Abstract
Abstract
Subacute exposure to manganese (Mn) produced Parkinson’s disease-like syndrome called Manganism. Chronic onset and progression are characteristics of Manganism, therefore, this study aimed to examine Mn toxicity following chronic exposures. Male Sprague-Dawley rats were injected Mn2+ 1 and 5 mg/kg, every 10 days for 150 days (15 injections). Animal body weight and behavioral activities were recorded. At the end of experiments, the brain and liver were collected for morphological and molecular analysis. Chronic Mn exposure did not affect animal body weight gain, but the high dose of Mn treatment caused 20% mortality after 140 days of administration. Motor activity deficits were observed in a dose-dependent manner at 148 days of Mn administration. Immunofluorescence double staining of substantia nigra pars compacta (SNpc) revealed the activation of microglia and loss of dopaminergic neurons. The chronic neuroinflammation mediators TNFα, inflammasome Nlrp3, Fc fragment of IgG receptor IIb, and formyl peptide receptor-1 were increased, implicating chronic Mn-induced neuroinflammation. Chronic Mn exposure also produced liver injury, as evidenced by hepatocyte degeneration with pink, condensed nuclei, indicative of apoptotic lesions. The inflammatory cytokines TNFα, IL-1β, and IL-6 were increased, alone with stress-related genes heme oxygenase-1, NAD(P)H:quinone oxidoreductase-1 and metallothionein. Hepatic transporters, such as multidrug resistant proteins (Abcc1, Abcc2, and Abcc3) and solute carrier family proteins (Slc30a1, Slc39a8 and Slc39a14) were increased in attempt to eliminate Mn from the liver. In summary, chronic Mn exposure produced neuroinflammation and dopaminergic neuron loss in the brain, but also produced inflammation to the liver, with upregulation of hepatic transporters.
Graphic Abstract
Electronic supplementary material
The online version of this article (10.1007/s11064-020-03059-2) contains supplementary material, which is available to authorized users.
Keywords: Chronic manganism, Substantia nigra pars compacta (SNpc), Microglia, Dopaminergic neuron loss, Liver inflammation, Hepatic transporters
Introduction
Manganese (Mn) is widely used in modern industries. Occupational exposures to Mn occur primarily through the steel industry, smelters and welding [4, 10, 15, 24]. Overexposure to Mn is known to cause clinical syndromes, including the neurologic disorder like Parkinsonism, dystonia, neuropsychiatric symptoms, motor activity impairment, subclinical liver dysfunction and increased serum bilirubin levels [14, 27, 34, 40]. Mn neurotoxicity is known to specifically damage the basal ganglia in humans [1, 34, 35]. Chronic exposure to Mn results in Manganism, a disorder displays nonmotor dysfunction and motor impairment that resembling, but not identical to Parkinson’s disease (PD, [4, 35].
Intrastriatal injection of MnCl2 into rats produces acute Manganism, with reduction of dopaminergic neurons, activation of microglia, and elevation of inflammatory cytokines [44]. Subacute intraperitoneal injections of MnCl2 or MnO2 are often used as models to study neurotoxic effects of Mn. For example, high dose of MnCl2 injections (100 mg/kg, for 3–7 days) produced oxidative damage, mitochondria dysfunction, and neuroinflammation [28],moderate dose of MnCl2 injections (6–15 mg/kg, 5/week for 4 weeks) altered brain dopamine levels and disrupted dopamine metabolism [30], and low dose of MnCl2 (2.5 mg/kg) or MnO2 (1.22 mg/kg) injections for 8–12 weeks altered brain neurotransmitters including decreased dopamine and its metabolites [29]. MnCl2 (15–50 mg/kg) injections every 2 days for 12 weeks reduced tyrosine hydrolase (TH) expression in a dose-dependent manner [43]. Given the facts that Mn has a relatively shorter half-life in blood, yet fairly long half-lives in tissues [31] and chronic progressive feature of Manganism and PD, a longer recovery time between injections, and longer observation periods are desired to define chronic toxicity effects of Mn exposure.
Manganism and hepatic encephalopathy are the most common pathologies from Mn exposures [35]. Liver dysfunction and injury are frequently observed in Mn exposed workers [4, 14, 35, 40], and Manganism patients are often associated with liver diseases [23]. Chronic liver diseases can result in an excessive accumulation of Mn in brain with ensuing signs and symptoms clinically called Mn hepatic encephalopathy [27]. With weakened liver function, there is also an increased risk of neurodegeneration with continued Mn exposure [40]. Experimentally, subacute intravenous MnCl2 administration to dogs caused severe hepatotoxicity with periportal hemorrhage, hepatocellular necrosis, and biliary epithelial hyperplasia [18],oral MnCl2 (20 mg/ml via the drinking water) to rats for 30 days increased serum enzyme activities and oxidative damage with reduced antioxidants, resulting in DNA fragmentation, apoptosis and necrosis [8]. Injections of MnCl2 (6 mg/kg for 30–90 days) to rats produced liver injury with mitochondria dysfunction [16]. Mn is thought to be metabolized and removed by the liver [23, 35). Hepatobiliary excretion of Mn represents a primary route of Mn clearance from the body, accounting for 80% of Mn elimination [19, 31]. Thus, the normal liver function is essential to maintain homeostasis of Mn in the body, including the brain.
Occupational Mn exposure occurs in Zunyi, China. In collaboration with Purdue University, we have conducted studies of Mn smelter workers on early behavioral changes and biomarkers, and identified the blood Mn/Fe ratio as a novel biomarker of Mn exposure [9, 10], we have discovered the association of divalent metal transporter-1, transferrin and hepcidin in blood and Mn exposures [12], and demonstrated reduced PARK2 expression in Mn smelter workers [13]. A transportable in vivo neutron activation analysis system was designed and utilized to assess bone Mn as accumulative Mn exposure, and found bone Mn and fingurenail Mn are associated with cognitive dysfunction in Mn-exposed workers [36, 37]. In conjunction with these human studies, we have also performed experimental studies on Mn toxicity following oral [38] and peripheral exposures [25]. Subacute Mn injections produced motor deficits and brain damage, but also disrupted circadian clock in the hippocampus and liver [25]. The present study further used the low-dose (Mn2+ 1 and 5 mg/kg), long recovery intervals between injections (every 10 days) for a longer period of exposure (150 days) to examine chronic toxicity of Mn exposure to the brain and liver to provide a whole picture for better understanding chronic Mn exposure and health effects.
