Abstract
Infectious diseases, both established and emerging, impose a significant burden globally. Successful management of infectious diseases requires considerable effort and a multidisciplinary approach to tackle the complex web of interconnected biological, public health and economic systems. Through a wide range of problem-solving techniques and computational methods, operational research can strengthen health systems and support decision-making at all levels of disease control. From improved understanding of disease biology, intervention planning and implementation, assessing economic feasibility of new strategies, identifying opportunities for cost reductions in routine processes, and informing health policy, this paper highlights areas of opportunity for operational research to contribute to effective and efficient infectious disease management and improved health outcomes.
Keywords: OR in health services, Infectious disease, Decision support systems
1. Introduction
It was only forty years ago that improvements in health and hygiene led to infectious diseases being considered an insignificant threat to global health (Heesterbeek et al., 2015). The spread of HIV, tuberculosis and malaria as well as recent outbreaks of Ebola and the coronavirus disease (COVID-2019) are a few examples of the danger that infectious diseases still pose to human health and mortality. Though many infectious diseases are preventable and treatable, they continue to impose a significant health burden, especially in resource-constrained settings. In 2016, more than half of all deaths in low-income countries were caused by infectious diseases, conditions arising during pregnancy and childbirth, and nutritional deficiencies, compared to 7% in high-income countries (World Health Organization, 2018).
A global movement towards minimising the threat posed by infectious diseases requires considerable effort and a multidisciplinary approach to tackle the complex web of interconnected biological, public health and economic systems. Operational research (OR), as a discipline that uses analytical and soft methods to better understand complex systems, is well-equipped to unravel infectious disease dynamics and support decision-making in health policy. The purpose of this paper is to provide an overview of components of infectious disease management where OR tools have been and can be used to answer key public health questions. These components include disease biology, planning and implementing interventions, economic feasibility and cost minimisation, and policy formulation. Each of these components are discussed in the context of OR tools and methodologies that could be applied, with examples if available. This paper is not intended to be a comprehensive review of all OR applications in infectious disease management but rather a synopsis of areas of the complex system where OR can contribute to the global agenda against infectious diseases.
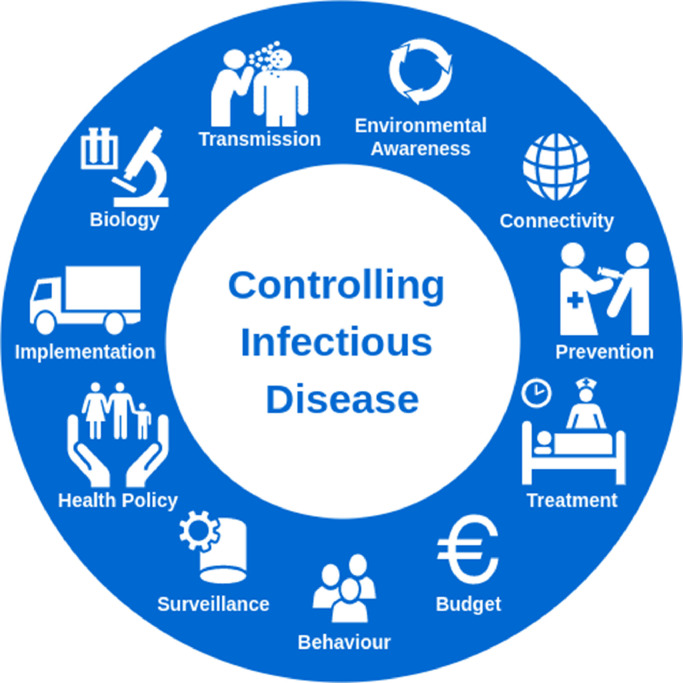
The path to controlling infectious diseases is challenging owing to the potential for the diseases to adapt and evolve, volatile environmental conditions, an unstable financial landscape and behavioural changes in the target populations. The conditions favouring transmission are varied and diverse to the extent that decisions cannot be made on the basis of a single condition (The malERA Consultative Group on Modelling, 2011). Fig. 1 describes components of the infectious disease system that need to be accounted for, to effectively intervene against disease. The biology of the disease needs to be well understood in terms of the contagiousness, transmissibility and environmental vulnerability. Malaria is one such example where the mode of transmission is through the Anopheles mosquito, repeated infections can lead to immunity against the disease, and transmissibility varies with changes in both rainfall and temperature (World Health Organization, 2020b). Connectivity between populations can lead to local outbreaks becoming public health emergencies of global concern; a case in point being COVID-19 (World Health Organization, 2020a). Intervening against disease through preventative and reactive measures needs to be shaped into health policy and is dependent on available financial and human resources. The distribution of financial resources is embedded within the infrastructure of the health system with country-specific procurement and spending policies. The impact of interventions is reliant on effective implementation and supportive patient behaviour. In grouping these components into disease biology, intervention planning and implementation, economic feasibility and cost minimisation, and health policy, while recognising areas of overlap, this paper will provide an overview of opportunities for OR methods to support decision-making at all levels of disease control.
