Abstract
Background
Obesity was recently identified as a major risk factor for worse COVID-19 severity, especially among the young. The reason why its impact seems to be less pronounced in the elderly may be due to the concomitant presence of other comorbidities. However, all reports only focus on BMI, an indirect marker of body fat.
Aim
To explore the impact on COVID-19 severity of abdominal fat as a marker of body composition easily collected in patients undergoing a chest CT scan.
Methods
Patients included in this retrospective study were consecutively enrolled among those admitted to an Emergency Department in Rome, Italy, who tested positive for SARS-Cov-2 and underwent a chest CT scan in March 2020. Data were extracted from electronic medical records.
Results
150 patients were included (64.7% male, mean age 64 ± 16 years). Visceral fat (VAT) was significantly higher in patients requiring intensive care (p = 0.032), together with age (p = 0.009), inflammation markers CRP and LDH (p < 0.0001, p = 0.003, respectively), and interstitial pneumonia severity as assessed by a Lung Severity Score (LSS) (p < 0.0001). Increasing age, lymphocytes, CRP, LDH, D-Dimer, LSS, total abdominal fat as well as VAT were found to have a significant univariate association with the need of intensive care. A multivariate analysis showed that LSS and VAT were independently associated with the need of intensive care (OR: 1.262; 95%CI: 1.0171–1.488; p = 0.005 and OR: 2.474; 95%CI: 1.017–6.019; p = 0.046, respectively).
Conclusions
VAT is a marker of worse clinical outcomes in patients with COVID-19. Given the exploratory nature of our study, further investigation is needed to confirm our findings and elucidate the mechanisms underlying such association.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Covid-19, COronaVIrus Disease 19; RT-PCR, real-time reverse transcriptase; ICU, Intensive Care Unit; IRB, Institutional Review Board; LSS, Lung Severity Score; ROI, region of interest; VAT, visceral adipose tissue; TAT, total adipose tissue; SAT, subcutaneous adipose tissue; EFT, epicardial fat thickness
Keywords: Covid-19, SARS-CoV-2, Obesity, Fat, Risk factor, Body composition
Highlights
-
•
High BMI is a risk factor of COVID-19 severity especially among the young.
-
•
BMI in an indirect marker of body fat excess.
-
•
COVID-19 patients routine chest CT scan may be leveraged to directly quantify body fat.
-
•
Visceral fat deposition is higher in COVID-19 patients accessing ICU.
-
•
Visceral fat is associated with the need of intensive care (OR = 2.474).
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly caused a pandemic of Coronavirus Disease 19 (COVID-19), forcing public health authorities to lockdown. Its relatively high lethality rate seems to be influenced by several factors, such as age, male gender and presence of hypertension [1]. When the epicenter of the pandemic shifted from China to Italy, Europe and then the United States, body weight was then suggested as an additional risk factor, with a retrospective study showing that 76% of patients admitted in intensive care for SARS-CoV-2 were obese [2], a finding confirmed by other reports [[3], [4], [5]]. This delay in such observation could be due to the difference in the prevalence of obesity in these countries: China, and the most affected Hubei area, counts less than 5% prevalence, where Italy features one above 10%, and the US is now over 40% [6,7]. Interestingly, obesity has been shown to be a major risk factor for COVID-19 worse prognosis especially among the young, whereas its role seems less striking in older subjects [[8], [9], [10], [11]]. This could be in part because of additional risk factors building up with aging, with the impact of obesity being diluted. However, it should be noted that the World Health Organization defines obesity as a condition characterized by abnormal or excessive fat accumulation that presents a risk to health, with no mention to the BMI [6]. BMI is, in fact, an indirect marker of excess body fat, and changes in body composition take place upon aging [12], with progressive muscle mass loss together with a shift from subcutaneous to visceral adipose tissue (VAT) and an increase in total fat [13]. An abnormal fat distribution, and in particular VAT expansion, is re-known to be associated with low grade inflammation and with the secretion of pro-inflammatory cytokines, such as interleukin 6 (IL-6) [14]. An excessive inflammatory response to SARS-CoV-2, as reflected by increased circulating cytokines, profound lymphopenia and mononuclear cell infiltration in the lungs, is thought to be a major cause of disease severity and death in patients with COVID-19 [15]. Increased VAT expansion may therefore play a crucial role in driving the excessive inflammation described in patients developing severe complications from SARS-CoV-2 infection, and further investigating its relationship with clinical outcomes across all ages is now of utmost importance.
Patients with a clinical suspicion for SARS-Cov-2 infection undergo CT scans to allow diagnosis and monitoring of the disease [16]. With the BMI possibly telling just one part of the story, and body composition being hard to assess in many ill patients, identifying easily collectable markers of fat deposition associated with worse prognosis might aid in a better stratification of patients at increased risk of developing complications. Moreover, such findings could pave the way to better understanding the mechanisms underlying the established link between body weight and COVID-19 severity. We therefore aimed at investigating the association of VAT deposition with clinical outcomes such as the need of intensive care among patients with COVID-19.
2. Methods
2.1. Population and study design
Patients included in this single center retrospective study were consecutively enrolled among those admitted to the Emergency Department of Sant'Andrea Hospital, Rome, Italy, who tested positive for SARS-Cov-2 and underwent a chest CT scan in March 2020. All patients were subjected to two naso- and oro-pharyngeal swabs at a 24-hour interval, and the specimens were tested to confirm SARS-COV2 diagnosis through real-time reverse transcriptase (RT-PCR) (Charitè, Berlin, Germany). Deidentified data about demographic and clinical characteristics were collected, together with laboratory data on presentation. Data were extracted from electronic medical records. Based on the clinical and radiological assessment, the patients were subsequently addressed from the Emergency Department towards home discharge or admitted to sub-intensive or Intensive Care Unit (ICU), and such clinical outcome was recorded for the purposes of this study. All patients accessing the ICU were intubated and underwent invasive mechanical ventilation, whereas those in subintensive care did not need intubation.