Material and Method
Reagent
Manganese(II) chloride tetrahydrate (MnCl2·4H2O) was purchased from Sigma Chemical Co. (St. Louis, USA) and was dissolved in normal saline. All other chemicals were of reagent grade. Antibodies against tyrosine hydroxylase (TH, rabbit: AB152) was from EMD Millipore (Temecula, CA), and against Iba-1 was from Wako Chemicals (Richmond, VA).
Animals and Treatment
Adult male Sprague-Dawley rats, weighting 220 ± 15 g, were purchased from the Experimental Animal Center of Third Military Medical College (Chongqing, China) and maintained in the special pathogen free (SPF)-grade animal facilities at the Key Lab of Pharmacology of Minister of Education at Zunyi Medical University. Rats were fed standard rodent chow and drinking water with 21 ± 2 °C and the light on from 8:00 to 20:00. Animal studies were conducted in accordance with the Chinese Guidelines of Animal Care and Welfare. The protocol was approved by the Zunyi Medical College Animal Care and Use Committee (2015-11).
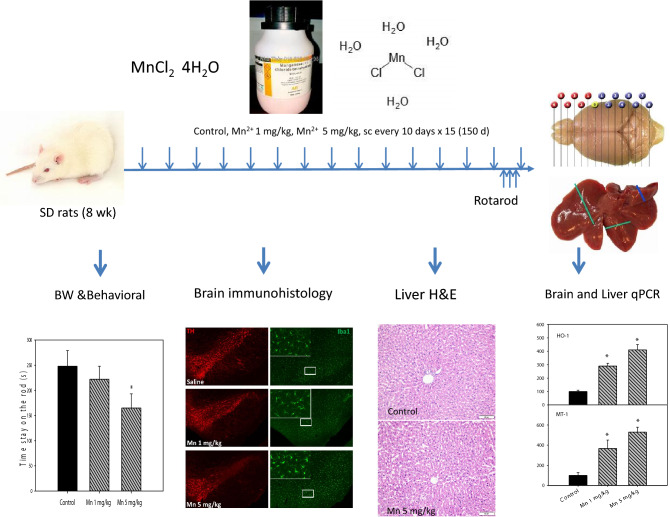
Thirty rats were randomly divided into three groups (n = 10). The low dose group was given Mn2+ 1 mg/kg, converted to MnCl2·4H2O of 3.6 mg/kg; and the high dose group was given Mn2+ 5 mg/kg, converted to MnCl2·4H2O as 18 mg/kg, and control group was injected with the same volume of saline. Rats were intraperitoneal injected every 10 days for 150 days (15 injections in total). The dose of Mn selection was based on our recent publication [25]. The experimental design is illustrated below.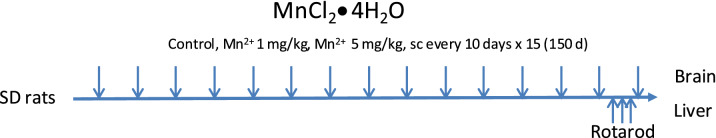
Animal Body Weight and Rotarod Test
Animal body weights were recorded every 10 days after Mn administration. The general health, animal behavioral activity and mortality were monitored. Rotarod test was conducted to evaluate the damage of Mn on motor function. Rats were placed on the rotating rod accelerating from 10 to 40 rpm (increasing 10 rotations/min every 30 s) until the fall from the rod [25]. Each rat was subject to training for three days. The data of 148 days after Mn2+ administration was used for calculation.
Tissue Collection
Twenty-four hours after the last dose (150 days from starting Mn administration), rats were euthanized with 6% choral hydrate. Under deep anesthesia, rats (3 per group) were transcardially perfused with PBS, followed by perfusion with 4% paraformaldehyde (PFA). Brains were further post-fixed in 4% PFA for 24 h, then transferred to ice-cold 10% sucrose solution and incubated at 4 °C overnight. The 10% sucrose was then replaced with ice-cold 30% sucrose, and brains were allowed to equilibrate completely at 4 °C, as evidenced by their sinking to the bottom of the container. Brains were then sectioned immediately on a freezing sliding microtome and processed for immune-staining as described previously [39]. The brains and livers of the remaining rats were removed, and SNpc-enriched midbrain was isolated and snap-frozen in liquid nitrogen and stored at − 80 °C until analysis.
Immunohistochemistry
Free-floating 35 μm coronal sections containing the SNpc were cut on a horizontal sliding microtome. A total of 8 sections were sampled at 105 µm intervals for each brain region. The free-floating sections were immune-blocked with 4% goat serum in 0.25% Triton/PBS for 2 h and then incubated with Iba-1 antibody (1:2000, Wako Chemicals, Richmond, VA) overnight at 4 °C. On the second day, the sections were washed by 1% BSA in 0.25% Triton/PBS before the incubation with anti-tyrosine hydroxylase (TH) antibody (1:2000, EMD Millipore, Temecula, CA) overnight at 4 °C. The double-label immunofluorescence pictures were taken under the confocal microscope by using Alexa-488 (green) and Alexa-594 (red) conjugated secondary antibodies (1:1000) to visualize the TH immune reactive (THir) or Iba-1 positive cells. Stereological counts of TH+ SNpc neurons were estimated using an optical fractionator method on an Olympus BX50 stereological microscope within user-defined boundaries. Samples were countered in a double-blind manner. Data were expressed as percentage to saline-injected controls [26, 39].
Histopathology
Liver samples were fixed in 10% buffered formaldehyde for 48 h, and processed with standard histological procedures. Livers were embedded in paraffin cassettes, and cut into 4 µm slices by using a RM2235 microtome (Leica, Germany), and stained with hematoxylin and eosin (H&E) for blinded examination under a light microscope (Olympus, Japan).
