There is high mortality in coronavirus disease 2019 (COVID-19)-infected individuals with chronic inflammatory diseases, like obesity, diabetes, and hypertension. A cytokine storm in some patients after infection contributes to this mortality. In addition to lungs, the intestine is targeted during COVID-19 infection. The intestinal membrane serves as a barrier to prevent leakage of microorganisms and their products into the bloodstream; however, dietary fats can affect the gut microbiome and may increase intestinal permeability.
KEYWORDS: COVID-19, cytokine storm, diet, endotoxin, gut bacteria, intestine
ABSTRACT
There is high mortality in coronavirus disease 2019 (COVID-19)-infected individuals with chronic inflammatory diseases, like obesity, diabetes, and hypertension. A cytokine storm in some patients after infection contributes to this mortality. In addition to lungs, the intestine is targeted during COVID-19 infection. The intestinal membrane serves as a barrier to prevent leakage of microorganisms and their products into the bloodstream; however, dietary fats can affect the gut microbiome and may increase intestinal permeability. In obese or diabetic individuals, there is an increase in the abundance of either Gram-negative bacteria in the gut or their product, endotoxin, in systemic circulation. We speculate that when the COVID-19 infection localizes in the intestine and when the permeability properties of the intestinal membrane are compromised, an inflammatory response is generated when proinflammatory endotoxin, produced by resident Gram-negative bacteria, leaks into the systemic circulation. This review discusses conditions contributing to inflammation that are triggered by microbially derived factors from the gut.
OBSERVATION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the CoV disease 2019 (COVID-19) pandemic, in which there is high mortality in individuals with underlying chronic inflammatory conditions. Vulnerable populations include the elderly and those with obesity, diabetes, and hypertension (1). The viral infection is characterized by an overproduction of various cytokines in severe cases, indicating that multiple inflammatory response systems are activated (2). The production of excess cytokines is thought to explain why some COVID-19 patients unexpectedly take a turn for the worse and do not survive (3). The mechanism underlying the cytokine storm is the subject of numerous hypotheses. We suggest that endotoxin, produced by Gram-negative gut bacteria, leaks out of a damaged gut and plays a role in the development of the cytokine storm.
It is now clear that the intestinal tract is likely to be a target for COVID-19 infection. Patients may experience diarrhea and vomiting during infection (4), and SARS-CoV-2 viral RNA has been detected in feces (5). The viral receptor angiotensin-converting enzyme-2 (ACE-2), required for viral entry into susceptible cells, has been found not only in the lung but also in the esophagus and the enterocytes of the ileum and colon (6). During the infection process, enterocytes are presumably infected, and the function of the intestinal membrane is likely compromised. One function of the intestine, as it relates to the cytokine storm, is that it serves as a barrier to prevent the leakage of microorganisms and their products into the bloodstream (7). The bacteria in the gut produce structurally diverse molecules called pathogen-activated molecular patterns (PAMPs), which can stimulate an immune response through Toll-like receptors (8). The PAMPs produced by Gram-negative bacteria include a glycolipid called lipopolysaccharide (LPS) or endotoxin. Endotoxin is part of the outer membrane of the bacterium and is shed during growth and bacterial cell death/lysis (9). It is detected in mouse and human feces (10, 11). Endotoxin stimulates the production of interleukin 6 (IL-6), IL-1, IL-8, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), cytokines that are also found in COVID-19 patients (12, 13). The inflammatory activity of endotoxin is structure dependent and varies by bacterial species and strain (14). For example, LPS of Bacteroides thetaiotaomicron, a prevalent fecal bacterium in the phylum Bacteroidetes, differs structurally from LPS of Escherichia coli and does not activate an inflammatory response (15). LPS produced by E. coli, a proteobacterium, is hexa-acylated, which accounts for its potent inflammatory activity, mediated via TLR4 (16). Endotoxin produced by gut bacteria is presumed to leak into the blood system and contribute to the development of an inflammatory response called metabolic endotoxemia (17). Further study of the gut microbiome is warranted to understand the variable inflammatory response to COVID-19 and whether intestinal lipopolysaccharide-producing Gram-negative bacteria are involved.
