Abstract
Muller glia are the predominant glial cell type in the retina, and they structurally and metabolically support retinal neurons. Wnt/β-catenin signaling pathways play essential roles in the central nervous system (CNS), including glial and neuronal differentiation, axonal growth and neuronal regeneration. We previously demonstrated that Wnt signaling activation in retinal ganglion cells (RGC) induces axonal regeneration after injury. However, whether Wnt signaling within the adjacent Muller glia plays an axongenic role is not known. In this study, we characterized the effect of Wnt signaling in Muller glia on RGC neurite growth. Primary Muller glia and RGC cells were grown in transwell co-cultures and adenoviral constructs driving Wnt regulatory genes were used to activate and inhibit Wnt signaling specifically in primary Muller glia. Our results demonstrated that activation of Wnt signaling in Muller glia significantly increased RGC average neurite length and branch site number. Additionally, the secretome of Muller glia after induction or inhibition of Wnt signaling was characterized using protein profiling of conditioned media by Q Exactive mass spectrometry. The Muller glia secretome after activation of Wnt signaling had distinct and more numerous proteins involved in regulation of axon extension, axon projection and cell adhesion. Furthermore, we showed highly redundant expression of Wnt signaling ligands in Muller glia and Frizzled receptors in RGCs and Muller glia. Therefore, this study provides new information about potential neurite growth promoting molecules in the Muller glia secretome, and identified Wnt-dependent target proteins that may mediate axonal growth.
Keywords: Wnt signaling, Retinal ganglion cells (RGC), Muller glia, neurite growth, secretome, proteomics
Introduction
The optic nerve is formed from axons of retinal ganglion cells (RGC) and transmits visual stimuli from the retina to the brain. Optic neuropathies and trauma cause progressive atrophy of the optic nerve and RGC death, leading to irreversible blindness (You, Gupta, Li, Klistorner, & Graham, 2013). An important therapeutic goal for optic neuropathies is to promote regeneration of RGC axons after injury. Numerous cytokines, neurotrophins and signaling pathways such as Wnt/β-catenin, mTOR and STAT3 have been reported to significantly enhance RGC axon regeneration in rodent optic nerve injury models and in primary RGC cultures (A. L. Garcia, Udeh, Kalahasty, & Hackam, 2018; Pernet & Schwab, 2014). However, stimulating these signaling pathways rarely result in extended regeneration from the eye to the visual cortex, indicating that much remains to be understood regarding the cellular and molecular mechanisms of axonal regrowth.
Muller cells are the predominant glial cell type in the retina, and they structurally and metabolically support retinal neurons (Araujo, Santos, & Silva, 2018; Vecino, Rodriguez, Ruzafa, Pereiro, & Sharma, 2016). Co-cultures of primary rat and porcine Muller glia improve the survival of RGCs and protect them from harmful effects of glutamate, high glucose concentration and NMDA treatment (Furuya, Pan, & Kashiwagi, 2012; Kashiwagi et al., 2004; Matteucci et al., 2014; Skytt et al., 2016). Muller glia secrete numerous trophic factors, such as CNTF, osteopontin, basigin, clusterin and PEDF, some of which support neuronal survival or induce axon regeneration in RGCs (Ruzafa, Pereiro, Lepper, Hauck, & Vecino, 2018; Unterlauft et al., 2014; Vecino et al., 2016). Over-expression of CNTF in Muller glia in vivo induced RGC axonal outgrowth after injury (Pernet et al., 2013) and extracellular matrix (ECM) proteins such as laminins, fibronectin and tenascin-c (TN-C), which are often secreted by Muller glia, regulate neuritogenesis and axon extension in RGC and play a crucial role in axon pathfinding (Siddiqui, Horvat-Brocker, & Faissner, 2008; Vecino et al., 2016). Muller glia-derived proteins were shown to enhance RGC survival and neurite elongation using co-cultures of porcine Muller glia and RGC, and by transferring conditioned media from mouse Muller glia onto RGCs, although the specific proteins involved were not determined (de Melo Reis, Cabral-da-Silva, de Mello, & Taylor, 2008; M. Garcia, Forster, Hicks, & Vecino, 2002). Therefore, mechanisms that mediate growth promoting effects of Muller glia on RGC neurites are not well understood.
The Wnt/β-catenin signaling pathway plays important roles in the embryonic and adult central nervous system (CNS), including neuronal differentiation, dendritic out-growth, axonal pathfinding, synaptic assembly and neurite regeneration (Garcia et al., 2018). The canonical Wnt pathway is activated by Wnt ligands binding to Frizzled (Fzd) receptors and co-receptor low-density lipoprotein receptor-related protein 5/6 (LRP5/6). The formation of this ligand-receptor complex leads to β-catenin stabilization in the cytoplasm and translocation into the nucleus, followed by activation of TCF/LEF transcription factor mediated target gene expression. In the neural retina, canonical Wnt signaling pathway proteins are expressed, and signaling activation is observed, in RGCs, Muller glia, microglia and amacrine cells (Liu, Mohamed, Dufort, & Wallace, 2003; Yi, Nakamura, Mohamed, Dufort, & Hackam, 2007). Our group recently demonstrated that Wnt/β-catenin signaling induced by intravitreal injection of recombinant Wnt3a significantly improved RGC survival, axon regeneration and RGC function after optic nerve crush injury in mice, which was associated with Wnt signaling activation in both Muller glia and RGCs (Patel, Park, & Hackam, 2017). Additionally, Wnt signaling activation in Muller glia led to enhanced photoreceptor survival and function (Patel et al., 2015), indicating an important neuroprotective activity of Wnt signaling in Muller glia. Wnt3a also promoted neurite outgrowth of primary RGC cells grown in culture (Udeh, Dvoriantchikova, Carmy, Ivanov, & Hackam, 2019). However, the contribution of Wnt signaling within Muller glia to RGC neurite growth is not yet known.
The purpose of this study was to investigate the role of Wnt signaling in Muller glia on RGC neurite growth, to identify Wnt pathway components expressed by Muller glia and RGCs and to characterize the secretome of Muller glia when Wnt signaling is induced and inhibited. Our results demonstrate that activation of Wnt signaling specifically in Muller glia induced significant RGC axon growth and neurite complexity, which was equivalent to the effect of Wnt signaling activated within RGCs. In addition, analysis of the Muller glia secretome identified numerous potential neurite growth promoting factors induced by activation of canonical Wnt signaling. Therefore, this study provides new information regarding neurite growth induction in RGC from manipulating extrinsic signaling mechanisms in glia.
Materials and Methods
Animals
All experiments using mice were carried out in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision research and were approved by the Animal Care and Use Committee at the University of Miami. To establish primary Muller glia and RGC cultures, retinas from male and female C57BL/6J pups (Jackson Laboratory Bar Harbor, Maine. Stock # 000664) were used. The breeding pairs and pups were housed under 12 hour light-dark cycle with ad libitum access to food and water.
Isolation of primary Muller glia
We used our previously described protocol for isolation of Muller glia (Yi et al., 2007). Eyes from post-natal day (p) 7–8 pups were enucleated after euthanization and the retinas were carefully isolated then dissociated with scissors in Dulbecco Phosphate Buffer Saline (DPBS). To make single cell suspensions, retinas were incubated in DPBS containing papain (16.5 U/mL; Worthington Biochemical Corp, Lakewood, NJ), L-cystine hydrochloride (2 mg/mL; Sigma-Aldrich, St. Louis, MO) and DNAse (125 U/mL; Sigma-Aldrich) for 30 min at 37 °C. The retina fragments were triturated gently for complete dissociation, followed by the addition of ovo mucoid (1.5 mg/mL; Sigma). The isolated retinal cell suspension was centrifuged (800 rpm for 5 min) then plated in plastic dishes with DMEM containing 10% fetal bovine serum (Thermo Fisher Scientific, Grand Island, NY) for 16 hours. The dishes were shaken to remove non-attached cells and the attached Muller glia were allowed to grow for 7 to 10 days in a T-75 flask at 37°C, 5% CO2, to eliminate any remaining retinal neurons and reach sufficient density. The purity of the Muller glial cultures was determined by their morphology and immunodetection with anti-glutamine synthase antibody (Abcam, Cambridge, MA; 1:1000).
