Abstract
This study examined trajectories of daily living skills, behavior problems, body mass index (BMI), and health conditions spanning nearly a decade in adolescents and adults with fragile X syndrome (N = 134; age range at study end = 19 – 49 years), examining the influences of sex and autism spectrum disorder (ASD) symptoms. Hierarchical linear modeling revealed that daily living skills and behavior problems showed early improvement that deteriorated at later ages, and BMI and health conditions increased linearly, worsening over time. Fewer ASD symptoms were associated with greater independence in daily living skills and fewer behavior problems at study start. This study offers some of the first prospective quantitative analyses of life course trajectories of behavior and health in FXS.
Keywords: Fragile X syndrome, behavioral phenotype, health phenotype, adolescents, adults
Fragile X syndrome (FXS) is a neurodevelopmental disorder caused by an expansion of CGG repeats on the FMR1 gene, which inhibits production of FMRP, a protein crucial for cognitive development (Brown, 2002). FXS is the most common inherited cause of intellectual disability and the most common genetic cause of autism spectrum disorder (ASD). Individuals with FXS often display behavior problems and functional limitations across the lifespan (Smith et al. 2012a; Smith et al. 2016). In addition, they experience physical health problems, such as being overweight and being in poor overall health (Raspa et al. 2010; Hartley et al. 2011). Although clinicians have made acute observations of FXS during adolescence and adulthood (Lee et al. 2018; Hersh and Saul 2011), very few prospective analyses of the behavioral phenotype and health associated with this disorder have been conducted. Much of our current empirical understanding about the behavioral and health phenotype in FXS comes from cross-sectional studies investigating relatively small samples of children and young adolescents. In the present study, longitudinal data spanning nearly a decade was utilized to examine trajectories of behavior (daily living skills and behavior problems) and health (body mass index [BMI] and number of health conditions) in individuals with FXS ranging from 19 to 49 years of age at the end of the study, accounting for sex and ASD symptoms.
Trajectories of Behavioral Characteristics
Only a few prior studies have applied longitudinal methods to examine behavioral profiles of adolescents and adults with FXS. Hustyi and colleagues found increases over six years in adaptive behavior and decreases in several behavior problem domains, including hyperactivity, inappropriate speech, and social withdrawal, in individuals with FXS ranging from 2 to 26 years of age (Hustyi et al. 2014). In a longitudinal examination of adaptive skills in a large sample of children and adolescents with FXS aged 2 to 18 years, Klaiman and colleagues observed increasing adaptive skills during adolescence and suggested that research using a sample with older participants could provide support for this trend (Klaiman et al. 2014). Cross-sectional work similarly suggests that possible age-related improvement in daily living skills during adolescence and adulthood (Raspa et al., 2018).
A great deal of previous research indicates that behavior problems are the most significant source of stress for family caregivers of adolescents or adults with developmental disabilities in general and FXS in particular (Hartley et al. 2012; Woodman 2014; Chan et al. 2017; Baker et al. 2012). A longitudinal study of behavior problems in boys with FXS suggest that challenging behavior may remain stable over a three year period during childhood (Hatton et al., 2002). However, there is a dearth of literature on trajectories of daily living skills and behavior problems across adolescence and adulthood in individuals with FXS.
Trajectories of Health
There are phenotypic health problems associated with FXS that may be stable across the lifespan, such as macrocephaly and strabismus (Rajaratnam et al. 2017), but there may also be other non-syndrome specific health problems that co-occur and may change over time. Notably, no previous study has examined longitudinal trajectories of health in individuals with FXS. Cross-sectional studies have suggested that increased body weight and poor overall health may be associated with the FXS phenotype. For example, obesity has been identified as a health problem in individuals with FXS at different points in the lifespan (McLennan et al. 2011; Utari et al. 2010; Raspa et al. 2010). The relation between obesity and FXS is multifaceted, as the FMR1 deficiency in FXS has a molecular association with obesity and individuals who take medications for associated health problems may be susceptible to medication-linked weight gain (McLennan et al. 2011). In addition, high food selectivity and low physical exercise rates may play a role (Raspa et al. 2010), which have also been found in individuals with ASD with and without intellectual disability (Fodstad and Matson 2008; Kuschner et al. 2015; Eaves and Ho 2008). These findings suggest the importance of examining the longitudinal trajectory of obesity in individuals with FXS.
In addition to obesity, other health problems non-specific to FXS may co-occur in individuals with a diagnosis of FXS for several reasons. Among people with intellectual disability, there is a higher level of health problems and higher rates of mortality than in the general population (van Schrojenstein Lantman-De Valk et al. 2000; Fisher 2004), possibly the result of barriers to and lower rates of health care utilization (Heller and Sorensen 2013). Over the course of time, these factors may have a cumulative effect of increasing the number of health problems that people experience. However, there is limited longitudinal research on non-syndrome specific health phenotypes in FXS.
There are additional factors that may impact trajectories of behavior and health over time for those with FXS. The prevalence of ASD that co-occurs with FXS has been estimated around 43–75% for males and 16–45% for females (Bailey et al. 2008; Demark et al. 2003; Harris et al. 2008; Hernandez et al., 2009; Klusek et al., 2014). Previous literature on the behavioral phenotype has demonstrated differences between adolescents and adults with FXS with and without co-occurring ASD. For example, in a cross-sectional study from our group, three sets of adolescents and adults were compared: Adolescents and adults with FXS were compared with individuals with FXS and ASD as well as individuals with ASD only (Smith, Barker, Seltzer, Abbeduto, & Greenberg, 2012). Those with FXS and ASD had higher levels of behavior problems than did those with ASD only or FXS only. Other studies provide evidence of an association between ASD symptoms and increases in problem behavior over time in individuals with FXS (H. Crawford et al. 2018). It also has been shown that adolescents and adults with FXS who have higher ASD symptom severity had lower levels of independence in daily living skills (Hustyi et al. 2015). Increased health care use in individuals with ASD suggests that ASD symptoms may have the potential to negatively impact health in FXS (Gurney et al. 2006). Taken together, these findings suggest that across outcomes such as daily living skills, behavior problems, and physical health, FXS with ASD may be associated with more symptomatic profiles than those of individuals with a single disorder of either FXS or ASD only.
In addition to ASD symptoms and age, sex is a factor that may impact mean levels and trajectories of behavior and health in individuals with FXS. In the context of FXS, males are generally more severely affected because of X-inactivation, in which one of a female’s two X chromosomes is inactivated (D. C. Crawford et al. 2001). Females with FXS display higher levels of adaptive behavior and lower levels of both behavior problems and ASD symptoms than males during adolescence and adulthood (Smith et al. 2016; Hartley et al. 2011). Decreases in problem behavior over time have been found to be significantly greater for females than males with FXS (Hustyi et al. 2014). Females with FXS are often less severely affected in terms of cognitive, developmental, and psychiatric profiles (Freund et al. 1993), which may have an impact on both starting points and trajectories across the lifespan.
Present Study
In this study, we utilized longitudinal data to investigate trajectories of daily living skills, behavior problems, BMI, and the number of health conditions experienced by adolescents and adults with FXS. In a prior report focused on the same sample, we examined trajectories of change in phenotypic characteristics in adolescents and adults with FXS over a three-year period (Smith, Hong, Greenberg & Mailick, 2016). The present study extended the duration of follow-up to nearly a decade, thus incorporating measures of individuals with FXS in midlife, and expanded the phenotypic features under analysis to include measures of health. In addition, the present study also examined the effects of lifetime ASD symptoms and sex on behavioral and health trajectories during adulthood.
