Abstract
Two modes of oviparity are known in cartilaginous fishes, (1) single oviparity where one egg case is retained in an oviduct for a short period and then deposited, quickly followed by another egg case, and (2) multiple oviparity where multiple egg cases are retained in an oviduct for a substantial period and deposited later when the embryo has developed to a large size in each case. Sarawak swellshark Cephaloscyllium sarawakensis of the family Scyliorhinidae from the South China Sea performs a new mode of oviparity, which is named “sustained single oviparity”, characterized by a lengthy retention of a single egg case in an oviduct until the embryo attains a sizable length. The resulting fecundity of the Sarawak swellshark within a season is quite low, but this disadvantage is balanced by smaller body, larger neonates and quicker maturation. The Sarawak swellshark is further uniquely characterized by having glassy transparent egg cases, and this is correlated with a vivid polka-dot pattern of the embryos. Five modes of lecithotrophic (yolk-dependent) reproduction, i.e. short single oviparity, sustained single oviparity, multiple oviparity, yolk-sac viviparity of single pregnancy and yolk-sac viviparity of multiple pregnancy were discussed from an evolutionary point of view.
Subject terms: Ecology, Evolution, Zoology
Introduction
The reproductive strategies of the Chondrichthyes (cartilaginous fishes) are far more diverse than those of the other animal groups. Reproduction in chondrichthyan fishes is divided into two main modes, oviparity (egg laying) and viviparity (live bearing). Oviparity is restricted to the orders Carcharhiniformes (ground sharks), Heterodontiformes (bullhead sharks), Orectolobiformes (carpet sharks), Rajiformes (skates) and Chimaeriformes (chimaeras). Viviparity is prevailing in all chondrichthyan orders except in Heterodontiformes and Chimaeriformes whose members are all oviparous.
The morphology of members of the order Carcharhiniformes is less diverse compared to those of orders Lamniformes, Orectolobiformes and Squaliformes1, but this order is far more speciose, containing more species than in all the other eight shark orders combined. Moreover, the reproductive strategies of carcharhiniform sharks are more diverse than in any other chondrichthyan orders, having two modes of oviparity1–7 and three or four modes of viviparity6,7.
The catsharks (families Pentanchidae and Scyliorhinidae) are the largest group within the order Carcharhiniformes with about 150 species. Their reproduction is mostly oviparous, with several yolk-sac viviparous species. Among the oviparous catsharks, most of them perform single oviparity, in which one egg case is kept in each oviduct only for a short time and deposited soon after the egg case is completed. The other mode multiple oviparity is found in some catsharks, where several egg cases are retained in an oviduct for months prior to being deposited with a developing embryo in each egg case.
Recently, we found a new mode of oviparity in Sarawak swellshark Cephaloscyllium sarawakensis (family Scyliorhinidae, order Carcharhiniformes) from Taiwan that does not match the two currently known modes of oviparity, and it is herein termed “sustained single oviparity”. In addition, the egg cases of this species were found to be glassy transparent, which is quite unique among oviparous cartilaginous fishes. The main aims of present study are to describe the new mode of oviparity and transparent egg case, to propose a new set of technical terms for oviparity in the cartilaginous fishes, to discuss the importance of the new oviparity from the biological aspects of reproduction, and to refer to the evolution of lecithotrophic (yolk-dependent) reproduction in sharks.
Results
Egg cases and embryos
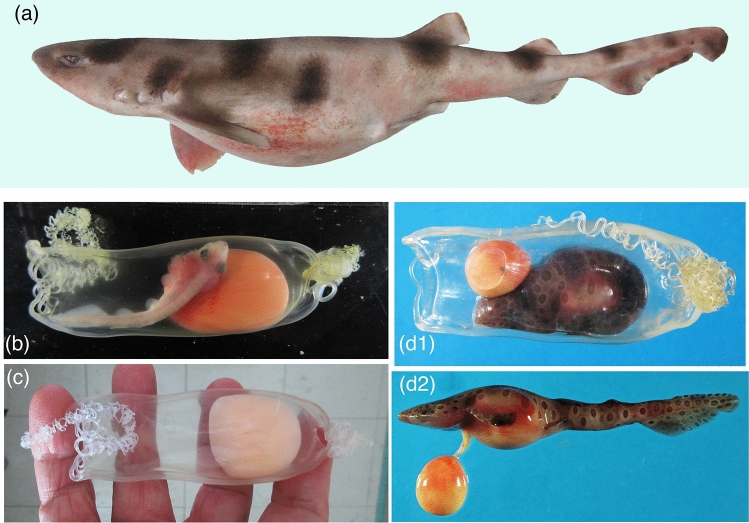
All the pregnant specimens of Sarawak swellshark Cephaloscyllium sarawakensis examined (Fig. 1a) had one egg case in each oviduct, i.e. two egg cases in each individual. Egg cases examined were 75.0‒85.9 mm in length, excluding tendrils (ECL) (16.5‒20.1% TL) and 30.0‒39.5 mm in width (7.4‒8.7% TL) (Table 3), and occupied most of the available space of the oviduct. Egg cases had a smooth surface and with tough, thick shell, roughly quadrangular in shape and rather flattened, with a truncate anterior end and a rounded posterior end (Fig. 1b‒d). The egg case was completely transparent, and the yolk and embryo (when present) were clearly visible through the casing. A very long and strong tendril was present on the horn at four corners of the egg case. There were four openings, or corner slits, one at the base of each horn. Corner slits at anterior horns were linear and long, and those at posterior horns were curved. Corner slits were closed in the egg cases without developing embryo, and were open in the egg cases with 43 mm TL and larger embryos.
Figure 1.
Cephaloscyllium sarawakensis and transparent egg cases. (a) 420 mm TL female with egg cases (NMMB-P 30853), (b) egg case and 64.8 mm TL embryo, from left oviduct, with external gills (NMMB-P30888, 81.6 mm ECL), (c) egg case from oviduct (NMMB-P30887, 79.9 mm ECL), showing transparency, (d) largest embryo 102 mm TL (NMMB-P 30991) in egg case (d1) and lateral view (d2).
Table 3.
Measurements of egg cases and embryos, and sampling data of Cephaloscyllium sarawakensis.
