Abstract
Diagnosing stable ischemic heart disease (IHD) is challenging, especially in females. Currently, no blood test is available. Plasma extracellular vesicles (EV) are emerging as potential biomarker source. We therefore aimed to identify stress induced ischemia due to stable IHD with plasma extracellular vesicle protein levels in chest pain patients. We analyzed 450 patients suspected for stable IHD who were referred for 82Rb PET/CT in the outpatient clinic. Blood samples were collected before PET/CT and plasma EVs were isolated in 3 plasma subfractions named: TEX, HDL, LDL. In total 6 proteins were quantified in each of these subfractions using immuno-bead assays. CD14 and CystatinC protein levels were independent significant predictors of stress-induced ischemia in the LDL and the HDL subfraction and SerpinC1 and SerpinG1 protein levels in the HDL fraction. Subgroup-analysis on sex revealed that these associations were completely attributed to the associations in women. None of the significant EV proteins remained significant in men. Plasma EV proteins levels are associated with the presence of stable IHD in females presenting with chest pain. This finding, if confirmed in larger cohort studies could be a crucial step in improving diagnostic assessment of women with suspected IHD.
Subject terms: Biochemistry, Cell biology, Biomarkers, Cardiology, Risk factors
Introduction
Ischemic heart disease (IHD) remains one of the most common causes of death worldwide1. IHD comprises two most prevalent clinical syndromes: acute coronary syndrome (ACS) and stable angina/stable IHD. ACS can be quickly diagnosed in most cases by either ST elevation on ECG or elevated troponin levels. Diagnosing stable IHD is more complex with a wide variety of non-invasive tests1,2. Although imaging modalities provide reasonable sensitivity and specificity (80–90%) they become more and more subject of debate because of high costs, radiation exposure and increasing use in inappropriate low-risk patients3,4. A recently performed cost-effectiveness analysis of non-invasive imaging showed a prevalence of obstructive coronary artery disease (CAD) in only 25% of suspected stable IHD patients5. Another concern that merit consideration in IHD is the evolving knowledge regarding sex differences in pathophysiology, symptoms, diagnostic test performance, and prognosis. Women less frequently have obstructive CAD, yet higher mortality rates compared to men within equal age range6–8. Since only 25% of patients with suspected IHD appear to have IHD, the need for a novel blood based biomarker to detect stable IHD is evident.
Plasma extracellular vesicles (EVs) are relatively unexplored as biomarker source. EVs have a bilipid membrane layer. Vesicles are ~ 50–1000 nm in size and include exosomes, micro vesicles and micro particles9. EVs can be produced by any cell type and consist of proteins, mRNA, miRNA and lipid particles derived from the cell of origin. EVs contain bioactive content that may influence (patho)physiological processes10,11. Previous studies showed associations between EV protein levels and future cardiovascular risk12,13. It is, however, unknown whether specific EV proteins could be used as biomarker to diagnose stable IHD. We therefore investigated if plasma EV protein levels in 3 subfractions are associated with stress-induced ischemia, in patients presenting with chest pain in the outpatient clinic.
Materials and methods
Study population
The MYOMARKER (MYOcardial ischaemia detection by circulating bioMARKERS) study is a prospective single-centre observational cohort study of consecutively enrolled patients (> 18 years) with suspected CAD who presented at the outpatient clinic of the Meander Medical Centre (Amersfoort, the Netherlands) between August 2014 and September 2016. All patients underwent a Rubidium-82 PET/CT. The complete cohort consists of 1,265 patients. For the purpose of this study a random sample of 450 patients was selected for the analysis (Fig. 1). The study (NL5078) was approved by the Medical Ethics Committee-United (MEC_U) and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Figure 1.
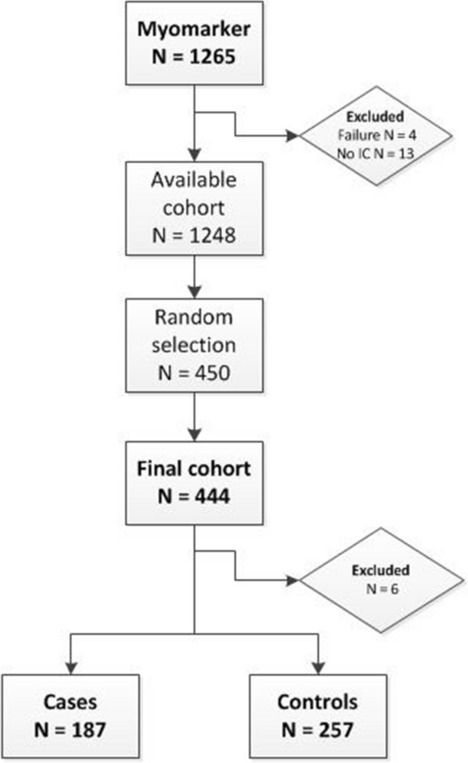
Flowchart patient selection. Original data from myomarker cohort. IC informed consent.
