Abstract
Background:
Mitral valve repair (MVr) for severe, degenerative mitral regurgitation (MR) is the gold standard, as medical management carries poor prognosis. However, despite clear benefit of MVr, many eligible patients are untreated. This study investigated whether MVr restores patients to normal life expectancy, at any age of operation, by comparing long-term survival of post-MVr patients to the life expectancy of the general United States (U.S.) population.
Methods:
This retrospective study investigated 1011 patients with degenerative MR who underwent isolated MVr between 2003 and 2017. Parametric distribution analysis was applied to long-term post-MVr mortality data, and Weibull probability plots provided the best-fit distribution by Anderson-Darling Goodness-of-Fit testing. Confidence intervals of the estimated distribution were used to compare additional life expectancy post-MVr to the general U.S. population across multiple decades of life. Post-MVr patients were categorized by age into decade (range 20–89 years).
Results:
The life expectancy of post-MVr patients matched the life expectancy of the general U.S. population at any age between 40–89. Lower-bound one-sided 95% confidence intervals for additional life expectancy were not significantly different from corresponding median additional life expectancy of the general population. There were very few deaths in the 20–39 year-old group limiting predictability, but survival also appeared normative.
Conclusions:
These findings suggest that degenerative MVr restores anticipated life expectancy to that of the general population, regardless of age. While our findings underscore the importance of repair for degenerative mitral disease, larger studies with longer-term follow-up are needed to reinforce this finding, particularly for younger patients.
Classifications: Mitral regurgitation, mitral valve repair, outcomes, statistics, survival analysis
Introduction
Mitral regurgitation (MR) is the most common valvular disease in the United States (U.S.) and affects more than two million Americans [1]. According to The Framingham Study, the prevalence of MR ≥ mild severity in the general population was 19% [2], which illustrates the wide-reaching scope of this disease process. Mitral valve surgery is the only treatment for severe MR shown to improve symptoms and prevent heart failure, as medical management alone carries a poor prognosis with 1-year mortality as high as 20% and 5-year mortality as high as 50% [3]. Among those that undergo surgery, mitral valve repair (MVr) is the treatment of choice for severe, degenerative mitral regurgitation [4, 5], as mitral repair has been repeatedly proven superior to replacement for degenerative mitral disease and prolapse [6–14]. In comparison with replacement, mitral valve repair has been shown to substantially improve outcomes, reducing mortality of patients with severe, degenerative MR by about 70% [1]. In addition, MVr has been linked with reduced risk of thromboembolism and improved survival [7, 11–14], even for elderly patients [8, 9].
While the optimal timing of surgical intervention in patients with severe, degenerative MR remains controversial [15–17], early surgical intervention has been shown to improve clinical outcomes compared with medical management alone [18–21]. In fact, operative mortality for isolated, elective MVr for degenerative disease has been reported to be less than 1% [22, 23], and successful repair has produced excellent long-term outcomes with very low incidence of reoperation [6, 7, 9–13, 24]. Yet, despite the clear benefit of mitral surgery over medical management for severe MR, up to 50% of eligible patients are not undergoing surgery [25]. In fact, despite definitive recommendations for intervention in the setting of severe, symptomatic, degenerative MR with left ventricular dilation or systolic dysfunction, prevalence studies suggest that it is common for patients not to undergo surgery [2, 26]. In addition, a survey of cardiologists treating adults in Canada showed that only 57.2% recognized an ejection fraction of 50% to 60% and only 15.6% recognized functional class II symptoms as indications for mitral intervention [27]. These findings underscore the disparity between the need for repair of degenerative mitral valve pathology and the performance of adequate repair.
Given the wide-reaching scope of degenerative MR and poor outcomes associated with failure to adequately treat this disease process, it is important to address this practice gap and mitigate concerns that referring providers may have regarding recommending mitral surgery. Our study therefore investigates the long-term survival of patients with severe, degenerative MR after MVr compared to the general population. In order to determine if MVr restores patients to normal life expectancy, at any age, this study compared long-term survival of a post-MVr cohort to the Center for Disease Control (CDC) national life expectancy at each decade of life.
Patients and Methods
Patient Population
This study is a single-center, retrospective analysis of 1011 patients with severe, degenerative MR who underwent routine, elective MVr between April 2003 and March 2017 at the University of Michigan. Patients were identified using the University of Michigan Research Database and the Society of Thoracic Surgery (STS) National Cardiac Surgery Database (NCSD). Figure 1 shows our overall study population. The Institutional Review Board at the University of Michigan approved this retrospective study and also waived the requirement for patient consent (HUM00148119).
Figure 1.