Fig. 1.
Components of the infectious disease system.
2. Understanding disease biology
Operational research can be useful in understanding disease at cellular, whole-body and systems levels. Mechanistic modelling can be used to understand the biochemical principles of disease and epidemiological modelling can be used to understand disease dynamics in a population. The growing availability of data from cellular levels to health systems enables operational researchers to understand disease pathogenesis within an individual as well as disease transmission in a population, leading to the development of more effective prevention and treatment methods (Motta & Pappalardo, 2013).
For example, mechanistic models have been developed by operational researchers to respond to the increasing concern over drug resistant strains of malaria. These models aided in the discovery that the malaria parasite is dependent on the glycolosis pathway for survival in the human body and thus, several pathway inhibitors have been proposed as new drug targets for development (Van Niekerk, Penkler, Du Toit, & Snoep, 2016). The same approach has been extended to the whole-body level, where the effect of inhibiting the glycolosis pathway is being tested in malaria patients (Penkler, Du Toit, Adams, Rautenbach, Palm, Van Niekerk, et al., 2015, Slater, Okell, Ghani, 2017). Similar approaches are being used to understand other infectious disease pathogenesis, including HIV and TB, in order to increase the effectiveness of current treatments.
On a systems level using context-specific epidemiological data, models (characterised as e.g. dynamic, compartmental, mechanistic, stochastic, mathematical, probabilistic and epidemiological models) developed by operational researchers can be useful in forecasting the future incidence of a disease in a population. These models can also be adapted to discover how the spread of a disease might change with population or virus changes. Where epidemiological data on a disease is missing, models can be useful in estimating the number of infections in a population based on the clinical disease parameters that are known from the setting and globally, a practice that is helpful in quantifying the burden of disease. As an example of infectious disease forecasting, epidemiological models have been developed to predict the incidence of malaria with and without the implementation of different interventions (Silal, Little, Barnes, & White, 2014). This modelling approach allows for the impact of an intervention to be explored before it is invested in and rolled out in a population, a methodology that is especially relevant in resource-limited settings. Furthermore, modelling has allowed operational researchers to estimate the impact of migration on imported malaria cases, a variable which lacks hard data and is relevant to policy for malaria elimination (Silal, Little, Barnes, & White, 2015). Mechanistic modelling can also be used to explore the limits of transmissibility of infection. For example, the boundary conditions required to sustain endemic TB transmission at the population level were explored allowing for the identification of super-spreaders (Issarow, Mulder, & Wood, 2018).
3. Intervention planning and implementation
Operational research has played an important role in the design and delivery of interventions for infectious diseases. Considerations of effectiveness, safety, cost and equity are crucial in rolling out any intervention for disease management (Royston, 2011). In designing an effective intervention, there is a need to estimate both the direct and indirect effects, especially in a population that is heterogeneous in terms of individual susceptibilities and in variability of the pathogen in question. Epidemiological study designs are not well adapted to these complexities and computer simulation techniques are often better suited (Halloran et al., 2017). Consider an intervention to control HIV. Given the complexity of the disease and how behaviour influences disease propagation, evaluating the effect of any intervention is not straightforward. Operational research can be used to design and evaluate complex interventions (Liberatore & Nydick, 2008). For example, in an HIV intervention study to determine the most effective strategy of scaling up condom use, a simulation model of sexually transmitted disease was developed to clearly demonstrate that the most effective strategy would be to increase condom use only in the high risk groups irrespective of the sexual behavioral patterns in the population (van Vliet et al., 2001). This study would otherwise not be possible using cohort study designs. This is firstly because of ethical reasons and secondly because the study would not capture the indirect effect of the intervention due to the complexity of behavioural and/or sexual contact patterns.
Assessing the optimal intervention package amongst other competing interventions is often difficult using epidemiological studies. The most appropriate and effective package might involve a combination of interventions that could result in complex interactions (Halloran et al., 2017). Operational research has been used extensively, not only looking at epidemiologically-effective intervention packages, but also identifying the most cost-effective intervention combinations. Malaria transmission intervention models and non-linear production functions have been used to assess the relative cost-effectiveness of introducing RTS,S (the only available malaria vaccine currently under trial in a few countries in Africa) in the presence of existing interventions like indoor residual spraying, insecticide treated nets (ITNs), and seasonal malaria chemoprevention (Winskill, Walker, Griffin, & Ghani, 2017). This study showed that ITNs are the most cost-effective intervention in averting malaria among populations in sub-Saharan Africa and other interventions only become cost-effective after a very high coverage of ITNs is achieved. Decision tree models have been used to show the cost-effectiveness of sputum smear microscopy over serological tests in India (Dowdy, Steingart, & Pai, 2011).