| NMMB-P | Mother (mm TL) | Egg case from | Embryo | Egg case length | Egg case width | Locality | Date | ||
|---|---|---|---|---|---|---|---|---|---|
| mm TL | mm ECL | %TL | mm ECW | %TL | |||||
| 30890 | 405 | Right oviduct | 0 | 80.0 | 19.8 | 30.0 | 7.4 | KTL | 2015/6/11 |
| Left oviduct | 0 | 80.0 | 19.8 | 31.0 | 7.7 | ||||
| 30883 | 440 | Right oviduct | 0 | 75.0 | 17.0 | 35.0 | 8.0 | HD | 2019/3/19 |
| Left oviduct | 0 | 75.0 | 17.0 | 34.0 | 7.7 | ||||
| 30887 | 443 | Right oviduct | 0 | 79.9 | 18.0 | 34.5 | 7.8 | HD | 2019/3/19 |
| Left oviduct | 0 | 80.4 | 18.1 | 35.7 | 8.1 | ||||
| 30965 | 455 | Right oviduct | 0 | 79.6 | 17.5 | 35.5 | 7.8 | HD | N/A |
| Left oviduct | 0 | 75.0 | 16.5 | 35.3 | 7.8 | ||||
| 30888 | 420 | Right oviduct | 61.7 | 83.0 | 19.8 | 33.0 | 7.9 | HD | 2019/3/19 |
| Left oviduct | 64.8 | 81.6 | 19.4 | 33.9 | 8.1 | ||||
| 30968 | 420 | Right oviduct | 61.5 | 82.1 | 19.5 | 36.7 | 8.7 | HD | N/A |
| Left oviduct | 68.4 | 84.4 | 20.1 | 36.4 | 8.7 | ||||
| 30884 | 435 | Right oviduct | 46.0 | 75.0 | 17.2 | 33.0 | 7.6 | HD | 2019/3/19 |
| Left oviduct | 43.0 | 76.0 | 17.5 | 36.0 | 8.3 | ||||
| 30970 | 439 | Right oviduct | 66.6 | 85.1 | 19.4 | 35.7 | 8.1 | HD | N/A |
| Left oviduct | 65.4 | 84.1 | 19.4 | 34.8 | 7.9 | ||||
| 30969 | 452 | Right oviduct | 63..3 | 80.0 | 17.7 | 33.9 | 7.5 | HD | N/A |
| Left oviduct | 64.2 | 80.2 | 17.7 | 35.3 | 7.8 | ||||
| 30967 | 455 | Right oviduct | 57.6 | 82.4 | 18.1 | 36.2 | 8.0 | HD | N/A |
| Left oviduct | 65.6 | 83.0 | 18.2 | 37.4 | 8.2 | ||||
| 30851 | 495 | Right oviduct | not collected | N/A | N/A | N/A | N/A | HD | 2019/3/19 |
| Left oviduct | 70.6 | 85.9 | 17.4 | 39.5 | 7.9 | ||||
| 30819 | Not collected | Right oviduct | 68.0 | 82.0 | N/A | 34.0 | N/A | KTL | 2019/1/11 |
| Left oviduct | not collected | N/A | N/A | N/A | N/A | ||||
| 30991 | Not collected | Right oviduct | 102 | 77.0 | N/A | 33.0 | N/A | KTL | N/A |
| Left oviduct | not collected | N/A | N/A | N/A | N/A | ||||
| 32979A | Unknown | Seabed | 94.5 | 80.0 | N/A | 30.0 | N/A | KTL | 2020/1 |
| 32979B | Unknown | Seabed | 65.0 | 80.0 | N/A | 30.0 | N/A | KTL | 2020/1 |
KTL Ketzuliao, Kaohsiung, south western Taiwan, SD Hsinda Port, Kaohsiung, south western Taiwan.
Eight egg cases from four females had only yolk, without developing embryos. The egg cases from nine females each contained a developing embryo. The largest embryo observed in the egg case (77.0 mm ECL) was 102 mm TL, with many polka-dot markings (Fig. 1d) and the smallest one was 43 mm TL without markings. External yolk was present on all these embryos.
Two already deposited egg cases were collected from trawl catch landings (Fig. 2a). One egg case (NMMB-P32979A, 80.0 mm ECL, Fig. 2a1) contained an embryo of 94.5 mm TL, and the other (NMMB-P32979B, 80.0 mm ECL, Fig. 2a2) had an embryo of 65.0 mm TL. Both embryos had dark polka-dot markings and external yolk that were clearly visible through the transparent casing. These egg cases were firmly tied to the tube of a tubeworm Paradiopatra sp. (family Onuphidae, order Eunicida) by posterior tendrils. The anterior tendrils of one egg case (NMMB-P32979A) were loosely entangled with the tube, and those of the other egg case (NMMB-P32979A) were broken and missing.
Figure 2.
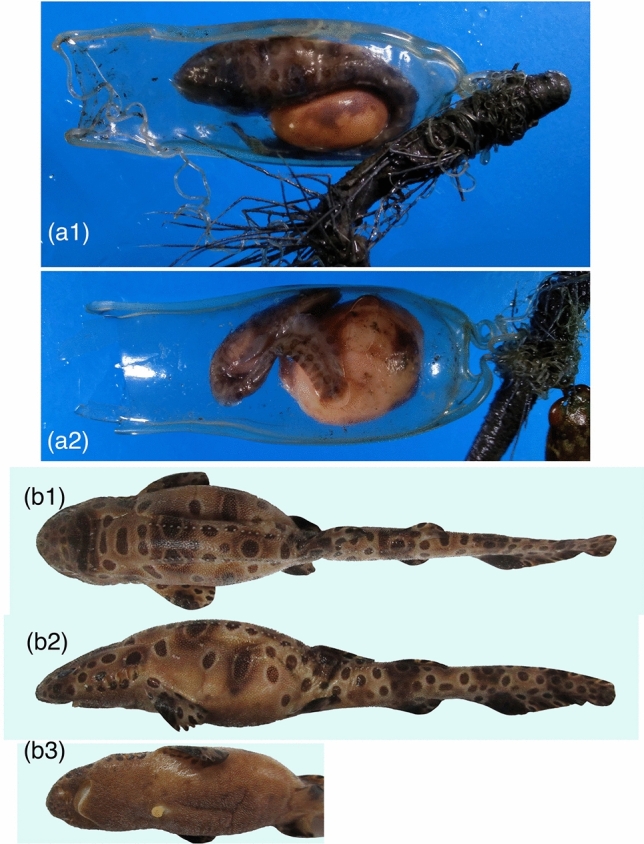
Egg case and embryo/juvenile of Cephaloscyllium sarawakensis collected by trawl net. (a) Egg cases laid on the tube of a tubeworm (a1 NMMB-P32797A, a2 NMMB-P32797B, both 80.0 mm ECL), (b) smallest juvenile (NMMB-P22719, 125 mm TL female) in dorsal (b1), lateral (b2) and latero-ventral view showing remnant of external yolk sac (b3).
Juveniles
The smallest, free-swimming specimen was a 125 mm TL female (NMMB-P22719) with a remnant of the external yolk sac (Fig. 2b). The body is light brownish with many dark polka-dot markings on lateral and dorsal surfaces of body. The second and third smallest juveniles were a 134 mm TL male (NMMB-P24872) and a 143 mm TL male (NMMB-P17143), both polka-dotted and with a reduced external yolk sac and a distinct scar.
Discussion
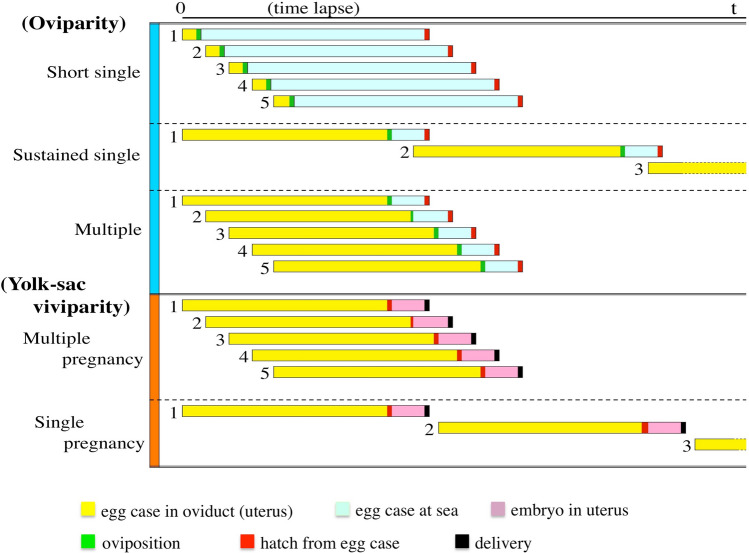
Two modes of oviparity, i.e. single and multiple oviparity, are currently recognized in chondrichthyans1–3,5–12. Single oviparity (Fig. 3a) is a mode where each oviduct in pregnant females contains one egg case, i.e. a pair of egg cases in a pregnant female. These egg cases are retained in the oviduct only for a short time and deposited immediately before the embryo has begun developing. The embryos are not recognizable at oviposition and become visible in a few weeks. Oviposition is repeatedly performed, and each mature female can deposit tens of egg cases over the course of a spawning season6 (“Short single” oviparity in Fig. 4). Multiple oviparity (Fig. 3b) is a mode where several egg cases accumulate in each oviduct and are retained for several months before oviposition, in which time embryos begin development in the oviduct and the egg cases are deposited later when the embryos grow large to a certain developmental stage (“Multiple” oviparity in Fig. 4).
Figure 3.
