Abstract
Sources of dietary phosphate differentially contribute to hyperphosphatemia in maintenance haemodialysis (MHD) patients. This cross-sectional study in Malaysia investigated association between dietary patterns and serum phosphorus in MHD patients. Dietary patterns were derived by principal component analysis, based on 27 food groups shortlisted from 3-day dietary recalls of 435 MHD patients. Associations of serum phosphorus were examined with identified dietary patterns. Three dietary patterns emerged: Home foods (HFdp), Sugar-sweetened beverages (SSBdp), and Eating out noodles (EO-Ndp). The highest tertile of patients in HF (T3-HFdp) pattern significantly associated with higher intakes of total protein (p = 0.002), animal protein (p = 0.001), and animal-based organic phosphate (p < 0.001), whilst T3-SSBdp patients had significantly higher intakes of total energy (p < 0.001), inorganic phosphate (p < 0.001), and phosphate:protein ratio (p = 0.001). T3-EO-Ndp patients had significantly higher intakes of total energy (p = 0.033), total protein (p = 0.003), plant protein (p < 0.001), but lower phosphate:protein ratio (p = 0.009). T3-SSBdp patients had significantly higher serum phosphorus (p = 0.006). The odds ratio of serum phosphorous > 2.00 mmol/l was significantly 2.35 times higher (p = 0.005) with the T3-SSBdp. The SSBdp was associated with greater consumption of inorganic phosphate and higher serum phosphorus levels.
Subject terms: Nephrology, Kidney diseases, Nutrition
Introduction
Hyperphosphatemia is associated with increased mortality and morbidity in maintenance haemodialysis (MHD) patients1,2. The high incidence of hyperphosphatemia in MHD patients is attributed to imbalance between phosphate intake and clearance, as dialysis remains the main route for removing excessive phosphate in patients with minimal kidney function3. Therefore, restriction of dietary phosphate intake in conjunction with the use of phosphate binders, is an important aspect of therapy for hyperphosphatemia4. However, restricting dietary phosphate often leads to a concomitant reduction of protein-rich foods intake, which inadvertently compromises dietary protein adequacy, thus increasing risk for protein energy wasting5.
There is a growing awareness in the variability of dietary phosphate bioavailability, both by source (plant or animal) and type (organic or inorganic). As such, bioavailability is lowest for phosphate derived from plants (10–30%), followed by phosphate derived from animals (40–60%), while it is highest (100%) for inorganic phosphates found in food additives6. The phosphate derived from plants is not readily digested and absorbed, as humans lack the necessary enzyme phytase to hydrolyse phytate to release phosphate. On the other hand, inorganic phosphates are salts that readily disassociate in the stomach, and as a result > 90% are absorbed7. Based on this understanding, the current approach in patient education should focus on choosing fresh food that contains phosphate with lower bioavailability, whereas avoiding food products with phosphate-containing additives4,8. It is also known that potential nutrient interactions in the digestive tract can affect dietary phosphate bioavailability9.
Dietary pattern is defined by the quantity, variety, or combination of different foods and beverages in a diet, and the frequency with which they are habitually consumed10. Dietary pattern analysis has emerged as a valuable approach to investigate associations between overall diet and health outcomes in human nutrition studies. In contrast to a singular nutrient/food-based approach, the dietary pattern-based approach takes into account that human food consumption falls into patterns reflecting a complex combination of dietary components and nutrients that are likely to have synergistic and competitive interactions11.
There are two approaches in dietary pattern analysis, namely à priori and à posteriori. An à priori approach evaluates the overall diet quality using scores or indices based on guidelines for a healthy diet or diets known to confer health benefits, whilst an à posteriori approach uses statistical methods to identify existing dietary patterns within a study population12. The à posteriori approach generates information on the population-specific dietary patterns, which facilitates a better understanding of health outcomes associated with locally relevant dietary patterns and development of culturally tailored nutrition intervention13. Appropriately, some studies have examined the association between the à posteriori derived dietary patterns and health related outcomes in MHD patients, such as nutritional risk14 and mortality15,16. A controlled feeding study in a non-chronic kidney disease (CKD) population has also reported that dietary phosphate bioavailability assessed using urinary phosphate excretion varies by dietary patterns17.
It is clear that the possible association between dietary patterns and serum phosphorus level in MHD patients remains unknown. Therefore, this study aimed to investigate the association between à posteriori derived dietary pattern and serum phosphorus level in MHD patients.
Methods
Study design and population
This was a cross-sectional study, which utilized data collected between October 2015 and November 2018 during screening of MHD patients for recruitment into the Palm Tocotrienols in Chronic Haemodialysis study, as described elsewhere14,18. Patients were recruited from 14 dialysis centres within the urban area of Klang Valley, Malaysia. Inclusion criteria included MHD patients aged at least 18 years old and dialyzed for at least 3 months. Exclusion criteria were patients with poor adherence toward haemodialysis treatment, unfit for assessment due to physical or mental disability, or with terminal illness such as HIV/AIDS or malignancy. Ethical approval was obtained from the Medical Research and Ethics Committee, Ministry of Health, Malaysia (reference number: NMRR-15-865-25260). All eligible patients gave written informed consent and all research procedures were conducted in accordance with relevant guidelines and regulations.
Variables and data collection
Patients’ sociodemographic data, medical history, and the most recent drug prescription were retrieved from medical records. Patients’ self-reported compliance to phosphate binder prescriptions was assessed via face-to-face interviews. Biochemical results were extracted from in-centre patient laboratory reports relevant to within 2 weeks of collection of dietary data information. All analyses were performed by accredited laboratories19 in accordance with the operating procedures mandated by the Ministry of Health, Malaysia.
Dietary assessment was performed by research dieticians using the 3-day dietary recall (3-DDR) method, which included a dialysis day, a non-dialysis day, and a weekend day20. Common household measurement tools (bowls, spoons, and glasses) were used to optimize the portion size estimation. In addition, patients were asked about their weekly frequency of eating out. Food and beverages consumed in household units were transformed into absolute weight (g) and volume (ml) before analysis for nutrient composition using the Nutritionist Pro Software (First DataBank Inc., USA), which references the Malaysian Food Composition21 and Singapore Food Composition22 databases. The Goldberg’s index was used to identify 3DDRs of acceptable reporters to ensure the quality of dietary data. In brief, patients’ basal metabolic rate (BMR) was estimated using the Harris–Benedict equation23. Based on the reported energy intake (EI), EI:BMR ratios of < 1.2, 1.2–2.4, and > 2.4 were considered as under-, acceptable-, and over-reporting of 3DDRs respectively24.
Food items from the 3DDRs were classified into either animal or plant protein categories25. The animal protein group consisted of the following food items: fish, shellfish, eggs, poultry, red meat, milk, dairy products, processed or preserved meat, seafood, and eggs. The food sources of plant protein group were rice, cereals, beans, legumes, fruits, leafy vegetables, and starchy vegetables. An individual food item was directly assigned to the respective food group. For cooked dishes with a mixture of ingredients consisting of animal and plant proteins, recipes from the Malaysian Food Composition21 were referenced to determine the protein content of each ingredient, which was then assigned to the corresponding protein group.