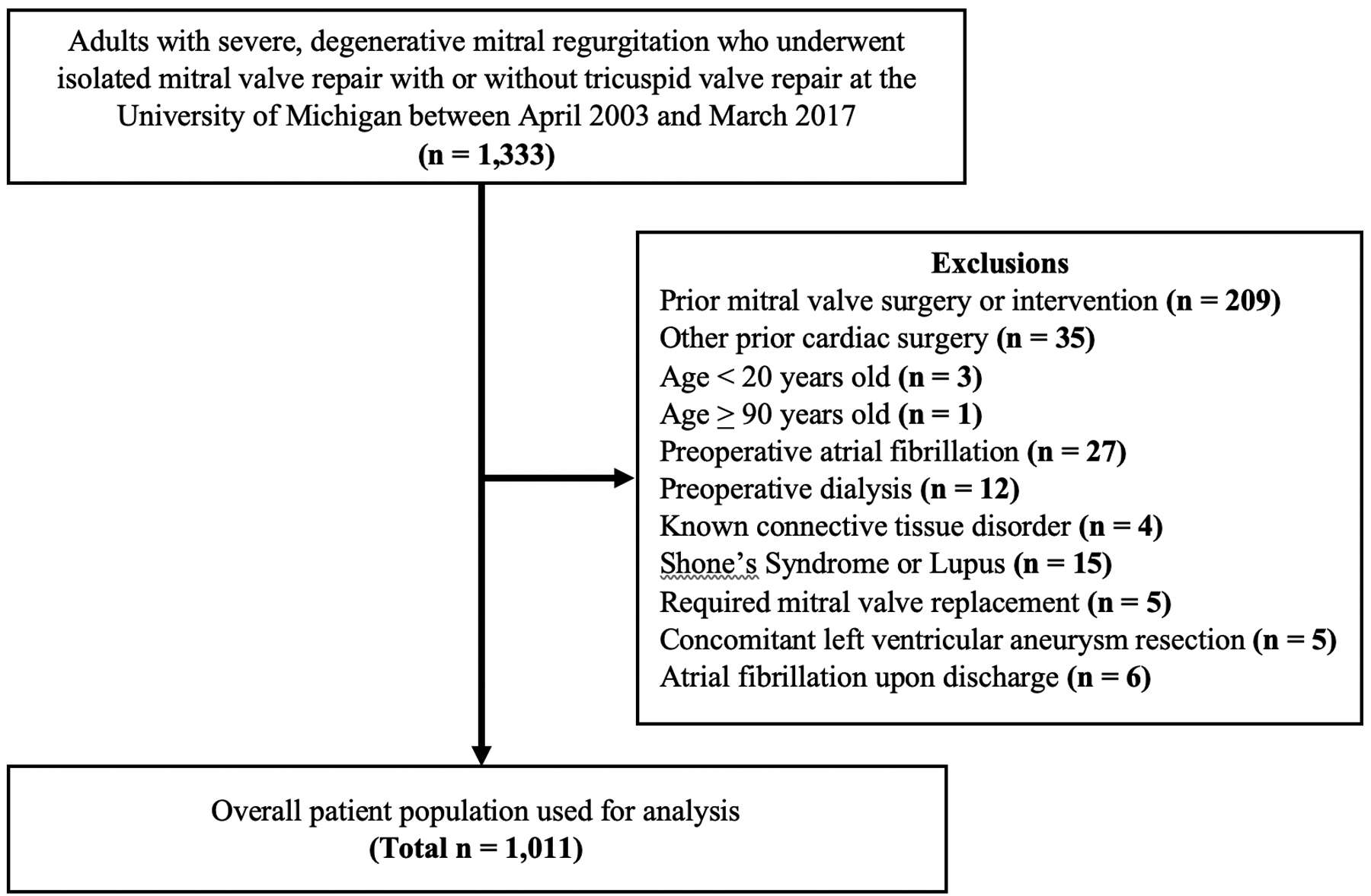
CONSORT Diagram of Patient Population
Inclusion and Exclusion Criteria
Patients 20 to 89 years old with severe, degenerative MR who underwent isolated MVr with or without tricuspid valve repair were selected. Patients who had undergone previous cardiac surgery, including coronary artery bypass grafting, surgical ablation, or aortic valve replacement were excluded. In addition, patients with pre-existing conditions that would otherwise impact their longevity, independent of MVr, were excluded. These conditions included preoperative atrial fibrillation, dialysis, or history of Marfan Syndrome, Lupus, Ehlers-Danlos, Loeys-Dietz, or Shone’s Syndrome.
Data Collection
Clinical data for the post-MVr cohort were collected using a combination of the STS NCSD, the University of Michigan Research Database, and chart review. Preoperative characteristics for the post-MVr cohort were collected including age, height, weight, sex, ejection fraction, valve morphology, current smoking status, and history of hypertension, hyperlipidemia, diabetes, cerebrovascular disease, peripheral vascular disease, liver disease, cancer, endocarditis, and immunosuppression. Intraoperative and postoperative characteristics were collected including cross-clamp time, cardiopulmonary bypass time, and postoperative length of stay. Patient death was confirmed through at least one of three methods: institution electronic health records, STS database file, or the National Death Index, which was utilized to indicate death but not to confirm alive status.
Life expectancy norms for each decade of life for the general U.S. population were collected from the 2011 Complete Annual United States Life Tables, provided by the CDC. The CDC’s published life expectancy data use vital statistics and census data to calculate death rates for ages less than 85 years and uses Medicare data for ages 85 years and older.
Primary Endpoint
The primary endpoint of this study was to examine the additional life expectancy of patients who underwent MVr compared with the general U.S. population across multiple decades of life.
Statistical Analysis
Baseline characteristics and comorbidities of the post-MVr cohort were compared to that of the general U.S. population using a one-sample t-test for quantitative variables and a proportion test for categorical variables. Normality assumption for the one sample t-test was verified using the Anderson-Darling and Ryan-Joiner Goodness-of-Fit statistics.