Primary outcome
Primary outcome of the study was stress-induced ischemia. The adjudication of the presence of the primary outcome was based on the results of both myocardial perfusion imaging (MPI), and coronary angiography (CAG) data if available. Rubidium-82 PET/CT MPI results were assessed according to the 17-segment model of the American Heart Association14. All scans were evaluated by 2 experienced observers. In short, the summed difference score (SDS) was the total difference between the stress and rest score for each of the 17 segments. Cases (patients with stable IHD) were defined as patients with SDS score ≥ 2 and visual agreement by both observers. Patients were considered as control if their SDS score was < 2. Based on a previously performed, comparable study, we decided to add available CAG data to the MPI results to improve the diagnostic accuracy of MPI15. CAG images were interpreted with quantitative coronary angiography (QCA) by 2 experienced clinicians using Cardiovascular Angiography Analysis System software (CAAS 7.3, Pie Medical Imaging, Maastricht, The Netherlands). CAG data was available in 146 patients. In total 27 (6%) patients were reclassified from stress-induced ischemia to no ischemia and 11 (2%) patients were reclassified from no ischemia into having stress-induced ischemia.
Identification of proteins
Previously performed proteomics analyses was used to select the proteins12,16. Selected proteins were Serpin C1 (SC1), CD14, Serpin G1 (SG1), Cystatin C (CC), Plasminogen (PLG) and Serpin F2 (F2). Protein levels were determined in in blood plasma and in all three EV subfractions.
Isolation of extracellular subfractions
Venous blood was collected in EDTA tubes directly before MPI from the peripheral intravenous cannula. Blood tubes were centrifuged 10 min at 1850×g at room temperature (RT) within 30 min after collection. Plasma was aliquoted and directly stored at − 80 °C. Plasma extracellular vesicle subfractions were isolated using a modified protocol based on the publication of Burstein et al.17. Detailed description of the isolation protocol used can be found in the supplemental materials. In short, a subset of EVs co-precipitated with Low-Density Lipid particles (LDL) while others co-precipitate with High-Density Lipid particles (HDL), which allows separation. In addition, one subfraction is analysed without the LDL and HDL subfractionation and therefore referred to as TEX subfraction. For the sequential isolation of the subfractions Dextran Sulphate (DS) (MP Biomedicals), Manganese (II) Chloride (MnCl2) (Sigma-Aldrich) solutions and Xtractt buffer (1:4) (Cavadis BV) were used (Supplemental Fig. 1).
Characterization of extracellular vesicles
Both the modified protocol which was used as well as extracellular vesicle characterization are described in detail in two previously published paper (especially in the supplemental materials of Zhang et al.)16,18. In short, we used density gradient centrifugation of the 3 plasma subfractions, all density fractions were characterized by CD9 western blot analysis as EV specific antibody. Lipid particles were identified with ApoB in all density gradient fractions. The presence of EVs was confirmed also visually with electron microscopy (EM) showing the typical bilayer EVs separated from lipid particles. The proteins studied in this manuscript (SC1, CD14, SG1, PLG, CC and SF2) were shown in the density gradient fractions that were shown with CD9 western blotting and EM, and, absent in the density gradient fractions with lipid particles. To get easy access to these data an EV-track ID was created: EV200044, in which the data is structured in a uniform way as suggested by Sluijter et al.19.
Additional to the previous performed experiments to characterize EVs in all three subfractions, we performed a size characterization analysis using Nanoparticle Tracking Analyzer (NTA) (supplemental materials and supplemental Fig. 2). This showed relatively small EVs in the TEX fraction (mean 84 nm), slightly larger EVs in the LDL fraction (mean 101 nm), and the largest particles in the HDL fraction (mean 120 nm). All three fraction show much larger particles then LDL (22–29 nm) or HDL (7–12 nm) particles20.