Isolation of primary retinal ganglion cells
To establish primary RGC cultures, we followed a two-step immunopanning protocol as described previously (Dvoriantchikova, Degterev, & Ivanov, 2014; Udeh et al., 2019). Briefly, single cell retinal suspensions from p10-p12 mouse pups were prepared as described above and macrophages and endothelial cells were removed by immunopanning with an anti-macrophage antibody (Accurate Chemical, Westbury, NY). RGCs were isolated from retinal cell suspensions by incubating for 45 min in a 100 mm petri-dish coated with anti-Thy1.2 antibody. After sequential washes with DPBS, the bound RGCs were released by trypsinization and plated on glass coverslips coated with 10 μg/mL poly-D-lysine and 2 μg/mL laminin in 24 well plates at a density of 50,000 cell per well in serum-free neurobasal/B27 media (Thermo Fisher Scientific, Grand Island, NY) and grown at 37°C, 5% CO2. Through this method, we obtained >95% pure RGC cultures, as determined by immunodetection using RGC-specific antibodies (anti-RBPMS antibody; PhosphoSolutions, Aurora, CO, USA; 1:500).
Muller glia and RGC co-cultures
To study the effects of activation or inhibition of canonical Wnt signaling in Muller glia on RGC neurite growth, 25,000 primary Muller glia cells were seeded on a membrane with 0.4 μM pores in a trans-well insert (ThinCert Greiner bio-one, Monroe, NC) with 200 μL of neurobasal/B27 medium without serum. Each trans-well insert was placed in a well of a 24 well plate, with 15 mm distance from the bottom and allowing contact with the neurobasal/B27 medium in the lower chamber. Muller glia cells were infected with 100,000 MOI of adenoviral constructs containing genes for the canonical Wnt pathway activator β-catenin-S33A, Wnt inhibitor β-eng or the control GFP, as previously described (Patel et al., 2015). These genes are under the control of a 2.2 kb long form of the GFAP promoter that directs expression specifically in Muller glia (de Leeuw et al., 2006; Su et al., 2004). The viral constructs also contain the GFP gene driven by the chicken β-actin promoter to allow visual confirmation of viral infection. After confirming viral infection in at least 80% of Muller glia, RGCs were plated onto coverslips and co-cultured underneath the transwells containing the Muller glia for 48 hours. In parallel experiments, canonical β-catenin signaling was induced in both Muller glia and RGC by adding 50 ng/mL recombinant Wnt3a (R & D systems, Minneapolis, MN) to the media of both chambers. The co-culture experiments were performed three times on 2 coverslips per experiment.
Immunostaining
After 48 hours of co-culturing, RGCs were fixed with 4% paraformaldehyde and immunostained, as described previously (Udeh et al., 2019). Fixed cells were permeabilized in 0.3% Triton X-100/PBS for 30 min then blocked with a buffer containing 2% bovine serum albumin (BSA), 5% goat serum and 0.3% Triton X-100 in PBS for 30 min. The coverslips were then incubated in the primary antibody rabbit anti-βIII-tubulin (Abcam, Cambridge, MA; 1:1000) diluted in blocking buffer, overnight at 4°C. The coverslips were then washed gently three times in Tween 20/PBS solution each for 3 min, incubated in Alexa 546 goat anti-rabbit IgG secondary antibody (1:500, Invitrogen, Carlsbad, CA) for 1 hour at room temperature, followed by three washes and then mounted onto glass slides using DAPI-containing mounting medium. The cells were imaged using a Zeiss LSM 700 confocal microscope. Neurite number, neurite length and branch site number were measured in at least 10 random fields per replicate, with neurites from 100–200 RGC measured in each replicate. Investigators were masked to the treatment identity during imaging and measurements.
Muller glia conditioned medium
To characterize the secretome of Muller glia after induction or inhibition of Wnt signaling, primary Muller glia cells were plated at a density of 20,000 cells/cm2 in T25 flasks with DMEM containing 10% FBS. The cells were infected with 100,000 MOI of the adenoviral constructs containing the canonical Wnt pathway activator β-catenin-S33A or Wnt inhibitor β-eng genes. Uninfected cells were used as a control for the secretome of Muller glia under normal conditions. After confirming 80% of cell infection using GFP expression, the cells were washed three times with DPBS and the media was replaced with serum free opti-MEM. After incubation for 48 hours at 37°C the conditioned media was collected, centrifuged at 1000 rpm for 5 min to remove cell debris, and then stored at −80°C. The conditioned medium was collected from three individual experiments.
Mass spectrometry and data analysis
Proteins were analysed in the conditioned media following our previously published papers (Edwards, Mesa, Vazquez-Padron, Kowalski, & Bhattacharya, 2017; Trzeciecka, Pattabiraman, Piqueras, Toris, & Bhattacharya, 2018) with the following modifications. Proteins were precipitated from conditioned media samples by adding 4 volumes of acetone and incubating overnight at −20°C. The proteins were pelleted by centrifugation (18,000 × g for 15 min) and the pellet was re-suspended in 50 mM ammonium bicarbonate. A bicinchonic assay (BCA) was performed using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) to determine the protein concentration. A 100 μg aliquot was made and was reduced using 0.5 M dithiothreitol (DTT) and subsequently alkylated using 0.55 M iodoacetamide (GE Healthcare Life Sciences, Logan, UT) with incubation at 56°C for 20 minutes and 15 minutes at room temperature respectively. The proteins were digested with 0.1 μg/μL sequence-grade modified trypsin (Promega Corporation, Madison, WI) and incubated at 37 °C overnight. Formic acid (50%) was added to stop the reaction and the samples were dried in a vacuum concentrator and then stored at −20 ˚C. Before LC-MS analysis, peptides were re-suspended in 30 μL of 2% acetonitrile in water with 0.1 % formic acid.
High Performance Liquid Chromatography (HPLC)
Reversed-phase chromatographic separation was performed using an Easy-nLC 1000 system (Thermo Fisher Scientific) with an Acclaim PepMap RSLC 75 μm × 15 cm, nanoViper column (Thermo Fisher Scientific). The solvents were LC-MS grade water and acetonitrile as solvent A and B respectively. The gradient ran from 2–98% solvent B for 84 minutes with a flow rate of 450 nL/min and an injection volume of 5 μL.
Mass Spectrometry
The peptides were analyzed using a Q Exactive mass spectrometer (Thermo Fisher Scientific) equipped with a heated electrospray ionization source (HESI) operating in positive ion mode, as described previously (Trzeciecka & Bhattacharya, 2019). The spray voltage was 1.8 kV, all gases were set to 0, the capillary temperature was 250 ˚C and the S-lens radio frequency level was 50. Full scan used a mass range of 150–1500 m/z with 70,000 resolution, automatic gain control (AGC) target of 1 × 106 ions, and maximum injection time (IT) of 100 ms. Data dependent MS-MS used 17,500 resolution, AGC target of 1 × 105 ions, maximum IT of 75 ms, and collision energy of 28.
Protein Identification
Protein identifications from MS/MS data utilized the Proteome Discoverer 2.2 software (Thermo Fisher Scientific) using Sequest HT and MS Amanda search engines. The data was searched against Mus musculus entries in the Uniprot protein sequence database. The search parameters included: precursor mass tolerance 10 ppm and 0.02 Da for fragments, 2 missed trypsin cleavages, oxidation (Met) and acetylation (protein N-term) as variable modifications, carbamidomethylation (Cys) as a static modification (Trzeciecka & Bhattacharya, 2019). Protein annotation analysis was performed on GO terms using Uniprot KB and DAVID bioinformatics databases.
RNA isolation, cDNA preparation and Polymerase Chain Reaction:
Total RNA was extracted from trypsinized primary Muller glial cells (after 7 days in culture) or primary RGC (after 2 days in culture) using Qiagen RNeasy mini kit (Qiagen, Valencia, CA, USA) and cDNA was prepared with the iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s recommendations. PCR was performed for all 19 Wnt ligands and 10 Fzd receptors using cDNA from Muller glia and RGC, using oligonucleotides listed in Supplemental Tables 1 and 2. At least one oligonucleotide in each pair spanned an intron-exon junction. Expression of Wnt ligands and Fzd receptors were determined by the presence of the predicted size PCR product in a 2% agarose gel and confirmed by Sanger sequencing (Genewiz, South Plainfield, NJ) of the eluted DNA from the agarose gel. Positive controls for primer amplification used cDNA derived from mouse whole eye, brain, kidney, spleen and/or liver tissues.