The total dietary phosphate intake derived from the nutrient composition analysis of 3DDRs was categorized into organic phosphates (plant and animal foods), or inorganic phosphates (processed food or beverages). This categorization was based on the assumption that organic phosphate is naturally found in food, while inorganic phosphate is an additive in processed foods26. Accordingly, all food items from 3DDRs were divided into two food groups according to the source of phosphate. Food groups as the source of organic phosphate included cooked rice, soda crackers, fresh or frozen vegetables and fruits, eggs, beans, legumes, nuts, milk, fresh or frozen poultry, seafood, meats, and plain tea or coffee. The phosphate content of these food groups was further subdivided into either organic phosphate from plant or animal, based on the protein category as mentioned previously. Food groups as sources of inorganic phosphate were processed cheese, frozen meals, ready-to-eat cereals, cookies, canned, processed, and luncheon meat, poultry or seafood, canned soups, fast food, cola beverages, and beverages added with sweetened condensed milk. As these food groups might contain a combination of organic and inorganic phosphates, an assumption model was used to derive the added inorganic phosphate based on the difference of total phosphate content and protein content of foods in the unprocessed form. For composite dishes, standard recipes were referred to determine the content and source of phosphate of each constituent ingredient, which was then assigned to the corresponding group.
As the 3DDR method was chosen to assess dietary intake instead of a food frequency questionnaire, aggregation of food items into food groups was first carried out as previously described14 prior to performing the dietary pattern analysis. All food items from 3DDRs were extracted and sorted by alphabetical order. Then, duplicates were removed, and the food items were grouped based on similarity, food preparation method, and nutrient content. Initially, 47 food groups were developed and then, based on the consumption of each food group, food groups with consumption by less than 5% of patients were either excluded or collapsed with similar food groups. This narrowed the final food listing to 27 food groups.
The Diet Monotony Index (DMI) was calculated as described by Zimmerer et al.27 to assess food variety. From the 27-food group listing developed, the total quantity of each food group consumed was converted into servings according to the Malaysian Dietary Guideline 201028. A diet consumed with a wide variety of food groups resulted in smaller proportions of total servings for each food group, and a smaller index value was derived, or vice versa.
Statistical analyses
Continuous variables were presented as mean ± SD or median [interquartile range (IQR)], while categorical variables were presented as frequency (percentages). Non-normally distributed variables were log-transformed before statistical analyses. Chi-square test was used to determine associations between two categorical variables. Simple linear regression was used to determine the correlation between serum phosphorus (dependent variable) and clinical and dietary parameters (independent variables). Variables with p-value < 0.1 on univariate analysis were included in the subsequently multiple linear regression analysis. Tolerance and variance inflation factor were used to check for multicollinearity.
Dietary patterns were derived using à posteriori approach as previously described14. The input variable for factor analysis was the weight of each food group. Principal component analysis (PCA) was used to derive dietary patterns and the derived patterns were orthogonally rotated (varimax rotation) to enhance the difference between loadings to improve the interpretability of factors. The number of dietary patterns retained was determined based on eigenvalue > 2.029, scree plot examination, and interpretability of the derived patterns30. The eigenvalue indicates the total variance explained by a given factor30. Dietary patterns were named in accordance to the food group with the highest factor loading. Patients were assigned to factor scores computed for each pattern identified, which indicated adherence to that pattern. Based on the factor score, patients were categorized into tertiles (T1–T3) for each dietary pattern, where tertile 1 (T1) represented the lowest adherence while tertile 3 (T3) was the highest adherence to that pattern.
One-way ANOVA was used to compare nutrient intakes by tertiles of identified dietary patterns, with Bonferroni test for post hoc analyses. Kruskal–Wallis with Dunn’s post hoc test was used for comparison of non-normally distributed variables. One-way analysis of covariance adjusted for covariates was used to compare serum phosphorus by tertiles of each dietary pattern, and Bonferroni test was used for multiple comparisons. Multiple logistic regressions were used to estimate the odds ratios (ORs) of hyperphosphatemia (serum phosphorus above 1.78 mmol/l and 2.00 mmol/l) associated with T3 of each dietary pattern. Missing covariate data (less than 2%) were imputed using the mean of the existing value of all patients. All analyses were computed using the SPSS version 25 (IBM, Chicago, IL, USA). Statistical significance was set at p-value < 0.05 for all evaluated parameters.
Results
The flow of patient enrolment is shown in Fig. 1. Of 800 eligible patients, 497 consented and completed all assessments. The 303 eligible patients who refused consent were reluctant to commit time for research procedures, as they were exposed to many research study in their settings. In addition, they were also experiencing fatigue when reporting dietary data. Out of 497 patients who completed the assessment, 62 (12.5%) were excluded due to implausible dietary data. Therefore, the final data analyses included 435 MHD patients, whose characteristics are shown in Table 1. This was a multiethnic population with Malay (42.3%), Chinese (39.8%), and Indian (17.9%) patients, with a mean age of 55 ± 13 years, median dialysis vintage of 56 months, and with 54.8% males. All patients were dialyzed thrice weekly for four hours per session as per the clinical practice guideline by the Ministry of Health, Malaysia31. Majority of the patients were recruited from dialysis centres of non-governmental organizations (46.4%). The mean serum phosphorus was 1.78 ± 0.50 mmol/l and 47.8% patients had serum phosphorus above 1.78 mmol/l. Patients with serum phosphorus level above 1.78 mmol/l were mainly from non-governmental dialysis centres (p = 0.024) and did not comply to using phosphate binder (p = 0.002) (Supplementary Table S1). Phosphate binders were prescribed to 98.2% patients and the type of phosphate binder was mainly calcium-based (92.9%). However, only 59.5% reported adhering to the phosphate binder prescription. The median frequency of eating out weekly was 8 (IQR = 10).
Figure 1.
Study flow chart for patient recruitment.
Table 1.
Characteristics of patients (n = 435).
| Characteristics | Mean ± SD | n (%) |
|---|---|---|
| Age (year) | 54.6 ± 13.4 | |
| Sex | ||
| Male | 239 (54.9) | |
| Female | 196 (45.1) | |
| Ethnicity | ||
| Malay | 184 (42.3) | |
| Chinese | 173 (39.8) | |
| Indian | 78 (17.9) | |
| Sector of dialysis provider | ||
| Government | 165 (37.9) | |
| Non-governmental organization | 202 (46.5) | |
| Private | 68 (15.6) | |
| Anthropometry | ||
| Weight (kg) | 62.5 ± 14.2 | |
| Height (cm) | 158.1 ± 8.8 | |
| Body mass index (kg/m2) | 24.9 ± 5.0 | |
| Biochemistry | ||
| Pre-dialysis urea (mmol/l) | 19.2 ± 5.4 | |
| Pre-dialysis creatinine (μmol/l) | 820 (271)* | |
| Serum potassium (mmol/l) | 5.0 ± 0.8 | |
| Serum phosphorus (mmol/l) | 1.78 ± 0.50 | |
| <1.18 mmol/l | 47 (10.8) | |
| 1.18–1.78 mmol/l | 180 (41.4) | |
| > 1.78 mmol/l | 208 (47.8) | |
| Serum corrected calcium (mmol/l) | 2.26 ± 0.24 | |
| Serum alkaline phosphatase (IU/l) | 103 (76)* | |
| Serum albumin (g/l) | 39.3 ± 4.0 | |
| Medical background | ||
| Co-morbidities | ||
| Diabetes mellitus | 190 (43.7) | |
| Hypertension | 349 (80.2) | |
| Hepatitis B/C | 47 (10.8) | |
| Dialysis vintage (month) | 56 (73)* | |
| Kt/V | 1.6 ± 0.4 | |
| nPNA (g/kg) | 1.0 ± 0.3 | |
| Parathyroidectomy history | 69 (15.9) | |
| Medication prescriptions | ||
| Phosphate binder prescription | 427 (98.2) | |
| Calcium based phosphate binder | 404 (92.9) | |
| Lanthanum carbonate | 17 (3.9) | |
| Sevelamer carbonate | 6 (1.4) | |
| Activated vitamin D prescription | 299 (68.7) | |
| Self-reported adherence to phosphate binder | 254 (59.5) | |
| Dietary intake | ||
| Energy (kcal) | 1524 ± 343 | |
| Energy (kcal/kg) | 25.2 ± 6.5 | |
| Total protein (g) | 52.5 (20.6)* | |
| Total protein (g/kg) | 0.9 (0.4)* | |
| Animal protein (g) | 28.2 (19.5)* | |
| Plant protein (g) | 23.7 (10.0)* | |
| Total phosphate (mg) | 634.8 (268.0)* | |
| Animal organic phosphate (mg) | 236.6 (206.0)* | |
| Plant organic phosphate (mg) | 269.7 (160.0)* | |
| Inorganic phosphate (mg) | 80.7 (137.0)* | |
| Phosphate to protein ratio (mg/g) | 12.0 (3.4)* | |
| Total fluid (ml) | 1246 (830)* | |
| Eating out frequency (per week) | 8 (10)* | |
| Diet Monotony Index | 30 (12)* | |
nPNA normalization of protein nitrogen appearance.
