Abstract
The pancreatic islet is a complex mini organ composed of a variety of endocrine cells and their support cells that work in concert to tightly control blood glucose homeostasis. Changes in glucose concentration are commonly regarded as the chief signal controlling insulin-secreting beta cells, glucagon-secreting alpha cells, and somatostatin-secreting delta cells. However, each of these cell types is highly responsive to a multitude of endocrine, paracrine, nutritional, and neural inputs, which collectively shape the final endocrine output of the islet. Here we review the principal inputs for each islet cell type and the physiological circumstances in which these signals arise through the prism of the insights generated by transcriptomes of each of the major endocrine cell types. A comprehensive integration of the factors that influence blood glucose homeostasis is essential if we are to succeed in improving therapeutic strategies to better manage diabetes.
Introduction
Over the past four decades, the number of adults with diabetes has nearly quadrupled with over 420 million individuals estimated to be affected by the disease worldwide1. As these numbers are expected to continue to rise, it is evident that improved therapeutic strategies to manage diabetes are necessary. Diabetes is a disease of chronically high blood glucose stemming principally from insulin impairment. However, defects in glucagon secretion – inappropriate hyperglucagonaemia as well as impaired counterregulation – are also inextricably intertwined with the etiology of diabetes2. This places the source of insulin and glucagon – the pancreatic islets – in the crosshairs of researchers’ attempts to understand and ameliorate the disease. A better appreciation for the mechanisms controlling islet hormone secretion is imperative to developing better strategies for dealing with diabetes.
The pancreatic islets are a heterogeneous mixture of endocrine cells and non-endocrine support cells that maintain homeostatic blood glucose levels via balanced hormone secretion. The beta cells make up (50–75%) of the islet cell mass in humans, and 60–80% in mice [Figure 1]3–5, and are the sole source of insulin in the body6. Insulin release, triggered by increased blood glucose7,8, lowers glycaemia through the net effect of decreased glycogenolysis and gluconeogenesis at the liver and skeletal muscle and increased uptake of glucose in the liver, skeletal muscle, and adipose tissue9,10 [Figure 2]. Insulin further stimulates nutrient uptake and triglyceride (TG) synthesis in adipocytes. Collectively these insulin actions restore normoglycaemia following a meal. Alpha cells are the second most abundant islet cell type, accounting for approximately 15–20% and of the endocrine cells in mice, and 25–35% in humans [Figure 1] 3–5. Alpha cells secrete glucagon as a counterregulatory signal in response to hypoglycaemia, and is additionally potentiated (amplified) by adrenergic stimulation and circulating amino acids. Glucagon increases hepatic glucose production primarily via increased glycogenolysis and gluconeogenesis [Figure 2]11. Delta cells make up 5–10% of the islet3 and release somatostatin dose-dependently in response to high glucose12,13. While insulin and glucagon are true hormones that are released into the circulation to elicit effects on target cells distant from site of release, somatostatin instead provides local inhibitory control over alpha and beta cells14–16. Nevertheless, this local regulation helps determine the homeostatic set point for plasma glucose17.