Method
Participants
Study participants were drawn from a multi-wave, longitudinal study of 147 individuals with FXS and their families (Mailick, Greenberg, Smith, Sterling, Brady, Warren, & Hong, 2014). Families in the study had a son or daughter diagnosed with FXS who was 12 years of age or older who either lived in the parental home or or had at least weekly contact with the mother, who was the primary respondent for the study. In families with multiple children with FXS, the target adolescent or adult (i.e., focus of study) was selected based on the following criteria and order: the son or daughter who was ≥ 12 years of age, who resided with mother, and who was the most severely affected as judged by the mother.
The current study utilized four waves of data, which were collected from 2008 to 2017. Of the original sample, 13 individuals were excluded from this analysis because they did not have intellectual disability, as having or not having intellectual disability could influence trajectories, and there were too few individuals without intellectual disability in the sample to investigate them as a separate group. An additional 9 individuals were excluded from hierarchical linear models because of missing data on key variables, resulting in a sample of 125 individuals. Individuals with FXS were mostly males (85.1%), around 20 years of age on average at the start of the study (M = 20.19, SD = 6.92, range 12–49 years), and most lived with their mothers (90.3%). As noted, the study participants who lived away from the parental home had at least weekly contact with their mothers. About one quarter (24.2%) of the sample had a co-occurring diagnosis of ASD based on confirmation of maternal report using a consensus procedure involving review of medical and educational records (DaWalt, Usher, Greenberg, & Mailick, 2017).
Mothers were age 50 on average (M = 49.98 years, SD = 7.36, range 35–79 years), and nearly all were white/non-Hispanic (94.8%). The majority had some college education or more (85.8%) and were married or living with a partner (82.9%). The median household income at the beginning of the study was $80,000 –89,999 (range $1,000 – $160,000+). Most mothers in the study had the FMR1 premutation (92.5%) with the remaining mothers having the full mutation of FXS (2.2%), mosaicism (2.2%), normal CGG repeats (0.7%), or missing CGG repeat information (2.2%).
Procedure
Mothers provided data on their sons or daughters with FXS through self-administered questionnaires and telephone interviews at four time points, unless otherwise noted below. The study spanned nearly a decade, with individual families’ participation spanning 7 to 9 years between Time 1 and Time 4. Time 1 and Time 2 were separated by an average of two years (M=2.13 years; SD=.11 years); Time 2 and Time 3 were separated by an average of 18 months (M =1.45 years; SD=.08 years), and Time 3 and Time 4 were separated by an average of four years (M=3.98 years; SD=.23 year). The Institutional Review Board at the University of Wisconsin-Madison approved the data collection protocol. Informed consent was obtained from all individual participants included in the study.
Measures
Daily Living Skills. The Waisman Activities of Daily Living scale (W-ADL; Maenner et al. 2013) was used to index independence in daily living skills. Mothers rated their son or daughter’s level of independence on 17 items relating to personal care, housekeeping, meal-related activities, and community activities such as dressing and grooming, making one’s bed, preparing a meal, and managing money. Each item was rated on a 3-point scale: 0 (does not perform the task at all), 1 (performs the task with help), or 2 (performs the task independently), and items were summed. Thus, a score of 34 indicates complete independence. Coefficient alphas for the total score in the current sample ranged from .87 to .91 for Times 1–4. This measure has strong psychometric properties and is sensitive to change over time (Maenner et al., 2013; Smith et al. 2012b). This measure is strongly associated with the daily living skills score in the Vineland (r = .82; Maenner et al., 2013) and takes less than 10 minutes to administer.
Behavior Problems. The Behavior Problems subscale of the Scales of Independent Behavior-Revised (SIB-R; Bruininks et al. 1996) assessed eight behavior problem types: (a) self-injurious behavior, (b) unusual or repetitive behaviors, (c) withdrawn or inattentive behavior, (d) behavior that is hurtful to others, (e) property destruction, (f) disruptive behavior, (g) socially offensive behavior, and (h) uncooperative behavior. If a behavior problem was endorsed, mothers rated frequency from 1 (less than once a month) to 5 (one or more times per hour) and severity from 1 (not serious) to 5 (extremely serious). Standardized algorithms (Bruininks et al., 1996) were used to create a general maladaptive behavior summary score, with higher scores indicating more severe maladaptive behaviors. A score of 110 or above indicates clinically significant behavior problems. Reliability and validity of this measure have been established by Bruininks et al. (1996). This measure is sensitive to change over time (Smith et al., 2016; Woodman et al., 2015).
Body Mass Index. Body Mass Index (BMI) was calculated for all participants using height and weight. BMI is a ratio of weight to height, calculated as weight in kilograms divided by the square of height in meters, that is used as an index of healthy versus unhealthy body weight (National Institutes of Health 2006). BMI of less than 18.5 indicates underweight, 18.5–24.9 indicates normal weight, 25–29.9 indicates overweight, and BMI of 30 or greater indicates obesity.
Number of Health Conditions. Mothers were asked whether their son or daughter had experienced or been treated for any of a list of 36 health conditions within the past 12 months. This list was adapted from the checklist of chronic conditions developed for the Midlife in the United States (MIDUS) Study (Midlife in the United States, n.d.) and included conditions including but not limited to allergies, persistent skin trouble, hypertension, sleep apnea, and thyroid disease. The number of health conditions experienced was collected at Times 1–3.
Lifetime Autism Spectrum Disorder Symptoms. A measure of lifetime ASD symptoms at Time 1 was included as a predictor of longitudinal outcomes. Lifetime ASD symptoms were measured using the Social Communication Scale (SCQ; Rutter et al. 2007), a parent report instrument for screening symptoms related to ASD. The SCQ was developed from 40 critical items of the Autism Diagnostic Interview (Lord et al. 1994). For the Lifetime SCQ measure, the mother chooses “yes” or “no” in response to questions asking whether behaviors have ever been present for items 2–19 and whether behaviors were present between 4 and 5 years of age for items 20–40. Scores are summed, with higher scores indicating greater numbers of autism symptoms.
Biological Sex
Sex (male = 1) was also examined as a predictor of outcomes.
Analytic Plan
We first examined descriptive data over time and visually inspected the individual trajectories using plots generated in R version 3.4.1 (R Core Team 2014). See Figures 1–4. In this visual examination, we plotted outcome variables by chronological age to understand individual trajectories in each outcome domain.