*Data is presented as median with interquartile range (IQR).
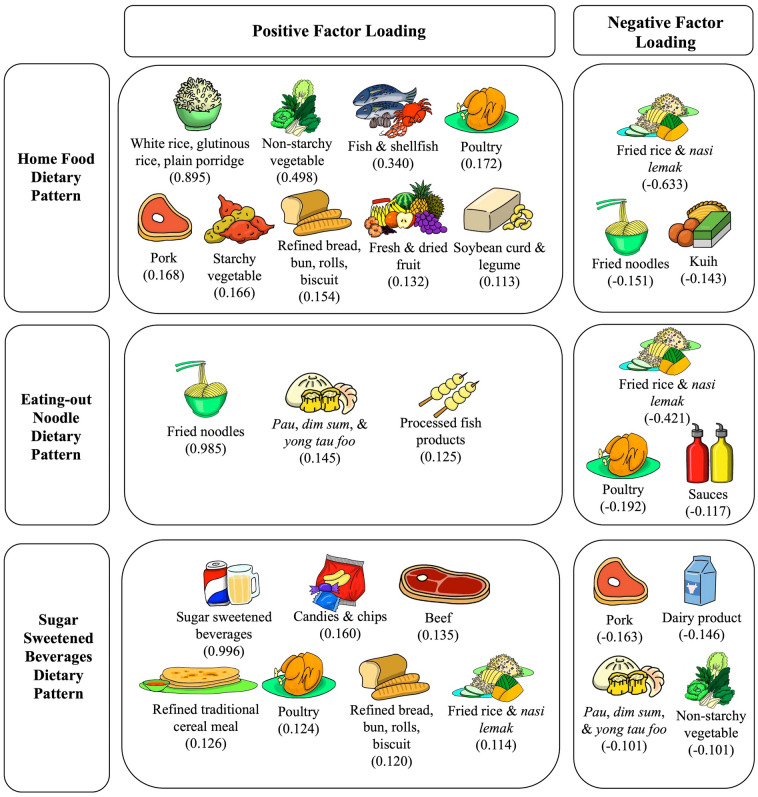
Three dietary patterns emerged with a total of 27 food groups using PCA (Table 2). The Kaiser–Meyer–Olkin value of PCA was 0.538, indicating acceptable sampling adequacy for factor analysis. The first dietary pattern was labelled as “Home FoodDP” as it represented a high intake of white rice, non-starchy vegetables, fish and shellfish, poultry, pork, refined bread, bun, roll, and biscuit, fresh and dried fruit, and soybean curd and legume, along with a low intake of fried rice and coconut milk rice (Fig. 2). The second dietary pattern was labelled as “Eating-out NoodlesDP” as it reflected a high intake of fried noodle dishes, pau, dim sum and yong tau foo, and processed fish products, along with a low intake of fried rice and nasi lemak. The third dietary pattern was characterized by a high intake of sugar-sweetened beverages, along with candies and chips, beef, refined traditional cereal meal, poultry, refined bread, bun, roll and biscuit, and fried rice and nasi lemak. This pattern was labelled as “Sugar-Sweetened BeveragesDP (SSBDP)”. These three dietary patterns explained 72.1% of the total variance among 27 food groups, with the SSBDP accounting for the highest variance (45.3%), followed by Home FoodDP (13.8%), and Eating-out NoodlesDP (13.0%) patterns.
Table 2.
Factor loadings for three dietary patterns derived by principal component analysis.
| Food groups | Components | ||
|---|---|---|---|
| Home food | Eating out noodles | Sugar sweetened beverages | |
| Beef | − 0.022 | − 0.044 | 0.135 |
| Candies and chips | − 0.013 | − 0.010 | 0.160 |
| Chicken egg | 0.093 | − 0.038 | 0.025 |
| Dairy products | − 0.021 | − 0.027 | − 0.146 |
| Fish and shellfish | 0.340 | 0.011 | − 0.027 |
| Fresh and dried fruit | 0.132 | − 0.061 | − 0.092 |
| Fried rice and nasi lemak | − 0.633 | − 0.421 | 0.114 |
| Kuih | − 0.143 | − 0.088 | 0.003 |
| Non-starchy vegetables | 0.498 | − 0.053 | − 0.101 |
| Noodles, fried | − 0.151 | 0.958 | − 0.010 |
| Noodles, soup | 0.079 | 0.056 | − 0.026 |
| Pau, dim sum and yong tau foo | − 0.046 | 0.145 | − 0.110 |
| Pork | 0.168 | 0.058 | − 0.163 |
| Poultry | 0.172 | − 0.192 | 0.124 |
| Preserved fish, shell fish, poultry, egg, and meat | 0.067 | − 0.001 | 0.016 |
| Preserved vegetables | 0.048 | 0.074 | − 0.059 |
| Processed chicken and red meat products | − 0.023 | 0.003 | − 0.030 |
| Processed fish products | − 0.008 | 0.125 | 0.053 |
| Refined bread, bun, roll and biscuit | 0.154 | − 0.079 | 0.120 |
| Refined traditional cereal meal | 0.039 | − 0.083 | 0.126 |
| Sauces | − 0.023 | − 0.117 | 0.066 |
| Soybean curd and legume | 0.113 | 0.014 | − 0.006 |
| Spreads (fat) | 0.077 | − 0.024 | 0.008 |
| Spreads (sweet) | − 0.027 | − 0.010 | 0.076 |
| Starchy vegetables | 0.166 | − 0.025 | − 0.100 |
| Sugar sweetened beverages | − 0.028 | − 0.080 | 0.996 |
| White rice, glutinuous rice, and plain rice porridge | 0.895 | − 0.043 | 0.074 |
Data expressed as factor loading (correlation coefficient between each food group and dietary pattern); foods groups with factor loadings ≥ 0.1 were bolded to indicate main food groups in each factor; principal component analysis with eigenvalue > 2.0 and orthogonal rotation for derivation of dietary patterns.
Local ethnic-based food names are Kuih—local sweet or savory bite sized snacks; Pau—filled steamed bun; Dim Sum—bite-sized dumpling filled with meat or seafood; Yong Tau Hoo—soybean curd or vegetables filled with fish paste or ground meat; Nasi Lemak—rice cooked in coconut milk.
Figure 2.
Food groups with factor loading within each dietary pattern. The factor loading indicates correlations of the food group with the dietary pattern. Local ethnic-based food names are Kuih—local sweet or savoury bite sized snacks; Pau—filled steamed bun; Dim Sum—bite-sized dumpling filled with meat or seafood; Yong Tau Hoo—soybean curd or vegetables filled with fish paste or ground meat; Nasi Lemak—rice cooked in coconut milk.