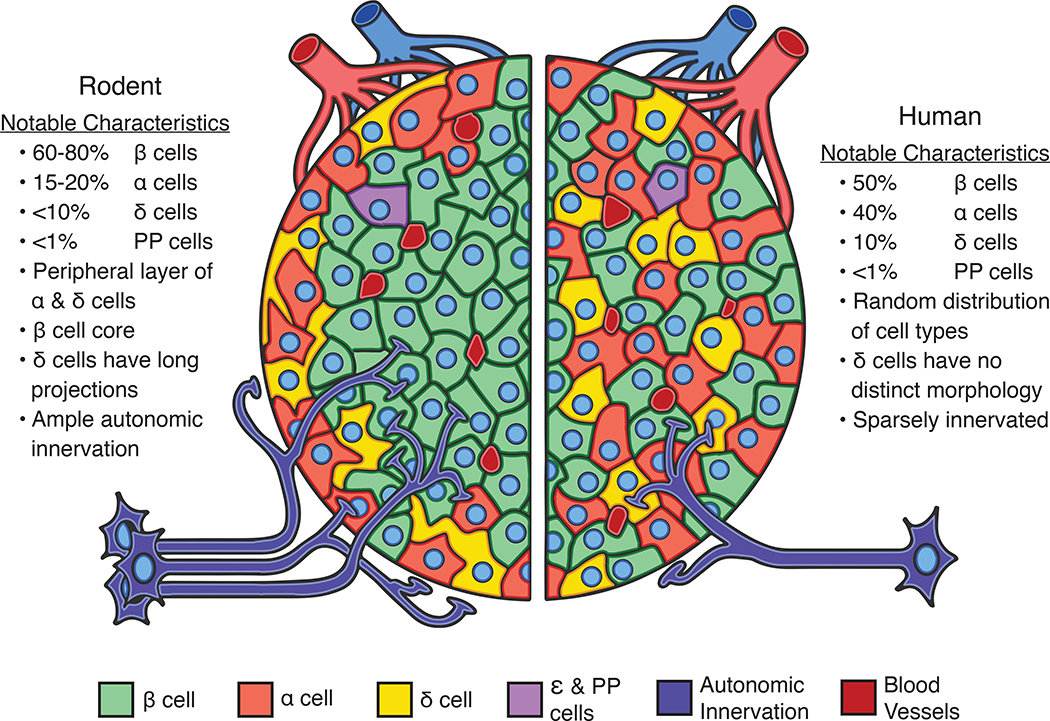
Figure 1. Comparative architecture of pancreatic islets of mice and humans.
Pancreatic islets of mice and humans differ in important ways, but also share many features in common. These shared features make mouse islets useful experimental models to study many aspects of human islet biology. The relative proportions of endocrine cell types in mouse (left) and human islets (right) are similar with beta cells (β; green) comprising the majority of the islet cell mass followed by alpha (α; light red) and delta cells (δ; yellow). Other islet endocrine cells such as pancreatic polypeptide and epsilon cells (PP and ε; purple) are more sparse in number. Human islets occur in a wide variety of sizes and conformations that range from highly structured to more random distributions of cells. Mouse islets exhibit a more uniform architecture with alpha and delta cells at the islet periphery surrounding a beta cell core. Islets in both species are vascularised (dark red) and innervated (dark blue) for rapid sensing of changing energy needs, although mouse islets are more densely innervated than humans.
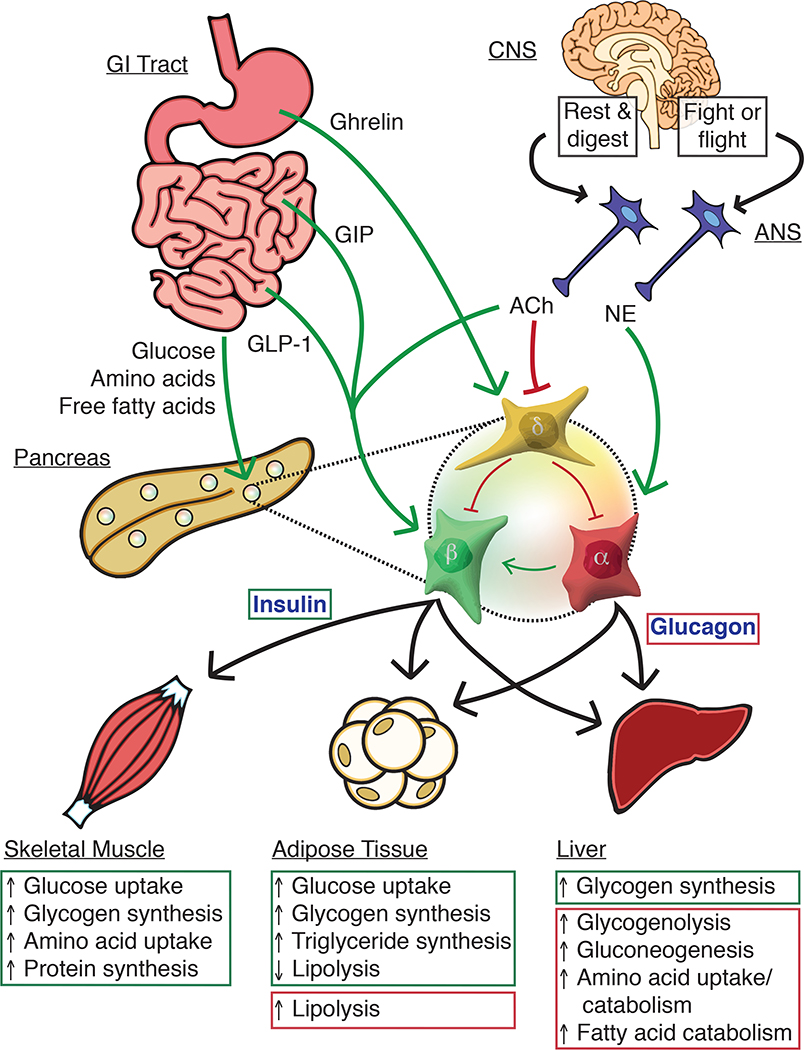
Figure 2. Inter-organ signaling from nutrient sensing to islet-mediated metabolic effects.
