Abstract
Fetal growth restriction (FGR) is the most common pregnancy complication in developed countries. Pregnancies affected by FGR, frequently concur with complications and high risk of neonatal morbidity and mortality. To date, no approved treatment is available for pregnant women affected with FGR. The objective of this study was to investigate the contribution of galectin-3 (gal-3), a β-galactoside binding protein involved in pregnancy, placental function and fetal growth. We demonstrated that lack of gal-3 during mouse pregnancy leads to placental dysfunction and drives FGR in the absence of a maternal preeclampsia syndrome. Analysis of gal-3 deficient dams revealed placental inflammation and malperfusion, as well as uterine natural killer cell infiltration with aberrant activation. Our results also show that FGR is associated with a failure to increase maternal circulating gal-3 levels during the second and third trimester in human pregnancies. Placentas from human pregnancies affected by FGR displayed lower gal-3 expression, which correlated with placental dysfunction. These data highlight the importance of gal-3 in the promotion of proper placental function, as its absence leads to placental disease and subsequent FGR.
Subject terms: Reproductive disorders, Endocrine reproductive disorders
Introduction
During the establishment of a mammalian pregnancy, the placenta plays a critical role in controlling fetal-maternal resource allocation and mediating fetal programming of future disease. Placental function depends on a healthy decidual environment, and inadequate endometrial receptivity or inappropriate implantation increase the incidence of anomalous placentation. Defects in placentation often result in fetal growth restriction (FGR), one of the most complex pregnancy complications without currently available treatment aside delivery, most often preterm1. Affecting up to 8% of pregnancies worldwide, FGR is also associated with significant perinatal complications, such as increased risk of stillbirth, neonatal long-term morbidity, obesity, type 2 diabetes, and coronary disease later in life1–4. When not attributable to structural or genetic defects of the fetus, FGR is primarily caused by placental insufficiency5,6, but the biological processes that promote placental insufficiency and the subsequent progression to FGR are currently poorly understood. To date, no treatment options are available1.
Galectins, a family of soluble glycan-binding proteins are increasingly recognized as powerful modulators of pregnancy-associated processes including proper placental development. Among the galectin family members, the chimera-type galectin-3 (gal-3, encoded by the Lgals3 gene) is highly expressed at the fetal-maternal interface7–10. Gal-3 knock-down in mouse endometrium results in substantially less implanted embryos11, and dysregulation of gal-3 is associated with several obstetrical complications resulting from placental dysfunction. These include preeclampsia (PE), hemolysis elevated liver enzymes and low platelets (HELLP) syndrome, small-for-gestational age, and gestational trophoblastic disease12–16. At present, the impact of dysregulated gal-3 expression in placental development and pregnancy outcome has not been addressed.
In this study, we found that gal-3 loss of function during gestation altered the decidual compartment favoring a pro-inflammatory milieu, which was accompanied by a decrease in progesterone (P4) in the maternal circulation. In the placental compartment, lack of gal-3 compromised placental vascularization and perfusion resulting in placental insufficiency and the subsequent development of asymmetric FGR in mice. Using the prospective birth cohort PRINCE (Prenatal Determinants of Children’s Health), we showed that development of FGR is accompanied by an altered kinetics of circulating maternal gal-3 levels during gestation. We also found that placental gal-3 expression is downregulated in human pregnancies complicated with FGR, demonstrating that gal-3 also plays a role in human FGR pathology. Finally, using reciprocal matings, we showed that gal-3 within the maternal compartment is required for proper placental development and fetal growth. Our findings identify gal-3 as a key component of the molecular program of decidual/placental development and offspring health, as well as a potential target for future strategies aimed at minimizing adverse outcomes in pregnancies at high risk for FGR.
Results
Gal-3 deficiency in pregnant mice leads to FGR
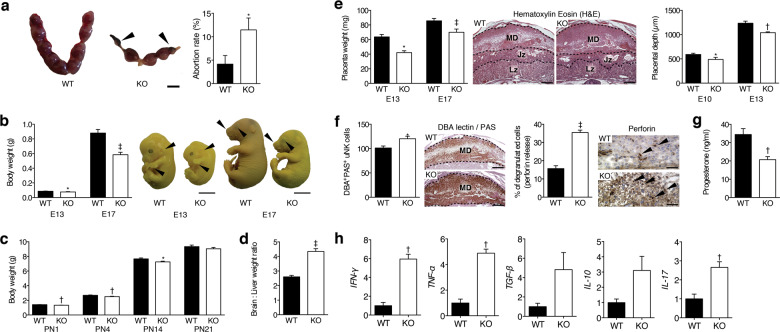
To evaluate the contribution of gal-3 during gestation, we analysed pregnancy outcome and fetal growth in gal-3 deficient mice. Although gestational length was similar between gal-3 wild type (Lgals3+/+) and deficient (Lgals3−/−) dams, gal-3 deficient dams displayed an increased frequency of fetal demise when compared to their wild type counterparts (Fig. 1a), which is in agreement with observations from other group11. Fetuses carried by Lgals3−/− dams showed a weight reduction of 20–40%, compared with Lgals3+/+ fetuses during pregnancy, assesed on embryonic day (E)13 and E17 (Fig. 1b). The reduced fetal weight was accompanied by a delay in fetal development, as the majority of Lgals3−/− fetuses only reached the Theiler stage (TS)21 on E13, whereas Lgals3+/+ offspring reached the TS22, which is appropriate for the gestational age. The immaturity of gal-3 deficient fetuses was still present on E17, evident as fewer skin wrinkles, smaller whiskers, and eyes visible through the eyelids corresponding to TS25 (Fig. 1b). In addition, we confirmed that Lgals3−/− fetuses suffered from asymmetric FGR, as denoted by an increased brain-to-liver weight ratio (Fig. 1d). The reduction of fetal weight carried over into the postnatal period, as Lgals3−/− offspring also showed a significantly reduced body weight until P14 (Fig. 1c). Upon weaning at 3 weeks of age, both Lgals3−/− and Lgals3+/+ offspring displayed similar body weights (Fig. 1c).