Statistical analysis
Statistical analysis was performed with ANOVA or Student’s t-test with GraphPad Prizm. P values <0.05 were considered to be statistically significant.
Results
Wnt signaling in Muller glia increased RGC neurite growth and neurite complexity
We previously identified Wnt signaling activation in both Muller glia and RGCs in vivo (Patel et al., 2017). To determine whether canonical Wnt signaling within Muller glia is sufficient to induce RGC neurite formation and regulate neurite length and complexity, we used a co-culture approach with trans-wells to separate the two cell types, with pores of sufficient size to allow diffusion of secreted factors. As shown in Figure 1A, activating Wnt signaling in Muller glia using constitutively active β-catenin-S33A significantly increased RGC average neurite length and average branch site number compared to Muller glia incubated with GFP control or the Wnt inhibitor β-eng. The average neurite length for RGCs co-cultured with Muller glia expressing β-catenin-S33A was 101.5 μm (±21.3) (mean ± SD), whereas neurite length for GFP was 44.2 μm (±3.5) and β-eng was 34 μm (±2.7) (ANOVA, GFP vs β-catenin-S33A, p=0.0006, n=3). Induction of Wnt signaling by Wnt3a, which activates the pathway in Muller glia and RGC simultaneously, had equivalent neurite length (110.1 μm ± 5) as β-catenin-S33A expressed in Muller glia.
Figure 1.
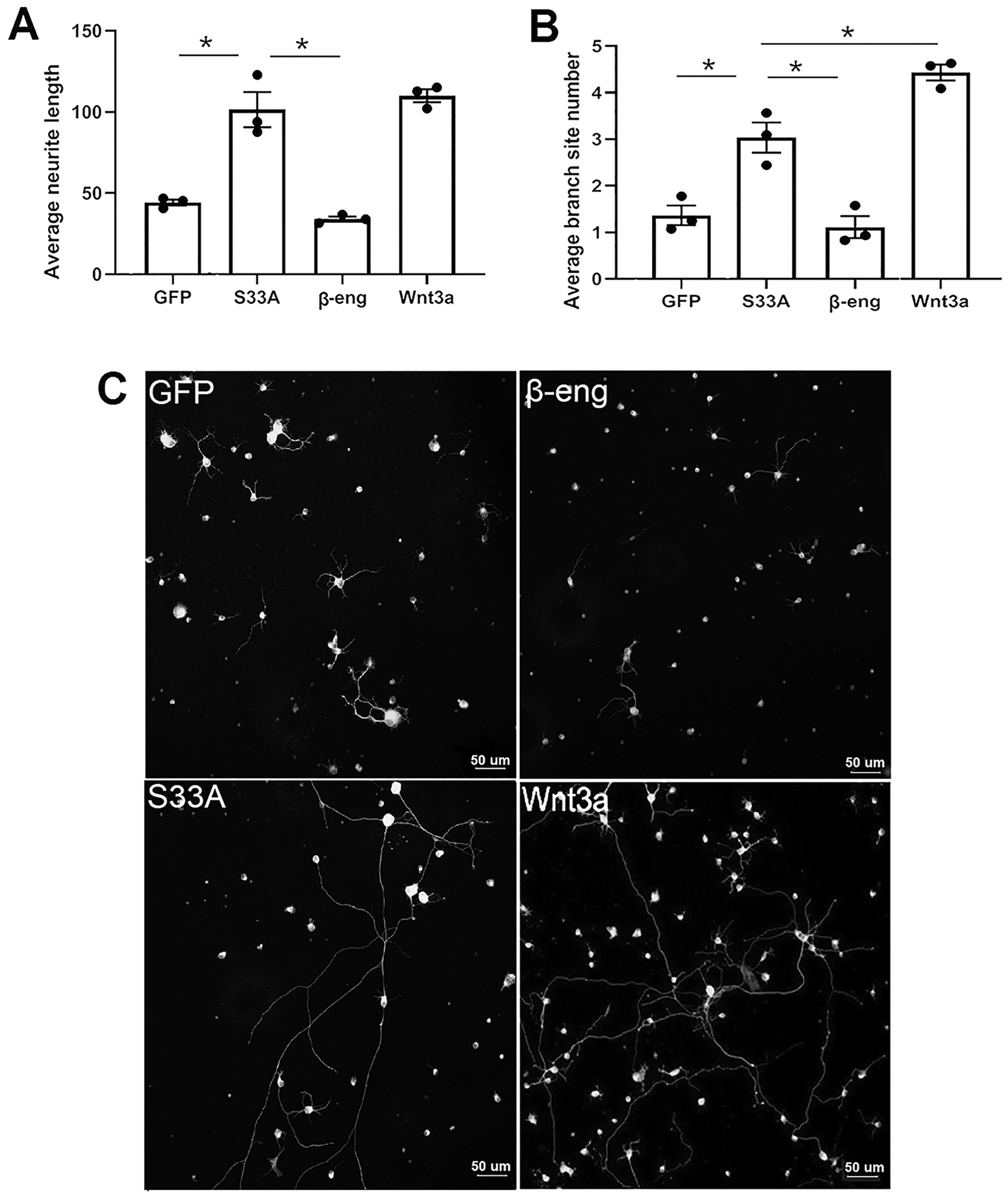
Wnt signaling in Muller glia increases RGC neurite growth and neurite complexity in co-cultures. (A) & (B) Bar diagrams indicate that induced Wnt signaling in Muller glia (β-catenin-S33A) increases RGC neurite growth and neurite complexity (branch site number) compared to inhibited Wnt signaling (β-eng) and control Muller glia (GFP) cells. Induction of Wnt signaling in both Muller glia and RGC further increases neurite complexity of RGC. Average neurite length and average branch site number per cell is represented as mean ± SD. Each point is an experimental replicate that indicates an average of neurite length or average of branch site number of approximately 100–200 RGCs. *p<0.05 (ANOVA, Tukey’s multiple comparisons test). (C) Representative images showing increased RGC neurite growth and neurite complexity when co-cultured with Muller glia with induced Wnt signaling compared to inhibited Wnt signaling and in control Muller glia. Soma and neurites of RGCs were detected with anti-β-III tubulin antibody staining.
Average branch site number was used as a measure of neurite complexity. As shown in Figure 1B, average branch site number was also significantly enhanced when Wnt signaling was induced in Muller glia compared to when Wnt signaling was inhibited. The average branch site numbers of β-catenin-S33A was 3.0 (± 0.6), which was significantly higher than β-eng and GFP control, which were 1.1 (± 0.4) and 1.3 (± 0.3), respectively (ANOVA, GFP vs β-catenin-S33A: p=0.005; β-eng vs β-catenin-S33A: p=0.002, n=3). Interestingly, the average branch site number in Wnt3a group was 4.4 (± 0.3), which is significantly higher than the β-catenin-S33A group (ANOVA, Wnt3a vs β-catenin-S33A; p=0.01, n=3). In contrast, there was no significant difference in neurite number between the different groups. These results demonstrate that extrinsic Wnt signaling in Muller glia induces neurite growth and complexity in RGC.
Identification of Wnt ligand and Frizzled receptors in primary Muller glia and primary RGC
PCR with gene-specific primers was used to amplify all 19 Wnt ligands and 10 Fzd receptors. Based on the expected size bands and Sanger sequencing of the PCR products, we confirmed expression of Wnt2, Wnt4, Wnt5a, Wnt5b, Wnt9a, Wnt10b and Wnt16 in Muller glia (Table 1), Fzd1, 3, 4, 5, and 7 expression in RGC, and Fzd1–7 and 9–10 in Muller glia (Table 2). In contrast, Wnt1, Wnt2b, Wnt3, Wnt3a, Wnt6a Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt9b, Wnt10a, Wnt11 were not expressed in Muller glia, Fzd2, 6 8, 9, 10 were not expressed in RGC, and Fzd8 was not expressed in Muller glia.