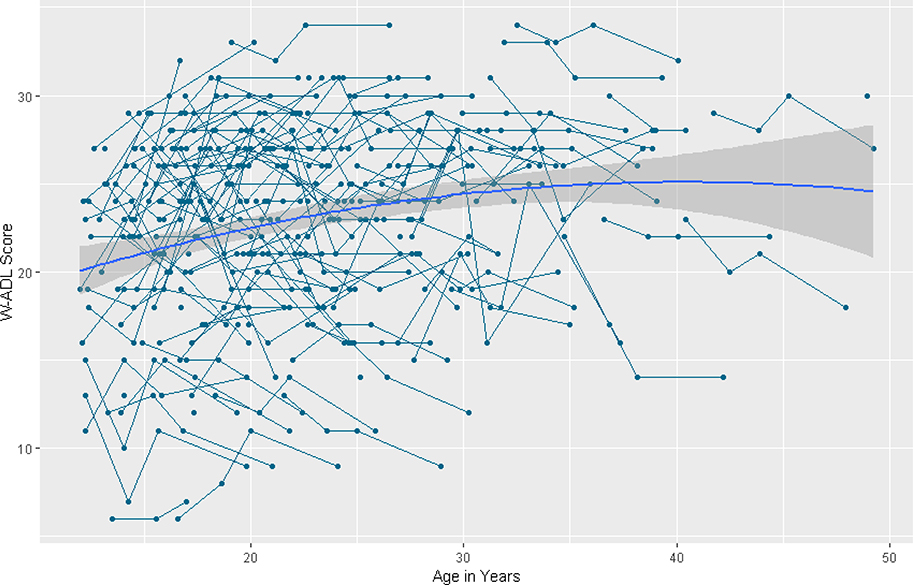
Figure 1.
Longitudinal individual and group trajectories of Waisman Activities of Daily Living scores.
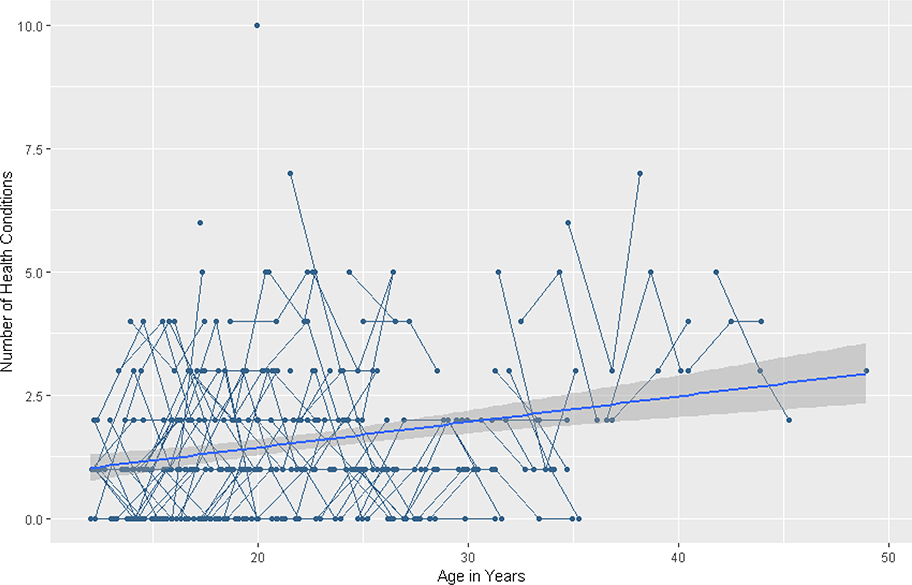
Figure 4.
Longitudinal individual and group trajectories of the number of health conditions.
Next, multilevel modeling was used to describe change in the behavioral and health phenotype of adolescents and adults over the course of nearly a decade and to examine associations of between-person differences in sex and ASD symptoms on phenotype trajectories. Separate growth curve models were evaluated using Hierarchical Linear Modeling (HLM) software version 7.03 for four different outcomes: daily living skills, behavior problems, BMI, and number of health conditions (Raudenbush et al. 2011). Change was modeled as a function of age.
We report findings from two models for each outcome: In an initial set of models, baseline linear growth models were used to describe patterns of change over time. To address within-person change, age was entered at level 1 and was centered at 12 years of age so that intercepts would represent initial levels of functioning. When a slope was non-significant in the baseline model, it was not included in the subsequent model. For the second set of models, to address between-person differences, sex and lifetime ASD symptoms at Time 1 (grand-mean centered) were entered at level 2 as predictors of the intercept and the slopes to examine whether ASD symptoms and sex interacted with the intercepts and slopes for each outcome. Non-significant predictors were removed from models. Finally, model fit statistics were examined to determine the final models with the best fitting results. Specifically, the change in the deviance statistic (−2 log likelihood) was compared across two nested models relative to the change in the number of parameters added to the model (Raudenbush and Bryk 2002).
Results
Descriptive Findings
See Table 1 for descriptive statistics across the four time points and Table 2 for a matrix of bivariate associations among variables at Time 1. Notably, at the end of the study period, 65.0% of the sample were in their 20s, 30.1% were in their 30s, and 4.9% were in their 40s. The average daily living score at each time point was approximately 23 on a scale in which 34 reflects complete independence. The percentage of the sample above the clinical cutoff for behavior problems (110 or greater) ranged from 52.4% at Time 1 to 27.8% at Time 4. In the current sample, BMIs at each wave ranged from 14 to 50. 13.5% were underweight at the start of the study, and 22.6% were overweight at that point. On average, sample members’ BMI increased over the study, with 32.2% being overweight by Time 4. The number of health conditions at each time point was less than 1.
Table 1.
Descriptive statistics.
| Time Point | Age M (SD), Range | Lifetime Autism Spectrum Disorder Symptoms M (SD) | Sex % Male | Daily Living Skills M (SD) | Behavior Problems M (SD) | % Above Behavior Problems Cutoff | Body Mass Index M (SD) | % Over-weight | % Obese | Number of Health Conditions M (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20.19 (6.92), 12.0 – 48.9 | 18.88 (7.86) | 82.3 | 22.54 (5.45) | 112.47 (10.37) | 52.4 | 25.15 (6.34) | 22.6 | 21.8 | 0.88 (1.34) |
| 2 | 22.16 (6.71), 14.1 – 43.8 | - | - | 23.05 (5.52) | 111.42 (10.21) | 45.4 | 25.83 (6.43) | 30.3 | 20.2 | 0.83 (0.94) |
| 3 | 23.88 (6.80), 15.5 – 45.3 | - | - | 23.41 (5.70) | 110.05 (8.65) | 41.3 | 26.66 (6.10) | 34.0 | 23.3 | 0.90 (1.19) |
| 4 | 27.79 (6.88), 19.3 – 49.3 | - | - | 22.67 (6.23) | 107.83 (9.10) | 27.8 | 28.32 (6.97) | 32.2 | 34.4 | - |
Table 2.
Bivariate correlations among variables of interest.
| Age | Lifetime ASD Symptoms | Sex (1 = Male) | Daily Living Skills | Behavior Problems | Body Mass Index | Number of Health Conditions | |
|---|---|---|---|---|---|---|---|
| Age | - | ||||||
| Lifetime ASD Symptoms | −.11 | - | |||||
| Sex (1 = Male) | .11 | .25** | - | ||||
| Daily Living Skills | .38*** | −.58*** | −.21* | - | |||
| Behavior Problems | −.17* | .50*** | .21* | −.54*** | - | ||
| Body Mass Index | .27** | .07 | .09 | .09 | .02 | - | |
| Number of Health Conditions | .31*** | .17+ | .12 | .05 | .16+ | .23** | - |
p <.10
p < .05
p < .01
p < .001.
Longitudinal Trajectories
Daily Living Skills. HLM results for daily living skills are summarized in Table 3. The baseline model for daily living skills revealed a significant quadratic slope, indicating that across adolescence and adulthood daily living skills changed in an inverted-U shape over time (β = −.01, SE = .003, p < .001). See Figure 1. There was significant variability in the intercept (χ2 = 111.50, df = 38, p < .001). However, there was no significant variance in linear slope (χ2 = 42.49, df = 38, p = .283) or quadratic slope (χ2 = 40.87 df = 38, p = .345).