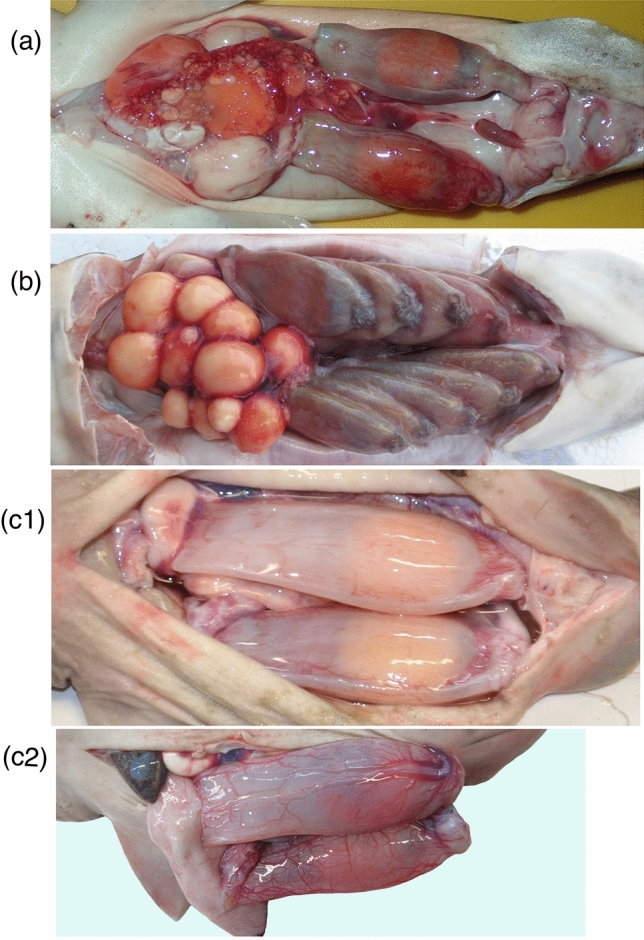
Three modes of oviparity in catsharks, showing egg cases in oviduct. (a) Short single oviparity (Galeus sauteri from Taiwan, uncatalogued), (b) multiple oviparity (Halalelurus buergeri, 410 mm TL from Kagoshima, Japan, uncatalogued), (c) Sustained single oviparity (Cephaloscyllium sarawakensis), (c1) egg cases without developing embryo (NMMB-P30890, 80.0 mm ECL), (c2) egg cases with a developing embryo in each (NMMB-P 30888, 81.6 mm, 83.0 mm ECL). Top three photographs (a,b,c1) cover whole abdominal cavities, showing difference of relative sizes of egg cases in three modes of oviparity. Cephaloscyllium sarawakensis (c1) has huge egg cases occupying most of the abdominal cavity.
Figure 4.
Schematic diagram of five modes of reproduction in catsharks, showing differences of succession, duration and condition of egg cases/ embryos per one oviduct/uterus. Numerals show order of egg case produced in the oviduct/uterus.
Cephaloscyllium sarawakensis does not fit the classic single oviparity, nor multiple oviparity. Pregnant females of this species always have a single egg case, never two or more, in each oviduct (Fig. 3c), and keep it until embryo attains a certain developmental stage (“Sustained single” oviparity in Fig. 4). These facts indicate that reproduction of C. sarawakensis represents a new mode of oviparity, which is herein termed “sustained single oviparity”. A 450 mm TL female of Cephaloscyllium silasi from the Indian Ocean had one egg case with a well-developed embryo in each oviduct13. Although they reported only one female specimen, this species possibly also displays the sustained single oviparity.
Various technical terms have been used for oviparity. Single oviparity has at least three alternative names, “extended” oviparity3,4,6,12,14, “external” oviparity5,15, and “simple” oviparity5. Multiple oviparity has been also termed “retained” oviparity3–6,12,14,16. These terms are used for the same reproductive mode, and this could lead to confusion and misunderstanding about the oviparity. Therefore, we herein summarized the terms of oviparity and proposed a new set of technical terms as follows: (1) “short single oviparity” for the single oviparity previously known; (2) “sustained single oviparity” for the new type of oviparity reported in this study; and (3) “multiple oviparity” instead of the former “retained” oviparity. The new definition of oviparity in the cartilaginous fishes was summarized, with additions of two modes of yolk-sac viviparity in catsharks (Table 1).
Table 1.
New definitions of oviparity and yolk-sac viviparity in lecithotrophic (yolk-dependent) cartilaginous fishes.
| Parity | Mode proposed | Definition | Order | Family | Genus and/or species | Sources |
|---|---|---|---|---|---|---|
| OVIPARITYa | Short single oviparity (syn. short, extended, external and simple oviparity) | One egg case per oviduct is kept for a short time, and is laid immediately before embryo develops | Carcharhiniformes | Pentanchidae | Apristurus, Asymbolus, Figaro, Haploblepharus, Holohalaelurus, Parmaturus | 1,2,37,39,56 |
| Bythaelurus bachi, B. canescens, B. dawsoni, B. naylori, B. vivaldi | 28,38,53 | |||||
| Galeus antilensis, G. arae, G. cadenati, G. eastmani, G. mincaronei, G. murinus, G. nipponensis, G.sauteri | 1,2,8,25,35,57 | |||||
| Scyliorhinidae | Atelomycterus, Cephaloscyllium (excluding C. sarawakensis and C. silasi), Poroderma, Schroederichthys, Scyliorhinus | 1,2,39 | ||||
| Proscylliidae | Proscyllium | 1,39,present paper | ||||
| Orectolobiformes | Parascylliidae | Cirrhoscyllium, Parascyllium | 4 | |||
| Hemiscylliidae | Chiloscyllium, Hemiscyllium | 4 | ||||
| Heterodontiformes | Heterodontidae | Heterodontus | 1,39,present paper | |||
| Rajiformes | All genera in Arhynchobatidae, Anacanthobatidae, Rajidae | 58 | ||||
| Chimaeriformes | all genera | |||||
| Sustained single oviparity | One egg case per oviduct is retained for a long time, and is laid after embryo grows large | Carcharhiniformes | Scyliorhinidae | Cephaloscyllium sarawakensis, C. silasi | 13,present paper | |
| Multiple oviparity (syn. retained oviparity) | Plural egg cases per oviduct are retained for a long time, and are laid after embryos grow large | Carcharhiniformes | Pentanchidae | Halaelurus boesemani, H. buergeri, H. lineatus, H. maculosus, H. natalensis, H. quagga, H. sellus | 1,25,33–35,59,60 | |
| Galeus atlanticus, G. melastomus, G. piperatus | 30,39,61 | |||||
| Orectolobiformes | Stegostomatidae | Stegostoma fasciatum | 4 | |||
| VIVIPARITYb | yolk-sac viviparity (single pregnancy) | One embryo per uterus is retained until delivery | Carcharhiniformes | Pentanchidae | Bythaelurus clevai, B. hispidus, B. lutarius, B. stewarti |
1 |
| Cephalurus cephalus | ||||||
| yolk-sac viviparity (multiple pregnancy) | Plural embryos per uterus are retained until delivery | Carcharhiniformes | Pentanchidae | Galeus polli | 1 |
aOviparous genus Aulohalaelurus (Scyliorhinidae, Carcharhiniformes) is excluded, because the mode is not determined yet.
bOnly catsharks are given.
Typically in the catsharks displaying short single oviparity, the shell of egg case is tough and thick, with two pairs of long strong tendrils on its anterior and posterior ends (Fig. 5a‒c,e,f), although tendrils are very short or replaced by fine silky materials (Fig. 5d) or absent in some species. The posterior pair of tendrils is used to attach the egg case to the substrate on the sea bottom, to pull it out from the oviduct, and coil it around the substrate, together with anterior pair of tendrils. The egg case is firmly secured on the substrate until juvenile hatches. The tendrils in the multiple oviparous species (Fig. 5f) tend to be thinner and shorter than those of the short single oviparous species. Cephaloscyllium sarawakensis of the sustained single oviparity has a thick shell and long tendrils (Fig. 1b,c,d1). The two egg cases (Fig. 2a) collected from fishery landings can be inferred to have been deposited intentionally on the tube of a tubeworm Paradiopatra sp. based on the fact that the posterior tendrils are firmly twined around it.