The linear regression analyses of serum phosphorus are shown in Table 3. In the model accounting for all variables, dialysis adequacy (Kt/V, β = − 0.223, p = 0.001), adherence to phosphate binders (β = − 0.184, p < 0.001), BMI (β = 0.016, p = 0.001), and age (β = − 0.005, p = 0.009) were negatively correlated to serum phosphorus level, whilst normalization of protein nitrogen appearance (β = 0.242, p = 0.005) was positively correlated to serum phosphorus level.
Table 3.
Factors associated with serum phosphate levels using linear regression analyses.
| Variables | Simple linear regression | Multiple linear regression† | ||
|---|---|---|---|---|
| β (95% CI) | P value | adjusted β (95% CI) | P value | |
| Age (year) | − 0.004 (− 0.008, − 0.001) | 0.016 | − 0.005 (− 0.008, − 0.001) | 0.009 |
| Sex (male) | 0.071 (− 0.024, 0.165) | 0.144 | − 0.010 (− 0.110, 0.091) | 0.852 |
| BMI (kg/m2) | 0.018 (0.009, 0.027) | < 0.001 | 0.016 (0.006, 0.026) | 0.001 |
| Dialysis vintage (month)* | 0.018 (− 0.030, 0.065) | 0.465 | – | |
| Parathyroidectomy (yes) | − 0.006 (− 0.136, 0.123) | 0.923 | – | |
| Adherence to phosphate binder (yes) | − 0.158 (− 0.252, − 0.063) | 0.001 | − 0.184 (− 0.276, − 0.092) | < 0.001 |
| Kt/V | − 0.214 (− 0.327, − 0.101) | < 0.001 | − 0.223 (− 0.357, − 0.089) | 0.001 |
| nPNA (g/kg) | 0.164 (0.000, 0.329) | 0.050 | 0.242 (0.073, 0.411) | 0.005 |
| Total protein (g)* | 0.205 (0.063, 0.346) | 0.005 | – | |
| Animal protein (g)* | 0.031 (0.003, 0.059) | 0.029 | 0.018 (− 0.034, 0.071) | 0.498 |
| Plant protein (g)* | 0.052 (− 0.016, 0.121) | 0.135 | 0.033 (− 0.034, 0.101) | 0.331 |
| Total phosphate (mg)* | 0.124 (− 0.010, 0.258) | 0.069 | 0.015 (− 0.126, 0.157) | 0.830 |
| Animal organic phosphate (mg)* | 0.016 (− 0.004, 0.037) | 0.122 | − 0.005 (− 0.043, 0.033) | 0.782 |
| Plant organic phosphate (mg)* | 0.007 (− 0.048, 0.062) | 0.810 | – | |
| Inorganic phosphate (mg)* | 0.000 (− 0.015, 0.014) | 0.946 | – | |
| Phosphate to protein ratio (mg/g)* | − 0.131 (− 0.334, 0.072) | 0.205 | – | |
nPNA, normalization of protein nitrogen appearance.
*Data was log-transformed.
“–” indicates variables not included in the multiple linear regression analysis; only variables with p-value < 0.25 in the simple linear regression were carried into the multiple linear regression analysis, “total protein” was excluded as it was a sum of both “animal protein” and “plant protein” while “phosphate to protein ratio” was excluded in the analysis due to collinearity. Bold values denote statistical significance at p-value < 0.05.
†The highest variance inflation factor was 3.960.
The nutrient profiles of three identified dietary patterns are presented in Table 4. The upper tertile (T3) of Home FoodDP indicated significantly higher intakes of total protein (p = 0.002), animal protein (p = 0.001), animal-based organic phosphate (p < 0.001), and total fluid (p = 0.003). On the other hand, the T3 of SSBDP indicated significantly higher intakes of total energy (p < 0.001), inorganic phosphate (p < 0.001), phosphate to protein ratio (p = 0.001), and total fluid (p = 0.010). The T3 of Eating-out NoodlesDP indicated significantly greater intakes of total energy (p = 0.033), total protein (p = 0.003), plant protein (p < 0.001) but lower phosphate to protein ratio (p = 0.009). Of note, the frequency of eating out was significantly greater in T3 of SSBDP (p = 0.002) and Eating-out NoodlesDP (p = 0.009). Patients of T3 of Eating-out noodleDP were mostly Chinese and from non-governmental dialysis centers while patients of T3 of SSBDP were younger, consisted of mostly men and Malay, and from governmental dialysis centers (Supplementary Table S2). The Home FoodDP was not associated with age, gender, ethnic, and sector of dialysis provider.
Table 4.
Comparison of dietary parameters between tertiles for each dietary pattern.
| Home FoodDP | P trend | P value* T1vsT3 | |||
|---|---|---|---|---|---|
| Tertile 1 (n = 142) |
Tertile 2 (n = 144) |
Tertile 3 (n = 149) |
|||
| Energy (kcal) | 1490 ± 359 | 1564 ± 366 | 1519 ± 301 | 0.183 | > 0.999 |
| Energy (kcal/kg) | 24.6 ± 6.0 | 25.9 ± 7.4 | 25.0 ± 5.9 | 0.244 | > 0.999 |
| Total protein (g) | 48.7 (23.0) | 52.6 (21.9) | 54.2 (17.9) | 0.002 | 0.002 |
| Total protein (g/kg) | 0.8 (0.5) | 0.8 (0.4) | 0.9 (0.4) | 0.010 | 0.007 |
| Animal protein (g) | 24.0 (22.0) | 26.7 (19.6) | 32.1 (16.7) | 0.001 | 0.001 |
| Plant protein (g) | 22.7 (11.2) | 23.9 (9.2) | 24.8 (9.6) | 0.474 | – |
| Total phosphate (mg) | 625 (284) | 632 (328) | 648 (237) | 0.206 | – |
| Animal organic phosphate (mg) | 196 (201) | 251 (186) | 282 (198) | < 0.001 | < 0.001 |
| Plant organic phosphate (mg) | 262 (183) | 273 (161) | 274 (133) | 0.247 | – |
| Inorganic phosphate (mg) | 89 (147) | 91 (148) | 67 (108) | 0.026 | 0.055 |
| Phosphate to protein ratio (mg/g) | 12.1 (4.1) | 12.2 (3.3) | 11.9 (2.9) | 0.249 | – |
| Total fluid (ml) | 1177 (730) | 1258 (966) | 1336 (776) | 0.004 | 0.003 |
| Dietary monotony index | 30 (13) | 30 (11) | 29 (13) | 0.803 | – |
| Eating out frequency (per week) | 8 (10) | 9 (11) | 7 (10) | 0.027 | > 0.999 |
| Sugar Sweetened BeveragesDP | P trend | P value* T1vsT3 | |||
|---|---|---|---|---|---|
| Tertile 1 (n = 145) |
Tertile 2 (n = 145) |
Tertile 3 (n = 145) |
|||
| Energy (kcal) | 1431 ± 334 | 1491 ± 332 | 1651 ± 328 | < 0.001 | < 0.001 |
| Energy (kcal/kg) | 23.5 ± 5.9 | 25.6 ± 6.8 | 26.5 ± 6.4 | < 0.001 | < 0.001 |
| Total protein (g) | 53.3 (20.6) | 51.5 (22.3) | 51.1 (21.2) | 0.583 | – |
| Total protein (g/kg) | 0.9 (0.4) | 0.9 (0.5) | 0.8 (0.4) | 0.525 | – |
| Animal protein (g) | 29.9 (22.0) | 27.7 (19.7) | 27.4 (17.0) | 0.411 | – |