Table 3.
Summary of Hierarchical Linear Model Results for Daily Living Skills (W-ADL).
| Baseline Model | Final Model | |
|---|---|---|
| Parameter | b (SE) | b (SE) |
| Intercept | 21.46 (0.59)*** | 22.24 (0.83)*** |
| Male | - | −0.96 (0.86) |
| Lifetime ASD Symptoms | - | −0.36 (0.04)*** |
| Linear Slope | 0.34 (0.08)*** | 0.34 (0.08) |
| Male | - | - |
| Lifetime ASD Symptoms | - | - |
| Quadratic Slope | −0.01 (.003)*** | −0.01 (0.003)*** |
| Male | - | - |
| Lifetime ASD Symptoms | - | - |
| Model Fit | ||
| Deviance (−2 Log Likelihood) | 2208.58 | 2147.94 |
| Δ Deviance | - | 60.64*** |
p <.10
p < .05
p < .01
p < .001.
Note: ASD = autism spectrum disorder.
The final model indicated significant quadratic change in daily living skills over time, (β = −.01, SE = .003, p < .001) in the shape of an inverted-U. Independence in daily living skills increased as people aged, but the rate of increase slowed over time and there was suggestion of decline in the oldest sample members. Higher levels of lifetime ASD symptoms were significantly associated with lower intercepts of daily living skills (β = −.36, SE = .04, p < .001). Sex was not significantly associated with the intercept or slope.
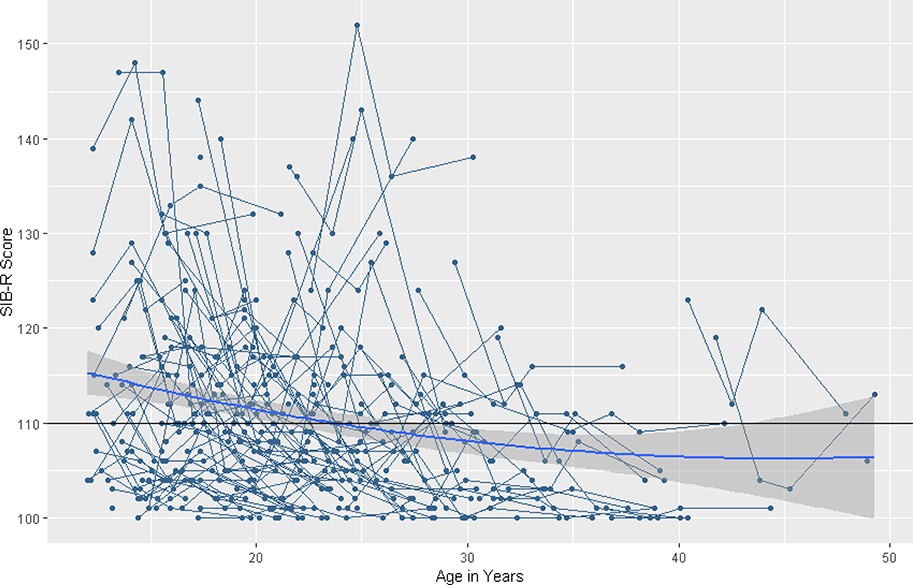
Behavior Problems. HLM results for behavior problems are summarized in Table 4. The baseline model for behavior problems revealed a significant quadratic slope in a U-shape, indicating that across adolescence and adulthood behavior problems changed in a curvilinear pattern over time (β = .01, SE = .01, p = .027). See Figure 2, noting the horizontal line indicating the clinical cutoff for the SIB-R behavior problems measure. There was significant variability in the intercept (χ2 = 96.07, df = 38, p < .001). However, there was no significant variability in the linear slope (χ2 = 34.83, df = 38, p < .500) or the quadratic slope (χ2 = 33.62, df = 38, p > .500).
Table 4.
Summary of Hierarchical Linear Model Results for Behavior Problems (SIB-R).
| Baseline Model | Final Model | |
|---|---|---|
| Parameter | b (SE) | b (SE) |
| Intercept | 116.86 (1.69)*** | 114.14 (2.11)*** |
| Male | - | 2.84 (1.83) |
| Lifetime ASD Symptoms | - | 0.41 (0.09)*** |
| Linear Slope | −0.78 (0.20)*** | −0.74 (0.19)*** |
| Male | - | - |
| Lifetime ASD Symptoms | - | - |
| Quadratic Slope | 0.01 (0.01)* | 0.01 (0.01)* |
| Male | - | - |
| Lifetime ASD Symptoms | - | - |
| Model Fit | ||
| Deviance (−2 Log Likelihood) | 2860.82 | 2833.59 |
| Δ Deviance | - | 27.23*** |
p <.10
p < .05
p < .01
p < .001.
Note: ASD = autism spectrum disorder.
Figure 2. Longitudinal individual and group trajectories of Scales of Independent Behavior-Revised Behavior Problem Subscale scores.
Note. The horizontal line indicates the clinical cutoff of 110 for the SIB-R.
The final model indicated significant quadratic change in behavior problems over time, (β = .01, SE = .01, p = .037), in a U-shape. Behavior problems decline as people age, but the rate of decline slowed down over time and there was suggestion of increase in the oldest sample members. Higher levels of lifetime ASD symptoms were significantly associated with higher intercepts of behavior problems (β = .41, SE = .09, p < .001). Sex was not significantly associated with the intercept or slope.
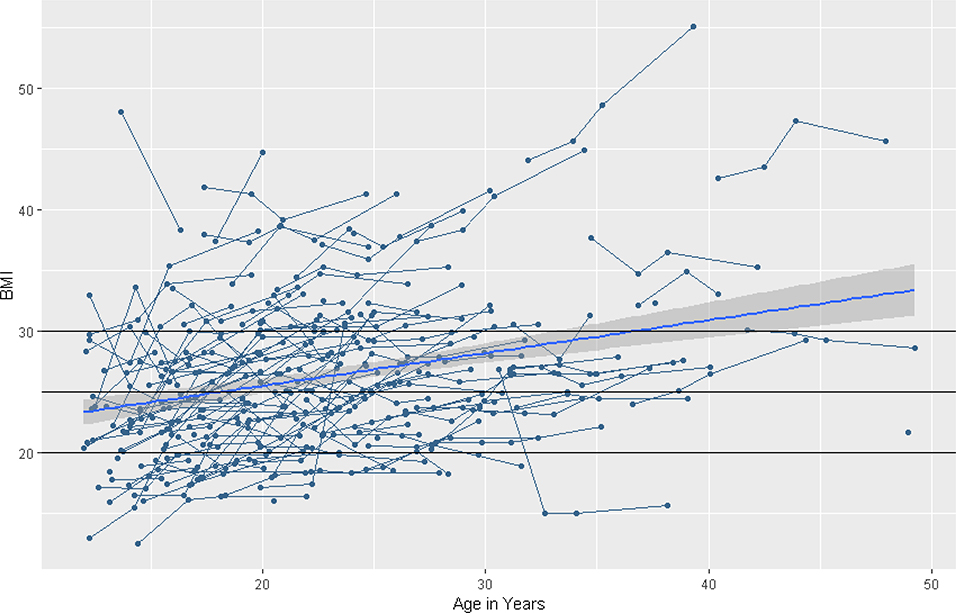
Body Mass Index. HLM results for BMI are summarized in Table 5. The baseline model indicated that BMI tended to increase in a linear pattern over time, on average (β = .37, SE = .05, p < .001). There was no evidence for curvilinear change over time. See Figure 3, noting the horizontal lines indicating the clinical cutoffs for BMI: <18.5 indicates underweight, 18.5–24.9 indicates normal weight, 25–29.9 indicates overweight, and ≥ 30 indicates obesity. There was significant variability in the intercept (χ2 = 699.37, df = 110, p < .001) and the linear slope (χ2 = 316.70, df = 110, p < .001).