It is well known that diet modulates the gut microbiota and influences host health. The most abundant members of the bacterial communities in human feces belong to the phyla Bacteroidetes and Firmicutes (18). Under conditions of high dietary saturated fat, a second taxon of Gram-negative organisms, Proteobacteria, is detected in human feces in some individuals (19), while in other studies they were not (20). This may be related to the amount of fat or the vulnerability of the individual exposed. Increases in the abundances of Proteobacteria were reported in a study using humanized gnotobiotic mice fed a high-fat diet consisting of a mixture of saturated, monounsaturated, and polyunsaturated fats (21). In a meta-analysis of studies examining the effect of a high-fat diet of the mouse fecal microbiome, 15 of 25 murine studies showed that an increase in the Firmicutes-to-Bacteroidetes ratio was predictive of consumption of a high-fat diet (22). There were changes in three major clades identified: Lachnospiraceae and Ruminococcaceae within the Firmicutes and Muribaculaceae within the Bacteroidetes. Increases in abundances of fecal Proteobacteria are reported in studies examining the effect of a high-fat diet (23–25) in the mouse. The increase was accounted for by an increase in Desulfovibrio spp. (24, 25), an organism that produces a hexa-acylated LPS molecule, which is expected to have high inflammatory activity (26). Although the Proteobacteria in mouse feces are detected at low abundance compared to Firmicutes and Bacteroidetes, we hypothesize that the proinflammatory endotoxins produced by Proteobacteria may functionally contribute to an inflammatory response during a COVID-19 infection.
The observed effects of dietary fats on the gut microbiota, specifically the Proteobacteria, may be variable because Proteobacteria are not the dominant taxa in feces (and the large intestine) and therefore may be overlooked in results based on analysis of fecal samples. Results of early studies examining the microbiota of the human small intestine indicate that the relative abundance of Proteobacteria may be higher in the small intestine than in feces (27). For example, stomach, duodenal, jejunum, and stool samples were collected from 8 heathy subjects; Proteobacteria were not detected in the stool but were present in the small intestinal samples (27). Other studies of small intestinal microbiota relied on the use of subjects undergoing esophagogastroduodenoscopy (28), such as for gastroesophageal reflux disease (29). Proteobacteria were detected in the duodenal samples, but it is not clear what role the medical condition may have played in these individuals. It is, however, important to recognize that the abundance of bacteria is several orders of magnitude higher in the large intestine than in the small intestine. Clearly, these studies represent the first steps in the development of methods to understand microbial communities in the small intestine.
The detection of Proteobacteria in the small intestine raises the possibility that it is from this section of the intestine that endotoxin molecules with high proinflammatory activity translocate from the gut and contribute to the inflammatory response during a COVID-19 infection rather than from the endotoxin produced by the Gram-negative bacteria of the large intestine. In vulnerable populations, such as in obese individuals, it is known that postprandial endotoxemia is higher than in lean subjects (30) and that postprandial inflammation is higher in lean individuals after consuming cream compared to water (31). The increase in postprandial endotoxemia occurs within hours of meal ingestion, suggesting that absorption of endotoxin occurs after gastric emptying into the proximal small intestine (32). The increases in postprandial endotoxemia and inflammation may be due to chronic high intake of fat, which induces changes in the intestinal membrane permeability properties (33). Identifying the bacterial communities in the small intestines of lean versus obese individuals may lead to a better understanding of how intestinal bacteria might play a role in the cytokine storm that many COVID-19 patients experience. The use of a humanized mouse gut microbiota model to study the effect of diet and other exogenous factors might be an ideal way to understand the specific effects of different fatty acids on the gut microbiota. To study an endotoxin-mediated inflammatory response, developing model conditions with an animal species that is more sensitive to endotoxin, like humans, than mice are (34) could help unravel the role that different Gram-negative bacteria in the intestinal tract might play in inflammatory diseases.
There is an increasing number of studies examining the gut microbiota in at-risk populations for COVID-19 infection, such as those with diabetes and those who are obese. For example, a comparison of the fecal microbiomes of treatment-naive (TN) type 2 diabetic (T2D), prediabetic, and normal glucose-tolerant subjects (35) showed increases in multiple genera within the Gram-negative Bacteroidetes phylum only in the TN T2D patients. Interestingly, higher levels of Escherichia coli were detected in the pre-T2D subjects. E. coli, depending on strain, produces a highly proinflammatory endotoxin molecule (36). These results suggest that the Gram-negative communities in the intestinal tract of diabetic subjects may be enriched with bacterial strains/species that produce the most proinflammatory endotoxin molecules, which warrants further study. In obese individuals, there is lower diversity in the fecal bacterial communities and the Firmicutes-to-Bacteroidetes ratio is higher than in lean individuals (18), although there is some question as to whether an increased Firmicutes-to-Bacteroidetes ratio is a reproducible marker of obesity in humans (22).
