Abstract
Work during the last decade has led to a novel hypothesis for a question that is half a century old: how is the secretory activity of GnRH neurons synchronized to produce episodic GnRH secretion. This hypothesis posits that a group of neurons in the arcuate nucleus (ARC) that contain kisspeptin, neurokinin B (NKB), and dynorphin (known as KNDy neurons) fire simultaneously to drive each GnRH pulse. Kisspeptin is proposed to be the output signal to GnRH neurons with NKB and dynorphin acting within the KNDy network to initiate and terminate each pulse, respectively. This review will focus on the importance of neuroanatomical studies in general and, more specifically, on the work of Dr. Marcel Amstalden during his post-doctoral fellowship with the authors, to the development and testing of this hypothesis. Critical studies in sheep that laid the foundation for much of the KNDy hypothesis included the report that a group of neurons in the ARC contain both NKB and dynorphin and appear to form an interconnected network capable of firing synchronously, and Marcel’s observations that the NKB receptor is found in most KNDy neurons, but not in any GnRH neurons. Moreover, reports that almost all dynorphin-NKB neurons and kisspeptin neurons in the ARC contained steroid receptors led directly to their common identification as “KNDy” neurons. Subsequent anatomical work demonstrating that KNDy neurons project to GnRH somas and terminals, and that the kisspeptin receptors are found in GnRH, but not KNDy, neurons provided important tests of this hypothesis. Recent work has explored the time course of dynorphin release onto KNDy neurons, and begun to apply new approaches, such as RNAscope in situ hybridization, and the use of whole tissue optical clearing with light sheet microscopy. Together with other approaches, these anatomical techniques will allow continued exploration of the functions of the KNDy population as well as the possible role of other ARC neurons in generation of GnRH pulses.
Keywords: NKB, kisspeptin, dynorphin, GnRH pulses
1. Introduction
It has now been 50 years since the episodic nature of tonic LH secretion was first described (1), which led to the hypothesis that GnRH release also occurred episodically. This hypothesis was directly verified by measurements of GnRH in hypophysial portal blood (2) and push-pull perfusates of the median eminence (3). These observations raised the intriguing question of how the activity of the anatomically-dispersed population of GnRH neurons (4) was synchronized to produce brief bursts of release. The neural elements responsible became known as the GnRH pulse generator and, as reviewed elsewhere (5,6) several mechanisms have been proposed to account for this phenomenon. However, it is only in the last decade that the current hypothesis for GnRH pulse generation was developed and tested.
This hypothesis, in its most basic form, proposes that a group of neurons in the arcuate nucleus (ARC) that contain kisspeptin, neurokinin B (NKB), and dynorphin (now known as KNDy neurons) form an interconnected network that fires synchronously to drive GnRH release during a pulse (7–10). The hypothesis also proposed that kisspeptin was the output signal from KNDy neurons that drives GnRH secretion and NKB and dynorphin act as start and stop signals, respectively, within the KNDy network. A variety of different approaches in a number of species provided vital support for the development and testing of this hypothesis. These include genetic studies in humans (11–13), knock-out (14) and optogenetic (15,16) work in rodents, electrophysiological recordings of single-cell activity in murine slices (17,18) and multi-unit activity in goats (19), use of receptor antagonists (20–22), and neuroanatomical data (23) from rodents (7), ruminants (19,24) and primates (25,26).
This review will focus on the neuroanatomical data from domestic animals (primarily sheep) that contributed to this hypothesis. We chose this topic for a review honoring the life and work of Dr. Marcel Amstalden for two reasons. First, Marcel recognized the importance of neuroanatomical studies and large animal models to understanding the physiological role of different systems within the hypothalamic-hypophysial unit and contributed to some of the work described here. Second, neuroanatomical studies in domestic animals and other species, that some might consider “descriptive in nature”, played a critical role in the development of the KNDy hypothesis for GnRH pulse generation. We will first describe early work that led to the discovery of KNDy neurons and provided key information on their characteristics. We will next describe neuroanatomical information that was important for the test of this hypothesis. Finally, we will briefly consider current work and possible future directions. It is important to note that this the work in domestic animals complemented similar studies in rodents and non-human primates. While we will not describe these in detail due to space limitations, we have included references to this literature where appropriate. These constraints also preclude a consideration of the limitations of neuroanatomical approaches, which are described in detail elsewhere (27).