Figure 5.
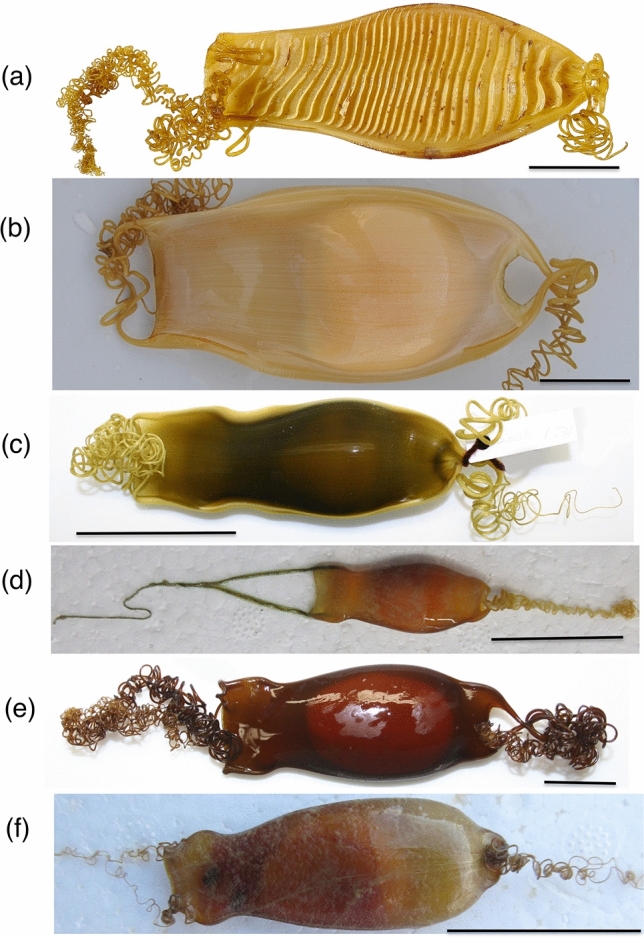
Egg cases of catsharks. (a) Cephaloscyllium laticeps from Australia, (b) Cephaloscyllium umbratile from Japan, (c) Poroderma africanum from Ibaraki Prefectural Oarai Aquarium, Japan, (d) Galeus sauteri from Taiwan, (e) Haploblepharus fuscus from Ibaraki Prefectural Oarai Aquarium, Japan, (f) Halaelurus buergeri from Japan. (a‒e) short single oviparous species, (f) multiple oviparous species. Scales 30 mm.
The egg cases in the species of sustained single oviparity are very large, with its length (ECL) ranging 16.5‒20.1% TL in Cephaloscyllium sarawakensis and 18.9‒19.2% TL in C. silasi13, while the egg cases of short single oviparous C. umbratile are 10.6‒15.1% TL2,17,18. The egg cases of other short single oviparous species are also small, with lengths 8.6‒9.0% TL in Holohalaelurus regani19, 9.2% TL in Galeus sauteripresent study, 9.2‒14.8% TL in four species of Apristurus20–23, 10.6‒14.9% TL in Atelomycterus marmoratus24, 11.4% TL in Schroederichthys maculatus25, 11.5‒11.9% TL in Scyliorhinus torazame26,presentstudy, 11.8% TL in S. capensis27, 12.0% TL in Bythaelurus dawsoni28 and 13.5‒18.9% TL from Fig. 10d29 of Parmaturus xaniurus. The egg case lengths of multiple oviparous species are about 11% TL in Halaelurus buergeripresent study and 10.4‒10.9% TL in H. quagga30. As seen in Fig. 3, the egg cases of C. sarawakensis (Fig. 3c1) are far larger than those of G. sauteri (Fig. 3a) and H. buergeri (Fig. 3b), occupying most of the available space of oviduct and abdominal cavity. Thus, the two species of sustained single oviparity have much larger egg cases than short single oviparous and multiple oviparous species, suggesting larger neonates at hatching in C. sarawakensis and C. silasi.
The embryos in the egg cases recorded from the oviduct indicate that Cephaloscyllium sarawakensis retains the egg case until the embryo attains about 102 mm TL (Fig. 1d; largest embryo in the egg inside the oviduct), and then oviposition occurs later. However, the two egg cases (both 80.0 mm ECL) from the seabed contained a 65 mm TL and a 94.5 mm TL embryo each (Fig. 2a). These facts suggest the timing of oviposition is rather wide in this species, and the egg cases are laid when the embryo grows roughly 6‒10 + cm TL, stimulated by some internal or external factors. The smallest free-swimming juvenile collected was 125 mm TL with a remnant of external yolk-sac (Fig. 2b), suggesting the hatching size from egg case being around 120 mm TL in this species. These evidences indicate C. sarawakensis keeps the egg case in the oviduct until embryo has developed to 50 ~ 80+ % of its hatching size. Therefore, the retention of egg case in the oviduct continues for an extended period, perhaps several months or more, in C. sarawakensis and probably also in C. silasi.
The other remarkable characteristic of Cephaloscyllium sarawakensis is the glassy transparent egg cases (Fig. 1b‒d), and the transparency is completely maintained even after oviposition (Fig. 2a). The egg cases of oviparous cartilaginous fishes are opaque, usually yellowish to dark brownish (Fig. 5), and sometimes with longitudinal or transverse ridges (Fig. 5a). The functional role of egg case is to protect the embryo from physical, physiological and biological hazards from the environment. The colored egg cases can be also effective to conceal the embryo in it. However, the egg case of C. sarawakensis is transparent and never cryptic that the orange yellow yolk would clearly be recognizable through transparent egg case, if the egg case is deposited immediately after egg case being formed.
As shown in this study, C. sarawakensis retains an egg case in each oviduct until the embryo is developed with a distinct dark polka-dot color pattern on light brownish body (Figs. 1d, 2a), typically seen in the juveniles (Fig. 2b). One of the reasons for transparent egg case may be related to their vivid body color patterns. Benthic and reef-dwelling sharks have complicated color patterns, which are effective to blend the body into their background or for camouflage31. The present egg cases of C. sarawakensis (Fig. 2a) were deposited around the tube of a tubeworm sticking out from the seabed. Their vivid polka-dots and light brownish body coloration could function as more effective camouflage against the complex and dark background through the transparent egg case. Thus, the long retention of transparent egg cases and vivid embryonic coloration could suggest a new method of reproductive tactics in cartilaginous fishes.
The oviparity is advantageous as a method to increase the fecundity in small elasmobranchs that have limited space in body cavity for care and storage of the embryos6. The species of short single oviparity (Fig. 3a) repeatedly deposits two egg cases immediately after the cases are completed and laid, resulting in 20‒100 eggs per season6. The captive Cephaloscyllium umbratile was recorded as depositing two egg cases at intervals of 11‒38 days (20 days in average) for whole year32, which means a single female deposited about 36 egg cases a year. Similarly, the captive C. laticeps laid two egg cases at intervals of up to 28 days throughout whole year11, equating more than 26 egg cases being deposited annually.
The multiple oviparous species retains a number of egg cases in each oviduct (Fig. 3b) for several months until the embryos have developed to a certain stage. All the species of Halaelurus are multiple oviparous, and H. buergeri has been recorded to deposit 8 egg cases one by one at a stage when the embryos inside have attained 70 mm TL33, or 10 egg cases at one time34. One specimen of H. buergeri we (KN) collected (Fig. 3b) had 10 egg cases in the oviducts with a developing embryo in each. A captive H. maculosus deposited 11 egg cases containing 50‒70 mm TL embryos in five days (personal communication with Mr. K. Tokunaga of Ibaraki Prefectural Oarai Aquarium). The fecundity of H. lineatus is up to 16 eggs at a time35.