Since blood levels of endotoxin are higher in obese individuals than in lean individuals (37), the results of human dietary intervention studies are of potential interest. In a 6-month randomized controlled-feeding trial using primarily soybean oil, a source of mono- and polyunsaturated fatty acids, increases in Bacteroides spp. were reported (38). There was no mention of an effect on Proteobacteria. In contrast, a small study consisting of healthy men fed a high-saturated-fat diet for 7 days reported an increase in Betaproteobacteria in a subset of individuals (19). Consumption of a high-fat diet (mixed fatty acids) by mice changes the fecal microbiome to raise the Firmicutes-to-Bacteroidetes ratio (39), while a diet high in saturated fats is associated with an increase in Proteobacteria (23–25). Results from murine studies (40, 41) indicate that the consumption of a diet rich in monounsaturated fatty acids, supplied as extra virgin olive oil, changes the fecal microbiome in a manner that is expected to lead to a reduction in endotoxins with proinflammatory activity. A decrease in the abundance of Desulfovibrionaceae was reported (40), and a decrease in the relative abundances of bacteria identified as aerobes and facultative anaerobes and bacteria likely to produce proinflammatory endotoxins was noted (41). These studies highlight the need for research to understand how dietary fats might modulate the types of bacteria that may produce highly inflammatory endotoxin molecules.
The reduction of gut Proteobacteria may be one way to reduce the level of inflammatory signals and thereby reduce the severity of a COVID-19 infection. In situations in which Proteobacteria in the gut are in high abundance, the leakage of proinflammatory endotoxin from the gut is hypothesized to add to the TLR4-mediated inflammation that the host develops in response to the viral infection. The acute lung injury that develops in SARS and in other conditions is mediated by host-derived oxidized phospholipid generated by NADPH oxidase-dependent production of reactive oxygen species as part of the immune response (42). Oxidized phospholipid is a potent stimulator of TLR4 (42). Interestingly, the lung pathology induced by influenza is reversed with a TLR4 antagonist, eritoran (43). It is thus of interest to understand whether high levels of circulating endotoxin in combination with host-derived TLR4 agonists (activator) are involved in triggering a more intense cytokine storm in vulnerable populations.
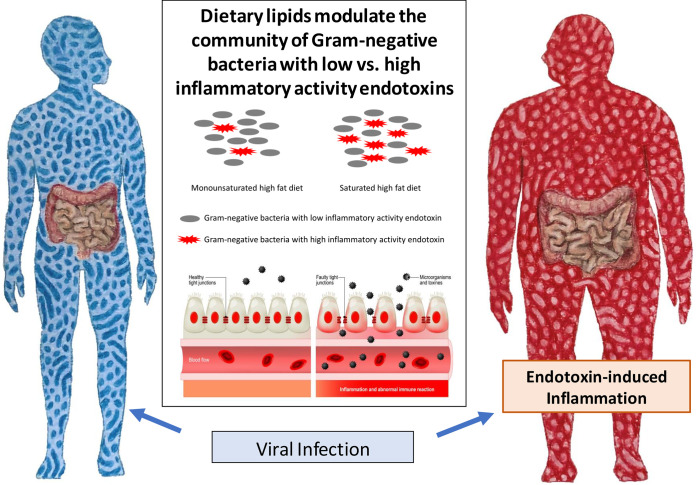
In conclusion, we suggest that as we prepare to live with COVID-19, individuals with chronic inflammatory diseases should consider changing their diets before they are infected to attenuate the development of the most severe symptoms. A standard dietary intervention approach to mitigate chronic diseases is to decrease total fats, and while most agree that reducing the ratio of saturated fatty acids to monounsaturated fatty acids is beneficial, this is still debated (44). Here, we speculate, as depicted in Fig. 1, that shifting from a diet high in saturated fats to one with monounsaturated fats will reduce the numbers of those bacteria that produce the most inflammatory endotoxin molecules and thereby reduce the severity of the inflammatory response to a COVID-19 infection in vulnerable individuals, such as in obese individuals. Finally, although COVID-19 gains access to cells via the ACE-2 receptor, translocation of the virus from the gut to the systemic circulation should be considered if the intestinal membrane is compromised prior to the COVID-19 infection. The use of animal models to study the pathogenesis of COVID-19 will provide opportunities to more fully understand why this novel virus has devastating complications in some individuals but not in others.
FIG 1.
Intestinal permeability, altered gut microbiome, and fatty acid intake can raise the risk of endotoxin-induced inflammation. It is hypothesized that a viral infection in a patient with a high-risk condition exacerbates the inflammatory response.