Fig. 1. Gal-3 deficiency leads to FGR.
a Macroscopic appearance of the implantation sites on embryonic day (E)13. The pictures show the normal phenotype of Lgals3+/+ (WT) compared to Lgals3−/− (KO) dams, which exhibit resorbed fetuses (left panel, bar = 1 cm). Abortion rate (%) was calculated on E13 as follows: abortion rate = (fetal resorptions x 100)/total number of implantations (right panel, n = 19–24). b Fetal body weight (in grams) of E13 and E17 fetuses carried by Lgals3+/+ (WT) or Lgals3−/− (KO) dams (left panel, n = 13–21). Theiler stage developmental analysis on E13, upper arrows show that pinna is not turned forward and lower arrows show absence of separated fingers in KO fetuses (bar = 0.25 cm). On E17, upper arrows display fewer skin wrinkles, smaller whiskers, and lower arrows show that eyes are clearly visible through the eyelids in gal-3 KO fetuses (bar = 0.5 cm) (right panel). c Mean body weights of pups from Lgals3+/+ and Lgals3−/− dams. Reduced neonatal body weight was observed in Lgals3 KO offspring, which persisted until postnatal day (P)14 (n = 55–161). d E17 asymmetrical FGR as identified by fetal brain-to-liver weight ratio (n = 15–30). e Placental weight (mg) observed on E13 and E17 (left panel, n = 13–21). H&E histological analysis of E10 and E13 implantation sites shows an enlarged decidua in Lgals3−/− mice (middle panel, bar = 500 μm). Isolectin B4 (IB4) analysis of placental depth related to the decidua was significantly lower in placentas of Lgals3 KO mice (right panel, n = 7–8). f Accumulation of mature NK cells (PAS+DBA+) were significantly elevated at E13 (left panel, bar = 500 μm, n = 15) and their perforin granules (arrows) were released in gal-3 deficient pregnancy (right panel, bar = 50 μm, n = 8–11). g Progesterone levels measured in E13 serum by ELISA (n = 5). h Quantitative PCR (qPCR) analysis of decidual IFN-Ƴ, TNF-α, TGF-β, IL-10, and IL-17 gene expression in Lgals3+/+ (WT) and Lgals3−/− (KO) dams at E7 (n = 4–9). In all figures, data are expressed as mean ± SEM. *P < 0.05, †P < 0.01, and ‡P < 0.001 using two-tailed t test.
Lack of gal-3 during murine gestation is linked to features of placental insufficiency
Since asymmetric fetal growth primarily arises from placental pathologies17, we next investigated whether the lack of gal-3 causes placental insufficiency. We observed reduced placental weight on E13 and E17 in Lgals3−/− mice (Fig. 1e). Furthermore, in contrast to wild type dams, the placental disc in E10 and E13 Lgals3−/− placentas displayed reduced trophoblast layers with a correspondingly enlarged maternal decidua (Fig. 1e). Improper placental development in Lgals3−/− mice was accompanied by a significantly increased abundance of uterine NK (uNK) cells in the decidua. Here a higher frequency of uNK cells contained cell-free, perforin-reactive granules (Fig. 1f). As shown in Fig. 1g, gal-3 deficiency was associated with a significant reduction in P4 levels. In line with this finding, mRNA expression of inflammatory cytokines (e.g. INF-γ, TNF-α, and IL-17) was significantly increased in Lgals3−/− decidual tissue (Fig. 1h).