The maternal environment offers the best protection to the developing embryos and can shorten the exposed period of time on the substrate. Therefore, the survival rate could be expected to be much higher in the sustained single and multiple oviparous species than the short single oviparous species. The species of the sustained single oviparity (Fig. 3c) and those of the multiple oviparity (Fig. 3b) are the same in that the egg cases have long maternal protection, but the number of the egg cases deposited at a time is considerably less in the sustained single oviparous species, i.e. 2 eggs vs. 4‒16 eggs per mother, respectively. Hence, the fecundity of Cephaloscyllium sarawakensis could be very low, 1/8–1/2 of the multiple oviparous species (see “Oviparity” in Fig. 4).
It is crucial to produce a certain number of offspring to maintain a sustainable population, and the very low fecundity in C. sarawakensis could be decisively disadvantageous for the species. Similar issues exist in the yolk-sac viviparous species. The yolk-sac viviparous catsharks, such as Bythaelurus clevai, B. hispidus, B. lutarius, B. stewarti and Cephalurus cephalus retain only one embryo per uterus or per mother1,28,36–38,KN pers.obs, which is hence termed here “single pregnancy”. These species of single pregnancy would have also lower fecundity than the species of “multiple pregnancy” seen in Galeus polli (see “Yolk-sac viviparity” in Fig. 4).
Species of the genus Cephaloscyllium are generally large in body sizes, mostly growing to more than 70 cm TL and some species (C. isabellum, C. laticeps, and C. umbratile) attain more than 100 cm TL39, whereas C. sarawakensis and C. silasi are dwarf species within the genus. Cephaloscyllium sarawakensis attains a maximum of only 39.7 cm TL in males and 49.5 cm TL in females40,present study, and matures at the sizes less than 32.5 cm TL and 35.4 cm TL in males and females, respectively41. Similarly, Cephaloscyllium silasi attains only 50 cm TL13 and reaches its maturity at less than 36.8 cm TL in males1 and less than 45 cm TL in females13. Bythaelurus clevai, B. hispidus and B. lutarius attain 42 cm TL, 36 cm TL and 39 cm TL, respectively37,39 and these species are also the smallest species for the genus. Cephalurus cephalus reaches 30 cm TL36,39, and this is known as one of the smallest sharks1.
The ratio of length at maturity to the largest total length was reported at around 0.73 for elasmobranchs42, and this indicates the smaller species could reach their maturity at smaller sizes than the larger species. Actually, the captive Cephaloscyllium umbratile which grows up to 118 cm TL hatches at lengths of 16–22 cm TL32 and attains its full maturity at 96‒104 cm TL17. In contrast, C. sarawakensis which produces very large egg cases relative to the mother size is expected to hatch at about 12 cm TL and mature at less than 35 cm TL. Therefore, C. sarawakensis grows only about 23 cm in length until maturity is attained, whereas C. umbratile needs to grow 75–90 cm to its maturity. Cephalurus cephalus produces 7–9 cm TL neonates and attains maturity at 18–22 cm TL43, thus 9–15 cm in length to grow to attain maturity. Eridacnis radcliffei gives birth 10.5‒12.8 cm TL neonates36, and attains maturity at 18.3 cm TL, only 5.5‒7.8 cm in length to the maturity.
Hence, these dwarf sharks of the sustained single oviparity and the yolk-sac viviparity of single pregnancy likely attain their maturity in a shorter time frame than the larger species do, enabling them to reproduce at an earlier age and keep their life-time fecundity high. Other factors to increase their lifetime fecundity include quick repetition of reproduction, longer lifetime reproduction and higher proportion of females, but these will not be referred to here.
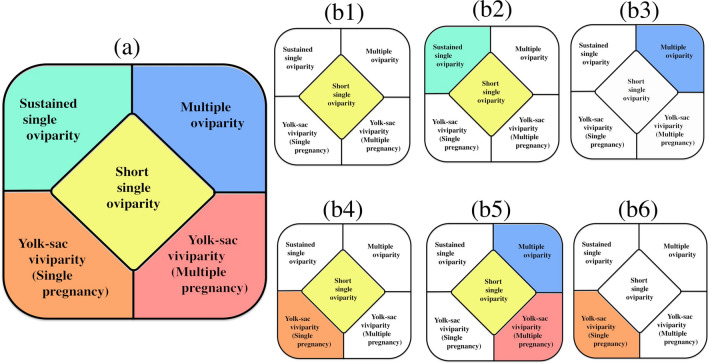
Figure 6a shows five modes of lecithotrophic (yolk-dependent) reproduction in the cartilaginous fishes, and Fig. 6b1–6 denote six combinations of these reproductive modes in the catsharks. Figure 6b1 (short single oviparity) represents ten genera such as Apristurus, Asymbolus, Atelomycterus, Figaro, Haploblepharus, Holohalaelurus, Parmaturus, Poroderma, Scyliorhinus and Schroederichthys. Figure 6b3 (multiple oviparity) and Fig. 6b6 (yolk-sac viviparity of single pregnancy) are Halaelurus and Cephalurus, respectively. However, Fig. 6b2,b4,b5 include two or three modes of reproduction. Cephaloscyllium (Fig. 6b2) performs short single oviparity + sustained single oviparity, Bythaelurus (Fig. 6b4) involves short single oviparity + yolk-sac viviparity of single pregnancy, and Galeus (Fig. 6b5) performs short single oviparity + multiple oviparity + yolk-sac viviparity of multiple pregnancy. These facts could suggest rather facile diversification of reproductive mode in closely related species, or may suggest necessity of some taxonomic reconsideration of them, as indicated for Bythaelurus38.
Figure 6.
Modes of reproduction (a) and combination of the modes at generic level in catsharks (b). (a) Five modes of lecithotrophy (yolk-dependent reproduction) in cartilaginous fishes, (b1) Apristurus, Asymbolus, Atelomycterus, Figaro, Haploblepharus, Holohalaelurus, Parmaturus, Poroderma, Scyliorhinus and Schroederichthys, (b2) Cephaloscyllium, (b3) Halaelurus, (b4) Bythaelurus, (b5) Galeus, and (b6) Cephalurus.
Oviparity has been suggested to be the ancestral mode of reproduction for vertebrates7,44, and it has also traditionally been believed as the ancestral mode for chondrichthyan fishes3,14,45. However, recent studies5,6,12,46,47 suggest that viviparity is ancestral for all chondrichthyans, with many reversions to oviparity and secondary reversions to viviparity. Phylogenetic interrelationships for the Galeomorphi (orders Heterodontiformes, Orectolobiformes, Lamniformes and Carcharhiniformes)4,45,48‒50 show that short single oviparity is the ancestral mode for the Galeomorphi, and also for the orders Heterodontiformes, Orectolobiformes and Carcharhiniformes. Multiple oviparity is generally considered to have evolved from short single oviparity5, or evolved intermediately between the short single oviparity and the yolk-sac viviparity1,7,14,51.
