Abstract
The COVID-19, coronavirus disease is an infectious disease caused by a novel virus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). By March 2020 the novel coronavirus known to cause a pandemic has infected nearly about 119 thousand people and killed more than 4,300 around 114 countries. Apart from the current controversial opinions about the origin, spreading, and sociological impact, it is much more imperative to put a halt to this current situation. Understanding, testing, and early to rapid diagnosis may be now the only key that can contain COVID-19 by “flattening the curve”. Biosensing is the platform that allows rapid, highly sensitive, and selective detection of analytes which in turn can serve the purpose for fast and precise detection of COVID-19. In this article, based on recently reported miniaturized sensing strategies, we hereby propose a promising personalized smartphone assisted electrochemical sensing platform for diagnosis of COVID-19.
Keywords: COVID-19, Biosensors, Smart phone, Personalized diagnosis, Coronavirus
1. The need for rapid diagnosis and point-of-care (POC) detection devices
The indications of COVID-19 disease are highly non-specific. Similar to the common flu, the preliminary symptoms of coronavirus disease are related to respiratory illness including cough, fever, difficulty in breathing [1]. Coronavirus disease can spread primarily through direct or indirect contact from an infected person. To date the disease has been reported to spread over 210 countries and territories, affecting over 11.3 million people (5 July, [2,3]). From the very beginning of the outbreak scientists all over the world are working extensively to understand the etiology of COVID-19. Though apart from the conventional molecular techniques [4,5], serological immunoassays [6,7] and chest CT imaging [8], there are no relevant methods of detection commercially available yet [9]. Moreover, these techniques have also testified with various faults and limitations in their respective applications [[10], [11], [12]]. The rapid lateral flow-based immunosensing assays detect the immunoglobulin M (IgM) and immunoglobulin G (IgG) produced in patients in response to SARS-CoV-2. However, the performance of this assays still demands critical evaluation for the clinical diagnosis of COVID-19. Thus, it is a crucial need indeed to develop a system that can sense and diagnose the SARS-CoV-2 infection as well by targeting it's specified-potent biomarkers in a label-free format. Electrochemical biosensors with a nano-engineered surface in recent years have become a critical area of research interest [[13], [14], [15]]. Scientists from all over the world are focusing on the exceptional atomic and molecular properties of engineered nanomaterials and their composites for better biological/diagnostic applications [[16], [17], [18], [19]]. Engineered nanomaterials integrated with functional nanoscale material can provide a new aspect towards the development of POC based modern immunosensors and other diagnostics platforms [20,21]). Recently, along with the development of nanostructured materials, a range of nanomaterials with diverse sizes and shapes have been utilized as the substrates for biorecognition element (BREs) immobilization [[22], [23], [24]]. It has been interpreted that the biomolecules immobilized on the nanostructured materials have various advantages over the bulk solid substrates. However, to proficiently immobilize biomolecules on nanostructured, material surfaces required labored work to modify/functionalize the substrate surface. Additionally, for some of the nanostructured materials, it is difficult to fully characterize their surfaces using conventional surface analytical tools. This eventually limits the detailed understanding of the immobilization mechanism. Hence, new nanostructured materials design and application towards BREs interfacing should be explored for various diseases including development of labelfree platforms or COVID-19 diagnosis.
