Abstract
It has been shown that bamboo leaf flavone (BLF) displays biological and pharmacological activities in mammals. However, the effects of BLF on broiler gut microbiota and related immune function have not been investigated. The aim of this study was to test our hypothesis that BLF can improve the health status of broilers by modulating the gut microbiota. A total of 300 one-day-old Arbor Acres (AA) broilers were used to characterize their gut microbiota and immune status after feeding diet supplemented with BLF. The V4 hypervariable region of the 16S rRNA gene from cecal bacteria was sequenced via the Illumina MiSeq platform. The Immune status and related parameters were assessed, including the immune organ index (the spleen, thymus, and bursa), serum concentrations of IL-2 and INF-γ, and spleen IL-2 and INF-γ gene expressions. The results showed the BLF diet had an Immune enhancement effect on broilers. In addition, BFL caused the changes of the gut microbial community structure, resulting in greater proportions of bacterial taxa belonging to Lactobacillus, Clostridiales, Ruminococcus, and Lachnospiraceae. These bacteria have been used as probiotics for producing short chain fatty acids in hosts. These results indicate that BLF supplement improves immune function in chicken via modulation of the gut microbiota.
Subject terms: Pharmacology, Immunology, Microbiology
Introduction
Flavonoids are polyphenolic compounds that ubiquitously present in plants. Flavonoid-rich foods and flavonoid supplements have been found to modulate immune function by inducing cytoprotective effects1, restoring lymphocyte proliferation2, and preventing cells apoptosis in mammals3. The flavonoid extracted from bamboo leaves (so-called bamboo leaf flavone, BLF), mainly including orientin, homoorientin, vitexin, and isovitexin4, have been confirmed to have multiple biological activities, such as scavenging oxygen radicals5, enhancing immunity6, and possessing anticancer7, antibacterial8, antiviral9, and antioxidant functions10. So, it has been widely used as drug substances, anti-aging products, cosmetics, and feed stuffs in human. However, its uses and roles in livestock production are limited11. The ban and restrictions on the use of antibiotics in livestock production have directed researches to investigate natural or organic alternatives to antibiotics in plants (e.g. BLF) with the potential roles to promote growth and health as well as production performance such as meat, milk, egg production11.
The microbiota communities in the gut play significant roles in promoting health status and productivity in chickens12 by modulating their immune system, inhibiting pathogenic colonization, and promoting detoxification and digestion13. Recent studies have reported that gut microbiota involve in transforming flavonoid into phenolic acids, which can be easily absorbed in mice14. Huang et al.15 reported that flavonoids extracted from plants including quercetin, catechin, and puerarin had impact on the relative viability of the gut microbiota, which implies that dietary flavonoids supplements play an important role in reshaping the gut microbial community, providing beneficial effects such as Immunoenhancement in hosts. To our knowledge, few reports on the use of BLF to regulate the gut microbiota and immune function in animals have been investigated.
Therefore, the aim of this study was to investigate effect of BLF on broiler cecal microbiota and related immunity index during various developing stages that are day 14 (starter stage), 28 (grower stage), and 42 (finisher stage). The results indicated that BFL is applicable as a natural feed additive for chickens to boost their health and production.
Results
Immune organs index, serum concentrations of IL-2 and INF-γ and their gene expression abundances in spleen
The positive effects of BFL on broiler vital immune organs (the thymus, spleen, and bursa of fabricius) are presented in Table 1. Compared to controls, spleen index was increased in all BLF fed broilers at day 42 although the significant increase was found in the M group only (P < 0.05). Similarly, the index of bursa of fabricius was increased in both M and H groups at day 28 (P < 0.05). The thymus index was increased in both L and H groups at day 14 (P < 0.05), and continuously increased in H group at day 24 (P < 0.05).
Table 1.
Effect of bamboo leaf flavone supplementation on broiler immune organs index.
| Organs | Growth point (day) | Treatments | |||
|---|---|---|---|---|---|
| Control | L | M | H | ||
| Spleen | 14 | 0.93 ± 0.14 | 0.97 ± 0.15 | 1.00 ± 0.07 | 1.07 ± 0.20 |
| 28 | 0.82 ± 0.06 | 0.91 ± 0.15 | 1.06 ± 0.22 | 1.05 ± 0.17 | |
| 42 | 1.09 ± 0.13b | 1.31 ± 0.20ab | 1.35 ± 0.06a | 1.22 ± 0.15ab | |
| Thymus | 14 | 2.48 ± 0.48b | 3.64 ± 1.96a | 3.10 ± 1.23ab | 3.98 ± 0.88a |
| 28 | 3.56 ± 0.49b | 5.20 ± 1.89ab | 5.35 ± 1.88ab | 5.63 ± 0.99a | |
| 42 | 5.53 ± 0.92 | 5.61 ± 0.63 | 5.90 ± 1.26 | 5.73 ± 0.99 | |
| Bursa of fabricius | 14 | 2.19 ± 0.61 | 2.02 ± 0.33 | 2.42 ± 0.27 | 2.38 ± 0.22 |
| 28 | 2.25 ± 0.36c | 2.36 ± 0.53bc | 3.23 ± 0.28b | 3.93 ± 0.54a | |
| 42 | 2.55 ± 0.20 | 2.71 ± 0.27 | 3.09 ± 0.49 | 2.88 ± 0.45 | |
Data were presented as mean ± SD.
a–cValues in the same row without the same uppercase differed significantly (P < 0.05).