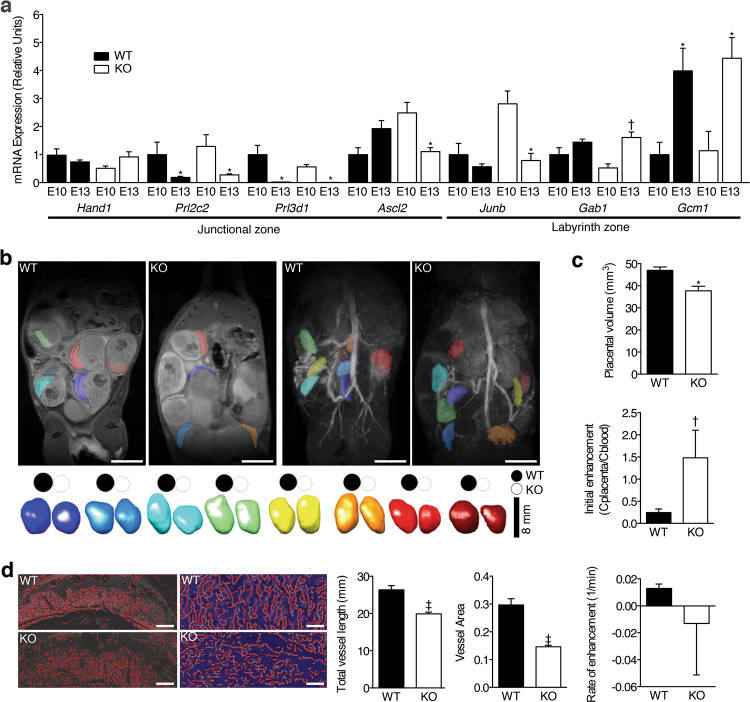
Placental gal-3 deficiency may also affect trophoblast differentiation during placentation. To address this possibility, we examined the expression of trophoblast cell markers between E10 and E13. Our analysis revealed altered expression of the trophoblast Junb, Gab1, and Gcm1 genes, a phenomenon that likely contributed to the failure of placental labyrinth differentiation in Lgals3−/− placentas (Fig. 2a). To further examine the placental labyrinth structure, we performed morphological and functional magnetic resonance imaging (MRI) during E13. As illustrated in Fig. 2b, placental volume was reduced in Lgals3−/− compared to Lgals3+/+ implantations. After injection of an albumin-bound contrast agent, initial contrast enhancement was significantly higher for Lgals3−/− mice but contrast material dynamics described by rate of enhancement were similar (Fig. 2c). Interestingly, the variability of both parameters was much higher in Lgals3−/− animals. Finally, we used Isolectin B4 staining to further characterize the Lgals3−/− placental labyrinth zone. As expected, gal-3 deficiency resulted in reduced placental labyrinth total vessel length and vessel area (Fig. 2d).
Fig. 2. Gal-3 deficiency results in placenta insufficiency.
a Relative mRNA expression of trophoblast differentiation genes determined by quantitative PCR (qPCR) on E10 and E13 placentas (n = 6–8). b 2D pre-contrast anatomical overview of MR images through abdomen of representative Lgals3+/+ (WT) and Lgals3−/− (KO) dams. Placentae labeled with false color transparent overlay (top left panel). 3D post-contrast maximum intensity projection angiograms of the mouse abdomen to determine placental volumes based on contrast enhancement (top right panel). 3D reconstruction of individual placentas ordered by volume (largest to smallest from left to right) are shown pair wise for Lgals3+/+ (WT) and Lgals3−/− (KO) animals. For better visual comparison, placental volumes were projected onto discs shown above each reconstruction illustrating smaller placentas in the Lgals3−/− animals (bottom panel, bar = 8 mm). c Group comparison of placental volumes over all animals confirmed a decrease in Lgals3−/− animals (top panel). Series of post-contrast images were acquired and mean signal was quantified repetitively for each placenta over ~10 min for functional MRI phenotyping. Initial enhancement, representative of maternal fractional blood volume in percent was increased in Lgals3−/− placentae whereas the rate of enhancement representative of several functional parameters such as perfusion, contrast agent inflow, clearance, and uptake was unchanged. Notably, a higher variability was found in KO animals for both, initial (middle panel) and rate (bottom panel) of enhancement (n = 8–16). d Isolectin B4 (IB4) staining showed poor development of fetal capillaries in the labyrinth of placentae of Lgals3−/− KO mice as revealed by AngioTool analysis (Total vessel length and vessel area; left bar = 500 μm and right = 150 μm, n = 25–34). In all figures, data are expressed as mean ± SEM. *P < 0.05, †P < 0.01, and ‡P < 0.001 using two-tailed t test.
Reduced maternal levels of gal-3 are linked to human FGR
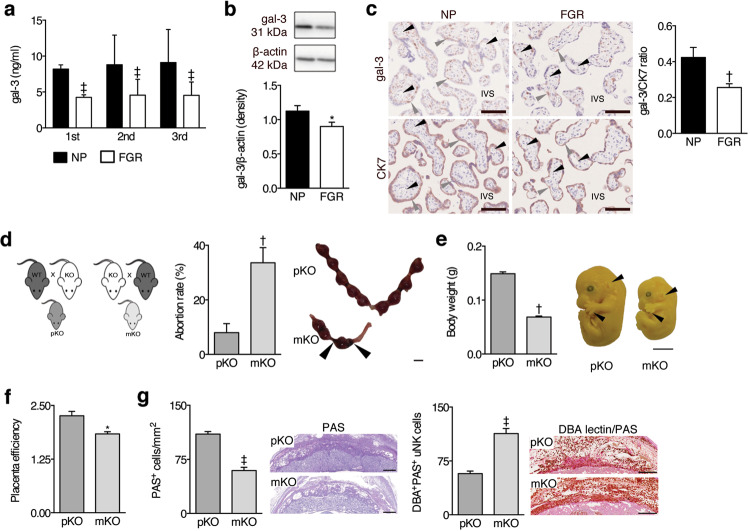
We next sought to asses the translational relevance of our findings in mice and determined gal-3 levels in human pregnancies using biological samples from two independent patient cohorts. In a subgroup of women participating in the prospective pregnancy cohort PRINCE carried out in Germany (clinical data shown in Supplementary information), we observed that serum gal-3 levels continuosly increased in normally progressing pregnancies (n = 33) (range 3.02–16.70 ng/ml, Fig. 3a). Conversely, in pregnant women affected by FGR (n = 32), levels of serum gal-3 were significantly lower as early as the first trimester and levels stalled throughout gestation (range 1.00–9.79 ng/ml). In order to asses the interaction between serum gal-3 levels in human pregnancies and placental function, we analysed its correlation with uterine artery perfusion (Ut PI). As suspected, gal-3 peripheral levels were found to correlate inversely with Ut PI values (rs = −0.5065, P < 0.05) in pregnancies complicated with FGR. In addition, we used placental biopsies taken at birth from uneventful (n = 27) and FGR pregnancies with placental pathology (n = 19) in an independent cohort recruited in Australia (clinical data shown in Supplementary information,18). Similar to our observations in mice, FGR placentas expressed decreased protein levels of gal-3 in general as revealed by western blot (Fig. 3b), or when specifically assessed within the extravillous CK7+ trophoblasts (Fig. 3c, Supplementary Fig. 1).