Patients whose CT could not be evaluated due to severe motion artifact or other technical issues such as restricted field of view for adipose tissue quantification or CT acquired with contrast medium were excluded, as well as those for whom investigated clinical outcomes were unavailable.
The study was approved by the local institutional review board (IRB), and written informed consent was obtained from all study participants. When patients were unable to provide informed consent, this was received by the next-of-kin or admitting physician.
2.2. Sample size and study power
Based on a preliminary pilot study of 20 patients, the mean ± SD VAT in the patient population was 14,000 ± 8500 mm2. Assuming a power of 0.80 and alpha of 0.05, 142 patients were considered appropriate to highlight an expected difference of 2000 mm2 in the VAT value. The number of subjects was further increased to 150 to prevent possible missing data. Assuming an alpha of 0.05, the post-hoc power calculated considering the mean ± SD VAT in the investigated groups with the smaller number of patients (i.e., 23) was 89.2%.
2.3. CT acquisition technique and Lung Severity Score evaluation
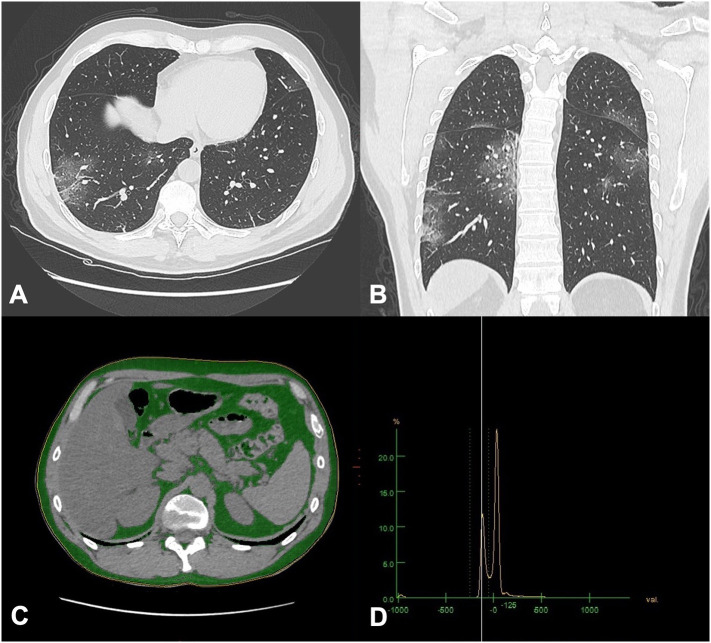
COVID-19 positive patients underwent a chest CT scan to determine the pulmonary involvement. The acquisitions were obtained on a 128-slice CT (GE Revolution EVO, GE Medical Systems, Milwaukee, WI, USA). Lung impairment was evaluated through a Lung Severity Score (LSS) calculated through visual assessment by two expert radiologists in consensus (DC and MZ with 10 and 5 years of experience respectively) ranging from 0 to 40 (Fig. 1A–B) [17]. Briefly, each lung was divided in 10 regions as already described [18], and for each region a visual score of parenchymal involvement was assigned (ground-glass opacities and consolidation), with a score 0 for absence, 1 for <50% and 2 for >50% lung involvement.
Fig. 1.
Pulmonary involvement and fat analysis in a chest CT scan of a COVID-19 patient. (A–B) Ground glass opacities can be seen peripherally in the lower lobes, typical radiological pattern of COVID-19; (C) the first slice where lung bases are no more visible at the thoracoabdominal level where visceral adipose tissue (VAT), and total adipose tissue (TAT) are quantified: fat is identified in green. Subcutaneous adipose tissue was calculated by subtraction. (D) Histogram analysis of CT numbers, with a range of CT numbers classified as fat was −50 to −250.
2.4. CT fat deposition assessment
Measurements were performed by two radiologists in consensus (MP and FP with 6 and 7 years of experience respectively) on a commercially available workstation computer were the chest CTs had been transferred (Advantage Windows Workstation, version 4.7, GE Healthcare). To quantify abdominal visceral and subcutaneous fat, the first slice where lung bases were no more visible at the thoracoabdominal level was selected, adapting from a previously described method (Fig. 1C) [19]. A region of interest (ROI) of the body circumference avoiding subcutaneous tissue was identified; then a HU range corresponding to the CT histogram of adipose tissue from −250 to −50 HU was set to automatically calculate the visceral adipose tissue (VAT) area expressed in mm2 on the selected slice (Fig. 1D). The procedure was repeated drawing a ROI including the subcutaneous tissue and obtaining the total adipose tissue (TAT) area expressed in mm2. Subcutaneous adipose tissue (SAT) area expressed in mm2 was obtained as the subtraction between TAT and VAT. Finally, epicardial fat thickness (EFT) was measured at the level of the right coronary artery origin on the axial plane. To reduce bias, measurements were assessed three times and a mean of the values was recorded, expressed in millimeters.