| Plant protein (g) | 22.7 (10.4) | 24.4 (9.6) | 24.3 (11.2) | 0.233 | – |
| Total phosphate (mg) | 604 (263) | 639 (290) | 683 (278) | 0.168 | – |
| Animal organic phosphate (mg) | 230 (230) | 242 (217) | 237 (195) | 0.670 | – |
| Plant organic phosphate (mg) | 264 (163) | 274 (156) | 270 (157) | 0.595 | – |
| Inorganic phosphate (mg) | 63 (106) | 64 (116) | 128 (167) | < 0.001 | < 0.001 |
| Phosphate to protein ratio (mg/g) | 11.4 (3.5) | 12.0 (3.1) | 12.7 (3.2) | 0.001 | 0.001 |
| Total fluid (ml) | 1183 (1032) | 1173 (632) | 1350 (795) | < 0.001 | 0.010 |
| Dietary monotony index | 28 (10) | 30 (15) | 30 (10) | 0.411 | – |
| Eating out frequency (per week) | 7 (10) | 7 (10) | 10 (9) | 0.002 | 0.002 |
| Eating-out NoodleDP | P trend | P value* T1vsT3 | |||
|---|---|---|---|---|---|
| T1 (n = 202) |
T2 (n = 116) |
T3 (n = 117) |
|||
| Energy (kcal) | 1491 ± 360 | 1549 ± 323 | 1557 ± 331 | 0.166 | 0.293 |
| Energy (kcal/kg) | 23.9 ± 6.7 | 26.6 ± 6.4 | 25.8 ± 5.9 | 0.001 | 0.033 |
| Total protein (g) | 49.1 (20.1) | 53.9 (15.6) | 53.1 (27.2) | 0.016 | 0.062 |
| Total protein (g/kg) | 0.8 (0.3) | 0.9 (0.4) | 1.0 (0.5) | < 0.001 | 0.003 |
| Animal protein (g) | 26.9 (18.8) | 30.0 (17.4) | 28.0 (24.4) | 0.476 | – |
| Plant protein (g) | 22.1 (9.9) | 23.2 (10.6) | 26.1 (9.1) | < 0.001 | < 0.001 |
| Total phosphate (mg) | 634 (264) | 634 (257) | 638 (327) | 0.847 | – |
| Animal organic phosphate (mg) | 238 (215) | 248 (167) | 226 (241) | 0.517 | – |
| Plant organic phosphate (mg) | 253 (169) | 273 (169) | 289 (150) | 0.390 | – |
| Inorganic phosphate (mg) | 77 (149) | 85 (151) | 79 (106) | 0.725 | – |
| Phosphate to protein ratio (mg/g) | 12.4 (3.6) | 12.1 (2.9) | 11.4 (3.1) | 0.004 | 0.003 |
| Total fluid (ml) | 1347 (1007) | 1209 (833) | 1222 (571) | 0.207 | – |
| Dietary monotony index | 30 (11) | 28 (9) | 31 (13) | 0.044 | 0.851 |
| Eating out frequency (per week) | 8 (10) | 8 (9) | 9 (10) | 0.011 | 0.009 |
Data is expressed as mean ± standard deviation or median with interquartile range (IQR). Bold values denote statistical significance at p-value < 0.05.
*Significance values had been adjusted for multiple tests.
“–” The Dunn’s post hoc test was not performed if the overall test was not significant.
T1 tertile 1, T2 tertile 2, T3 tertile 3.
Serum phosphorus level by tertiles for each dietary pattern is presented in Table 5. After adjustment for confounders, serum phosphorus levels were found to be significantly higher for T3 vs T1 of SSBDP (p = 0.006). Although, no T3 of any dietary pattern significantly was associated with a higher OR for serum phosphorus level > 1.78 mmol/l (p > 0.05), still a non-significant trend (p = 0.067) was observed with the SSBDP (OR = 1.64; 95% CI 0.97, 2.79), which could be considered significant for p value < 0.10. We found, however, that the OR for elevating serum phosphorous > 2.00 mmol/l, a cut-off that is clinically relevant, significantly increased by 2.35 times (95% CI 1.30, 4.28, p = 0.005) with T3 of SSBDP. Coffee or tea added with sugar was the most common SSB (39.0%) consumed by patients in T3 of SSBDP, followed by coffee or tea added with condensed milk or evaporated milk (24.3%), and syrup or cordial or tetra pak beverages (13.3%) (Fig. 3).
Table 5.
Serum phosphorus and odds ratio of hyperphosphatemia (serum phosphorus > 1.78 mmol/l and 2.00 mmol/l) by tertiles of dietary patterns.
| Dietary pattern | Serum phosphorus (mmol/l)* | Serum phosphorus > 1.78 mmol/l† | Serum phosphorus > 2.00 mmol/l† | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Home food | ||||||
| Tertile 1 | 1.76 ± 0.04 | 1.80 ± 0.05 | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 1.72 ±0.05 | 1.77 ± 0.05 | 0.68 (0.42, 1.09) | 0.67 (0.41, 1.09) | 0.78 (0.46, 1.33) | 0.81 (047, 1.39) |
| Tertile 3 | 1.74 ± 0.04 | 1.78 ± 0.05 | 0.70 (0.43, 1.13) | 0.69 (0.42, 1.13) | 0.91 (0.54, 1.54) | 0.87 (0.51, 1.50) |
| P trend | 0.829 | 0.830 | 0.208 | 0.119 | 0.661 | 0.737 |
| P value for T1 vs T3 | > 0.999 | > 0.999 | 0.141 | 0.137 | 0.735 | 0.622 |
| Sugar sweetened beverages | ||||||
| Tertile 1 | 1.67 ± 0.05 | 1.70 ± 0.05 | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 1.71 ± 0.04 | 1.75 ± 0.05 | 1.00 (0.61, 1.62) | 1.03 (0.62, 1.71) | 1.12 (0.64, 1.96) | 1.29 (0.72, 2.31) |
| Tertile 3 | 1.85 ± 0.05 | 1.88 ± 0.05 | 1.61 (0.97, 2.67) | 1.64 (0.97, 2.79) | 1.99 (1.14, 3.48) | 2.35 (1.30, 4.28) |
| P trend | 0.008 | 0.005 | 0.097 | 0.108 | 0.030 | 0.013 |
| P value for T1 vs T3 | 0.011 | 0.006 | 0.066 | 0.067 | 0.015 | 0.005 |
| Eating out noodles | ||||||
| Tertile 1 | 1.73 ± 0.04 | 1.77 ± 0.04 | 1.00 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 1.74 ± 0.05 | 1.78 ± 0.06 | 1.04 (0.64, 1.71) | 1.04 (0.63, 1.72) | 1.14 (0.66, 1.96) | 1.20 (0.69, 2.09) |
| Tertile 3 | 1.77 ± 0.06 | 1.82 ± 0.06 | 1.00 (0.60, 1.66) | 1.03 (0.61, 1.73) | 1.10 (0.63, 1.92) | 1.18 (0.66, 2.11) |
| P trend | 0.868 | 0.745 | 0.985 | 0.987 | 0.881 | 0.756 |
| P value for T1 vs T3 | > 0.999 | > 0.999 | 0.993 | 0.918 | 0.744 | 0.568 |
Model 1 is adjusted for age, gender, ethnic, sector of dialysis provider; Model 2 is adjusted for all confounders in model 1 and dialysis vintage, Kt/V, normalization of protein nitrogen appearance, history of parathyroidectomy, prescription of activated vitamin D, and self-reported compliance to phosphate binder.
*Data is presented as mean ± standard error, †data is presented as odds ratio (95% confidence interval). Bold values denote statistical significance at p-value < 0.05.
Figure 3.

Types of beverages consumed by tertile 3 patients (n = 145) within the sugar sweetened beverages dietary pattern.