Quantification of EV protein levels
EV concentrations of selected proteins were measured using the Bio-plex 200 systems (Bio-Rad). Briefly, magnetic Magplex-C Microspheres (Luminex) were conjugated with antibodies specific for the proteins of interest. 50 μl of the protein lysates from the EV subfractions were added to the bead-antibody complexes and were incubated for 45 min. Subsequently, biotin-labelled antibodies were added and again incubated for 45 min. Dilution of the biotinylated antibodies differed for each of the six proteins, respectively; SG1 1:200, SF2 1:400, SC1 1:100, CC 1:200, PLG 1:400 and for CD14 1:400. Bio-Plex 200 system was used for the sample analysis and calibrated each day before use, in accordance to the specifics of the manufacturer. Streptavidin–phycoerythrin (1:500) (Moss) was used to quantify the concentration. Calibration lines were created for each of the six proteins using recombinant proteins (supplemental Fig. 3). Washing steps were executed with a Hydrospeed plate washer (Tecan). SC1 and CD14 biotinylated antibodies were diluted in assay buffer 1x (Thermo Fischer Scientific) with human anti mouse antibody (HAMA) blocking reagent (1:90) (Fitzgerald). All other dilutions were prepared in assay buffer 1x. Data analysis was performed using Bio-Plex Manager Software version 6.1.1 (Bio-Rad). Antibodies and recombinant proteins are listed in supplementary Table 1. Protein concentration were measured in pg/mL. Non-specific binding of the detection antibodies to the bead was verified as follows: capture antibodies of the six proteins were conjugated to six different beads (CD14 + Bead 77; SC1 + Bead 57; PLG + Bead 67; SF2 + Bead68; CC + Bead 27 and SG1 + Bead 55) and mixed. This beadmix was incubated with one of the recombinant protein (the amount was depended on the protein: CD14 and SF2 = 2222 pg/ml; or SC1, PLG and SG1 = 8333 pg/ml; or CC = 5555 pg/ml) in six wells for each recombinant protein (total 36wells). In each of the six wells with the same recombinant protein one biotinylated detection antibody of the six proteins was added. No non-specific binding of the detection antibodies was found. A signal was only seen in the containing the correct combination of recombinant protein with the detection antibody for that specific protein, while other wells had a value comparable to the blanc (supplemental Table 2).
Statistical analysis
Continuous data are expressed as mean ± standard deviation or median ± interquartile range, categorical data as frequencies with corresponding percentages. Differences in continuous variables were compared by independent t-test or Mann–Whitney were appropriate. Dichotomous variables were compared by Chi-square or Fisher’s exact test were appropriate. All EV proteins were standardized with the use of synthetic liposomes (SVs) to serve as internal control (detailed description can be found in the supplemental materials). To provide insight in raw data we provided baseline levels of EV proteins as measured with Bio-Plex.
Distribution of all EV proteins were visually inspected with boxplots and histograms. Because of the skewed distribution of all proteins, we logarithmically transformed them to achieve normal distributions. After transformation all distributions were visually inspected again. The SV standardized and logarithmically transformed variables were used for the logistic regression analyses. All EV proteins were tested in a univariable logistic regression model as well as in a multivariable model adjusted for known cardiovascular risk factors (sex, age, smoking, hypertension, hypercholesterolemia, diabetes mellitus and coronary artery disease). Additional adjustment was performed for cardiovascular medication separately.
We performed exploratory subgroup analyses based on sex and history of coronary revascularization, either percutaneously or with coronary bypass surgery. All subgroup analyses were adjusted for known cardiovascular risk factor (were possible). All hypotheses tests were two-sided with a critical significance level of < 0.05. We did not correct for multiplicity in our study because of the clear exploratory nature of this study, as suggested by Rothman21. Statistical analysis was performed with R software (R software, version 3.5.1).
Results
In total 444 out of the total 450 patients were analysed. Baseline characteristics are summarized in Table 1. Sex and generally accepted risk factors were equally distributed between cases and controls. Patients with stable IHD were slightly older compared to patients without stable IHD (67.65 vs. 69.47, p value 0.043). History of CAD and previous complaints of angina were more common among cases (respectively 69.5% vs 37.0%, p value < 0.001 and 58.8% vs. 35.4%, p value < 0.001).
Table 1.