Nutrition-related signals from the gastrointestinal (GI) tract combine with neuronal input from the autonomic nervous system (ANS) to direct insulin and glucagon secretion from pancreatic islets. Changes in blood glucose levels are sensed by alpha, beta, and delta cells, which respond by restoring blood glucose to homeostatic levels. Alpha cells release glucagon at low glucose to increase hepatic glucose production. During hyperglycemia, beta cells lower blood glucose by releasing insulin to increase glucose storage in the liver, skeletal muscle, and adipose tissue. Insulin release is amplified by the incretin hormones GLP-1 and GIP from the small intestine as well as by glucagon from neighbouring alpha cells. Delta cells secrete somatostatin across a range of glucose levels, but most prominently in response to hyperglycemia. Amino acids and free fatty acids (FFAs) stimulate both alpha and beta cells, and the peripheral effects of both glucagon and insulin result in reduced circulating amino acids and FFAs. The central nervous system (CNS) can augment islet secretion in conditions such as the “rest and digest” state where direct insulin secretion is further facilitate by a suppression of somatostatin secretion by acetylcholine (ACh) associated with parasympathetic innervation. Glucagon secretion is increased during the “fight or flight” response by norepinephrine (NE) released by sympathetic nerves.
However, beyond glucose, multiple levels of paracrine, endocrine, neuronal, and nutritional inputs collectively determine islet cell activity. In this review, we focus on emerging themes with regards to control of islet endocrine function. Part of this discussion will incorporate insights gained from islet cell transcriptomes that have provided a wealth of information on the inputs that do, and do not, impinge on the islet cell types they were long thought to act upon. It is not our intent to cover all possible inputs that have been attributed to islets over many years. We refer the interested reader to comprehensive descriptions of important topics such as species differences10,18, islet innervation19, and islet cell receptors20–22 that have been published elsewhere. Additionally, non-endocrine islet cells such as macrophages, endothelial, and stellate cells make important contributions to the islet as a functional unit [Box 1] that we will not address in detail.
Box 1. The contributions of non-canonical endocrine cells and non-endocrine cells to proper islet function.
What follows are brief descriptions of the various other cells within the islet and their relation to intra-islet signaling and diabetes.
PP/gamma cells
Pancreatic polypeptide (PP) cells are the fourth islet endocrine cell type, which comprise <5% of human and <2% of mouse islet mass167. PP cells are found in the islet and sparingly throughout the gastrointestinal tract, and release PP in response to meals168. PP regulates satiety and decreases appetite and food intake in rodents and humans with no apparent paracrine effect on insulin and glucagon levels169.
Epsilon cells
Epsilon cells are the fifth endocrine cell type and are defined by the expression of ghrelin, classically known as the “hunger hormone”. The epsilon cells increase in number throughout development reaching as high as 30% of the islet mass before decreasing to <5% in neonatal and <1% in adult islets170. Whether they play a role in both developing and adult islets is currently undefined.
Endothelial cells
Endothelial cells that make up the microvasculature of the islet are essential to proper endocrine function as the islet cells require high blood flow and blood volume to effectively sense nutrients and distribute their hormones. Beta cells (and to some extent alpha cells) produce a number of angiogenic and angiostatic factors that target endothelial cell receptors including VEGF-A and Angiopoietin 1171. Defects in beta cell-endothelial cell crosstalk in mice result in impaired GSIS and angiogenesis is vital for successful integration of transplanted islets172.