2. Early work that laid the groundwork for the KNDy hypothesis
The discovery of KNDy neurons represented the integration of three largely independent lines of study. The earliest of these were studies on the role of endogenous opioid peptides (EOP) in the control of pulsatile LH secretion. The EOP can be considered the kisspeptin of the 1980s: they were first identified (28) as important for other physiological systems (e.g., pain perception) and work in humans (with an EOP receptor antagonist) was the first to indicate that they were involved in the control of episodic GnRH secretion (29). Early studies in humans (30), primates (31), and sheep (32,33) implicated EOP in the negative feedback actions of progesterone, so our initial work focused on which of the three EOP (β-endorphin, dynorphin, and/or enkephalin) mediated the inhibitory actions of progesterone in sheep. We first tested the effects of local administration of antagonists to the three different EOP receptors (κ, μ, and δ) into the ovine medial basal hypothalamus (MBH), where the GnRH neurons that drive LH pulses are located (34). Because the antagonist to the dynorphin receptor (the κ-EOP receptor [KOR]), but not antagonists to μ- or δ-EOP receptors, increased LH pulse frequency in luteal phase ewes (35), subsequent studies concentrated on MBH dynorphin neurons that are found largely in the ARC. This study also found that almost all GnRH neurons in the MBH received synaptic input (based on correlative light microscopic and electron microscopic analyses) from dynorphin neurons (35), which was the first evidence that these ARC neurons projected to GnRH cells. Another important neuroanatomical observation on ovine ARC dynorphin neurons was that over 90% of them contained progesterone receptors (PR) (36). This not only supported their proposed role in progesterone negative feedback, but was also the first of three specific data sets that led to the identification of KNDy neurons.
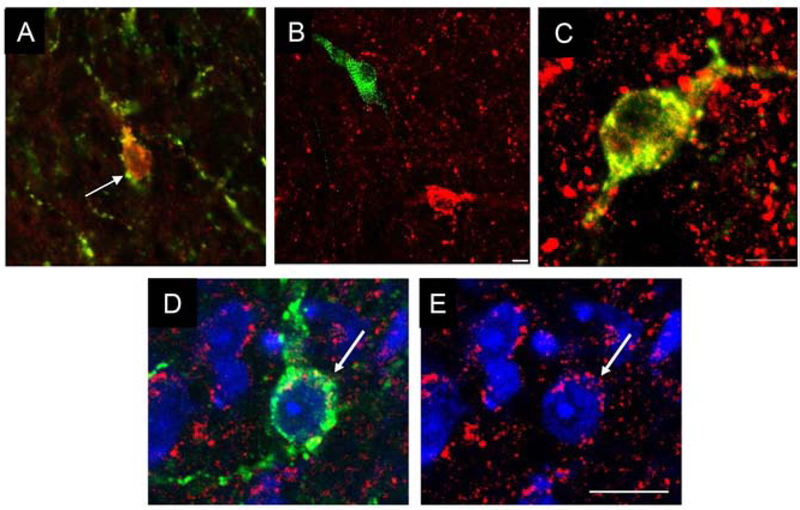
The second line of study focused on NKB neurons in the ARC. The initial work in sheep was also based on work in humans, specifically the report that expression of mRNA for NKB in the human ARC increased in post-menopausal women (37). This led Goubillon et al., to characterize NKB-ir neurons in the ovine ARC (38). They observed that this population was sexually dimorphic, with more cells in females than males, and that 97% of them contained estrogen receptor-α (ERα). The very high levels of steroid receptors in ARC NKB and dynorphin neurons contrasted with the fairly low (10–20%) co-localization of these receptors in other ARC neurons (i.e., β-endorphin (39), neuropeptide Y (40), and dopaminergic (39) cells). This led Marcel and Chad Foradori to hypothesize that there was a single population of neurons that contained both NKB and dynorphin in the ovine ARC. They tested this hypothesis using dual-immunohistochemistry (IHC) and demonstrated that 85% of NKB-positive ARC neurons also contained dynorphin and 88% of dynorphin-ir cells contained NKB (41). Similar, but not as extensive, co-localization of NKB and dynorphin in the ARC of rats was reported at the same time as this work in sheep (42,43), suggesting this might be seen across many species. Foradori et al., and one of the rodent studies (42), also observed that almost all of these neurons were contacted by vesicles that contained both dynorphin and NKB (Fig. 1A), which provided the initial evidence that these neurons form an interconnected network. Marcel then followed-up this study by examining expression of the NKB receptor (NK3R) in both NKB and GnRH neurons (44). This study found no instance of co-localization of NK3R with GnRH in either the preoptic area or median eminence, even though NK3R-ir cells and fibers were evident in the same section (Fig 1B). In contrast, 64% of ARC NKB neurons also contained NK3R-ir (Fig 1C). These anatomical observations were the primary evidence that led to the hypothesis that NKB acts within the KNDy network, and not directly on GnRH neurons. A similar co-localization of NK3R and NKB was observed in rats (42), but in contrast to sheep, extensive co-localization of NK3R-ir and GnRH was found in fiber tracts in this species and 16% of GnRH cell bodies also contained NK3R (45).