Estimating the effects of interventions on disease incidence where host heterogeneity (such as variation in immunity levels and pathogen variation) is involved is a difficult task to accomplish. Considering heterogeneity in host and pathogens is particularly important in infectious disease control when the goal of the intervention is to reduce the cases to an almost negligible amount or eliminate the disease. Dynamic compartmental models have the potential of taking into account these variations when estimating effects of a control effort. An example is the utilisation of stochastic, non-linear, ordinary differential equations to predict the path to and cost of elimination of malaria in the 22 countries of the Asia-Pacific region (Silal et al., 2019).
Exploring the impact of intervention design is something that can usually only be completed in silico due to ethical and logistical challenges. Yet this problem is highlighted in outbreak situations where efficient study design is critical to decreasing the size of the epidemic. The 2014/2015 Ebola outbreak in Guinea and Sierra Leone is an example of simulation modelling being used to optimise trial design. Bellan et al. (2015) compared the impact of two vaccine trial designs aimed at health workers and Hitchings, Grais, and Lipsitch (2017) simulated the target sample size for vaccine roll-out accounting for the non-obvious effects on transmission dynamics.
With limited internal resources and external financing, optimisation techniques have also found their place in guiding the choice and implementation of interventions. To roll-out ARV-based microbicides in South Africa, optimisation techniques and geospatial modelling were used to compare two competing roll-out plans. Optimisation showed that ARV-based microbicides intervention in South Africa can be successful only when decisions regarding the geographical resource allocation and selection of intervention sites are made before the roll-out (Gerberry, Wagner, Garcia-Lerma, Heneine, & Blower, 2014).
Stakeholders require more than forecasts and cost breakdowns. They need to know that the implementation of interventions meet their expectations. Therefore the use of surveillance systems is critical for ensuring maximal impact of disease prevention and control programmes. With the advent of digital technology, society is more capable of real time surveillance than it has ever been. This enables transparency and accountability as well as the opportunity to swiftly refine models and correct mistakes. Operational research techniques offer rapid and cost-effective ways to answer questions relevant to the implementation of infectious disease interventions.
4. Economic feasibility and cost minimisation
Cost considerations are central to several facets of infectious disease management including planning and selecting interventions, informing policy decisions, and optimising the implementation of new interventions as well as routine processes. In the developing world where resources are often severely constrained, incorporating cost-focused analyses (for which there are a range of OR and economic evaluation methods) into an infectious disease management agenda is especially important. For new interventions, combining cost-effectiveness (or cost-benefit) analyses with dynamic compartmental mathematical disease models is valuable for assessing whether the proposed intervention is financially feasible or worthwhile in relation to its estimated impact. These epidemiological-economic models have been used across a range of infectious diseases and intervention types, e.g. to assess the impact and cost-effectiveness of introducing new vaccines (Drolet, Bénard, Jit, Hutubessy, Brisson, 2018, Fernandes, Rodrigues, Sartori, De Soárez, Novaes, 2019), to determine the optimal coverage of ITNs for malaria control (Drake, Devine, Yeung, Day, White, Lubell, 2016, White, Conteh, Cibulskis, Ghani, 2011), and to determine the resource requirements of different tuberculosis case-finding approaches (Sumner et al., 2019).
This type of OR modelling can also help inform national policy, budget and priority setting. An example is the use of a dynamic, multispecies mathematical and economic model to project the cost of malaria elimination by 2030 for the 22 countries in the Asia-Pacific region (Shretta, Silal, Celhay, Gran Mercado, Kyaw, Avancena, et al., 2020, Silal, Shretta, Celhay, Gran Mercado, Saralamba, Maude, et al., 2019). The Malaria Elimination Transmission and Costing for the Asia-Pacific (METCAP) study was commissioned in 2017 by the Asia-Pacific Malaria Leaders Alliance. Eighty scenarios were modelled to generate robust estimates of the optimal coverage and components of malaria elimination packages and the likely resources needed to implement them. The studies predicted that malaria elimination in the Asia-Pacific region would have a 6:1 return on investment.