Baseline characteristics.
| Control | Case | P value | |
|---|---|---|---|
| n | 257 | 187 | |
| Demographics | |||
| Age | 67.65 (9.10) | 69.47 (9.63) | 0.043 |
| %Women | 65 (25.3) | 46 (24.6) | 0.956 |
| BMI | 27.47 (4.42) | 27.18 (4.49) | 0.508 |
| Previous history | |||
| Cardiovascular disease | 237 (92.2) | 176 (94.1) | 0.557 |
| Coronary artery disease | 95 (37.0) | 130 (69.5) | < 0.001 |
| Acute myocardial infarction | 54 (21.0) | 87 (46.5) | < 0.001 |
| Coronary revascularization | 93 (36.2) | 112 (59.9) | < 0.001 |
| Angina pectoris | 91 (35.4) | 110 (58.8) | < 0.001 |
| Kidney disease | 4 (3.9) | 6 (8.1) | 0.393 |
| Risk factor | |||
| Smoking | 37 (14.5) | 38 (20.4) | 0.127 |
| Diabetes Mellitus | 55 (21.4) | 47 (25.1) | 0.419 |
| Hypertension | 171 (66.5) | 120 (64.2) | 0.677 |
| Hypercholesterolemia | 151 (59.0) | 113 (60.4) | 0.835 |
| Family history CAD | 75 (29.5) | 54 (29.3) | 1.000 |
| Medication | |||
| Platelet inhibitors | 149 (58.0) | 136 (72.7) | 0.002 |
| Oral anticoagulants | 50 (19.5) | 42 (22.5) | 0.514 |
| Blood pressure lowering agents | 209 (84.6) | 170 (92.9) | 0.013 |
| Lipid-lowering agents | 166 (67.2) | 139 (76.0) | 0.062 |
Values are displayed as mean ± SD or frequency (%), Case = patient with a SDS score ≥ 2 on myocardial perfusion imaging (MPI), and/or functionally relevant coronary artery disease on coronary angiogram. CAD Coronary artery disease. CVD history of CAD or peripheral vascular disease or history of ischemia CVA, Kidney disease = eGFR < 30.
Raw EV-biomarker levels are shown for cases and controls (supplemental table 3). Univariable analysis showed significant differences between cases and controls for SC1 HDL (odds ratio (OR) 1.31, 95% CI: 1.04–1.66), CD14 HDL (OR 1.46, 95 CI: 1.02–2.11), CD14 LDL (OR 1.54, 95% CI: 1.09–2.16), SG1 HDL (OR 1.37, 95% CI: 1.04–1.80), CC HDL (OR 1.35, 95% CI: 1.04–1.76), CC LDL (OR 1.70, 95% CI: 1.17–2.46). Adjusted for both cardiovascular risk factors and additional for cardiovascular medication, all biomarkers with statistically significant impact in the unadjusted analysis remained significant. Results can be found next to the univariable analysis in Table 2. As can be seen in supplemental Fig. 4, none of the selected proteins measured in whole plasma would have the ability to distinguish between cases and controls.
Table 2.
Logistic regression analysis for stress-induced myocardial ischemia.
| Biomarker | Unadjusted | RF adjusteda | RF + Med Adjustedb | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Serpin C1 HDL | 1.31 (1.04–1.66) | 0.024 | 1.38 (1.05–1.80) | 0.017 | 1.39 (1.06–1.82) | 0.017 |
| Serpin C1 LDL | 1.35 (0.91–2.01) | 0.135 | 1.33 (0.86–2.05) | 0.204 | 1.29 (0.83–2.01) | 0.252 |
| Serpin C1 TEX | 0.87 (0.52–1.44) | 0.581 | 0.79 (0.46–1.37) | 0.400 | 0.80 (0.46–1.39) | 0.428 |
| CD14 HDL | 1.46 (1.01–2.11) | 0.042 | 1.56 (1.04–2.35) | 0.032 | 1.62 (1.07–2.45) | 0.023 |
| CD14 LDL | 1.54 (1.09–2.16) | 0.014 | 1.62 (1.11–2.35) | 0.013 | 1.64 (1.12–2.41) | 0.011 |
| CD14 TEX | 1.52 (0.83–2.78) | 0.172 | 1.43 (0.74–2.75) | 0.288 | 1.50 (0.77–2.92) | 0.230 |
| Serpin G1 HDL | 1.37 (1.04–1.80) | 0.027 | 1.42 (1.05–1.92) | 0.024 | 1.41 (1.04–1.92) | 0.028 |
| Serpin G1 LDL | 1.04 (0.76–1.43) | 0.810 | 1.24 (0.87–1.75) | 0.230 | 1.27 (0.90–1.81) | 0.179 |
| Serpin G1 TEX | 1.08 (0.68–1.73) | 0.737 | 1.27 (0.74–2.17) | 0.393 | 1.25 (0.72–2.19) | 0.432 |
| Cystatin C HDL | 1.35 (1.04–1.76) | 0.026 | 1.38 (1.03–1.85) | 0.034 | 1.41 (1.04–1.91) | 0.025 |
| Cystatin C LDL | 1.70 (1.17–2.46) | 0.006 | 1.69 (1.11–2.55) | 0.014 | 1.70 (1.12–2.60) | 0.014 |
| Cystatin C TEX | 1.45 (0.81–2.58) | 0.210 | 1.14 (0.59–2.21) | 0.694 | 1.13 (0.58–2.20) | 0.726 |
| Serpin F2 HDL | 1.00 (0.82–1.22) | 0.967 | 1.07 (0.87–1.32) | 0.519 | 1.07 (0.87–1.32) | 0.521 |
| Serpin F2 LDL | 0.99 (0.82–1.21) | 0.945 | 1.03 (0.84–1.27) | 0.772 | 1.02 (0.83–1.26) | 0.825 |
| Serpin F2 TEX | 0.89 (0.63–1.24) | 0.480 | 0.92 (0.63–1.30) | 0.582 | 0.94 (0.65–1.36) | 0.738 |
| Plasminogen LDL | 1.16 (0.78–1.71) | 0.468 | 1.28 (0.83–1.96) | 0.262 | 1.32 (0.85–2.04) | 0.212 |
| Plasminogen TEX | 1.02 (0.63–1.64) | 0.950 | 0.92 (0.55–1.54) | 0.755 | 0.94 (0.56–1.57) | 0.804 |
Biomarker levels are log-transformed and standardized per synthetic vesicle and shown as mean ± SD. Original assay units are pg/ml.