Fig. 1.
Key anatomical observations supporting the KNDy hypothesis. A) Dual-immunolabelling in the sheep ARC showing an NKB (green)/dynorphin (red) terminal in direct contact with a NKB/dynorphin cell body, i.e., evidence for reciprocal contacts among KNDy cells (from Foradori et al. [40]); B, C) Dual-immunolabeled sections showing the lack of co-localization of NK3R (red) and GnRH (green) in the sheep POA but striking co-localization of NK3R and NKB (yellow) in the ARC (from Amstalden et al., 2010 [41]); D, E) internalization of KOR (red) in a kisspeptin-labeled (green) ovine KNDy neuron during onset of a GnRH/LH pulse. DAPI-labelled nuclei are blue in D and E. Green channel is removed in E to show cytoplasmic localization of internalized KOR-positive particles (from Weems et al. [62]).
The final line of study began, of course, with the observations that loss-of-function mutations in the gene for the kisspeptin receptor disrupted GnRH secretion and thus produced infertility in humans (11,12). Neuroanatomical studies soon demonstrated that 99% of murine ARC kisspeptin cells contained mRNA for ERα (46)and 93% of ovine ARC kisspeptin-ir neurons expressed ERα (47). Applying the same logic as was used to develop the hypothesis of NKB-dynorphin co-localization led to our report that 94% of ovine ARC kisspeptin neurons contained dynorphin and 80% of them contained NKB (24). Given the major focus of reproductive neuroendocrinologists on kisspeptin, this observation, which has been confirmed in mice (7), rats (48), and goats (19), generated considerable interest in the other two peptides, and particularly NKB. This interest was soon reinforced by the report that mutations disrupting NKB-NK3R signaling in humans produced infertility (13). The evidence that: 1) all three KNDy peptides played a major role in the control of GnRH pulses, 2) two of them were necessary for fertility in humans, 3) KNDy neurons formed an interconnected network, 4) recording sites of the bursts in MUA that correlated with LH pulses were adjacent to KNDy neurons in goats (49), and 5) the known cellular distribution of NK3R and Kiss1r led four groups, working largely independently, to propose the KNDy hypothesis for pulse generation in 2009 (7) and 2010 (8–10).
3. Testing the KNDy hypothesis
The results of neuroanatomical studies also provided useful support for this hypothesis. Specifically, two lines of evidence tested the proposal that kisspeptin was the output signal from KNDy neurons to GnRH cells. One took advantage of previous work demonstrating that close contacts observed using confocal microscopy are synapses based on electron microscopy (50) and data that the three KNDy peptides and vGLUT (as a marker for glutamate (43)) represent a unique combination of transmitters found only in KNDy neurons (51). Thus triple-label IHC for GnRH and two of the KNDy peptides identified closed contacts on GnRH cell bodies in both the POA and MBH, as well as KNDy fibers in close proximity to GnRH fibers the median eminence in sheep (51). The latter have been confirmed by tract tracing studies in sheep (52), and dual immunoelectron microscopic analysis of tissue from goats indicated that kisspeptin-ir terminals contacted GnRH-containing nerve terminals in this region (53). The second important observation was that mRNA for Kiss1r was found in almost all GnRH neurons, but not in any KNDy neurons in sheep (52) and mice (54,55). It should be noted that other ARC neurons contained Kiss1r (52,54), and there is some pharmacological evidence that they may be involved in the generation of GnRH pulses (21,22). However, what role if any these non-KNDy ARC neurons have in episodic GnRH secretion remains to be resolved.
Tract-tracing work has also provided convincing evidence that KNDy neurons on each side of the third ventricle project to each other via axons that run under the ventricle in the internal zone of the median eminence (56). This anatomical study also received important functional support from evidence that MUA was synchronized between the two halves of the ARC (56). Taken together these data, which have been replicated in rodents (16,57), provide a simple explanation on how KNDy neural activity in each half of the ARC can be synchronized.
A final anatomical tool that has been used to test this hypothesis relies on use of c-Fos as an index of an increase in neural activity. Thus, the report that Fos expression in KNDy, but not POA kisspeptin, neurons increased in parallel with LH pulse frequency following OVX of ewes (58) is consistent with the KNDy hypothesis. Interestingly, an increase in Fos expression in KNDy cells was also seen in luteal phase ewes, but only if tissue was collected shortly after an endogenous pulse (59). Thus, Fos expression, and presumably KNDy neural activity, correlates specifically with the occurrence of a GnRH/LH pulse. Finally, the observation that an icv NKB treatment that induced an LH pulse also increased Fos expression in ovine KNDy neurons, supports (60) the proposed role for NKB in pulse initiation.