RNA Isolation and qPCR Analysis
Real-time qPCR analysis was performed as described [26]. Briefly, total RNA was extracted with TRIzol and reverse transcribed to cDNA with TaKaRa RT kits (Dalian, China). Real-time PCR was performed with the SYBR Green PCR Kit (Bio-Rad Laboratories, Hercules, CA, USA) on Bio-Rad CFX-96 real-time PCR system. The primers were designed with Primer3 software and listed in Supplementary Table 1. The Ct values were used to calculate the relative expression by the 2−ΔΔCt method and normalized with β-actin, setting control as 100%.
Statistical Analysis
Data were expressed as mean ± SEM. Comparisons were made using one-way ANOVA analysis followed by the Statistical Package for Social Sciences (SPSS) software (version 17.0). The significant criteria were set at p < 0.05.
Results
General Health and Animal Body Weights
Adult male SD rats were injected saline, Mn2+ 1 mg and 5 mg/kg, every 10 days for 150 days. Animal body weights were recorded every 10 days after Mn administration. No apparent differences in body weights were observed during the treatment period. At the end of 150 days of administration, the body weights were 369 ± 15, 391 ± 13, and 387 ± 9 for Control, Mn2+ 1 mg/kg, Mn2+ 5 mg/kg, respectively. Two rats of Mn2+ 5 mg/kg group died after 140 days of administration (mortality rate 20%), and the general activity of the high dose group decreased at the end of experiment. The general health of Mn2+ 1 mg/kg group was not different from Controls. In the previous repeated Mn intoxication study (30 injections in 30 days), Mn accumulation in the brain was 520, 594, and 811 ng/g in Control, Mn2+ 1 mg and Mn2+ 5 mg/kg groups, respectively; Mn accumulation in the liver was 1179, 1216, and 1726 ng/g in Control, Mn2+ 1 mg and Mn2+ 5 mg/kg groups, respectively [25].
Chronic Mn Treatment Impaired Motor Activity
The behavioral activities were examined at 148 days after Mn administration. Rotarod test is one of classic methods to measure the muscular coordination in PD animals. As shown in Fig. 1, Mn administration decreased Rotarod activity, especially in the high dose group. The time stay on the rod was 248 ± 31, 222 ± 26, and 165 ± 28 for Control (n = 10), Mn2+ 1 mg/kg (n = 10), and Mn2+ 5 mg/kg (n = 8), respectively.
Fig. 1.
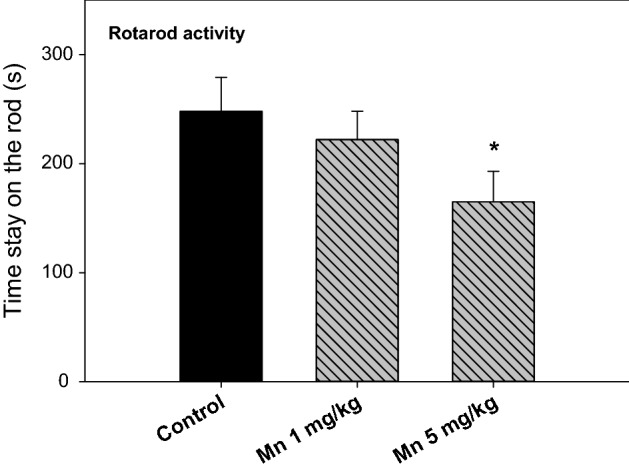
Behavioral (Rotarod, time stay on the rod) tests. Rats were given Mn2+ 1 mg/kg, 5 mg/kg every 10 days. Rotarod tests were performed at 148 days after Mn administration (14 injections). Data are mean + SEM of 8–10 rats. *Significantly different from Controls, p < 0.05
Chronic Mn Treatment Produced Neuroinflammation and Dopaminergic Neuron Loss
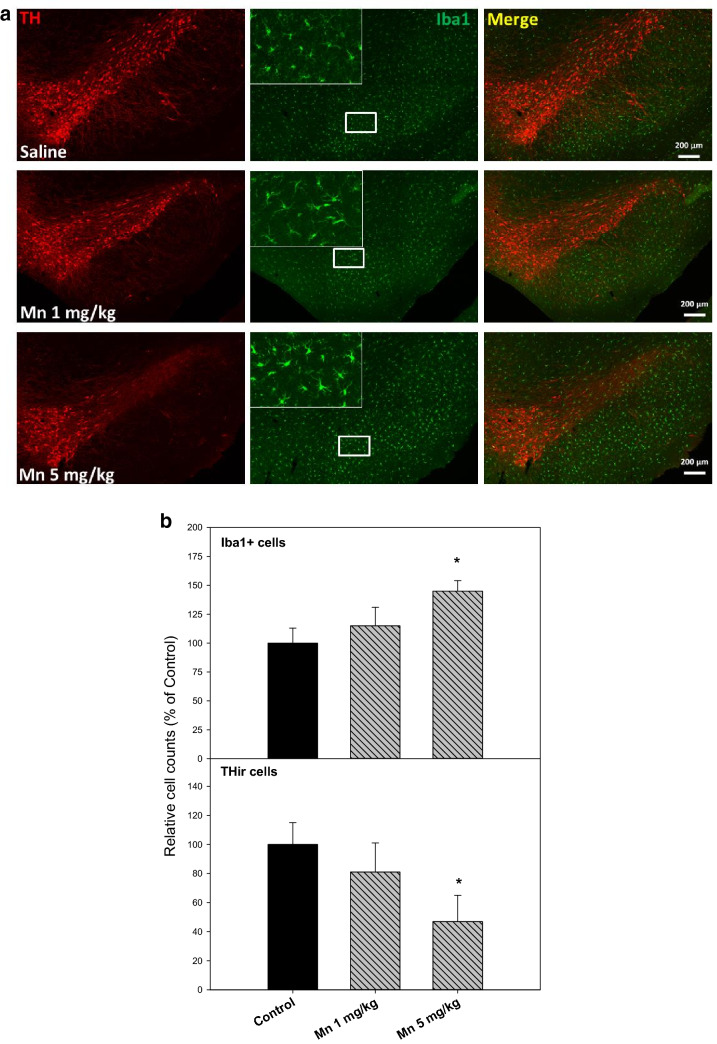
Subacute Mn2+ injections (30 days) produced chronic neuroinflammation and dopaminergic neuron loss in rats [25]. Whether chronic injection of Mn2+ every 10 days for 150 days could also produce similar pathology changes in rats was examined. As illustrated in Fig. 2a, 150 days after 15 injections of Mn2+ 1 mg and 5 mg/kg, Mn2+ induced microglia activation in a dose-dependent manner, characterized by hypertrophied morphology and intensified Iba1 staining (green). The enlarged photo showed ramified microglia and the numbers of microglia were increased by 15 and 45% after Mn2+ 1 mg and 5 mg/kg, respectively (Fig. 2b). Upon microglia activation, dopamine neurons loss was evidenced by reduced THir neurons (red). The numbers of THir cells were decreased by 19 and 53% after Mn2+ 1 mg and 5 mg/kg, respectively (Fig. 2b). These results demonstrated that Mn injections induced chronic neuroinflammation in rats causing delayed loss of THir neurons in SNpc, a brain region sensitive to Mn and LPS intoxication [25, 26]. It should be noted that in this study, 20% rats in the Mn2+ 5 mg/kg group died, which could have more severe injury than surviving rats.
