Abstract
Objectives
Patients with rheumatologic diseases might be more susceptible to COVID-19 and carry a poorer prognosis. The aim of this study is to examine the incidence and outcomes of all COVID-19 patients with rheumatologic conditions in Hong Kong.
Methods
This is a population-based retrospective study. All patients tested positive for SARS-CoV-2 by PCR with a previous diagnosis of rheumatologic diseases were reviewed. The incidence of COVID-19 in patients with rheumatologic conditions was calculated and compared to the general population in Hong Kong. Descriptive data of those rheumatologic patients with COVID-19 and the clinical course of the index infection were presented.
Results
Up till 27 May 2020, there were 1067 cases of COVID-19 diagnosed in Hong Kong which had a population of 7.5 million. Out of the 39,835 patients with underlying rheumatologic diseases, we identified 5 PCR confirmed COVID-19 cases. The estimated incidence of COVID-19 was 0.0126% patients with rheumatologic diseases, compared to 0.0142% in the general population. All 5 patients had inflammatory arthropathies. One patient was on hydroxychloroquine and sulphasalazine, and one was on methotrexate. None of the 3534 patients on b/tsDMARDs was infected. Four patients had leucopenia/lymphopenia and stool viral PCR was positive in 3 patients. All patients made uneventful recovery without complications or flare of underlying diseases.
Conclusions
We found no alarming signals of increased frequency or severity of COVID-19 in patients with rheumatologic diseases, although extrapolation of the results to other populations with different infection control strategies should be made with caution.
Keywords: COVID-19, SARS-CoV-2, Incidence, Outcome
Introduction
On 12th March 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a pandemic [1]. Patients with rheumatologic diseases are at increased risk of infection due to the underlying systemic inflammation and the use of immunosuppressive therapies [2]. They might be more likely to contract the virus. The increased prevalence of comorbidities, such as cardiovascular and pulmonary diseases, in rheumatologic patients have also been reported to be the poor prognostic factors of COVID-19 [3,4]. In contrast, rheumatologic patients might be unexpectedly protected, by virtue of the medications that they are using. Much attention has been paid to the controversial anti-SARS-CoV-2 effect of hydroxychloroquine [5]. The other immune-modulators used in our patients may counteract the overwhelming pro-inflammatory cytokines release in the cytokine storm associated with COVID-19 [6]. On the other hand, rheumatologic diseases might be affected by the autoimmunity triggers due to the SARS-CoV-2 [7].
At the present time, there are mainly self-reported survey or registry data. Large scale population-based studies are lacking. It remains uncertain how rheumatologic diseases or medications will affect the susceptibility and disease course of COVID-19, as well as how SARS-CoV-2 impacts patients with rheumatologic conditions. We aimed to study the incidence, baseline characteristics, disease course and outcomes of COVID-19 patients with rheumatologic diseases in Hong Kong.
Patients and methods
Study design and patients
This is a territory-wide multi-centered retrospective observational study. An extensive surveillance scheme was implemented in Hong Kong early in the outbreak. All patients presented to the hospitals with fever, respiratory symptoms or chest x-ray pneumonic changes were tested for SARS-CoV-2 by polymerase chain reaction (PCR) via nasapharyngeal aspiration (NPA). Patients with fever or respiratory symptoms seen in the clinics were also encouraged to undergo the test. Asymptomatic inbound travelers from high risk areas were also tested. All patients tested positive were admitted to the hospitals for further management. The treatments the patients received were at the discretion of the treating physicians.
Data source
Patient identification was done using the Clinical Data Analysis and Reporting System (CDARS), which was constructed for recordkeeping and research purposes in Hong Kong [8]. It recorded and centralized information on patient demographics, laboratory results, diagnoses, and dates of hospital admissions and discharges. Data validation in CDARS demonstrated a high coding accuracy and the system has been extensively used in large-scale epidemiological studies [9], [10], [11], [12]. Patients with a positive SARS-CoV-2 PCR test and any rheumatologic diagnosis were identified from the CDARS. The investigators of the study would then review the records of each patient to verify the diagnoses and collect the clinical data. The total numbers of living patients with International Classification of Diseases Ninth Revision (ICD-9) diagnostic codes for rheumatoid arthritis (RA), systemic lupus erythematosus, ankylosing spondylitis (AS), psoriatic arthropathy (PsA), scleroderma, dermatomyositis, polymyositis, systemic vasculitis, adult onset Still's disease and mixed connective tissue disease who have been registered in the CDARS were retrieved. The data search and analysis was up to 26th May 2020.