While valuable for estimating the disease impact and cost-effectiveness of potential interventions, these advanced mathematical models can be challenging for non-experts to interpret and implement. The use of interactive web-based applications as decision support systems can improve the applicability and acceptability of modelling to inform real-world public health decision-making. As part of the METCAP study, a user-friendly, open-source application was developed that allowed policy-makers to explore different scenarios, calibrate the model to local data, run scenario analysis and conduct cost evaluations (Celhay et al., 2019).
In addition to planning and selecting new interventions, OR also provides useful methods for cost optimisation of existing interventions and routine processes. Methods such as cost minimisation and cost variance analysis can aid management decisions for reducing costs and maximising budget value. Performing aggregate demand forecasting and census prediction also helps determine the demand and supply side considerations of efficient resource allocation (Pierskalla & Brailer, 1994). This informs decisions on staffing, service provision and scheduling, support service planning, and medical equipment and drug procurement across all levels of the healthcare system. Operational research methods are particularly valuable for informing effective resource schedules, which can significantly reduce operational costs without reducing the quality or level of care provided (Burdett, Kozan, 2018, Jain, Shah, Sadh, Marfatia, Khandelwal, 2018). Developing models for effective resource allocation is important for appropriately responding to epidemics and outbreaks, where maximising the budget’s impact is crucial. Many OR studies have addressed the optimal allocation of resources for the control of infectious diseases, showing the value of this approach (Brandeau, 2006, Dasaklis, Pappis, Rachaniotis, 2012, Rachaniotis, Dasaklis, Pappis, 2012, Zaric, Brandeau, 2001, Zaric, Brandeau, 2002).
Optimising the supply chain to maximise drug distribution and availability, while minimising stock-outs and wastage is another opportunity for OR methods to introduce cost saving processes and checks (Iqbal, Geer, Dar, 2016, Vledder, Friedman, Sjöblom, Brown, Yadav, 2019). Cameron, Ewen, Ross-Degnan, Ball, and Laing (2009) estimated that the average availability of medicines in public sector health facilities ranged from 29.4% to 54.4% across 36 low- and middle-income countries. Therefore not only does effective procurement and supply chain management have the potential to reduce costs, it can also improve health outcomes.
Disease outbreaks place a considerable and often sudden burden on the healthcare system. Discrete event simulation and agent-based modelling can provide effective ways to encapsulate and account for the details of a system that is to be optimised. The allocation of hospital beds to patients is one area where such simulation has proved useful. This can be formulated as an assignment problem and solved as a binary integer program as in Schmidt, Geisler, and Spreckelsen (2013). Bloem (2015) presented a solution using agent-based methods to evaluate different allocation strategies. This presented hospitals with a set of rules to optimise bed allocation. Acknowledging that agent-based methods generally have larger computational power and data requirements than top down methods and have only become possible with improvements in technology, it is not suitable for all country settings.
5. Shaping health policy
The use of OR is becoming increasingly important in the development of public health policies at global, regional and national levels. The insights that OR methods offer are useful for priority setting, resource mobilisation, situational analyses, and capacity building (Bosu, 2014). These improve our understanding of diseases as well as the population-level impacts and cost-effectiveness of interventions.
Understanding the present and projected future epidemiology of a disease is an important part of demand forecasting that allows for the quantification of how many people in a population will require treatment over a selected period of time (Xiong et al., 2008). For example, epidemiological modelling has been largely successful in the efforts to scale-up access to HIV treatment. Demand forecasting research has helped to estimate the number of HIV patients that will need access to antiretrovirals (ARVs) – findings that have driven policy-makers to mobilise resources to ensure that the necessary quantity of ARVs exists in the global market, in national supply-chains and at the local point-of-care (Xiong et al., 2008). Additionally, OR has been useful in improving the efficiency of national supply-chains for delivery of treatment at the point-of-care (Xiong et al., 2008).
Operational research has also been useful in guiding capacity building in health systems. Forecasting of disease epidemiology helps to understand the treatment burden that will likely be placed on a health system and can help estimate the human resources and health service capacity needed to deliver care to patients (Xiong et al., 2008). These findings aid policy-makers in allocating resources to building the health care workforce.
In cases where more than one intervention exists for the prevention or control of a disease, OR has been useful in selecting the most appropriate intervention or combination of interventions for a specific setting to be included in national strategic plans. In the example of malaria, with the development of new prevention technologies, OR has been useful in modelling the projected effectiveness of these new interventions compared to the standard of care in order to ensure that resources are deployed in a cost-effective way given budget and equity considerations (Ramsay, Olliaro, & Reeder, 2016).