While neuroanatomical data was a foundation for the KNDy hypothesis and a useful initial test of it, pharmacological approaches in domestic animals provided stronger support for it as well as insights into possible underlying mechanisms. For example, icv administration of either a KISS1R (61) or a NK3R (62) antagonist inhibited LH pulses in OVX ewes, as did local administration of these to the ARC (22). In contrast, antagonists to KOR increased LH pulse frequency when given either icv (19) or into the ARC (22). Not surprisingly, the opposite effects were observed with agonists to NK3R and KOR (19).
Although they are beyond the scope of this review, it is important to point out that pharmacological, genetic, and optogenetic studies in rodents have also produced strong support for the KNDy hypothesis, with one study providing a definitive test that KNDy neurons are the GnRH pulse generator (15). However, this work has also raised the possibility of significant species differences. For example, there appears to be considerable redundancy in tachykinin signaling in rodents so that neurokinin A and/or substance P can compensate for the loss of NKB-NK3R signaling (63). In contrast, anatomical and pharmacological data indicate that similar redundancy does not occur in sheep (64). The role of dynorphin in pulse termination has also been questioned in other species (26). These species differences are beyond the scope of this discussion and interested readers are directed to other recent reviews (63,65).
4. Current work and future directions
One of the key components of the KNDy hypothesis of pulse generation has been the role of dynorphin as the stop signal responsible for terminating each GnRH/LH pulse, acting via KOR in the MBH. Although the presumption was that this occurred directly via KOR in KNDy neurons, initial in situ hybridization (ISH) studies in mouse suggested that only a small percentage of KNDy neurons co-expressed KOR mRNA (7). Marcel carried out early preliminary work examining the question of KOR localization in the sheep hypothalamus, successfully using single-label ISH with radiolabeled probes to determine the distribution of KOR in the ovine preoptic area and hypothalamus (66). However, the low abundance of KOR mRNA, together with long times of exposure to photographic emulsion solutions required for this procedure, made detection of KNDy peptide immunoreactivity in the same sections problematic. However, these ISH data provided valuable information that was used for the validation of a polyclonal antibody which allowed reliable, multiple-label IHC localization of KOR in KNDy and other neurons in the sheep brain (67). Using this antibody, KOR was found co-localized in a large majority (94%) of KNDy neurons and surprisingly, also in a majority (75%) of GnRH neurons in the sheep as well as in the rat (67). The unexpected presence of KOR in GnRH neurons provided provocative evidence that, in addition to acting upon KNDy cells in sheep, and perhaps other species, dynorphin may be acting directly at the level of GnRH neurons to influence neuroendocrine release.
An added benefit of the ability to detect KOR with IHC was that it could be used to visualize receptor both at the cell surface and internalized in the cytosol (67). Internalization of G-protein coupled receptors, such as KOR, occurs rapidly following ligand binding and receptor phosphorylation, and can be used as a marker for endogenous release of ligand upon the surface of individual neurons during behavioral and physiological events (68,69). For example, visualization of internalization of the μ-opioid receptor had been used to examine EOP release onto preoptic neurons during components of male sexual behavior in rats (69). Using this marker, we then addressed the question of when is dynorphin released upon KNDy neurons during a GnRH pulse. In this study, we used a sheep model in which NKB was delivered icv to induce a single GnRH/LH pulse, and within the discrete time period of that pulse, analyzed KOR internalization using IHC at both pulse onset and termination (70). The results showed striking internalization of KOR in the cytoplasm of KNDy neurons at both pulse onset (Fig. 1D, E) and termination, suggesting that dynorphin is released at, or very soon, after pulse onset and continues to be released for its duration. This time course of dynorphin action is consistent with an earlier report that iv infusion of the non-specific EOP receptor antagonist, naloxone, increased the amount of GnRH secreted during each pulse (71). Interesting, we found that KOR was also internalized in MBH GnRH neurons during an individual pulse; however, in contrast to the timing of internalization in KNDy neurons, KOR was internalized only at the end of a pulse and not during its onset (70). This evidence provided further support for the possibility that dynorphin acts upon both GnRH and KNDy neurons to terminate each pulse, and has led us to slightly modify the original KNDy hypothesis to incorporate this potential, additional site of action in the regulation of GnRH pulses (65).
