive therapy only for patients with severe coronavirus disease 2019 (COVID-19). A total of 115 patients with laboratory-confirmed COVID-19 were retrospectively analysed. All patients received supportive care as well as regular laboratory and clinical monitoring. The 115 patients comprised 44 patients who received intravenous ribavirin (treatment group) and 71 who did not (control group). Baseline laboratory and clinical characteristics were similar between the two groups. The negative conversion time for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR in the ribavirin group was 12.8 ± 4.1 days compared with 14.1 ± 3.5 days in the control group (P = 0.314). Moreover, 7/41 patients (17.1%) in the ribavirin group died compared with 17/69 (24.6%) in the control group (P = 0.475). Adverse effects were similar between the two groups. In conclusion, in patients with severe COVID-19, ribavirin therapy is not associated with improved negative conversion time for SARS-CoV-2 test and is not associated with an improved mortality rate. Further assessment in designed randomised controlled trials is recommended.
1. Introduction
The 2019 novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease that is causes, named coronavirus disease 2019 (COVID-19), which was first discovered in Wuhan, China, in December 2019, is a serious concern worldwide. Patients with COVID-19 show respiratory diseases and severe pneumonia. Since the detection of the outbreak, the number of cases has been increasing worldwide; as of early April 2020, more than 9000 cases and more than 3000 deaths have been reported in China alone, and more than 950 000 cases in other countries. Treatment of patients with COVID-19 consists of supplemental oxygen, admit to an isolation ward, supportive care, antimicrobial therapy and respiratory support [1,2]. To date, no antiviral therapy has been approved for the treatment of COVID-19.
Several therapeutic interventions for coronavirus infection were studied during the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) outbreaks in 2003 and 2012, respectively [3,4]. Reviews of the literature suggest that ribavirin might be of benefit in patients with coronavirus infection [4]. However, other studies have shown that ribavirin may not improve outcomes [5,6]. In addition, ribavirin has potential adverse effects [7], therefore its clinical use should be carefully assessed.
The aim of this study was to evaluate and compare the efficacy of ribavirin in the treatment of patients with severe COVID-19 pneumonia.
2. Study population and methods
2.1. Setting
This single-centre, retrospective cohort study included patients diagnosed with laboratory-confirmed SARS-CoV-2 infection in Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) from January–February 2020.
2.2. Informed consent
This study was approved by the Ethics Commission of Union Hospital. Owing to the rapid emergence of this infectious disease, the need for written informed consent was waived.
2.3. PCR assay
SARS-CoV-2 infection was confirmed by positive reverse transcription PCR (RT-PCR) of respiratory tract samples. Two RT-PCR tests were used for SARS-CoV-2 diagnostic confirmation. Nucleocapsid protein (N) and open reading frame 1ab (ORF1ab) were amplified during RT-PCR [8].
2.4. Patients
Patients were excluded if they met the following exclusion criteria: (i) age <18 years or >80 years; and (ii) transferred to another hospital (after the first consultation, some patients with special medical insurance types were transferred to the corresponding hospitals for treatment). Patients were considered severe COVID-19 patients if they met any of the following condition: (i) oxygen saturation ≤93% at resting state; (ii) respiratory rate ≥30 breaths/min; and (iii) arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FiO2) ≤300 mmHg [9].
2.5. Procedure
The experimental group comprised patients who received intravenous ribavirin 500 mg every 12 h (Zhengzhou Cheuk-fung pharmaceutical Co., Ltd., Xinzheng City, Zhengzhou, China), whereas those who did not receive ribavirin therapy formed the control group. The control group received no other potential antiviral treatments. All patients received appropriate antimicrobial therapy and supportive care. In addition to routine clinical monitoring, liver enzymes, renal function, electrolytes and blood cell counts were assessed regularly throughout the course of treatment.
2.6. Outcomes
The outcomes for the study were (i) the negative conversion time for SARS-CoV-2 RT-PCR test and (ii) mortality rate in the two groups.