RF Risk factor, Med medication.
aRF adjusted; age, sex, hypertension, hypercholesterolemia, smoking, diabetes mellitus and coronary artery disease.
bRF + Med adjusted: platelet inhibitors and blood pressure lowering agents.
We performed an exploratory adjusted subgroup analysis based on sex. Raw baseline EV biomarker levels were stratified on sex are provided in the supplemental data (supplemental table 4). Figure 2 shows the OR with corresponding confidence intervals for all EV biomarker levels stratified on sex. In addition to the proteins with significant impact for the complete study population three additional proteins reach significance among women. All proteins became insignificant among men. Supplemental Fig. 5 shows an additional exploratory subgroup analysis on previous coronary revascularization. Most important differences were found between patients who underwent a coronary artery bypass graft (CABG). Consistent with the results from the complete study cohort SC1 HDL, CD14 LDL and CC LDL remained significant adjusted predictors of stress-induced ischemia.
Figure 2.
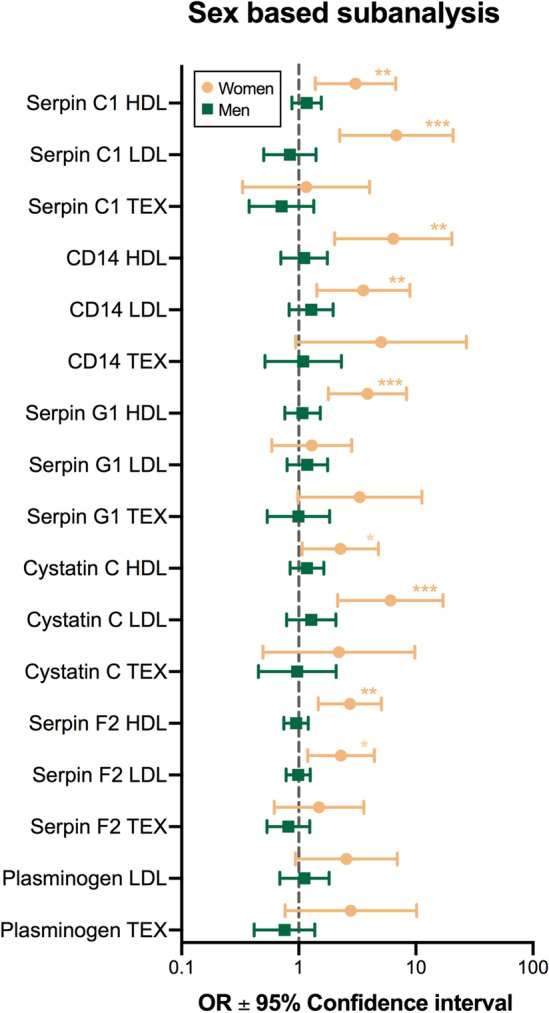
Forestplot of multivariable logistic regression subanalysis stratified on sex. Horizontal bars indicate adjusted* odds ratios and corresponding 95% CI for ischemia. Biomarker levels are log-transformed and standardized per synthetic vesicle. Original assay units are pg/ml. *Adjusted for: age, hypertension, smoking, hypercholesterolemia, diabetes mellitus and coronary artery disease.