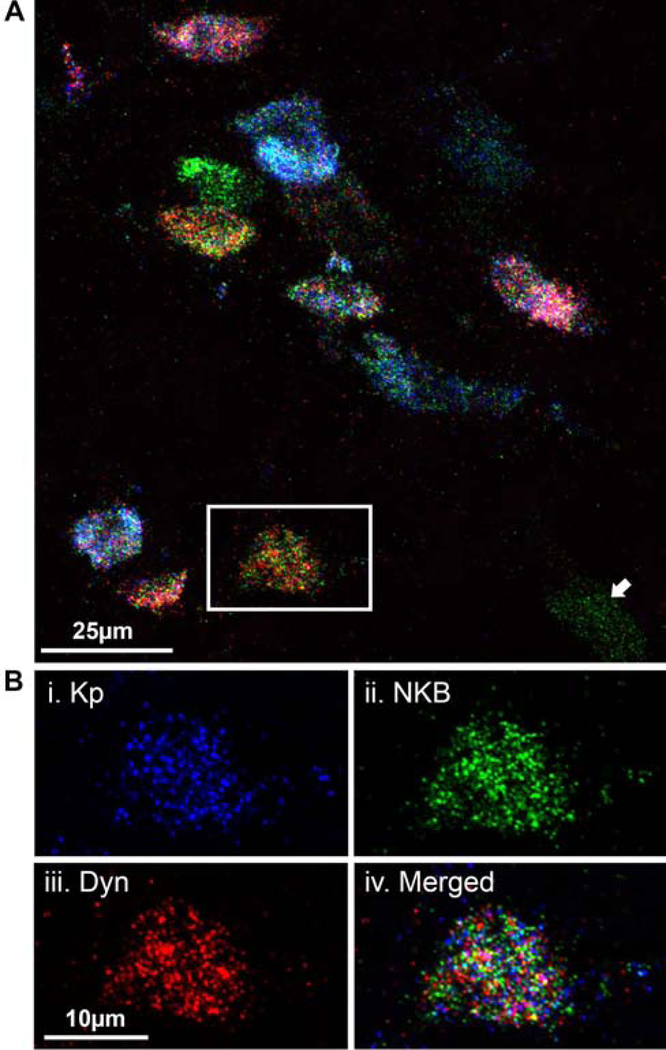
Ongoing work has continued to incorporate innovative, new techniques and approaches into anatomical studies of the sheep reproductive neuroendocrine system responsible for GnRH pulses. One example is the use of newly developed, highly sensitive techniques for ISH such as RNAscope; using this technique, we have been able to simultaneously visualize mRNAs for kisspeptin, NKB and dynorphin in individual KNDy neurons (Fig. 2). This new technique allows for quantitative measurement of multiple transcripts in response to steroid treatments or other protocols at a high level of anatomical detail. In addition to the ease of multiplex mRNA detection, RNAscope ISH also has the advantage of being able to process sections within a single day, as opposed to the weeks or months required for probe incubation in conventional ISH protocols. Another new technique recently incorporated into studies in the sheep is that of whole tissue optical clearing in conjunction with multiple-label IHC and light sheet microscopy to visualize the complete KNDy neuron population in the intact 3D hypothalamus (72). Whole brain optical clearing (modified iDISCO) allows us to map out and analyze the entire distribution of KNDy neurons in the sheep hypothalamus in the intact brain. Our initial use of this technique, in fact, revealed a previously undescribed kisspeptin cell population in the ventrolateral hypothalamus; this small population of cells had been previously missed using analysis of sectioned material due to the few numbers of cells in it (72). The use of whole brain optical clearing with multiple-label IHC and light sheet microscopy can potentially include detection of markers of cell activation, such as Fos, to identify functional sets of KNDy and other neurons in the intact brain active during episodic GnRH secretion. One major limitation to important anatomical studies in sheep, and other large animals, is the absence of models with Cre-drivers in specific neuronal populations. We hope that in the future, generation of transgenic sheep incorporating KNDy cell specific Cre-drivers will allow for the use of transgenic trans-synaptic tracing techniques, which combined with whole brain optical clearing and light sheet microscopy, could extend these analyses to examination of the synaptic connectivity of specific sets of neurons regulating neuroendocrine function (73).
Fig. 2.
Multiple-label fluorescent ISH using RNAscope demonstrating co-localization of mRNAs for kisspeptin (blue), NKB (green), and dynorphin (red) in KNDy neurons in the sheep. Neuron in box, Panel A, is shown in more detail in Panel B (from Moore at al. [57]).