2.7. Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (S.D.) and categorical variables were expressed as number (%). The χ2 test and t-test were used to determine any statistical difference between the proportions and means of the two groups. Single-factor analysis was used for comparison of clinical and laboratory features in the different groups. Univariate and multiple logistic regression models were used to evaluate the associations between patients receiving ribavirin or not and the risk of death. All analyses were performed with EmpowerStats (http://www.empowerstats.com; X&Y Solutions, Inc., Boston, MA, USA) and the statistical software package R (http://www.R-project.org; The R Foundation). A P-value of <0.05 (two-sided) was considered statistically significant.
3. Results
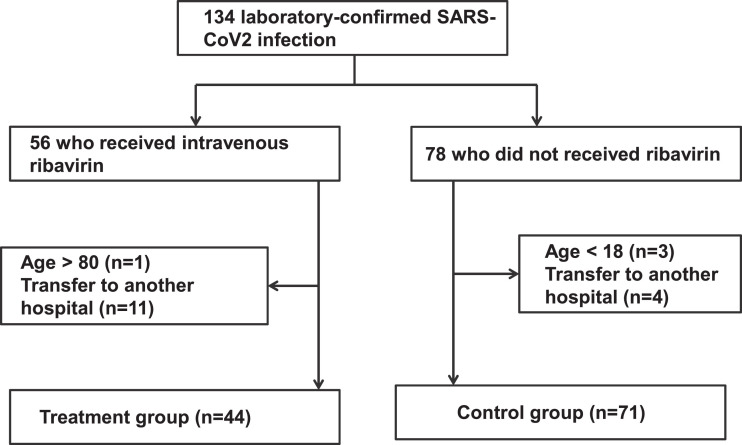
A total of 134 individuals were diagnosed with severe COVID-19 between January–February 2020. Baseline characteristics were similar between patients who received ribavirin and those who did not. After excluding ineligible patients, 115 patients were included in the current study, comprising 71 in the control group and 44 in the treatment group (Fig. 1 ). The mean ± S.D. age of all 115 patients was 54.9 ± 15.1 years and 62 (53.9%) were male (Table 1 ). Mean ± S.D. laboratory values on the day of SARS-CoV-2 diagnosis were haemoglobin 125.5 ± 18.2 g/L, peripheral white blood cell count 9.55 × 109/L ± 6.03 × 109/L, absolute neutrophil count 5.7 × 109/L ± 3.5 × 109/L, lymphocyte count 0.9 × 109/L ± 0.5 × 109/L, platelet count 194.1 × 109/L ± 83.8 × 109/L, alanine aminotransferase (ALT) 38.3 ± 32.6 U/L, aspartate aminotransferase (AST) 40.1 ± 20.3 IU/L, blood urea nitrogen (BUN) 4.8 ± 3 mmol/L and serum creatinine 73.1 ± 25.1 μmol/L. Of the 115 patients overall, 28 (24.3%) required non-invasive ventilation support and 9 patients (7.8%) required invasive ventilation support. All patients received broad-spectrum antibiotic therapy. In the treatment group, ribavirin was initiated within a median of 4 days (range 1–12 days) of the SARS-CoV-2 diagnosis and within a median of 8 days (range 1–18 days) from symptom onset. There were no statistically significant differences in clinical characteristics (Table 1) or support measures (Table 2 ) between the two groups. The negative conversion time for SARS-CoV-2 test in patients who received ribavirin was 12.8 ± 4.1 days compared with 14.1 ± 3.5 days in the control group (P = 0.314). The overall mortality rate was 20.9% (24/115). The mortality rate was 17.1% (7/41) in the ribavirin group and 24.6% (17/69) in the control group; there was no significant difference in mortality between the two groups (P = 0.475).
Fig. 1.