Post-MVr patients were categorized into age groups at the time of surgery. Decades 2–3 and 7–8 were combined due to smaller sample size and fewer number of deaths that occurred in these subgroups, limiting the power and predictability of the statistical modeling. Mortality data for each age group was evaluated to identify an appropriate distribution to perform subsequent survival analyses. In particular, a parametric distribution analysis of right-censored data assessed the fit of fourteen types of distributions including: normal, log-normal, exponential, and Weibull. The Weibull probability distribution provided the best fit among a variety of potential distributions for each age group of the post-MVr cohort. A Weibull distribution was applied to each decade allowing the parameters to vary, which is consistent with sampling subsets of a Weibull parent distribution. Anderson-Darling Goodness-of-Fit testing was used to evaluate how well the Weibull distribution fit the mortality data for each age group.
The parameter chosen to make the comparison between the post-MVr cohort and the general U.S. population was the median additional life expectancy for each age group. The fitted Weibull distributions for additional life expectancy after MVr yielded the values for the expected median and its 95% lower confidence bound. Confidence intervals for the median additional life expectancy of the post-MVr cohort were compared to the median additional life expectancy of the general U.S. population for each age group. Since the null hypothesis was that MVr does not restore life expectancy, the appropriate confidence intervals are one-sided, lower bound. Given the relatively few uncensored values, the estimated Weibull distributions are right-skewed, so the upper bound for confidence intervals is non-physiologically large. A Kaplan-Meier survival analysis was used to assess the survival probability of post-MVr patients for each age group.
Results
The study cohort consisted of 1011 patients with mean age 57±13 (range 20–88) years, 44% of whom were female. Table 1 describes the post-MVr cohort preoperative characteristics stratified by age compared with that of the general U.S. population, as well as intraoperative and postoperative case characteristics. Many of the characteristics listed for our post-MVr patients in Table 1 are not available for the general U.S. population through the CDC census data. In the post-MVr cohort, there were 103 patients in the 20–39 year-old group, 206 patients in the 40–49 year-old group, 272 patients in the 50–59 year-old group, 254 patients in the 60–69 year-old group, and 176 patients in the 70–89 year-old age group. Mean ejection fraction for the post-MVr cohort was 56±12% and was similar among age groups (Table 1). There were 156 patients (15.4%) who underwent concomitant tricuspid valve repair. Among all post-MVr patients, 30-day mortality was 0.4%, 6-month mortality was 1.3%, 1-year mortality was 1.8%, 5-year mortality was 7.9%, and 10-year mortality was 13.9%. To date, 30 patients in the study (3%) have undergone mitral reoperation, 19 (63%) of whom had mitral valve replacements, and 11 (37%) of whom had repeat repairs. Mean time to mitral reoperation was 23±3 (range 0–77) months. Median time to follow-up was 5.3 years (interquartile range 6.5 years).
Table 1.
Patient and Case Characteristics by Age
| Mitral Valve Repair Patients | U.S. Population | ||||||
|---|---|---|---|---|---|---|---|
| 20–39 yrs (n=103) | 40–49 yrs (n=206) | 50–59 yrs (n=272) | 60–69 yrs (n=254) | 70–89 yrs (n=176) | All (n=1011) | ||
| Height, cm ± SD | 172 ± 11 | 172 ± 12 | 173 ± 10 | 170 ± 10 | 168 ± 10 | 171 ± 11 | 169 cm |