2.5. Statistics
The Statistical Package for Social Sciences (SPSS), v.20, and R-package (Vienna, Austria) were used for statistical analysis. Results are presented as mean ± standard deviation (SD). Normality was assessed with the Kolmogorov–Smirnov test. Variables not normally distributed were log-transformed. Patients were categorized in three groups based on the type of treatment (Home discharge, Sub-intensive Care, and ICU). The primary endpoint was admission to ICU, which includes requirement for intubation.
For continuous variables, a one-way ANCOVA was conducted to compare the distribution of the clinical, biochemical and CT-derived adiposity parameters and LSS among the three different levels of care controlling for age and gender, and a post-hoc multiple comparison LSD adjusted was conducted. Chi-Square test/Fisher's exact test was used for categorical variables. Pearson's correlation coefficient was used to investigate the level of association between continuous variables.
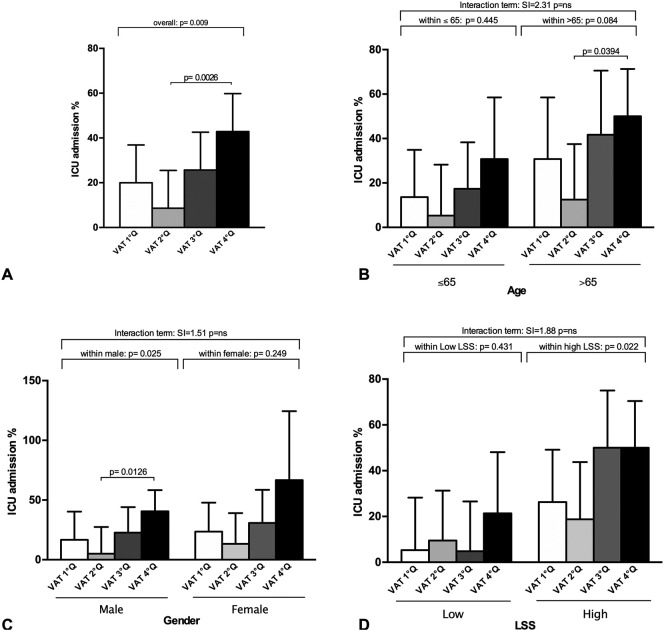
To assess the presence of additive interaction of investigated variables on ICU admission, three indices were calculated [20] using the R-package “interactionR”: (1) the relative excess risk due to interaction (RERI), (2) the proportion of disease among those with both exposures that is attributable to their interaction (AP), and (3) the synergy index (SI). A RERI of one means no interaction or perfect additivity. A RERI of greater than one means positive interaction or more than additivity. A RERI of less than one means negative interaction or less than additivity. RERI ranges from zero to infinity. An AP of zero means no interaction or perfect additivity. An AP greater than zero means positive interaction or more than additivity. An AP of less than zero means negative interaction or less than additivity. AP ranges from −1 to +1. The SI is the ratio of the combined effects and the individual effects. An SI of one means no interaction or perfect additivity. An SI of greater than one means positive interaction or more than additivity. An SI of less than one means negative interaction or less than additivity. SI ranges from zero to infinity. The confidence interval for the three additive interaction measures was calculated according Hosmer and Lemeshow [21]. In Fig. 2B–D only the SI value has been represented as estimate of the interaction term; SI and the other indexes (AP and RERI) are represented in the Supplementary Tables 1, 2 and 3.
Fig. 2.
ICU admission requirement (%) per visceral adipose tissue area quartile (VAT Q) in the study population (A), stratified by age group (≤65, >65 years) (B), gender (C), and lung severity score (LSS) (high or low based on the population LSS median value) (D). Data are expressed as % and SD. P for interaction term, interaction of VAT accumulation and age (figure B), gender (figure C), and lung severity score (LSS) (figure D) on ICU admission, is from a synergy index (SI) analysis. The SI is the ratio of the combined effects and the individual effects. An SI of one means no interaction or perfect additivity. An SI of greater than one means positive interaction or more than additivity. An SI of less than one means negative interaction or less than additivity. SI ranges from zero to infinity (figures B, C and D). P for within group analysis is from a Chi-square test (figure A; subgroups for figures B, C and D).
Univariate and multivariate logistic regression models were performed to analyze the relationship between the admission to ICU as the dependent variable and clinical, biochemical, CT-derived adiposity parameters and LSS as independent variables. Univariate analysis was performed by converting continuous variables into dummy dichotomic variables based on median values, while continuous variables were used for multivariate analyses. To build a multivariate logistic regression model with ICU admission as the dependent variable, we used a forward stepwise approach as previously reported [5] and investigated the following models/variables; model 1: multivariate analysis including age (quartiles) and gender, together with variables with significant univariate association (p value ≤0.05) analyzed one by one as regressors (age + gender + lymphocytes; age + gender + CRP; age + gender + LDH; age + gender + D-dimer; age + gender + LSS; age + gender + TAT; age + gender + VAT; the variable age was adjusted for gender only); model 2: multivariate analysis including all statistically significant variables of the univariate analysis as regressors in one single model; model 3: multivariate analysis including all the variables of model 2 with the addition of hypertension, ACEi or ARB use prior to admission and diabetes as clinically important variables. The forward stepwise selection method does not provide ORs and 95% CI for the variables not retained in the model because they do not significantly improve prediction. Therefore, only the variables with statistically significant results were added in the table, reporting their OR and 95% CI, [R2]. For the forward stepwise analysis, a P-IN = 0.05 and a P-OUT = 0.10 were used. The effect estimate is reported as Nagelkerke's R2, which informs on how much the model explains the variance of the dependent variable. The ORs represent the mean change in the dependent variable per one unit of change in the independent variable while holding other predictors in the model constant. Variance inflation factor (VIF) values were lower than 4.0, suggesting the absence of multicollinearity between included variables [22]. The results were considered statistically significant when p < 0.05.