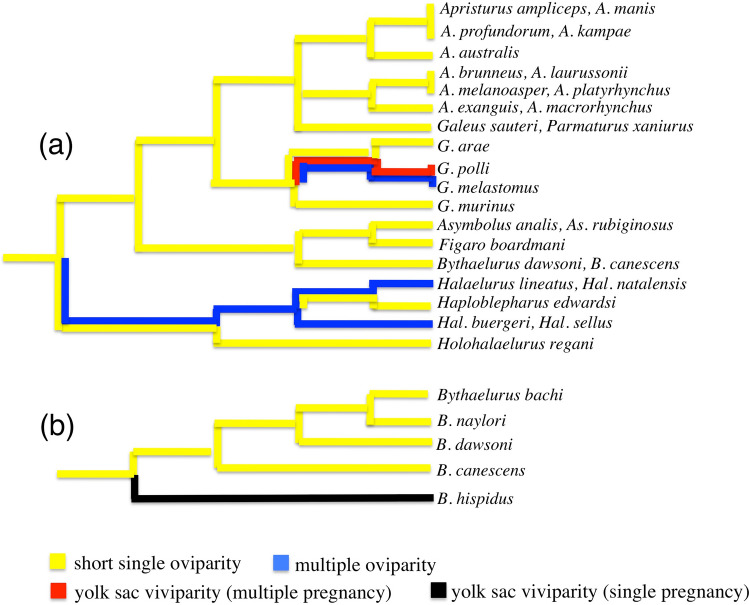
The catsharks (now Pentanchidae and Scyliorhinidae) in the Carcharhiniformes are separated into a few isolated groups, based on genetic works49,50,52. Mapping of the reproductive modes on their phylogenetic relationships suggests that the short single oviparity is ancestral for each group. According to the phylogenetic result50 which covers more catshark taxa than the other works, their Scyliorhinidae I50 (Fig. 7a) includes nine genera of the family Pentanchidae. Six genera of these, i.e. Apristurus, Asymbolus, Figaro, Haploblepharus, Holohalaelurus and Parmaturus, display short single oviparity. The multiple oviparous genus Halaelurus is sister to the groups of short single oviparous catsharks, and the relationships suggest the multiple oviparity has derived from the short single oviparity.
Figure 7.
Reproductive modes mapped on simplified relationship of (a) Scyliorhinidae I50 and (b) Bythaelurus53, and suggested evolution.
The genus Bythaelurus, which is also deeply merged in groups of short single oviparous species in the Scyliorhinidae I50 (Fig. 7a), is currently comprised of 14 species38,53,54, with five short single oviparous species (B. bachi, B. canescens, B. dawsoni, B. naylori and B. vivaldi) and four yolk-sac viviparous species of single pregnancy (B. clevai, B. hispidus, B. lutarius and B. stewarti) (Fig. 6b4). Interrelationships of five Bythaelurus species53 (Fig. 7b) show they are clearly separable in two groups, i.e. short single oviparous species (B. bachi, B. naylori, B. dawsoni and B. canescens) and yolk-sac viviparous species of single pregnancy (B. hispidus). The short single oviparous B. dawsoni and B. canescens have a sister relation with short single oviparous genera Asymbolus + Figaro in the Scyliorhinidae I50 (Fig. 7a). These facts suggest that the short single oviparity is ancestral for Bythaelurus and the yolk-sac viviparity of single pregnancy could have derived from the short single oviparity1, maybe via sustained single oviparity.
The genus Galeus contains 18 species with three reproductive modes (Fig. 6b5), i.e. short single oviparity (G. antillensis and seven other species), multiple oviparity (G. atlanticus, G. melastomus and G. piperatus) and yolk-sac viviparity of multiple pregnancy (G. polli). The genus Galeus is deeply embedded within groups of short single oviparous species in the Scyliorhinidae I50 (Fig. 7a), and the short single oviparity is considered to be ancestral for the genus Galeus, and the yolk-sac viviparity of multiple pregnancy in G. polli could have derived from short single oviparity via multiple oviparity.
Their Scyliorhinidae II50 includes three genera Atelomycterus, Schroederichthys of short single oviparity and oviparous Aulohalaelurus55. Scyliorhinidae III50 includes three genera Cephaloscyllium, Scyliorhinus and Poroderma, and they all display short single oviparity. Cephaloscyllium sarawakensis and C. silasi of sustained single oviparity were not treated in their analysis50, but the relationships of Cephaloscyllium in the Scyliorhinidae III50 that is composed of short single oviparous species could suggest derivation of the sustained single oviparity directly from short single oviparity by longer retention of one egg case in an oviduct.
The modes of reproduction in the catsharks were summarized in Table 2, and the phylogenetic evidences mentioned above suggest: (1) short single oviparity is ancestral for the catsharks; (2) more diverse modes of reproduction evolved in the family Pentanchidae than family Scyliorhinidae; (3) sustained single oviparity in Cephaloscyllium was derived directly from short single oviparity; (4) multiple oviparity in Halaelurus was derived from short single oviparity; (5) multiple oviparity in Galeus was derived from short single oviparity, and originated yolk-sac viviparity of multiple pregnancy; (6) yolk-sac viviparity of single pregnancy in Bythaelurus was derived from short single oviparity, possibly via sustained single oviparity; and (7) yolk-sac viviparity of single pregnancy in Cephalurus was derived possibly from short single oviparity via sustained single oviparity.
Table 2.
Modes of reproduction and evolution in catsharks.
| Parity | Mode | Pentanchidae | Scyliorhinidae | |||||
|---|---|---|---|---|---|---|---|---|
| Apristurus, Asymbolus, Figaro, Haploblepharus, Holohalaelurus, Parmaturus | Bythaelurus | Galeus | Halaelurus | Cephalurus | Atelomycterus, Poroderma, Scyliorhinus, Schroederichthys | Cephaloscyllium | ||
| Oviparitya | Short single | ⦿ | ⦿ | ⦿ | ○ | ○ | ⦿ | ⦿ |
| Sustained single | ○ | ○ | ⦿ | |||||
| Multiple | ⦿ | ⦿ | ||||||
| Yolk sac viviparity | Single pregnancy | ⦿ | ⦿ | |||||
| Multiple pregnancy | ⦿ | |||||||
⦿Actual mode, ○ possible ancestral or transitional mode.
aOviparous Aulohalaelurus in the Scyliorhinidae is not included, because the mode is unknown.
Materials and methods
All specimens of Cephaloscyllium sarawakensis examined were bycatch from commercial bottom trawlers operating in the South China Sea off southwest Taiwan, and were collected at Hsin-da port (HD) and Ke-tzu-liao (KTL) in Kaohsiung. Specimens were fixed in 4% formalin and then transferred to 70% Ethanol or 50% Isopropanol ethanol. All specimens were deposited at Pisces collection of the National Museum of Marine Biology & Aquarium, Pingtung, Taiwan (NMMB-P). Total length (TL) was measured using a ruler or digital caliper, to nearest 1 or 0.1 mm, respectively.
Egg cases (Table 3): a total of 8 egg cases without visible embryo on the yolk, and 15 egg cases with a developed embryo each were collected from the oviduct of thirteen specimens of C. sarawakensis. Two egg cases with a developed embryo tied on the tube of a tubeworm Paradiopatra sp. (family Onuphidae, order Eunicida) were collected from the fishery landings by fishers, and were kept frozen. Egg cases were deposited and catalogued in NMMB-P collection. Length (excluding tendrils, ECL) and width (ECW) of the egg case were measured by a ruler or digital caliper.
Juveniles: NMMB-P22719, 125 mm TL female, KTL, 2 Apr. 2015; NMMB-P17143, 143 mm TL male; NMMB-P24872 (1 of 6 specimens), 134 mm TL male, KTL, 18 Mar. 2016.
Acknowledgements
This study is supported by the Ministry of Science and Technology, Executive Yuan, Taiwan and the National Museum of Marine Biology and Aquarium, Pingtung, Taiwan. We thank Kun-Hsuan Lee for identification of the tubeworm; Chao-Chan Huang, Yu-Yun Hsu, Yu-Tsung Hsu and Ying-Min Huang for collecting specimens for this study; Kazuya Kofuji and Kohtaro Tokunaga at Ibaraki Prefectural Oarai Aquarium for photographs of egg cases of Poroderma africanum and Haploblepharus fuscus, and reproductive information of Halaelurus buergeri and H. maculosus; Rou-Rong Chen at National Museum of Marine Biology & Aquarium for curatorial assistance; Kouichi Hoshino for providing literature information.