Bamboo leaf flavone effects on serum IL-2 and INF-γ concentrations in broilers were summarized in Table 2. Compared to controls, IL-2 concentrations were significantly increased in both M and H groups at day 28 (P < 0.05), which were continuously increased in all treatment groups on day 42 (P < 0.05). Moreover, compared to control group, the concentrations of IFN-γ were increased in all the experimental groups at day 28 (P < 0.05); while at day 42, the upregulation was seen in M group only (P < 0.05).
Table 2.
Treatment effects on the serum concentrations of IL-2 and IFN-γ.
| Immune factors (ng/L) | Growth point (day) | Treatments | |||
|---|---|---|---|---|---|
| Control | L | M | H | ||
| IL-2 | 14 | 42.13 ± 2.32 | 44.2 ± 3.15 | 45.5 ± 2.11 | 44.4 ± 2.12 |
| 28 | 45.43 ± 3.13b | 46.5 ± 4.21b | 55.4 ± 4.14a | 58.7 ± 3.11a | |
| 42 | 26.12 ± 3.14b | 32.2 ± 4.28a | 34.2 ± 1.50a | 31.1 ± 2.10a | |
| IFN-γ | 14 | 11.21 ± 0.88 | 11.56 ± 0.56 | 12.01 ± 0.72 | 11.44 ± 0.91 |
| 28 | 8.21 ± 0.65b | 9.82 ± 0.44a | 10.16 ± 0.57a | 9.72 ± 0.61a | |
| 42 | 5.63 ± 0.43c | 6.31 ± 0.45bc | 7.32 ± 0.41a | 6.84 ± 0.37ab | |
a–cValues in the same row without the same uppercase differed significantly (P < 0.05).
The gene expressions of IL-2 and INF-γ were examined in the spleen (Table 3). Compared to controls, the expression of spleen IL-2 gene was increased in both M and H groups at day 28 (P < 0.05), then returned toward the control level in H group (P > 0.05) but not in M group (P < 0.05) at day 42. Spleen IFN-γ gene were higher expressed in both M and H groups at day 14 (P < 0.05), which was continuously to day 28 (P < 0.05), compared to control group.
Table 3.
Treatment effects on the gene expression abundances of immune factors in the spleen.
| Immune factors | Growth point (day) | Treatments | |||
|---|---|---|---|---|---|
| Control | L | M | H | ||
| IL-2 | 14 | 0.40 ± 0.14 | 0.45 ± 0.12 | 0.49 ± 0.11 | 0.46 ± 0.09 |
| 28 | 0.34 ± 0.08b | 0.32 ± 0.06b | 0.62 ± 0.18a | 0.58 ± 0.07a | |
| 42 | 0.48 ± 0.14b | 0.39 ± 0.13b | 0.71 ± 0.07a | 0.59 ± 0.08ab | |
| IFN-γ | 14 | 2.54 ± 0.42b | 2.64 ± 0.24b | 3.32 ± 0.67a | 3.23 ± 0.08a |
| 28 | 2.08 ± 0.06b | 2.22 ± 0.52ab | 3.14 ± 0.13a | 2.89 ± 0.28a | |
| 42 | 1.08 ± 0.23 | 1.19 ± 0.17 | 1.26 ± 0.33 | 1.16 ± 0.15 | |
a–cValues in the same row without the same uppercase differed significantly (P < 0.05).
Diversity and structure of cecal microbiota