ICU admission was used as the gold standard for nonparametric clustered receiver operating characteristic (ROC) analysis to evaluate the predictive utility of the models. By comparing observed and calculated ICU admission, sensitivity and specificity were plotted in ROC form. When a perfect correlation of predicted versus observed ICU admission was found, the ROC area under curve (AUC) was equal to 1, whereas random assignment of outcome led to an AUC of 0.5.
3. Results
One hundred and fifty patients were enrolled, 64.7% male, with a mean ± SD age of 64 ± 16 years, ranging from 22 to 97 years old. Over a half (58.6%) was affected by hypertension, 22% was diabetic, 16.8% had a history of cardiovascular disease, 10.3% had kidney failure, and 9.3% had a recent or current history of malignancy. Of those with a diagnosis of hypertension, 61% was on either Angiotensin Converting Enzyme inhibitors (ACEis) or Angiotensin II Receptor Blockers (ARBs). Following the access to the emergency department, 15.3% were discharged for home monitoring, 61.3% were admitted to Sub-intensive Care, and 23.3% required intubation and therefore accessed ICU (Table 1 ).
Table 1.
Baseline demographic and clinical characteristics of the study population.
| All patients |
No intubation |
Intubation |
p | ||
|---|---|---|---|---|---|
| n (%) |
150 (100) |
115 (76) |
35 (23) |
||
| Home therapy (a) |
Subintensive CU (b) |
Intensive CU (c) |
|||
| n (%) | 23 (15) | 92 (61) | 35 (23) | ||
| Demographic and clinical characteristics | |||||
| Age (years) | 64.15 ± 15.69 | 62.91 ± 19 | 61.95 ± 14.87c | 70.77 ± 13.91†,b | 0.009 |
| Male gender N(%) | 97 (64.7) | 17 (11.3) | 57 (38) | 23 (15.3) | 0.556 |
| Lymphocytes (∗103/μL)§ | 1.28 ± 1.46 | 1.27 ± 0.72 | 1.21 ± 0.57 | 1.49 ± 2.85 | 0.654 |
| CRP (mg/dL) | 8.74 ± 8.32 | 7.42 ± 8.89c | 6.83 ± 7.01c | 14.62 ± 8.61†,a,b | <0.0001 |
| LDH (U/L) | 339.5 ± 129.7 | 343.1 ± 124.6 | 311.2 ± 103.5c | 408.6 ± 165.5†,b | 0.003 |
| D-dimer (ng/mL) | 570.15 ± 952.5 | 407.5 ± 422.6 | 451.19 ± 589.8 | 975.18 ± 1625.5 | 0.073 |
| Temperature (°C) | 37.6 ± 0.98 | 37.5 ± 1.11 | 37.5 ± 0.89 | 37.8 ± 1.09 | 0.104 |
| Hypertension N(%) | 65 (58.6) | 12 (66.7) | 41 (59.4) | 12 (50.0) | 0.54 |
| Cardiovascular Disease n(%) | 18 (16.8) | 1 (5.6) | 15 (22.4) | 2 (9.1) | 0.13 |
| ACEi/ARB N(%) | 25 (61.0) | 3 (33.3) | 15 (68.2) | 7 (70.0) | 0.15 |
| Diabetes N(%) | 24 (22.0) | 3 (16.7) | 18 (26.5) | 3 (13.0) | 0.34 |
| Malignancy N(%) | 10 (9.3) | 4 (22.2) | 5 (7.5) | 1 (4.5) | 0.11 |
| Kidney failure N(%) | 11 (10.3) | 0 (0) | 10 (14.9) | 1 (4.5) | 0.11 |
| Radiological characteristics | |||||
| LSS | 16.70 ± 8.37 | 13.83 ± 7.79c | 14.76 ± 7.21c | 23.69 ± 7.92†,a,b | <0.0001 |
| TAT (mm2) | 28,076.90 ± 14,016.29 | 24,877.85 ± 13,813.71 | 26,936.17 ± 13,415.86 | 32,844.06 ± 14,866.94 | 0.067 |
| VAT (mm2) | 14,331.51 ± 8372.32 | 12,014.00 ± 7585.72c | 13,679.66 ± 7807.05 | 17,343.56 ± 9561.3†,a | 0.032 |
| EFT (mm)§ | 6.93 ± 2.54 | 6.41 ± 2.66 | 6.86 ± 2.04 | 7.45 ± 3.48 | 0.713 |
| SAT (mm2) | 13,745.39 ± 8506.76 | 12,863.85 ± 8598.83 | 13,256.51 ± 8122.65 | 15,500.50 ± 9382.40 | 0.186 |
Data expressed as mean ± SD, or N (%); p is from a one-way ANCOVA with multiple comparison LSD adjusted for age and gender or Chi-Square test/Fisher's exact test for categorical variables. Letters denote the columns with which a statistically significant pairwise comparison exists. The results were considered statistically significant when p < 0.05. Percentage of patients on ACEi/ARB is calculated on the hypertensive subpopulation. p < 0.05 are highlighted in bold. CU, Care Unit, ICU, Intensive Care Unit; CRP, C reactive protein; LDH, Lactate dehydrogenase; ACEi, Angiotensin-Converting-Enzyme inhibitor; ARB, Angiotensin II Receptor Blocker; TAT, Total Fat Area; VAT, Visceral Fat Area; SAT, Subcutaneous Fat Area; EFT, Epicardial Fat Thickness; LSS, Lung Severity Score.