2. Development of impedimetric immunosensor for diagnosing COVID-19
In the last few years, our laboratory has developed various POC-immunosensors based on electrochemically engineered nanocomposites modified sensor surface [[25], [26], [27]]. In a recent work, we have demonstrated a miniaturized label-free electrochemical impedance spectroscopy-based detection of biomarkers using metallic nanoparticles (NPs), electrochemically engineered nano-dendroids, and graphene oxide (GO) nanocomposites. These materials were sequentially deposited over the screen-printed carbon electrode (SPCE) and antibodies against the specific biomarker were immobilized using a bioconjugation process [27]. GO, a wonder material with exceptional electrical property can be used for the development of high performance COVID-19 biosensing system utilizing such analytical platform. Besides, the incredibly large specific surface area (two accessible sides), the abundant oxygen-containing surface functionalities, such as epoxide, hydroxyl, and carboxylic groups, and the high water solubility afford GO sheets a great promise for many more sensing applications [[28], [29], [30]]. It is possible to improvise these sensing systems by integrating the detection platform with newly discovered markers which are specific to COVID-19. Recently various molecular biology techniques have used to identify several genes thought to be specific for SARS-CoV-2 detection. In a recent study, an RNA-dependent RNA polymerase (RdRp)/helicase (H) genes of SARS-CoV-2, an important marker that does not show any cross-reactivity with other human coronaviruses or respiratory viruses has been utilized for diagnostic purpose [31]. Thus, if anti-RdRp helicase is successfully immobilized on our recently designed highly conducting surface [27] it may pave a new way to detect the infection inevitably, due to its robustness and high analytical performance (Fig. 1 ).
Fig. 1.
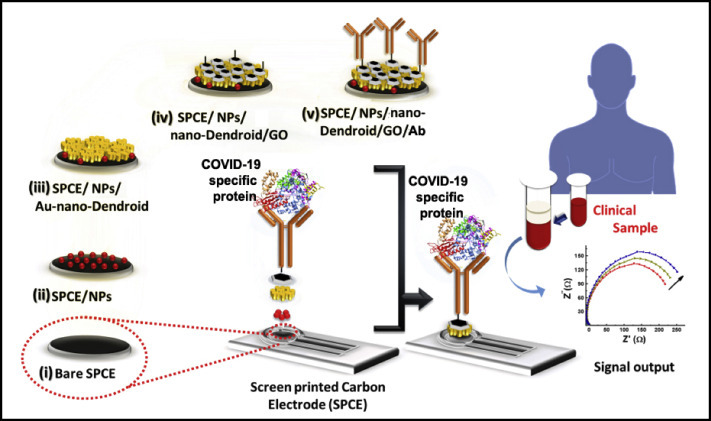
Conceptualised schematic representation of the SPCE/NPs/nano-Dendroids/GO/Ab probe fabrication for POC diagnosis of COVID-19 infection (Modified with permission from Ref. [27]).
In another study, our group has developed a nonenzymatic electrochemical nanoprobe for the rapid determination of chemical analyte present in the blood samples [32]. A gold sputtered nano-hierarchical 3D dendritic structure is used to attain high surface area, high conductivity, and different degrees of surface roughness possessing a greater catalytic potential. Multi-walled carbon nanotube (MWCNT) integrated dendrite modified electrode is another promising sensing surface with very high conductivity and electrocatalysis [33]. Conducting monomers and polymers can also be used by integrating with the dendritic system for the development of label-free sensors utilizing their high electron transfer capacity [34,35]. Surfaces with such high conductivity can be functionalized using suitable chemistry and additional nanomaterials to accommodate BREs and used for label-free sensing of specific protein targets of COVID-19. These cost-effective nanoprobes can be useful as a platform to capture and analyze the various reported biomarkers i.e. nucleocapsid protein (N), spike protein (S), envelope protein (E), and membrane protein (M) or the open reading frame 1b (ORF1b) of the viral RNA expressed in COVID-19 disease [36]. The advantage of both these sensors are in their robustness and label-free mode of operation, which is one of the most critical parameters when applied in clinical diagnostics in hospitals and/on in personalized disease monitoring. Another important feature of such sensors are data accusation in terms of electronic signals, that can be linked with the smartphone-based analytical systems [15,37].
3. Smartphone-assisted sensing platform
Recently, smartphone-based sensing systems have gained wider attention as it offers a semi-automated user interface which can be used by common people without extensive training or technical knowledge [18,26]. With a tailored hardware and sensing software inbuilt, sensing systems can be developed within a smartphone to miniaturize the system to be carried out to any locations and can be operated by any semitrained personnel. Smartphone-driven monitoring and diagnostic devices might provide a cost-effective alternative for expensive stand-alone technologies. It may be possible to develop a COVID-19 biosensor by integrating a complete disposable sensing module as discussed before to a smartphone-based application platform for personalized diagnosis (Fig. 2 ). Sensing surface can be optimized according to the marker molecules and can further be improvised for POC diagnosis in real clinical samples. Such a miniaturized system can provide a fast and affordable sensing not only to detect, but also to monitor the outbreak on a large scale.