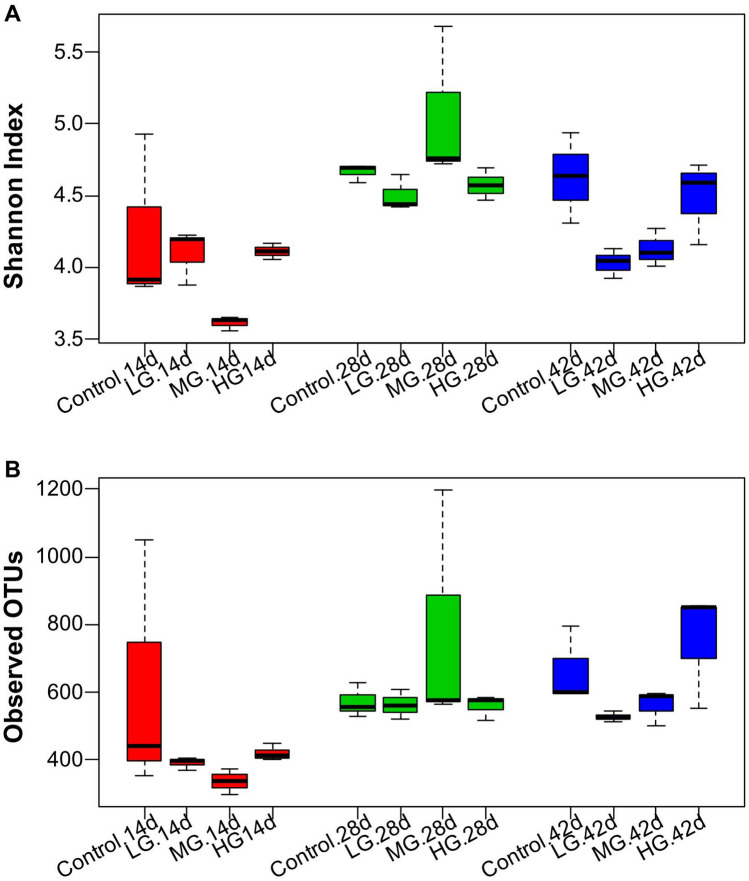
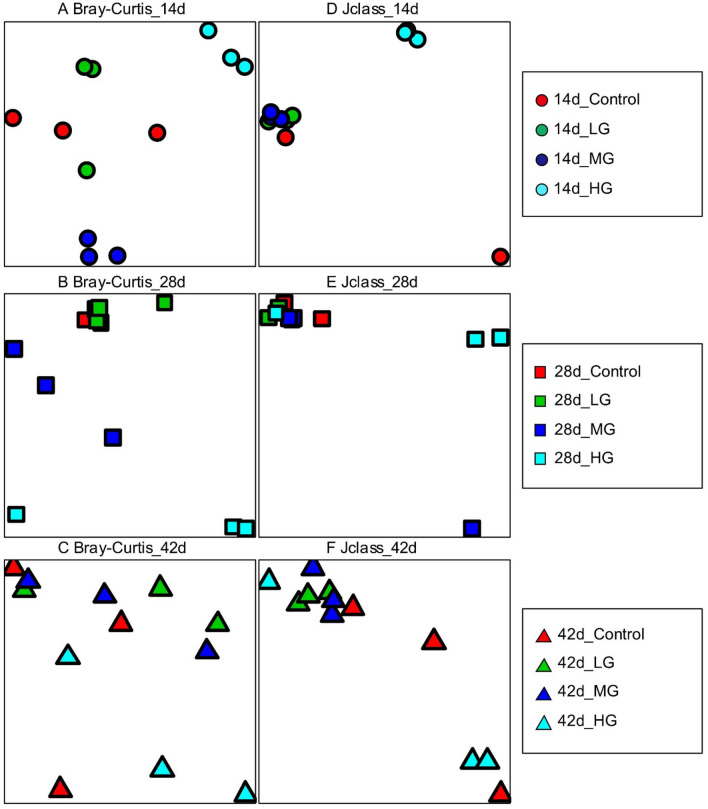
The effects of BLF dietary supplement on the composition and diversity of the cecal microbiota of broilers were measured using the next generation sequencing. A total of 1,196,537 high quality reads was generated from 22,374 operational taxonomic units (OTUs), based on > 97% sequencing, which was similar from all 36 samples. Enough sequencing coverage was achieved after attaining a coverage range from 90.10 to 97.95% by normalizing the number of reads to the smallest for each sample (6,919) (Supplementary Table S2). Both the community richness index of Sobs (Observed OTUs) and Chao1 and community diversity index of Shannon and Inverse Simpson are presented in Fig. 1; and it showed there was no treatment effect on the bacterial community diversity and richness during all examined time points (P > 0.05, Fig. 1A, B). The dissimilarities between community structure and membership were examined by principal coordinate analysis (PCoA) based on Bray–Curtis and Jclass distances. On the PCoA plots, each symbol represented the bacterial community of a bird (Fig. 2). The community structure of M and H groups on both day 14 and 28 formed distinct clusters compared to control group (Fig. 2A, B), while H group showed clear separation on day 42 (Fig. 2C). The separations were clearly showed in the community membership in all the age groups (Fig. 2D–F). These findings illustrated that the microbial community was influenced by the BLF supplement.
Figure 1.
Differences in the gut bacterial community diversity (A) and richness (B) in chickens fed different levels of bamboo leaf flavone. Alpha diversities were measured by Shannon index and Observed OTUs. The top and bottom boundaries of each box plot indicate the 75th and 25th quartile values, respectively. The horizontal lines within each box represent the median values.
Figure 2.
Differences in the gut bacterial community membership and structure in chickens fed different levels of bamboo leaf flavone. Principal Coordinate Analysis (PCoA) of bacterial community structures (A) and memberships (E) of the microbiota of three age groups with different concentrations of bamboo leaf flavone. PCoA indicated distinct bacterial communities on day 14 (B) and (F), 28 (C) and (G), and 42 (D). Each symbol represents each sample, red: day 14; green: day 28; and blue: day 42.
