Abstract
Airborne infectious diseases such as the new Coronavirus 2019 (COVID-19) pose serious threat to human health. Indoor air pollution is a problem of global environmental concern as well. Singlet oxygen (1O2) is a reactive oxygen species that plays important role in bacteria/virus inactivation and pollutant degradation. In this study, we found that commercially available filters typically deployed in air purifier and air conditioning units, when impregnated with Rose Bengal (RB) as a 1O2 sensitizer, can be used for heterogeneous gas-phase generation of 1O2. It was confirmed that irradiation of the RB filter under oxygen gas stream produced 1O2, which was measured using furfuryl alcohol trapping method followed by HPLC analysis. It was also observed that the amount of 1O2 generated increases as the light intensity increased. Similarly, the sensitizer loading also positively influenced the 1O2 generation. The heterogeneous gas-phase generation of 1O2 can find potential applications in air purifier and air conditioning units for the purpose of bacteria/virus inactivation and/or pollutant degradation thereby improving indoor air quality.
Keywords: Singlet oxygen, Indoor air pollution, Bacteria/virus inactivation, Air purifier, Rose Bengal, Membrane filter
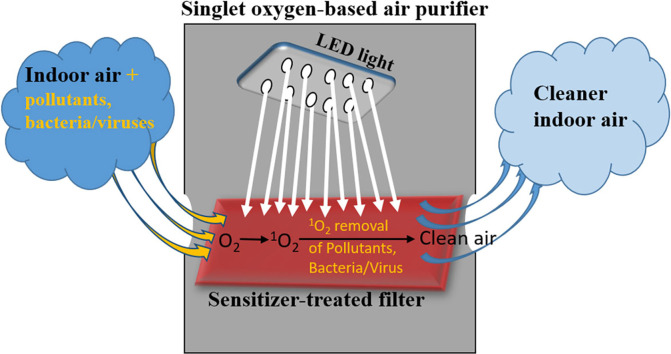
Graphical abstract
Highlights
-
•
Gas-phase singlet oxygen (1O2) generation from Rose Bengal-impregnated filter
-
•
A simple experimental set-up for gas phase 1O2 estimation from impregnated filter
-
•
Sensitizer loading and light intensity were the most important factors.
-
•
Potential application of 1O2 for indoor bacteria/virus inactivation in air
1. Introduction
Air pollution is a problem of global concern, with indoor air pollution particularly worrisome. Indoor air can be polluted with both chemical and microbial contaminants thereby posing a great threat to human health. The outbreak of airborne infectious diseases such as the recent Coronavirus disease (COVID-19) requires methods for virus inactivation in indoor air. SARS-CoV-2 (the virus responsible for COVID-19) remained viable in aerosols for up to 3 h under environmental conditions (van Doremalen et al., 2020). Therefore, poor ventilation may enable the spread when infected and uninfected people are in close contact or in a closed environment. The involvement of reactive oxygen species (ROS) such as singlet oxygen (1O2) in bacteria/virus inactivation and pollutant degradation can be explored for the purpose of improved indoor air quality.
Singlet oxygen, which is molecular oxygen in its lowest excited electronic state, is a reactive oxygen species (ROS) that is photochemically formed by the action of an irradiation light on a photosensitizer in the presence of molecular oxygen. It is involved in the degradation of pollutants (Latch et al., 2003; Liu & Sun, 2011; Kim et al., 2012; Xie et al., 2018; Blacha-Grzechnik et al., 2020) and inactivation of bacteria/virus (Banks et al., 1985; Dahl et al., 1987, Dahl et al., 1988; Lenard et al., 1993; Nitzan et al., 1995; Wainwright et al., 1998; Usacheva et al., 2001; Villen et al., 2006; Bartusik et al., 2012a; Costa et al., 2012; Felgenträger et al., 2014; Kim et al., 2020). Photodynamic inactivation (PDI), which involves generating ROS such as 1O2 upon irradiation of a sensitizer, has been deployed to inactivate a wide range of bacteria and viruses. PDI occurs via two mechanisms, namely: Type I mechanism involving the generation of free radicals, and Type II mechanisms involving 1O2. Type II mechanism involving generation of 1O2 has been shown to be the predominant mechanism of bacteria/virus inactivation (Costa et al., 2012). Comprehensive reviews highlighting the various bacteria (Hamblin and Hasan, 2004; Liu et al., 2015) and viruses (Costa et al., 2012) inactivated by 1O2 are available in literature. Viruses including human immunodeficiency virus (HIV), influenza virus, Sendai virus (Lenard et al., 1993), dengue virus (Huang et al., 2004), vaccinia virus (Turner and Kaplan, 1968), adenovirus (Schagen et al., 1999), enterovirus (Wong et al., 2010) etc. have all been reported to be inactivated by 1O2. In addition, it was showed that herpesvirus and influenza virus (enveloped type) and adenovirus and poliovirus (unenveloped type) were inactivated by 1O2 using a spray of Rose Bengal (RB) solution as mist and air scavenging system (Masaoka et al., 2013). Furthermore, a recent report by Eickmann et al. (2018) showed that both UVC and methylene blue/light (MB/L) systems were effective in inactivating Ebola virus (EBOV) and Middle East respiratory syndrome coronavirus (MERS-CoV) in blood plasma. The MB/L systems involves irradiation of methylene blue by visible light to produce 1O2. The inactivation of MERS-CoV, which belongs to the same genus and family with Covid-19 virus, suggests that 1O2 may also work for the inactivation of COVID-19 virus.