Denotes a statistically significant difference between intubation and no intubation.
Denotes a Ln transformed variable to achieve normal distribution (Lymphocytes and EFT: Mean ± SD = 0.0316 ± 0.2229; 18,605 ± 0,41,905, respectively).
When evaluating patients based on the clinical outcome and adjusting by gender, age was unsurprisingly significantly different across groups, being increasingly higher from home monitoring to ICU. Different from what previously reported, male gender proportion was not significantly different across groups. Among biochemical markers of inflammation, age- and gender- adjusted C Reactive Protein (CRP) and Lactate dehydrogenase (LDH) were significantly higher in patients with worse clinical outcomes, D-dimer levels trended higher (p = 0.073), whereas lymphocyte absolute count as well as body temperature on presentation were not different. LSS was worse in those accessing ICU as expected (p < 0.0001). TAT was not significantly different upon clinical outcome categorization, although trend higher levels were observed at increasing clinical severity (p = 0.067). Patients requiring intubation did not therefore show a significantly higher TAT compared to those not in need of intubation. Conversely, VAT was significantly different across groups, and post-hoc analysis revealed it being higher in those requiring intubation and thus accessing ICU compared to those discharged for home monitoring and those admitted to sub-intensive care, both groups not needing intubation. The same could not be affirmed regarding SAT and EFT, as patients did not show any difference based on clinical outcome (Table 1).
Upon categorization by VAT quartiles, a rise in ICU admission was observed with increasing VAT accumulation (p = 0.009; Fig. 2A), and patients belonging to the highest VAT quartile required ICU admission significantly more than those in the second VAT quartile (p = 0.026) (Fig. 2A).
Stratifying by age, 65 years being the median age of the study population, RERI, AP, and SI, reflecting the interaction between age and VAT accumulation in contributing to ICU admission, consistently indicated a positive interaction on an additive scale, meaning that the combined effect of age and VAT seems to be more than the sum of the effects (1.75, 0.43, and 2.32, respectively, Supplementary Table 1). However, this interaction was not significant (p = ns, Fig. 2B). On an exploratory basis, we further performed a subgroup analysis and observed a trend increased need of ICU admission per higher VAT accumulation in the age group >65 years (p = 0.084, Fig. 2B); within this subgroup, a significant difference was observed between the fourth and second VAT quartile (p = 0.0394, Fig. 2B).
Similarly, upon gender stratification, AP, and SI, but not RERI, indicated a positive interaction on an additive scale between VAT accumulation and gender (0.18, 1.51 and 0.41, respectively, Supplementary Table 2), however, once again the interaction term was not significant (p = ns, Fig. 2C). The exploratory subgroup investigation showed that male subjects confirmed what observed in the general population (p = 0.025, Fig. 2C); conversely, female subjects did not confirm this finding (p = 0.249, Fig. 2C). Within the male subgroup, subjects with VAT belonging to the fourth quartile were significantly more in need of ICU admission compared to those in the second quartile (p = 0.0126, Fig. 2C).
Upon stratification by LSS, RERI, AP, and SI, suggested a positive interaction on an additive scale between VAT accumulation and LSS (3.67, 0.42, and 1.88, respectively, Supplementary Table 3), but once again the interaction term was not significant (p = ns, Fig. 2D). However, the explorative subgroup analysis suggested that patients in the high LSS group (when LSS was higher than the LSS median value) and belonging to the highest quartile of VAT deposition were admitted to ICU more frequently (0.022 for high LSS, Fig. 2D). Patients in the low LSS group did not confirm this finding (p = 0.431, Fig. 2D).
Uncorrected correlation assessment of continuous variables showed that, as expected, VAT and TAT were directly correlated with other adiposity markers, but also with LSS and biochemical inflammation markers. SAT and EFT, despite showing a correlation with some inflammation markers on top of the expected one with the other fat markers, failed to be significantly correlated with LSS. Age was found to be directly correlated with LSS, PCR and D-Dimer among radiological and biochemical markers of inflammation and disease. EFT was also positively correlated, whereas temperature showed a negative association (Table 2 ).
Table 2.
Pearson's correlation matrix between investigated continuous variables.
| VAT | TAT | SAT | LSS | EFT§ | CRP | LDH | D-Dimer | Temperature | Lymphocytes§ | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.094 | 0.032 | −0.040 | 0.285⁎⁎ | 0.260⁎⁎ | 0.195⁎ | 0.069 | 0.225⁎⁎ | −0.169⁎ | −0.020 |
| VAT | 0.827⁎⁎ | 0.379⁎⁎ | 0.241⁎⁎ | 0.183⁎ | 0.243⁎⁎ | 0.247⁎⁎ | 0.130 | 0.167 | 0.051 | |
| TAT | 0.833⁎⁎ | 0.237⁎⁎ | 0.263⁎⁎ | 0.209⁎ | 0.271⁎⁎ | 0.209⁎ | 0.150 | 0.100 | ||
| SAT | 0.153 | 0.253⁎⁎ | 0.105 | 0.200⁎ | 0.213⁎ | 0.083 | 0.114 | |||
| LSS | 0.145 | 0.509⁎⁎ | 0.593⁎⁎ | 0.181⁎ | 0.055 | −0.136 | ||||
| EFT§ | −0.040 | 0.143 | 0.188⁎ | −0.133 | 0.108 | |||||
| CRP | 0.409⁎⁎ | 0.141 | 0.084 | −0.118 | ||||||
| LDH | 0.233⁎⁎ | 0.106 | −0.184⁎ | |||||||
| D-Dimer | 0.067 | −0.016 | ||||||||
| Temperature | 0.031 |
Correlations are reported as Pearson correlation coefficient. p < 0.05 are highlighted in bold. TAT, Total Fat Area; VAT, Visceral Fat Area; SAT, Subcutaneous Fat Area; EFT, Epicardial Fat Thickness; LSS, Lung Severity Score. CRP, C Reactive Protein; LDH, Lactate Dehydrogenase.
