Abstract
Cancer stem cells are initiating cells of cancer and propagate its growth through self-renewal and differentiation of its daughter cells. CD133 is a cell surface antigen that is present on glioma stem cells and has been used to prospectively isolate glioma stem cells. We hypothesized that a major histocompatibility complex (MHC)-independent and long-lasting immune response against CD133 could be generated by transfecting CD133 mRNA into dendritic cells and vaccinating animals with experimental gliomas. To test this hypothesis, we developed a novel humanized mouse model using CD34-positive hematopoietic stem cells. We confirmed the robust simultaneous activation of CD8- and CD4-positive T cells by dendritic cell vaccination with modified CD133 mRNA leading to a potent and long-lived immune response, with subsequent abrogation of CD133-positive glioma stem cell propagation and tumor growth. This study for the first time demonstrates in both a humanized mouse model and in a syngeneic mouse model of glioblastoma that targeting a glioma stem cell-associated antigen is an effective strategy to target and kill glioma stem cells. This novel and simple humanized mouse model for immunotherapy is a significant advance in our ability to test human-specific immunotherapies for glioblastoma.
Keywords: CD133, GBM, dendritic cell vaccine, humanized mouse model
Graphical Abstract

CD133 is a cell surface antigen on glioma stem cells. Do et al. show that dendritic cells transfected with CD133 mRNA are able to stimulate activation of both CD8 and CD4 cells in an MHC-independent method. The T cells that were elicited showed abrogation of CD133-positive glioma stem cell propagation and tumor growth in both humanized and syngeneic mouse models.
Introduction
Glioblastoma (GBM) represents the most lethal form of brain cancer and comprises 15.4% of primary malignant brain tumors, which have a yearly incidence rate of 7.25/100,000.1 Current treatments, such as surgical resection, radiation, and chemotherapy, have done little to improve the median survival time of GBM patients, which now stands at approximately 14.6 months.2 Brain tumor stem cells (BTSCs) are prospectively isolated by their cell-surface marker CD133.3, 4, 5, 6 Enrichment of CD133 BTSCs (CD133-positive) has indicated a highly tumorigenic population7,8 that is negatively correlated with patient survival,9 making these cells an ideal population for targeted immunotherapy. We previously identified histocompatibility leukocyte antigen (HLA)-A2-restricted epitopes for CD13310 and tested its safety in HLA-A2 transgenic mice. We considered the use of CD133 mRNA for the following potential benefits: the absence of HLA restriction, which enables treatment of all patients; the use of multiple potential cytotoxic and helper epitopes, generating both helper and cytotoxic T cell responses; and the ability to test both in a syngeneic as well as a humanized model of intracranial GBM.
The challenge with targeted immunotherapy is that several approaches have been developed exclusively using ex vivo analysis, non-invasive procedures, or moving immediately to clinical trials.11 Such approaches have been deemed necessary largely because animal modeling has been hindered by differences in mammalian biology, particularly within the immune system where many aspects are species specific. This problem has been exacerbated by the fact that new therapeutic and immunomodulatory agents are human specific. Although humanized mouse models have been previously created,12, 13, 14 in this study, we use a novel modification of a CD34-positive stem cell-generated immune system in a humanized mouse model, where dendritic cells (DCs) can supply the necessary interleukin (IL)-2 to generate an anti-tumor cellular immune response. We test the efficacy of this vaccine approach and suggest that this study lays the foundation for pre-clinical testing of human-specific immunologic interventions for GBM.
Results
CD133 Is Highly Expressed on BTSCs
We first determined whether our BTSCs (murine GL261 and human BTSC5) had the hallmark features of BTSCs (i.e., self-renewal and differentiation) that have been previously described by us and others.3, 4, 5, 6 GL261 and BTSC5 cultured in stem cell media resulted in neurosphere formation. CD133 expression was observed on neurosphere-forming cells by immunofluorescence staining (Figure S1). Fluorescence-activated cell sorting (FACS) analysis indicated that CD133 is highly expressed on BTSCs, with 79.04% of BTSC5 cells and 20.1% of GL261 cells being positive for CD133 expression (Figure S2).
DCs Transfected with Modified CD133 mRNA Showed Increased T Cell Activation
Using an attached signal sorting (SS) fragment and a transmembrane–cytoplasmic (TM/cyto) domain fragment juxtaposed on either side of CD133 (Figure S3), human or mouse, depending on which mouse model was used, we were able to allow for cross-presentation of major histocompatibility complex (MHC) class I- and class II-restricted antigens, thereby enhancing the immune response. The SS fragment and TM/cyto domain fragments promoted the transport of CD133 protein efficiently not only to MHC class I compartments but also to MHC class II compartments on DCs for eventual cross-presentation.15,16
To evaluate DC function for antigen presentation, as well as the potential for activation of T cells, we analyzed DC IL-12 production. DCs transfected with modified human CD133 mRNA demonstrated increased secretion of IL-12 at 24 and 48 h after maturation as compared to DCs without RNA transfection. At 24 h, DCs that were transfected showed 318 pg/mL versus 170 pg/mL in non-transfected DCs. This effect on IL-12 release was maintained in DCs that were transfected at 48 h, measuring 305 pg/mL (Figure 1A), showing that transfected DCs are more efficient at activating T cells.