| Weight, kg ± SD | 79 ± 21 | 83 ± 20 | 84 ± 18 | 77 ± 16 | 75 ± 17 | 80 ± 19 | 80 kg |
| Male, n (%) | 55 (53) | 116 (56) | 173 (64) | 131 (52) | 95 (54) | 570 (56) | 49% |
| Hypertension, n (%) | 22 (21) | 77 (37) | 112 (41) | 134 (53) | 112 (64) | 457 (45) | 33% |
| Hyperlipidemia, n (%) | 54 (52) | 85 (41) | 56 (21) | 139 (55) | 95 (54) | 429 (42) | N/A |
| Diabetes, n (%) | 3 (3) | 24 (12) | 24 (9) | 25 (10) | 23 (13) | 99 (10) | 9% |
| Cerebrovascular Disease, n (%) | 6 (6) | 11 (5) | 11 (4) | 22 (9) | 22 (13) | 72 (7) | N/A |
| Peripheral Vascular Disease, n (%) | 0 (0) | 6 (3) | 4 (1) | 7 (3) | 7 (4) | 24 (2) | N/A |
| Current Smoker, n (%) | 15 (19) | 47 (27) | 52 (21) | 38 (17) | 33 (21) | 185 (18) | 16% |
| Liver Disease, n (%) | 1 (1) | 0 (0) | 0 (0) | 5 (2) | 2 (1) | 8 (1) | 2% |
| Cancer, n (%) | 7 (7) | 4 (2) | 7 (3) | 9 (4) | 4 (2) | 31 (3) | N/A |
| Endocarditis, n (%) | 7 (6.80) | 8 (3.88) | 20 (7.35) | 9 (3.54) | 17 (9.66) | 61 (6.03) | N/A |
| Immunosuppressed, n (%) | 3 (3) | 6 (3) | 7 (3) | 6 (2) | 2 (1) | 24 (2) | N/A |
| Ejection Fraction, % ± SD | 55 ± 9 | 55 ± 14 | 58 ± 12 | 57 ± 12 | 56 ± 12 | 56 ± 12 | N/A |
| Valve Morphology | |||||||
| Isolated Anterior, n (%) | 4 (4) | 4 (2) | 3 (1) | 8 (3) | 5 (3) | 24 (2) | N/A |
| Bileaflet, n (%) | 15 (15) | 98 (48) | 94 (35) | 29 (11) | 27 (15) | 263 (26) | N/A |
| Isolated Posterior, n (%) | 84 (82) | 104 (50) | 175 (63) | 217 (85) | 144 (82) | 724 (72) | N/A |
| Cross-Clamp Time, min ± SD | 54 ± 19 | 54 ± 13 | 62 ± 33 | 53 ± 14 | 88 ± 33 | 62 ± 27 | N/A |
| Cardiopulmonary Bypass Time, min ± SD | 75 ± 24 | 75 ± 18 | 85 ± 38 | 73 ± 17 | 112 ± 41 | 84 ± 33 | N/A |
| Postop Length of Stay, days ± SD | 5 ± 4 | 5 ± 4 | 6 ± 4 | 5 ± 4 | 5 ± 4 | 5 ± 4 | N/A |
Figure 2 represents Weibull probability plots for additional life expectancy by age group in the post-MVr cohort. As demonstrated by Anderson-Darling Goodness-of-Fit tests, there is high confidence about the fit of the Weibull survival distribution for decades 4, 5, 6, and 7. However, in decades 2, 3, and 8 there have not yet been enough deaths in these age groups to develop reliable survival probability predictions. These Weibull probability plots have more curved lower confidence bands with more variation in the tails of the distributions. Consequently, to generate more reliable parameter estimates for the fitted Weibull distributions, decades 2 and 3 and decades 7 and 8 were combined for subsequent comparison analyses with the general U.S. population.
Figure 2.
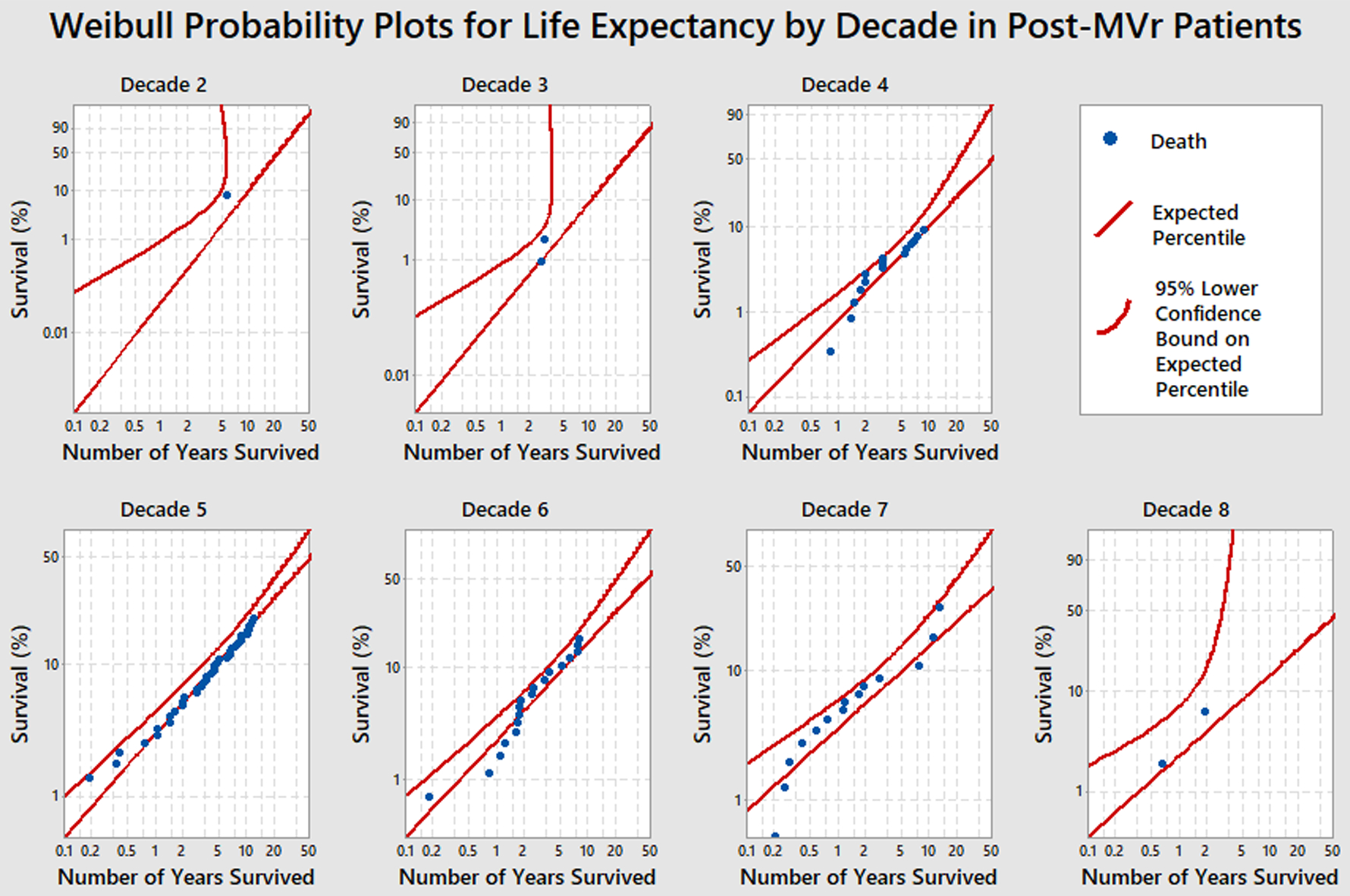
Weibull Probability Plots for Life Expectancy by Decade in Post-MVr Patients
Figure 3 illustrates the additional years of life expectancy by age group for the post-MVr cohort compared with the general U.S. population. The Weibull survival distributions were applied to the post-MVr cohort to determine the estimated median and corresponding 95% lower confidence bound for the median of each age group. The additional life expectancy of post-MVr patients was non-inferior to and matched the median additional life expectancy of the general U.S. population at any age between 40–89 at the alpha=0.05 level. The lower-bound one-sided 95% confidence intervals for the median additional life expectancy in post-MVr patients were not significantly different from the corresponding median additional life expectancy for the general U.S. population. In fact, there was a slight statistical improvement in additional life expectancy in the post-MVr cohort for ages 50–89, but this difference may not have clinical significance. Very few deaths occurred in the 20–39 year-old age group, limiting predictability; however, survival in this group also appeared to be normative.