Fig. 3. Maternal gal-3 is necessary for normal placental and fetal development.
a Circulating maternal gal-3 evaluated by ELISA in PRINCE cohort of patients (n = 65) during first, second, and third trimester, where some women subsequently development FGR (n = 32). b Western blot analysis of gal-3 expression in placental tissue from normal, healthy human pregnancies (NP) (n = 27) and pregnancies complicated by FGR (n = 19). Densitometry was performed to quantify changes in protein expression using ImageJ. c Representative images of gal-3 and cytokeratin (CK) 7 immunohistochemistry in NP and FGR placentae (n = 14, bar = 100 μm; left panel). The percent staining area of gal-3 and CK7 slides was quantified, and a ratio was calculated by dividing the percent area of gal-3 to CK7 staining in slides from the same placental tissue sample (right panel). d Schematic diagram showing the lack of paternal (mating of Lgals3−/− (KO) male with Lgals3+/+ (WT) female, pKO) and maternal (mating of Lgals3+/+ (WT) male with Lgals3−/− (KO) female, mKO) gal-3. The offspring is heterogeneous (HT) for gal-3 in both mating combinations (left panel). The abortion rate was calculated on E13 as follows: abortion rate = (fetal resorptions x 100)/total number of implantations (middle panel, n = 6–8). Pictures display whole implantation sites on E13. Arrows point to fetal resorptions (right panel, bar = 1 cm). e The embryo body weight was determined on E13 to identify potential fetal growth restriction (FGR) (n = 6). To assess the fetal development, Theiler stage (TS) analysis was performed. Normally developed embryos on E13 are in TS22 (pKO). FGR embryos are in a lower stage (e.g., TS21 or 21.5) and are characterized by a pinna that is not turned forward and non-separated fingers (arrows; mKO) (bar = 0.25 cm). f Placental efficiency was calculated as fetal/placental weight ratio on E13 (n = 14–21). g Glycogen cells in the spongiotrophoblast of the placenta were stained with periodic acid Schiff (PAS) and counted in 3 squares (1 × 1 mm) per mouse on E13 (n = 14–21). The number of mature uterine natural killer (uNK) cells in the decidua basalis were counted in 3 to 4 squares (1 × 1 mm) per mouse (n = 19–26). In all figures, data are expressed as mean ± SEM. *P < 0.05, †P < 0.01, and ‡P < 0.001 using two-tailed t test.
Since placental insufficiency is not exclusive to FGR, we wondered whether gal-3 dysregulation also occurs in pregnancies complicated by preeclampsia. Placental expression and circulating levels of gal-3 in preeclamptic women (n = 36) from the Oslo study were not different from those of uneventful pregnancies (n = 23) (Supplementary Fig. 2a–d; clinical data shown in Supplementary information). Supporting this, gal-3 deficient mice did not develop preeclampsia-like features (increased blood pressure, new-onset proteinuria, production of Angiotensin II receptor type 1 autoantibody (AT1AA) or circulating anti-angiogenic factors (Supplementary Fig. 2e–h). In addition, no differences were found in E10 and E13 decidual spiral artery wall thickness between WT and KO dams (Supplementary Fig. 2i). We conclude that gal-3 deficiency is involved in the placental-fetal axis and confined to the fetal phenotype downstream of placental pathology without significantly affecting maternal vascular health in pregnancy.
Maternally-derived gal-3 drives proper placental function and fetal growth
We next investigated whether maternal gal-3 plays a role in placental and fetal development. As shown in Fig. 3d, absence of maternal gal-3 increased fetal demise. Most notably, we observed that the body weight of Lgals3+/− fetuses derived from Lgals3−/− dams (maternal KO, mKO) was significantly reduced compared to Lgals3+/− fetuses carried by Lgals3+/+ dams (paternal KO, pKO). Also, an immature Theiler stage (TS21), characterized by a backwards pinna and unseparated fingers (Fig. 3e), was detected in fetuses derived from Lgals3−/− dams. In addition, Lgals3+/− implantations carried by Lgals3−/− dams displayed a decreased fetal/placental weight ratio, indicative of placental insufficiency (Fig. 3f), along with a reduction of PAS+ glycogen cells in the junctional zone (Fig. 3g, Supplementary Fig. 3a). We also identified an increased uNK cell abundance in the decidua of Lgals3−/− dams, compared to the reciprocal matings (Fig. 3g, Supplementary Fig. 3b). These findings highlight that maternally-derived gal-3 maintains placental function. If maternal gal-3 expression is low, the incidence of developing FGR is increased.