Statistical analysis
Due to the small number of cases, only descriptive data were presented. The incidence of rheumatologic patients with COVID-19 in the Hong Kong population was calculated. The incidence of the COVID-19 infection in rheumatologic patients was deduced using a crude estimation of the number of patients with rheumatologic conditions in Hong Kong. It was numerically compared with the overall Hong Kong incidence of COVID-19 infection.
Ethical approval
The study was approved by the Institutional Review Board of the University of Hong Kong and the Hospital Authority Hong Kong West Cluster (UW20–389), Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (NTEC-2020–0248) and Kowloon Central Cluster REC / Kowloon East Cluster REC (20–0121/ER-1). This study was conducted according to the principles of the Declaration of Helsinki.
Results
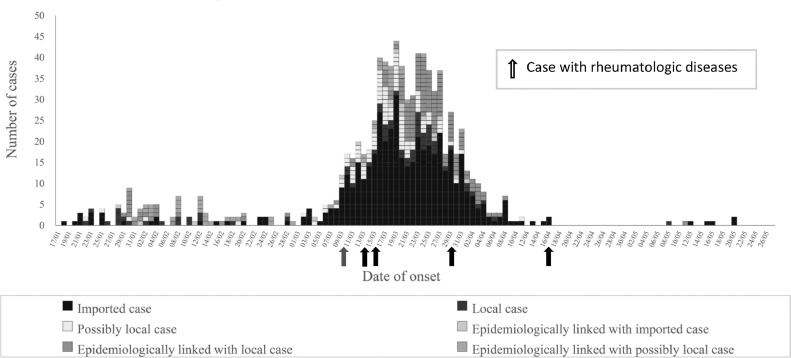
Since 23rd January when the first case was confirmed to the time of writing on 27th May, there were 1067 cases of COVID-19 diagnosed in Hong Kong. There was a downward trend of new cases reported as shown in Fig. 1 . So far there were 4 deaths and one patient who was still in critical condition. We identified 5 COVID-19 patients with underlying rheumatologic diseases during this period, making the incidence rate of rheumatologic patients with COVID-19 in the Hong Kong, which has a population of 7.5 million as of end of 2019, 0.67 cases per one million inhabitants. There were 39,835 patients with rheumatologic diseases in Hong Kong from CDARS search. The estimated incidence of COVID-19 was 0.0126% in patients with rheumatologic diseases, compared to 0.0142% of the general Hong Kong population. None of the 3534 patients (at the time of writing) in the Hong Kong Biologics Registry who were on biologic or targeted synthetic disease modifying anti-rheumatic drugs (b/tsDMARDs) was infected. Of note, 4 out of the 5 confirmed cases were believed to be imported.
Fig. 1.
1067 confirmed cases of COVID-19 in Hong Kong (as of 27th May 2020).
The baseline characteristics and clinical features of the 5 patients were presented in Table 1 . All of the patients had arthritides (3 AS, 1 RA and 1 PsA). No patient with other connective tissue diseases was diagnosed to have COVID-19. Of the 5 infected patients, one was on hydroxychloroquine/sulphasalazine combination and another patient was on methotrexate. Only methotrexate was stopped due to the infection after hospitalization. All of them presented with respiratory symptoms, and two had diarrhea and vomiting. Besides NPA, 2 patients with diarrhea and 1 without were tested positive for stool viral PCR. Four out of 5 patients had leucopenia (<5,000/mm3) and lymphopenia (<1,500/mm3). They mostly had mild COVID-19 disease without developing any significant complication. Only one patient required supplementary oxygen therapy temporarily. Remdesivir was given in 2 patients including the one requiring oxygen therapy. Lopinavir/ritonavir in combination with interferon beta were given in another patient. Their symptoms resolved in 7 to 14 days and viral clearance (-ve PCR for 2 consecutive times) was documented in 19 to 31 days. There was no obvious flare of the underlying rheumatologic diseases before discharge from the hospital which was no later than the date of viral clearance.