Figure 1.
Dendritic Cells Transfected with Modified CD133 mRNA Showed Increased T Cell Activation
(A) Graph depicting IL-12 releasing ability from immature dendritic cells (DCs), non-transfected mature DCs, and from DCs transfected with modified human CD133 mRNA at 24 h after maturation and at 48 h after maturation. (B) Graph depicting IL-2 production from T cells only, DCs transfected with CD133 only, T cells cultures with non-transfected DCs, and T cells cultured with DCs transfected with CD133. (C) Graph depicting IFN-γ releasing ability from DCs cultured with human BTSCs and various other cell groups. (D) Graph depicting IFN-γ releasing ability from DCs cultured with murine BTSCs and various other cell groups.
To further examine the immune response elicited by DCs, we measured IL-2 production as a means of evaluating cell proliferation and T cell activation to effector cells. As shown in Figure 1B, there was a 2-fold higher production of IL-2 when T cells were co-cultured with DCs transfected with modified mRNA versus T cells co-cultured with non-transfected DCs (116 pg/mL versus 55 pg/mL), indicating that transfected DCs not only activate T cells but that there is a corresponding T cell response. DCs transfected with modified mRNA without T cells and T cells cultured without DC stimulation had IL-2 production of 33 and 32 pg/mL/104 cells, respectively.
Next, we determined whether transfected DCs, cultured with T cells, would mount an immune response to BTSCs. As a measure of T cell activation, we determined the interferon (IFN)-γ production from T cells stimulated with DCs. DCs transfected with CD133 mRNA co-cultured with CD4-positive T cells and CD8-positive T cells with BTSC5 elicited the highest IFN-γ release (137 pg/mL/104 cells) compared to all other conditions (Figure 1C). In contrast, DCs transfected with modified human CD133 mRNA co-cultured with only CD8-positive T cells and BTSC5 showed 84 pg/mL/104 cells of IFN-γ releasing ability. However, when DCs without RNA transfection were co-cultured with CD4- and CD8-positive T cells and BTSC5, DCs showed only 1.6 pg/mL of IFN-γ releasing ability. DCs transfected and cultured with either CD8-positive T cells only or both CD4- and CD8-positive T cells without BTSC5 showed little IFN-γ releasing ability, that is, 2.5 and 30.3 pg/mL, respectively.
Similarly, when mouse DCs were transfected with modified mouse CD133 mRNA and co-cultured with CD4-positive T cells, CD8-positive T cells, and GL261, DCs showed the highest IFN-γ release of 125 pg/mL/104 cells (Figure 1D). In contrast, DCs transfected with modified mouse CD133 mRNA co-cultured with only CD8-positive T cells and GL261 showed 66 pg/mL of IFN-γ releasing ability. However, when DCs without RNA transfection were co-cultured with CD4- and CD8-positive T cells and GL261 and the DCs showed only 33 pg/mL of IFN-γ releasing ability. DCs transfected and cultured with either CD8-positive T cells only or both CD4- and CD8-positive T cells without GL261 showed little IFN-γ releasing ability. Therefore, in the presence of BTSCs with CD133, transfected DCs and T cells released more cytokines to further activate the immune system than did non-transfected DCs or T cells alone.
DCs Transfected with CD133 mRNA Induced Tumor Killing Activity In Vitro
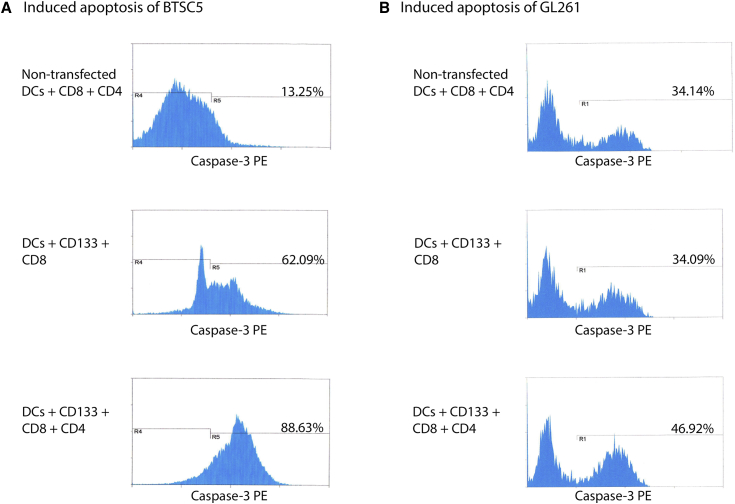
To confirm that DCs were able to elicit cytotoxic T lymphocytes (CTLs), CTL killing assays were performed with both human and murine cancer stem cells. The greatest proportion of cells were killed when CD4- and CD8-positive T cells were stimulated with DCs transfected with modified human CD133 mRNA with 88.63% of BTSC5 cells induced to apoptosis (Figure 2A). This was in comparison to CD8 cells alone (62.09%) or CD8 and CD4 cells stimulated with non-transfected DCs (13.25%). As for GL261 cells, when co-cultured with CD4- and CD8-positive cells stimulated by DCs transfected with modified murine CD133 mRNA, 46.92% of GL261 cells were induced to apoptosis. However, when cultured with only CD8-positive cells stimulated by DCs transfected with modified murine CD133 mRNA or CD4-positive cells and CD8-positive cells stimulated by DCs without RNA transfection, 34.09% and 34.14% of GL261 cells were induced to apoptosis, respectively (Figure 2B). These results confirm the elicitation of both CD4 helper and CD8 cytotoxic T cell responses as a result of vaccination with DCs transfected with CD133 mRNA.