Fig. 2.
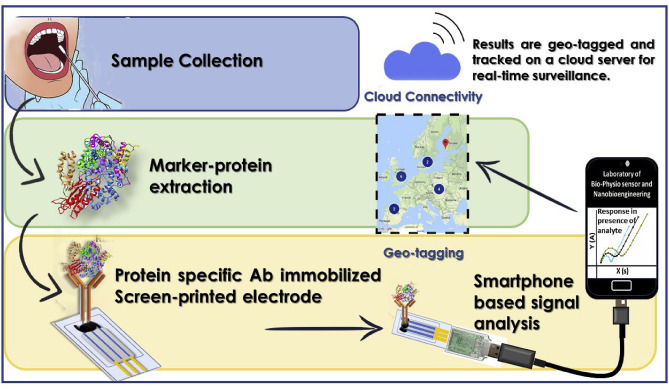
Proposed smartphone assisted signal analysis for COVID-19 detection and cloud-based real-time surveillance.
A smartphone-based “cloud” directory can also provide real-time surveillance by the means of geo-tagging. Geo-tagging constitutes the process of defining, creating, and provisioning a set of geolocation information to a computing device securely [38]. Thus, geolocation can enable identification of a cloud server's approximate location by adding contagion/outbreak information to the server's root of trust. Moreover, In the case of novel outbreaks or pandemics, location and time are indispensable for appropriate clinical administration, infection deterrence, and control. The information can be accessed using secure protocols to assert the integrity of the platform and confirm the location of the host [39]. Once this system is employed within a community, tracking of the outbreak will be enabled and updated in real-time to comprehend epidemiological studies through simplifying the restraining strategies.
4. Conclusion
As the race to develop a vaccine for COVID-19 is the primary concern now, it is essential to understand the epidemiological characteristics to stand against this outbreak. Moreover, it is suggested to identify the potent intermediate hosts those who can carry the disease, to prevent as well as to eradicate it from the near future. These steps require a compelling approach for the detection of the disease. Hence, POC based label-free sensing techniques integrated with smartphones can not only track the disease spreading around the globe, but also allow to form a library of data and facts required for future preparation to endure such pandemic. If alarms are raised early in infected areas, physicians can rapidly decide on the appropriate treatment for each new patient and public health organizations can manage the spread of the disease. It is anticipated that powerful integrative electrochemical label-free technologies can be escalated to develop a personalized analytical system to combat COVID-19 and other infectious diseases.
Declaration of competing interest
Author have no conflict of interest to declare.
Acknowledgments
Dr. Pranjal Chandra thanks Prof. Pramod Kumar Jain, Director IIT(BHU) for encouragement and providing the necessary facility for completion of this work.