Relative abundances of the dominant phyla and genera in the cecal microbiota
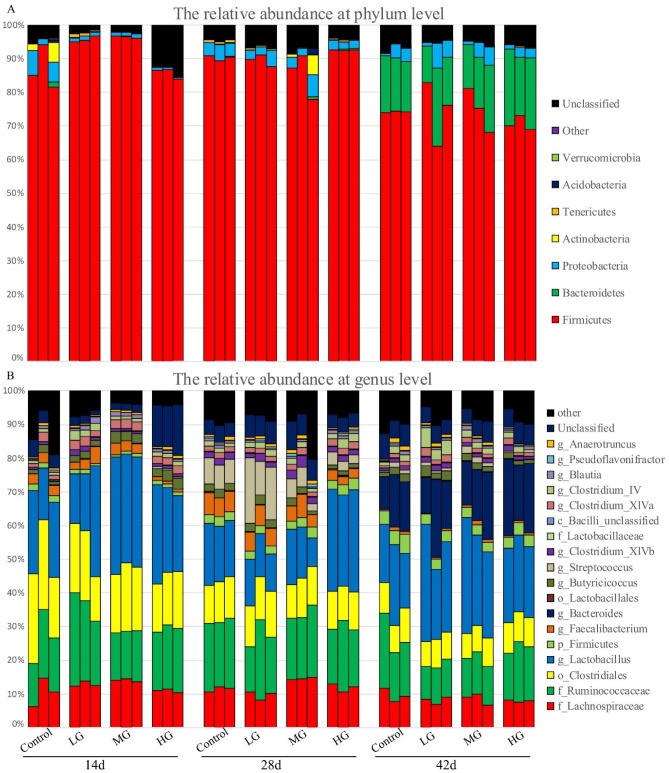
A demonstration was carried out to determine the effect of BFL on bacterial composition and related bacterial community. Therefore, their sequences were binned out into phylotypes according to taxonomic classification of RPD project. After the demonstration by crossed all samples, more than 7 phyla of bacteria were identified, of which Bacteroidetes, Firmicutes, and Proteobacteria were the most dominant ones (Fig. 3A) and 10 unclassified phyla were also found. The greatest change was occurred on post-treatment day 42 with higher levels of Bacteroidetes but less Firmicutes, compared with those on day 14 and 28. The distribution of the community composition at genus level is illustrated in Fig. 3B. Unclassified Lachnospiraceae, Ruminococcaceae, Clostridiales, and Lactobacillus, as the four most abundant genera, were also generated. In addition, the levels of both Streptococcus and Bacteroides were improved on day 28 and 42, respectively.
Figure 3.
Microbial composition affected by chicken age chicken and the levels of bamboo leaf flavone. The levels of phylum (A) and genus (B). Each bar represents the relative abundance of each bacterial taxon. The top 5 abundant phylum and 28 abundant genera are listed.
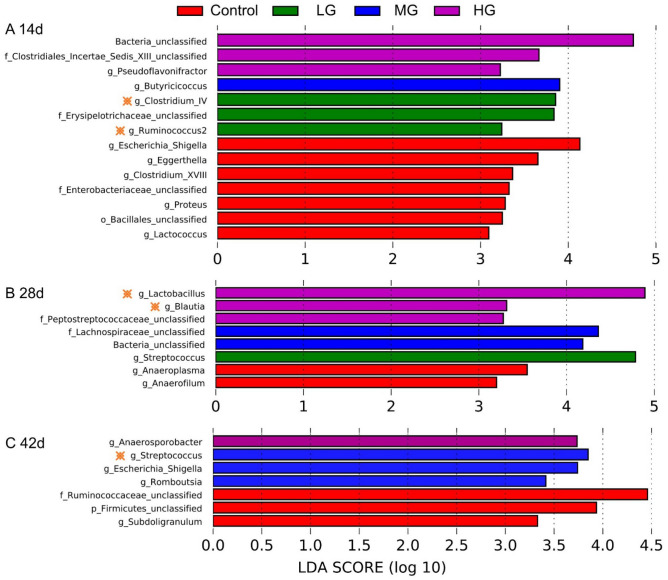
To further identify specific bacterial taxa associated with the BLF supplement, the linear discriminant analysis effect size (LEfSe) was performed to determine the features of phylotypes based on the logarithmic LDA using the score of 2.0 as the cutoff, with the emphasis on both statistical significance and biological consistency. The results showed several phylotypes were differentially represented in each group among the growth phases (Fig. 4). On day 14 (Fig. 4A), 14 genera (e.g. Clostridiales_Incertae_Sedis_XIII, Clostridium_XVIII, and Eggerthella) were significantly differentially represented among the four groups, while 8 (Fig. 4B) and 7 genera (Fig. 4C) (e.g. Lactobacillus and Anaerosporobacter) were identified on day 28 and 42, respectively.
Figure 4.
Bacterial taxa affected by chicken age identified by linear discriminant analysis coupled with effect size (LEfSe). The changes of bacterial taxa at day 14 (A); day 28 (B); and day 42 (C).
Gut microbiome associated with age and bamboo leaf flavone
The bacterial taxa at the OTUs level associated with age and the BLF supplement were examined by using MaAslin online analysis (https://huttenhower.sph.harvard.edu/galaxy/). A total of 14 bacterial taxa was discovered to be associated with both age and BLF treatment. Especially, one was observed to associate with the BLF dosage (Supplementary Table S2). Among the taxa, the OTUs including Otu147_o_Clostridiales, Otu192_f_Ruminococcaceae, and Otu266_f_Ruminococcaceae were positively coefficient with age and BLF treatment, while Otu002_g_Lactobacillus, Otu012_f_Lachnospiraceae, Otu031_g_Clostridium_XlVa, Otu066_g_Butyricicoccus, Otu168_g_Faecalibacterium, and one OTU belonging to unclassified phylum Firmicutes were either positively or negatively associated with age and BLF treatment. Otu001_g_Lactobacillus and Otu036_f_Ruminococcaceae were negatively associated with both age and the concentration of BLF.