Discussion
We showed that EV protein levels in plasma subfractions differ between patients with and without stress-induced ischemia. Exploratory subgroup analysis revealed that the differences in biomarker levels were only seen in women. This finding emphasizes the difference between men and women and the need for sex-specific diagnostic strategies. The role of previous revascularization also needs consideration. Subgroup analysis on CABG imply that EV proteins might have a limited role in CABG patients. This could be the result of the complicated interpretation of MPI results in CABG patients, however, it is an important finding which should be considered for future studies.
Biomarkers to detect stress-induced ischemia are studied extensively. However, only few biomarkers have shown some evidence as potential biomarker. Best known are; high-sensitive cardiac troponin (hs-cTn) and BNP22–24. Both have shown significantly higher blood levels in patients with myocardial ischemia compared to those without. Nevertheless, their diagnostic performance remains limited. Differences in EV protein levels depict differences upon cell-level. EV content might change already in a very early stage of the disease, whereas hs-cTn and BNP are end-products of cell damage. This difference might be the explanation for the great potential of plasma EV proteins as diagnostic biomarker. Larger studies are needed to build a reliable plasma EV-based biomarker model to test whether EV proteins are able to improve clinical decision making. However, the differences of protein levels in women observed in this cohort are remarkable and may lead directly to improved patient care for women.
EV proteins levels and ischemic heart disease
Serpin C1, known as anti-thrombin is an anticoagulant protein. Its main function is inhibition of thrombus formation25. In our study plasma EV SC1 in the HDL subfraction was associated with stress-induced ischemia. Only one, however not comparable, previously performed study was done on plasma EV SC1, this study showed no effect of statin therapy on plasma EV protein levels of SC126. CD14 is a membrane anchored protein known from its function in the innate immune system as TLR4 co-receptor27. Both the HDL and LDL fractionated plasma EV CD14 protein was associated with stress-induced ischemia. This is in line with previous studies on plasma EVs where CD14′s role in thrombotic and inflammatory processes during CVD was shown12,18,28.
Serpin G1, also known as C1-inhibitor, is an acute phase protein which regulates the complement activation29,30. Its main function is the inhibition of coagulation and atherosclerotic plaque formation31. In our study population plasma EV Serpin G1 in the HDL subfraction was associated with stress-induced ischemia. Previous research on plasma EV content in cardiovascular disease showed a strong correlation between Serpin G1 and low-grade inflammation, which is known as keystone in atherosclerotic disease28,32.
Cystatin C is an inhibitor of proteases that play a key role in inflammation. It is produced and secreted by cardiomyocytes and its synthesis is elevated when the myocardium experiences ischemia33. Plasma Cystatin C is known as an important marker for renal dysfunction and also for its close relationship with CAD34–36. De Hoog et al. showed that plasma EV-Cystatin C was associated with an acute coronary syndrome in the TEX subfraction in male patients37. Interestingly we also found an association of EV CC but only in the HDL and LDL subfractions. ACS significantly differs pathophysiologically from stable IHD which might explain this difference. It might also be that plasma EV protein concentrations differ between EV subfractions depending on the atherosclerotic burden and plaque stability. Future studies should provide more insight in this.
Serpin F2, known as alpha-2-antiplasmin, is a protease inhibitor and best known from its function in inhibition of plasmin, which has an important role in fibrinolysis 38,39. The sex based subgroup analysis revealed F2 in both LDL and HDL subfraction as adjusted significant predictor for stress-induced ischemia. Plasminogen is known as precursor of plasmin, which plays a role in fibrinolysis. No association of PLG with stress-induced ischemia was found. To our knowledge only one study has been performed on EV-plasminogen, which looked into the effect of statin use on plasma EV-plasminogen levels. They found an strong association with EV-plasminogen levels after statin treatment26.
Sex differences
Our exploratory subgroup analysis on sex showed the differences in biomarker levels between cases and controls were only seen in women. Sex differences in CVD risk are well known, but not well understood6. It has been proposed that inflammation, metabolic syndrome and adiposity contribute more significantly to the pathophysiology of CVD in females compared with males40. Our results contribute to this statement since plasma EV-Cystatin C, CD14 and Serpin G1 are known to be important proteins within the inflammatory cascade. This was also found in a previous study on EVs which showed their relation with obesity and metabolic complications28. The same was seen in 2 studies about the relation between plasma inflammatory markers and the development of CVD in women41,42. Recently, E. Lau et al. published an article on sex difference in biomarkers within the CVD field43. They performed a large study on > 7,000 patients (54% female) and examined in total 71 proteins, of which 61 differed between men and women. The sex differences observed in our study are in line with these results. The associations found in women could be a crucial step in improving the diagnostic assessment of ischemia in this subgroup. However, these findings should be interpreted with caution and seen as hypothesis generating since this is based on a subgroup analysis with low numbers. Future studies with large numbers are needed to test this hypothesis and explore the role of an EV based model improves clinical care.