References
- 1.WHO . WHO; 2020. Q&A on Coronaviruses (COVID-19) [Google Scholar]
- 2.Worldometer . Worldometer; 2020. Coronavirus Update (Live): 472,907 Cases and 21,315 Deaths from COVID-19 Virus Outbreak - Worldometer. [Google Scholar]
- 3.Shetti N.P., Srivastava R.K., Sharma S., Basu S., Aminabhavi T.M. Invasion of novel corona virus ( COVID-19 ) in Indian territory. Sensors Int. 2020;1:100012. doi: 10.1016/j.sintl.2020.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S., Basu S., Shetti N.P., Aminabhavi T.M. Current treatment protocol for COVID-19 in India. Sensors Int. 2020;1:100013. doi: 10.1016/j.sintl.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of A rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vashist S.K. 2020. In Vitro Diagnostic Assays for COVID-19 : Recent Advances and Emerging Trends. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai W., Zhang H., Yu J., Xu H., Chen H., Luo S., Zhang H., Liang L., Wu X., Lei Y., Lin F. CT imaging and differential diagnosis of COVID-19. Can. Assoc. Radiol. J. 2020 doi: 10.1177/0846537120913033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahapatra S., Chandra P. Clinically practiced and commercially viable nanobio engineered analytical methods for COVID-19 diagnosis. Biosens. Bioelectron. 2020 doi: 10.1016/j.bios.2020.112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L., Pan I., Shi L.-B., Wang D.-C., Mei J., Jiang X.-L., Zeng Q.-H., Egglin T.K., Hu P.-F., Agarwal S., Xie F., Li S., Healey T., Atalay M.K., Liao W.-H. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia X., Zhang P., Tian Y., Wang J., Zeng H., Wang J., Jiao L., Chen Z., Zhang L., He H., He K., Liu Y. Clinical significance of IgM and IgG test for diagnosis of highly suspected COVID-19 infection. medRxiv. 2020 doi: 10.1101/2020.02.28.20029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Yao L., Li J., Chen L., Song Y., Cai Z., Yang C. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahato K., Purohit B., Bhardwaj K., Jaiswal A., Chandra P. Novel electrochemical biosensor for serotonin detection based on gold nanorattles decorated reduced graphene oxide in biological fluids and in vitro model. Biosens. Bioelectron. 2019 doi: 10.1016/j.bios.2019.111502. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A., Purohit B., Mahato K., Roy S., Srivastava A., Chandra P. Design and development of ultrafast sinapic acid sensor based on electrochemically nanotuned gold nanoparticles and solvothermally reduced graphene oxide. Electroanalysis. 2020 doi: 10.1002/elan.201900406. [DOI] [Google Scholar]
- 15.Chandra P. 2016. Nanobiosensors for Personalized and Onsite Biomedical Diagnosis, Nanobiosensors for Personalized and Onsite Biomedical Diagnosis. [DOI] [Google Scholar]
- 16.Chandra P., Tan Y.N., Singh S.P. 2017. Next Generation Point-of-care Biomedical Sensors Technologies for Cancer Diagnosis. [DOI] [Google Scholar]
- 17.Kumar A., Sharma S., Pandey L.M., Chandra P. Nanoengineered material based biosensing electrodes for enzymatic biofuel cells applications. Mater. Sci. Energy Technol. 2018;1:38–48. doi: 10.1016/j.mset.2018.04.001. [DOI] [Google Scholar]
- 18.Purohit B., Kumar A., Mahato K., Chandra P. Smartphone-assisted personalized diagnostic devices and wearable sensors. Curr. Opin. Biomed. Eng. 2020 doi: 10.1016/j.cobme.2019.08.015. [DOI] [Google Scholar]
- 19.Prasad A., Mahato K., Chandra P., Srivastava A., Joshi S.N., Maurya P.K. Bioinspired composite materials: applications in diagnostics and therapeutics. J. Mol. Eng. Mater. 2016;4:1640004. [Google Scholar]
- 20.Kumar A., Purohit B., Mahato K., Chandra P. RSC Detection Science. 2019. Chapter 11: advance engineered nanomaterials in point-of-care immunosensing for biomedical diagnostics. [DOI] [Google Scholar]