Table 5.
Summary of Hierarchical Linear Model Results for Body Mass Index (BMI).
| Baseline/Final Model | Full Model | |
|---|---|---|
| Parameter | b (SE) | b (SE) |
| Intercept | 22.71 (0.70)*** | 21.26 (1.71)*** |
| Male | - | 1.77 (1.90) |
| Lifetime ASD Symptoms | - | −0.04 (0.09) |
| Linear Slope | 0.37 (0.05)*** | 0.48 (0.14)*** |
| Male | - | −0.13 (0.15) |
| Lifetime ASD Symptoms | - | −0.002 (0.01) |
| Quadratic Slope | - | - |
| Male | - | - |
| Lifetime ASD Symptoms | - | - |
| Model Fit | ||
| Deviance (−2 Log Likelihood) | 2174.82 | 2173.76 |
| Δ Deviance | - | 1.06 |
p <.10
p < .05
p < .01
p < .001.
Note: ASD = autism spectrum disorder.
Figure 3. Longitudinal individual and group trajectories of Body Mass Index.
Note. The horizontal lines indicate the cutoffs for BMI: <18.5 indicates underweight, 18.5–24.9 indicates normal weight, 25–29.9 indicates overweight, and ≥ 30 indicates obesity.
When ASD symptoms and sex were entered as predictors of BMI in the full model, the linear slope remained significant (β = .48, SE = .14, p < .001). However, lifetime ASD symptoms and sex were not significantly associated with the intercept for BMI, ps > .05. Thus, we retained the baseline model as the final model for describing BMI trajectories.
Number of Health Conditions
HLM results for number of health conditions are summarized in Table 6. The baseline model for health conditions revealed that overall, the number of health conditions increased in a linear pattern over time (β = .04, SE = .01, p < .001). There was no evidence for curvilinear change over time. See Figure 4. There was no significant variability in intercepts (χ2 = 101.20, df = 109, p > .500) or in linear slope (χ2 = 100.98, df = 109, p > .500).
Table 6.
Summary of Hierarchical Linear Model Results for Number of Health Conditions.
| Baseline/Final Model | Full Model | |
|---|---|---|
| Parameter | b (SE) | b (SE) |
| Intercept | 0.48 (0.16)** | 0.15 (0.17) |
| Male | - | 0.40 (0.22)+ |
| Lifetime ASD Symptoms | - | 0.03 (0.02) |
| Linear Slope | 0.04 (0.01)*** | 0.08 (0.02)*** |
| Male | - | −0.04 (0.02)+ |
| Lifetime ASD Symptoms | - | 0.00002 (0.002) |
| Quadratic Slope | - | - |
| Male | - | - |
| Lifetime ASD Symptoms | - | - |
| Model Fit | ||
| Deviance (−2 Log Likelihood) | 955.29 | 947.80 |
| Δ Deviance | - | 7.49 |
p <.10
p < .05
p < .01
p < .001.
Note: ASD = autism spectrum disorder.
When lifetime ASD symptoms and sex were entered as predictors of the number of health conditions in the full model, the linear slope remained significant (β = .08, SE = .03, p = .012). Neither lifetime ASD symptoms nor sex were significantly associated with the intercept or linear slope for the number of health conditions. We retained the baseline model as the final model for describing trajectories of the number of health conditions.
Discussion
This was the first study to examine longitudinal trajectories of the behavioral and physical health phenotype in adolescents and adults with FXS over nearly a decade, accounting for sex and ASD symptoms. Trajectories indicated increases in daily living skills and decreases in behavior problems over time, with deceleration at later ages. Trajectories for BMI and the number of health problems suggested patterns of weight gain and poorer health over time in a linear fashion. Higher levels of lifetime ASD symptoms were associated with lower levels of daily living skills and higher levels of behavior problems at the initial time point. Sex was not a significant predictor of these outcomes. All of these patterns indicate areas for support and intervention.
Adults with FXS pose significant challenges to family caregivers as well as to the service system. Their limitations in independence in daily living skills, the large sub-group with clinically-significant behavior problems, and their increased number of health problems has the potential to tax families in which multiple members are genetically affected. In this study, most mothers had either the premutation of the FMR1 gene or the full mutation. Moreover, a substantial proportion of families in the sample (over one-third) have more than one child with FXS and about 14% have one or more other children with ASD (Usher, DaWalt, Greenberg, & Mailick, 2019). In this context, the longitudinal patterns detected by the nearly decade-long research cast the family challenges in sharp relief. The need for long-term care planning is only underscored by these patterns.
Every intellectual and developmental disability has its own characteristic behavioral and health phenotype as manifested across the life course. A great deal is known about age-related changes in Down syndrome (Esbensen et al. 2010; Rice et al. 2015; Ghezzo et al. 2014; Carfì et al. 2014; Grieco et al. 2015) and increasingly in ASD (Woodman et al. 2015; Esbensen et al. 2010; Smith et al. 2012b). With regards to FXS, clinical reports have described life course behavior and health phenotypes (Lee et al. 2018; Hersh and Saul 2011). The present study offers quantitative insights into life course development in adolescents and adults with FXS.
In our previous research on the present sample, we found evidence of increasing independence in daily living skills for individuals with FXS over shorter periods of time (Smith, Hong, Greenberg, Mailick, 2016). However, by extending the follow-up period, we observed that the pattern of increasing independence slowed in individuals in their 30s and possibly reversed in midlife. In a longitudinal study of adolescents and adults with ASD, improvement was found in the same measure of daily living skills during adolescence and early adulthood that plateaued in the late 20s and showed a pattern of declining independence in midlife. However, this pattern was not characteristic of adults with Down syndrome (Smith et al. 2012b). Independence in daily living skills has been found to be a significant predictor of overall independence in adult life in terms of residential living, social contact with friends, and vocational independence for adults with ASD (Esbensen et al. 2010), emphasizing the significance of an increase in daily living skills over the lifespan. The deceleration in trajectories of daily living skills at later ages and the suggestion of decline in skills observed in the present study indicates that maintenance of these abilities is of critical importance for individuals with FXS. As individuals with FXS age, interventions are needed both to promote continued independence in previously-acquired skills as well as to provide supports to individuals and families in cases were the adult may have experienced a loss of skills.
Higher levels of lifetime ASD symptoms were associated with higher starting levels of behavior problems, similar to the association observed with daily living skills. In the present study, regardless of ASD symptom level, adolescents and adults displayed decreasing behavior problems over time. Although behavior problem severity reduced over time, almost one third of the sample remained above the clinical cutoff for behavior problems over the course of a decade. This pattern underscores the point that behavior problems remain a concern for individuals with FXS, even though they often are less severe as individuals age. There has been a much effort to develop behavioral and pharmacological treatments for some of the behavior problems associated with FXS. For example, behavioral interventions designed for use with young children with ASD have been modified for use in small groups of children with FXS with success (Vismara et al. 2018). Still, researchers acknowledge that there is a need for controlled studies of these treatments with larger samples to better understand potential effects on behavior in adolescents and adults and the implications of large observed placebo effects (Hagerman et al. 2009; Erickson et al. 2018).