Discussion
FGR remains one of the “great obstetrical syndromes”, and is a leading contributor worldwide to perinatal mortality and morbidity, as well as playing an important role in mediating non-communicable diseases in adult life, including cardiovascular disease, diabetes, metabolic syndrome, and obesity. Although significant advances have been made on its understanding, there is still no definitive cure or treatment for pregnancies complicated by FGR. Thus, the current knowledge gap emphasizes the need for a better understanding of how fetal development is normally achieved and how it is dysregulated in FGR. Since normal placental development and functional integrity are essential for normal fetal growth, the identification of alterations during critical windows of placental development could help to optimize antepartum monitoring and timely delivery of FGR infants. Our study unveils that a dysregulation of gal-3, especially at the maternal compartment, is a significant factor involved in abnormal placentation and development of FGR. This phenotype appears to be mirrored in human FGR patients, suggesting that gal-3 can assert similar effects in the human placenta.
Galectin-3 was initially reported to be a factor related to endometrial receptivity due to its increased expression in the endometrium and trophoblast during embryo implantation19,20. Subsequent studies have revealed that endometrial gal-3 is involved in embryo implantation21 and dysregulation of placental gal-3 is associated with adverse pregnancy outcomes12–16,22,23. The present study demonstrates that gal-3 is critical for orchestrating healthy fetal-maternal interface interactions and its dysregulation results in asymmetric FGR. Affecting approximately 70% of growth-restricted fetuses, the asymmetric type of FGR occurs when brain growth and maturity is maintained while the visceral organs (especially the liver) have relatively reduced weights24, and represents a later timing of embryonic damage that results from maternal vascular factors and/ or placental insufficiency. The fact that gal-3 deficiency-induced asymmetric growth restriction followed by relatively rapid catch-up growth is of significant importance since human babies with these characteristics, particularly those with accelerated postnatal weight gain after in utero growth restriction, have a greatly enhanced risk of developing type 2 diabetes, obesity and cardiovascular disease25–27.
Studies in gal-3 deficient dams enabled us to identify this chimera lectin as a key player in regulating decidualization and maternal immune adaptation during early gestation. The absence of gal-3 per se induced decidual enlargement, inflammatory cytokine gene expression, and NK cell infiltration with aberrant activation. Supporting our observations, these features were also found in a different mouse FGR model established by independent researchers28. Gal-3 is known to regulate immune responses, acting as a pro-inflammatory signal with diverse innate immune cell targets to promote their activation, degranulation, and cytokine production29,30. In this context, recent in vitro studies have shown that endogenous expression of gal-3 in human NK cells can be stimulated by activating cytokines (IL-2, IL-15) and correlates functionally with their degree of cytotoxic degranulation31. While similar functions remain to be examined in the context of pregnancy, the identification of an endogenous gal-3 ligand localizing specifically to mouse uNK perforin granules32 implies a potential role of this lectin in controlling the activation status of this particular lymphocyte subset, which has been ascribed important roles in the modulation of decidual development, trophoblast invasion, and maternal vascular remodeling during early pregnancy.
Gal-3 deficiency impacts placental function by means of intrinsic effects on trophoblast biology. Specifically, we showed that gal-3 deficiency during gestation alters trophoblast differentiation, vascularization of the labyrinth zone and subsequently causes changes in placental perfusion. While the structural organization of the fetal-maternal interface differs between human and mouse (villous versus labyrinthine placenta), the syncytium separates fetal and maternal blood in both species and serves analogous functions. Therefore, it is not surprising that gal-3 deficiency compromised the labyrinth layer during mouse placentation. Since inflow and clearance of the contrast agent strongly depend on perfusion, we speculate on more heterogeneous maternal circulation in gal-3 deficient compared to control placentas. Indeed, gal-3 deficiency caused reduced total vessel length and vessel area in the placental labyrinth, suggesting that absence of this lectin during gestation alters vascularization of the labyrinth and subsequently results in placental malperfusion. On the other hand, maternal deficiency of gal-3 also provoked alterations in placental structure, particularly in glycogen cells of the junctional zone, emphasizing the importance of an interplay between fetal and maternal sources of gal-3 expression for normal placental development. Alterations in placental energy storage (glycogen cells) are a typical feature of FGR models and likely to contribute to the detrimental maternal effect on fetal growth33,34. A reduced placental glycogen reserve will definitely impact on placental well-being and fetal growth as the main source of energy for the placenta comes from glucose and also, the glycogen stores may be destined to the fetal demands35. In addition, glycogen trophoblasts may serve other functions likely to impact fetal growth, as these cells invade the decidua congregating near spiral arteries to support their remodeling and also have an endocrine role, being the source of several mediators including retinoic acid, prolactin-like hormones and particularly IGF-2, a crucial modulator of placental and fetal growth33,36.