The above-mentioned reports majorly involved homogeneous 1O2 generation. However, homogeneous generation in aqueous phase cannot be easily deployed to provide 1O2 needed in the gas phase for potential indoor air purification. 1O2 can also be generated from heterogeneous systems where a solid-state sensitizer, either in isolation or deposited by physical and/or chemical modification onto another solid substance, is irradiated in the presence of oxygen gas to generate 1O2, which flows along with the gas stream into a collecting solution where it reacts with a substrate. Reaction of the formed 1O2 with the selective substrate in solution provides evidence of 1O2 generation from the solid-gas heterogeneous system. In addition to substrate solution, involvement of 1O2 in the deactivation of bacteria and viruses in air has also been reported. Kim et al. (2020) showed that 1O2 inactivated micro-organisms in air up to a distance of 10–15 cm away from the source. Several researchers have presented different experimental set-ups based on this heterogeneous system and demonstrated 1O2 generation from them with further application of the generated 1O2 for substrate degradation or bacteria/virus inactivation (Bartusik et al., 2012a, Bartusik et al., 2012b; Zamadar et al., 2009; Aebisher et al., 2010; Carpenter et al., 2015; Zhao et al., 2014; Hettegger et al., 2015). Nevertheless, the potential application of 1O2 for the purpose of improved air quality has not been well explored. One way of achieving that is to develop products that can generate 1O2 in the gas phase. Such products will help to inactivate micro-organisms in the indoor environment and may find applications as air purifiers in places like small rooms or offices, on automobiles, trains and even hand dryers. This will make a significant contribution towards cleaner indoor air by removing bacteria/virus or pollutants present in the air.
In this study, we present a simple, inexpensive set-up, designed from commonly available materials, for gas-phase 1O2 generation. A filter material typically employed in the air purifier and air conditioning units was impregnated with Rose Bengal and irradiated using a panel of LED lights. Furfuryl alcohol (FFA) in solution was used as a substrate to provide qualitative and quantitative evidence of 1O2 generation from the filter.
2. Materials and methods
2.1. Filter impregnation with Rose Bengal
The reagents and materials used are listed in supplementary information (S1). RB stock was prepared by dissolving 10 g RB in 150 g of the supplied gel. Two pieces of filters of the same dimension (L × W: 7 × 2.2 cm) were cut-out from the same filter material and their initial weights were obtained. RB was physically impregnated into one of the filters by completely immersing it in the RB-dissolved gel for 5 min. The gel was necessary to help impregnate RB in the filter because of the hydrophobic nature of the filter. The other filter was treated with a blank gel and used as the control filter. Both filters were dried overnight using cold air from an air drier. Treating the control filter with the blank gel helped to account for the contribution of the gel to the weight of the filters after drying. The RB-treated filter was further subjected to air blowing to remove any loosely adhered RB particles on the filter. The images of the RB-treated and control filters are presented in Fig. S2. Thereafter, the amount of RB impregnated into the filter was determined gravimetrically from the difference in the weight of the filter before impregnation and weight after impregnation (dry, RB-impregnated filter). The amount of RB per area of filter (mg/ cm2) was then calculated.
To investigate the effect of sensitizer loading on 1O2 generation, different filters were impregnated with varying amounts of RB. This was done by firstly preparing serial dilutions (10, 50 and 1000-fold dilutions) of the RB stock using the blank gel. Thereafter, filters were treated with these dilute solutions (Fig. S2). The amount of RB impregnated into the filters from treatment with different solutions of RB was also calculated.