Trajectories for BMI indicated weight gain over time, highlighting an area for support and intervention. In the general population, BMI increases over time for both men and women (Newby et al. 2003). Previous cross-sectional research indicates higher levels of obesity in individuals with FXS than typically developing peers in childhood, but similar rates by adulthood (McLennan et al. 2011; Utari et al. 2010; Raspa et al. 2010). In the present study we found lower rates of obesity than those of the general population (Utari et al. 2010) at the start of the study but higher rates of being overweight by Time 4. Some medications used to treat behavior problems can lead to significant weight gain in adolescents and adults with intellectual disability (Hellings et al. 2001), and individuals with FXS have high rates of medication use (Laxman et al. 2017). Physical activity and nutrition health promotion interventions for adults with intellectual disability have been found effective in increasing physical activity and reducing weight gain (Heller et al. 2011; Heller et al. 2004). Findings from this study suggest that these interventions, or a modification of them, could be useful for individuals with FXS, especially during adolescence and adulthood when service use decreases from that of childhood (Raspa et al. 2017).
The number of health conditions experienced by adolescents and adults with FXS displayed a linear pattern of change, with health conditions increasing over time. Although the present study lacked a control group, this pattern is consistent with findings from studies indicating that people with ASD and those with intellectual disability experience increasing health problems as they age. In our prior work we found that adolescents and adults with FXS with a higher number of health problems use more medications than those with lower numbers of health problems, and once taking medications are not likely to stop taking them over time (Laxman et al. 2017). In addition to the physical activity interventions discussed above, health and nutrition education programs have been utilized successfully to increase health behavior attitudes in people with developmental disabilities (Heller and Sorensen 2013). Further research is needed to evaluate these interventions ameliorating health problems in adolescents and adults with FXS.
In contrast with prior work indicating that females may have less severe phenotypes (Smith et al. 2016; Hartley et al. 2011), in the present research sex did not have a robust effect on intercepts or slopes in the multilevel models of daily living skills, behavior problems, or BMI. There was a marginal impact of sex on health, with a trend for males to have a greater number of health conditions. However, the number of females with FXS in the sample was small, limiting the generalizability of the findings to women with FXS. In addition, by including only those individuals who had ID, many of the sex differences associated with FXS were not evident here. Future studies would benefit from oversampling of females with FXS to obtain results that apply to females with the full mutation, who may have different starting levels and patterns of change for constructs of interest.
Findings from the present study indicate a need for continuing clinical and behavioral treatment approaches across the life course for people with FXS. However, the availability of clinicians with specialized expertise in FXS treatment during adulthood is limited (McMillan et al. 2017; Bridgemohan et al. 2018; Marrus et al. 2014). Furthermore, since FXS is a rare disorder (Brown, 2002) with fewer affected individuals in any single geographic area relative to more common IDD conditions such as Down syndrome and ASD, the delivery of services to them poses logistical challenges. A possible remedy, given the trajectory of continued impairments and limitations in adulthood, is delivery of services via telehealth. Telehealth interventions have shown promise for individuals with FXS (Nelson et al. 2018) and researchers and clinicians have called for telehealth programs focused on reducing behavior problems (McDuffie et al. 2016). Parent-delivered telehealth interventions have successfully reduced behavioral challenges in children with FXS (Diez-Juan et al. 2014) as well as in individuals in the broader IDD population (Ramdoss et al. 2012; Kagohara et al. 2013). Further research and clinical work in this area will illuminate strengths and challenges related to remote delivery of intervention as well as family acceptability of these strategies.
Strengths and Limitations
This study had several strengths, including analysis of four points of data collection spanning a nearly decade in time in a sample that extended into midlife at the end of the study period. These data allowed for the examination of both linear and quadratic change across adolescence and adulthood. This is also the first study to investigate longitudinal BMI and health phenotypes in adolescents and adults with FXS. Findings may be used to inform future research studies as well as treatment and intervention approaches for this population. A strength of the multilevel modeling approach is that this technique capitalizes on the sample size by including all participants in statistical analyses. As FXS is a rare disorder with few studies of affected individuals in adulthood, research that reveals linear as well as curvilinear patterns of lifespan development contributes novel understanding of how this disorder unfolds over time.
These strengths should be considered alongside the limitations to this study. The sample had too few individuals without intellectual disability to analyze as a separate group, so they were excluded from the present study. Further examination of these individuals would inform knowledge about the life course for this unique group. Related, the sample size in the present study was too small to employ a growth mixture model framework; however, given the heterogeneity of the disorder, it will be valuable for future work with larger samples to test for possible subgroups who may change in different ways over time (e.g., some increasing, some decreasing). Mothers reported on all variables of interest, potentially introducing reporter bias. Multiple reporters or observations of individuals with FXS could potentially offer nuanced information on behavior and health outcomes of interest. Finally, we interpret the findings with regards to trends in older age with caution, due to the smaller number of cases above the age of 40 years.
Conclusions
This study provides the first examination of trajectories of change in behavioral and physical health phenotypes for adolescents and adults with fragile X syndrome spanning nearly a decade. Although independence in daily living skills increased during adolescence and early adulthood, by the early 30s and beyond there was a plateau or a decline in independence. Similarly, individuals with FXS displayed a plateau or increase in behavior problems later in life. Lower levels of lifetime ASD symptoms were associated with greater independence in daily living skills and fewer behavior problems at study start. Body mass index also increased over time, with increasing percentages of adolescents and adults categorized as overweight or obese over the course of the study, and the number of health conditions that individuals experienced increased over time as well. Further longitudinal research is needed to inform treatment and support options for individuals with FXS and their families.
Acknowledgments
Funding: This work was supported by the National Institute of Child Health and Human Development to the IDDRC at the University of North Carolina (P30 HD003100-S1) to support a Fragile X Research Center at three additional sites (Research Triangle Institute International, the University of Wisconsin-Madison and University of Kansas). The present analysis was based on data collected by the UW-Madison Waisman Center site (M. Mailick, PI). Additional support was provided by the National Institute of Child Health and Human Development (T32 HD07489 and R01 HD082110, M. Mailick, PI) and the Waisman Center IDDRC (P30 HD03352 and U54 HD090256, A. Messing, PI).
Footnotes
Conflict of Interest: All authors declare that they have no conflicts of interest.