Table 1:
Wnt ligands expressed in mouse primary Muller glia
| Gene name | mRNA ID | Expression in primary Muller glia |
|---|---|---|
| Wnt1 | NM_021279.4 | − |
| Wnt2 | NM_023653.5 | + |
| Wnt2b | NM_009520.3 | − |
| Wnt3 | NM_009521.2 | − |
| Wnt3a | NM_009522.2 | − |
| Wnt4 | NM_009523.2 | + |
| Wnt5a | NM_009524.4 | + |
| Wnt5b | NM_009525.3 | + |
| Wnt6 | NM_009526.3 | − |
| Wnt7a | NM_009527.4 | − |
| Wnt7b | NM_009528.3 | − |
| Wnt8a | NM_009290.2 | − |
| Wnt8b | NM_011720.3 | − |
| Wnt9a | NM_139298.2 | + |
| Wnt9b | NM_011719.4 | − |
| Wnt10a | NM_009518.2 | − |
| Wnt10b | NM_011718.2 | + |
| Wnt11 | NM_001285792.1 | − |
| Wnt11 | NM_001285795.1 | − |
| Wnt16 | NM_053116.4 | + |
The presence or absence of expression of different Wnt ligands in primary Muller glia is indicated by + or − respectively. The reference mRNA sequences used to design the oligonucleotides are listed.
Table 2:
Frizzled receptors expressed in mouse primary RGCs and in mouse primary Muller glia
| Gene name | mRNA ID | Expression in primary RGCs | Expression in primary Muller glia |
|---|---|---|---|
| Fzd1 | NM_021457.3 | + | + |
| Fzd2 | NM 020510.2 | − | + |
| Fzd3 | NM_021458.2 | + | + |
| Fzd4 | NM_008055.4 | + | + |
| Fzd5 | NM_022721.3 | + | + |
| Fzd5 | NM_001042659.1 | + | + |
| Fzd6 | NM_008056.3 | − | + |
| Fzd7 | NM_008057.3 | + | + |
| Fzd8 | NM_008058.2 | − | − |
| Fzd9 | NM_010246.1 | − | + |
| Fzd10 | NM_175284.3 | − | + |
The presence or absence of expression of different Fzd ligands in primary RGC and in primary Muller glia is indicated by + or − respectively. The reference mRNA sequences used to design the oligonucleotides are listed.
Characterization of the Muller glia secretome after induction and inhibition of canonical Wnt signaling
Media was obtained from three individual experiments of Muller glia expressing β-catenin-S33A, β-eng or an untreated control. After analysis of the spectra obtained from mass spectrometry and excluding the low and medium confidence peptides, we identified an average of 3810 proteins (range: 3511–4175) in the three β-catenin-S33A conditioned media samples, 3675 (range: 3294–3947) proteins in the three β-eng media samples and 3001 proteins (range: 469–4274) in untreated Muller glia media. We further filtered proteins that were observed in at least two out of the three replicates, resulting in 1864 proteins in the β-catenin-S33A group (Table S3), 1737 proteins in the β-eng group (Table S4) and 1253 in the untreated controls (Table S5). Of these filtered proteins, we sorted proteins that were in common in all three groups (263 proteins) and proteins in common between β-catenin-S33A and β-eng groups (570 proteins) (Figure 2). Furthermore, we identified 1104 proteins that were exclusively in the media of the β-catenin-S33A group, and 1001 that were exclusively in the β-eng group (Table S6).
Figure 2.
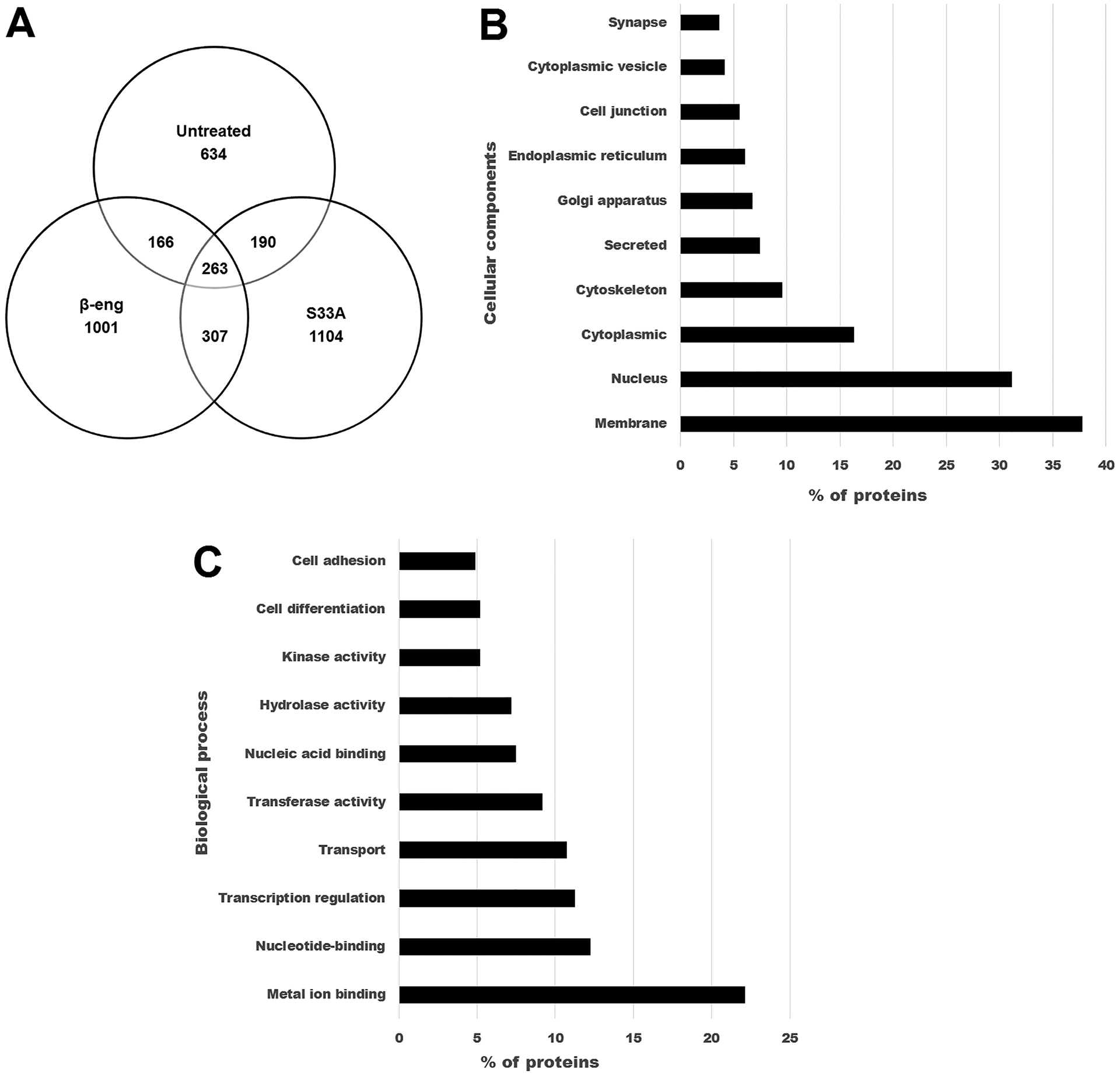
Characterization of conditioned media collected from untreated Muller glia and Muller glia after induction or inhibition of Wnt/β-catenin signaling. A) Venn diagram shows the proteins identified exclusively in untreated, β-catenin-S33A and β-eng groups and in common with the other groups. (B) & (C) Enrichment analysis of proteins exclusively identified in conditioned media collected from the β-catenin-S33A group of Muller glia cells. B) The top 10 enriched cellular components are shown. C) The top 10 enriched biological process are shown.