Discussion
Flavonoids, a kind of natural polyphenolic compounds in plants16, have been proven to have a beneficial effect on the immune system of animals17. In this study, we confirmed that BLF can improve the immune performance in broilers. The immune organ index has been used as an indicator of the capability of lymphoid cells to produce immune factors in response to stimulations18, and our data indicated that BLF has a positive effect on the immune performance of broilers via stimulating the development of the bursa, thymus and spleen. Similar to our findings, previous research has reported that flavonoids modulate immune function in animals. For example, supplementation of alfalfa flavonoids in diets improves growth performance, spleen and bursa weights, and aspartate transaminase activity of Yangzhou geese19. In addition, cytokine profile is an important marker of immune status in animals. IFN-γ and IL-2 are two important cytokines in the immune system19,20. Both enhance the activity of T and natural killer (NK) cells, playing a major role in the host defense system. The current results further revealed that BLF enhances IFN-γ and IL-2 secretion in bloodstream and IL-2 and INF-γ gene expressions in the spleen to exert immunoregulatory effects. Therefore, the BLF as a feed additive could possess health-promoting properties in chickens.
Numerous studies have reported that chicken intestinal tract harbors complex microbiota which can be altered by various stimuli including dietary factors such as protein levels and feed additives (e.g. probiotics, organic acid, and feed enzymes)21–23. Reed et al.24 reported that Zn biofortified wheat based diet increased microbial diversity in chickens, particularly increased lactic acid bacteria producing short chain fatty acids. Supplementation of raffinose and stachyose in diets also increased beneficial bacterial populations to promote health in broilers25. In addition, the gut microbial ecology plays an important role in the development of the immune response in animals24,25. Similarly, we have detected that broilers harbor complex and dynamic bacterial phyla, which dominantly are Firmicutes, Bacteroidetes, and Proteobacteria. Healthy animals show a harmonious interaction between the gastrointestinal tract and resident microbiota to keep biological homeostasis. However, a dysbiosis in their composition and/or function may result in intestinal and extraintestinal diseases26. For instance, changes of Firmicutes and Bacteroidetes contribute to host metabolic disorders through changing energy harvest, lipid metabolism, endocrine function, and inflammatory response27.
There is a great interest in the study of small intestinal microbiota as well as the microbiota occupied in the anaerobic cecum in chickens, as the gut microbiota displays a great impact on chicken growth and health28. The results from several studies have suggested that plant extracts may modify the composition of the intestinal microbiota to improve the host health29. Vidanarachchi et al.30 reported that water-soluble carbohydrate extracted from the Cabbage trees (Cordyline australis, Acacia, and Undaria seaweed) increased the number of Lactobacilli and reduced harmful bacteria, such as pathogenic coliforms and C. perfringens. Li et al.31 also reported that flavonoid isoorientin inhibited potential pathogenic bacteria such as Helicobacter and Alistipes in mice. In addition, it has also suggested that flavonoid extracted from pumelo peel inhibits the growth of pathogens, such as Escherichia coli and Pseudomonas32. Our data showed BLF caused a major difference in bacterial community structure as increased the level of genus Lactobacillus and Ruminococcus etc. Lactobacillus has be used as probiotics with beneficial effects on promoting poultry performance33,34 and immunity via modulation of the intestinal microbiome35,36. In addition, Ruminococcus is commonly found in chicken faeces37 with functions in degrading mucin to provide carbon and energy for animals. Taken together, the improved immune functions in BLF fed chickens could be modulated by increasing abundances of beneficial bacterial communities.
The bacterial taxa identified in this study mainly belong to Clostridiales and Lactobacillus genera; and the families of Ruminococcaceae and Lachnospiraceae were affected by both age and BLF diet. In addition, the genus of Faecalibacterium showed higher abundances on post-treatment day 14 and 28, which is one of the most prominent butyrate producers to provide energy39. Lachnospiraceae, as a family of anaerobic, spore-forming bacteria, are known to degrade complex polysaccharides to short chain fatty acids38. These two bacterial families have been found to be associated with the maintenance of gut health38. We observed BLF supplement in chicken also enhanced the presence of Erysipelotrichaceae and Streptococcus. Lu et al.40 demonstrated that the OTUs belonging to Streptococcus were more prevalent in the ileum segment than that in the cacum, while Ptak et al.41 found that Streptococcus abundance was reduced in diets supplemented with Ca, P, and phytase. Generally, findings from the current and previous studies provide a new perspective for understanding the effect of flavones on the chicken gut microbiota and related its funtions. The BLF as a feed additive may inhibit colonization of pathogenic bacteria and increase the abundances of beneficial bacteria to improve the chicken immune response.
Overall, BLF, one of the Chinese herbs, may improve immune function and change the gut microbiota structure in chickens to protect them from colonization of pathogenic bacteria and related inflammatory bowel diseases. The effect of the cecal microbiota on chicken growth performance and production safety has attracted considerable interests since the European Union banned (2006) the use of antimicrobials in livestock production42. Particularly, BLF, considered as “prebiotic-like” effects, shapes the gut microbiota to favor other specific gut microbial species that provide healthy benefits to the hosts, such as Faecalibacterium and Lactobacillus in this study. The current results provided evidence which BLF could be an alternative to antibiotics for improving chicken health and production.