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Bailey DB, Raspa M, Olmsted MY, Holiday DB (2008). Co-occurring conditions associated with FMR1 gene variations:Findings from a national parent survey. American Journal of Medical Genetics: Part A. 146, 2060–2069. [DOI] [PubMed] [Google Scholar]
- Baker JK, Seltzer MM, & Greenberg JS (2012). Behaviour problems, maternal internalising symptoms and family relations in families of adolescents and adults with fragile X syndrome. Journal of Intellectual Disability Research, 56(10), 984–995, doi: 10.1111/j.1365-2788.2012.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, Movaghar A, Greenberg JS, Page D, DaWalt LS, Brilliant MH, et al. (2018). Using machine learning to identify patterns of lifetime health problems in decedents with autism spectrum disorder. Autism Research, 11(8), 1120–1128, doi:doi: 10.1002/aur.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgemohan C, Bauer NS, Nielsen BA, DeBattista A, Ruch-Ross HS, Paul LB, et al. (2018). A workforce survey on developmental-behavioral pediatrics. Pediatrics, 141(3), doi: 10.1542/peds.2017-2164. [DOI] [PubMed] [Google Scholar]
- Bruininks RH, Bradley HK, Weatherman RF, & Woodcock RW (1996). SIB-R: Riverside Publishing Company. [Google Scholar]
- Carfì A, Antocicco M, Brandi V, Cipriani C, Fiore F, Mascia D, et al. (2014). Characteristics of adults with down syndrome: Prevalence of age-related conditions. [Original Research]. Frontiers in Medicine, 1(51), doi: 10.3389/fmed.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W, Smith LE, Greenberg JS, Hong J, & Mailick MR (2017). Executive functioning mediates the effect of behavioral problems on depression in mothers of children with developmental disabilities. American Journal on Intellectual and Developmental Disabilities, 122(1), 11–24, doi: 10.1352/1944-7558-122.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, & Sherman SL (2001). FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine, 3(5), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford H, Moss J, Stinton C, Singla G, & Oliver C (2018). Overactivity, impulsivity and repetitive behaviour in males with fragile X syndrome: contrasting developmental trajectories in those with and without elevated autism symptoms. Journal of Intellectual Disability Research. [DOI] [PubMed] [Google Scholar]
- DaWalt LS, Usher LV, Greenberg JS, & Mailick MR (2017). Friendships and social participation as markers of quality of life of adolescents and adults with fragile X syndrome and autism. Autism, 23(2):383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark JL, Feldman MA, & Holden JJA (2003). Behavioral relationship between autism and fragile X syndrome. American Journal on Mental Retardation, 108(5), 314–326, doi:. [DOI] [PubMed] [Google Scholar]
- Diez-Juan M, Schneider A, Phillips T, Lozano R, Tassone F, Solomon M, et al. (2014). Parent-delivered touchscreen intervention for children with fragile X syndrome. Intractable & Rare Diseases Research, 3(4), 166–177, doi: 10.5582/irdr.2014.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LC, & Ho HH (2008). Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders, 38(4), 739–747. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Kaufmann WE, Budimirovic DB, Lachiewicz A, Haas-Givler B, Miller RM, et al. (2018). Best practices in fragile X syndrome treatment development. Brain Sciences, 8(12), doi: 10.3390/brainsci8120224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen A, Bishop S, Seltzer M, Greenberg J, & Taylor JL (2010). Comparisons between individuals with autism spectrum disorders and individuals with Down syndrome in adulthood. American Journal of Intellectual and Developmental Disabilities, 115(4), 277–290, doi: 10.1352/1944-7558-115.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K (2004). Health disparities and mental retardation. Journal of Nursing Scholarship, 36(1), 48–53, doi: 10.1111/j.1547-5069.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- Fodstad JC, & Matson JL (2008). A comparison of feeding and mealtime problems in adults with intellectual disabilities with and without autism. Journal of Developmental and Physical Disabilities, 20(6), 541–550. [Google Scholar]
- Freund LS, Reiss AL, & Abrams MT (1993). Psychiatric disorders associated with fragile X in the young female. Pediatrics, 91(2), 321–329. [PubMed] [Google Scholar]
- Ghezzo A, Salvioli S, Solimando MC, Palmieri A, Chiostergi C, Scurti M, et al. (2014). Age-related changes of adaptive and neuropsychological features in persons with Down Syndrome. PLoS ONE, 9(11), e113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco J, Pulsifer M, Seligsohn K, Skotko B, & Schwartz A (2015). Down syndrome: Cognitive and behavioral functioning across the lifespan. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 169(2), 135–149. [DOI] [PubMed] [Google Scholar]
- Gurney JG, McPheeters ML, & Davis MM (2006). Parental report of health conditions and health care use among children with and without autism: National survey of children's health. Archives of Pediatrics & Adolescent Medicine, 160(8), 825–830, doi: 10.1001/archpedi.160.8.825. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, et al. (2009). Advances in the treatment of fragile X syndrome. Pediatrics, 123(1), 378–390, doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, et al. (2008). Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation, 113(6), 427–438, doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Hong J, Greenberg J, Smith LE, Almeida DM, et al. (2012). Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. International Journal of Behavioral Development, 36(1), 53–61, doi: 10.1177/0165025411406857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, & Bailey DB Jr (2011). Exploring the adult life of men and women with fragile X syndrome: results from a national survey. American Journal of Intellectual and Developmental Disabilities, 116(1), 16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, & Wheelter A (2002). Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics, 108, 105–116. [DOI] [PubMed] [Google Scholar]
- Health N. I. o. (2006). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health; National Heart, Lung, and Blood Institute; September 1998. Publication No. 98–4083. [Google Scholar]
- Heller T, Hsieh K, & Rimmer JH (2004). Attitudinal and psychosocial outcomes of a fitness and health education program on adults with Down syndrome. American Journal on Mental Retardation, 109(2), 175–185. [DOI] [PubMed] [Google Scholar]
- Heller T, McCubbin JA, Drum C, & Peterson J (2011). Physical activity and nutrition health promotion interventions: What is working for people with intellectual disabilities? Intellectual and Developmental Disabilities, 49(1), 26–36. [DOI] [PubMed] [Google Scholar]
- Heller T, & Sorensen A (2013). Promoting healthy aging in adults with developmental disabilities. Developmental Disabilities Research Reviews, 18(1), 22–30, doi: 10.1002/ddrr.1125. [DOI] [PubMed] [Google Scholar]
- Hellings JA, Zarcone JR, Crandall K, Wallace D, & Schroeder SR (2001). Weight gain in a controlled study of risperidone in children, adolescents and adults with mental retardation and autism. Journal of Child and Adolescent Psychopharmacology, 11(3), 229–238, doi: 10.1089/10445460152595559. [DOI] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, & Kaufmann WE. (2009). Autism spectrum disorder in fragile X syndrome: A longitudinal evaluation. American Journal of Human Genetics, 149, 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh JH, & Saul RA (2011). Clinical report—health supervision for children with Fragile X syndrome. American Academy of Pediatrics. [DOI] [PubMed] [Google Scholar]