Footnotes
Citation Onishi JC, Häggblom MM, Shapses SA. 2020. Can dietary fatty acids affect the COVID-19 infection outcome in vulnerable populations? mBio 11:e01723-20. https://doi.org/10.1128/mBio.01723-20.
Contributor Information
Dominique Soldati-Favre, University of Geneva.
Jack Gilbert, UC San Diego.
Peter Turnbaugh, University of California, San Francisco.
Clett Erridge, Anglia Ruskin University.
REFERENCES
- 1.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP, the Northwell COVID-19 Research Consortium. 2020. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. 2012. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brumfiel G. 2020. Why some COVID-19 patients crash: the body's immune system might be to blame. NPR Health News, Washington, DC. https://www.npr.org/sections/health-shots/2020/04/07/828091467/why-some-covid-19-patients-crash-the-bodys-immune-system-might-be-to-blame.
- 4.Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. 2020. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Parker J, Smits S, Underwood J, Dolwani S. 2020. Persistent viral shedding of SARS-CoV-2 in faeces—a rapid review. Colorectal Dis 22:611–620. doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Li Z, Cui X, Xiao J, Zhan J, Meng T, Zhou W, Liu J, Xu H. 2020. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut Epub 69:1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- 7.Groschwit KR, Hogan SP. 2009. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124:3–22. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutler B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 9.Kaparakis-Liaskos M, Ferrero R. 2015. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol 15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura S, Hirayam K, Mishima M, Miyaji K, Akiyama T, Mitsuoka T. 1990. Endotoxic activity in faeces of mice from different microbiological environments. Res Microbiol 141:1095–1101. doi: 10.1016/0923-2508(90)90083-3. [DOI] [PubMed] [Google Scholar]
- 11.Van Saene JM, Stoutenbeek CP, van Saene H. 1992. Faecal endotoxin in human volunteers: normal values. Microb Ecol Health Dis 5:179–184. doi: 10.3109/08910609209141584. [DOI] [Google Scholar]
- 12.Cavaillon JM. 2018. Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon 149:45–53. doi: 10.1016/j.toxicon.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Schett G, Sticherling M, Neurath MF. 2020. COVID-19: risk for cytokine targeting in chronic inflammatory diseases. Nat Rev Immunol 20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raetz CRH, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson AN, Choudhury BP, Fischbach MA. 2018. The biosynthesis of lipooligosaccharide from Bacteroides thetaiotaomicron. mBio 9:e02289-17. doi: 10.1128/mBio.02289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haimovich B, Reddell MT, Calvano JE, Calvano SE, Macor MA, Coyle SM, Lowry SF. 2010. A novel model of common Toll-like receptor 4- and injury-induced transcriptional themes in human leukocytes. Crit Care 14:R177. doi: 10.1186/cc9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastelli M, Knauf C, Cani PD. 2018. Gut microbes and health: a focus on the mechanisms linking microbes, obesity, and related disorders. Obesity (Silver Spring) 26:792–800. doi: 10.1002/oby.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 19.Ott B, Skurk T, Lagkouvardos L, Fischer S, Büttner J, Lichtenegger M, Clavel T, Lechner A, Rychlik M, Haller D, Hauner H. 2018. Short-term overfeeding with dairy cream does not modify gut permeability, the fecal microbiota, or glucose metabolism in young healthy men. J Nutr 148:77–85. doi: 10.1093/jn/nxx020. [DOI] [PubMed] [Google Scholar]
- 20.Lang JM, Pan C, Cantor RM, Tang WHW, Garcia-Garcia JC, Kurtz I, Hazen SL, Bergeron N, Krauss RM, Lusis AJ. 2018. Impact of individual traits, saturated fat, and protein source on the gut microbiome. mBio 9:e01604-18. doi: 10.1128/mBio.01604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ. 2019. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 26:265–272.e264. doi: 10.1016/j.chom.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahim RS, Bushman F, Wu GD. 2009. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Zhang M, Pang X, Zhao Y, Wan L, Zhao L. 2012. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J 6:1848–1857. doi: 10.1038/ismej.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onishi JC, Campbell S, Moreau M, Patel F, Brooks AI, Zhou YX, Häggblom MM, Storch J. 2017. Bacterial communities in the small intestine respond differently to those in the caecum and colon in mice fed low- and high-fat diets. Microbiology 163:1189–1197. doi: 10.1099/mic.0.000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang-Sun W, Augusto LA, Zhao L, Caroff M. 2015. Desulfovibrio desulfuricans isolates from the gut of a single individual: structural and biological lipid A characterization. FEBS Lett 589:165–171. doi: 10.1016/j.febslet.