Fig. 2.
Chronic Mn-induced chronic neuroinflammation and loss of dopaminergic neurons. Adult male SD rats were given injections of saline, Mn2+ 1 and 5 mg/kg, i.p. every 10 days for 150 days. The brains were collected and SNpc region was double stained for dopaminergic neurons (THir-strain, red) and microglia (Iba1-stain, green). Representative photos were shown. b Relative cell counts in chronic Mn-induced chronic neuroinflammation and loss of dopaminergic neurons. Adult male SD rats were given injections of saline, Mn2+ 1 and 5 mg/kg, i.p. every 10 days for 150 days. The activation of microglia (Iba1+ cells) and loss of dopaminergic neurons (THir cells) were quantified. Data are mean + SEM of 3 rats. *Significantly different from Controls, p < 0.05
Chronic Mn Treatment Increased Inflammatory Gene Expression in the Brain
At the end of the experiment, brains were collected (n = 7, 7 and 5 for Control, Mn2+ 1 mg and 5 mg/kg, respectively). The SNpc-enriched midbrain was isolated to extract RNA for real-time RT-qPCR analysis (Fig. 3). The expression of TNFα in the SNpc-enriched midbrain was 100, 121 and 147% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; The expression of inflammasome Nlrp3 in the SNpc-enriched midbrain was 100, 109 and 141% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; The expression of Fcgr2b in the SNpc-enriched midbrain was 100, 113 and 144% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; The expression of formyl peptide receptor 1 (Fpr1) was markedly increased (100, 298 and 510% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively). However, there were no changes in the expression of acute inflammatory mediators IL-1β and iNOS (data not shown).
Fig. 3.
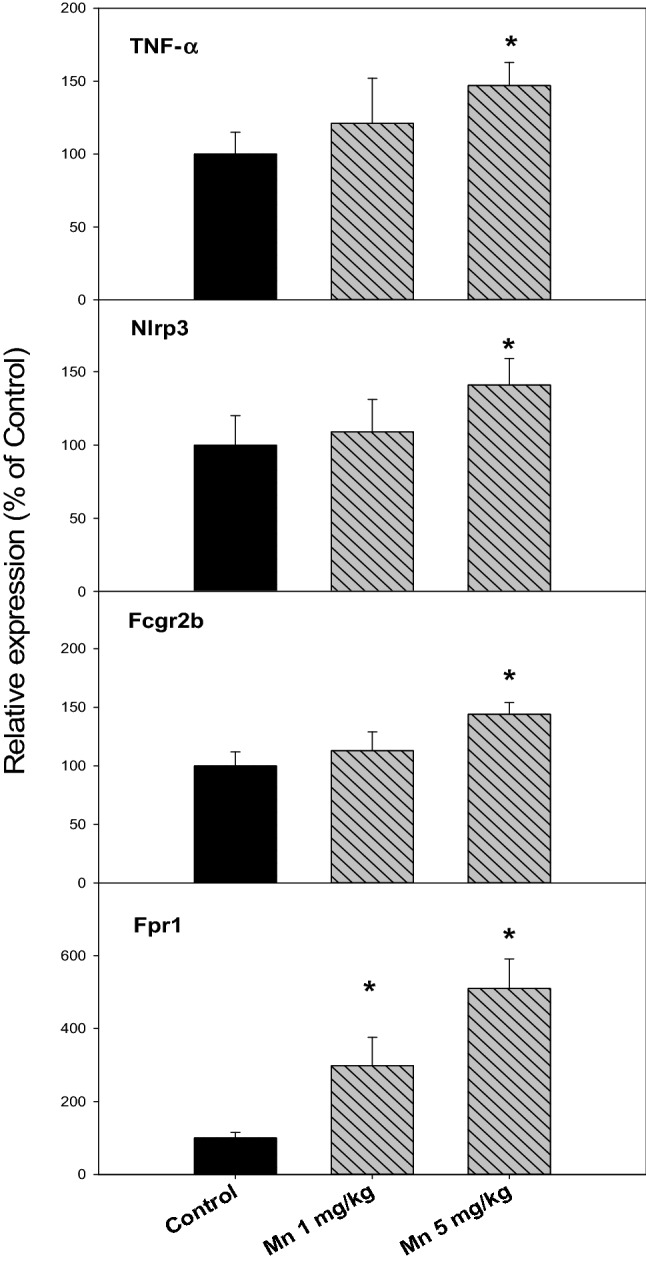
Inflammatory gene expressions in the SNpc-enriched midbrain. Rats were given injections of saline, Mn2+ 1 and 5 mg/kg, i.p. every 10 days for 150 days (15 injections), and the SNpc-enriched midbrain was isolated for RNA extraction, and the expression of inflammatory genes was examined via qPCR. Data are mean ± SEM of 5–7 rats. *Significantly different from Controls, p < 0.05
Chronic Mn Treatment Produced Hepatocyte Degeneration and Apoptotic Lesions
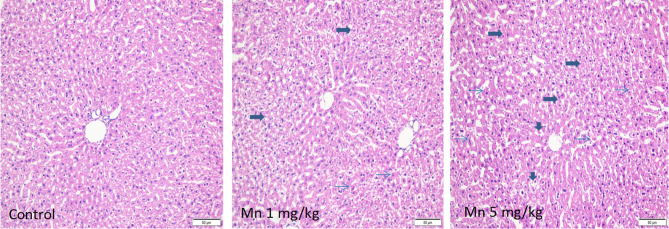
Liver is a target of Mn toxicity [16, 25, 35]. Representative H&E microphotos are shown in Fig. 4. In livers of rats receiving Mn2+ 1 mg/kg, foci of liver degeneration and apoptotic lesions could be seen, but the liver injury was mild; In comparison, in livers of rats given high dose of Mn2+, widespread hepatocyte vacuolation and degeneration (arrows), apoptotic lesions (pink, condensed nuclei were observed (thin arrows), and focal necrosis (vertical arrowheads) could be observed.