Finally, our results showing dysregulated gal-3 expression in human pregnancies affected by impaired fetal growth are consistent with the role played by this lectin in pregnancy orchestration as demonstrated in our mouse studies. Particularly, the fact that besides sharing a common placental histopathology, FGR and PE placentas differed in their pattern of regulation of gal-3 expression is of great importance. Our clinical and experimental findings demonstrated that gal-3 loss of function is not sufficient to provoke a maternal PE-like syndrome in mice, and women with both early and late-onset PE showed no alterations in gal-3 expression. Because gal-1 and gal-3 are the most prominent galectins associated with critical processes in early gestation, we hypothesize that unique properties exerted by gal-3 in supporting placental development, fetal growth, and pregnancy cannot be substituted by gal-1. Our study is clinically relevant as it provides important insights into the role of gal-3 during gestation, highlighting its requirement for proper placental development and function and fetal growth. It also reinforces the concept that unique functional properties in support of healthy pregnancy are specific to each of the different members of the placental galectin network.
Materials and methods
Mice
C57BL/6 Lgals3+/+ and Lgals3−/− mice were purchased from Jackson Laboratories. The presence of a vaginal plug after mating was denoted as embryonic day (E) 0. Timed pregnant Lgals3+/+ and Lgals3−/− mice were evaluated at E7, E10, E13, E17, P4, and P21 (n = 7–9 mice per group) and approved by Charité and Berlin authority for Animal Use in Research and Education. A second experiment used Lgals3+/+or Lgals3−/− females mated with Lgals3−/− or Lgals3+/+ males respectively to create the “paternal” (pKO) or “maternal” (mKO) gal–3 deficiency. On E13, after euthanizing the mice, the percentage of failed implantations was calculated (non-viable implantations/total implantation ×100) and whole implantation sites were frozen for histological sectioning and isolation of total protein or total RNA according to our published procedures7. Embryos were fixed in Bouin’s solution and subsequently cleared in 70% v/v ethanol for body weight measurement and Theiler stage analysis37. Placenta efficiency was calculated as “fetal/placental weight ratio” and used to measure nutrient transfer capacity from the placenta to the fetus.
Human samples
Three patient cohorts were used in this study. The prospective human low-Risk pregnancy cohort PRINCE (Prenatal Identification of Children’s Health, Ethics Committee: PV3694) is based at the university medical center in Hamburg, Germany. Since 2012 a total number of 721 women have been included during early pregnancy and examined a total of three times during the course of the pregnancy (14th, 24th, and 36th week of pregnancy). In addition to collecting demographic details and psychosocial factors, these prenatal study visits involved extensive ultrasound examinations of fetal growth and placental function, nutritional behavior, medication intake, and vaccinations during pregnancy. Blood was drawn at each of the three-time points.
For analyses of gal–3 expression in pregnancy affected only by FGR, patient samples from the research bio-bank collection at the Kolling Institute, Sydney, Australia were used. Use was approved by the Northern Sydney Local Health District Human research Ethics Committee, (St Leonards, NSW, Australia) and was assigned the site-specific assessment number 0912-348 M and the Australian national ethics application form number HREC/09/HARBR/165 as described18.
For analyses of gal–3 expression in pregnancy affected by preeclampsia, patient samples from the Oslo Pregnancy Biobank research collection at Oslo University Hospital, Oslo, approved by the Regional Committee of Medical Research Ethics in Eastern Norway, were used as described38. Circulating soluble fms-like tyrosine kinase-1 (sFlt-1) and placenta growth factor (PlGF) levels were determined by commercial ELISA from R&D Systems following the manufacturer’s recommendations. The clinical characteristics of the human cohorts are summarized in Supplementary information.
Histological analysis
Serial paraffin-embedded uterine sections from Lgals3+/+ and Lgals3−/− mice at E13 were cut into 4 μm-thick sections and stained with hematoxylin-eosin (H&E), PAS, Masson-Goldner’s trichrome, Dolichos biflorus agglutinin (DBA) lectin/PAS and Isolectin B4 as previously described39. Whole implantation sites were stained and digitally scanned by a high-resolution bright field and fluorescence slide scanner (Pannoramic MIDI BF/FL, 3DHISTECH Ltd.), and staining was evaluated on virtual slides using Pannoramic Viewer 1.15.4 (3DHISTECH Ltd.) by two examiners blinded to the pregnancy outcome.
Magnetic resonance imaging
MRI measurements for in vivo phenotype of Lgals-3-deficient mice at E13 were performed in a dedicated 7 T small animal scanner (Biospec, Bruker BioSpin, Ettlingen, Germany). The imaging protocol was adapted from a previous study by Plaks and coworkers40. Full details on the MRI protocol is provided in the Supplementary information.
Galectin-3 ELISA
Gal-3 concentrations in the serum of pregnant patients were determined by ELISA as described previously41. Briefly, immunolon 2 ELISA plates (Dynatech Laboratories, USA) were covered with polyclonal antihuman gal-3 antibody (1 μg/ml; AF1154, R&D Systems, USA) and washed with washing buffer (0.5% Tween-20 in PBS). Plates were blocked with 3% BSA in PBS. Individual wells were incubated with serial dilutions of gal-3 (1154-GA, R&D Systems, USA) or serum samples (diluted 1/10) for 2 h at RT. Wells were washed and incubated with biotinylated polyclonal antihuman gal-3 antibody (0.25 μg/ml in PBS 0.1% BSA; BAF1154, R&D Systems, USA). Plates were washed six times and incubated with horseradish peroxidase (HRP)-conjugated streptavidin (Calbiochem, USA). After eight additional washes, a colorimetric reaction was developed with the 3,3,5,5′-tetramethyl benzidine (TMB) substrate (Pierce Biotechnology, USA). The reaction was stopped by adding one volume of 4 N H2SO4. Absorbance at 450 nm was recorded. Each reported value is the mean of triplicate assays.