Figure 2.
DCs Transfected with CD133 mRNA Induced Tumor Killing Activity In Vitro
(A) CTL assays showing percentage of BTSC5 cells induced to apoptosis after culture with the various groups of cells. (B) CTL assays showing percentage of GL261 cells induced to apoptosis after culture with the various groups of cells.
Successful Humanization of Immune-Deficient Mice with Stem Cell Transplant
To develop an appropriate animal mouse model for immunotherapy that could recapitulate the human microenvironment, and yet be conducive to brain tumor formation, we assessed the potential of humanization of immuno-deficient NOG mice.17 CD34-positive hematopoietic stem cells isolated from human peripheral blood mononuclear cells (PBMCs) that have the same HLA phenotype as BTSCs were implanted into NOG mice. NOG mice received a busulfan injection (for suppression of their immune system), and CD34-positive hematopoietic stem cells were implanted via tail vein injection. Blood collections were performed 4 and 8 weeks after CD34-positive hematopoietic stem cell implantation. Blood samples collected at 4 weeks after humanization showed that 11.80% were CD45-positive and of those 31.42% were human CD3-positive cells. Furthermore, 0.19% of human CD45-positive cells were human CD4-positive cells, and 13.29% of human CD45-positive cells were CD8-positive cells.
DC Vaccination Modified with CD133 mRNA Induces Immune Response
In order to test the efficacy of our cancer vaccine in our humanized mouse model, we separated mice into three groups at 40 days after humanization. Group 1 received a vaccination of DCs transfected with modified human CD133 mRNA. Group 2 received a vaccination of DCs without mRNA transfection. Group 3 received an injection of phosphate-buffered saline (PBS). Blood samples were collected from all groups at 8 weeks after humanization. The population of human CD3-positive cells (gated from CD45-positive cells) was 18.54% in group 1. Human CD3-positive cells were 0.7% and 4.96% in groups 2 and 3, respectively. In group 1, the population of CD8- and CD4-positive T cells within the CD3 cell population was 69.97% and 33.33%, respectively. In groups 2 and 3, CD8- and CD4-positive cells could not be detected (Figure 3).
Figure 3.
DC Vaccination Modified with CD133 mRNA Induces Immune Response in Humanized Mouse Model
(A) Population of CD8-positive T cells in blood samples collected from three groups of humanized mice at 8 weeks after humanization. Group 1: vaccinated with DCs transfected with modified human CD133 mRNA. Group 2: vaccinated with DCs without mRNA transfection. Group 3: vaccinated with phosphate-buffered saline. (B) Population of CD4-positive T cells in the aforementioned groups.
Humanized Mice Given Transfected DC Vaccination Were Able to Generate Specific CTLs
To confirm the generation of CD133-specific CTLs in the humanized mouse models, we used a tetramer assay and evaluated blood samples collected from humanized mice that were vaccinated. Two tetramers were developed and previously described to detect T cells that respond to two HLA-A2-restricted 9-aa epitopes of CD133. The numbers of CD8-positive cells that responded to tetramers of HLA-A2 combined with CD133-405 peptide, HLA-A2 combined with CD133-753 peptide, and HLA-A2 alone were 0.96%, 26.3%, and 4.11%, respectively, in blood samples from group 1 (Figure 4). Non-transfected DCs (without CD133 mRNA) or the injection of PBS instead of DCs (i.e., groups 2 and 3) was unable to generate enough CD8-positive cells to perform the tetramer assay.
Figure 4.
Humanized Mice Given Transfected DC Vaccination Were Able to Generate Specific Cytotoxic T Lymphocytes
Shown are the amounts of CD8-positive cells that responded against HLA-A2 combined with CD133-405 peptide, HLA-A2 combined with CD133-753 peptide, and non-combined HLA-A2 tetramer. Note that results are from the group 1 humanized mice. There were not enough CD8-positive cells to perform the tetramer assay in groups 2 and 3.