Our main focus in this analysis is secreted proteins and shed membrane proteins that are involved in axonogenesis, neurite growth and axon guidance. Membrane proteins were considered because some ligands that are present on Muller glia, such as Notch and semaphorin family members, may interact with receptors on neuronal cells to induce or inhibit signaling mechanisms that regulate neurite growth. Therefore, we mapped the identified protein profiles based on information about signal peptide, transmembrane domain and cellular localization, and performed enrichment analysis based on GO terms using Uniprot KB and DAVID bioinformatics databases (Huang da, Sherman, & Lempicki, 2009; UniProt Consortium, 2018). In the profile of 1253 proteins (Uniprot accession numbers) of the untreated Muller glia secretome, 1157 IDs (unique proteins) were identified using DAVID, which mainly includes secreted proteins (119 unique proteins; 10.2% of total), membrane proteins (422 unique proteins; 36.4%) and extracellular region-containing proteins (139 unique proteins; 12%). The secreted proteins are predominantly enriched with extracellular matrix proteins (55 unique proteins) such as collagens, laminin, mucin, SPARC like protein 1 and tenascin. The secreted proteins include multiple growth factors, such as BMP1, FGF4, PDGF, VEGF-D, GDF-5, Neuregulin-2, Fibrosin and Inhibin beta C, and cell growth associated proteins such as Wnt10b and Thrombospondin-3. Also, numerous proteins were identified that are involved in neuron development (7 unique proteins, eg. Plexin B2 and Semaphorin 5B), neuron differentiation (11 unique proteins, eg: Notch1, Notch 2, Notch 3 and Jag 1, Jag 2), axon guidance (3 unique proteins, eg: Neuropilin1, Slit homolog 3) and synapse organization (10 unique proteins, eg: Neuroligin2, Neurexin-III and Piccolo). Additionally, proteins involved in cell adhesion (68 unique proteins), and EGF-like domain containing (65 unique proteins) molecules were significantly enriched in the Muller glia secretome.
The profile of proteins that were exclusively identified in β-catenin-S33A (1104 proteins) and β-eng (1001 proteins) groups were predominantly enriched with metal ion binding proteins, membrane proteins and secreted proteins. Furthermore, expression of distinct sets of proteins involved in axon extension, axon guidance, neuronal projection and synapse organization were identified. For example, in the β-catenin-S33A group, we identified more proteins that are involved in cell adhesion (60 unique proteins), axon guidance (23 unique proteins; eg. Sema3E, Sema6D, Slit homolog 1, EphrinA4), regulation of axon extension (4 unique proteins; eg. Ntrk3, Gprin1, SLIT and NTRK like protein 4), neuron projection development (12; eg. Contactin 2, Plexin-A3, Slit homolog 1) and growth regulation (7 unique proteins; Gap43, Ntrk3) compared to the β-eng group (Figure 3) (Table S7). In contrast, in the β-eng group, we observed fewer proteins involved in cell adhesion (28 unique proteins), axon guidance (10; eg. Sema3A, Sema6A, Ephrin A5), regulation of axon extension (0 unique proteins), neuron projection development (7 unique proteins) and growth regulation (0 unique proteins). Interestingly, canonical Wnt signaling in Muller glia leads to the expression of different Wnt ligands (Wnt10a and Wnt7b) in the β-catenin-S33A group compared to untreated (Wnt10b and Wnt4) and β-eng groups (no Wnt ligands were identified).
Figure 3.
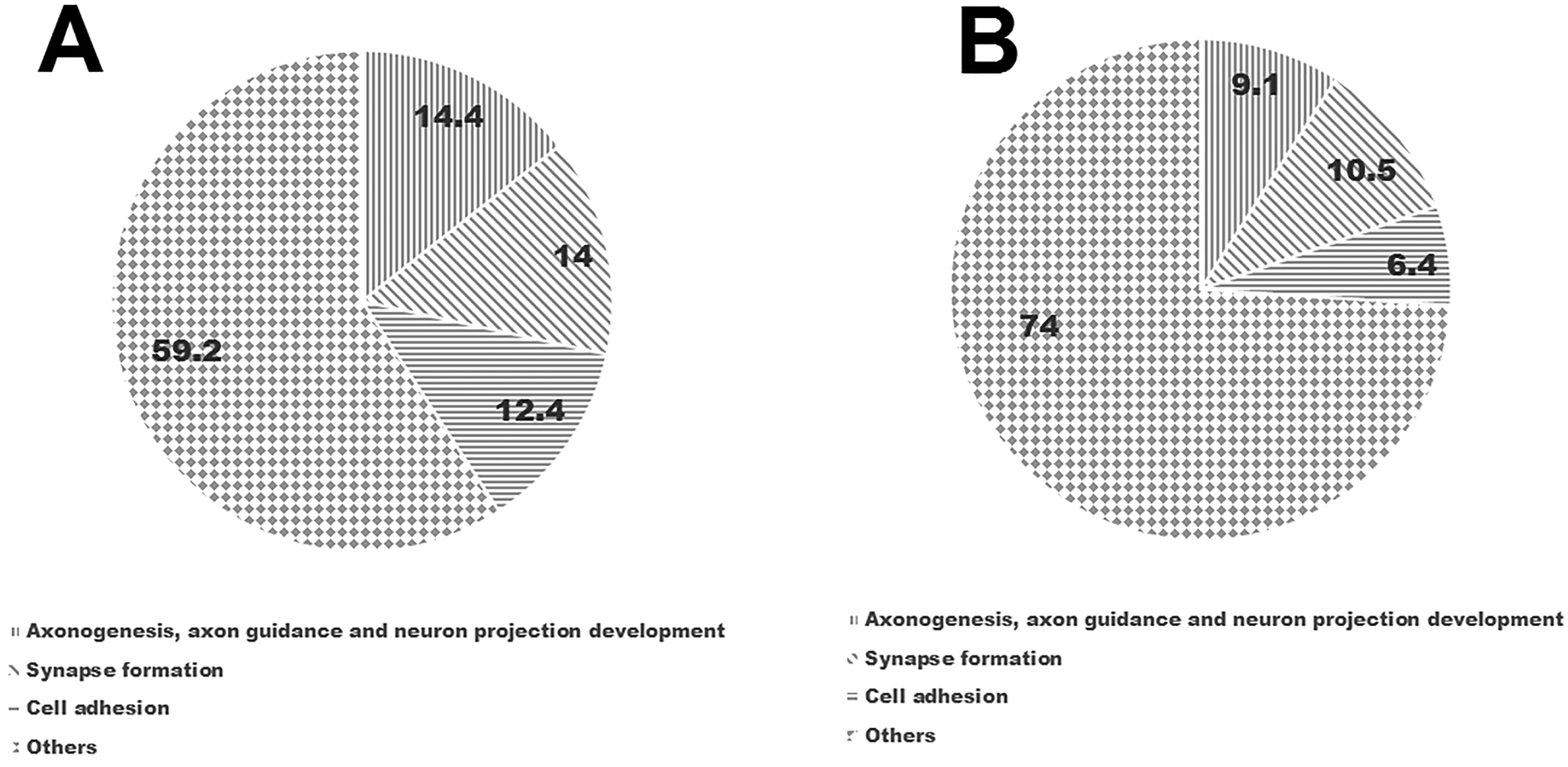
Exclusive proteins in the β-catenin-S33A and β-eng groups. (A) & (B) Pie charts represent percent of proteins involved in axonogenesis, axon guidance, neuron projection development, synapse formation and cell adhesion that were identified in β-catenin-S33A and β-eng groups, respectively.
Discussion
The main objective of this study was to explore the role of extrinsic Wnt/β-catenin signaling in Muller glia in RGC neuritogenesis and identify potential neurite growth factors induced by Wnt/β-catenin signaling. Our results demonstrated that Wnt/β-catenin signaling in Muller glia significantly induces RGC neurite growth and neurite complexity in primary co-cultures. Furthermore, we demonstrated distinct axon growth and guidance proteins after inducing Wnt/β-catenin signaling compared to inhibited Wnt/β-catenin signaling in Muller glia. Therefore, we identified potential new candidate inducers of RGC axonal growth and demonstrated that Muller glia could be a cellular target to enhance regeneration after injury.