Gal-3 western blotting
Protein was extracted from frozen placental tissue and 10 µg of protein lysate was separated by SDS-PAGE as described previously19. Immunoblotting was performed for gal-3 (1:1000, Santa Cruz Biotechnology SC-20157) or β-actin (loading control) and protein expression was detected using the appropriate HRP-conjugated secondary antibodies (1:3000, Bio-Rad).
Galectin-3 staining in human samples
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded placental tissues from both normal and FGR pregnancies as previously described19. Slides were incubated with gal-3 (0.5 µg/ml; Santa Cruz Biotechnology sc-32790), cytokeratin 7 (0.09 µg/ml; Abcam ab68459) or isotype controls at equivalent concentrations, visualized using NovaRed peroxidase HRP substrate kit (Vector Laboratories) and staining quantitated using ImageJ.
Statistical analysis
Data are expressed as mean ± SEM. Mouse and human data were analysed using the non-parametric Mann–Whitney U-test. GraphPad Prism 8.0 (GraphPad Software, Inc.) was used to analyse the data and a P value <0.05 was considered as statistically significant.
Supplementary information
Acknowledgements
We thank P. Moschansky and G. Koch (Blois’s lab), L. Øhra Levy (Staff’s lab), and S. Mueller (Boehm-Sturm’s lab) for their excellent technical assistances in generating this work. We are grateful to Dr. Herse and Dr. Dechend for discussion and assistance with PE experiments. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) BL1115/2-1, Heisenberg Program (BL1115/3-1-BL1115/7-1) to S.M.B. MRI studies are supported by the Federal Ministry of Education and Research (grant: 01EO0801-Center for Stroke Research Berlin) and DFG, Excellence Cluster NeuroCure to P.B.S. The PRINCE study was supported by grants of the German Research Foundation within the Clinical Research Unit 296 “Feto-maternal immune cross talk” to A.D. (DI 2103/3-2) and P.C.A. (AR232/25-2). A.D. and P.C.A. would like to thank all the PRINCE participants for allowing us to study them during their pregnancy, Gudula Hansen, Mirja Pagenkemper for their support in recruitment and Thomas Andreas, Christopher Urbschat and Agnes Wiezorek for their technical assistance. Open Access Funding provided by Projekt DEAL.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by H-U Simon
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nancy Freitag, Irene Tirado-Gonzalez
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-02791-5).
References
- 1.Lees, C. C. et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet (Lond., Engl.)385, 2162–2172, 10.1016/s0140-6736(14)62049-3 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Lees, C. et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol.42, 400–408, 10.1002/uog.13190 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Bilardo, C. M. et al. Severe fetal growth restriction at 26-32 weeks: key messages from the TRUFFLE study. Ultrasound Obstet. Gynecol.50, 285–290, 10.1002/uog.18815 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Suzuki, K. The developing world of DOHaD. J. Dev. Orig. Health Dis.9, 266–269, 10.1017/s2040174417000691 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Burton, G. J. & Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol.218, S745–s761, 10.1016/j.ajog.2017.11.577 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Nawathe, A. & Lees, C. Early onset fetal growth restriction. Best. Pract. Res. Clin. Obstet. Gynaecol.38, 24–37, 10.1016/j.bpobgyn.2016.08.005 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Blois, S. M. et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med13, 1450–1457 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Than, N. G. et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta10.1016/j.placenta.2014.07.015 (2014). [DOI] [PMC free article] [PubMed]
- 9.Tirado-Gonzalez, I. et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Human Reprod.10.1093/molehr/gas043 (2013). [DOI] [PubMed]
- 10.Blois, S. M., Conrad, M. L., Freitag, N. & Barrientos, G. Galectins in angiogenesis: consequences for gestation. J. Reprod. Immun.10.1016/j.jri.2014.12.001 (2015). [DOI] [PubMed]
- 11.Yang, H., Lei, C. & Zhang, W. Expression of galectin-3 in mouse endometrium and its effect during embryo implantation. Reprod. Biomed. Online24, 116–122, 10.1016/j.rbmo.2011.09.003 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Bozic, M. et al. Galectin-1 and galectin-3 in the trophoblast of the gestational trophoblastic disease. Placenta25, 797–802 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Hu, R., Jin, H., Zhou, S., Yang, P. & Li, X. Proteomic analysis of hypoxia-induced responses in the syncytialization of human placental cell line BeWo. Placenta28, 399–407, 10.1016/j.placenta.2006.07.005 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Jeschke, U. et al. Expression of galectin-1, -3 (gal-1, gal-3) and the Thomsen-Friedenreich (TF) antigen in normal, IUGR, preeclamptic and HELLP placentas. Placenta10.1016/j.placenta.2007.06.006 (2007). [DOI] [PubMed]