- 21.Mahato K., Maurya P.K., Chandra P. Fundamentals and commercial aspects of nanobiosensors in point-of-care clinical diagnostics. Biotechnology. 2018;3 doi: 10.1007/s13205-018-1148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreescu S., Njagi J., IspasC . The New Frontiers of Organic and Composite Nanotechnology. 2008. Nanostructured materials for enzyme immobilization and biosensors. [DOI] [Google Scholar]
- 23.Meryam S R.A. Enzyme immobilization: an overview on nanoparticles as immobilization matrix. Biochem. Anal. Biochem. 2015 doi: 10.4172/2161-1009.1000178. [DOI] [Google Scholar]
- 24.Siqueira J.R., Caseli L., Crespilho F.N., Zucolotto V., Oliveira O.N. Immobilization of biomolecules on nanostructured films for biosensing. Biosens. Bioelectron. 2010 doi: 10.1016/j.bios.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Akhtar M.H., Hussain K.K., Gurudatt N.G., Chandra P., Shim Y.B. Ultrasensitive dual probe immunosensor for the monitoring of nicotine induced-brain derived neurotrophic factor released from cancer cells. Biosens. Bioelectron. 2018 doi: 10.1016/j.bios.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 26.Mahato K., Chandra P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image colorimetry integrated with smartphone. Biosens. Bioelectron. 2019 doi: 10.1016/j.bios.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Mahato K., Purohit B., Kumar A., Chandra P. Clinically comparable impedimetric immunosensor for serum alkaline phosphatase detection based on electrochemically engineered Au-nano-Dendroids and graphene oxide nanocomposite. Biosens. Bioelectron. 2020 doi: 10.1016/j.bios.2019.111815. [DOI] [PubMed] [Google Scholar]
- 28.Chandra P., Prakash R. 2020. Nanobiomaterial Engineering: Concepts and Their Applications in Biomedicine and Diagnostics. [DOI] [Google Scholar]
- 29.Lee J., Kim J., Kim S., Min D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016;105:275–287. doi: 10.1016/j.addr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu X., Cheng H., Zhang M., Zhao Y., Qu L., Shi G. Graphene-based smart materials. Nat. Rev. Mater. 2017;2:17046. doi: 10.1038/natrevmats.2017.46. [DOI] [Google Scholar]
- 31.Chan J.F.-W., Yip C.C.-Y., To K.K.-W., Tang T.H.-C., Wong S.C.-Y., Leung K.-H., Fung A.Y.-F., Ng A.C.-K., Zou Z., Tsoi H.-W., Choi G.K.-Y., Tam A.R., Cheng V.C.-C., Chan K.-H., Tsang O.T.-Y., Yuen K.-Y. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol JCM. 2020 doi: 10.1128/JCM.00310-20. 00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purohit B., Mahato K., Kumar A., Chandra P. Sputtering enhanced peroxidase like activity of a dendritic nanochip for amperometric determination of hydrogen peroxide in blood samples. Microchim Acta. 2019 doi: 10.1007/s00604-019-3773-2. [DOI] [PubMed] [Google Scholar]
- 33.Purohit B., Mahato K., Kumar A., Chandra P. Novel sensing assembly comprising engineered gold dendrites and MWCNT-AuNPs nanohybrid for acetaminophen detection in human urine. Electroanalysis. 2020;32:561–570. doi: 10.1002/elan.201900551. [DOI] [Google Scholar]
- 34.Naveen M.H., Gurudatt N.G., Noh H.B., Shim Y.-B. DealloyedAuNi dendrite anchored on a functionalized conducting polymer for improved catalytic oxygen reduction and hydrogen peroxide sensing in living cells. Adv. Funct. Mater. 2016;26:1590–1601. doi: 10.1002/adfm.201504506. [DOI] [Google Scholar]
- 35.Valera A.E., Nesbitt N.T., Archibald M.M., Naughton M.J., Chiles T.C. On-chip electrochemical detection of cholera using a polypyrrole-functionalized dendritic gold sensor. ACS Sens. 2019;4(3):654–659. doi: 10.1021/acssensors.8b01484. [DOI] [PubMed] [Google Scholar]
- 36.Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purohit B., Kumar A., Mahato K., Chandra P. Electrodeposition of metallic nanostructures for biosensing applications in health care. J. Sci. Res. 2020 doi: 10.37398/jsr.2020.640109. [DOI] [Google Scholar]
- 38.Yeluri R., Castro-Leon E., Yeluri R. Building the Infrastructure for Cloud Security. 2014. Boundary control in the cloud: geo-tagging and asset tagging. [DOI] [Google Scholar]
- 39.Web Reference 1 http://nist.gov\publications\drafts\ir7904\draft_nistir_7904.pdf