Pericytes
Pericytes associate closely with islet capillaries, and dynamically regulate blood flow by constricting or dilating capillaries in response to signals from beta cells, endothelial cells and peripheral nerves173. The pericytes exhibit a certain amount of plasticity as vascular damage to islets during type 1 diabetes increases pericyte density as a possible healing response. Conversely, in type 2 diabetes the opposite occurs and vascular coverage decreases174. This likely contributes to impaired diabetic GSIS due to compromised in blood flow. Interestingly, pericytes also regulate beta cell function independent from their role in controlling vasculature. Beta cell insulin content and expression are reduced and GSIS is impaired when pericytes are ablated suggesting that pericytes sustain beta cell maturity in a paracrine fashion175.
Glial/Schwann cells
Glial cells are peripheral neuronal cells that have been shown to both penetrate the core of islets and form a peripheral sheath around the islet mantle. This sheath becomes more dense in response to injury such as stress or autoimmune attack during type 1 diabetes, reflecting a protective role of the glial cells173. The glial cells also serve a paracrine role: glial-derived neurotrophic factor increases beta cell mass and insulin content, which improves glucose tolerance176.
Resident macrophages
The islet also contains resident macrophages, which, under non-inflammatory conditions, contribute to endocrine cell development in mice by supporting normal alpha and beta cell expansion177,178.
Stellate cells
Fibroblasts and myofibroblasts are uncommon in healthy islets, but contribute to fibrosis seen in pancreatic diseases like pancreatitis and pancreatic cancer. Stellate cells are quiescent myofibroblast-like cells that secrete fibrous extracellular matrix proteins upon activation. Stellate cells are primarily responsible for fibrosis that is occasionally observed in type 2 diabetes, which has been linked to reduced insulin expression and apoptosis among beta cells179.
Acinar cells
The exocrine pancreatic acinar tissue releases digestive enzymes and is affected not just by nutrient status following food intake, but also by local signaling from the endocrine islet. Insulin potentiates amylase release while somatostatin and pancreatic polypeptide both inhibit exocrine secretion180.
Goal of Review
Our goal with this review is to focus on areas where recent insights challenge us to reconsider traditional views of the physiological mechanisms that control islet hormone release, and we discuss specific differences between rodent and human islets where appropriate. One major theme is the renewed appreciation for amino acids as significant contributors to nutrient-stimulated alpha cell secretion. With regards to intra-islet crosstalk, increased evidence of alpha cell-mediated beta cell potentiation now compels us to reconsider the view of glucagon as a predominantly counterregulatory hormone in favor of a model where glucagon also makes significant physiological contributions to glucose-stimulated insulin secretion. Additionally, delta cells have emerged as physiologically important modulators of insulin and glucagon secretion. Finally, we discuss how the inputs that coordinate insulin and glucagon release from healthy islets are affected by diabetes and how a better understanding of the physiological inputs into the healthy islets may be leveraged towards improved management in disease.
The complexities of studying islet endocrine cells
Multiple layers of nutrient, paracrine, endocrine, and neuronal signals modulate the islet cell hormone secretion that is triggered by glucose levels. The mammalian islet is highly vascularised23, allowing for both rapid sensing of changes in nutritional status or circulating hormones, and for swift delivery of insulin or glucagon to peripheral tissues. Islets are also tightly innervated by autonomic neurons23 which supports sympathetic and parasympathetic modulation of insulin, glucagon, and somatostatin release [Figure 2]. Briefly, the net effect of sympathetic stimulation is an increase in glucagon release and a decrease in insulin and somatostatin release19,24. Net parasympathetic signaling activates both insulin and glucagon secretion while decreasing somatostatin. Interestingly, islet cells also synthesise a number of classic neurotransmitters such as GABA, acetylcholine, and serotonin for intra-islet signaling independent of innervation (detailed below)25–27. And while mouse islets have historically been suggested to be more highly innervated than human islets28, there are also reports showing prominent autonomic innervation of humans islets23.