Flow diagram for study inclusion. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline characteristics on the day of diagnosis of SARS-CoV-2 infection and outcome of severe COVID-19 patients a
| Characteristic | Control group (n = 71) | Ribavirin group (n = 44) | P-value |
|---|---|---|---|
| Age (years) | 55.1 ± 16.2 | 54.6 ± 13.3 | 0.862 |
| Sex | 0.069 | ||
| Male | 43 (60.6) | 19 (43.2) | |
| Female | 28 (39.4) | 25 (56.8) | |
| Temperature (°C) | 38.7 ± 0.6 | 38.8 ± 0.6 | 0.302 |
| Cough | 0.583 | ||
| No | 44 (62.0) | 25 (56.8) | |
| Yes | 27 (38.0) | 19 (43.2) | |
| Pharyngalgia | 0.935 | ||
| No | 68 (95.8) | 42 (95.5) | |
| Yes | 3 (4.2) | 2 (4.5) | |
| Dyspnoea b | 0.961 | ||
| No | 60 (85.7) | 37 (86.0) | |
| Yes | 10 (14.3) | 6 (14.0) | |
| Fever b | 0.231 | ||
| No | 2 (3.3) | 0 (0.0) | |
| Yes | 59 (96.7) | 43 (100.0) | |
| Lymphocyte count (× 109/L) | 0.9 ± 0.5 | 1.0 ± 0.6 | 0.592 |
| Leukocyte count (× 109/L) | 4.9 ± 3.8 | 3.8 ± 2.6 | 0.113 |
| Haemoglobin (g/L) | 127.6 ± 18.7 | 122.0 ± 17.2 | 0.119 |
| Platelet count (× 109/L) | 197.3 ± 88.8 | 188.9 ± 75.9 | 0.611 |
| Prothrombin time (s) | 13.5 ± 1.3 | 14.0 ± 2.1 | 0.140 |
| aPTT (s) | 41.1 ± 11.5 | 39.8 ± 6.5 | 0.503 |
| Procalcitonin (μg/L) | 0.2 ± 0.3 | 0.3 ± 0.5 | 0.324 |
| C-reactive protein (mg/L) | 67.4 ± 49.1 | 65.4 ± 61.6 | 0.853 |
| ESR (mm/h) | 47.8 ± 32.7 | 48.3 ± 25.6 | 0.934 |
| Serum creatinine (μmol/L) | 75.5 ± 27.9 | 69.3 ± 19.4 | 0.213 |
| BUN (mmol/L) | 5.2 ± 3.1 | 4.3 ± 2.8 | 0.132 |
| Glomerular filtration rate () | 93.1 ± 25.9 | 94.6 ± 18.1 | 0.733 |
| ALT (U/L) | 41.6 ± 38.5 | 32.6 ± 18.3 | 0.159 |
| AST (U/L) | 41.7 ± 21.1 | 37.4 ± 18.9 | 0.289 |
| Lactate dehydrogenase (U/L) | 377.4 ± 166.6 | 381.9 ± 160.2 | 0.901 |
| CT imaging findings b | 0.804 | ||
| No lesion | 2 (2.9) | 1 (2.4) | |
| Ground-glass opacity | 53 (77.9) | 30 (73.2) | |
| Consolidative pulmonary opacities | 13 (19.1) | 10 (24.4) | |
| No. of infected lung lobes b | 0.509 | ||
| 0 | 2 (3.3) | 1 (3.2) | |
| 1 | 6 (9.8) | 1 (3.2) | |
| 2 | 8 (13.1) | 3 (9.7) | |
| 3 | 4 (6.6) | 0 (0.0) | |
| 4 | 4 (6.6) | 2 (6.5) | |
| 5 | 37 (60.7) | 24 (77.4) | |
| Smoking history | 0.906 | ||
| Never smoked | 65 (91.5) | 40 (90.9) | |
| Smokes or previously smoked | 6 (8.5) | 4 (9.1) | |
| Drinking history | 0.892 | ||
| Never drinks | 64 (90.1) | 40 (90.9) | |
| Drinks or previously drank | 7 (9.9) | 4 (9.1) | |
| Hypertension | 0.238 | ||
| No | 55 (77.5) | 38 (86.4) | |
| Yes | 16 (22.5) | 6 (13.6) | |
| Cardiovascular disease | 0.072 | ||
| No | 66 (93.0) | 44 (100.0) | |
| Yes | 5 (7.0) | 0 (0.0) | |
| Diabetes mellitus | 0.946 | ||
| No | 61 (85.9) | 38 (86.4) | |
| Yes | 10 (14.1) | 6 (13.6) | |
| Cancer | 0.424 | ||
| No | 65 (91.5) | 42 (95.5) | |
| Yes | 6 (8.5) | 2 (4.5) | |
| Cerebrovascular disease | 0.730 | ||
| No | 70 (98.6) | 43 (97.7) | |
| Yes | 1 (1.4) | 1 (2.3) | |
| Negative conversion time for SARS-CoV-2 test (days) | 14.1 ± 3.5 | 12.8 ± 4.1 | 0.314 |
| Deathc | 0.475 | ||
| No | 52 (75.4) | 34 (82.9) | |
| Yes | 17 (24.6) | 7 (17.1) | |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; aPTT, activated partial thromboplastin time; ESR, erythrocyte sedimentation rate; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography.