A total of 105 patients of T3 of Home FoodDP and 101 patients of T3 of SSBDP were identified and comparison between these two groups was made (Table 6). The T3 patients of SSBDP were younger (p = 0.001); mainly men (p = 0.037) and likely Malay patients (p < 0.011) compared to T3 patients of Home FoodDP. The total energy intake (p < 0.011), inorganic phosphate intake (p < 0.011) and phosphate to protein ratio (p = 0.001) were significantly higher while total protein intake (p = 0.044), animal protein intake (p = 0.004), and organic phosphate from animal sources (p = 0.003) were significantly lower in T3 patients of SSBDP compared to T3 patients of Home FoodDP. The serum phosphorus level of T3 patients of SSBDP was significantly greater than T3 patients of Home FoodDP (1.87 ± 0.49 vs. 1.71 ± 0.47 mmol/l, p = 0.021), and remained significant (p = 0.014) after adjustment for confounding factors.
Table 6.
Comparison of characteristics between patients of tertile 3 of home food and sugar sweetened beverages dietary patterns.
| Tertile 3 Home FoodDP (n = 105) |
Tertile 3 SSBDP (n = 101) |
P value | |
|---|---|---|---|
| Age (year) | 58.6 ± 12.6 | 52.6 ± 12.1 | 0.001 |
| Gender | |||
| Male | 58 (55.2%) | 70 (69.3%) | 0.037 |
| Female | 47 (44.8%) | 31 (30.7%) | |
| Sector | |||
| Government | 36 (34.3%) | 45 (44.6%) | 0.050 |
| NGO | 54 (51.4%) | 35 (34.7%) | |
| Private | 15 (14.3%) | 21 (20.8%) | |
| Ethnic | |||
| Malay | 34 (32.4%) | 59 (58.4%) | < 0.001 |
| Chinese | 54 (51.4%) | 26 (25.7%) | |
| Indian | 17 (16.2%) | 16 (15.8%) | |
| Compliance to binder | |||
| Yes | 39 (37.5%) | 42 (42.9%) | 0.437 |
| No | 65 (62.5%) | 56 (57.1%) | |
| Kt/V | 1.66 ± 0.43 | 1.65 ± 0.27 | 0.909 |
| Energy (kcal/kg) | 1456 ± 266 | 1644 ± 327 | < 0.001 |
| Protein (g/kg)* | 54.1 (17.4) | 49.5 (20.2) | 0.044 |
| Phosphate (mg)* | 607 (228) | 632 (280) | 0.584 |
| Plant protein (g)* | 24.4 (8.6) | 23.5 (9.5) | 0.688 |
| Animal protein (g)* | 31.9 (18.6) | 24.7 (16.4) | 0.004 |
| Organic plant phosphate (mg)* | 267 (131) | 265 (164) | 0.353 |
| Organic animal phosphate (mg)* | 283 (199) | 231 (186) | 0.003 |
| Inorganic phosphate (mg)* | 45 (98) | 147 (158) | < 0.001 |
| Phosphate protein ratio* | 11.6 (3.1) | 12.9 (3.6) | 0.001 |
| Serum phosphorus (mmol/l) | 1.71 ± 0.47 | 1.87 ± 0.49 | 0.021 |
| 1.75 ± 0.06† | 1.92 ± 0.06† | 0.014† | |
| Category of serum phosphorus | |||
| ≤ 1.78 mmol/l | 61 (58.1%) | 45 (44.6%) | 0.052 |
| > 1.78 mmol/l | 44 (41.9%) | 56 (55.4%) | |
Continuous data was presented as mean ± SD and independent t test was used for analyses while categorical data was presented as frequency (%) and chi-square test was used for analysis.
DP dietary pattern, NGO non-governmental organization, SSB sugar sweetened beverages.
*Data was presented as median with interquartile range (IQR) and Mann–Whitney test was used for analyses. Bold values denote statistical significance at p-value < 0.05.
†Data was presented as mean ± SE and the general linear model was used for analysis with adjustment for age, gender, ethnic, sector of dialysis provider, dialysis vintage, Kt/V, normalization of protein nitrogen appearance, history of parathyroidectomy, prescription of activated vitamin D, and self-reported compliance to phosphate binder.
Forty-four patients were excluded in these analyses as they were categorized into tertile 3 of both Home FoodDP and SSBDP.
Discussion
In this cross-sectional study of 435 MHD patients, three dietary patterns emerged through the à posteriori approach, namely Home FoodDP, SSBDP, and Eating-out NoodleDP. The empirically derived dietary patterns reflected the habitual dietary intake within this MHD population. Similar dietary patterns were also reported in an earlier study with a smaller population14. In the data reported by Sulaheen et al.14, the first 382 patients enrolled were used in the primary analyses, which focused on malnutrition. In the present study, we assessed the subsequent 497 enrolled patients for hyperphosphatemia risk. However, dietary patterns reported were different due to sample size, ethnicity proportion, and analyses. In the study by Sualeheen et al.14, four dietary patterns were identified, namely Home Food, Eating-out Noodle, Eating-out Rice, and Eating-out SSB. The dietary pattern with the highest variance was Eating-out Noodle (17.0%). Contrarily, we used eigenvalue > 2.0 in the PCA for the present study, which led to retention of only three dietary patterns, with the SSBDP accounting for the highest variance (45.3%). It should be also noted that the patients in the study by Sualeheen et al.14 were mainly Chinese (45%), followed by Malay (36%) and Indian (19%) whilst an almost equal number of Malay and Chinese patients were recruited in the present study. The trend of studies examining dietary patterns for CKD populations does note that these patterns reflect local food habits. The DIET-HD study identified “fruits and vegetables” and “Western” dietary patterns for 8,110 MHD patients across 10 European countries and Argentina16. These dietary patterns are also consistent with reported patterns for the general population within these same countries32. The Reasons for Geographic and Racial Differences in Stroke study conducted for CKD populations in the South-eastern United States, identified a “Southern” dietary pattern characterized by fried foods, organ meats, and sweetened beverages, which is typical of Southern cuisine33. Similarly, the PROGREDIR study conducted among pre-dialysis CKD patients in São Paulo, Brazil identified a “traditional” dietary pattern (composed of white rice, beans, and coffee) typical to the local community34.
In this study, we did not observe any significant association between serum phosphorus level and any individual nutrient, including dietary phosphate. There are some possible explanations for this null finding. Firstly, total dietary phosphate intake does not reflect the actual amount of phosphate absorbed in the intestinal tract, as phosphate bioavailability varies by food source7. Secondly, patients’ dietary phosphate intakes were estimated from 3DDRs with reference to food composition databases. Other studies have indicated discrepancies in phosphate content estimated from diet recalls using food composition databases with direct laboratory analyses of duplicate portions of diet samples35,36. The wide use of phosphate additives in food manufacturing adds to actual burden of dietary phosphate intake as opposed to the lower estimations from referencing the food composition databases37. In addition, we observed variables such as dialysis adequacy (Kt/V) and adherence to phosphate binder prescription were inversely associated with serum phosphorus level. This implies that management of hyperphosphatemia in MHD patients is beyond limiting total amount of dietary phosphate intake alone. In fact, Lynch et al.38 have shown that prescribed dietary phosphate restrictions could not be associated with improving survival. In the present study, more patients from non-governmental dialysis centers had serum phosphorus level above 1.78 mmol/l compared to patients from government or private centers. This finding is consistent with our previous study, which reported the prevalence of hyperphosphatemia in MHD patients based on the data from Malaysian National Renal Registry39. A possible explanation is that government and private centers have more resources in terms of manpower and treatment options for achieving the target serum phosphorus level.