Table. 1.
The baseline characteristics and clinical features of the 5 COVID-19 patients with rheumatologic diseases.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age | 59 | 23 | 66 | 61 | 25 |
| Gender | Male | Female | Male | Female | Male |
| Race | Chinese | Chinese | Chinese | Chinese | White |
| Smoking | Ex-smoker | Smoker | No | No | Unknown |
| Comorbidities | None | None | DM, HT | None | None |
| Rheumatologic diagnosis | Ankylosing spondylitis | Ankylosing spondylitis | Psoriatic arthropathy | Rheumatoid arthritis | Ankylosing spondylitis |
| Disease activity | Moderate | Low | Low | Low | Remission |
| Treatment received (stopped/continued after COVID-19) | None | None | MTX (stopped) | HCQ (continued), SSZ (Continued) | None |
| Other medications | None | None | NSAID, PD5i | COX2i | None |
| COVID-19 features | |||||
| Diagnosis date | 13/3/2020 | 18/3/2020 | 23/3/2020 | 5/4/2020 | 20/4/2020 |
| Diagnosis location | Government outpatient | Private outpatient | Inpatient | Quarantine services | Government outpatient |
| Diagnosis method (sites) | PCR (NPA/NPS, deep throat sputum, stool) | PCR (NPA/NPS, deep throat sputum, stool) | PCR (NPA/NPS, deep throat sputum, stool) | PCR (NPA/NPS, deep throat sputum) | PCR (NPA/NPS, deep throat sputum) |
| Epidemiological link | Confirmed cases in same building | From United Kingdom | From Macau | From Nigeria | From United Kingdom |
| Symptoms | Fever, sore throat, cough, diarrhea/ vomiting | Sore throat, cough, myalgia | Fever, cough, SOB, diarrhea/vomiting | Rhinorrhea, anosmia | Anosmia |
| Treatment for COVID-19 | Remdesivir | None except supportive | Remdesivir | Kaletra, interferon beta | None except supportive |
| Maximal level of care | No need oxygen | No need oxygen | Supplemental oxygen (3 L nasal cannula) | No need oxygen | No need oxygen |
| Complications | None | None | None | None | None |
| Co-infection | No | No | No | No | No |
| Anemia < 9.2g/dL | No | No | No | No | No |
| Leukopenia < 5,000/mm3 | Yes | Yes | Yes | Yes | No |
| Lymphopenia <1,500/mm3 | Yes | Yes | Yes | Yes | No |
| Thrombocytopenia <110,000/mm3 | No | No | No | No | No |
| D-dimer > ULN | N/A | N/A | Yes | Yes | No |
| AST/ALT > ULN | No | No | No | Yes | No |
| Deceased | No | No | No | No | No |
| Duration till symptoms resolution | 14 days | 7 days | 10 days | 7 days | 12 days |
| Duration from symptom onset to viral clearance (-ve PCR x 2) | 31 days | 26 days | 19 days | 27 days | 28 days |
DM: diabetes mellitus, HT: hypertension, MTX: methotrexate, HCQ: hydroxychloroquine, SSZ (sulphasalazine), NSAID: non-steroidal anti-inflammatory drugs, PD5i: phosphodiesterase-5 inhibitor, COX2i: cyclooxygenase-2 inhibitor, PCR: polymerase chain reaction, NPA: nasopharyngeal aspirate, NPS: nasopharyngeal swab, SOB: shortness of breath, ULN: upper limit of normal.