The plethora of input signals that target the islet as a functional unit has made it a challenge to distinguish direct versus indirect mechanisms that modulate alpha and beta cell activity. In recent years, islet cell type-specific reporter mice29 and antibodies30, supported by advances in RNA-Seq approaches, have made it possible to disentangle how multiple layers of external and intra-islet signals affect each individual cell type. These efforts have generated comprehensive, high-quality bulk and single cell transcriptomes of mouse and human islet cells31–34. Advances in functional imaging and electrophysiology have similarly made characterising islet responses much more cell-type specific: Genetically-encoded calcium indicators have improved upon traditional calcium dyes by enabling targeted functional imaging of populations of a single cell type33. In parallel, patch-clamp recordings aggregated across hundreds of islet cells provide us with cell type-specific electrophysiological fingerprints35. And Patch-Seq provides a unique approach to validate single cell transcriptomes with direct functional correlates acquired by patch-clamp measurements on the same single cell36,37. These technical advances now make it more feasible than ever to distinguish direct versus indirect actions on islet cells with single cell resolution.
As detailed in this review, the collective inputs that influence islet secretion are sufficiently similar between mice and humans that mouse models, with their ease of experimental manipulation, offer unparalleled advantages in understanding islet biology. Nevertheless, species differences do exist. Mouse islets are organised as a core of beta cells surrounded by a mantle of alpha and delta cells [Figure 1]3. This same architecture is seen in islets from young humans, but adult human islets exhibit a variable assortment of islet architecture from the rodent-like mantle-core organisation to a more intermingled distribution of alpha, beta, and delta cells – a setup well suited for paracrine signaling through the interstitial space10,38,39. And while many islet paracrine signals are shared between species, although some such as islet amyloidogenic polypeptide (IAPP) and peptide YY (PYY) are notably enriched in mouse over human islets22,40. Morphologically, human delta cells are relatively compact while mouse delta cells have long, neuron-like projections, which may help them overcome the distance from mantle to core when releasing somatostatin to inhibit beta cell activity15. In spite of these differences, mouse and human islets share responses to many external factors and intra-islet paracrine signals that shape the final islet output.
Nutrient stimulation of alpha, beta and delta cells
Most textbooks offer the glucose-centric view that insulin secretion is triggered when glucose values rise over a threshold of 5 mM (7 mM in mice)41. Meanwhile, alpha cells release most glucagon under hypoglycemic conditions, and demonstrate modest glucagon secretion under hyperglycemic conditions. However, insulin and glucagon play a large role in the metabolism of not only carbohydrates, but also of lipids (free fatty acids)9,42 and proteins (amino acids)9,43 as detailed in this section.
Glucose-Stimulated Insulin and Somatostatin Secretion
Glucose is arguably the single most important signal that controls insulin release, although glucose fluctuations in healthy subjects are relatively modest and would not by themselves elicit robust insulin secretion. Full insulin secretion in vivo requires glucose stimulation that is potentiated by the combined actions of other nutrients, endocrine, and paracrine factors. Glucose-stimulated insulin secretion (GSIS) is initiated when beta cells sense increases in blood glucose via glucose transporters (GLUT1 in humans and Glut2 in mice)44,45 [Figure 3]. This glucose serves as a substrate for glycolysis and oxidative phosphorylation, generating ATP and leading to an accompanying drop in ADP levels. This shift in ATP/ADP ratio closes ATP-sensitive potassium channels (KATP channels), which causes membrane depolarisation. This in turn opens L-type voltage-gated Ca2+ channels (VGCCs), leading to a Ca2+ influx and calcium-induced calcium release that triggers exocytosis of insulin secretory granules18. While L-type VGCCs are responsible for the majority of Ca2+ currents in mouse beta cells, in humans P/Q-type VGCCs are about equally as involved as L-type channels41.