Priority-setting is rarely best solved quantitatively as optimisation of interventions is typically from a policy-makers perspective, yet successful implementation depends on both the provider and the patient. Soft OR methods such as Strategic Choice Approach, Cognitive Mapping and Multi-Criteria Decision Analysis (MCDA) offer solutions for structuring problems and incorporating human behaviour into decision-making. In Thailand a study was carried out using MCDA to rank 40 HIV/AIDS interventions based on the priority-setting criteria put forward by policy-makers, people living with the disease, and volunteers in the community (Youngkong, Teerawattananon, Tantivess, & Baltussen, 2012). Policy-makers gave high priority to the interventions that target high risk groups, volunteers gave high priority to interventions that targeted youth, while people living with HIV/AIDS ranked all interventions equally.
In recent years, OR is increasingly being required as part of public health resourcing for funding applications to external donor organisations. For example, the Global Fund to Fight Aids, Tuberculosis and Malaria recommends and often solicits epidemiological modelling, costing and optimisation to provide target countries with information about which interventions and service delivery models may have the greatest impact (World Health Organization & The Global Fund, 2008). The Global Fund refers to OR as the “science of better” as it helps to identify issues with programme quality, efficiency and effectiveness helping to maximise the impact of donor funds (World Health Organization & The Global Fund, 2008).
6. Discussion
This paper has described several examples of how OR can be used to support and improve the management of infectious diseases, particularly in the areas of understanding disease biology, planning and implementing interventions, assessing economic feasibility and opportunities for cost minimisation, and informing health policy. However, to maximise impact, it is important to bridge the gap between research and policy or practice. Bradley et al. (2017) discuss three key factors that can contribute to successful research translation. The first is engaging with stakeholders and decision-makers during model design and validation stages. Given the multidisciplinary nature of applying OR to infectious disease management, involving stakeholders from different levels of the health system, including clinicians, management professionals and patients enables a holistic approach that encourages ownership, prioritises policy relevant questions, identifies appropriate methodology, and integrates local knowledge. The second is using contextually representative data, which helps produce results that are relevant and implementable in the setting. The third is prioritising the communication of research findings to appropriate stakeholders. For this it is necessary to go beyond peer-review publication, and communicate findings directly to relevant audiences. User-friendly interfaces and visual simulation environments can support effective, non-technical communication of modelling processes and results.
While there are many opportunities for OR to improve the implementation of infectious disease programmes in low- and middle-income countries, several challenges exist. Limited data availability, weak surveillance and monitoring systems, and inadequate access to computing power can undermine the use of hard OR methods. To overcome this in settings with limited data availability, soft OR methods may be useful. One possible approach is the integration of goals and benchmarks identified in international guidelines, such as those provided by the World Health Organization, into mathematical models and simulations. Balawanth et al. (2019) provide a detailed assessment on KwaZulu-Natal’s (a province in South Africa) progress towards malaria elimination using these guidelines as a metric. Another challenge is the lack of an enabling research policy framework as national health research systems are weak in many developing countries. A recent review from 44 African countries reported that only 36% had a functional national health research governance mechanism and 20% had a functional national health research management forum (Mbondji et al., 2014). Developing supportive legislative environments as well as building capacity for OR and management approaches within the health system can encourage its routine use (Royston, 2011).
There is an ever-present hazard that novel human pathogens will emerge, posing a risk to global human health. In late 2019, COVID-19 emerged in Wuhan City in the Hubei Province of China. A month later, the WHO declared the outbreak to be a Public Health Emergency of International Concern (World Health Organization, 2020c). It is at times like these that the international community of scientists and researchers come together to study and act against the disease. Already operational researchers have performed dynamic disease modelling to estimate the spread of the epidemic through computing serial intervals, incubation periods and periods of infectivity (Lai, Bogoch, Ruktanonchai, Watts, Li, Yu, et al., Nishiura, Linton, & Akhmetzhanov, Shi, Cao, & Feng) with several researchers estimating the impact of imposed restrictions such as travel bans and social distancing, along with quarantining and active contact tracing (Chen, Yang, Yang, Wang, Bärnighausen, 2020, Gostic, Gomez, Mummah, Kucharski, & Lloyd-Smith, Hellewell, Abbott, Gimma, Bosse, Jarvis, Russell, et al., 2020, Li, Guan, Wu, Wang, Zhou, Tong, et al., 2020). Infectious diseases have become one of the world’s most pressing issues (Global Issues Network, 2020). Operational research as a field can make a multidisciplinary contribution to the management of infectious diseases by providing robust, evidence-based insights to collectively shape local and global public health policies.