Discussions
In this territory-wide population-based study, we found an apparently low incidence of laboratory proven COVID-19 in patients with rheumatologic disease in Hong Kong, which was similar to that of the general public. Monti and colleagues provided an early account of patients with arthritis in Lombardy, Italy, one of the most heavily hit regions [13]. Out of the 320 surveyed patients, 4 (1.25%) were diagnosed of COVID-19 infection. In a study conducted in Milan, out of 530 patients on b/tsDMARDs, 3 (0.57%) were diagnosed to have COVID-19 by PCR [14]. In another Italian cohort from a different region, only 2 (0.21%) of the 916 patients with rheumatologic diseases were virologically diagnosed to have COVID-19 and the incidence was not significantly different from the general population [15]. A similar finding of comparable COVID-19 incidence rate between rheumatologic patients and the general population (0.48% and 0.58% respectively) was again reported in a Spanish study [16]. However, all of the above studies were conducted in the form of patient self-reported survey which might affect the validity of the results.
Whether rheumatologic disease is a poor prognostic factor for COVID-19 is debatable. In our study, the disease courses of the 5 patients with COVID-19 were largely uneventful. A similarly reassuring message was conveyed from the above mentioned cohorts. On the other hand, early data from the COVID-19 Global Rheumatology Alliance, the international initiative to collect data of rheumatologic patients with COVID-19, revealed an overall death rate of 5% in 110 cases [17]. In a French report of 17 systemic lupus erythematosus patients with COVID-19, 5 patients required invasive mechanical ventilation and 2 patients died [18]. In the latest report from the Global Rheumatology Alliance, corticosteroids use was found to be the poor prognostic factor, and patients on anti-TNFs had a decreased odds of hospitalization [19]. However, as these are survey or registry data, they are subject to selection or reporting bias.
Leucopenia and gastro-intestinal (GI) symptoms were common in our patients and might be a unique finding. A systemic review showed a high frequency of lymphopenia but not leucopenia in patients with COVID-19 [20]. Lymphocytes, the major antiviral cells, were found to be prone to decrease continually in patients with severe COVID-19 [21]. At the same time, as the illness progressed, neutrophilia emerged and was an indicator of poor prognosis [22]. This might explain the relatively low total leucocyte counts in our patients with benign disease courses. On the other hand, another review suggested that 12% of patients with COVID-19 would manifest GI symptoms, although viral shedding was observed in 40.5% of patients [23]. The diagnostic, prognostic and infection control implications of these findings need to be further studied.
Anti-endemic measures, including border restrictions, quarantine, and social distancing were implemented early to curb the spread of the infection in Hong Kong [24]. All these, but without complete society lock-down, together with a 96.6% compliance of mask usage of the general public were believed to the reasons for the low overall incidence of COVID-19 in Hong Kong [25]. The potentially even more stringent behavioral measures adopted by our rheumatologic patients due to the awareness of an increased risk, may lead to underestimation of the true incidence. The different testing strategies applied could have equally affected the rate of COVID-19 diagnosis. The number of COVID-19 PCR tests performed in Hong Kong was 27,057 per million population, which was comparable to most of the developed countries [26,27]. It is also possible that the COVID-19 infections presented to us are only the tip of the iceberg, as the many of cases are asymptomatic or minimally symptomatic. We extended the testing to asymptomatic inbound travelers. No asymptomatic COVID-19 patient with rheumatologic diseases was found. Unfortunately, wide-spread serological testing was not done which might provide a better picture of the epidemiology of the disease. Another major limitation of our study is the small sample size of the COVID-19 patients which hampered the inference of our results.
To conclude, our findings could provide further reassurance about the incidence and possibly outcome of COVID-19 in patients with rheumatologic diseases. Nevertheless, these should be interpreted in the context of all the mitigation measures implemented in Hong Kong, and may not be applicable to other parts of the world adopting different strategies. The results also support avoidance of interrupting immunosuppressive therapies, at least before COVID-19 is diagnosed. The implications of the apparent high frequency of leucopenia and positive stool viral PCR require further investigations.
Contributors
All authors have substantially contributed to all aspects of this study. HS, JWM, GL and LT: conceptualization, formal analysis and writing - original draft, review & editing; JS, FL, JL, SC, CH, JMC, SK and WN: data curation and writing - review & editing.g All authors critically revised and approved the final version of the manuscript.
Data availability statement
Data can be shared upon request.
Patient consent for publication
Not required.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of Competing Interests
None declared.