2.2. Experimental set-up for 1O2 photogeneration and monitoring
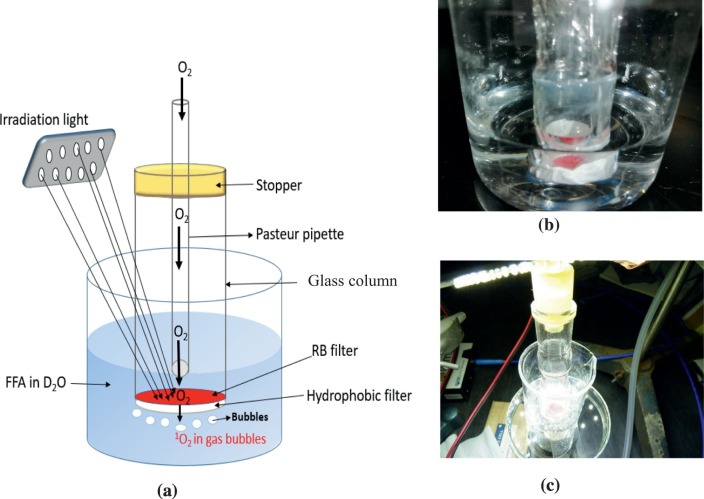
A schematic diagram of the set-up in this study is shown in Fig. 1a while Fig. 1b and c are pictures taken in the dark and during irradiation respectively. The experimental set-up employed involved immersion of the RB-filter, protected by a PTFE hydrophobic filter, in the trapping solution with oxygen flowing through it during irradiation. The RB-impregnated filter was attached to a glass column (length × base i.d.; 8 × 1.3 cm) with an acrylate adhesive. The base of the glass column where the RB-impregnated filter had been attached was then covered by a hydrophobic PTFE filter which was glued to the edges of the glass column and secured tightly to the sides of the column using transparent tapes. The column was stoppered at the top using a silicon stopper with hole. A capillary tube was inserted into the glass column through the hole of the stopper. The tube delivered oxygen gas to the surface of the RB-impregnated filter at a rate of 0.1–0.2 L/min. The stopper ensured that the top of the column was air-tight preventing any loss of oxygen through the top. The stability of this assembly and monitoring potential leakage or damage was ensured by immersing the assembly firstly in ultrapure water for about 5 min prior to immersion in the substrate solution. This preliminary immersion test showed that there was no leakage or leaching of RB into the solution. This rules out any possibility of aqueous phase contribution to 1O2 generation. The test also showed that at the immersion depth, the gas stream was sufficiently contacting the solution and was not just directly escaping into the air in the laboratory. This was necessary to ensure optimal contact between the 1O2 in the gas stream and the substrate solution. This shows that potential 1O2 exiting the filter along with oxygen flow will contact the reacting solution.
Fig. 1.
(a) A schematic diagram of the experimental set-up for 1O2 generation from RB-treated filter. (b) Picture showing the immersion of the assembly in water. This is used to check for potential failure of the assembly, leakage or leaching of RB prior to immersion in the substrate solution and irradiation. (c) Picture of a typical irradiation process for gas-phase 1O2 generation.
The irradiation light was suspended above the glass column. The light was from a locally fabricated LED panel (Excel Co. Ltd., Fukuyama, Japan) consisting of 60 LED bulbs emitting white light with a power output of up to 144 W when the full irradiation mode is adopted. The spectrum of the irradiation light was obtained using a miniature spectrophotometer device (Flame spectrophotometer, Ocean optics, U.S.A.) while the light intensity was measured using a pyranometer (LI-COR LI-189, U.S.A). As shown in Fig. S1, the LED light has peaks around 445 nm and 550 nm which matches the λmax of RB at about 550 nm.
Four mL solution of 100 μM FFA in D2O was employed as a substrate to demonstrate 1O2 generation from the irradiation of impregnated filter. 1O2 reacts with FFA to produce 6-hydroxyl-2H-pyran-3(6H)-one (6-HP-one) as a major product (Haag et al., 1984). 1O2 arriving in the solution was monitored indirectly through the degradation of FFA and corresponding formation of 6-HP-one by HPLC analysis (Supplementary info. S2). Although 1O2 was not generated in solution in this experiment, but in the gas-phase from the irradiation of RB-treated filter, the determined photoformation rate (calculated in solution) is indicative of the amount of 1O2 arriving in the substrate solution. This is expected to be lesser than that generated from the filter due to quenching of some 1O2 as it travels through the pores of the filter, or by the gas stream before successfully contacting the substrate solution. Therefore, the obtained information on 1O2 generation from the filter can be regarded as an estimate.
3. Results and discussion
3.1. Monitoring 1O2 formation
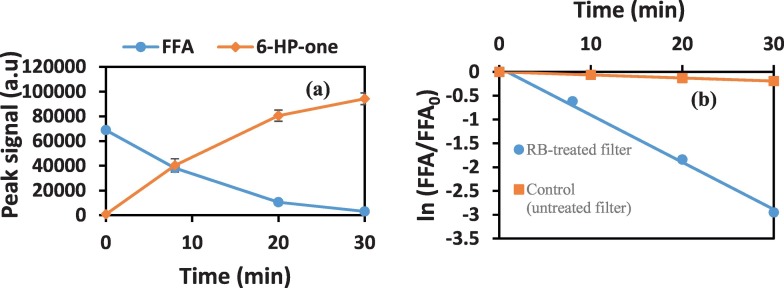
The 1O2 in the oxygen gas stream passing through the filter and arriving in the substrate solution was monitored indirectly by following the peaks of FFA degradation and 6-HP-one formation. Monitoring 6-HP-one formation confirms that the degradation of FFA is due to chemical reaction with 1O2 arriving from the filter into the substrate solution. A typical plot showing the degradation of FFA and corresponding formation of 6-HP-one is shown in Fig. 2a. Furthermore, the degradation of FFA due to 1O2 followed a first order kinetics. The first order plot of FFA degradation during irradiation of RB-treated filter and untreated (blank) filter is shown in Fig. 2b. Irradiation of the RB-treated filter caused a significant degradation of FFA compared to the blank filter. The value obtained for the blank filter was similar to that obtained when the FFA solution was directly irradiated with oxygen flowing into the solution in the absence of the filter assembly. This suggests that the blank filter had no significant contribution to 1O2 formation and that the RB-treated filter was solely responsible for the formed 1O2. In addition, there was no leaching of RB into solution during the irradiation. This is because the hydrophobic PTFE filter shielded the RB-treated filter from directly contacting the solution. This is a very important precaution because RB is highly soluble (100 mg/mL) in water. Therefore, any contact between the RB-treated filter and the solution will encourage a massive leaching or dissolution of RB. Under such scenario, there will be aqueous phase contribution to 1O2 generation. No leaching of RB, in part or whole, was observed during our experiments. This shows that the only source of 1O2 is that generated from the irradiation of the RB-treated filter which reaches the solution through the gas stream. Immersion of the filter assembly, with continuous oxygen flow, in the dark did not result in any appreciable loss of FFA or formation of 6-HP-one.