Methods
Experimental design and sample collection
The animal experiment was performed at the Poultry Farm of Sichuan Agricultural University. All of the procedures was conducted in accordance with the national standard “Laboratory Animal Requirements of Environment and Housing Facilities” (GB 14925-2001) and approved by the Sichuan Agricultural University’s Institutional Animal Care and Use Committee (Approval number DYY-2018203007).
A total of 300, one-day-old male Arbor Acres broilers (AA) was obtained from Wenjiang Zhengda livestock and poultry Co., Ltd (Sichuan, China), and randomly assigned into 1 of 4 groups in 3 replicates of 25 birds per replicate: A regular diet with BLF supplement at 0 (control), 200 (Low group, L), 400 (Medial group, M), and 800 mg/(kg d) (High group, H). The product of BLF was received from the Zhejiang Saint's Biotechnology Co., Ltd (Zhejiang, China), with the ingredient includes 85% flavonoids of bamboo leaf (mainly contained orientin, homoorientin, vitexin, and isovitexin) and 15% starch (Certificate No: 20130314). Bamboo leaf flavone was extracted by followed the enzyme-assistant extraction method, described previously43.
The chickens were vaccinated against the Marek’s disease (at day 1), H9 avian influenza (at day 8), and H5 avian influenza (at day 24), respectively. The corn-soy diet with 21.4% CP and 3,015 kcal of ME/kg was fed from day 1 to day 28, followed by the diet with 19.9% CP and 3,100 kcal of ME/kg from day 29 to day 42. Feed and water were provided ad libitum.
The chickens were inspected their activity and healthy status daily. A 5 mL blood was collected from each of 16 sampled birds per group on day 14, 28, and 42, then killed by exsanguination. The spleen, thymus, and bursa from each sampled bird were collected and weighted, respectively. The immune organ index was calculated as organ weight divided by body weight. Blood serum and spleen samples were collected to analyze the concentrations of IL-2 and INF-γ proteins and their gene expressions, respectively. The cecal contents were collected from each replicate from all groups. Considering normalizing the dissimilarity in gut microbiota, three samples per repetition (0.5 g per sample) were mixed. Then, a total of 36 cecal samples was collected in sterilized tubes, and immediately stored at − 80 °C for further microbial DNA extraction.
DNA extraction and the V4 region of 16S rRNA gene sequencing
Total microbial DNA from each cecal sample was isolated by using the E.Z.N.A.® Stool DNA Kit (Omega, USA) according to the manufacturer’s instructions. The DNA quality was detected by electrophoresis on a 1% agarose gel and then stored at − 20 °C. The bacterial V4 hypervariable region of 16S rRNA gene was amplified by using broadly conserved primer pairs 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GACTACHVGGGTWTCTAAT-3′). The PCR reaction was performed in a total of 25 μL volume including 1 μL (100 mg) DNA template, 0.5 μL each primers, 10 μL Five Prime Hot Master Mix (5 prime: Item No 2200410) and 13 μL PCR Grade H2O (MoBio: Item No 17000-11) under the following conditions: an initial denaturation cycle at 94 °C for 3 min, followed by 35 cycles at 94° C for 45 s , 50° C for 60 s annealing, 72° C for 90 s extension, and a final extension at 70° C for 10 min. PCR products were purified by using the QIAGEN Gel Purification Kit (QIAGEN, Dusseldorf, Germany) and quantified by using Qubit. At last, 20 purified amplicons were pooled followed the Qubit value and sequenced on the Illumina MiSeq 2 × 250 platform conducted by Novogene company (Beijing, China). Raw sequences were submitted to NCBI Sequence Read Archive (SRA accession: PRJNA601604).
Sequence processing and bioinformatics analysis
The quality of Miseq pyrosequencing data were evaluated and analyzed using mothur v1.39.544 followed Miseq SOP (https://www.mothur.org/wiki/MiSeq_SOP) described by Kozich et al.45. Briefly, (1) the reads with ambiguous bases and any longer than 275 bp were removed; (2) the chimeric sequences were removed by using UCHIME46; (3) the sequences were aligned with the SILVA reference database v12847, and the sequences that could not be aligned due to short overlap were removed; and 4) the sequences were pre-clustered to remove sequences that contain possible pyrosequencing errors. At last, the high-quality sequences were assigned to operational taxonomic units (OTUs) based on a sequence identity threshold of 97% and were classified using the RDP classifier48. The obtained high-quality data was used for the subsequent analysis.
To reduce the biases caused by sequencing effort in downstream alpha and beta diversity analysis, the reads of each sample were randomly subsampled to 6,918. The OTU-level alpha diversity indices such as Shannon and Sobs (observed OTUs) index were calculated to measure the community diversity and community richness. The beta diversity indices based on Bray–Curtis and Jaccard distance were used to estimate the dissimilarity in community structure and membership, which was applied by the principal coordinate analysis (PCoA) to visualize the dissimilarity of microbial communities.