Acknowledgments
None
References
- 1.World Health Organization. Coronavirus disease (COVID-19) outbreak. [Cited2020May 26]. Available from: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/.
- 2.Doran M.F., Crowson C.S., Pond G.R., O'Fallon W.M., Gabriel S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 3.Kuo C.F., Chou I.J., Rees F. Temporal relationships between systemic lupus erythematosus and comorbidities. Rheumatology. 2019;58:840–848. doi: 10.1093/rheumatology/key335. [DOI] [PubMed] [Google Scholar]
- 4.Chen D., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taccone F.S., Gorham J., Vincent J.-.L. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunnol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamriz O., Shoenfeld Y. Infections: a double-edge sword in autoimmunity. Curr Opin Rheumatol. 2018;30:365–372. doi: 10.1097/BOR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 8.Hospital Authority. Introduction: caring for our community's health. [cited2020May 26]. Available from: https://www.ha.org.hk/visitor/ha_visitor_index.asp?Content_ID=10008&Lang=ENG&Dimension=100&Parent_ID=10004/.
- 9.Sing C.W., Woo Y.C., Lee A.C.H., Lam J.K.Y., Chu J.K.P., Wong I.C.K. Validity of major osteoporotic fracture diagnosis codes in the clinical data analysis and reporting system in Hong Kong. Pharmacoepidemiol Drug Saf. 2017;26:973–976. doi: 10.1002/pds.4208. [DOI] [PubMed] [Google Scholar]
- 10.Chiu S.S., Lau Y.L., Chan K.H., Peiris J.S.M. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 11.Lau W.C., Chan E.W., Cheung C.L., Sing C.W., Man K.K.C., Lip G.Y.H. Association between dabigatranvs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. 2017;317:1151–1158. doi: 10.1001/jama.2017.1363. [DOI] [PubMed] [Google Scholar]
- 12.Yip T.C., Chan L.H., Wong W.V., Tse Y., Lam K.L., Wong G.L. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol. 2017;67:902–908. doi: 10.1016/j.jhep.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Monti S., Balduzzi S., Delvino P., Bellis E., Quadrellia V.S., Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79:667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favalli E.G., Ingegnoli F., Cimaz R., Caporali R. What is the true incidence of COVID-19 in patients with rheumatic diseases? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217615. [DOI] [PubMed] [Google Scholar]
- 15.Zen M., Fuzzi E., Astorri D., Saccon F., Padoan R., Ienna L. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in Northeast Italy: a cross-sectional study on 916 patients. J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102502. Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michelena X., Borrell H., Lopez-Corbeto M., Lopez-Lasanta M., Moreno E., Pascual-Pastor M. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2020;50:564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianfrancesco M.A., Hyrich K.L., Gossec L., Strangfeld A., Carmona L., Mateus E.F. Rheumatic disease and COVID-19: initial data from the COVID-19 global rheumatology alliance provider registries. Lancet Rheumatol. 2020 Apr 16 doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathian A., Mahevas M., Rohmer J., Roumier M., Cohen-Aubart F., Amador-Borrero B. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217566. [DOI] [PubMed] [Google Scholar]
- 19.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M., Gossec L. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Zhang R., Ge G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;3:1–8. doi: 10.1007/s00277-020-04103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D.W., Hu B., Hu C., Zhu F.F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 23.Parasa S., Desai M., Chandrasekar V.T., Patel H.K., Kennedy K.F., Roesch T. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowling B.J., Ali S.T., Ng T.W.Y., Tsang T.K., Li J.C.M., Fong M.W. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020 doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng V.C.C., Wong S.C., Chuang V.W.M., So S.Y., Chen J.H., Sridhar S. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centre for Health Protection, The government of the Hong Kong special administrative region. Statistics on testing for COVID-19 in Hong Kong. [Cited2020May 26] Available from: https://www.chp.gov.hk/files/pdf/statistics_on_covid_19_testing.pdf.
- 27.Statista. Rate of COVID-19 testing in most impacted countries worldwide as of May 29, 2020. [Cited 2020 May 26]. Available from: https://www.statista.com/statistics/1104645/covid19-testing-rate-select-countries-worldwide/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be shared upon request.