Denotes a Ln transformed variable to achieve normal distribution (Lymphocytes and EFT: Mean ± SD = 0.0316 ± 0.2229; 1.8605 ± 0.41905, respectively).
p < 0.05.
p < 0.01.
Univariate associations with the need of ICU admission were assessed for all baseline demographic and clinical characteristics, together with available biochemical and radiological parameters. Increasing age (expressed in quartiles), all investigated inflammation markers (Lymphocytes, CRP, LDH, D-Dimer), LSS, TAT as well as VAT were found to have a significant univariate association (Table 3 ). Multivariate logistic regression analyses showed that, when correcting for age and gender, of the variables found to have an association with the need of ICU admission, lymphocytes and D-dimer lost significance (Table 3, Multivariate model 1). A second multivariate logistic regression analysis model including in one model all variables found to be significantly associated with the need of ICU admission in the univariate logistic regressions showed that CRP levels and the LSS explained 36.8% of the clinical outcome (R2 = 0.368) (Table 3, Multivariate model 2). When adding to the model variables deemed to be clinically relevant based on previous reports (presence of diabetes, the use of ACEi/ARB, and hypertension) [[23], [24], [25], [26]], it was found that the log likelihood for the model including LSS and VAT z-score (R2 = 0.533) better explained the clinical outcome compared to the log likelihood for a baseline model, with VAT showing a stronger association with the need of ICU admission compared to the LSS (OR: 2.474 vs 1.262, respectively) (Table 3, Multivariate model 3). The diagnostic efficacy of this model was quantified by receiver operating characteristic (ROC) curve, and the area under the curve (AUC) was 0.654 (95% CI: 0.572–0.73, p = 0.0056).
Table 3.
Univariate and multivariate logistic regression analyses for ICU admission.
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| (1) |
(2) |
(3) |
||
| OR (95% CI) p-value [R2] | OR (95% CI) p-value [R2] | OR (95% CI) p-value R2 = 0.368 | OR (95% CI) p-value R2 = 0.533 | |
| Demographic and clinical variables | ||||
| Gender (male) | 1.062 (0.48–2.35) p = 0.882 [0.000] | |||
| Age (years) | 1.7 (1.21–2.49) p = 0.003 [0.094] | 1.733 (1.206–1.491) p = 0.003 [0.094] | ||
| Lymphocytes (∗103/μL) | 0.49 (0.22–1.06) p = 0.034 [0.01] | |||
| CRP (mg/dL) | 12.1 (0.22–1.06) p = 0.0001 [0.274] | 1.107 (1.053–1.163) p = 0.0001 [0.256] | 1.091 (1.019–1.169) p = 0.012 | |
| LDH (U/L) | 2.53 (1.08–5.93) p = 0.033 [0.055] | 1.005 (1.002–1.009) p = 0.003 [0.183] | ||
| D-dimer (ng/mL) | 4.62 (1.91–11.17) p = 0.001 [0.134] | |||
| Temperature (°C) | 1.84 (0.84–4.05) p = 0.13 [0.125] | |||
| Hypertension | 1.56 (0.63–3.87) p = 0.338 [0.013] | |||
| Cardiovascular disease | 2.32 (0.49–10.95) p = 0.288 [0.019] | |||
| ACEi/ARB | 0.77 (0.25–2.43) p = 0.658 [0.005] | |||
| Diabetes | 2.15 (0.58–7.98) p = 0.251 [0.021] | |||
| Malignancy | 2.49 (0.30–20.75) p = 0.400 [0.013] | |||
| Kidney failure | 2.8 (0.34–23.14) p = 0.339 [0.017] | |||
| Radiological variables | ||||
| LSS | 5.79 (2.34–14.35) p = 0.0001 [0.165] | 1.173 (1.101–1.249) p = 0.026 [0.312] | 1.121 (1.042–1.206) p = 0.002 | 1.262 (1.071–1.488) p = 0.005 |
| TAT (mm2) | 2.22 (0.99–4.93) p = 0.049 [0.041] | 1.59 (1.057–2.392) p = 0.026 [0.167] | ||
| VAT (mm2) | 3.13 (1.36–7.19) p = 0.007 [0.081] | 1.577 (1.051–2.365) p = 0.028 [0.168] | 2.474 (1.017–6.019) p = 0.046 | |
| EFT (mm) | 1.29 (0.60–2.76) p = 0.504 [0.005] | |||
| SAT (mm2) | 1.60 (0.73–3.50) p = 0.239 [0.015] | |||
Notes: Univariate analysis was performed by converting continuous variables into dummy dichotomic variables based on median values, while continuous variables were used for multivariate analyses. To build a multivariate logistic regression model with ICU admission as the dependent variable, we used a forward stepwise approach and investigated the following variables/models: (1) multivariate analysis including age (quartiles) and gender, together with variables with significant univariate association (p value ≤.05) analyzed one by one as regressors (age + gender + lymphocytes; age + gender + CRP; age + gender + LDH; age + gender + D-dimer; age + gender + LSS; age + gender + TAT; age + gender + VAT; the variable age was adjusted for gender only). (2) Multivariate analysis including all statistically significant variables of the univariate analysis as regressors in one single model. (3) Multivariate analysis including all the variables of model 2 with the addition of hypertension, ACEi or ARB use prior to admission and diabetes as clinically important variables. The forward stepwise selection method does not provide ORs and 95% CI for the variables not retained in the model because they do not significantly improve prediction. Therefore, only the variables with statistically significant results were added in the table, reporting their OR and 95% CI, [R2]. For the forward stepwise analysis, a P-IN = 0.05 and a P-OUT = 0.10 were used. The effect estimate is reported as Nagelkerke's R2, which informs on how much the model explains the variance of the dependent variable. The ORs represent the mean change in the dependent variable per one unit of change in the independent variable while holding other predictors in the model constant. The results were considered statistically significant when p < 0.05. Age in years. Pearson coefficient values are highlighted in bold when correlation was statistically significant at the p < 0.05 level and below. OR, Odds ratio; CI, confidence interval; CRP, C reactive protein; LDH, Lactate dehydrogenase; ACEi angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; TAT, Total Fat Area; VAT, Visceral Fat Area; SAT, Subcutaneous Fat Area; EFT, Epicardial Fat Thickness; LSS, Lung Severity Score.