The relative abundances of the recovered phyla, classes, orders, families, genera, and species were statistically compared among the samples and groups. Specific microbiota species among the different treatment groups were identified using LEfSe (Linear discriminant analysis effect size) (https://huttenhower.sph.harvard.edu/galaxy/49, which was performed under the threshold on the logarithmic LDA score > 2.0 for discriminative features. The association between the microbial community (genus) abundance with age and concentration of bamboo leaf flavone was evaluated by MaAsLin (Multivariate Analysis by Linear Models) online.
Serum IL-2 and INF-γ concentration analysis
The blood sample was kept at room temperature for 1 h and then centrifuged at 4,550×g at 4 °C for 5 min. Supernatant liquid was collected and stored at −20 °C for further analysis. The concentrations of IL-2 and INF-γ were determined by ELISA (Nanjing Jiancheng Biological Reagent co., LTD, China) followed the company’s instruction.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA from the spleen was extracted by using Trizol reagent (TIANGEN, Beijing, China) according to the manufacturer’s protocol. The RNA quality was examined by electrophoresis on a 1% agarose gel. One microgram of total RNA from each sample was reverse transcribed into cDNA using the PrimeScript RT reagent kit with cDNA eraser (TaKaRa, Dalian, China). The following PCR reaction program was applied: 3 min heating at 95 °C, followed by 40 cycles of denaturation (10 s at 95 °C), annealing (20 s at 57 °C), and extension (15 s at 72 °C). Primer pairs used for the reverse transcription were listed in Supplementary Table S1. Relative mRNA expression levels of each target gene were calculated using the 2−∆∆Ct method.
Statistical analysis
The effects of BLF were analyzed using one-way ANOVA. The major factors were with or without BLF and BLF concentrations. The differences of alpha diversity indices were analyzed by using Kruskal Wallis test among treatment groups. The results were presented as: mean + SEM. Statistical significance was considered at P < 0.05.
Supplementary information
Acknowledgements
The authors would like to thank Prof. Hengwei Cheng from Department of Animal Sciences, Purdue University, USA for his great comments on this paper. This study was supported by the National Natural Science Foundation of China (31872347) and the Modern Agricultural Industry Technology System (CARS 41) of Ministry of Agricultural and Rural Areas, China.
Author contributions
The study and experiments were conceived and designed by W Ky and Z Xl. Birds raising, reagents preparation, sample collection, and data analysis were contributed by X D, Y Lz, H Cl, L Jc, T Yf, and F Hl. Data analysis and interpretation were performed by K Fl and S G. The manuscript was written and prepared by K Fl, S G, Z Xl and W Ky. All authors read and approved the final manuscript. All the data obtained in this study are available based on requested.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gang Shu, Fanli Kong and Dan Xu.
Supplementary information
is available for this paper at 10.1038/s41598-020-69010-1.
References
- 1.Carrero P, Ortega H, Martínez-Botas J, Gómez-Coronado D, Lasuncion M. Flavonoid-induced ability of minimally modified low-density lipoproteins to support lymphocyte proliferation. Biochem. Pharmacol. 1998;55:1125–1129. doi: 10.1016/s0006-2952(97)00635-7. [DOI] [PubMed] [Google Scholar]
- 2.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 3.Park EJ, Jhon DY. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. LWT Food Sci. Technol. 2010;43:655–659. [Google Scholar]
- 4.Wang L, et al. Application of ionic liquid-based ultrasonic-assisted extraction of flavonoids from bamboo leaves. Molecules. 2018;23:2309. doi: 10.3390/molecules23092309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajendran M, Manisankar P, Gandhidasan R, Murugesan R. Free radicals scavenging efficiency of a few naturally occurring flavonoids: A comparative study. J. Agric. Food Chem. 2004;52:7389–7394. doi: 10.1021/jf0400718. [DOI] [PubMed] [Google Scholar]
- 6.Middleton E. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 7.Ghasemzadeh A, Jaafar HZE, Rahmat A, Devarajan T. Evaluation of bioactive compounds, pharmaceutical quality, and anticancer activity of curry leaf (Murraya koenigii L.) Evid. Based Complement. Altern. Med. 2014;6:803–873. doi: 10.1155/2014/873803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottigli F, et al. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomedicine. 2001;8:302–305. doi: 10.1078/0944-7113-00036. [DOI] [PubMed] [Google Scholar]
- 9.Ghasemzadeh A, Jaafar HZ, Karimi E, Rahmat A. Optimization of ultrasound-assisted extraction of flavonoid compounds and their pharmaceutical activity from curry leaf (Murraya koenigii L.) using response surface methodology. BMC Complement. Altern. Med. 2014;14:318. doi: 10.1186/1472-6882-14-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahiro S, Hiroshi M. Cancer preventive effect of Kumaizasa bamboo leaf extracts administered prior to carcinogenesis or cancer inoculation. Anticancer Res. 2010;30:111–118. [PubMed] [Google Scholar]
- 11.Chen Y, et al. Effects of dietary alfalfa flavonoids extraction on growth performance, organ development and blood biochemical indexes of Yangzhou geese aged from 28 to 70 days. Anim. Nutr. 2016;2:70–74. doi: 10.1016/j.aninu.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei S, Morrison M, Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- 13.Dragana S, Hughes RJ, Moore RJ. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- 14.Lin W, Wang W, Yang H, Wang D, Ling W. Influence of intestinal microbiota on the catabolism of flavonoids in mice. J. Food Sci. 2016;81:H3026. doi: 10.1111/1750-3841.13544. [DOI] [PubMed] [Google Scholar]