Fig. 4.
Representative H&E images of liver. Adult male SD rats were given injections of saline, Mn2+ 1 and 5 mg/kg, i.p. every 10 days for 150 days. Arrows indicate hepatocyte vacuolation and degeneration, and thin arrows indicate pink, condensed nuclei, indicative of apoptotic lesions. Magnitude × 100
Chronic Mn Treatment Produced Inflammatory and Stress Gene Expression in the Liver
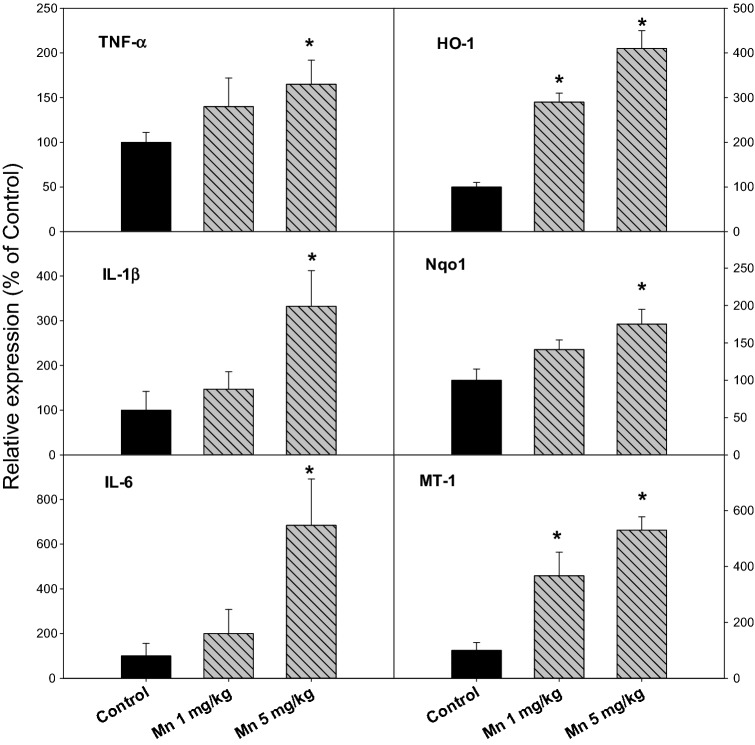
At the end of the experiment, livers were collected (n = 10, 10 and 8 for Control, Mn2+ 1 mg and 5 mg/kg, respectively). Total RNA was extracted for real-time RT-qPCR analysis (Fig. 5). For inflammatory cytokines, the expression of TNFα in the liver was 100, 116 and 155% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; The expression of IL-1β was 100, 147 and 332% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; the expression of IL-6 was 100, 200 and 684% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively. For stress genes, the expression of heme oxygenase-1(HO-1) was markedly increased (100, 290 and 410% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively), the expression of NAD(P)H:quinone oxidoreductase-1 (Nqo1) was 100, 141, and 175% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; and the expression of metal-binding protein gene metallothionein-1 (MT-1) was 100, 367, and 530% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively.
Fig. 5.
Pro-inflammatory gene and stress gene expression in the liver. Rats were given injections of saline, Mn2+ 1 and 5 mg/kg, i.p. every 10 days for 150 days (15 injections), and liver RNA was isolated, and subjected to qPCR analysis. Data are mean ± SEM of 8–10 rats. *Significantly different from Controls, p < 0.05
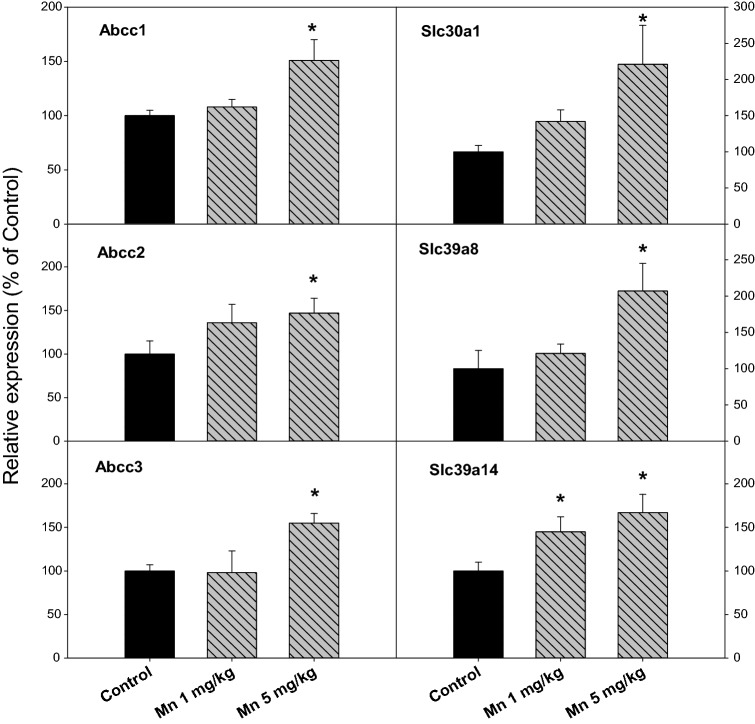
Chronic Mn Treatment Induced Hepatic Transporter Expressions
Mn is thought to be metabolized and removed by the liver [31, 35]. The expression of genes encoding hepatic transporters was further examined. Figure 6 illustrates the induction of hepatic transporters following chronic Mn exposure. Multidrug resistance proteins (MRPs) are main hepatic efflux transporters [20]. The expression of MRP1 (Abcc1) was 100, 108, and 151% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; the expression of MRP2 (Abcc2) was 100, 136, and 147% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; and the expression of MRP3 (Abcc3) was 100, 98, and 155% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively. The expression of ZnT1 (Slc30a1), Mn specific transporter Slc39a8 and Slc39a14 were also examined. The expression of ZnT1 (Slc30a1) was 100, 142, and 221% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; the expression of Slc39a8 was 100, 121, and 207% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively; and the expression of Slc39a14 was 100, 145, and 167% for Control, Mn2+ 1 mg/kg, and 5 mg/kg, respectively.