Figure 3.
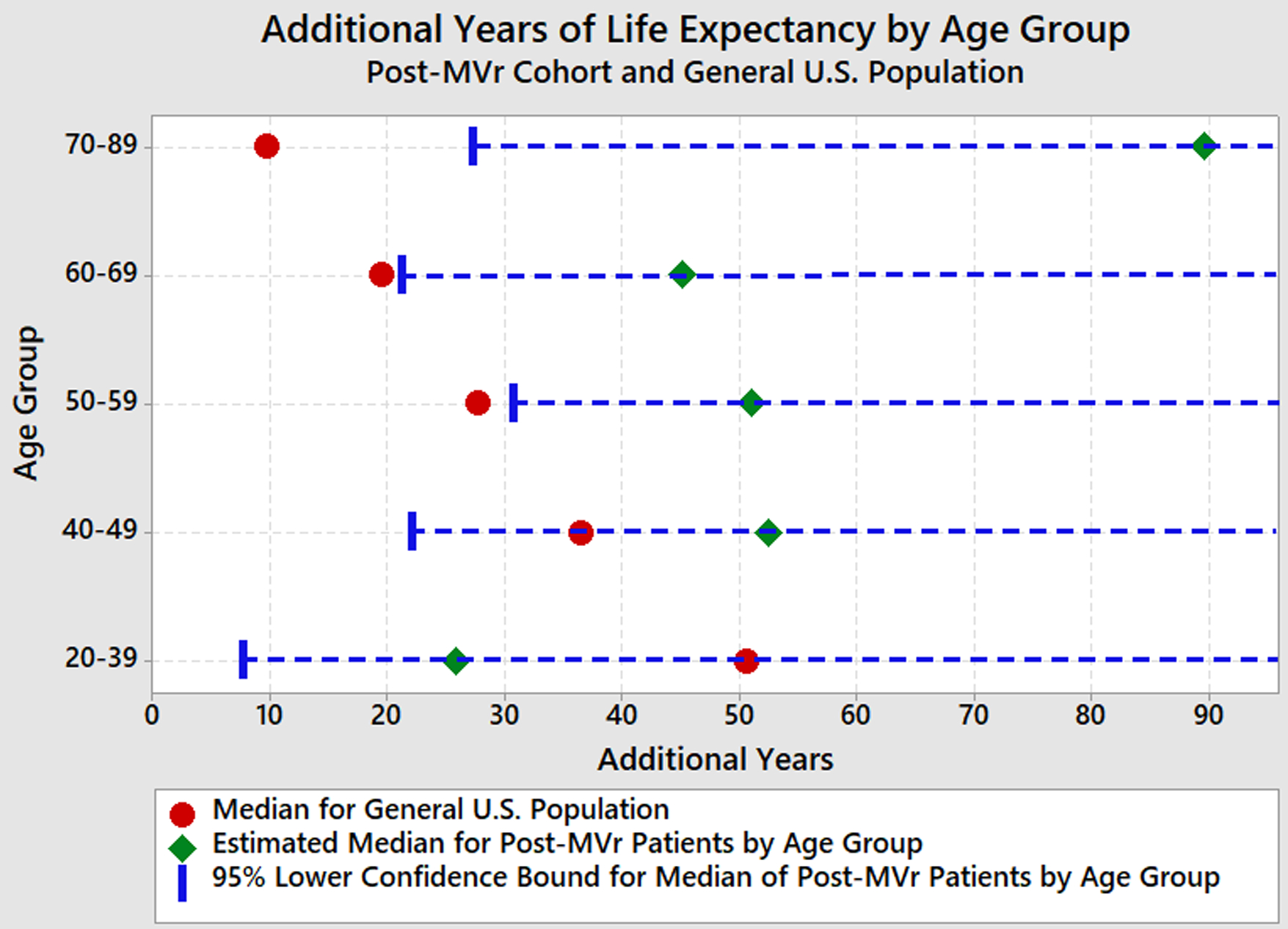
Additional Years of Life Expectancy by Age Group
Figure 4 depicts the Kaplan-Meier survival analysis of the post-MVr patient cohort by age group for 14 years after surgery. Survival estimates at 10 years after surgery for the 20–39 year-old age group was 94.6%, for the 40–49 year-old age group was 90.5%, for the 50–59 year-old age group was 83.7%, for the 60–69 year-old age group was 82.3%, and for the 70–89 year-old age group was 89.5%.