Strengths and limitations
This study was a retrospective single centre analysis with myocardial perfusion analysis as reference standard for stable IHD. Since patients were included in the study after referral for perfusion imaging, indicating relative high suspicion, there will be referral and selection bias. This study has a relatively small sample size and results from subgroup analysis could only be interpreted as hypothesis generating. There are several strengths of this study. The cohort consists of real-world data in a centre with high number of outpatient clinic visits and myocardial perfusion imaging.
Conclusion
We showed associations between EV protein levels and stress-induced ischemia. Subgroup analysis on sex showed that all significant associations were completely attributed to women and none of them remained significant in men. Larger studies are needed to confirm our findings, but EV proteins should be considered as promising future tool to improve the diagnostic process for women with suspected stable IHD.
Supplementary information
Acknowledgements
Prof. D.P.V de Kleijn, dr. L. Timmers, prof. D.E. Grobbee and prof. R.J. de Winter received a research grant by the Dutch Heart Foundation, CVON 2017–05 pERSUASIVE.
Abbreviations
- IHD
Ischemic heart disease
- EV
Extracellular vesicle
- ACS
Acute coronary syndrome
- CAD
Coronary artery disease
- MPI
Myocardial perfusion imaging
- CAG
Coronary angiography
- SDS
Summed Difference Score
- CABG
Coronary artery bypass graft
- PCI
Percutaneous coronary intervention
Author contributions
M.D., F.W., A.M., L.T. and D.dK. contributed to the conception of the design. J.B., I.B., A.S., R.S. and G.P. contributed to the performed analysis. M.D. wrote the manuscript. N.T., M.S., J.W., C.M., D.G., R.W. contributed in the first interpretation of the results and critically reviewed the manuscript. All authors reviewed the final manuscript and all authors agreed on this final version of the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
CM has received research support and speakers/consulting honoraria from several diagnostic companies. IP was filed and owned by the UMCU Holding: PCT application (N2015924). None of the authors had any conflict or financial interest to mention.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-69297-0.
References
- 1.Knuuti J, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2019 doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 2.Iannaccone M, et al. Diagnostic accuracy of functional, imaging and biochemical tests for patients presenting with chest pain to the emergency department: A systematic review and meta-analysis. Eur. Hear. J. Acute Cardiovasc. Care. 2019;8:412–420. doi: 10.1177/2048872617754275. [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ. Medical imaging in the 21st Century—getting the best bang for the Rad. N. Engl. J. Med. 2010;362:943–945. doi: 10.1056/NEJMe1000802. [DOI] [PubMed] [Google Scholar]
- 4.Ladapo JA, Blecker S, Douglas PS. Physician decision making and trends in the use of cardiac stress testing in the United States. Ann. Intern. Med. 2014;161:482. doi: 10.7326/M14-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenzoni V, et al. Cost-effectiveness analysis of stand-alone or combined non-invasive imaging tests for the diagnosis of stable coronary artery disease: Results from the EVINCI study. Eur. J. Heal. Econ. 2019 doi: 10.1007/s10198-019-01096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bairey Merz CN, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular cor. J. Am. Coll. Cardiol. 2006;47:S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J. Am. Coll. Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 8.Shaw LJ, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of cardiology-national cardiovascular data registry. Circulation. 2008;117:1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 9.Boulanger CM, Loyer X, Rautou P-E, Amabile N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017;14:259–272. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 10.Martinez MC, Tual-Chalot S, Leonetti D, Andriantsitohaina R. Microparticles: Targets and tools in cardiovascular disease. Trends Pharmacol. Sci. 2011 doi: 10.1016/j.tips.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Loyer X, Vion A-C, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 12.Kanhai DA, et al. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int. J. Cardiol. 2013;168:2358–2363. doi: 10.1016/j.ijcard.2013.01.231. [DOI] [PubMed] [Google Scholar]
- 13.Sinning JM, et al. Circulating CD31 +/Annexin V + microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011;32:2034–2041. doi: 10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira MD, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int. J. Cardiovasc. Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 15.Puelacher C, et al. Combining high-sensitivity cardiac troponin and B-type natriuretic peptide in the detection of inducible myocardial ischemia. Clin. Biochem. 2018;52:33–40. doi: 10.1016/j.clinbiochem.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Wang JW, et al. Lowering low-density lipoprotein particles in plasma using dextran sulphate co-precipitates procoagulant extracellular vesicles. Int. J. Mol. Sci. 2018;19:2. doi: 10.3390/ijms19010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970;11:583–595. [PubMed] [Google Scholar]