Notwithstanding continued technical advances, several important theoretical questions remain concerning the KNDy hypothesis in the sheep with relevance to other species including humans. One of these is the functional role of Kiss1R neurons in the ARC. As noted above, Kiss1R neurons are a subset of ARC cells distinct from KNDy neurons, and delivery of Kiss1R antagonists into the ovine ARC significantly reduces LH pulse frequency (22), suggesting these cells may function as part of the local ARC circuitry regulating KNDy activity and thus the output signal to GnRH neurons. Additionally, observations that kisspeptin can reset the human GnRH/LH pulse generator (74) suggest this subpopulation may be functionally important for pulse generation in human health and disease. Another question related to the specific roles of KNDy peptides, and complementing our studies of KOR described above, is when does NK3R internalization occur during an endogenous GnRH/LH pulse? As the start signal hypothesized to be responsible for synchronization of KNDy neurons on both sides of the hypothalamus, we expect that NK3R will be internalized at the start of each pulse but it may be that, as is the case for KOR, the sites of action are more complex and wider than anticipated. Supporting this possibility are observations that non-KNDy cells containing both NK3R and KOR are numerous in the sheep ARC (75), and may serve as either an additional, intrinsic component of the GnRH pulse generator or as outputs for other functions associated with KNDy neurons (73).
5. Summary
Neuroanatomical work in sheep, and other species, even before the discovery of KNDy neurons, provided critical evidence that laid the foundation for the development of the KNDy hypothesis for GnRH pulse generation. Subsequent studies, together with complementary pharmacological and electrophysiological approaches, not only provided important tests of this hypothesis, but also identified species variations in some aspects of it and led to proposed modifications of the original hypothesis. The latter included a possible role for non-KNDy neurons within the ARC and recent technical innovations will enable workers to assess the importance of the anatomical organization of KNDy cells and the functional role of local circuitry within the ARC. We would like to close by emphasizing the important contributions that Marcel Amstalden made to the early development of this hypothesis. He played a major role contributing to the demonstration that dynorphin and NKB are found in the same cells in the ovine ARC and form an interconnected network, and his description of the distribution of NK3R expression in the ovine hypothalamus, including its colocalization in NKB cells of the ARC, was key to the proposed role of NKB in pulse initiation as foundation for the KNDy hypothesis.
Highlights.
Discovery of ARC NKB-dynorphin cells set stage for identification of KNDy neurons
NK3R distribution suggested that NKB acts on interconnected KNDy neurons
KNDy neurons project to GnRH soma and terminals
Dynorphin acts during pulse on KNDy cells, but only at end of pulse on GnRH cells
RNAscope and whole tissue optical clearing are important new approaches
Acknowledgements
We thank all the high school and undergraduate students, graduate students, post-doctoral fellows, and colleagues without whose efforts the work on which this review is based could not have been done. In particular, we greatly appreciate the insights and hard work of Drs. Marcel Amstalden, Chad Foradori, Christina Merkley, and Peyton Weems, who played major roles in the results highlighted in this review.
The work from our laboratories described here was supported by grants from the National Institutes of Health (NIH R01HD033916 and R01HD082135).
Grant support: NIH R01 HD039916 and R01 HD082135
Footnotes
The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma lh levels in the ovariectomized rhesus monkey. Endocrinology 1970;87:850–853. [DOI] [PubMed] [Google Scholar]
- 2.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (gnrh) and luteinizing hormone (lh) secretion in ovariectomized ewes. Endocrinology 1982;111:1737–1739. [DOI] [PubMed] [Google Scholar]
- 3.Levine JE, Pau KY, Ramirez VD, Jackson GL. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 1982;111:1449–1455. [DOI] [PubMed] [Google Scholar]
- 4.Herbison A. Physiology of the adult gonadotropin-releasing hormone neuronal network In: Knobil and neill’s physiology of reproduction, fourth edition, Plant TM, Zeleznik AJ (Eds.), Elsevier, 2015, pp 399–467. [Google Scholar]
- 5.Goodman RL, Coolen LM, Lehman MN. Unraveling the mechanism of action of the gnrh pulse generator: A possible role for kisspeptin/neurokinin b/dynorphin (kndy) neurons In: Cellular endocrinology in health and disease Conn MD (Ed.), Elsevier, 2015, pp 133–152. [Google Scholar]
- 6.Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology 2018;159:3723–3736. [DOI] [PubMed] [Google Scholar]
- 7.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin b neurons in the arcuate nucleus of the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/neurokinin b/dynorphin (kndy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying gnrh pulse generation by the hypothalamus. Brain research 2010;1364:103–115. [DOI] [PubMed] [Google Scholar]