Mice Given Transfected DC Vaccination Had Improved Survival
To assess the anti-tumor effect of DC vaccination against an orthotropic GBM mouse model, we generated a DC vaccine where we transfected either mouse or human CD133 mRNA into DCs for vaccination against mice bearing intracranial human GBMs. We performed a Kaplan-Meier analysis on the humanized mouse model. Those treated with DCs transfected with human CD133 mRNA had median survival times of more than 60 days, whereas those mice treated with non-transfected DCs had median survival times of 40 days and 38 days for the PBS control group (Figure 5A). Results of the Wilcoxon test of equality for the three groups were significantly different with a p value of 0.0013.
Figure 5.
Kaplan-Meier Analysis on the Humanized and Syngeneic Mouse Model Showed That Mice Given a Transfected DC Vaccination Had Improved Survival
(A) Kaplan-Meier curves in the humanized mouse model for the various treatment groups. (B) Kaplan-Meier curves in the syngeneic mouse model for the various treatment groups.
We also performed a Kaplan-Meier analysis on the syngeneic mouse model. Mice treated with DCs transfected with mouse CD133 mRNA had median survival times of 38 days versus those treated with non-transfected DCs, which had median survival times of only 20 days. The PBS control group had a median survival time of 22 days (Figure 5B). Results of the Wilcoxon test of equality for the three groups were significantly different with a p value of 0.007. These results revealed that vaccination using DCs transfected with modified CD133 mRNA can prolong the survival of GBM-bearing mice.
Immunohistochemistry Analysis of Mouse Models Revealed Infiltration in Mice Treated with Transfected DC Vaccine
To confirm the generation of human immunocompetent cells in humanized mice, spleen and bone marrow were collected from humanized mice that had been vaccinated with DCs transfected with modified CD133 mRNA after humanization or from normal NOG mice. H&E staining revealed the presence of granulocytes in the spleen and bone marrow from humanized mice (Figures 6A and 6B). Using immunohistochemical (IHC) staining, human CD34-positive cells were also confirmed in spleen and bone marrow from humanized mice (Figures 6C and 6D). IHC staining further revealed numerous human CD45-positive cells that infiltrated into lymphoid follicles in the spleens of humanized mice (Figure 6E). Human CD3-, CD4-, and CD8-positive cells were among the human CD45-positive cells (Figures 6F, 6G and 6H). Human immunocompetent cells were not found in the spleen or bone marrow from normal NOG mice (data not shown). IHC staining revealed that human CD45, CD3, CD4, and CD8 were also confirmed in the bone marrow of humanized mice (data not shown). However, there was a paucity of those cells compared to the numbers found in the spleen.
Figure 6.
Immunohistochemical Analysis
(A and B) H&E staining of bone marrow (A) and of spleen (B) of a humanized mouse that received vaccination using DCs transfected with CD133 mRNA revealed granulocytes in spleen and bone marrow of the mouse. (C and D) H&E staining from spleen (C) and bone marrow (D) from a humanized mouse that received vaccination using DCs transfected with CD133 mRNA (8 weeks after humanization) revealed human CD34-positive cells. (E) Immunohistochemical staining also revealed numerous amounts of human CD45-positive cells in the spleen. (F–H) Within the CD45-positive cells were CD3-positive cells (F), CD4-positive cells (G), and CD8-positive cells (H). (I) Immunofluorescence analysis of humanized mouse brain (removed 30 days after tumor implantation). DAPI (blue) showed that an implanted GBM had high cell density and involved the necrosis area, with CD133 positivity (green) found on the extracellular membrane. (J) There was no tumor found in NOG mice vaccinated with DCs transfected with CD133 mRNA. (K) Brain tissues from a humanized mouse that received PBS as a control showed a large tumor. (L) Brain tissues from a humanized mouse that received vaccination using DCs without transfection showed a large tumor.
Brains of humanized mice were collected at 30 days after BTSC5 implantation in order to confirm CD133 expression and T cell infiltration. Immunofluorescence staining revealed that brain tissue from non-treated humanized mice has large brain tumors and high cellularity, including necrotic lesions with CD133-positive cells confirmed at the border of necrotic lesions, and CD133 proteins were shown to exist on the extracellular membrane of tumor cells (Figure 6I). Alternatively, CD133-positive cells could not be confirmed in normal brain (data not shown).
IHC staining revealed that there was no tumor growth in brain tissue from humanized mice that received vaccination using DCs transfected with CD133 mRNA (Figure 6J). IHC staining of humanized mouse brains that received PBS vaccination showed large tumors, and human CD3-positive cells infiltrated into brain tumor (Figure 6K). Infiltration of human CD4-positive cells and human CD8-positive cells were rarely seen by IHC analysis of non-treated humanized mice (data not shown). Mouse brains of animals vaccinated with DCs without mRNA transfection demonstrated large tumor growth and infiltration of human CD3-positive cells into mouse brain (Figure 6L). However, there was an absence of CD4- and CD8-positive cell infiltration (data not shown).