Author contributions
SPS conceived the study and all authors contributed equally to the literature search and drafting of the manuscript. All authors read and approved the final manuscript.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejor.2020.07.037.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- Balawanth R., Ba I., Qwabe B., Gast L., Maharaj R., Raman J. Assessing Kwa-Zulu-Natal’s progress towards malaria elimination and its readiness for sub-national verification. Malaria Journal. 2019;18(1):108. doi: 10.1186/s12936-019-2739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellan S.E., Pulliam J.R.C., Pearson C.A.B., Champredon D., Fox S.J., Skrip L. Statistical power and validity of Ebola vaccine trials in Sierra Leone: A simulation study of trial design and analysis. The Lancet Infectious Diseases. 2015;15(6):703–710. doi: 10.1016/S1473-3099(15)70139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem C. Improving hospital bed utilisation through simulation and optimisation in South African Public Hospitals. 2015. Ph.D. Thesis, University of Pretoria, Faculty of Engineering. https://repository.up.ac.za/handle/2263/45144. [Google Scholar]
- Bosu W.K. Learning lessons from operational research in infectious diseases: Can the same model be used for noncommunicable diseases in developing countries? Advances in Medical Education and Practice. 2014;5:469–482. doi: 10.2147/AMEP.S47412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B.D., Jung T., Tandon-Verma A., Khoury B., Chan T.C.Y., Cheng Y.-L. Operations research in global health: a scoping review with a focus on the themes of health equity and impact. Health Research Policy and Systems. 2017;15(1):15–32. doi: 10.1186/s12961-017-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeau M.L. Operations research and health care. Kluwer Academic Publishers; 2006. Allocating resources to control infectious diseases; pp. 443–464. [DOI] [Google Scholar]
- Burdett R.L., Kozan E. An integrated approach for scheduling health care activities in a hospital. European Journal of Operational Research. 2018;264(2):756–773. doi: 10.1016/j.ejor.2017.06.051. [DOI] [Google Scholar]
- Cameron A., Ewen M., Ross-Degnan D., Ball D., Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: A secondary analysis. The Lancet. 2009;373(9659):240–249. doi: 10.1016/S0140-6736(08)61762-6. [DOI] [PubMed] [Google Scholar]
- Celhay O.J., Silal S.P., Maude R.J., Gran Mercado C.E., Shretta R., White L.J. An interactive application for malaria elimination transmission and costing in the Asia-Pacific. Wellcome Open Research. 2019;4:61. doi: 10.12688/wellcomeopenres.14770.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Yang J., Yang W., Wang C., Bärnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020:1–3. doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasaklis T.K., Pappis C.P., Rachaniotis N.P. Epidemics control and logistics operations: A review. International Journal of Production Economics. 2012;139(2):393–410. doi: 10.1016/j.ijpe.2012.05.023. [DOI] [Google Scholar]
- Dowdy D.W., Steingart K.R., Pai M. Serological testing versus other strategies for diagnosis of active tuberculosis in India: A cost-effectiveness analysis. PLoS Medicine. 2011;8(8):e1001074. doi: 10.1371/journal.pmed.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T.L., Devine A., Yeung S., Day N.P., White L.J., Lubell Y. Dynamic transmission economic evaluation of infectious disease interventions in low- and middle-income countries: A systematic literature review. Health Economics. 2016;25:124–163. doi: 10.1002/hec.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M., Bénard É., Jit M., Hutubessy R., Brisson M. Model comparisons of the effectiveness and cost-effectiveness of vaccination: A systematic review of the literature. Value in Health. 2018;21(10):1250–1258. doi: 10.1016/j.jval.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Fernandes E.G., Rodrigues C.C.M., Sartori A.M.C., De Soárez P.C., Novaes H.M.D. Economic evaluation of adolescents and adults’ pertussis vaccination: A systematic review of current strategies. Human Vaccines and Immunotherapeutics. 2019;15(1):14–27. doi: 10.1080/21645515.2018.1509646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerberry D.J., Wagner B.G., Garcia-Lerma J.G., Heneine W., Blower S. Using geospatial modelling to optimize the rollout of antiretroviral-based pre-exposure HIV interventions in Sub-Saharan Africa. Nature Communications. 2014;5(1):5454. doi: 10.1038/ncomms6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Issues Network (2020). Global Infectious Diseases. https://globalissuesnetwork.org/learn-about-our-global-issues/global-infectious-diseases/.