Role of extrinsic Wnt/β-catenin signaling in Muller glia in RGC neuritogenesis, neurite growth and neurite complexity
Our findings are supported by previous studies that demonstrate that secreted factors from Muller glia support retinal neurons (Garcia et al., 2002; Ruzafa et al., 2018; Unterlauft et al., 2014; Vecino et al., 2016; von Toerne et al., 2014) and induce RGC neurite growth (de Melo Reis et al., 2008; M. Garcia et al., 2002). Furthermore, Pernet et al. (Pernet et al., 2013), demonstrated that overexpressing CNTF in Muller glia in vivo induced significant, albeit aberrant, RGC axonal growth. Interestingly, CNTF and its downstream mediator STAT3 are also direct targets of Wnt signaling (Patel et al., 2015). Additionally, we showed that neurite growth in the β-catenin-S33A group was equivalent to Wnt signaling induced in both Muller glia and RGC using Wnt3a. However, neurite complexity was significantly higher in the Wnt3a group compared to the β-catenin-S33A group, indicating that induction of Wnt/β-catenin signaling in both cell types may be required for neurite complexity whereas induction of signaling in Muller glia is sufficient to induce maximum effects on neurite growth. The ~30% higher branch site number in the Wnt3a group provides information about the relative contribution of Wnt signaling in Muller glia vs RGCs, although further studies using a combination of Wnt3a and β-eng would directly test the relative contribution of the two cell types to neurite growth. Also, it is possible that the differential effect on complexity may also be related to different signals induced by β-catenin-S33A bypassing Wnt receptors on the plasma membrane, in contrast to Wnt3a, which binds to Wnt receptors. Interestingly, we did not observe significant differences in neurite growth and neurite complexity between β-eng and GFP groups, indicating that basal levels of neurite growth were independent of Wnt signaling.
Wnt ligands expressed by primary Muller glia and Frizzled (Fzd) receptors expressed by primary RGC and Muller glia
Several studies have characterized the expression of Wnt ligands and Fzd receptors in the neural retina, but retinal cell-type specific expression of Wnt ligands and Fzd receptors has not been reported. We identified multiple Wnt ligands and Fzd receptors in primary RGC and Muller glia (Table 1 and 2), consistent with previous studies in adult mouse neural retina (Liu et al., 2003; Osakada et al., 2007; Van Raay & Vetter, 2004). Because we detected expression through PCR, we cannot rule out the possibility of very low expression of remaining Wnt ligands and Fzd receptors in Muller glia and RGC.
The identification of multiple Wnt ligands in Muller glia and multiple Fzd receptors in RGC and Muller glia indicates the complexity of Wnt signaling in neural retina. Furthermore, expression of multiple Fzd receptors suggests the possibility of activation of distinct Wnt signaling mechanisms that may function redundantly. Furthermore, expression of canonical (Wnt2, Wnt4 and Wnt10b) (Lyons et al., 2004; Paik et al., 2015; Sousa et al., 2010) and non-canonical Wnt ligands (Wnt5a, Wnt5b and Wnt16) (Blakely et al., 2011; Hutchins, Li, & Kalil, 2011; Mazzotta et al., 2016; Teh et al., 2007) by Muller glia and their receptors in RGC suggests the activation of both canonical and non-canonical Wnt signaling pathways. Although various studies reported a role for non-canonical Wnt signaling in neurite growth and neurite complexity of CNS neurons (Blakely et al., 2011; Hutchins et al., 2011; Pino, Choe, & Pleasure, 2011), there are no studies reporting their activation in neural retina. It was also demonstrated that some of the non-canonical Wnt ligands function antagonistically to canonical Wnt ligands and repel axons (Keeble et al., 2006; Topol et al., 2003). Further studies are warranted to identify non-canonical Wnt signaling mechanisms that are activated in RGCs and to determine their synergistic or antagonistic effects on canonical Wnt signaling, their role in RGC survival, neurite growth and axon guidance. The roles of different Wnt ligands and Fzd receptors expressed by Muller glia and RGC in neurite growth and regeneration will be an important focus of future studies.
Identification of neuroprotective and neurite growth promoting factors in the secretome of Muller glia after induction or inhibition of Wnt/β-catenin signaling
We demonstrated different types of proteins involved in axon extension, neuron projection, development and axon guidance in the exclusive proteins of the β-catenin-S33A compared with the β-eng group. Also, there were more total proteins involved in cell adhesion, axon guidance, axon extension, neuron projection and growth regulation in the β-catenin-S33A group than the β-eng group. However, although mass spectrometry is a highly sensitive method for identifying low abundant proteins, our experiments do not provide information on the expression levels of these identified proteins among the treatment groups. Future studies are needed to quantify the proteins in the secretome in the Wnt activated and inhibited treatments, and to compare protein levels during regeneration in vivo.
It was reported that glia cells in brain, retina and peripheral nervous system secrete various growth factors, extracellular matrix proteins, Adams (A disintegrin and metalloprotease), Adamts (A disintegrin and metalloproteinase with thrombospondin motif) neurite growth regulating proteins and proteins involved in synaptogenesis, in addition to proteins involved in regulating vasculogenesis and blood retina or blood brain barrier (Araujo et al., 2018; Jha et al., 2018; Ruzafa et al., 2018; Schira et al., 2019; Vecino et al., 2016; von Toerne et al., 2014). The protein profile of the porcine retinal Muller glia secretome were reported earlier (Ruzafa et al., 2018; von Toerne et al., 2014), and were mainly focused on identifying growth factors that support the survival of photoreceptors and RGC. Notably, although the majority of the proteins we identified in the normal Muller glia secretome corroborates previous published data, we did not detect growth factors that were reported previously secreted by Muller glia such as CNTF, PEDF, IGF-1, osteopontin and Leukemia inhibitory factor (Ruzafa et al., 2018; Unterlauft et al., 2014; Vecino et al., 2016; von Toerne et al., 2014). This discrepancy might be due to technical variation, and/or to the data analysis and filtration that we used. The untreated Muller glia secretome data is also in agreement with the secretome of other types of glial cell such as Schwann cells and astrocytes (Jha et al., 2018; Schira et al., 2019).
We identified the growth factors BMP1, FGF4, PDGF-C, TGF-β, GDF-5, Neuregulin-2, fibrosin and inhibin beta C and VEGF-D in conditioned media from untreated Muller glia. Several of these growth factors support neuron survival and/or neurite growth (Brecknell et al., 1996; Hegarty et al., 2014; Li, Gu, & Yi, 2017; Nakano, Kanekiyo, Nakagawa, Asahi, & Ide, 2016; Namwanje & Brown, 2016). In addition to these growth factors, we identified developmental cell growth associated proteins such as thrombospondins (eg. Thbs1 and Thbs3), Wnt ligands, growth factor binding proteins (eg: Igfbpl-1, Igfbp-2, Igfbp-6, Ltbp1, Ltbp2 and Ltbp4), axon guidance (eg. Nrp1, Slit3), neurite growth promoting proteins such as semaphorins and their receptors (eg. Sema5b), Adams (eg. Adam3, 5, 9, 11, 23 and 32), Adamts (eg. Adamts6, 9, 12, 14 and 19) and proteins involved in synapse organization (eg. Nlgn2, Nrxn3, and Sparcl1). The thrombospondin family members and some of the family members of ADAMS, ADAMTS and insulin growth factor biding proteins play a crucial role in inducing neurite out-growth and synaptogenesis (Bray et al., 2019; Hamel et al., 2008; Hsia et al., 2019; Risher & Eroglu, 2012; Yu et al., 2008). This suggests that Muller glia secrete neurotrophic and neurite growth promoting molecules even in the absence of neuronal cells. An important caveat is that the primary Muller glia and RGCs used in this study are from early post-natal animals due to the technical limitations of culturing these cell types from adult tissue, and additional studies are needed to confirm whether these molecules are secreted from adult glia.
Chen et al. (2007) reported that Wnt/β-catenin signaling in NIH3T3 cells induces cell adhesion protein expression (Chen, McLean, Carter, & Leask, 2007). Some of the cell adhesion molecules that we identified in the β-catenin-S33A group such as f-spondin and nidogen induce neuron survival or neurite growth in CNS neurons (Burstyn-Cohen, Frumkin, Xu, Scherer, & Klar, 1998; Lee, Seo, Suh, & Park, 2009). In addition to neurite growth promoting molecules, we observed various proteins involved in synapse organization. Similar to brain astrocytes, Muller glia are also involved in formation of synapses in-between different neurons in the neural retina. Although these signaling pathways and neurite growth promoting molecules are known to induce neuronal survival and neurite growth in CNS neurons, further studies are needed to identify their role in context of RGC survival and neurite growth.