Data are presented as the n (%) or mean ± standard deviation.
There were missing data for dyspnoea, fever, CT imaging findings and number of infected lung lobes.
Two participants in the control group and three participants in the treatment group were still receiving treatment in hospital at the end of the study.
Table 2.
Support measures offered during the course of SARS-CoV-2 infection a
| Support measure | Control group (n = 71) | Ribavirin group (n = 44) | P-value |
|---|---|---|---|
| Immunoglobulin therapy | 0.143 | ||
| No | 46 (64.8) | 33 (75.0) | |
| Yes | 25 (35.2) | 11 (25.0) | |
| Non-invasive ventilation support | 0.750 | ||
| No | 53 (74.6) | 34 (77.3) | |
| Yes | 18 (25.4) | 10 (22.7) | |
| Invasive ventilation support | 0.302 | ||
| No | 64 (90.1) | 42 (95.5) | |
| Yes | 7 (9.9) | 2 (4.5) | |
| Corticosteroid therapy b | 0.288 | ||
| No | 45 (65.2) | 30 (75.0) | |
| Yes | 24 (34.8) | 10 (25.0) | |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are presented as n (%).
There were missing data for immunoglobulin therapy and corticosteroid therapy.
Ribavirin treatment was well tolerated and there were no early discontinuations due to adverse effects. There were no significant differences in laboratory parameters (haemoglobin, leukocyte count, lymphocyte count, C-reactive protein, platelet count, serum creatinine, BUN, ALT and AST) between the two groups after the treatment course (Table 3 ).
Table 3.
Laboratory parameters following therapy for severe COVID-19 a
| Parameter | Control group (n = 71) | Ribavirin group (n = 44) | P-value |
|---|---|---|---|
| Haemoglobin (g/L) | 115.8 ± 19.8 | 116.0 ± 16.7 | 0.952 |
| Haemoglobin change (g/L) | –10.4 ± 12.6 | –5.3 ± 13.5 | 0.051 |
| Leukocyte count (× 109/L) | 6.4 ± 3.6 | 5.7 ± 3.0 | 0.283 |
| Lymphocyte count (× 109/L) | 1.1 ± 0.6 | 1.1 ± 0.5 | 0.720 |
| C-reactive protein (mg/L) | 39.1 ± 48.1 | 28.2 ± 37.9 | 0.233 |
| Platelet count (× 109/L) | 243.3 ± 103.8 | 263.4 ± 128.2 | 0.367 |
| Serum creatinine (μmol/L) | 69.7 ± 26.8 | 63.3 ± 21.4 | 0.195 |
| BUN (mmol/L) | 5.8 ± 4.3 | 4.4 ± 2.7 | 0.068 |
| ALT (U/L) | 62.1 ± 187.3 | 35.8 ± 17.7 | 0.372 |
| AST (U/L) | 56.9 ± 145.9 | 34.3 ± 21.7 | 0.327 |
COVID-19, coronavirus disease 2019; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Data are presented as the mean ± standard deviation.
4. Discussion
With the global pandemic of COVID-19, there is an urgent need for effective therapeutic interventions in patients with severe SARS-CoV-2 infection. This study shows that ribavirin is not associated with reduced time to negative conversion time for SARS-CoV-2 test and does not provide a survival benefit compared with control treatment (supportive therapy only).