Discussion
Cancer stem cells from human brain tumors can be enriched by prospectively isolating CD133-positive tumor cells.3, 4, 5, 6,18 CD133-positive BTSCs have been investigated as a means of elucidating the tumorigenic process in the central nervous system and to develop therapies that target BTSCs.7,8,19, 20, 21 The exact cellular functions of CD133 are not well understood; however, its association with resistance to radiation and chemically induced cell death has been established. As a large protein with five transmembrane domains, CD133 may trigger a cascade of intracellular signals to activate a host of genes that are involved in cellular survival and DNA repair mechanisms. Our aim here was to exploit the glioma stem cell properties associated with CD133 through immunologic targeting of this stem cell marker.
Historically, DCs are powerful antigen-presenting cells that induce and maintain primary CTL responses directed against tumor antigens. Therefore, there has been a concerted effort to determine how to generate and enhance CTL responses through modification of DCs to express tumor antigens or immunostimulatory molecules via gene transfer, mRNA transfection, peptide stimulation, or protein antigen loading.22, 23, 24, 25 We previously identified CTL epitopes on CD133 that could target human CD133-bearing cancer stem cells in vitro; however, the HLA-restricted nature of this approach relied solely on the CD8 cytotoxic arm of the T cell response and limited the patient population that could be treated. We hypothesized that with the use of DCs transfected with mRNA, we would be able to induce a synergistic helper and cytotoxic T cell response. In this study, we enhanced the anti-tumor effect of DCs transfected with mRNA by enabling the efficient presentation of CD133 through both MHC class I and II compartments (cross-presentation) via a modified plasmid cassette.15,16,26 This was verified with blood samples in a humanized NOG mouse model showing large populations of CD4 and CD8 cells and immunohistochemistry analysis showing infiltration into GBMs induced in the mouse models. Furthermore, cytokine-releasing assays revealed that modifications to mRNA can boost the IL-12 and stimulatory effect of DCs to induce IFN-γ release from T cells. These results revealed that MHC class I-restricted antigen presentation to CD8 coincided with MHC class II-restricted antigen presentation to CD4 and would therefore be able to enhance the immune activity of not just MHC class I but class II as well.
The mechanism by which CD133 is presented is thought to be due to the ability to introduce the entire CD133 protein to the DC and allowing antigen processing to take place by both MHC class I and MHC class II pathways. The mechanism by which CD133 is presented is thought to be due to the ability to introduce the entire CD133 protein to the DC and allowing antigen processing to take place by both the MHC class I and MHC class II pathways. In our previous study, we showed that CD133 contains at least two HLA-A2-restricted cytotoxic T cell epitopes that show immunogenicity, which we converted to peptides, deemed CD133-405 (ILSAFSVYV) and CD133-753 (YLQWIEFSI). We showed that of the two peptides, CD133-405, when loaded into DCs and co-cultured, elicited a significant immune response.10 However, in this study, where CD133 mRNA was transfected into HLA-A2-bearing DCs, the 405 region of the protein did not elicit a significant immune response; instead, the 753 region did. We suspect that due to the hydrophobic nature of peptide CD133-405, which has been reported27 to contain the amino acid sequence AFS, that antigen processing alters the presentation to MHC molecules resulting in no significant immune response. Thus, when peptide loading of DCs is replaced with transfection of the entire CD133 mRNA sequence, antigen processing may impact the hydrophobic nature of peptide 405, resulting in a higher immune response in peptide CD133-753. Consistent with this hypothesis, it has been reported that the hydrophobic structure is known to be easily cleaved by endosomal immunoproteases in the course of antigen processing. In the present study, we showed that 26.3% of CD8-positive T cells responded against CD133-753 peptide by a tetramer assay. This value was extremely high compared with our previous data.28
The results of an in vitro killing assay also showed that antigen presentation to CD8 coincided with antigen presentation to CD4 by DCs transfected with modified CD133 mRNA, resulting in a substantial increase in tumor cell death. Co-culturing of human CD8- and CD4-positive T cells with human DCs transfected with modified CD133 mRNA resulted in 86.63% cell death by a caspase-3 assay. Interestingly, co-culturing with mouse CD8- and CD4-positive T cells induced by mouse DCs transfected with modified CD133 mRNA resulted in 46.92% cell death. The apparent decrease in cell death induction in GL261 compared to BTSC5 cells is consistent with the level of CD133 expression we see between the two BTSCs, where BTSC5 contained 79.04% of CD133-positive cells but GL261 neurosphere cells showed only 20.17% CD133-positive cells.
With the knowledge that DCs transfected with CD133 mRNA elicited cytokine release, and in vitro killing of BTSCs, we aimed to develop a targeted therapy to CD133 within a mouse model that was both efficacious and safe. Previously, we have demonstrated the safety of vaccinations with specific HLA-A2-restricted epitopes of CD133 in an HLA-A2 transgenic mouse model.10 However, we could not test this human therapy in an experimental animal tumor model. Hence, we focused on the development of a humanized mouse model to investigate the efficacy of targeting CD133 cancer stem cells in mice bearing intracranial tumors.