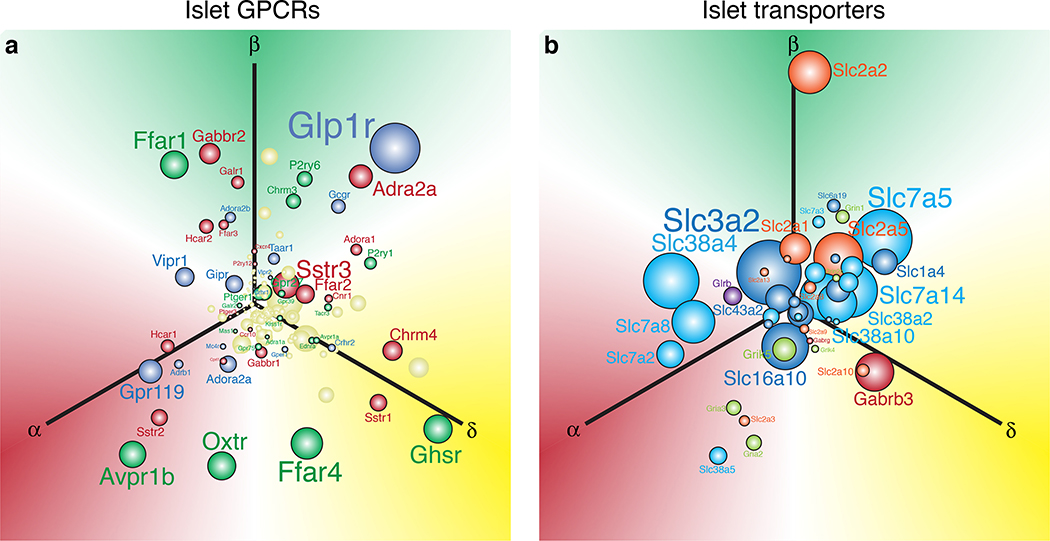
Figure 3. Visualisation of the abundance and selectivity of GPCR and transporter gene expression in alpha, beta, and delta cells.
We used the natural log of the normalised expression values for a gene (ln[RPKM]) to plot the relative position of that gene along three axes representing alpha, beta, and delta cells. These expression values are derived from transcriptomes of FACS-purified mouse alpha, beta, and delta cells described elsewhere33. a) Each of these three individual gene expression values are converted into x and y vectors and then consolidated into a single set of x, y coordinates that represents the overall selectivity of the expression of that gene. The origin represents equal expression (i.e. no enrichment) in each of the three islet cell types, whereas. placement in any direction along one of the axes reflects enrichment in the corresponding cell type. Sphere and font sizes are proportional to abundance of the gene based on the highest RPKM value for that gene in alpha, beta, or delta cells. b) The top 150 most abundant G protein-coupled receptors (GPCR) of the islet cells are color coded in accordance with the predominant signaling cascade associated with each receptor. Blue genes are Gαs-coupled, green are Gαq, red are Gαi, and yellow is ‘unknown’ or ambiguous based on receptor classifications from IUPHAR (International Union of Basic and Clinical Pharmacology)166. c) Non-GPCR receptors and transporters are colour-coded according to the class of signaling molecules utilised by each receptor/transporter, following IUPHAR classification for solute carriers.
The delta cell signal transduction for glucose-stimulated somatostatin secretion (GSSS) shares many features with beta cells15 albeit with a few distinctions. KATP channel closure and subsequent membrane depolarisation is required, but in contrast with beta cells, calcium-induced calcium release plays a larger role in GSSS than in GSIS46. Additionally, delta cells have been suggested to be electrically coupled to beta cells47. The propagation of depolarisation from glucose-activated beta cells to delta cells may help potentiate somatostatin release. However, delta cells are active at lower glucose concentrations than beta cells, possibly due to a difference in KATP activity48. Moreover, somatostatin secretion in response to hyperglycemia is synchronous with insulin secretion, albeit with a 30 second to 2 minute delay49. Such a delay is not readily reconciled with a model where delta cells operate in lock-step with beta cells mediated solely through gap junction-mediated coordination and suggests an important paracrine component to the coordination between beta and delta cells.
Glucose-Mediated Glucagon Secretion
The mechanism underlying glucose-mediated alpha cell activation remains incompletely understood. Alpha cells express analogous machinery to that used for GSIS in beta cells and similarly rely on KATP channels and VGCCs for secretion, yet alpha cells are active at low as opposed to high glucose. While there is no consensus paradigm for glucagon secretion, one current model is that increasing glucose induces a KATP-driven depolarisation to inactivate voltage-gated Na+ channels50. By driving Na+ channels to a non-conducting state, alpha cells are unable to reach the membrane potential necessary for VGCC-opening and cease to secrete glucagon. Conversely, under hypoglycemic conditions, alpha cell KATP channels operate at a low level that holds alpha cells in an electrically active state and causes small depolarisations that open P/Q-type VGCCs. While this model of alpha cell activation helps explain some of the dynamics of glucagon release, a full explanation of alpha cell activity in response to hypoglycemia likely involves a combination of alpha cell-intrinsic, paracrine, endocrine, and neural factors. Indeed, when stripped of the paracrine influence of delta cells, alpha cell glucagon release is uniformly increased across a gradient of glucose concentrations51. This suggests that paracrine factors such as somatostatin have significant influence on the glucose-responsiveness of alpha cells, which we will revisit in more detail.