Fig. 2.
(a) Degradation of FFA and corresponding formation of 6-HP-one, (b) First order FFA degradation during the irradiation of RB-treated filter. Values are mean of triplicate measurements. Error bars are embedded.
3.2. Solvent isotope effect and 1O2 scavenger
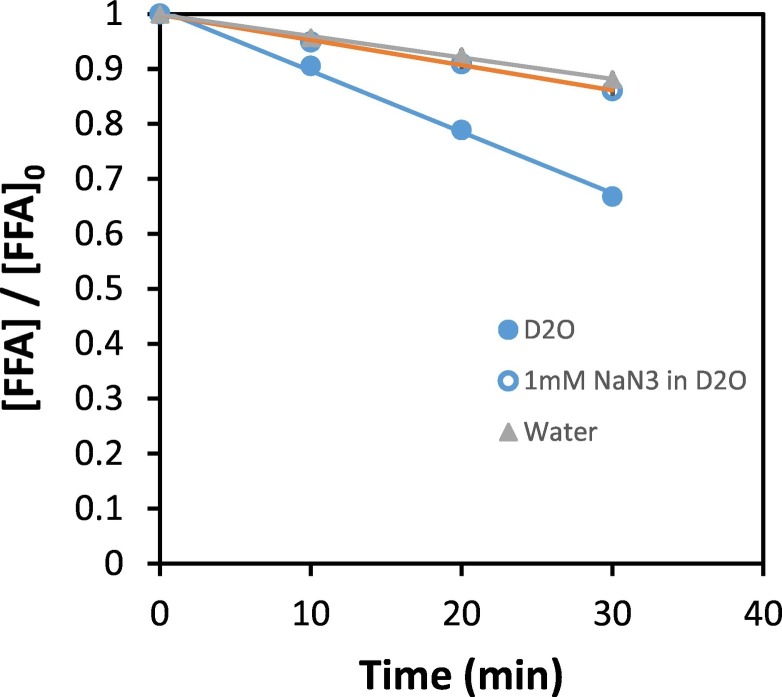
The solvent isotope effect and the influence of 1O2 scavengers are two important ways of demonstrating the generation and involvement of 1O2 in a particular reaction. To confirm the presence of 1O2 in the gas stream arriving in the substrate solution, the solvent isotope effect was studied by monitoring the degradation of FFA in D2O and H2O under identical experimental conditions using the same RB-treated filter. It is known that 1O2 has a lifetime in D2O that is about 13 times longer than in water (Lindig et al., 1980). The deactivation rate constant of 1O2 in water is 2.5 × 105 s−1(Rodgers and Snowden, 1982) and 1.6 × 104 s−1 in D2O (Lindig et al., 1980). According to Fig. 3 , the degradation of FFA in D2O was higher than in H2O. This can be associated with the longer lifetime of 1O2 in D2O, allowing more 1O2 to undergo chemical reaction with FFA. This confirms the involvement of 1O2 in the degradation of FFA. In the solvent isotope effect, it is important that the concentration of FFA does not contribute significantly to the physical quenching or deactivation of 1O2 in the solvents (D2O and H2O). From the product of FFA concentration (100 μM) and the reaction rate constant of 1O2 with FFA (8.3 × 107 M−1 s−1 in D2O) (Latch et al., 2003), FFA made a negligible 5% contribution to the deactivation rate constant of 1.6 × 104 s−1 for 1O2 in D2O. Similarly, using the reaction rate constant of 1.09 × 108 M−1 s−1 (Haag et al., 1984) for the reaction of 1O2 with FFA in H2O, the 100 μM FFA concentration only made an insignificant 4% contribution to the deactivation rate constant of 2.5 × 105 s−1 for 1O2 in H2O. This shows that the solvent isotope effect is mainly due to collision with the solvents, without any significant contribution from the physical quenching of 1O2 by FFA.