- 10.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin b and the hypothalamic regulation of reproduction. Brain research 2010;1364:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The gpr54 gene as a regulator of puberty. The New England journal of medicine 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 12.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the kiss1-derived peptide receptor gpr54. Proceedings of the National Academy of Sciences of the United States of America 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. Tac3 and tacr3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin b in the central control of reproduction. Nature genetics 2009;41:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colledge WH. Transgenic mouse models to study gpr54/kisspeptin physiology. Peptides 2009;30:34–41. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic gnrh pulse generator in mice. Proceedings of the National Academy of Sciences of the United States of America 2017;114:E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Ronnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites gnrh neurons. eLife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin b, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Croft S, Boehm U, Herbison AE. Neurokinin b activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 2013. [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin b and dynorphin a in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O’Byrne KT. Suppression of the gnrh pulse generator by neurokinin b involves a kappa-opioid receptor-dependent mechanism. Endocrinology 2012;153:4894–4904. [DOI] [PubMed] [Google Scholar]
- 21.Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates gnrh pulse generator frequency in the rat. PloS one 2009;4:e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy S, Millar R, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin b, and dynorphin act in the arcuate nucleus to control activity of the gnrh pulse generator in ewes. Endocrinology 2013;154:4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: Comparative and developmental aspects. Advances in experimental medicine and biology 2013;784:27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin a and neurokinin b. Endocrinology 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 25.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin b stimulates gnrh release in the male monkey (macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010;151:4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hrabovszky E, Sipos MT, Molnar CS, Ciofi P, Borsay BA, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin b, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the kndy neuron concept. Endocrinology 2012;153:4978–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman RL, Ohkura S, Okamura H, Coolen LM, Lehman MN. Kndy hypothesis for generation of gnrh pulses: Evidence from sheep and goats In: The gnrh neuron and its control, Herbison AE, Plant TM (Eds.), John Wiley and Sons, 2018, pp 289–324. [Google Scholar]
- 28.Kosterlitz HW, Hughes J. Opiate receptors and endogenous opioid peptides in tolerance and dependence. Advances in experimental medicine and biology 1977;85B:141–154. [DOI] [PubMed] [Google Scholar]
- 29.Quigley ME, Yen SS. The role of endogenous opiates in lh secretion during the menstrual cycle. The Journal of clinical endocrinology and metabolism 1980;51:179–181. [DOI] [PubMed] [Google Scholar]
- 30.Reid RL, Quigley ME, Yen SS. The disappearance of opioidergic regulation of gonadotropin secretion in postmenopausal women. The Journal of clinical endocrinology and metabolism 1983;57:1107–1110. [DOI] [PubMed] [Google Scholar]
- 31.Ferin M, Van Vugt D, Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent progress in hormone research 1984;40:441–485. [DOI] [PubMed] [Google Scholar]
- 32.Brooks AN, Lamming GE, Lees PD, Haynes NB. Opioid modulation of lh secretion in the ewe. Journal of reproduction and fertility 1986;76:693–708. [DOI] [PubMed] [Google Scholar]
- 33.Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biology of reproduction 1988;39:1032–1038. [DOI] [PubMed] [Google Scholar]
- 34.Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology 1999;140:5929–5936. [DOI] [PubMed] [Google Scholar]
- 35.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 2004;145:2959–2967. [DOI] [PubMed] [Google Scholar]
- 36.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 2002;143:4366–4374. [DOI] [PubMed] [Google Scholar]
- 37.Rance NE, Young WS, 3rd. Hypertrophy and increased gene expression of neurons containing neurokinin-b and substance-p messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 1991;128:2239–2247. [DOI] [PubMed] [Google Scholar]
- 38.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin b-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 2000;141:4218–4225. [DOI] [PubMed] [Google Scholar]
- 39.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the suffolk ewe. Endocrinology 1993;133:887–895. [DOI] [PubMed] [Google Scholar]
- 40.Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide y, and beta-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology 1997;138:2585–2595. [DOI] [PubMed] [Google Scholar]
- 41.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin b immunoreactivity in the arcuate nucleus and median eminence of the sheep. Journal of neuroendocrinology 2006;18:534–541. [DOI] [PubMed] [Google Scholar]
- 42.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin b immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. The Journal of comparative neurology 2006;498:712–726. [DOI] [PubMed] [Google Scholar]
- 43.Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 2006;141:1731–1745. [DOI] [PubMed] [Google Scholar]
- 44.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: Colocalisation in neurokinin b cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. Journal of neuroendocrinology 2010;22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin b modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. The Journal of comparative neurology 2005;489:372–386. [DOI] [PubMed] [Google Scholar]