Author contributions
K.N. collected the data, developed the study concept and wrote the initial manuscript. W.T.W. and H.C.H. interpreted the data, reviewed the manuscript and contributed to the discussion and improvement of the manuscript. W.T.W. checked English expression. All authors reviewed the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Compagno LJV. Sharks of the Order Carcharhiniformes 1–486. Princeton: Princeton University Press; 1988. [Google Scholar]
- 2.Nakaya K. Taxonomy, comparative anatomy and phylogeny of Japanese catsharks, Scyliorhinidae. Mem. Fac. Fish. Hokkaido Univ. 1975;23:1–94. [Google Scholar]
- 3.Dulvy NK, Reynolds JD. Evolutionary transitions among egg-laying, live-bearing and maternal inputs in sharks and rays. P. R. Soc. Lond. B. 1997;264:1309–1315. [Google Scholar]
- 4.Goto T. Comparative anatomy, phylogeny and cladistic classification of the order Orectolobiformes (Chondrichthyes, Elasmobranchii) Mem. Grad. Sch. Fish. Sci. Hokkaido Univ. 2001;48:1–100. [Google Scholar]
- 5.Musick JA, Ellis JK. Reproductive evolution of Chondrichthyes. In: Hamlett WC, editor. Reproductive Biology and Phylogeny of Chondrichthyes. Beijing: Science Publishers; 2005. pp. 45–79. [Google Scholar]
- 6.Conrath CL, Musick JA. Reproductive biology of elasmobranchs. In: Musick JC, Heithaus MR, editors. Biology of Sharks and Their Relatives Carrier. Boca Raton: CRC Press; 2012. pp. 291–311. [Google Scholar]
- 7.Lode T. Oviparity or viviparity? That is the question. Reprod. Biol. 2012;12:259–264. doi: 10.1016/j.repbio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Ebert DA, Compagno LJV, Cowley PD. Reproductive biology of catsharks (Chondrichthyes: Scyliorhinidae) of the west coast of southern Africa. ICES J. Mar. Sci. 2006;63:1053–1065. [Google Scholar]
- 9.Parsons GR, Hoffmayer ER, Frank J, Bet-Sayad W. A review of shark reproductive ecology: Life historyy and evolutionary implications. In: Rocha MJ, Arukwe A, Kapoor BG, editors. Fish Reproduction. Beijing: Science Publishers; 2007. pp. 435–469. [Google Scholar]
- 10.Rodda KR, Seymour RS. Functional morphology of embryonic development in the Port Jackson shark Heterodontus portusjacksoni (Myer) J. Fish. Biol. 2008;72:961–984. [Google Scholar]
- 11.Awruch CA, Pankhurst NW, Frusher SD, Stevens JD. Reproductive seasonality and embryo development in the draughtboard shark Cephaloscyllium laticeps. Mar. Freshw. Res. 2009;60:1265–1272. [Google Scholar]
- 12.Awruch, C. A. Reproduction strategies. In Physiology of Elasmobranch Fishes. Fish Physiology, vol. 34A (eds. Shadwick, R. E., Farrell, A. P. & Brauner, C. J.) 255‒310 (2016).
- 13.Akhilesh KV, Bineesh KK, Mishra SS, Ganga U, Pillai NGK. Notes on the Indian swellshark, Cephaloscyllium silasi (Scyliorhinidae: Carcharhiniformes) from deep waters off the west coast of India. Mar. Biodiv. Rec. 2014;7(e25):1–5. [Google Scholar]
- 14.Compagno LJV. Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes. 1990;28:33–75. [Google Scholar]
- 15.White WT. Aspects of the biology of carcharhiniform sharks in Indonesian waters. J. Mar. Biol. Assoc. UK. 2007;87:1269–1276. [Google Scholar]
- 16.Costa ME, Erzini K, Borges TC. Reproductive biology of the blackmouth catshark, Galeus melastomus (Chondrichthyes: Scyliorhinidae) o¡ the south coast of Portugal. J. Mar. Biol. Assoc. UK. 2005;85:1173–1183. [Google Scholar]
- 17.Taniuichi T. Aspects of reproduction and food habits of the Japanese swellshark Cephaloscyllium umbratile from Choshi, Japan. Nippon Suisan Gakkaishi. 1988;54:627–633. [Google Scholar]
- 18.Horie T, Tanaka S. Reproduction and food habits of the Japanese swellshark, Cephaloscyllium umbratile (Family Scyliorhinidae) in Suruga Bay, Japan. J. Sch. Mar. Sci. Tech. Tokai Univ. 2002;53:89–109. [Google Scholar]
- 19.Richardson AJ, et al. Abundance, distribution, morphometrics, reproductive aspects and diet of the catshark Holohalaelurus regani. J. Fish Biol. 2000;56:552–576. [Google Scholar]
- 20.Nakaya K, Sato K. Taxonomic review of Apristurus laurussonii (Saemundsson, 1922) from the eastern North Atlantic (Elasmobranchii: Scyliorhinidae) Cybium. 1998;22:149–157. [Google Scholar]
- 21.Iglesias SP, Nakaya K, Stehmann M. Apristurus melanoasper, a new species of deep-water catshark from the North Atlantic (Chondrichthyes: Carcharhiniformes: Scyliorhinidae) Cybium. 2004;28:345–356. [Google Scholar]
- 22.Kawauchi J, Sasahara R, Sato K, Nakaya K. Occurrence of the deep-water catsharks Apristurus platyrhynchus and A. pinguis in the Indian and Western South Pacific Oceans (Carcharhiniformes: Scyliorhinidae) In: Last PR, White WT, Pogonoski JJ, editors. Descriptions of New Australian Chondrichthyans. Canberra: CSIRO; 2008. pp. 75–91. [Google Scholar]
- 23.Sato K, Nakaya K, Yorozu M. Apristurus australis sp. nov., a new long-snout catshark (Chondrichthyes: Carcharhiniformes: Scyliorhinidae) from Australia. In: Last PR, White WT, Pogonoski JJ, editors. Descriptions of New Australian Chondrichthyans. Canberra: CSIRO; 2008. pp. 113–121. [Google Scholar]
- 24.Bor PHF, van Oijen MJP, Magenta C. The egg capsule of the coral cat shark, Atelomycterus marmoratus (Bennett, 1830) (Chondrichthyes: Scyliorhinidae) Zool. Med. Leiden. 2003;77:325–330. [Google Scholar]
- 25.Springer S. A revision of the catsharks, Family Scyliorhinidae. NOAA Tech. Rep. NMFS Circ. 1979;422:1–152. [Google Scholar]
- 26.Shirai S, Hagiwara S, Nakaya K. Scyliorhinus tokubee sp. nov. from Izu Peninsula, southern Japan (Scyliorhinidae, Elasmobranchii) Jpn. J. Ichthyol. 1992;39:9–16. [Google Scholar]
- 27.Soares KDA, de Carvalho MR. The catshark genus Scyliorhinus (Chondrichthyes: Carcharhiniformes: Scyliorhinidae): Taxonomy, morphology and distribution. Zootaxa. 2019;4601:1–147. doi: 10.11646/zootaxa.4601.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Francis MP. Distributuion and bilogy of the New Zealand endemic catshark Halaelurus dawsoni. Env. Biol. Fishes. 2006;64:295–306. [Google Scholar]
- 29.Flammang BE, Ebert DA, Cailliet GM. Egg cases of the genus Apristurus (Chondrichthyes: Scyliorhinidae): Phylogenetic and ecological implications. Zoology. 2007;110:308–317. doi: 10.1016/j.zool.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Akhilesh KV, Bineesh KK, Shanis CPR, Human BA, Ganga U. Rediscovery and description of the quagga shark, Halaaelurus quagga (Alcock, 1899) (Chondrichthyes: Scyliorhinidae) from the southwest coast of India. Zootaxa. 2011;2781:40–48. [Google Scholar]
- 31.Heithaus MR. Predator-prey interactions. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives. Boca Raton: CRC Press; 2004. pp. 487–521. [Google Scholar]