Fig. 3.
Effect of solvent and 1O2 scavenger on the reaction of 1O2 with FFA (100 μM). A RB-treated filter was irradiated at an oxygen flow rate of 0.125 L/min. The substrate solution as shown in the figure involve: 100 μM FFA in D2O, 100 μM FFA + 1 mM NaN3 in D2O, 100 μM FFA in water.
In addition, the effect of 1O2 scavenger on the degradation of FFA in D2O was studied using NaN3 as a scavenger. NaN3 is an efficient scavenger of 1O2 with a second order rate constant of 7.8 × 108 M−1 s−1 (Wilkinson and Brummer, 1981). The degradation of FFA in D2O was observed to reduce significantly in the presence of 1 mM NaN3 compared to its absence (FFA + D2O, no NaN3) (Fig. 3). This further confirms the generation of 1O2. The results of the solvent isotope effect and NaN3 scavenger experiment clearly confirm that 1O2 is present in the gas stream arriving in the substrate solution, and it is responsible for the degradation of FFA.
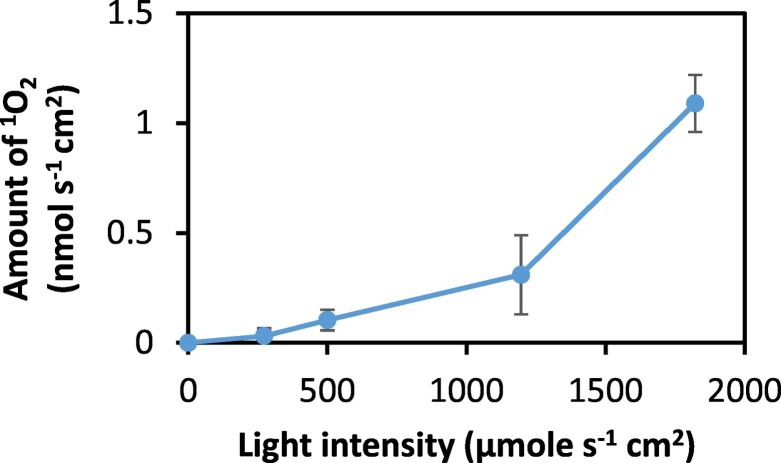
3.3. Influence of light intensity
To investigate how light intensity can influence the generation of 1O2, the intensity of the irradiation light was varied by employing the full-power mode and half-power mode of the irradiation light and/or increasing the distance between the filter and irradiation light. A plot of the amount of 1O2 detected in solution, during the irradiation of RB-treated filter, as a function of light intensity is shown in Fig. 4 . It is observed that the amount of 1O2 generated increased as the light intensity increases. The sharp increase between 1196 and 1861 μmole s−1 cm−2 suggest a regime of light intensity where higher production of 1O2 is observed. Based on the present experimental set-up, the distance between the irradiation light and filter is 11 cm. In an application where the light source is closer to the filter, the light intensity will be much higher and 1O2 generation can be expected to be much higher.
Fig. 4.
Effect of light intensity on generation of 1O2. The light intensity values are 0 (dark), 273, 501, 1196 and 1861 μmole s−1 cm2.
3.4. Effect of amount of sensitizer adsorbed in the filter
The effect of the amount of RB adsorbed by the filter on 1O2 production was investigated by treating blank filters with varying concentrations of RB dissolved in gel. The serial dilutions of the RB stock solution were made by addition of the blank gel to obtain approximately 10, 50 and 1000-fold diluted RB solutions. The rate of 1O2 generation upon irradiation of the filters is shown in Table 1 . It is observed that 1O2 generation increased as the amount of RB adsorbed on the filter increased. The maximum solubility of RB in the gel was 10 g RB in 150 g of gel. This was the same solution used as the undiluted stock in Table 1. Therefore, the effect of amount of adsorbed RB on 1O2 generation could not be studied beyond this point. Using the undiluted stock as the highest RB loading possible, the amount of 1O2 reaching the solution was calculated to be 12.8 μmole s−1 of 1O2 per mole of RB.
Table 1.
Effect of sensitizer loading on 1O2 generation.
| Treatment | RB adsorbed (mg/cm2) | R1O2 (x 10 −7 M s−1) |
|---|---|---|
| 1000-fold diluted stock | 0.082 | ⁎ND |
| 50-fold diluted stock | 0.26 | 0.232 |
| 10-fold diluted | 2 | 0.62 |
| Undiluted stock (10 g RB in 150 g of gel) |
9.4 | 3.32 |
ND: Not detectable as there was no observable difference from blank.