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Seekatz AM, Schnizlein MK, Koenigsknecht MJ, Baker J, Hasler WL, Bleske BE, Young VB, Sun D. 2019. Spatial and temporal analysis of the stomach and small-intestinal microbiota in fasted healthy humans. mSphere 4:e00126-19. doi: 10.1128/mSphere.00126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leite GGS, Morales W, Weitsman S, Celly S, Parodi G, Mathur R, Sedighi R, Barlow GM, Rezaie A, Pimentel M. 2019. Optimizing microbiome sequencing for small intestinal aspirates: validation of novel techniques through the REIMAGINE study. BMC Microbiol 19:239. doi: 10.1186/s12866-019-1617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nardelli C, Granata I, D'Argenio V, Tramontano S, Compare D, Guarracino MR, Nardone G, Pilone V, Sacchetti L. 2020. Characterization of the duodenal mucosal microbiome in obese adult subjects by 16S rRNA sequencing. Microorganisms 8:485. doi: 10.3390/microorganisms8040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vors C, Pineau G, Drai J, Meugnier E, Pesenti S, Laville M, Laugerette F, Malpuech-Brugère C, Vidal H, Michalsk MC. 2015. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose-effect trial. J Clin Endocrinol Metab 100:3427–3435. doi: 10.1210/jc.2015-2518. [DOI] [PubMed] [Google Scholar]
- 31.Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. 2010. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 33:991–997. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbain JL, Vekemans MC, Bouillon R, Van Cauteren J, Bex M, Mayeur SM, Van den Maegdenbergh V, Bataille G, Charkes ND, Malmud LS. 1993. Characterization of gastric antral motility disturbances in diabetes using a scintigraphic technique. J Nucl Med 34:576–581. [PubMed] [Google Scholar]
- 33.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. 2020. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nut 11:7–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redl H, Bahrami S, Schlag G, Traber DL. 1993. Clinical detection of LPS and animal models of endotoxemia. Immunobiology 187:330–345. doi: 10.1016/S0171-2985(11)80348-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhong H, Ren H, Lu Y, Fang C, Hou G, Yang Z, Chen B, Yang F, Zhao Y, Shi Z, Zhou B, Wu J, Zou H, Zi J, Chen J, Bao X, Hu Y, Gao Y, Zhang J, Xu X, Hou Y, Yang H, Wang J, Liu S, Jia H, Madsen L, Brix S, Kristiansen K, Liu F, Li J. 2019. Distinct gut metagenomics and metaproteomics signatures in prediabetics and treatment-naïve type 2 diabetics. EBioMedicine 47:373–383. doi: 10.1016/j.ebiom.2019.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dabbah R, Ferry E, Gunther DA, Hahn R, Mazur P, Neely M, Nicholas P, Pierce JS, Slade J, Watson S, Weary M, Anford RL. 1980. Pyrogenicity of E. coli 055:B5 endotoxin by the USP rabbit test—a HIMA collaborative study. PDA J Pharm Sci Technol 34:212–216. [PubMed] [Google Scholar]
- 37.Hawkesworth S, Moore SE, Fulford AJC, Barclay GR, Darboe AA, Mark H, Nyan OA, Prentice AM. 2013. Evidence for metabolic endotoxemia in obese and diabetic Gambian women. Nutr Diabetes 3:e83–e83. doi: 10.1038/nutd.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, Li H, Wang R, Tang J, Huang T, Zheng J, Sinclair AJ, Mann J, Li D. 2019. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 68:1417–1429. doi: 10.1136/gutjnl-2018-317609. [DOI] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. 2008. Marked alterations in the distal gut microbiome linked to diet-induced obesity. Cell Host Microbe 3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prieto I, Hidalgo M, Segarra AB, Martínez-Rodríguez AM, Cobo A, Ramírez M, Abriouel H, Gálvez A, Martínez-Cañamero M. 2018. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS One 13:e0190368. doi: 10.1371/journal.pone.0190368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abulizi N, Quin C, Brown K, Chan YK, Gill SK, Gibson DL. 2019. Gut mucosal proteins and bacteriome are shaped by the saturation index of dietary lipids. Nutrients 11:418. doi: 10.3390/nu11020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YHC, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JSM, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JCG, Vogel SN. 2013. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraus RM, Kris-Etherton PM. 3 June 2020. Public health guidelines should recommend reducing saturated fat consumption as much as possible: debate consensus. Am J Clin Nutr doi: 10.1093/ajcn/nqaa134. [DOI] [PubMed] [Google Scholar]