We went beyond the single nutrient approach and examined the effect of dietary patterns on serum phosphorus level. We found that the SSBDP was significantly associated with higher serum phosphorus levels, which could be attributed to greater consumption of inorganic phosphate as found in beverages added with condensed milk. It is well known that cola beverages contain phosphoric acid additives that contribute to substantial dietary phosphate burden40. However, our study showed that carbonated beverages were the least consumed SSB. Instead, tea or coffee added with sugar, sweetened condensed milk or evaporated milk were the most popular choice of SSB in our population as indicated by national trade estimates data41. Inorganic phosphate additives such as sodium phosphates, calcium phosphates, triphosphates, and polyphosphates are added as stabilizing salts during the manufacturing process of evaporated and sweetened condensed milk which according to CODEX standards may be between 0.2 to 0.3% of product content42,43. We examined the front-on-pack labelling of popular evaporated and sweetened condensed milk brands in the market, but found none disclosed the phosphate content or type of additives. The non-mandatory reporting of phosphate content in food labelling has made it difficult for MHD patients to identify potential hidden sources of phosphate44. In the United States, a cross-sectional analysis from the National Health and Nutrition Examination Survey revealed that consumption of dairy products and cereals or grains with inorganic phosphate additives were also associated with greater serum phosphorus in the general population26. Contrarily, a recent dietary pattern study on African American patients on MHD did not observe significant difference in serum phosphorus level between high SSBDP and low SBBDP, likely due to a smaller sample size, a homogenous population and different food selection45.
Although the Home FoodDP was associated with greater dietary protein intake, particularly animal protein, this pattern did not adversely affect serum phosphorus levels. Typical protein foods implicated in the Home FoodDP were fish and shellfish, poultry, pork, and soybean curd and legumes, which did not occur in the other two patterns. At the same time, the Home FoodDP also carried appreciable factor loadings for non-starchy vegetables (0.498), starchy vegetables (0.166), and fresh and dried fruit (0.132). The non-significant association between Home FoodDP and serum phosphorus level, despite higher protein intakes may be explained by the lower phosphate absorption from fruits and vegetables17. Achieving dietary protein adequacy without excessive phosphate intake is critical for MHD patients, yet challenging as phosphate is naturally found in protein-rich food. Shinaberger et al.5 showed that increased protein intake alongside with reduced serum phosphorus level was associated with better survival in MHD patients, but controlling serum phosphorus level by decreasing protein intake led to increased mortality. Thus the Home FoodDP reflecting a balanced diet with rice, protein foods, vegetables, and fruits, is clearly the answer to patients facing the phosphate-protein dilemma. In addition, we have previously shown that the Home Food pattern was associated with better nutritional status indicated by serum albumin, malnutrition inflammation score, and handgrip strength14.
The present study highlighted potential applications of the dietary pattern approach in nutritional intervention for MHD patients. The dietary pattern approach takes into account the multidimensional exposure and interactions that exist across complex combinations of dietary components and nutrients from foods12. As people consume foods instead of nutrients in isolation, dietary patterns can be readily translated into practical dietary advice. Nevertheless, we cannot refute the importance of recognizing and restricting inorganic phosphate intake. We have clearly shown that a dietary pattern containing beverages rich in inorganic phosphate was associated with hyperphosphatemia in this Malaysian population and this aspect should be communicated in patient education as well as incorporated into the local clinical practice guideline8. At the same time, it is also critical to emphasize to patients the value of a balanced diet.
There were some important limitations in this study. Firstly, this was a cross-sectional study with potential residual confounders and the causality cannot be established. Secondly, dietary intakes were assessed using the 3DDR method, which may be subject to measurement and recall bias. However, trained dieticians were involved in dietary data collection and expectedly minimized bias in recall data. Further, we excluded patients with implausible dietary data to minimize recall bias as per the standard protocol of dietary pattern analyses15,32, although this could also possibly lead to selection bias. The refusal rate for participation (37.9%) of this study could have also introduced selection bias. Thirdly, the process of dietary pattern derivation requires a high degree of subjective decision making such as aggregation of food items into food groups, the number of factors to retain, the method of rotation, and naming of patterns, which leads to inconsistency between researchers46. However, this limitation was overcome by consensus between researchers for the food listing and derivation of dietary patterns. A major limitation was the lack of organic and inorganic phosphate database or even its identification on processed food labels for us to accurately distinguish these two types of phosphate. Therefore, we had to use an assumption model to overcome this deficiency. Inclusion of parathyroid hormone as a parameter to examine phosphate resorption from bone and gut availability from dietary phosphate origin would have been ideal, but owing to its cost, we could not include this measurement for screening this population. The information on ultrafiltration volume was also not available in this study. Finally, this data-driven approach identified dietary patterns specific only to the study population and therefore, should not be generalized to other populations outside of Malaysia.
In conclusion, the SSBDP was associated with higher concentrations of serum phosphorus in MHD patients, possibly due to greater intakes of inorganic phosphate. The Home FoodDP was associated with greater amount of protein intake, but was not associated with the serum phosphorus level. Therefore, evaluation of the habitual dietary pattern could provide valuable insights for the dietary management of hyperphosphatemia in MHD patients. Future studies investigating the effectiveness of dietary pattern intervention on hyperphosphatemia in MHD patients are warranted.
Supplementary information
Acknowledgments
This study was supported by the Malaysian Government through the Malaysian Palm Oil Board (grant number: NN-2015-080). The funder was not involved in the study conceptualization and design, data collection, analysis, decision to publish, or preparation of this manuscript. B.H.K. has received Chancellor's Research Scholarship (Zamalah Yayasan Canselor) from Universiti Kebangsaan Malaysia for his postgraduate study and is now a postdoctoral researcher (MI-2020-004) of Universiti Kebangsaan Malaysia. S.S. has received scholarship (MyBrain15) from Ministry of Higher Education, Malaysia for her postgraduate study. We would like to thank Ng Hi Ming for assisting in preparing the figure in this manuscript.
Author contributions
Conceptualization and design of study: T.K., B.H.K., and A.S.; patient recruitment: A.H.A.G., S.B., B.L.G., and Z.M.; data acquisition: B.H.K. and S.S.; statistical analysis: K.B.H., K.C., and A.S.; drafting of manuscript: B.H.K. and T.K.; revision of manuscript: S.S., A.H.A.G., S.B., B.L.G., Z.M., Z.A.M.D., P.K., and A.Y.M.W. All authors have read and approved the submitted manuscript.
Data availability
The datasets generated and/or analysed during this study are available on reasonable request from the corresponding author, T.K.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Tilakavati Karupaiah, Email: tilly_karu@yahoo.co.uk.
PaTCH Investigators:
Boon Cheak Bee, Ghazali Ahmad, Soo Kun Lim, Mohammad Zaimi Abdul Wahab, Ravindran Visvanathan, and Rosnawati Yahya
Supplementary information
is available for this paper at 10.1038/s41598-020-68893-4.
References
- 1.Palmer SC, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 2.Yamada S, et al. Association between serum phosphate levels and stroke risk in patients undergoing hemodialysis: the Q-Cohort Study. Stroke. 2016;47:2189–2196. doi: 10.1161/STROKEAHA.116.013195. [DOI] [PubMed] [Google Scholar]
- 3.Copland M, Komenda P, Weinhandl ED, McCullough PA, Morfin JA. Intensive hemodialysis, mineral and bone disorder, and phosphate binder use. Am. J. Kidney Dis. 2016;68:S24–S32. doi: 10.1053/j.ajkd.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease Improving Global Outcomes CKD-MBD Update Work Group. KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int. Suppl.7, e1–e59 (2017). [DOI] [PMC free article] [PubMed]
- 5.Shinaberger CS, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J. Clin. Nutr. 2008;88:1511–1518. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waheed AA, Pedraza F, Lenz O, Isakova T. Phosphate control in end-stage renal disease: barriers and opportunities. Nephrol. Dial. Transpl. 2013;28:2961–2968. doi: 10.1093/ndt/gft244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010;5:519–530. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 8.Malaysian Society of Nephrology. Malaysian CKD-MBD and Parathyroidectomy Guidelines and SOP (Malaysian Society of Nephrology, 2018).