- 15.Jiacheng H, et al. Different flavonoids can shape unique gut microbiota profile in vitro. J. Food Sci. 2016;81:2273–2279. doi: 10.1111/1750-3841.13411. [DOI] [PubMed] [Google Scholar]
- 16.Ren W, Qiao Z, Wang H, Zhu L, Li Z. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 17.Steed AL, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.S.H.M. Jeurissen. Structure and function of the chicken spleen. Res. Immunol. (1991). [DOI] [PubMed]
- 19.Morser J. Interferon: Research, clinical application, and regulatory consideration. Trends Biotechnol. 1984;3:80–81. [Google Scholar]
- 20.Fisher K. Contemporary Topics in Molecular Immunology. Volume 10: Edited by S Gillis and F P Inman, pp 291. Plenum Press, New York. 1985. $42.50 0-306-41776-6. Biochem. Mol. Biol. Educ. 2010;14:90–90. [Google Scholar]
- 21.Rehman H, Hellweg P, Taras D, Zentek J. Effects of dietary inulin on the intestinal short chain fatty acids and microbial ecology in broiler chickens as revealed by denaturing gradient gel electrophoresis. Poult. Sci. 2008;87:783–789. doi: 10.3382/ps.2007-00271. [DOI] [PubMed] [Google Scholar]
- 22.Chambers JR, Gong J. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res. Int. 2011;44:3149–3159. [Google Scholar]
- 23.Elijah K, Romero LF, Nyachoti CM. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013;26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- 24.Spenser R, et al. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7:9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarina P, et al. Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut microflora populations. Nutrients. 2017;9:304. doi: 10.3390/nu9030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu H, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. Tem. 2011;22:117–123. doi: 10.1016/j.tem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Wen C, et al. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019;13:1422–1436. doi: 10.1038/s41396-019-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu N, et al. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front. Microbiol. 2019;10:1333. doi: 10.3389/fmicb.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidanarachchi JE, Mikkelsen L, Choct M, Paul I. Effect of some plant extracts on growth performance, intestinal morphology, microflora composition and activity in broiler chickens. Anim. Prod. Sci. 2010;50:880–889. [Google Scholar]
- 31.Yuan L, et al. Effects of natural flavonoid isoorientin on growth performance and gut microbiota of mice. J. Agric. Food Chem. 2018;66:9777–9784. doi: 10.1021/acs.jafc.8b03568. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, Duan C, Zang Y, Huang Z, Liu G. The flavonoid composition of flavedo and juice from the pummelo cultivar (Citrus grandis (L.) Osbeck) and the grapefruit cultivar (Citrus paradisi) from China. Food Chemistry. 2011;129:1530–1536. [Google Scholar]
- 33.Emmanouil A, Didier R. The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS ONE. 2010;5:e10463. doi: 10.1371/journal.pone.0010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerard H, Richard D, Filip VI. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Bian X, et al. Effect of lactobacillus strains on intestinal microflora and mucosa immunity in Escherichia coli O157:H7-induced diarrhea in mice. Curr. Microbiol. 2016;73:65–70. doi: 10.1007/s00284-016-1010-3. [DOI] [PubMed] [Google Scholar]
- 36.Thakur BK, et al. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int. Immunopharmacol. 2016;36:39–50. doi: 10.1016/j.intimp.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Asrore M, et al. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathogens. 2015;7:4. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drennan DM, et al. Organoheterotrophic bacterial abundance associates with zinc removal in lignocellulose-based sulfate-reducing systems. Environ. Sci. Technol. 2015;50:378–387. doi: 10.1021/acs.est.5b04268. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y, et al. Broilers fed dietary vitamins harbor higher diversity of cecal bacteria and higher ratio of Clostridium, Faecalibacterium, and Lactobacillus than broilers with no dietary vitamins revealed by 16S rRNA gene clone libraries. Poult. Sci. 2013;92:2358–2366. doi: 10.3382/ps.2012-02935. [DOI] [PubMed] [Google Scholar]
- 40.Jiangrang L, et al. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ptak, A., Bedford, M. R., Świątkiewicz, S., Żyła, K. & D. Józefiak. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. Plos ONE10, e0119770 (2014). [DOI] [PMC free article] [PubMed]
- 42.Castanon JIR. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Liang T, Wu Y, Du Y. Study on extraction process of flavonoids from bamboo leaves by enzymatic hydrolysis. Guizhou Agric. Sci. 2010;38:185–187. [Google Scholar]
- 44.Schloss PD, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgar RC, Haas BJ, Clemente JC, Christopher Q, Rob K. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfgang L, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiong W, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huse SM, et al. Comparison of brush and biopsy sampling methods of the ileal pouch for assessment of mucosa-associated microbiota of human subjects. Microbiome. 2014;2:5. doi: 10.1186/2049-2618-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.