- 18.Zhang YN, et al. Extracellular vesicle proteins associated with systemic vascular events correlate with heart failure: An observational study in a dyspnoea cohort. PLoS ONE. 2016;11:1–19. doi: 10.1371/journal.pone.0148073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sluijter JPG, et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018;114:19–34. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams PT, et al. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler. Thromb. Vasc. Biol. 2003;23:314–321. doi: 10.1161/01.ATV.0000053385.64132.2D. [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Sabatine MS, Morrow DA, De Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: Results from TIMI 35. Eur. Heart J. 2009;30:162–169. doi: 10.1093/eurheartj/ehn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staub D, et al. Use of B-type natriuretic peptide in the detection of myocardial ischemia. Am. Heart J. 2006;151:1223–1230. doi: 10.1016/j.ahj.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 24.Staub D, et al. Use of N-terminal pro-B-type natriuretic peptide to detect myocardial ischemia. Am. J. Med. 2005;118(1287):e9–1287.e16. doi: 10.1016/j.amjmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Perry DJ. Antithrombin and its inherited deficiencies. Blood Rev. 1994;8:37–55. doi: 10.1016/0268-960X(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 26.Verbree-Willemsen L, et al. LDL extracellular vesicle coagulation protein levels change after initiation of statin therapy. Findings from the METEOR trial. Int. J. Cardiol. 2018;271:247–253. doi: 10.1016/j.ijcard.2018.05.098. [DOI] [PubMed] [Google Scholar]
- 27.Wang JW, et al. Plasma extra cellular vesicle protein content for diagnosis and prognosis of global cardiovascular disease. Netherlands Hear. J. 2013;21:467–471. doi: 10.1007/s12471-013-0462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kranendonk MEG, et al. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc. Diabetol. 2014;13:1–11. doi: 10.1186/1475-2840-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis AE, Lu F, Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb. Haemost. 2010;104:886–893. doi: 10.1160/TH10-04-0252. [DOI] [PubMed] [Google Scholar]
- 30.Schousboe I. Binding of activated Factor XII to endothelial cells affects its inactivation by the C1-esterase inhibitor. Eur. J. Biochem. 2003;270:111–118. doi: 10.1046/j.1432-1033.2003.03367.x. [DOI] [PubMed] [Google Scholar]
- 31.Cai S, Davis AE. Complement regulatory protein C1 inhibitor binds to selectins and interferes with endothelial-leukocyte adhesion. J. Immunol. 2003;171:4786–4791. doi: 10.4049/jimmunol.171.9.4786. [DOI] [PubMed] [Google Scholar]
- 32.Kostner KM, et al. Inflammation, complement activation and endothelial function in stable and unstable coronary artery disease. Clin. Chim. Acta. 2006;365:129–134. doi: 10.1016/j.cca.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Negrusz-Kawecka M, et al. Evaluation of the significance of cystatin C levels in patients suffering from coronary artery disease. Adv. Clin. Exp. Med. 2014;23:551–558. doi: 10.17219/acem/37222. [DOI] [PubMed] [Google Scholar]
- 34.Shlipak MG, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 35.Keller T, et al. Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the AtheroGene study. Eur. Heart J. 2009;30:314–320. doi: 10.1093/eurheartj/ehn598. [DOI] [PubMed] [Google Scholar]
- 36.Kiyosue A, et al. Plasma cystatin c concentration reflects the severity of coronary artery disease in patients without chronic kidney disease. Circ. J. 2010;74:2441–2447. doi: 10.1253/circj.CJ-10-0158. [DOI] [PubMed] [Google Scholar]
- 37.de Hoog VC, et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur. Hear. J. Acute Cardiovasc. Care. 2013;2:53–60. doi: 10.1177/2048872612471212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 39.del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 40.Savji N, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Hear. Fail. 2018;6:701–709. doi: 10.1016/j.jchf.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam CSP, et al. Influence of sex and hormone status on circulating natriuretic peptides. J. Am. Coll. Cardiol. 2011;58:618–626. doi: 10.1016/j.jacc.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khera A, et al. Race and gender differences in c-reactive protein levels. J. Am. Coll. Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 43.Lau ES, et al. Sex Differences in circulating biomarkers of cardiovascular disease. J. Am. Coll. Cardiol. 2019;74:1543–1553. doi: 10.1016/j.jacc.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