- 32.Hagiwara S. Keeping and reproduction of chondrichthyans in captivity at Shimoda Floating Aquarium. Rep. Jpn. Soc. Elasmobranch Stud. 1993;30:1–18. [Google Scholar]
- 33.Kudo S. Studies on the sexual maturation of female and on the embryo of Japanese dog fish Halaelurus buergeri (Muller ete Henle) Rep. Nankai Reg. Fish. Res. Lab. 1959;11:41–45. [Google Scholar]
- 34.Makihata N. Development and nutrition in embryo and young in oviparous blackspotted dogfish Halaelurus buergeri (Muller et Henle) Rep Jpn. Soc. Elasmobranch Stud. 1984;18:12–25. [Google Scholar]
- 35.Bass AJ. Scyliorhinidae. In: Smith MM, Heemstra PC, editors. Smith’s Sea Fishes. Berlin: Springer; 1986. pp. 88–95. [Google Scholar]
- 36.Akhilesh KV, Bineesh KK, White WT, Pillai NG. Aspects of the biology of the pygmy ribbontail catshark Eridacnis radcliffei (Proscylliidae, Carcharhiniformes) from the south-west coast of India. J. Fish Biol. 2012;81:1138–1144. doi: 10.1111/j.1095-8649.2012.03379.x. [DOI] [PubMed] [Google Scholar]
- 37.Akhilesh KV, White WT, Bineesh KK, Ganga U, Pillai NG. Biological observations on the bristly catshark Bythaelurus hispidus from deep waters off the south-west coast of India. J. Fish Biol. 2013;82:1582–1591. doi: 10.1111/jfb.12087. [DOI] [PubMed] [Google Scholar]
- 38.Weigmann S, Kaschner CJ, Thiel R. A new microendemic species of the deep-water catshark genus Bythaelurus (Carcharhiniformes, Pentanchidae) from the northwestern Indian Ocean, with investigations of its feeding ecology, generic review and identification key. PLoS One. 2018;13:e0207887. doi: 10.1371/journal.pone.0207887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebert DA, Fowler S, Compagno L. Sharks of the World. Plymouth: Wild Nature Press; 2013. pp. 1–528. [Google Scholar]
- 40.Nakaya K, Inoue S, Ho HC. A review of the genus Cephaloscyllium (Chondrichthyes: Carcharhiniformes: Scyliorhinidae) from Taiwanese waters. Zootaxa. 2013;3752:101–129. doi: 10.11646/zootaxa.3752.1.8. [DOI] [PubMed] [Google Scholar]
- 41.Yano, K. et al. Sharks and Rays of Malaysia and Brunei Darussalam 1‒530 (SEAFDEC-MFRDMD, 2005).
- 42.Frisk MG, Miller TJ, Fogarty MJ. Estimation and analysis of biological parameters in elasmobranch fishes: A comparative life history study. Can. J. Fish. Aquatic Sci. 2001;58:969–981. [Google Scholar]
- 43.Aguirre-Villasenor H, Salas-Singh C. New records of the lollipop catshark Cephalurus cephalus (Scyliorhinidae) from the Gulf of California, Mexico. Rev. Mex. Biodiversid. 2012;83:298–300. [Google Scholar]
- 44.Blackburn DG. Viviparity and oviparity: Evolution and reproductive strategies. In: Knobil TE, Neill JD, editors. Encyclopedia of Reproduction. London: Academic Press; 1999. pp. 994–1003. [Google Scholar]
- 45.Wourms JP. Reproduction and development in chondrichthyan fishes. Am. Zool. 1977;17:379–410. [Google Scholar]
- 46.Blackburn DG. Evolution of vertebrate viviparity and specializations for fetal nutrition: A quantitative and qualitative analysis. J. Morph. 2015;276:961–990. doi: 10.1002/jmor.20272. [DOI] [PubMed] [Google Scholar]
- 47.Grogan ED, Lund R, Greenfest-Allen E. The origin and relationships of early chondrichthyans. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives. New York: CRC Press; 2012. pp. 3–29. [Google Scholar]
- 48.Heinicke MP, Naylor GJP, Hedges SB. Cartilaginous fishes (Chondrichthyes) In: Hedges SB, Kumar S, editors. Timetree of Life. Oxford: Oxford University Press; 2009. pp. 320–327. [Google Scholar]
- 49.Vélez-Zuazo X, Agnarsson I. Shark tales: A molecular species-level phylogeny of sharks (Selachimorpha, Chondrichthyes) Mol. Phyl. Evol. 2011;58:207–217. doi: 10.1016/j.ympev.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Naylor GJP, et al. Elasmobranch phylogeny: A mitochondrial estimate based on 595 species. In: Carrier JC, Musick JA, Heithaus MR, et al., editors. Biology of Sharks and Their Relatives. New York: CRC Press; 2012. pp. 31–56. [Google Scholar]
- 51.Carrier JC, Pratt HL, Jr, Castro JI. Reproductive biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of Sharks and Their Relatives. Boca Raton: CRC Press; 2004. pp. 269–286. [Google Scholar]
- 52.Iglesias SP, Lecointre G, Sellos DY. Extensive paraphylies within sharks of the order Carcharhiniformes inferred from nuclear and mitochondrial genes. Mol. Phyl. Evol. 2005;34:569–583. doi: 10.1016/j.ympev.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 53.Weigmann S, Ebert DA, Clerkin PJ, Stehmann MFW, Naylor GJP. Bythaelurus bachi n. sp., a new deep-water catshark (Carcharhiniformes, Scyliorhinidae) from the southwestern Indian Ocean, with a review of Bythaelurus species and a key to their identification. Zootaxa. 2016;4208:401–432. doi: 10.11646/zootaxa.4208.5.1. [DOI] [PubMed] [Google Scholar]
- 54.Weigmann S, Kaschner CJ. Bythaelurus vivaldii, a new deep-water catshark (Carchrhiniformes, Scyliorhinidae) from the northwestern Indian Ocean off Somalia. Zootaxa. 2017;4263:97–119. doi: 10.11646/zootaxa.4263.1.4. [DOI] [PubMed] [Google Scholar]
- 55.Cavanagh RD, Kyne PM, Fowler SL, Musick JA, Bennett MB. The Conservation Status of Australasian Chondrichthyans. Rep. IUCN Shark Specialist Group Australia and Oceania Regional Red List Workshop. Queensland: University of Queensland; 2003. [Google Scholar]
- 56.Kyne PM, Courtney AJ, Bennett MB. Observations on the reproductive biology of three catsharks (Carcharhiniformes: Scyliorhinidae: Asymbolus and Figaro) from the continental shelf of southern Queensland, Australia. J. Mar. Biol. Assoc UK. 2011;91:1157–1164. [Google Scholar]
- 57.Konstantinou H, McEachran JD, Woolley JB. The systematics and reproductive biology of the Galeus area subspecific complex (Chondrichthyes: Scyliorhinidae) Env. Biol. Fishes. 2000;57:117–129. [Google Scholar]
- 58.Ishihara H, Treloar M, Bor PHF, Senou H, Joong CH. The comparative morphology of skate egg capsules (Chondrichthyes: Elasmobranchii: Rajiformes) Bull. Kanagawa Prefect. Mus. Nat. Sci. 2012;41:17–33. [Google Scholar]
- 59.White WT, Last PR, Stevens JD. Halaelurus maculosus n. sp. and H. sellus n. sp., two new species of catshark (Carcharhiniformes: Scyliorhinidae) from the Indo-West Pacific. Zootaxa. 2007;1639:1–21. [Google Scholar]
- 60.Capape C, Guélorget O, Vergne Y, Reynaud C. Reproductive biology of the blackmouth catshark, Galeus melastomus (Chondrichthyes: Scyliorhinidae) off the Languedocian coast (southern France, northern Mediterranean) J. Mar. Biol. Assoc. UK. 2008;88:415–421. [Google Scholar]
- 61.Castro JI. The Sharks of North America 1–613. Oxford: Oxford University Press; 2011. [Google Scholar]