- 9.St-Jules DE, Jagannathan R, Gutekunst L, Kalantar-Zadeh K, Sevick MA. Examining the proportion of dietary phosphorus from plants, animals, and food additives excreted in urine. J. Ren. Nutr. 2017;27:78–83. doi: 10.1053/j.jrn.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sànchez-Villegas, A. & Sánchez-Tainta, A. A Healthy diet for your heart and your brain. In The Prevention of Cardiovascular Disease Through the Mediterranean Diet 169–197 (2018).
- 11.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ocké MC. Evaluation of methodologies for assessing the overall diet: dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 2013;72:191–199. doi: 10.1017/S0029665113000013. [DOI] [PubMed] [Google Scholar]
- 13.Paterson EN, et al. Dietary patterns and chronic kidney disease: a cross-sectional association in the Irish Nun Eye Study. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sualeheen A, et al. Habitual dietary patterns of patients on hemodialysis indicate nutritional risk. J. Ren. Nutr. 2019 doi: 10.1053/j.jrn.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Tsuruya K, et al. Dietary patterns and clinical outcomes in hemodialysis patients in Japan: a cohort study. PLoS ONE. 2015;10:e0116677. doi: 10.1371/journal.pone.0116677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saglimbene VM, et al. Dietary patterns and mortality in a multinational cohort of adults receiving hemodialysis. Am. J. Kidney Dis. 2019;75:361–372. doi: 10.1053/j.ajkd.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 17.McClure ST, et al. The percentage of dietary phosphorus excreted in the urine varies by dietary pattern in a randomized feeding study in adults. J. Nutr. 2019;149:816–823. doi: 10.1093/jn/nxy318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khor BH, et al. Dietary fatty acid intake in hemodialysis patients and associations with circulating fatty acid profiles: a cross-sectional study. Nutrition. 2019;63:14–21. doi: 10.1016/j.nut.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Department of Standard. Skim Akreditasi Makmal Malaysia. https://www.jsm.gov.my/skim-akreditasi-makmal-malaysia-samm-#.Xpr0sC-B3u0 (2020).
- 20.Fouque D, et al. EBPG guideline on nutrition. Nephrol. Dial. Transpl. 2007;22:45–87. doi: 10.1093/ndt/gfm020. [DOI] [PubMed] [Google Scholar]
- 21.Tee ES, Noor MI, Azudin MN, Idris K. Nutrient compositions of Malaysian foods. 4. Kuala Lumpur: Institute for Medical Research Malaysia; 1997. [Google Scholar]
- 22.Health Promotion Board. Energy and Nutrient Composition of Foods Singapore. Health Promotion Board. https://focos.hpb.gov.sg/eservices/ENCF/ (2011).
- 23.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. U.S.A. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000;24:1119–1130. doi: 10.1038/sj.ijo.0801376. [DOI] [PubMed] [Google Scholar]
- 25.Tharrey M, et al. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int. J. Epidemiol. 2018;47:1603–1612. doi: 10.1093/ije/dyy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore LW, Nolte JV, Gaber AO, Suki WN. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am. J. Clin. Nutr. 2015;102:444–453. doi: 10.3945/ajcn.114.102715. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerer JL, Leon JB, Covinsky KE, Desai U, Sehgal AR. Diet monotony as a correlate of poor nutritional intake among hemodialysis patients. J. Ren. Nutr. 2003;13:72–77. doi: 10.1053/jren.2003.50025. [DOI] [PubMed] [Google Scholar]
- 28.National Coordinating Committee on Food and Nutrition. Malaysian Dietary Guidelines. (Ministry of Health Malaysia, 2010).
- 29.Eng JY, Moy FM, Bulgiba A, Rampal S. Consistency and generalizability of dietary patterns in a multiethnic working population. J. Acad. Nutr. Diet. 2018;118:1249–1262. doi: 10.1016/j.jand.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Agnoli C, Pounis G, Krogh V. Dietary pattern analysis. In: Pounis G, editor. Analysis in Nutrition Research: Principles of Statistical Methodology and Interpretation of the Results. Kuala Lumpur: Charlotte Cockle; 2018. pp. 86–93. [Google Scholar]
- 31.Ministry of Health. Renal replacement therapy: clinical practice guidelines. (2011).
- 32.Martínez-Gonzàlez MA, et al. Empirically-derived food patterns and the risk of total mortality and cardiovascular events in the PREDIMED study. Clin. Nutr. 2015;34:859–867. doi: 10.1016/j.clnu.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Gutiérrez OM, et al. Dietary patterns and risk of death and progression to ESRD in individuals with CKD: a cohort study. Am. J. Kidney Dis. 2014;64:204–213. doi: 10.1053/j.ajkd.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado AD, et al. Dietary intake of non-dialysis chronic kidney disease patients: the PROGREDIR study. A cross-sectional study. Sao Paulo Med. J. 2018;136:208–215. doi: 10.1590/1516-3180.2017.0177141217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro-Alarcon M, Zambrano E, Moreno-Montoro M, Agil A, Olalla M. Duplicate portion sampling combined with spectrophotometric analysis affords the most accurate results when assessing daily dietary phosphorus intake. Nutr. Res. 2012;32:573–580. doi: 10.1016/j.nutres.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Zhang ZW, et al. Estimates of mineral intakes using food composition tables vs measures by inductively-coupled plasma mass spectrometry: Part 1. Calcium, phosphorus and iron. Eur. J. Clin. Nutr. 1999;53:226–232. doi: 10.1038/sj.ejcn.1600711. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J. Ren. Nutr. 2007;17:350–354. doi: 10.1053/j.jrn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch KE, Lynch R, Curhan GC, Brunelli SM. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011;6:620–629. doi: 10.2215/CJN.04620510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khor BH, et al. The state of nutrition care in outpatient hemodialysis settings in Malaysia: a nationwide survey. BMC Health Serv. Res. 2018;18:939. doi: 10.1186/s12913-018-3702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser M, et al. Phosphorus content of popular beverages. Am. J. Kidney Dis. 2015;65:969–971. doi: 10.1053/j.ajkd.2015.02.330. [DOI] [PubMed] [Google Scholar]
- 41.United Nations. United Nations Commodity Trade Statistics Database. https://comtrade.un.org (2016).
- 42.Bylund G. Dairy Processing Handbook. Lund: Tetra Pak Processing Systems AB; 2003. [Google Scholar]
- 43.Kalyankar SD, Deshmukh MA, Khedkar CD, Deosarkar SS, Sarode AR. Condensed milk. In: Caballero B, editor. The Encyclopedia of Food and Health. Oxford: Academic Press; 2016. pp. 291–295. [Google Scholar]
- 44.Calvo MS, Sherman RA, Uribarri J. Dietary phosphate and the forgotten kidney patient: a critical need for FDA regulatory action. Am. J. Kidney Dis. 2019;73:542–551. doi: 10.1053/j.ajkd.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Tallman DA, et al. Dietary patterns and health outcomes among African American maintenance hemodialysis patients. Nutrients. 2020;12:797. doi: 10.3390/nu12030797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tapsell LC, Neale EP, Probst Y. Dietary patterns and cardiovascular disease: insights and challenges for considering food groups and nutrient sources. Curr. Atheroscler. Rep. 2019;21:9. doi: 10.1007/s11883-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during this study are available on reasonable request from the corresponding author, T.K.