In mice, similar to humans, the primary lymphoid organs are the bone marrow and the thymus. Normal immunocompetent cells are produced in the bone marrow, and specific expansion of CD8-positive T cells occurs in the thymus under IL-2 secretion. However, our humanized mouse model (NOG.Cg-Prkdcscid Il2rgtm1Sug/JicTac mice) lacks a thymus, and therefore one would think that it lacks the capacity to produce CD8-positive T cells. Previous work has suggested that CD8-positive cells were present just after humanization but decreased gradually over time in a humanized mouse model.29,30 Other laboratories have used human fetal thymus implantation to help solve the decline in CD8-positive cells.31,32 However, human fetal thymus implantation has had several problems ethically, technically, and in cost. We think that CD8-positive T cells are generated by naive T cells (CD4) that are under the influence of IL-2 secretion produced with DC vaccination.25 This is evidenced by the fact that the CD8-positive T cell population 4 weeks after humanization of mice was 13.29% and, similar to findings in other studies, declined to undetectable levels by 8 weeks after humanization. However, when we introduced DCs transfected with modified CD133 mRNA after humanization, the level of CD8-positive T cells was maintained, unlike DCs introduced after humanization that were not transfected with mRNA, or with PBS injection after humanization where CD8-positive T cells were undetectable. Furthermore, DCs transfected with modified CD133 mRNA had 2-fold the levels of IL-2 secretion, which has been shown to be important for CD8 cell expansion, particularly for CD8 memory T cells. Although we have demonstrated the prolonged survival of the CD8-positive T cell population, we did not examine the long-term potential of CD8-positive T cells, as this was beyond the scope of our study. This result suggests that vaccination using DCs transfected with modified mRNA can induce a sufficient amount of CD8-positive T cells in the short-term, and also revealed that humanized mice lacking a human fetal thymus may have promise as an appropriate animal model for cancer immunotherapy.
We have shown the potential of a humanized mouse as a model for cancer immunotherapy. Under certain conditions, DCs transfected with modified mRNA can maintain CD8-positive T cells in a humanized mouse model for a significant period of time with the benefit of CD4-positive T cell activation. Our present study also showed that DCs transfected with modified mRNA could induce strong specific CTL activity against GBM cancer stem cells both in vitro and in vivo, and vaccination using DCs transfected with modified mRNA could prolong the survival of tumor-bearing mice. These results revealed that both MHC class I- and MHC class II-restricted antigen presentation caused by modified mRNA transfection can activate CD8- and CD4-positive T cells, acquiring a stronger immune response against GBM. Our data strongly suggest that modified mRNA transfection as a tumor antigen loading method using DCs transfected with modified CD133 mRNA should be pursued for its clinical therapeutic potential to target cancer stem cells.
Materials and Methods
Cell Culture
GL261 is an established mouse GBM cell line purchased from the Frederick National Laboratory’s Laboratory Services at the National Cancer Institute (NCI). Patient brain tumor samples classified as GBM based on the World Health Organization (WHO) criteria33 were obtained in accordance with the appropriate Institutional Review Boards and isolated as previously described. BTSCs (BTSC5) were cultured in neurobasal (NBE) media as previously described.4 BTSCs were generated at Cedars-Sinai Medical Center. BTSC5 was enriched for CD133 by FACS sorting using the CD133/2 (293C) antibody (Miltenyi Biotec, 130-090-854).
Mouse Model
C57BL/6 mice were obtained from Charles River Laboratories. NOG mice were obtained from Taconic and housed in a specific pathogen-free environment. All mice weighing between 25 and 35 g were used in this study. Free access to sterilized food and water was provided. This experiment was reviewed by the Institutional Animal Care and Use Committee at the Cedars-Sinai Medical Center.
Modified CD133 mRNA
Plasmid constructs have been described previously.15,16,26 Briefly, for in vitro transcription, the plasmids were cloned with pSP64 vector (Promega). A TRP-2 signal sequence fragment and TM/cyto domain were amplified from TRP-2 cDNA by using PCR (Ex Taq polymerase; Takara Bio). The PCR products were cloned as a HindIII-PstI signal sequence fragment and a BamHI-SmaI TM/cyto domain fragment into pSP64 (Figure S3) to allow in vitro transcription under the control of an SP6 promoter to transport the CD133 protein efficiently to MHC class II compartments for eventual cross-presentation by both classes I and II on DCs in a cognate manner.
The total RNAs of the BTSC5 and GL261 neurosphere cells were collected for reverse transcriptase PCR by using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Primers used can be found in Supplemental Materials and Methods.