It should be noted that the amount of 1O2 generated on the surface of the filter may be several orders of magnitude higher than the value reported here due to the heterogeneous distribution of 1O2 generation. It has been demonstrated for aqueous solutions that 1O2 generation in the DOM microenvironment could be up to three orders of magnitude higher than in the bulk solution due to physical quenching as the 1O2 diffuses into the bulk solution (Latch & McNeill, 2006; Grandbois et al., 2008). A similar explanation could be adopted here where the 1O2 generated on the surface of the filter may undergo significant quenching before arriving in the substrate solution. This quenching may be caused by the filters (both treated filters and hydrophobic PTFE filter) as the gas stream containing 1O2 migrates from the irradiated side of the filter to the substrate solution. Also, quenching by ground-state molecular oxygen may also arise. Therefore, the quantity of singlet oxygen reported here can only be considered as a minimum. Nevertheless, in the real applications, the filters will be directly exposed to light, while air is circulated or brought into contact with the filter. Under such circumstances, the amount of generated on the surface of the filter is > > than 1O2 migrating further away from the filter into the air. Therefore, contaminated air will encounter a large amount of 1O2 on the surface of the filter that will be significant towards bacteria/virus inactivation.
4. Conclusions
1O2 generation from the irradiation of a sensitizer-impregnated filter was successfully demonstrated in this study using simple, inexpensive laboratory and commercially-available materials. By using filters already deployed in air purifier and air conditioner units for this study, we have successfully demonstrated that 1O2-generating ability can be incorporated into impregnated filters. Such filters can find potential application for bacteria/virus inactivation in indoor environment by incorporating them into air purifiers. Current commercially available purifiers make use of ozone (O3) or hydroxyl radical (•OH) in their systems. Ozone is harmful to health. Also, some ozone-based purifiers have been shown to increase the background indoor ozone level. Consequently, the use of O3, especially in indoor environments has been discouraged. The •OH-based systems generate •OH by the high energy UVC irradiation (185 nm) of water vapor using UVC lamps containing mercury (Crosley et al., 2017) or by heterogeneous photocatalytic oxidation technology (Zhong and Haghighat, 2015). The disposal of such lamps constitutes an environmental concern and they pose health threats if carelessly handled by unsuspecting users. Also, the mechanisms of •OH generation also produces O3 (Crosley et al., 2017) which may increase the O3 levels in the indoor environment. Singlet oxygen-based purifiers as proposed in this study present a number of advantages. Firstly, the involvement of 1O2 in the inactivation of a wide range of bacteria and viruses has been well documented in literature (Banks et al., 1985; Lenard et al., 1993; Wainwright et al., 1998; Villen et al., 2006; Bartusik et al., 2012; Costa et al., 2012; Felgenträger et al., 2014; Kim et al., 2020). Similar evidence for •OH is insufficient. Therefore, 1O2-based air purifiers can potentially provide protection against a wider range of bacteria and viruses. Secondly, the lifetime in air is about 85 ms (Schweitzer and Schmidt, 2003) while that of •OH ranges between 0.01 and 1 s (Crosley et al., 2017). The lifetimes of both •OH and 1O2 may be lower depending on the nature of other substances present. Hence, these values can be considered to be their upper limit values at ground-levels in the atmosphere. Nevertheless, the use of RB as sensitizer, which has a high 1O2 quantum yield (0.76) (Wilkinson et al., 1992), can ensure relatively high production of 1O2. Therefore, more 1O2 can be available for bacteria/virus inactivation in 1O2-based air purifiers compared to •OH in •OH-based systems. In addition, the system proposed in this study makes use of simple, cheap and commercially-available LED light sources emitting white light in the visible region, in-line with the λmax of RB (550 nm). Such light source is relatively simple, easier to handle and maintain. The findings from this study can contribute significantly to the development of 1O2-based air purifiers with potential applications in small or large indoor environments in offices, at homes or even on automobiles and trains. Such products are necessary to contribute to improved indoor air quality considering the outbreak of airborne viral infections such as the present COVID-19. Although this is a preliminary study, the findings from this study have led to the development of a prototype where additional parameters necessary for its deployment are being studied.
CRediT authorship contribution statement
Michael Oluwatoyin Sunday: Investigation, Formal analysis, Writing - original draft. Hiroshi Sakugawa: Resources, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are grateful to Yokota Kogyo Shokai Co. Inc., Hiroshima, Japan for providing research funding for this study. We also appreciate Prof. Kazuhiko Takeda of the Graduate School of Biosphere Science, Hiroshima University, for the advice rendered during this study.
Editor: Prof. Pavlos Kassomenos
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.141186.