During optic neuropathies, Muller glial cells are resistant to cell death compared to RGCs and therefore these cells are potential intermediates to repurpose developmental signaling pathways to induce neurite growth promoting factors and guidance cues (Pernet & Schwab, 2014). Induction of multiple neurite growth promoting protein pathways, as we found for Wnt signaling in the Muller glia secretome, would likely be more beneficial compared to treatment with single molecule to induce the neurite growth. Future in vivo studies will increase our understanding about the effects of induction of extrinsic signaling mechanisms in Muller glia on RGC neurite regeneration.
Supplementary Material
Supplemental Table 3: S33A conditioned media protein profile (common proteins in at least 2 samples out of 3 samples)
Supplemental Table 4: β-eng conditioned media protein profile (common proteins in at least 2 samples out of 3 samples)
Supplemental Table 5: Untreated conditioned media protein profile (common proteins in at least 2 samples out of 3 samples)
Supplemental Table 6: Exclusive proteins to S33A and β-eng groups
Supplemental Table 7: Membrane secreted proteins involved in neurite growth synapse cell adhesion protein from S33A and β-eng
Main Points.
Wnt/β-catenin signaling within Muller glia induced significant RGC neurite growth and neurite complexity.
The protein profiles of mouse Muller glia secretomes were characterized after induction or inhibition of Wnt/β-catenin signaling using mass spectrometry.
This study provides new knowledge about potential axonal growth-promoting molecules secreted by Muller glia.
Acknowledgements
Support for this study was from the NIH/NEI R01 EY026546 (ASH), R01 EY027311 (DI) and U.S. Department of Defence (WHX81-16-0715) (SKB). Institutional support to BPEI was from a Research to Prevent Blindness Unrestricted Grant and an NEI Center Core Grant EY014801.
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
Data Availability Statement: The data that supports the findings of this study are available in the supplementary material of this article.
References
- Araujo RS, Santos DF, & Silva GA (2018). The role of the retinal pigment epithelium and Muller cells secretome in neovascular retinal pathologies. Biochimie, 155, 104–108. doi: 10.1016/j.biochi.2018.06.019 [DOI] [PubMed] [Google Scholar]
- Blakely BD, Bye CR, Fernando CV, Horne MK, Macheda ML, Stacker SA, … Parish CL (2011). Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS One, 6(3), e18373. doi: 10.1371/journal.pone.0018373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray ER, Yungher BJ, Levay K, Ribeiro M, Dvoryanchikov G, Ayupe AC, … Park KK (2019). Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells. Neuron, 103(4), 642–657 e647. doi: 10.1016/j.neuron.2019.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecknell JE, Du JS, Muir E, Fidler PS, Hlavin ML, Dunnett SB, & Fawcett JW (1996). Bridge grafts of fibroblast growth factor-4-secreting schwannoma cells promote functional axonal regeneration in the nigrostriatal pathway of the adult rat. Neuroscience, 74(3), 775–784. doi: 10.1016/0306-4522(96)00167-4 [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Frumkin A, Xu YT, Scherer SS, & Klar A (1998). Accumulation of F-spondin in injured peripheral nerve promotes the outgrowth of sensory axons. J Neurosci, 18(21), 8875–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, McLean S, Carter DE, & Leask A (2007). The gene expression profile induced by Wnt 3a in NIH 3T3 fibroblasts. J Cell Commun Signal, 1(3–4), 175–183. doi: 10.1007/s12079-007-0015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw B, Su M, ter Horst M, Iwata S, Rodijk M, Hoeben RC, … Brenner M (2006). Increased glia-specific transgene expression with glial fibrillary acidic protein promoters containing multiple enhancer elements. J Neurosci Res, 83(5), 744–753. doi: 10.1002/jnr.20776 [DOI] [PubMed] [Google Scholar]
- de Melo Reis RA, Cabral-da-Silva M, de Mello FG, & Taylor JS (2008). Muller glia factors induce survival and neuritogenesis of peripheral and central neurons. Brain Res, 1205, 1–11. doi: 10.1016/j.brainres.2008.02.035 [DOI] [PubMed] [Google Scholar]
- Dvoriantchikova G, Degterev A, & Ivanov D (2014). Retinal ganglion cell (RGC) programmed necrosis contributes to ischemia-reperfusion-induced retinal damage. Exp Eye Res, 123, 1–7. doi: 10.1016/j.exer.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Mesa A, Vazquez-Padron RI, Kowalski JM, & Bhattacharya SK (2017). Sample Preparation and Analysis for Imaging Mass Spectrometry. Methods Mol Biol, 1609, 43–50. doi: 10.1007/978-1-4939-6996-8_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T, Pan Z, & Kashiwagi K (2012). Role of retinal glial cell glutamate transporters in retinal ganglion cell survival following stimulation of NMDA receptor. Curr Eye Res, 37(3), 170–178. doi: 10.3109/02713683.2011.645105 [DOI] [PubMed] [Google Scholar]
- Garcia AL, Udeh A, Kalahasty K, & Hackam AS (2018). A growing field: The regulation of axonal regeneration by Wnt signaling. Neural Regen Res, 13(1), 43–52. doi: 10.4103/1673-5374.224359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Forster V, Hicks D, & Vecino E (2002). Effects of muller glia on cell survival and neuritogenesis in adult porcine retina in vitro. Invest Ophthalmol Vis Sci, 43(12), 3735–3743. [PubMed] [Google Scholar]
- Hamel MG, Ajmo JM, Leonardo CC, Zuo F, Sandy JD, & Gottschall PE (2008). Multimodal signaling by the ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) promotes neurite extension. Exp Neurol, 210(2), 428–440. doi: 10.1016/j.expneurol.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty SV, Collins LM, Gavin AM, Roche SL, Wyatt SL, Sullivan AM, & O’Keeffe GW (2014). Canonical BMP-Smad signalling promotes neurite growth in rat midbrain dopaminergic neurons. Neuromolecular Med, 16(2), 473–489. doi: 10.1007/s12017-014-8299-5 [DOI] [PubMed] [Google Scholar]
- Hsia HE, Tushaus J, Brummer T, Zheng Y, Scilabra SD, & Lichtenthaler SF (2019). Functions of ‘A disintegrin and metalloproteases (ADAMs)’ in the mammalian nervous system. Cell Mol Life Sci, 76(16), 3055–3081. doi: 10.1007/s00018-019-03173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, & Lempicki RA (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc, 4(1), 44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Hutchins BI, Li L, & Kalil K (2011). Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev Neurobiol, 71(4), 269–283. doi: 10.1002/dneu.20846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Kim JH, Song GJ, Lee WH, Lee IK, Lee HW, … Suk K (2018). Functional dissection of astrocyte-secreted proteins: Implications in brain health and diseases. Prog Neurobiol, 162, 37–69. doi: 10.1016/j.pneurobio.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Iizuka Y, Tanaka Y, Araie M, Suzuki Y, & Tsukahara S (2004). Molecular and cellular reactions of retinal ganglion cells and retinal glial cells under centrifugal force loading. Invest Ophthalmol Vis Sci, 45(10), 3778–3786. doi: 10.1167/iovs.04-0277 [DOI] [PubMed] [Google Scholar]
- Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, … Cooper HM (2006). The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci, 26(21), 5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Seo IA, Suh DJ, & Park HT (2009). Nidogen plays a role in the regenerative axon growth of adult sensory neurons through Schwann cells. J Korean Med Sci, 24(4), 654–659. doi: 10.3346/jkms.2009.24.4.654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Gu X, & Yi S (2017). The Regulatory Effects of Transforming Growth Factor-beta on Nerve Regeneration. Cell Transplant, 26(3), 381–394. doi: 10.3727/096368916X693824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, & Wallace VA (2003). Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn, 227(3), 323–334. doi: 10.1002/dvdy.10315 [DOI] [PubMed] [Google Scholar]
- Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, … McCrea PD (2004). Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp Cell Res, 298(2), 369–387. doi: 10.1016/j.yexcr.2004.04.036 [DOI] [PubMed] [Google Scholar]