- 46.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of kiss1 gene expression in the brain of the female mouse. Endocrinology 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 47.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neuroscience letters 2006;401:225–230. [DOI] [PubMed] [Google Scholar]
- 48.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin b neuronal projections and regulation during lactation in the rat. Journal of neuroendocrinology 2011;23:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. Journal of neuroendocrinology 2009;21:813–821. [DOI] [PubMed] [Google Scholar]
- 50.Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology 2006;147:4843–4851. [DOI] [PubMed] [Google Scholar]
- 51.Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto kndy and gnrh neurones during the preovulatory lh surge in the ewe. Journal of neuroendocrinology 2015;27:624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory lh surge and stimulates gnrh release from the isolated ovine median eminence. Endocrinology 2011;152:1001–1012. [DOI] [PubMed] [Google Scholar]
- 53.Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, Maeda K, Ichikawa M, Okamura H. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: An immunoelectron microscopic study. Neuroendocrinology 2011;94:323–332. [DOI] [PubMed] [Google Scholar]
- 54.Higo S, Iijima N, Ozawa H. Characterisation of kiss1r (gpr54)-expressing neurones in the arcuate nucleus of the female rat hypothalamus. Journal of neuroendocrinology 2017;29. [DOI] [PubMed] [Google Scholar]
- 55.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of kiss-1 mrna in the male rat. Neuroendocrinology 2004;80:264–272. [DOI] [PubMed] [Google Scholar]
- 56.Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized gnrh pulse generator activity among kisspeptin/neurokinin b/dynorphin a (kndy) neurons in goats. The Journal of reproduction and development 2013;59:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin b neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 2010;166:680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. Kndy (kisspeptin/neurokinin b/dynorphin) neurons are activated during both pulsatile and surge secretion of lh in the ewe. Endocrinology 2012;153:5406–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ezzat A, Pereira A, Clarke IJ. Kisspeptin is a component of the pulse generator for gnrh secretion in female sheep but not the pulse generator. Endocrinology 2015;156:1828–1837. [DOI] [PubMed] [Google Scholar]
- 60.Sakamoto K, Murata K, Wakabayashi Y, Yayou KI, Ohkura S, Takeuchi Y, Mori Y, Okamura H. Central administration of neurokinin b activates kisspeptin/nkb neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. The Journal of reproduction and development 2012;58:700–706. [DOI] [PubMed] [Google Scholar]
- 61.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29:3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that neurokinin b controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinology 2015;101:161–174. [DOI] [PubMed] [Google Scholar]
- 63.Fergani C, Navarro V. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reproduction 2016. [DOI] [PubMed] [Google Scholar]
- 64.Fergani C, Mazzella L, Coolen LM, McCosh RB, Hardy SL, Newcomb N, Grachev P, Lehman MN, Goodman RL. Do substance p and neurokinin a play important roles in the control of lh secretion in ewes? Endocrinology 2016;157:4829–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. Kndy cells revisited. Endocrinology 2018;159:3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amstalden M, Foradori CD, coolen LM, Lehman MN, Goodman RL. Distribution of kappa opioid receptor mrna in the preoptic area and hypothalamus of the ewe. Abstracts, Annual meeting of the Society of Reproduction, Quebec Cuty, Canada 2005. [Google Scholar]
- 67.Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. Kappa-opioid receptor is colocalized in gnrh and kndy cells in the female ovine and rat brain. Endocrinology 2016;157:2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science 2002;297:1566–1569. [DOI] [PubMed] [Google Scholar]
- 69.Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of mu opioid receptors in the medial preoptic area following copulation in male rats. Neuroscience 2004;124:11–21. [DOI] [PubMed] [Google Scholar]
- 70.Weems PW, Coolen LM, Hileman SM, Hardy S, McCosh RB, Goodman RL, Lehman MN. Evidence that dynorphin acts upon kndy and gnrh neurons during gnrh pulse termination in the ewe. Endocrinology 2018;159:3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology 1995;136:2412–2420. [DOI] [PubMed] [Google Scholar]
- 72.Moore AM, Lucas KA, Goodman RL, Coolen LM, Lehman MN. Three-dimensional imaging of kndy neurons in the mammalian brain using optical tissue clearing and multiple-label immunocytochemistry. Scientific reports 2018;8:2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore AM, Coolen LM, Lehman MN. Kisspeptin/neurokinin b/dynorphin (kndy) cells as integrators of diverse internal and external cues: Evidence from viral-based monosynaptic tract-tracing in mice. Scientific reports 2019;9:14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF, Jr., Ren C, Chan KK, Seminara SB. Kisspeptin resets the hypothalamic gnrh clock in men. The Journal of clinical endocrinology and metabolism 2011;96:E908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He W, Coolen LM, Goodman RL, Lehman MN. Co-localization of nk3r and kor mrna in kndy and non-kndy neurons in the ovine arcuate nucleus. Program Anual meeting of Society for Neuroscience 2019:Abstr 673.617. [Google Scholar]