4. Discussion
We report that visceral deposition of fat within the abdomen is independently associated with worse clinical outcomes upon SARS-CoV-2 infection. Interestingly, VAT accumulation seems to have a stronger link with the need of ICU admission and therefore intubation as opposed to other relevant parameters, such as severity of interstitial pneumonia, markers of inflammation, age, gender or comorbidities. Upon stratification, it seems that higher VAT is associated with an increased need of intensive care in subjects over 65 and in male gender, and among those with high LSS, having higher VAT is associated with increased ICU admission. However, it should be noted that the number of patients requiring ICU admission in each subgroup was small leading to non-significant interaction terms between subgroups (gender, age, severity score). Subgroup analysis needs therefore to be considered exploratory and should be taken with caution, but it can lay the path for future studies.
The pathophysiology underlying the suggested link is likely multi-stranded. First, obesity is associated with complement system hyperactivation, chronic inflammation, and presence of comorbidities such as diabetes and hypertension proposed in turn to be risk factors for COVID-19 poor prognosis [27]. Furthermore, visceral adipose tissue is capable of secreting Interleukin-6 [28], whose levels were found to be retrospectively increased in COVID-19 non-survivors [25]. SARS-CoV-2 seems to infect cells through an Angiotensin-converting enzyme 2 (ACE2)-dependent mechanism, a receptor also expressed by fat, including ectopic reservoirs [29], and an additional direct proinflammatory role of COVID-19 in the adipose tissue may therefore possibly contribute to the observed results.
Although the design of our study does not allow for a causal relationship to be found, our findings led us to hypothesize that VAT accumulation may work behind the scenes to cause some of the conditions currently suggested to be risk factors for poor prognosis. Noteworthy, our results are in line with a previous report suggesting that ectopic fat deposition within the liver is strongly associated to COVID-19 clinical severity among obese subjects [30]. However, Non-Alcoholic Fatty Liver Disease is hard to be diagnosed as a chest CT scan only is performed in COVID-19 patients, and our findings are therefore more likely to produce an immediate clinical impact.
Among the great variety of ectopic fat deposition sites, epicardial fat represents a marker of systemic inflammation [31], and a role has been hypothesized in the worse evolution of patients with COVID-19 and obesity [32,33]. Interestingly, no correlation was observed with pulmonary disease severity, hinting that this specific reservoir may have no role in the increased need of intubation correlated with obesity, possibly retaining one in cardiac manifestations related to COVID-19, which was not investigated in the present study.
Our study has several limitations. First, the sample size was relatively small, leading to small subgroup sizes upon stratification analysis by age or gender. However, the sample size exceeded that calculated based on a preliminary pilot study, and the post-hoc power was 89.2%. The nature of this study was retrospective, and only selected biochemical and clinical parameters were available, together with a partial clinical history, making correction for some possible confounders not possible. Also, other hard outcomes such as mortality could not be retrieved in the study population, as many patients were transferred to other COVID centers due to governmental directives. Moreover, anthropometric parameters (i.e. BMI) were unavailable, and a comparison in outcomes prediction was therefore not performed. Furthermore, this was not a mechanistic study, and the pathophysiology underlying the findings, possibly involving the inflammatory role of visceral adipocytes, could not be clarified.
Some strengths should also be acknowledged. The age distribution of our study population was wider than that of many reports. Moreover, not only the need of intubation was available, but also a categorization between patients in need of sub-intensive care as opposed to those whose clinical conditions were good enough to be discharged for home monitoring could be retrieved. Finally, to the best of our knowledge, this is the first study investigating fat deposition, thus indirectly body composition, in patients with COVID-19, and the assessed markers did not require additional instrumental tests, leading to a possible immediate clinical applicability.
In conclusion, our preliminary findings suggesting that increased visceral accumulation of fat is associated with worse COVID-19 severity might aid in further confirmatory studies, on the one hand investigating VAT as a useful clinical marker, and on the other hand elucidating the underlying pathophysiology.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Mikiko Watanabe:Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.Damiano Caruso:Investigation, Data curation, Methodology, Validation, Writing - original draft, Writing - review & editing.Dario Tuccinardi:Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.Renata Risi:Data curation, Formal analysis, Writing - review & editing.Marta Zerunian:Investigation, Data curation.Michela Polici:Investigation, Data curation.Francesco Pucciarelli:Investigation, Data curation.Mariarita Tarallo:Investigation, Data curation.Lidia Strigari:Formal analysis, Writing - review & editing.Silvia Manfrini:Investigation, Writing - review & editing.Stefania Mariani:Writing - original draft, Writing - review & editing.Sabrina Basciani:Writing - review & editing, Project administration.Carla Lubrano:Conceptualization, Writing - review & editing.Andrea Laghi:Supervision, Writing - review & editing.Lucio Gnessi:Supervision, Writing - review & editing.