- Hustyi KM, Hall SS, Jo B, Lightbody AA, & Reiss AL (2014). Longitudinal trajectories of aberrant behavior in fragile X syndrome. Research in Developmental Disabilities, 35(11), 2691–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustyi KM, Hall SS, Quintin E-M, Chromik LC, Lightbody AA, & Reiss AL (2015). The relationship between autistic symptomatology and independent living skills in adolescents and young adults with fragile X syndrome. Journal of Autism and Developmental Disorders, 45(6), 1836–1844, doi: 10.1007/s10803-014-2342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagohara DM, van der Meer L, Ramdoss S, O’Reilly MF, Lancioni GE, Davis TN, et al. (2013). Using iPods((R)) and iPads((R)) in teaching programs for individuals with developmental disabilities: a systematic review. Research in Developmental Disabilities, 34(1), 147–156, doi: 10.1016/j.ridd.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Klaiman C, Quintin E-M, Jo B, Lightbody AA, Hazlett HC, Piven J, et al. (2014). Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschner ES, Eisenberg IW, Orionzi B, Simmons WK, Kenworthy L, Martin A, et al. (2015). A preliminary study of self-reported food selectivity in adolescents and young adults with autism spectrum disorder. Research in Autism Spectrum Disorders, 15, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxman DJ, Greenberg JS, DaWalt LS, Hong J, Aman MG, & Mailick M (2017). Medication use by adolescents and adults with fragile x syndrome. Journal of Intellectual Disability Research, doi: 10.1111/jir.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Ventola P, Budimirovic D, Berry-Kravis E, & Visootsak J (2018). Clinical development of targeted fragile X syndrome treatments: An industry perspective. Brain Sciences, 8(12), 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Smith LE, Hong J, Makuch R, Greenberg JS, & Mailick MR (2013). Evaluation of an activities of daily living scale for adolescents and adults with developmental disabilities. Disability and Health Journal, 6(1), 8–17, doi: 10.1016/j.dhjo.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailick MR, Greenberg JS, Smith LE, Sterling A, Brady N, Warren SF, & Hong J (2014). Fragile X-associated disorders: How the family environment and genotype interact In Burack JA & Schmidt LA (Eds.), Cultural and contextual perspectives on developmental risk and well-being (pp. 221–253). New York, NY: Cambridge University Press; 10.1017/CBO9780511920165.015 [DOI] [Google Scholar]
- Marrus N, Veenstra-Vanderweele J, Hellings JA, Stigler KA, Szymanski L, King BH, et al. (2014). Training of child and adolescent psychiatry fellows in autism and intellectual disability. Autism, 18(4), 471–475, doi: 10.1177/1362361313477247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Oakes A, Machalicek W, Ma M, Bullard L, Nelson S, et al. (2016). Early language intervention using distance video-teleconferencing: A pilot study of young boys with fragile X syndrome and their mothers. American Journal of Speech-Language Pathology, 25(1), 46–66, doi: 10.1044/2015_ajslp-14-0137. [DOI] [PubMed] [Google Scholar]
- McLennan Y, Polussa J, Tassone F, & Hagerman R (2011). Fragile X syndrome. Current Genomics, 12(3), 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan JA, Land M Jr., & Leslie LK (2017). Pediatric residency education and the behavioral and mental health crisis: A call to action. Pediatrics, 139(1), doi: 10.1542/peds.2016-2141. [DOI] [PubMed] [Google Scholar]
- Midlife in the United States: A National Longitudinal Study of Health and Well-Being (2011). Retrieved from: http://midus.wisc.edu/scopeofstudy.php
- Nelson S, McDuffie A, Banasik A, Tempero Feigles R, Thurman AJ, & Abbeduto L (2018). Inferential language use by school-aged boys with fragile X syndrome: Effects of a parent-implemented spoken language intervention. Journal of Communication Disorders, 72, 64–76, doi: 10.1016/j.jcomdis.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby PK, Muller D, Hallfrisch J, Qiao N, Andres R, & Tucker KL (2003). Dietary patterns and changes in body mass index and waist circumference in adults. The American Journal of Clinical Nutrition, 77(6), 1417–1425. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing.
- Rajaratnam A, Shergill J, Salcedo-Arellano M, Saldarriaga W, Duan X, & Hagerman R (2017). Fragile X syndrome and fragile X-associated disorders. F1000Research, 6, 2112, doi: 10.12688/f1000research.11885.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdoss S, Machalicek W, Rispoli M, Mulloy A, Lang R, & O’Reilly M (2012). Computer-based interventions to improve social and emotional skills in individuals with autism spectrum disorders: a systematic review. Developmental Neurorehabilitation, 15(2), 119–135, doi: 10.3109/17518423.2011.651655. [DOI] [PubMed] [Google Scholar]
- Raspa M, Bailey DB Jr., Bishop E, Holiday D, & Olmsted M (2010). Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 115(6), 482–495, doi: 10.1352/1944-7558-115.6.482. [DOI] [PubMed] [Google Scholar]
- Raspa M, Franco V, Bishop E, Wheeler AC, Wylie A, Bailey DB (2018). A comparison of functional academic and daily living skills in males with fragile X syndrome with and without autism. Research in Developmental Disabilitys, 78, 1–14. [DOI] [PubMed] [Google Scholar]
- Raspa M, Wheeler AC, & Riley C (2017). Public health literature review of fragile X syndrome. Pediatrics, 139(Supplement 3), S153–S171, doi: 10.1542/peds.2016-1159C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS (2002). Hierarchical linear models: Applications and data analysis methods (Vol. 1): Sage. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong Y, Congdon R, & Du Toit M (2011). HLM 7 [Computer software]. Lincolnwood, IL: Scientific Software International [Google Scholar]
- Rice LJ, Gray KM, Howlin P, Taffe J, Tonge BJ, & Einfeld SL (2015). The developmental trajectory of disruptive behavior in Down syndrome, fragile X syndrome, Prader–Willi syndrome and Williams syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 169(2), 182–187. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C, Cianchetti C, & Fancello GS (2007). SCQ: Social Communication Questionnaire: manuale: Giunti OS. [Google Scholar]
- Smith LE, Barker ET, Seltzer M, Abbeduto L, & Greenberg J (2012a). Behavioral phenotype of fragile X syndrome in adolescence and adulthood. American Journal on Intellectual and Developmental Disabilities, 117(1), 1–17, doi: 10.1352/1944-7558-117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Hong J, Greenberg JS, & Mailick MR (2016). Change in the behavioral phenotype of adolescents and adults with FXS: Role of the family environment. Journal of Autism and Developmental Disorders, 46(5), 1824–1833, doi: 10.1007/s10803-016-2714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Maenner MJ, & Seltzer M (2012b). Developmental trajectories in adolescents and adults with autism: the case of daily living skills. Journal of the American Academy of Child & Adolescent Psychiatry, 51(6), 622–631, doi: 10.1016/j.jaac.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher LV, DaWalt LS, Greenberg JS, Mailick MR (2019). Unaffected siblings of adolescents and adults with fragile X syndrome: Direct and buffering effects on maternal health. Journal of Family Psychology, 33(4), 487–492. doi: 10.1037/fam0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utari A, Adams E, Berry-Kravis E, Chavez A, Scaggs F, Ngotran L, et al. (2010). Aging in fragile X syndrome. Journal of Neurodevelopmental Disorders, 2(2), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schrojenstein Lantman-De Valk HMJ, Metsemakers JFM, Haveman MJ, & Crebolder HFJM (2000). Health problems in people with intellectual disability in general practice: a comparative study. Family Practice, 17(5), 405–407, doi: 10.1093/fampra/17.5.405. [DOI] [PubMed] [Google Scholar]
- Vismara LA, McCormick CEB, Shields R, & Hessl D (2018). Extending the parent-delivered Early Start Denver Model to young children with fragile X syndrome. Journal of Autism and Developmental Disorders, doi: 10.1007/s10803-018-3833-1. [DOI] [PubMed] [Google Scholar]
- Woodman AC (2014). Trajectories of stress among parents of children with disabilities: A dyadic analysis. Family Relations: An Interdisciplinary Journal of Applied Family Studies, 63(1), 39–54, doi: 10.1111/fare.12049. [DOI] [Google Scholar]
- Woodman AC, Smith LE, Greenberg J, & Mailick M (2015). Change in autism symptoms and maladaptive behaviors in adolescence and adulthood: the role of positive family processes. Journal of Autism and Developmental Disorders, 45(1), 111–126, doi: 10.1007/s10803-014-2199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]