- 15.Demmert, M. et al. Galectin-3 in cord blood of term and preterm infants. Clin. Exp. Immunol.167, 246–251, 10.1111/j.1365-2249.2011.04509.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarilyo, G. et al. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J. Perinatol.31, 30–32, 10.1038/jp.2010.53 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Nardozza, L. M. et al. Fetal growth restriction: current knowledge to the general Obs/Gyn. Arch. Gynecol. Obstet.286, 1–13, 10.1007/s00404-012-2330-6 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Powell, K. L. et al. Role for the thromboxane A2 receptor beta-isoform in the pathogenesis of intrauterine growth restriction. Sci. Rep.10.1038/srep28811 (2016). [DOI] [PMC free article] [PubMed]
- 19.Lee, V. H., Lee, A. B., Phillips, E. B., Roberts, J. K. & Weitlauf, H. M. Spatio-temporal pattern for expression of galectin-3 in the murine utero-placental complex: evidence for differential regulation. Biol. Reprod.58, 1277–1282, 10.1095/biolreprod58.5.1277 (1998). [DOI] [PubMed] [Google Scholar]
- 20.von Wolff, M., Wang, X., Gabius, H. J. & Strowitzki, T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol. Hum. Reprod.11, 189–194 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Yang, H. et al. The antiapoptotic effect of galectin-3 in human endometrial cells under the regulation of estrogen and progesterone. Biol. Reprod.87, 39, 10.1095/biolreprod.112.099234 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Yang, H., Lei, C. & Zhang, W. Expression of galectin-3 in mouse endometrium and its effect during embryo implantation. Reprod. biomedicine online24, 116–122 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Bojić-Trbojević, Ž. et al. Human trophoblast requires galectin-3 for cell migration and invasion. Sci. Rep.9, 2136, 10.1038/s41598-018-38374-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson, A. M. & David, A. L. Animal models of fetal growth restriction: Considerations for translational medicine. Placenta10.1016/j.placenta.2015.03.003 (2015). [DOI] [PubMed]
- 25.Clausson, B., Cnattingius, S. & Axelsson, O. Outcomes of post-term births: the role of fetal growth restriction and malformations. Obstet. Gynecol.94, 758–762 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Resnik, R. Intrauterine growth restriction. Obstet. Gynecol.99, 490–496 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Barker, D. J. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol.49, 270–283 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Yougbare, I. et al. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat. Commun.8, 224, 10.1038/s41467-017-00269-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alves, C. M. et al. Galectin-3 plays a modulatory role in the life span and activation of murine neutrophils during early Toxoplasma gondii infection. Immunobiology215, 475–485 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Chen, H. Y., Liu, F. T. & Yang, R. Y. Roles of galectin-3 in immune responses. Archivum Immunol. et. therapiae experimentalis53, 497–504 (2005). [PubMed] [Google Scholar]
- 31.Brittoli, A., Fallarini, S., Zhang, H., Pieters, R. J. & Lombardi, G. “In vitro” studies on galectin-3 in human natural killer cells. Immunol. Lett.194, 4–12, 10.1016/j.imlet.2017.12.004 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Crider-Pirkle, S. et al. Cubilin, a binding partner for galectin-3 in the murine utero-placental complex. J. Biol. Chem.277, 15904–15912 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Akison, L. K., Nitert, M. D., Clifton, V. L., Moritz, K. M. & Simmons, D. G. Review: alterations in placental glycogen deposition in complicated pregnancies: Current preclinical and clinical evidence. Placenta54, 52–58, 10.1016/j.placenta.2017.01.114 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Kuhnel, E. et al. Placental-specific overexpression of sFlt-1 alters trophoblast differentiation and nutrient transporter expression in an IUGR mouse model. J. Cell. Biochem.118, 1316–1329, 10.1002/jcb.25789 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Coan, P. M., Conroy, N., Burton, G. J. & Ferguson-Smith, A. C. Origin and characteristics of glycogen cells in the developing murine placenta. Dev. Dyn.235, 3280–3294, 10.1002/dvdy.20981 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Constancia, M. et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature417, 945–948, 10.1038/nature00819 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Barrientos, G. et al. CXCR4(+) Dendritic cells promote angiogenesis during embryo implantation in mice. Angiogenesis, 10.1007/s10456-012-9325-6 (2012). [DOI] [PubMed]
- 38.Freitag, N. et al. Interfering with Gal-1-mediated angiogenesis contributes to the pathogenesis of preeclampsia. Proc. Nat. Acad. Sci. USA.10.1073/pnas.1303707110 (2013). [DOI] [PMC free article] [PubMed]
- 39.Freitag, N. et al. Influence of relative NK-DC abundance on placentation and its relation to epigenetic programming in the offspring. Cell Death Dis. 10.1038/cddis.2014.353 (2014). [DOI] [PMC free article] [PubMed]
- 40.Plaks, V. et al. Functional phenotyping of the maternal albumin turnover in the mouse placenta by dynamic contrast-enhanced MRI. Mol. Imag. Biol.13, 481–492, 10.1007/s11307-010-0390-1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng, W., Wang, H. Y., Miyahara, Y., Peng, G. & Wang, R. F. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res.68, 7228–7236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.