Amino Acids
Beta cells are sensitive to circulating amino acids and insulin signaling promotes both amino acid uptake and protein synthesis in skeletal muscle9. Beta cells express high levels of cationic amino acid transporters (CATs) and sodium-coupled neutral amino acid transporters (SNATs) [Figure 3c]32,33. The mechanisms by which amino acids stimulate insulin secretion vary18. Many amino acids including arginine, lysine, leucine, and glutamine depolarise beta cells upon import, either directly if they carry a positive charge52, or due to sodium co-transport53. The ensuing depolarisation triggers Ca2+ influx to stimulate insulin release54. In parallel, amino acids such as alanine, glutamic acid and glutamine can fuel components of mitochondrial metabolism, thus increasing the ATP/ADP ratio55. Other amino acids such as glycine potentiate insulin secretion via its ionotropic glycine receptor (GlyR) on the beta cell surface56 [Figure 3c]. Furthermore, paracrine interactions contribute to the effects of amino acids on insulin.
Alpha cells are highly sensitive to increases in amino acids, and are stimulated by 17 of the 20 natural amino acids57. This largely explains the post-prandial spike in glucagon in response to a normal mixed meal58. It is vital that glucagon is released along with insulin in response to amino acids as the two hormones will synergistically increase amino acid uptake in response to protein ingestion while effectively countering each other’s actions on carbohydrates. Glucagon signaling in the liver increases hepatic utilisation of amino acid substrates in gluconeogenesis43, leading to a decrease in circulating amino acids. This important safety mechanism ensures normoglycemia during protein-rich, carbohydrate-low diets58. The liver-alpha cell axis that connects alpha cells, amino acids, and the liver is one of the primary mediators of amino acid homeostasis, highlighting the important role of glucagon in the post-prandial state.
The exact mechanism by which most amino acids directly stimulate glucagon secretion is less characterised than in beta cells, but likely involves similar mechanisms. Arginine is a potent stimulator of glucagon secretion that directly depolarises the alpha cell upon cellular transport into the cell59. Other amino acids induce glucagon secretion following import via abundantly expressed CATs and SNATs [Figure 3c] followed by use as metabolic substrate32,33. Glycine, signaling through ionotropic GlyR, can increase intracellular Ca2+ and stimulate exocytosis in alpha cells independent of amino acid transporters60.
Much like peripheral insulin resistance causes hyperglycemia, which in turn contributes to beta cell hyperplasia (at least in mice), a similar relationship is emerging between hepatic glucagon signaling, amino acid levels and alpha cell mass. Chronically elevated amino acids can influence the total capacity for glucagon secretion by stimulating alpha cell proliferation through mTOR signaling61. Indeed, in glucagon receptor knockout mice or mice treated with glucagon receptor antagonists, interrupted hepatic glucagon signaling causes a marked accumulation of serum amino acids that triggers a remarkable alpha cell hyperplasia62. This expansion is largely driven by glutamine and alanine, which activate mTor signaling in alpha cells through SNAT5 (Slc38a5) in mice and an as of yet unknown amino acid transporter in humans61,63. These drastic alpha cell phenotypes are supported by clinical evidence. Hyperaminoacidemia is accompanied by hyperglucagonemia in patients with impaired liver function64,65. Interestingly, the liver-alpha cell axis appears to function independently of glucose levels as a comparison between diabetic and non-diabetic patients with non-alcoholic fatty liver disease revealed no correlation of glucose levels to elevated amino acids and glucagon64,65.