In this study, treatment with ribavirin was well tolerated. Anaemia is a common complication of ribavirin therapy and has been observed in previous studies investigating the treatment of MERS-CoV and SARS-CoV infection [4,10]. In the current study, the degree of change in haemoglobin values during admission was 5.3 g/L in the treatment group and 10.4 g/L in the control group. There was no statistical difference between the two groups (P = 0.051). In addition, there were no interruptions in treatment due to anaemia.
Whether to use ribavirin treatment was based on the doctor's clinical experience. Moreover, during a particular period at the peak of the outbreak, ribavirin was sometimes out of stock, which may also have led to treatment without ribavirin. Although use of ribavirin or not was not completely random, there were no statistically significant differences in clinical characteristics (included medical history, demographic data, physical examination, and haematological, biochemical and radiological results) or support measures (immunoglobulin therapy, ventilation support and corticosteroid therapy) between the ribavirin and control groups, making the two groups comparable.
It was thought that ribavirin might be useful for treating coronavirus infection because of its broad-spectrum inhibition of RNA viruses. Several studies have shown that ribavirin has useful activity against SARS-CoV in vitro [11,12]. However, other studies have found that ribavirin did not inhibit the virus in vivo and did not promote the recovery of patients infected with SARS-CoV [13,14]. A retrospective cohort study showed that ribavirin and interferon alfa-2a therapy improved survival at 14 days but not at 28 days in patients with severe MERS-CoV infection [4]. It should also be pointed out that a large, retrospective, multicentre study on different types of interferon with ribavirin to treat critically ill MERS cases did not improve survival [6]. Therefore, we should consider to remove the suggestion that patients with COVID-19 be treated with ribavirin.
This study is limited by its single-centre, retrospective and non-randomised nature. Inevitably, selection bias cannot be completely ruled out. Incontrovertibly, new interventions should be evaluated in randomised controlled clinical trials. However, such an approach is generally accepted in emerging diseases such as SARS-CoV-2 infection. In addition, the sample size required to achieve 90% power of test is approximately 1048 patients. Thus, the sample size in the current study is limited and it is possible that small effects were missed. Nevertheless, the results can provide a reference for further studies based on a larger sample size randomised clinical trial or other populations.
In conclusion, severe COVID-19 is associated with a relatively high mortality rate. Intravenous ribavirin therapy is not associated with improved negative conversion time for SARS-CoV2 test or a reduced mortality rate. Ribavirin therapy was well tolerated and there were no significant adverse effects. These results should be verified in randomised controlled clinical trials. The role of ribavirin in patients with mild SARS-CoV-2 infection also requires further study.
Acknowledgments
The authors thank all of the patients and their families involved in this study.
Funding: None.
Competing interests: None declared.
Ethical approval: This study was approved by Ethics Commission of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) [2020-0039]. Owing to the rapid emergence of this infectious disease, the need for written informed consent was waived.
Contributor Information
Sihua Wang, Email: sihua_wang@126.com.
Jinjun Jiang, Email: jiang.jinjun@zs-hospital.sh.cn.
References
- 1.Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Quasim E. Ribavirin and interferon therapy for critically ill patients with Middle East respiratory syndrome: a multicenter observational study. Clin Infect Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller MP, Dresser L, Raboud J, McGeer A, Rea E, Richardson SE. Adverse events associated with high-dose ribavirin: evidence from the Toronto outbreak of severe acute respiratory syndrome. Pharmacotherapy. 2007;27:494–503. doi: 10.1592/phco.27.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles SR, Phillips EJ, Dresser L, Matukas L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis. 2003;37:1139–1142. doi: 10.1086/378304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saijo M, Morikawa S, Fukushi S, Mizutani T, Hasegawa H, Nagata N. Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus. Antiviral Res. 2005;66:159–163. doi: 10.1016/j.antiviral.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res. 2006;71:53–63. doi: 10.1016/j.antiviral.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee N, Allen CK, Hui DS, Ng EK, Wu A, Chiu RW. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]