Figure 4.
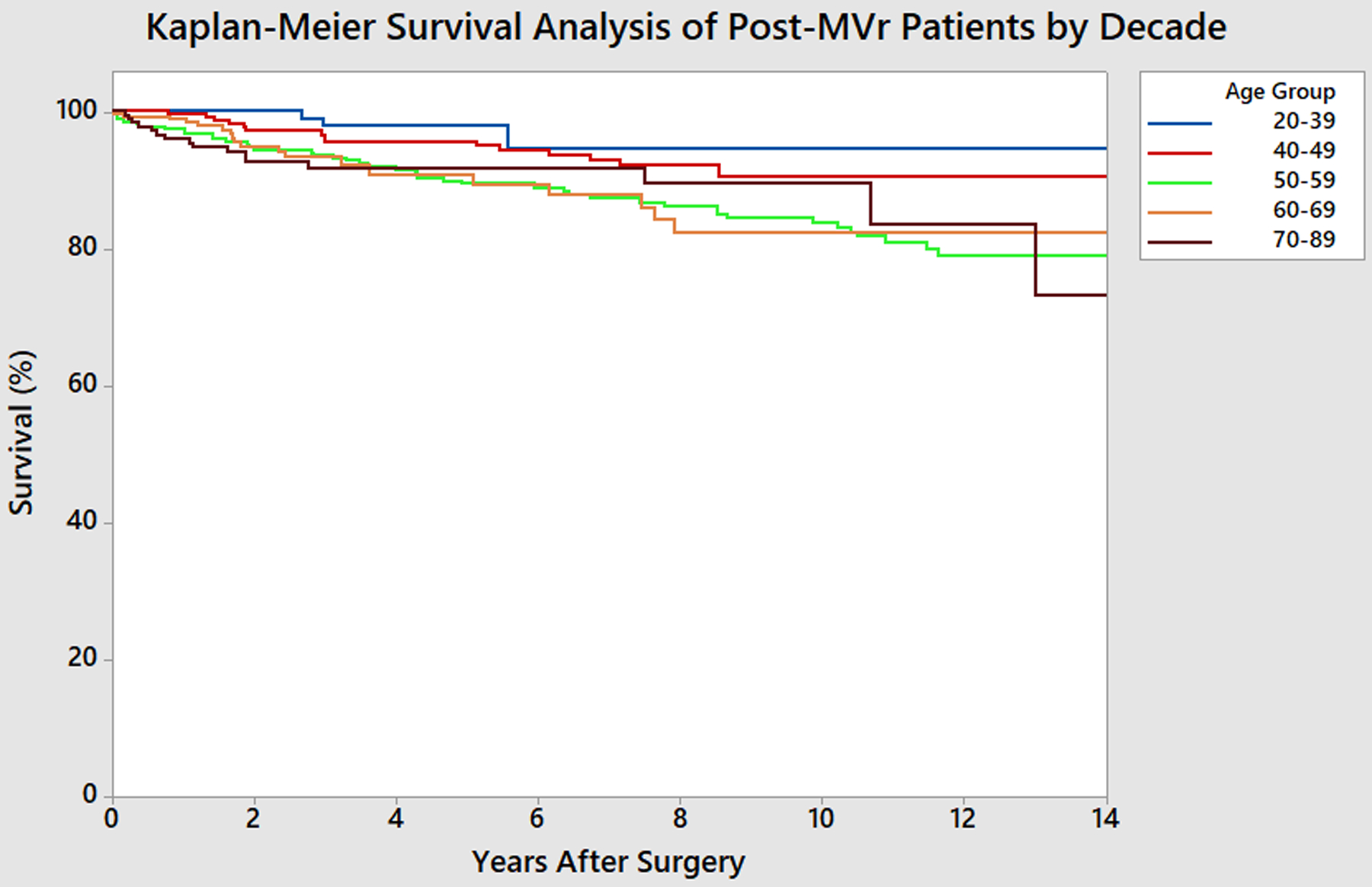
Kaplan-Meier Survival Analysis of Post-MVr Patients by Decade
Comment
In evaluating the long-term survival of a post-MVr cohort, this study found that life expectancy after MVr was non-inferior to and matched the median life expectancy of the general U.S. population at any age between 40–89. The lower-bound one-sided 95% confidence intervals for additional life expectancy in post-MVr patients were not significantly different from the corresponding median additional life expectancy for the general U.S. population. The few deaths to date in the 20–39 year-old cohort limit our ability to predict life expectancy at this time; however, survival in this group also appeared to be normative.