Appendix A. Supplementary data
Supplementary material
References
- Aebisher D., Zamadar M., Mahendran A., Ghosh G., McEntee C., Greer A. Fiber-optic singlet oxygen [1O2 (1Δg)] generator device serving as a point selective sterilizer. Photochem. Photobiol. 2010;86:890–894. doi: 10.1111/j.1751-1097.2010.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J.G., Board R.G., Carter J., Dodge A.D. The cytotoxic and photodynamic inactivation of micro-organisms by Rose Bengal. J. Appl. Bacteriol. 1985;58:391–400. doi: 10.1111/j.1365-2672.1985.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Bartusik D., Aebisher D., Lyons A.M., Greer A. Bacteria inactivation by a singlet oxygen bubbler. Identifying factors controlling the toxicity of 1O2 bubbles. Environ. Sci. Technol. 2012;46(21):12098–12104. doi: 10.1021/es303645n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartusik D., Aebisher D., Ghafari B., Lyons A.M., Greer A. Generating 1O2 bubbles: a new mechanism for gas-liquid oxidations in water. Langmuir. 2012;38:3053–3060. doi: 10.1021/la204583v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacha-Grzechnik A., Drewniak A., Walczak K.Z., Szindler M., Ledwon P. Efficient generation of singlet oxygen by perylene diimide photosensitizers covalently bound to conjugate polymers. J. Photochem. & Photobiol. A: Chemistry. 2020;388:112161. [Google Scholar]
- Carpenter B.L., Scholle F., Sadeghifar H., Francis A.J., Boltersdorf J., Weare W.W., Argyropoulos D.S., Maggard P.A., Ghiladi R.A. Synthesis, characterization and antimicrobial efficacy of photomicrobicidal cellulose paper. Biomacromolecules. 2015;16:2484–2492. doi: 10.1021/acs.biomac.5b00758. [DOI] [PubMed] [Google Scholar]
- Costa L., Faustino M.A.F., Neves M.G., Cunha A., Almeida A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses. 2012;4:1034–1074. doi: 10.3390/v4071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosley D.R., Araps C.J., Doyle-Eisele M., Mcdonald J.D. Gas-phase photolytic production of hydroxyl radicals in an ultraviolet purifier for air and surfaces. J. of air & waste mgt. assoc. 2017;67:231–240. doi: 10.1080/10962247.2016.1229236. [DOI] [PubMed] [Google Scholar]
- Dahl T.A., Midden W.R., Hartman P.E. Pure 1O2 cytotoxicity for bacteria. Photochem. Photobiol. 1987;46:345–352. doi: 10.1111/j.1751-1097.1987.tb04779.x. [DOI] [PubMed] [Google Scholar]
- Dahl T.A., Midden W.R., Neckers D.C. Comparison of photodynamic action by Rose Bengal in gram-positive and gram-negative bacteria. Photochem. Photobiol. 1988;48:607–612. doi: 10.1111/j.1751-1097.1988.tb02870.x. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:16. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmann M., Gravemann U., Handke W., Tolksdorf F., Reichenberg S., Muller T.H., Seltsam A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion. 2018;58:2202–2207. doi: 10.1111/trf.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgenträger A., Maisch T., Späth A., Schröder J.A., Bäumler W. 1O2 generation in porphyrin-doped polymeric surface coating enables antimicrobial effects of Staphylococcus aureus. Phys. Chem. Chem. Phys. 2014;2014(16):20598–20607. doi: 10.1039/c4cp02439g. [DOI] [PubMed] [Google Scholar]
- Grandbois M., Latch D.E., Mcneill K. Microheterogeneous concentrations of singlet oxygen in natural organic matter isolate solutions. Environ. Sci. Technol. 2008;42:9184–9190. doi: 10.1021/es8017094. [DOI] [PubMed] [Google Scholar]
- Haag W.R., Hoigne J.r., Gassman E., Braun A.M. Singlet oxygen in surface waters — part I: Furfuryl alcohol as a trapping agent. Chemosphere. 1984;13(5):631–640. [Google Scholar]
- Hamblin M.R., Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettegger H., Gorfer M., Sortino S., Fraix A., Bandian D., Rohrer C., Harreither W., Potthast A., Rosenau T. Synthesis, characterization and photo-bactericidal activity of silanized xanthene-modified bacteria cellulose membranes. Cellulose. 2015;2015(22):3291–3304. [Google Scholar]
- Huang Q., Fu W.-L., Chen B., Huang J.-F., Zhang X., Xue Q. Inactivation of dengue virus by methylene blue/narrow bandwidth light system. J. Photochem. Photobiol. B Biol. 2004;2004(77):39–43. doi: 10.1016/j.jphotobiol.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim W., MacKeyev Y., Lee G.-S., Kim H.-J., Tashikawa T., Hong S., Lee S., Kim J., Wilson L.J., Majima T., Alvarez P.J.J., Choi W., Lee J. Selective oxidative degradation of organic pollutants by singlet oxygen-mediated photosensitization: tin porphyrin versus C60 aminofullerene systems. Environ. Sci. Technol. 2012;46:9606–9613. doi: 10.1021/es301775k. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee H., Lee J.-Y., Park K.-H., Kim W., Lee J.H., Kang H.-J., Hong S.W., Park H.-J., Lee S., Lee J.-H., Park H.-D., Kim J.Y., Jeong Y.W., Lee J. Photosensitized production of singlet oxygen via C60 fullerene covalently attached to functionalized silica-coated stainless-steel mesh: remote bacterial and viral inactivation. Appl. Catal. B Environ. 2020;270 [Google Scholar]