- Gostic, K., Gomez, A. C. R., Mummah, R. O., Kucharski, A. J., & Lloyd-Smith, J. O. (2020). Estimated effectiveness of traveller screening to prevent international spread of 2019 novel coronavirus (2019-nCoV). Not peer-reviewed.medRxiv. 10.1101/2020.01.28.20019224
- Halloran M.E., Auranen K., Baird S., Basta N.E., Bellan S.E., Brookmeyer R. Simulations for designing and interpreting intervention trials in infectious diseases. BMC Medicine. 2017;15(1):223. doi: 10.1186/s12916-017-0985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesterbeek H., Anderson R.M., Andreasen V., Bansal S., DeAngelis D., Dye C. Modeling infectious disease dynamics in the complex landscape of global health. Science. 2015;347(6227) doi: 10.1126/science.aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. The Lancet Global Health. 2020 doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchings M.D.T., Grais R.F., Lipsitch M. Using simulation to aid trial design: Ring-vaccination trials. PLOS Neglected Tropical Diseases. 2017;11(3) doi: 10.1371/journal.pntd.0005470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M.J., Geer M.I., Dar P.A. Medicines management in hospitals: A supply chain perspective. Systematic Reviews in Pharmacy. 2016;8(1):80–85. doi: 10.5530/srp.2017.1.14. [DOI] [Google Scholar]
- Issarow C.M., Mulder N., Wood R. Environmental and social factors impacting on epidemic and endemic tuberculosis: A modelling analysis. Royal Society Open Science. 2018;5(1) doi: 10.1098/rsos.170726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M., Shah M., Sadh N., Marfatia N., Khandelwal N. Applications of operations research techniques in healthcare. International Journal of Scientific & Engineering Research. 2018;9(1):708–713. [Google Scholar]
- Lai, S., Bogoch, I., Ruktanonchai, N., Watts, A., Li, Y., Yu, J., et al. (2020). Assessing spread risk of Wuhan novel coronavirus within and beyond China, January-April 2020: A travel network-based modelling study. Not peer reviewed. medRxiv. 10.1101/2020.02.04.20020479
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirusinfected pneumonia. New England Journal of Medicine. 2020 doi: 10.1056/nejmoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore M.J., Nydick R.L. The analytic hierarchy process in medical and health care decision making: A literature review. European Journal of Operational Research. 2008;189(1):194–207. doi: 10.1016/j.ejor.2007.05.001. [DOI] [Google Scholar]
- Mbondji P.E., Kebede D., Zielinski C., Kouvividila W., Sanou I., Lusamba-Dikassa P.-S. Overview of national health research systems in sub-Saharan Africa: Results of a questionnaire-based survey. Journal of the Royal Society of Medicine. 2014;107(1 Suppl):46–54. doi: 10.1177/0141076814530600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta S., Pappalardo F. Mathematical modeling of biological systems. Briefings in Bioinformatics. 2013;14(4):411–422. doi: 10.1093/bib/bbs061. [DOI] [PubMed] [Google Scholar]
- Nishiura, H., Linton, N. M., & Akhmetzhanov, A. R. (2020). Serial interval of novel coronavirus (2019-nCoV) infections. Not peer-reviewed.medRxiv. 10.1101/2020.02.03.20019497
- Penkler G., Du Toit F., Adams W., Rautenbach M., Palm D.C., Van Niekerk D.D. Construction and validation of a detailed kinetic model of glycolysis in Plasmodium falciparum. FEBS Journal. 2015;282(8):1481–1511. doi: 10.1111/febs.13237. [DOI] [PubMed] [Google Scholar]
- Pierskalla W.P., Brailer D.J. Applications of operations research in health care delivery. Handbooks in Operations Research and Management Science. 1994;6(C):469–505. doi: 10.1016/S0927-0507(05)80094-5. [DOI] [Google Scholar]
- Rachaniotis N.P., Dasaklis T.K., Pappis C.P. A deterministic resource scheduling model in epidemic control: A case study. European Journal of Operational Research. 2012;216(1):225–231. doi: 10.1016/j.ejor.2011.07.009. [DOI] [Google Scholar]
- Ramsay A., Olliaro P., Reeder J.C. The need for operational research and capacity-building in support of the Global Technical Strategy for Malaria 2016–2030. Malaria Journal. 2016;15(1):1–3. doi: 10.1186/s12936-016-1302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston G. Meeting global health challenges through operational research and management science. Bulletin of the World Health Organization. 2011;89(9):683–688. doi: 10.2471/BLT.11.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Geisler S., Spreckelsen C. Decision support for hospital bed management using adaptable individual length of stay estimations and shared resources. BMC Medical Informatics and Decision Making. 2013;13:3. doi: 10.1186/1472-6947-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, P., Cao, S., & Feng, P. (2020). SEIR Transmission dynamics model of 2019 nCoV coronavirus with considering the weak infectious ability and changes in latency duration. Not peer-reviewed.medRxiv. 10.1101/2020.02.16.20023655