Declaration of competing interest
The authors of this manuscript have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2020.154319.
Appendix A. Supplementary data
Supplementary Table 1. Interaction term between age and visceral adipose tissue (VAT) on ICU admission. Continuous variables were converted into dummy dichotomic variables based on median values. RERI, relative excess risk due to interaction; AP, proportion of disease among those with both exposures that is attributable to their interaction; SI, synergy index; OR, odds ratio, CI, confidence interval.
Supplementary Table 2. Interaction term between gender and visceral adipose tissue (VAT) on ICU admission. Continuous variables were converted into dummy dichotomic variables based on median values. The variable GenderM0F1 was 0 for male and 1 for female. RERI, relative excess risk due to interaction; AP, proportion of disease among those with both exposures that is attributable to their interaction; SI, synergy index; OR, odds ratio, CI, confidence interval.
Supplementary Table 3. Interaction term between lung severity score (LSS) and visceral adipose tissue (VAT) on ICU admission. Continuous variables were converted into dummy dichotomic variables based on median values. RERI, relative excess risk due to interaction; AP, proportion of disease among those with both exposures that is attributable to their interaction; SI, synergy index; OR, odds ratio, CI, confidence interval.
References
- 1.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 4.ICNARC report on COVID-19 in critical care. 2020. [Google Scholar]
- 5.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Health topics. Obesity. https://www.who.int/topics/obesity/en/
- 7.Watanabe M., Risi R., De Giorgi F., Tuccinardi D., Mariani S., Basciani S. Obesity treatment within the Italian national healthcare system tertiary care centers: what can we learn? Eat Weight Disord. 2020 doi: 10.1007/s40519-020-00936-1. [pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busetto L., Bettini S., Fabris R., Serra R., Dal Pra C., Maffei P. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22918. [pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Morbid obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22913. [pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batsis J.A., Mackenzie T.A., Bartels S.J., Sahakyan K.R., Somers V.K., Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999-2004. Int J Obes (Lond) 2016;40:761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster B.H. Measuring body fat distribution and content in humans. Curr Opin Clin Nutr Metab Care. 2002;5:481–487. doi: 10.1097/00075197-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 15.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caruso D., Zerunian M., Polici M., Pucciarelli F., Polidori T., Rucci C. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;201237 doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso D., Parinella A.H., Schoepf U.J., Stroebel M.H., Mangold S., Wichmann J.L. Optimization of window settings for standard and advanced virtual monoenergetic imaging in abdominal dual-energy CT angiography. Abdom Radiol (NY) 2017;42:772–780. doi: 10.1007/s00261-016-0963-9. [DOI] [PubMed] [Google Scholar]
- 18.Yang R., Li X., Liu H., Zhen Y., Zhang X., Xiong Q. 2020. Chest CT severity score: an imaging tool for assessing severe COVID-19. https://doiorg/101148/ryct2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M.S., Choi Y.-J., Lee Y.H. 2020. Visceral fat measured by computed tomography and the risk of breast cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knol M.J., VanderWeele T.J., Groenwold R.H., Klungel O.H., Rovers M.M., Grobbee D.E. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26:433–438. doi: 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosmer D.W., Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hair J., Black W.C., Babin B.J., Anderson R.E. Pearson Education International; Upper Saddle River, New Jersey: 2010. Multivariate data analysis (7th Ed.) [Google Scholar]
- 23.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes/Metabolism Research and Reviews. n/a:e3325. [DOI] [PubMed]
- 28.Chait A., den Hartigh L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel V.B., Basu R., Oudit G.Y. ACE2/Ang 1-7 axis: a critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. 2016;5:306–311. doi: 10.1080/21623945.2015.1131881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;154244 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L. Obesity accompanying COVID-19: the role of epicardial fat. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22867. [pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Kim I.C., Han S. Epicardial adipose tissue: fuel for COVID-19-induced cardiac injury? Eur Heart J. 2020;41:2334–2335. doi: 10.1093/eurheartj/ehaa474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Interaction term between age and visceral adipose tissue (VAT) on ICU admission. Continuous variables were converted into dummy dichotomic variables based on median values. RERI, relative excess risk due to interaction; AP, proportion of disease among those with both exposures that is attributable to their interaction; SI, synergy index; OR, odds ratio, CI, confidence interval.
Supplementary Table 2. Interaction term between gender and visceral adipose tissue (VAT) on ICU admission. Continuous variables were converted into dummy dichotomic variables based on median values. The variable GenderM0F1 was 0 for male and 1 for female. RERI, relative excess risk due to interaction; AP, proportion of disease among those with both exposures that is attributable to their interaction; SI, synergy index; OR, odds ratio, CI, confidence interval.
Supplementary Table 3. Interaction term between lung severity score (LSS) and visceral adipose tissue (VAT) on ICU admission. Continuous variables were converted into dummy dichotomic variables based on median values. RERI, relative excess risk due to interaction; AP, proportion of disease among those with both exposures that is attributable to their interaction; SI, synergy index; OR, odds ratio, CI, confidence interval.