Experimental Design
NOG mice were injected with CD34-positive hematopoietic stem cells that were isolated from human PBMCs with the same HLA-A2 phenotype as BTSCs. Immunosuppression of NOG mice was aided by busulfan injection. Blood collections at 4 and 8 weeks were used to determine NOG mice humanization where CD45, CD3, CD4, and CD8 cell percentages were determined by FACS. DCs were then obtained from human PBMCs with the same HLA-A2 phenotype as BTSCs and evaluated by measuring IL-2, IFN-γ, and IL-12 production and were then used for introduction of human CD133 mRNA, which then served as a vaccine against experimentally generated mice bearing intracranial GBM tumors, with appropriate controls (i.e., PBS, no transfection). Survival analysis and evaluation of CD133, CD45, CD3, CD4, and CD8 to confirm the efficacy and efficiency of DC vaccination on intracranial GBM bearing mice were performed through immunofluorescence, immunohistochemistry, and Kaplan-Meier survival analysis. Studies on mice were carried out in accordance with protocols approved by Cedars-Sinai Medical Center Institutional Animal Care and Use Committee. In vitro analysis using CTL cell killing assays and CD133 peptide loading further confirmed the utility of CD133 as a target for immunotherapy. Similar results were obtained for a syngeneic mouse model. Further details about experimental procedures and statistical analysis is given in the Supplemental Materials and Methods.
Statistical Analysis
For determining sample size, we estimated mean ± standard deviation of 36 ± 2 days for survival in our untreated control group based on preliminary data. Therefore, our final study of five animals per group would have 80% power to detect an increase of at least 6 days in average survival times with the two-sided non-parametric Kruskal-Wallis rank test at the 0.05 significance level. Power analysis was performed using PASS v11 software. Survival times were tested across groups using the Gehan-Breslow-Wilcoxon test visualized via the Kaplan-Meier method with SAS 9.3 software. p values for pairwise comparisons were adjusted for multiple comparisons using the Tukey-Kramer method.
Author Contributions
T.A. initiated experiments. T.A. and J.S.Y. designed experiments. A.S.-M.S.D., T.A., L.A.E., and J.S.Y. wrote the manuscript. T.A., L.Z., and M.D.P.V. performed experiments. T.A., L.Z., and L.A.E. performed data analysis. T.A. and L.Z. provided experimental materials.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by a grant from FasterCures and the Lowell Milken Foundation (to J.S.Y.) and a grant from the National Institute for Neurological Disorders and Stroke (NIH) (2R01# NS 048959 to J.S.Y.).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2020.06.019.
Supplemental Information
References
- 1.Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J., Wolinsky Y., Kruchko C., Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncol. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 4.Yuan X., Curtin J., Xiong Y., Liu G., Waschsmann-Hogiu S., Farkas D.L., Black K.L., Yu J.S. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 5.Lee J., Kotliarova S., Kotliarov Y., Li A., Su Q., Donin N.M., Pastorino S., Purow B.W., Christopher N., Zhang W. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Piccirillo S.G., Reynolds B.A., Zanetti N., Lamorte G., Binda E., Broggi G., Brem H., Olivi A., Dimeco F., Vescovi A.L. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 7.Gallo M., Ho J., Coutinho F.J., Vanner R., Lee L., Head R., Ling E.K., Clarke I.D., Dirks P.B. A tumorigenic MLL-homeobox network in human glioblastoma stem cells. Cancer Res. 2013;73:417–427. doi: 10.1158/0008-5472.CAN-12-1881. [DOI] [PubMed] [Google Scholar]
- 8.Li Z., Bao S., Wu Q., Wang H., Eyler C., Sathornsumetee S., Shi Q., Cao Y., Lathia J., McLendon R.E. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kase M., Minajeva A., Niinepuu K., Kase S., Vardja M., Asser T., Jaal J. Impact of CD133 positive stem cell proportion on survival in patients with glioblastoma multiforme. Radiol. Oncol. 2013;47:405–410. doi: 10.2478/raon-2013-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji J., Judkowski V.A., Liu G., Wang H., Bunying A., Li Z., Xu M., Bender J., Pinilla C., Yu J.S. Identification of novel human leukocyte antigen-A∗0201-restricted, cytotoxic T lymphocyte epitopes on CD133 for cancer stem cell immunotherapy. Stem Cells Transl. Med. 2014;3:356–364. doi: 10.5966/sctm.2013-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brehm M.A., Shultz L.D., Greiner D.L. Humanized mouse models to study human diseases. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17:120–125. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcantar-Orozco E.M., Gornall H., Baldan V., Hawkins R.E., Gilham D.E. Potential limitations of the NSG humanized mouse as a model system to optimize engineered human T cell therapy for cancer. Hum. Gene Ther. Methods. 