Fig. 6.
Expression of Abcc and Slc transporters. Rats were given injections of saline, Mn2+ 1 and 5 mg/kg, i.p. every 10 days for 150 days, and liver RNA was isolated, and subjected to qPCR analysis. Data are mean ± SEM of 8–10 rats. *Significantly different from Controls, p < 0.05
Discussion
The present study clearly demonstrated that chronic (150 days) Mn administration with longer intervals (10 days) between injections produced neurotoxicity in rats, as evidenced by impaired Rotarod activity, activation of microglia and loss of dopaminergic neurons in the SNpc region of the brain. These neurotoxicity effects were accompanied by increased expression of neuro-inflammation mediators. Chronic Mn also produced liver injury, with hepatocyte degeneration and apoptotic lesions. Molecular analysis revealed the enhanced expression of proinflammatary cytokines and stress protein genes. Furthermore, the expressions of hepatic transporters including Abcc families and Slc families were increased in an attempt to remove Mn from the liver. This study could add to our understanding of chronic Mn toxicity to the brain and liver.
In laboratory studies, most of experiments utilized consecutive injections of Mn. However, the half-life of Mn is relatively short [31], and the body has a strong recovery capability after cessation of Mn exposure (Cao et al., manuscript in preparation). In the present study we choose 10 days of recovery time between injections, in an attempt to give animals enough time to recover from Mn-induced acute stress, but the exposure lasted much longer (150 days) to mimic chronic progression of Manganism. The low dose of Mn2+ (1 mg/kg) caused mild abnormalities, while the high dose of Mn2+ 5 mg/kg caused 20% mortality after 140 days of administration, demonstrating a dose-dependent chronic Mn toxicity. The threshold of Mn toxicity is narrow, the increased dose renders it from an essential metal to a toxic metal [31]. Rats receiving the high dose of Mn had significantly decreased Rotarod activity, consistent with subacute Mn injections in our earlier observations [25]. Mn intoxication decreases spontaneous locomotor behavior (open field test) and muscle strength (weight test) [11]. Mn also induces non-motor behavior deficits such as the memory and recognition deficits in water maze and step-down tests [38]. The developmental (PND 8–12) exposure to MnCl2 (5–20 mg/kg) could result in behavioral deficits during adulthood (PND 60–65) [33]. Thus, behavioral impairments are important aspects of chronic Mn intoxication in humans [34, 35] and in experimental animals [11, 25, 33, 38].
Mn exposure specifically damage the basal ganglia in humans [1, 34, 35]. Activation of microglia and loss of THir neurons are the characters of Mn neurotoxicity either from intrastriatal injection [44] or from intraperitoneal injection [25]. The present immunofluorescent double staining of the SNpc clearly demonstrated that loss of THir neurons was accompanied by activation of microglia, indicating the key role of microglia activation in Manganism. Chronic microglial activation may be fueled either by Mn accumulation in the brain or by dying/damaged neurons characterized by enhanced TNFα expression [3]. Accumulating evidence has suggested that factors like damage-associated molecular patterns (DAMPs, including inflammasome Nlrp3) released by stressed and dying neurons are likely involved in self-propelling activation of microglia [6]. The increased expression Fcgr2b in neurons mediates cell-to-cell transmission of α-synuclein contributing to PD [7]. Formyl-peptide receptors (FPRs) are increasingly recognized for their expression in diverse host cell types to interact with chemotactic DAMPs contributing to inflammation and many pathophysiologic diseases [5]. Thus, overexpression of inflammatory mediates contributes to chronic microglia activation leading to neurodegeneration, loss of dopaminergic neurons, Parkinsonism, and Manganism.
Liver is a major target organ of Mn exposures [4, 14, 35, 40]. Chronic liver diseases further contribute to excessive accumulation of Mn in the brain leading to Mn hepatic encephalopathy [17, 23]. Liver dysfunction could lead to neurodegeneration with continued Mn exposure [40]. Compared to severe hepatotoxicity following acute and subacute exposed to Mn [16, 18], the liver injury observed in the present study was relatively mild, probably due to the longer time of recovery intervals (10 days) between injections. Nonetheless, at the high dose of Mn2+ 5 mg/kg, hepatocyte degeneration and apoptotic lesions and focal necrosis were evident. At molecular levels, the expression of pro-inflammatory cytokines, TNFα, IL-1β, and IL-6, were coordinately increased, indicating that chronic Mn induced inflammation to the liver [32]. Together with the inflammatory responses, acute-phase stress proteins were increased. HO-1 and Nqo1 are oxidative stress markers, but also indicators of the Nrf2 antioxidant pathway activation to combat with Mn-induced stress to rescue the liver [22]. MT-1 is a metal-binding protein, its upregulation plays important roles in metal detoxication, including Mn [21]. The induction of HO-1, Nqo1, and MT-1 in the liver can be envisioned as adaptive responses to Mn-induced oxidative stress and liver injury. It should be mentioned that chronic Mn exposure increased oxidative stress biomarkers in the brain and liver, raising the possibility of antioxidant interventions, which deserves further investigation.