Of note, the estimated median additional life expectancy for some of the age groups in the post-MVr cohort were large, likely due to greater variation in the parameter estimates as well as the inherent right-skew of the Weibull distributions given that not all deaths have occurred yet. As a result, the appropriate 95% confidence intervals are one-sided and lower bound. While the additional life expectancy of these groups can be compared and determined equivalent, the actual number of additional years of life expectancy for each group may not translate clinically given the impact of incomplete death data on the parameter estimates. For example, in the 70–89 year age group, the additional years of life expectancy does not clinically translate due to a mathematical anomaly inherent in the statistical analysis caused by two factors: 1) there is a clinical plateau for maximum life expectancy not factored into the statistical analysis, and 2) there is appropriately no upper confidence bound on these estimates because many of the patients in the cohort have not died yet. While these results do not imply that these patients will live indefinitely, we can make the claim that the additional life expectancy for these groups is not significantly different than the general U.S. population. Similarly, even though there was slight statistical improvement in additional life expectancy of the post-MVr cohort for ages 50–89, this difference may not have clinical meaning due to the right-skew. However, we feel confident stating that the additional life expectancy was non-inferior and at least matched the corresponding life expectancy for the general U.S. population.
Interestingly, it is clinically plausible that the elderly patients who undergo MVr are actually healthier than their age-matched counterparts in the general U.S. population. Patients who are motivated and well enough to undergo mitral valve surgery at an old age are typically healthy, elderly patients without major comorbidities that may preclude surgery, committed to leading a healthy lifestyle with adequate social support to optimize recovery after surgery. One could speculate that these patients may actually live longer than similarly-aged elderly individuals who do not undergo mitral valve surgery.
Multiple prior studies have supported early MVr in order to improve long-term patient outcomes. Indeed, several studies have demonstrated that the risk associated with MVr in asymptomatic patients is quite low, and in a substantial proportion of patients watchful waiting is associated with subsequent development of indications for surgery [17–19, 21]. Another study of 192 asymptomatic patients with severe, degenerative MR found superior 10-year survival with early surgery compared with conservative management [20]. In addition, one study of 478 patients evaluated the impact of preoperative symptoms on postoperative outcomes after MVr and found that higher NYHA class was associated with excess mortality after MVr, providing more evidence in support of early surgery [28]. Furthermore, a retrospective study comparing 488 patients undergoing degenerative MVr to the age-matched general population found worse long-term survival after MVr; however, in a subset analysis of asymptomatic patients, long-term survival after MVr was comparable to the general population [6].
Despite these existing data, timing of surgical repair for severe MR remains controversial. To our knowledge, the current study is the first to demonstrate comparable long-term survival of post-MVr patients by age group compared with age-matched individuals in the general U.S. population. In addition, our analysis has a larger sample size (n=1011) than prior studies and our findings reinforce the importance of early MVr to avoid the complications associated with long-standing MR.
This study has several limitations. It is a retrospective study that reflects the clinical practice involving several surgeons at a single academic institution. We were unable to account for all elements of selection bias and changes in clinical practice over time. Although the overall sample size of this patient cohort is large, the relatively few deaths in the youngest and oldest patient subgroups limited survival predictability in those age groups. In addition, this study only evaluated long-term survival among patients who underwent MVr, and therefore was not able to draw any comparisons with survival of those who underwent mitral valve replacement. Furthermore, as a retrospective study spanning 14 years of data, we were unable to fully characterize preoperative symptomatology. Moreover, reoperation data was obtained through a combination of the STS NCSD, the University of Michigan Research Database, and chart review. Although it would be rare for patients to undergo reoperation at an institution that does not report outcomes to the STS database, we acknowledge this as a limitation of the scope of our data collection. Additionally, this study was unable to control for the quality of MVr performed and lacks long-term echocardiographic follow-up to evaluate for long-term repair durability. However, the standard of practice at our institution combined with the excellent long-term survival and very low mitral reoperation rate reported in this study suggest that the quality of MVr performed was generally very good.
In terms of generalizability, these results reflect the long-term survival of patients with degenerative MR who underwent MVr from a practice that reports excellent outcomes, with an institutional 7-year mitral repair rate of 99.1% (661/667) for degenerative mitral disease between 2012 and 2018. Importantly, the conclusions drawn from this study may not apply for other etiologies of MR, or when the quality of MVr is in question or repair rate is not as high. However, this point further emphasizes the importance of performing MVr at a high rate with excellent long-term outcomes. In fact, for asymptomatic patients with preserved heart function and chronic, severe, primary MR the 2014 AHA/ACC guidelines only recommend MVr if the likelihood of a successful and durable repair without residual MR is >95% with an expected mortality rate of <1% when performed at a Heart Valve Center of Excellence [29].
MVr remains underutilized despite explicit advantages when compared to prolonged medical management. Optimal medical management including beta-blockers and angiotensin converting enzyme (ACE) inhibitors may lessen symptoms but do not offer a long-term solution to degenerative mitral disease. These data validate the strong belief that MVr should be the treatment of choice for severe, degenerative MR, regardless of age at operation. Not only do these findings indicate that MVr may restore survival across multiple decades of life, but they also reinforce the importance of early MVr to avoid the complications associated with long-standing MR. This study has important implications not only for surgeons but also for referring providers. These excellent long-term survival data should help mitigate the concerns of providers for referring otherwise healthy patients for early degenerative mitral surgery. Larger studies with longer-term follow-up are needed to reinforce this finding, particularly for the younger age group.