2013;24:310–320. doi: 10.1089/hgtb.2013.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris D.T., Badowski M. Long term human reconstitution and immune aging in NOD-Rag (−)-γ chain (−) mice. Immunobiology. 2014;219:131–137. doi: 10.1016/j.imbio.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seung E., Tager A.M. Humoral immunity in humanized mice: a work in progress. J. Infect. Dis. 2013;208(Suppl 2):S155–S159. doi: 10.1093/infdis/jit448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukui M., Ueno K., Suehiro Y., Hamanaka Y., Imai K., Hinoda Y. Anti-tumor activity of dendritic cells transfected with mRNA for receptor for hyaluronan-mediated motility is mediated by CD4+ T cells. Cancer Immunol. Immunother. 2006;55:538–546. doi: 10.1007/s00262-005-0027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amano T., Kajiwara K., Yoshikawa K., Morioka J., Nomura S., Fujisawa H., Kato S., Fujii M., Fukui M., Hinoda Y., Suzuki M. Antitumor effects of vaccination with dendritic cells transfected with modified receptor for hyaluronan-mediated motility mRNA in a mouse glioma model. J. Neurosurg. 2007;106:638–645. doi: 10.3171/jns.2007.106.4.638. [DOI] [PubMed] [Google Scholar]
- 17.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 18.Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 19.Tunici P., Irvin D., Liu G., Yuan X., Zhaohui Z., Ng H., Yu J.S. Brain tumor stem cells: new targets for clinical treatments? Neurosurg. Focus. 2006;20:E27–E33. doi: 10.3171/foc.2006.20.4.17. [DOI] [PubMed] [Google Scholar]
- 20.Shigdar S., Qiao L., Zhou S.F., Xiang D., Wang T., Li Y., Lim L.Y., Kong L., Li L., Duan W. RNA aptamers targeting cancer stem cell marker CD133. Cancer Lett. 2013;330:84–95. doi: 10.1016/j.canlet.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Vlashi E., Kim K., Lagadec C., Donna L.D., McDonald J.T., Eghbali M., Sayre J.W., Stefani E., McBride W., Pajonk F. In vivo imaging, tracking, and targeting of cancer stem cells. J. Natl. Cancer Inst. 2009;101:350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melhem N.M., Gleason S.M., Liu X.D., Barratt-Boyes S.M. High-level antigen expression and sustained antigen presentation in dendritic cells nucleofected with wild-type viral mRNA but not DNA. Clin. Vaccine Immunol. 2008;15:1337–1344. doi: 10.1128/CVI.00154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponsaerts P., Van Tendeloo V.F., Berneman Z.N. Cancer immunotherapy using RNA-loaded dendritic cells. Clin. Exp. Immunol. 2003;134:378–384. doi: 10.1046/j.1365-2249.2003.02286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shurin M.R., Gregory M., Morris J.C., Malyguine A.M. Genetically modified dendritic cells in cancer immunotherapy: a better tomorrow? Expert Opin. Biol. Ther. 2010;10:1539–1553. doi: 10.1517/14712598.2010.526105. [DOI] [PubMed] [Google Scholar]
- 25.Holtkamp S., Kreiter S., Selmi A., Simon P., Koslowski M., Huber C., Türeci O., Sahin U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 26.Saka M., Amano T., Kajiwara K., Yoshikawa K., Ideguchi M., Nomura S., Fujisawa H., Kato S., Fujii M., Ueno K. Vaccine therapy with dendritic cells transfected with Il13ra2 mRNA for glioma in mice. J. Neurosurg. 2010;113:270–279. doi: 10.3171/2009.9.JNS09708. [DOI] [PubMed] [Google Scholar]
- 27.Corbeil D., Röper K., Weigmann A., Huttner W.B. AC133 hematopoietic stem cell antigen: human homologue of mouse kidney prominin or distinct member of a novel protein family? Blood. 1998;91:2625–2626. [PubMed] [Google Scholar]
- 28.Toes R.E., Nussbaum A.K., Degermann S., Schirle M., Emmerich N.P., Kraft M., Laplace C., Zwinderman A., Dick T.P., Müller J. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J. Exp. Med. 2001;194:1–12. doi: 10.1084/jem.194.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J., Liu Y., Liu Y., Liu M., Xiang Z., Lam K.-T., Lewis D.B., Lau Y.-L., Tu W. Human CD8<sup>+</sup> regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Sci. Transl. Med. 2013;5:168ra9. doi: 10.1126/scitranslmed.3004943. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y., Nagata S., Takiguchi M. Effective elicitation of human effector CD8+ T cells in HLA-B∗51:01 transgenic humanized mice after infection with HIV-1. PLoS ONE. 2012;7:e42776. doi: 10.1371/journal.pone.0042776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covassin L., Jangalwe S., Jouvet N., Laning J., Burzenski L., Shultz L.D., Brehm M.A. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin. Exp. Immunol. 2013;174:372–388. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudek T.E., Allen T.M. HIV-specific CD8+ T-cell immunity in humanized bone marrow-liver-thymus mice. J. Infect. Dis. 2013;208(Suppl 2):S150–S154. doi: 10.1093/infdis/jit320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.