Mn is mainly removed from the liver through bile [19], and multidrug resistant proteins (MRPs, ABCCs) play a critical role in the transport of xenobiotics from the liver to the bile [20]. In the present study, the increases of MRP transporters were observed in a Mn dose-dependent manner, which could be resulted from the activation of the Nrf2 pathway [22]. The increased MRPs transporters could help elimination of Mn into the bile as one of adaptive mechanisms. Slc30 subfamilies are implicated in zinc and Mn transport. For example, Slc30a10 (ZnT10) in the liver could regulate brain Mn concentration [42]. Slc39a8 (ZIP 8) and Slc39a14 (ZIP 14) are both involved in cellular transport of Mn, controlling tissue Mn levels [2, 41]. Upregulation of these MRPs and SLCs transporters could be envisioned as cellular adaptive mechanisms to remove Mn from the liver.
In summary, this study demonstrates that chronic Mn exposure with longer recover time between injections and longer exposure times produced activation of microglia, neuroinflammation and loss of dopaminergic neurons in the brain, but also produced inflammation in the liver leading to liver injury and activation of the Nrf2 antioxidant pathway and upregulation of efflux transporters as adaptive mechanisms against chronic Mn toxicity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (81860568).
Author Contributions
X-MF: Conceptualization, Methodology, Data curation, Writing: Original draft preparation. YL: Investigation, Data curation, Writing: Original draft preparation. Y-MC: Visualization, Investigation, qPCR. T-WX: Writing: Reviewing and Editing, Visualization. SS: Investigation, Immunofluorescence double staining, Visualization. JL: Conceptualization, Methodology, Data curation, Writing and editing. Q-YF: Supervision, Conceptualization, Writing and editing.
Funding
This study was funded by the National Natural Science Foundation of China (81860568).
Data Availability
All data are available upon request.
Code Availability
N/A.
Consent to Participate
N/A.
Consent for Publication
All authors have read and approved the final version of the manuscript.
Compliance with Ethical Standards
Conflicts of interest
We do not have conflict of interest.
Ethics Approval
Animal studies were conducted in accordance with the Chinese Guidelines of Animal Care and Welfare. The protocol was approved by the Zunyi Medical College Animal Care and Use Committee (2015-11).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jie Liu, Email: Jie@liuonline.com, Email: jieliu@zmu.edu.cn.
Qi-Yuan Fan, Email: 1719975989@qq.com, Email: fanqy@zunyiyizhuan.cn.
References
- 1.Asser A, Hikima A, Raki M, et al. Subacute administration of both methcathinone and manganese causes basal ganglia damage in mice resembling that in methcathinone abusers. J Neural Transm (Vienna Austria: 1996) 2019 doi: 10.1007/s00702-019-02110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aydemir TB, Cousins RJ. The multiple faces of the metal transporter ZIP14 (SLC39A14) J Nutr. 2018;148(2):174–184. doi: 10.1093/jn/nxx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 4.Bowler RM, Gocheva V, Harris M, et al. Prospective study on neurotoxic effects in manganese-exposed bridge construction welders. Neurotoxicology. 2011;32(5):596–605. doi: 10.1016/j.neuro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Bao Z, Gong W, Tang P, Yoshimura T, Wang JM. Regulation of inflammation by members of the formyl-peptide receptor family. J Autoimmun. 2017;85:64–77. doi: 10.1016/j.jaut.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen SH, Oyarzabal EA, Hong JS. Critical role of the Mac1/NOX2 pathway in mediating reactive microgliosis-generated chronic neuroinflammation and progressive neurodegeneration. Curr Opin Pharmacol. 2016;26:54–60. doi: 10.1016/j.coph.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YR, Cha SH, Kang SJ, Kim JB, Jou I, Park SM. Prion-like propagation of alpha-synuclein is regulated by the FcgammaRIIB-SHP-1/2 signaling pathway in neurons. Cell Rep. 2018;22(1):136–148. doi: 10.1016/j.celrep.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Chtourou Y, Garoui E, Boudawara T, Zeghal N. Therapeutic efficacy of silymarin from milk thistle in reducing manganese-induced hepatic damage and apoptosis in rats. Hum Exp Toxicol. 2013;32(1):70–81. doi: 10.1177/0960327112455674. [DOI] [PubMed] [Google Scholar]
- 9.Cowan DM, Fan Q, Zou Y, et al. Manganese exposure among smelting workers: blood manganese–iron ratio as a novel tool for manganese exposure assessment. Biomark Biochem Indic Expo Response Susceptibility Chem. 2009;14(1):3–16. doi: 10.1080/13547500902730672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan DM, Zheng W, Zou Y, et al. Manganese exposure among smelting workers: relationship between blood manganese–iron ratio and early onset neurobehavioral alterations. Neurotoxicology. 2009;30(6):1214–1222. doi: 10.1016/j.neuro.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Fari R, Abbaoui A, Bourziq A, et al. Neuroprotective effects of docosahexaenoic acid against sub-acute manganese intoxication induced dopaminergic and motor disorders in mice. J Chem Neuroanat. 2019;102:101686. doi: 10.1016/j.jchemneu.2019.101686. [DOI] [PubMed] [Google Scholar]
- 12.Fan Q, Zhou Y, Yu C, et al. Cross-sectional study of expression of divalent metal transporter-1, transferrin, and hepcidin in blood of smelters who are occupationally exposed to manganese. PeerJ. 2016;4:e2413. doi: 10.7717/peerj.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X, Luo Y, Fan Q, Zheng W. Reduced expression of PARK2 in manganese-exposed smelting workers. Neurotoxicology. 2017;62:258–264. doi: 10.1016/j.neuro.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge X, Liu Z, Hou Q, et al. Plasma metals and serum bilirubin levels in workers from manganese-exposed workers healthy cohort (MEWHC) Environ Pollut (Barking Essex 1987) 2019;258:113683. doi: 10.1016/j.envpol.2019.113683. [DOI] [PubMed] [Google Scholar]