Acknowledgements/Disclosures:
A.A.B. is supported by the National Research Service Award postdoctoral fellowship (No. 5T32HL076123).
Footnotes
Meeting Presentation:
This manuscript was presented at the 65th Annual Meeting of the Southern Thoracic Surgical Association, Amelia Island, Florida, November 7–10, 2018.
References:
- 1.Enriquez-Sarano M, Akins CW, and Vahanian A, Mitral regurgitation. Lancet, 2009. 373(9672): p. 1382–94. [DOI] [PubMed] [Google Scholar]
- 2.Singh JP, et al. , Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol, 1999. 83(6): p. 897–902. [DOI] [PubMed] [Google Scholar]
- 3.Goel SS, et al. , Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol, 2014. 63(2): p. 185–6. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, et al. , 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2017. 70(2): p. 252–289. [DOI] [PubMed] [Google Scholar]
- 5.Bonow RO, et al. , 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol, 2008. 52(13): p. e1–142. [DOI] [PubMed] [Google Scholar]
- 6.David TE, et al. , Late outcomes of mitral valve repair for floppy valves: Implications for asymptomatic patients. J Thorac Cardiovasc Surg, 2003. 125(5): p. 1143–52. [DOI] [PubMed] [Google Scholar]
- 7.Shuhaiber J and Anderson RJ, Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur J Cardiothorac Surg, 2007. 31(2): p. 267–75. [DOI] [PubMed] [Google Scholar]
- 8.Gillinov AM, et al. , Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg, 2008. 135(4): p. 885–93, 893 e1–2. [DOI] [PubMed] [Google Scholar]
- 9.Vassileva CM, et al. , Hospital volume, mitral repair rates, and mortality in mitral valve surgery in the elderly: an analysis of US hospitals treating Medicare fee-for-service patients. J Thorac Cardiovasc Surg, 2015. 149(3): p. 762–8 e1. [DOI] [PubMed] [Google Scholar]
- 10.David TE, et al. , A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg, 2005. 130(5): p. 1242–9. [DOI] [PubMed] [Google Scholar]
- 11.Braunberger E, et al. , Very long-term results (more than 20 years) of valve repair with carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation, 2001. 104(12 Suppl 1): p. I8–11. [PubMed] [Google Scholar]
- 12.Mohty D, et al. , Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation, 2001. 104(12 Suppl 1): p. I1–I7. [DOI] [PubMed] [Google Scholar]
- 13.Suri RM, et al. , Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg, 2006. 82(3): p. 819–26. [DOI] [PubMed] [Google Scholar]
- 14.Anyanwu AC, Bridgewater B, and Adams DH, The lottery of mitral valve repair surgery. Heart, 2010. 96(24): p. 1964–7. [DOI] [PubMed] [Google Scholar]
- 15.Bonow RO, et al. , Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation, 1998. 98(18): p. 1949–84. [DOI] [PubMed] [Google Scholar]
- 16.Vahanian A, et al. , Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J, 2007. 28(2): p. 230–68. [DOI] [PubMed] [Google Scholar]
- 17.Rosenhek R, et al. , Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation, 2006. 113(18): p. 2238–44. [DOI] [PubMed] [Google Scholar]
- 18.Kang DH, et al. , Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation, 2009. 119(6): p. 797–804. [DOI] [PubMed] [Google Scholar]
- 19.Enriquez-Sarano M, et al. , Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med, 2005. 352(9): p. 875–83. [DOI] [PubMed] [Google Scholar]
- 20.Montant P, et al. , Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg, 2009. 138(6): p. 1339–48. [DOI] [PubMed] [Google Scholar]
- 21.Adams DH and Anyanwu AC, Valve Disease: Asymptomatic mitral regurgitation: does surgery save lives? Nat Rev Cardiol, 2009. 6(5): p. 330–2. [DOI] [PubMed] [Google Scholar]
- 22.Gammie JS, et al. , Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg, 2009. 87(5): p. 1431–7; discussion 1437–9. [DOI] [PubMed] [Google Scholar]
- 23.Fedak PW, McCarthy PM, and Bonow RO, Evolving concepts and technologies in mitral valve repair. Circulation, 2008. 117(7): p. 963–74. [DOI] [PubMed] [Google Scholar]
- 24.David TE, et al. , Long-term results of mitral valve repair for myxomatous disease with and without chordal replacement with expanded polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg, 1998. 115(6): p. 1279–85; discussion 1285–6. [DOI] [PubMed] [Google Scholar]
- 25.Bach DS, et al. , Failure of guideline adherence for intervention in patients with severe mitral regurgitation. J Am Coll Cardiol, 2009. 54(9): p. 860–5. [DOI] [PubMed] [Google Scholar]
- 26.Avierinos JF, et al. , Natural history of asymptomatic mitral valve prolapse in the community. Circulation, 2002. 106(11): p. 1355–61. [DOI] [PubMed] [Google Scholar]
- 27.Toledano K, et al. , Mitral regurgitation: determinants of referral for cardiac surgery by Canadian cardiologists. Can J Cardiol, 2007. 23(3): p. 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tribouilloy CM, et al. , Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation, 1999. 99(3): p. 400–5. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura RA, et al. , 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg, 2014. 148(1): p. e1–e132. [DOI] [PubMed] [Google Scholar]