- Shretta R., Silal S.P., Celhay O.J., Gran Mercado C.E., Kyaw S.S., Avancena A. Malaria elimination transmission and costing in the Asia-Pacific: Developing an investment case. Wellcome Open Research. 2020;4:60. doi: 10.12688/wellcomeopenres.14769.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silal S.P., Little F., Barnes K.I., White L.J. Towards malaria elimination in Mpumalanga, South Africa: A population-level mathematical modelling approach. Malaria Journal. 2014;13:297. doi: 10.1186/1475-2875-13-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silal S.P., Little F., Barnes K.I., White L.J. Hitting a moving target: A model for malaria elimination in the presence of population movement. PLoS One. 2015;10(12):e0144990. doi: 10.1371/journal.pone.0144990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silal S.P., Shretta R., Celhay O.J., Gran Mercado C.E., Saralamba S., Maude R.J. Malaria elimination transmission and costing in the Asia-Pacific: A multi-species dynamic transmission model. Wellcome Open Research. 2019;4:62. doi: 10.12688/wellcomeopenres.14771.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater H.C., Okell L.C., Ghani A.C. Mathematical modelling to guide drug development for malaria elimination. Trends in Parasitology. 2017;33(3):175–184. doi: 10.1016/j.pt.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner T., Bozzani F., Mudzengi D., Hippner P., Houben R.M., Cardenas V. Estimating the impact of tuberculosis case detection in constrained health systems: An example of case-finding in South Africa. American Journal of Epidemiology. 2019;188(6):1155–1164. doi: 10.1093/aje/kwz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The malERA Consultative Group on Modeling A research agenda for malaria eradication: Modeling. PLoS Medicine. 2011;8(1):e1000403. doi: 10.1371/journal.pmed.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Niekerk D.D., Penkler G.P., Du Toit F., Snoep J.L. Targeting glycolysis in the malaria parasite Plasmodium falciparum. FEBS Journal. 2016;283(4):634–646. doi: 10.1111/febs.13615. [DOI] [PubMed] [Google Scholar]
- Vledder M., Friedman J., Sjöblom M., Brown T., Yadav P. Improving Supply chain for essential drugs in low-income countries: Results from a large scale randomized experiment in Zambia. Health Systems and Reform. 2019;5(2):158–177. doi: 10.1080/23288604.2019.1596050. [DOI] [PubMed] [Google Scholar]
- van Vliet C., Meester E.I., Korenromp E.L., Singer B., Bakker R., Habbema J.D. Focusing strategies of condom use against HIV in different behavioural settings: an evaluation based on a simulation model. Bulletin of the World Health Organization. 2001;79(5):442–454. doi: 10.1590/S0042-96862001000500011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.T., Conteh L., Cibulskis R., Ghani A.C. Costs and cost-effectiveness of malaria control interventions: A systematic review. Malaria Journal. 2011;10:337. doi: 10.1186/1475-2875-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winskill P., Walker P.G.G., Griffin J.T., Ghani A.C. Modelling the cost-effectiveness of introducing the RTS,S malaria vaccine relative to scaling up other malaria interventions in sub-Saharan Africa. BMJ Global Health. 2017;2(1):e000090. doi: 10.1136/bmjgh-2016-000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018). The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- World Health Organization (2020a). Coronavirus (COVID-19) events as they happen. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- World Health Organization (2020b). Malaria. https://www.who.int/news-room/fact-sheets/detail/malaria.
- World Health Organization (2020c). WHO | Coronavirus disease (COVID-2019) R&D. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/.
- World Health Organization, The Global Fund . Technical Report. World Health Organization; Geneva: 2008. Guide to operational research in programmes supported by the Global Fund. [Google Scholar]
- Xiong W., Hupert N., Hollingsworth E.B., O’Brien M.E., Fast J., Rodriguez W.R. Can modeling of HIV treatment processes improve outcomes? Capitalizing on an operations research approach to the global pandemic. BMC Health Services Research. 2008;8(1):166. doi: 10.1186/1472-6963-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngkong S., Teerawattananon Y., Tantivess S., Baltussen R. Multi-criteria decision analysis for setting priorities on HIV/AIDS interventions in Thailand. Health Research Policy and Systems. 2012;10 doi: 10.1186/1478-4505-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaric G.S., Brandeau M.L. Resource allocation for epidemic control over short time horizons. Mathematical Biosciences. 2001;171(1):33–58. doi: 10.1016/S0025-5564(01)00050-5. [DOI] [PubMed] [Google Scholar]
- Zaric G.S., Brandeau M.L. Dynamic resource allocation for epidemic control in multiple populations. IMA Journal of Mathematics Applied in Medicine and Biology. 2002;19(4):235–255. doi: 10.1093/imammb19.4.235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/