- Latch D.E., McNeill K. Micorheterogeneity of singlet oxygen distributions in irradiated humic acid solutions. Science. 2006;311:1743–1747. doi: 10.1126/science.1121636. [DOI] [PubMed] [Google Scholar]
- Latch D.E., Stender B.L., Packer J.L., Arnold W.A., McNeill K. Photochemical fate of pharmaceuticals in the environment: cimetidine and ranitidine. Environ. Sci. Technol. 2003;37(15):3342–3350. doi: 10.1021/es0340782. [DOI] [PubMed] [Google Scholar]
- Lenard J., Rabson A., Vanderoef R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: inhibition of fusion and synctia formation. Proc. Natl. Acad. Sci. 1993;90:158–162. doi: 10.1073/pnas.90.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindig B.A., Rodgers M.A.J., Schaap A.P. Determination of the lifetime of singlet oxygen in water‑d2 using 9,10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 1980;02(17):5590–5593. [Google Scholar]
- Liu N., Sun G. Production of reactive oxygen species by photoactive anthraquinone compounds and their applications in wastewater treatment. Ind. Eng. Chem. Res. 2011;50:5326–5333. [Google Scholar]
- Liu Y., Qin R., Zaat S.A.J., Breukink E., Heger M. Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clinical and Trans. Res. 2015;1(3):140–167. [PMC free article] [PubMed] [Google Scholar]
- Masaoka Y., Tagawa M., Takao S. 2013. Air Purification System. (Japanese Patent 424167, Japan Patent office, December 6, 2013) [Google Scholar]
- Nitzan Y., Dror R., Ladan H., Malik Z., Kimel S., Gottfried V. Structure-activity relationship of porphines for photoinactivation of bacteria. Photochem. Photobiol. 1995;1995(62):342–347. doi: 10.1111/j.1751-1097.1995.tb05279.x. [DOI] [PubMed] [Google Scholar]
- Rodgers M.A.J., Snowden P.T. Lifetime of oxygen (O2(1.DELTA.g)) in liquid water as determined by time-resolved infrared luminescence measurements. J. Am. Chem. Soc. 1982;104(20):5541–5543. [Google Scholar]
- Schagen F.H.E., Moor A.C.E., Cheong S.C., Cramer S.J., van Ormondt H., van der Eb A.J., Dubbelman T.M.A.R., Hoeben R.C. Photodynamic treatment of adenoviral vectors with visible light: an easy and convenient method for viral inactivation. Gene Ther. 1999;1999(6):873–881. doi: 10.1038/sj.gt.3300897. [DOI] [PubMed] [Google Scholar]
- Schweitzer C., Schmidt R. Physical mechanism of generation and deactivation of singlet oxygen. Chem. Rev. 2003;103:1685–1758. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- Turner G.S., Kaplan C. Photoinactivation of vaccinia virus with rose bengal. J. Gen. Virol. 1968;3:433–443. doi: 10.1099/0022-1317-3-3-433. [DOI] [PubMed] [Google Scholar]
- Usacheva M.N., Teichert M.C., Biel M.A. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 2001;29:165–173. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- Villen L., Manjon F., Garcia-Fresnadillo D., Orellana G. Solar water disinfection by photocatalytic singlet oxygen production in heterogeneous medium. Appl. Catal. B Environ. 2006;69:1–9. [Google Scholar]
- Wainwright M., Phoenix D.A., Laycock S.L., Wareing D.R., Wright P.A. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol. Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson F., Brummer L. Rate constants for the decay and reactions of the lowest electronically excited singlet-state of molecular oxygen in solutions. J. Phys. Chem. Ref. Data. 1981;10:809–1000. [Google Scholar]
- Wilkinson J.F., Helman P., Ross A. Quantum yields for the photosensitized formation of the lowest electronically excited singlet state of molecular oxygen in solution. J. Phys. Chem. Ref. Data. 1992;22:1–126. [Google Scholar]
- Wong T.-W., Huang H.-J., Wang Y.-F., Lee Y.-P., Huang C.-C., Yu C.-K. Methylene bluemediated photodynamic inactivation as a novel disinfectant of enterovirus 71. J. Antimicrob. Chemother. 2010;65:2176–2182. doi: 10.1093/jac/dkq301. [DOI] [PubMed] [Google Scholar]
- Xie X., Zhang Z., Hu Y., Cheng H.A. A mechanistic kinetic model for singlet oxygen mediated self-sensitized photo-oxidation of organic pollutants in water. Chem. Eng. J. 2018;334:1242–1251. [Google Scholar]
- Zamadar M., Aebisher D., Greer A. 1O2 delivery through the porous cap of a hollow-core fiber optic device. J. Phys. Chem. B. 2009;113:15803–15806. doi: 10.1021/jp907945c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Liu Y., Xu Q., Barahman M., Bartusik D., Greer A., Lyons A.M. 1O2 generation on porous superhydrophobic surfaces: effect of gas flow and sensitizer wetting on trapping efficiency. J. Phys. Chem. A. 2014;118:10364–10371. doi: 10.1021/jp503149x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Haghighat F. Photocatalytic air cleaners and materials technologies – abilities and limitations. Build. Environ. 2015;9:191–203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material