- Matteucci A, Gaddini L, Villa M, Varano M, Parravano M, Monteleone V, … Pricci F (2014). Neuroprotection by rat Muller glia against high glucose-induced neurodegeneration through a mechanism involving ERK1/2 activation. Exp Eye Res, 125, 20–29. doi: 10.1016/j.exer.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Mazzotta S, Neves C, Bonner RJ, Bernardo AS, Docherty K, & Hoppler S (2016). Distinctive Roles of Canonical and Noncanonical Wnt Signaling in Human Embryonic Cardiomyocyte Development. Stem Cell Reports, 7(4), 764–776. doi: 10.1016/j.stemcr.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Kanekiyo K, Nakagawa T, Asahi M, & Ide C (2016). NTAK/neuregulin-2 secreted by astrocytes promotes survival and neurite outgrowth of neurons via ErbB3. Neurosci Lett, 622, 88–94. doi: 10.1016/j.neulet.2016.04.050 [DOI] [PubMed] [Google Scholar]
- Namwanje M, & Brown CW (2016). Activins and Inhibins: Roles in Development, Physiology, and Disease. Cold Spring Harb Perspect Biol, 8(7). doi: 10.1101/cshperspect.a021881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, & Takahashi M (2007). Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci, 27(15), 4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik DT, Rai M, Ryzhov S, Sanders LN, Aisagbonhi O, Funke MJ, … Hatzopoulos AK (2015). Wnt10b Gain-of-Function Improves Cardiac Repair by Arteriole Formation and Attenuation of Fibrosis. Circ Res, 117(9), 804–816. doi: 10.1161/CIRCRESAHA.115.306886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Park KK, & Hackam AS (2017). Wnt signaling promotes axonal regeneration following optic nerve injury in the mouse. Neuroscience, 343, 372–383. doi: 10.1016/j.neuroscience.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Surapaneni K, Yi H, Nakamura RE, Karli SZ, Syeda S, … Hackam AS (2015). Activation of Wnt/beta-catenin signaling in Muller glia protects photoreceptors in a mouse model of inherited retinal degeneration. Neuropharmacology, 91, 1–12. doi: 10.1016/j.neuropharm.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, … Schwab ME (2013). Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis, 51, 202–213. doi: 10.1016/j.nbd.2012.11.011 [DOI] [PubMed] [Google Scholar]
- Pernet V, & Schwab ME (2014). Lost in the jungle: new hurdles for optic nerve axon regeneration. Trends Neurosci, 37(7), 381–387. doi: 10.1016/j.tins.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Pino D, Choe Y, & Pleasure SJ (2011). Wnt5a controls neurite development in olfactory bulb interneurons. ASN Neuro, 3(3), e00059. doi: 10.1042/AN20100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, & Eroglu C (2012). Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol, 31(3), 170–177. doi: 10.1016/j.matbio.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzafa N, Pereiro X, Lepper MF, Hauck SM, & Vecino E (2018). A Proteomics Approach to Identify Candidate Proteins Secreted by Muller Glia that Protect Ganglion Cells in the Retina. Proteomics, 18(11), e1700321. doi: 10.1002/pmic.201700321 [DOI] [PubMed] [Google Scholar]
- Schira J, Heinen A, Poschmann G, Ziegler B, Hartung HP, Stuhler K, & Kury P (2019). Secretome analysis of nerve repair mediating Schwann cells reveals Smad-dependent trophism. FASEB J, 33(4), 4703–4715. doi: 10.1096/fj.201801799R [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Horvat-Brocker A, & Faissner A (2008). The glia-derived extracellular matrix glycoprotein tenascin-C promotes embryonic and postnatal retina axon outgrowth via the alternatively spliced fibronectin type III domain TNfnD. Neuron Glia Biol, 4(4), 271–283. doi: 10.1017/S1740925X09990020 [DOI] [PubMed] [Google Scholar]
- Skytt DM, Toft-Kehler AK, Braendstrup CT, Cejvanovic S, Gurubaran IS, Bergersen LH, & Kolko M (2016). Glia-Neuron Interactions in the Retina Can Be Studied in Cocultures of Muller Cells and Retinal Ganglion Cells. Biomed Res Int, 2016, 1087647. doi: 10.1155/2016/1087647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa KM, Villaescusa JC, Cajanek L, Ondr JK, Castelo-Branco G, Hofstra W, … Arenas E (2010). Wnt2 regulates progenitor proliferation in the developing ventral midbrain. J Biol Chem, 285(10), 7246–7253. doi: 10.1074/jbc.M109.079822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Hu H, Lee Y, d’Azzo A, Messing A, & Brenner M (2004). Expression specificity of GFAP transgenes. Neurochem Res, 29(11), 2075–2093. [DOI] [PubMed] [Google Scholar]
- Teh MT, Blaydon D, Ghali LR, Briggs V, Edmunds S, Pantazi E, … Philpott MP(2007). Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. J Cell Sci, 120(Pt 2), 330–339. doi: 10.1242/jcs.03329 [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, & Yang Y (2003). Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol, 162(5), 899–908. doi: 10.1083/jcb.200303158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciecka A, & Bhattacharya SK (2019). Dataset of growth cone-enriched lipidome and proteome of embryonic to early postnatal mouse brain. Data Brief, 24, 103865. doi: 10.1016/j.dib.2019.103865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciecka A, Pattabiraman P, Piqueras MC, Toris C, & Bhattacharya SK (2018). Quantitative Proteomic Analysis of Human Aqueous Humor Using iTRAQ 4plex Labeling. Methods Mol Biol, 1695, 89–95. doi: 10.1007/978-1-4939-7407-8_9 [DOI] [PubMed] [Google Scholar]
- Udeh A, Dvoriantchikova G, Carmy T, Ivanov D, & Hackam AS (2019). Wnt signaling induces neurite outgrowth in mouse retinal ganglion cells. Exp Eye Res, 182, 39–43. doi: 10.1016/j.exer.2019.03.004 [DOI] [PubMed] [Google Scholar]
- UniProt Consortium T (2018). UniProt: the universal protein knowledgebase. Nucleic Acids Research, 46(5), 2699–2699. doi: 10.1093/nar/gky092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterlauft JD, Claudepierre T, Schmidt M, Muller K, Yafai Y, Wiedemann P, … Eichler W (2014). Enhanced survival of retinal ganglion cells is mediated by Muller glial cell-derived PEDF. Exp Eye Res, 127, 206–214. doi: 10.1016/j.exer.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, & Vetter ML (2004). Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci, 26(5–6), 352–358. doi: 10.1159/000082277 [DOI] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, & Sharma SC (2016). Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res, 51, 1–40. doi: 10.1016/j.preteyeres.2015.06.003 [DOI] [PubMed] [Google Scholar]
- von Toerne C, Menzler J, Ly A, Senninger N, Ueffing M, & Hauck SM (2014). Identification of a novel neurotrophic factor from primary retinal Muller cells using stable isotope labeling by amino acids in cell culture (SILAC). Mol Cell Proteomics, 13(9), 2371–2381. doi: 10.1074/mcp.M113.033613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Nakamura RE, Mohamed O, Dufort D, & Hackam AS (2007). Characterization of Wnt signaling during photoreceptor degeneration. Invest Ophthalmol Vis Sci, 48(12), 5733–5741. doi: 10.1167/iovs.07-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Gupta VK, Li JC, Klistorner A, & Graham SL (2013). Optic neuropathies: characteristic features and mechanisms of retinal ganglion cell loss. Rev Neurosci, 24(3), 301–321. doi: 10.1515/revneuro-2013-0003 [DOI] [PubMed] [Google Scholar]
- Yu K, Ge J, Summers JB, Li F, Liu X, Ma P, … Zhuang J (2008). TSP-1 secreted by bone marrow stromal cells contributes to retinal ganglion cell neurite outgrowth and survival. PLoS One, 3(6), e2470. doi: 10.1371/journal.pone.0002470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 3: S33A conditioned media protein profile (common proteins in at least 2 samples out of 3 samples)
Supplemental Table 4: β-eng conditioned media protein profile (common proteins in at least 2 samples out of 3 samples)
Supplemental Table 5: Untreated conditioned media protein profile (common proteins in at least 2 samples out of 3 samples)
Supplemental Table 6: Exclusive proteins to S33A and β-eng groups
Supplemental Table 7: Membrane secreted proteins involved in neurite growth synapse cell adhesion protein from S33A and β-eng