- 15.Hobson A, Seixas N, Sterling D, Racette BA. Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg. 2011;55(1):113–125. doi: 10.1093/annhyg/meq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang P, Chen C, Wang H, et al. Manganese effects in the liver following subacute or subchronic manganese chloride exposure in rats. Ecotoxicol Environ Saf. 2011;74(4):615–622. doi: 10.1016/j.ecoenv.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Zheng W, Long L, et al. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology. 2007;28(1):126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan KN, Andress JM, Smith PF. Toxicity of subacute intravenous manganese chloride administration in beagle dogs. Toxicol Pathol. 1997;25(4):344–350. doi: 10.1177/019262339702500402. [DOI] [PubMed] [Google Scholar]
- 19.Klaassen CD. Biliary excretion of metals. Drug Metab Rev. 1976;5(2):165–196. doi: 10.3109/03602537609029977. [DOI] [PubMed] [Google Scholar]
- 20.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- 22.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244(1):57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klos KJ, Ahlskog JE, Josephs KA, Fealey RD, Cowl CT, Kumar N. Neurologic spectrum of chronic liver failure and basal ganglia T1 hyperintensity on magnetic resonance imaging: probable manganese neurotoxicity. Arch Neurol. 2005;62(9):1385–1390. doi: 10.1001/archneur.62.9.1385. [DOI] [PubMed] [Google Scholar]
- 24.Li GJ, Zhang LL, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004;46(3):241–248. doi: 10.1097/01.jom.0000116900.49159.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Fan X, Luo Y, Song S, Liu J, Fan Q. Repeated manganese administration produced abnormal expression of circadian clock genes in the hypothalamus and liver of rats. Neurotoxicology. 2017;62:39–45. doi: 10.1016/j.neuro.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Song S, Wang Y, et al. Low-grade inflammation aggravates rotenone neurotoxicity and disrupts circadian clock gene expression in rats. Neurotox Res. 2019;35(2):421–431. doi: 10.1007/s12640-018-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long LL, Li XR, Huang ZK, Jiang YM, Fu SX, Zheng W. Relationship between changes in brain MRI and (1)H-MRS, severity of chronic liver damage, and recovery after liver transplantation. Exp Biol Med (Maywood NJ) 2009;234(9):1075–1085. doi: 10.3181/0903-rm-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240(2):219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen BS, Larsen EH, Ladefoged O, Lam HR. Subchronic, low-level intraperitoneal injections of manganese(IV) oxide and manganese(II) chloride affect rat brain neurochemistry. Int J Toxicol. 2017;36(3):239–251. doi: 10.1177/1091581817704378. [DOI] [PubMed] [Google Scholar]
- 30.O’Neal SL, Lee JW, Zheng W, Cannon JR. Subacute manganese exposure in rats is a neurochemical model of early manganese toxicity. Neurotoxicology. 2014;44:303–313. doi: 10.1016/j.neuro.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Neal SL, Zheng W. Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep. 2015;2(3):315–328. doi: 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng DJ, Zhang YW, Li ZC, et al. Preventive impacts of PAS-Na on the slow growth and activated inflammatory responses in Mn-exposed rats. J Trace Elem Med Biol Organ Soc Miner Trace Elem. 2019;54:134–141. doi: 10.1016/j.jtemb.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Peres TV, Eyng H, Lopes SC, et al. Developmental exposure to manganese induces lasting motor and cognitive impairment in rats. Neurotoxicology. 2015;50:28–37. doi: 10.1016/j.neuro.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology. 2012;33(4):881–886. doi: 10.1016/j.neuro.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera-Mancia S, Rios C, Montes S. Manganese accumulation in the CNS and associated pathologies. Biomet Int J Role Met Ions Biol Biochem Med. 2011;24(5):811–825. doi: 10.1007/s10534-011-9454-1. [DOI] [PubMed] [Google Scholar]
- 36.Rolle-McFarland D, Liu Y, Mostafaei F, et al. The association of bone, fingernail and blood manganese with cognitive and olfactory function in Chinese workers. Sci Total Environ. 2019;666:1003–1010. doi: 10.1016/j.scitotenv.2019.02.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolle-McFarland D, Liu Y, Zhou J, et al. Development of a cumulative exposure index (CEI) for manganese and comparison with bone manganese and other biomarkers of manganese exposure. Int J Environ Res Public Health. 2018 doi: 10.3390/ijerph15071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi XQ, Yan W, Wang KY, Fan QY, Zou Y. Protective effects of dietary fibre against manganese-induced neurobehavioral aberrations in rats. Arh hig rada toksikol. 2012;63(3):263–270. doi: 10.2478/10004-1254-63-2012-2149. [DOI] [PubMed] [Google Scholar]
- 39.Song S, Wang Q, Jiang L, et al. Noradrenergic dysfunction accelerates LPS-elicited inflammation-related ascending sequential neurodegeneration and deficits in non-motor/motor functions. Brain Behav Immun. 2019;81:374–387. doi: 10.1016/j.bbi.2019.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Squitti R, Gorgone G, Panetta V, et al. Implications of metal exposure and liver function in Parkinsonian patients resident in the vicinities of ferroalloy plants. J Neural Transm (Vienna Austria 1996) 2009;116(10):1281–1287. doi: 10.1007/s00702-009-0283-0. [DOI] [PubMed] [Google Scholar]
- 41.Steimle BL, Smith FM, Kosman DJ. The solute carriers ZIP8 and ZIP14 regulate manganese accumulation in brain microvascular endothelial cells and control brain manganese levels. J Biol Chem. 2019;294(50):19197–19208. doi: 10.1074/jbc.RA119.009371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor CA, Hutchens S, Liu C, et al. SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity. J Biol Chem. 2019;294(6):1860–1876. doi: 10.1074/jbc.RA118.005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian Y, Chen C, Guo S, Zhao L, Yan Y. Exploration of the establishment of manganese poisoning rat model and analysis of discriminant methods. Toxicology. 2018;410:193–198. doi: 10.1016/j.tox.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci Off J Soc Toxicol. 2009;107(1):156–164. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon request.
Code Availability
N/A.
Consent to Participate
N/A.
Consent for Publication
All authors have read and approved the final version of the manuscript.