Lipids
Insulin regulates lipid metabolism by promoting glucose uptake for conversion into triglycerides, while simultaneously inhibiting lipolysis9. The net effect of this is to promote glucose storage as triglycerides in adipocytes. Beta cell sensitivity to circulating fatty acids in addition to glucose is therefore important to nutrient balance. Beta cell secretion is stimulated by fatty acids of varying chain length and saturation level66,67. These effects are mediated by fatty acid receptor signaling as well as by signaling downstream of intracellular fatty acid metabolism68. The primary receptor for circulating fatty acids expressed by mammalian beta cells is free fatty acid receptor 1 (FFAR1/Ffar1; a.k.a. GPR40), a Gαq-coupled G protein-coupled receptor (GPCR)33,69 [Figure 3b]. FFAR1 supports medium- to long-chain saturated fatty acid as well as unsaturated fatty acid signaling. Most studies on FFAR1 have focused on the actions of palmitate – one of the most abundant circulating saturated fatty acids69,70. The Gαq signaling cascade activates phospholipase C (PLC) and induces 1,4,5 inositol-triphosphate (IP3) formation. This mobilises Ca2+ from the ER, which in beta cells triggers insulin secretion71. Acute Ffar1/FFAR1 activation on the minute-to-hour timescale in mouse72 and human73 islets increases insulin secretion at high glucose levels, and the strength of insulin secretion increases with the length of the FFA chain74. Conversely, chronic (multi-day) palmitate exposure induces dissociation between insulin granules and VGCCs that drive secretion, which results in insulin secretion being decreased by more than 50%73,75. Separate of IP3-mediated activation, fatty acids that diffuse into beta cells can be converted into triglycerides and diacylglycerol that feed into GSIS amplification pathways or directly induce insulin exocytosis68, independent of fatty acid receptors. Indeed, palmitate increases the calcium currents and increases the readily-releasable pool of insulin-containing granules76.
The degree to which free fatty acids directly affect glucagon secretion has been debated for decades. Early studies suggested that alpha cells were actually inhibited by free fatty acids77, but there is growing evidence of an activating role78,79. Similar to beta cells, human alpha cells express the activating receptor, FFAR132. In both mice and humans, glucagon release is directly stimulated by long-chain fatty acids via the resulting downstream increase in cytosolic Ca2+78,79. Glucagon’s role is primarily to modulate hepatic lipid catabolism. Glucagon signaling at the liver increases fatty acid beta oxidation42 and decreases lipoprotein synthesis and secretion80,81. At the adipose tissue glucagon stimulates lipolysis, although this is balanced by simultaneous lipolysis inhibition by insulin9. Alpha cell sensitivity to circulating free fatty acids is thus an important contributor to whole body lipid metabolism.
In both mice and humans, delta cells preferentially express free fatty acid receptor 4 (FFAR4/Ffar4; a.k.a. GPR120) [Figure 3b]33, which is a Gαi-coupled GPCR. Gαi signaling inhibits adenylyl cyclase, which catalyses the formation of cyclic AMP (cAMP). As cAMP potentiates hormone secretion in islet cells, activation of Ffar4 in delta cells inhibits somatostatin secretion by 50%79,82. Together, these fatty acid receptor profiles illustrate a system where, in the presence of high circulating free fatty acids, the combined activation of insulin and glucagon is augmented by disabling the inhibitory actions of delta cells to handle this increased lipid load.
Paracrine Signaling in the Islet
Although nutrients are significant inputs to stimulate insulin and glucagon release, the islet itself is a rich source of signals that engage in intra-islet crosstalk. Paracrine signaling provides an additional layer of control over islet endocrine output that is essential for maintaining establishing and maintaining the homeostatic glucose setpoint. In particular, we will focus on recent developments in the paracrine actions of alpha cells on beta cells – and vice versa – as well as the overarching role of intra-islet delta cell signaling.
Alpha Cell-Mediated Beta Cell Activation
It would seem intuitive for beta and alpha cell activity to be mutually suppressive given that insulin and glucagon are functional antagonists, at least when it comes to maintaining euglycemia. Yet, while beta cell activity suppresses alpha cells (mediated at least in part via delta cells as will be discussed later), glucagon from alpha cells has long been known to stimulate insulin secretion83. While an arrangement where alpha cells stimulate beta cells, beta cells inhibit alpha cells, but stimulate delta cells, and delta cells inhibit both may appear counterintuitive (Figure 4a), modeling studies validate that this is the most stable way to organise a 3-node interaction84. It is increasingly apparent that beta cells require alpha cell inputs for full insulin secretion and establishing normoglycemia85. This realisation warrants a paradigm shift in our thinking of alpha cells as not only mediators of counterregulatory hepatic glucose production, but also as an important local source beta cell potentiation. The following section highlights local alpha cell secretions that potentiate beta cell activation, namely glucagon as well as acetylcholine and corticotropin-releasing hormone.
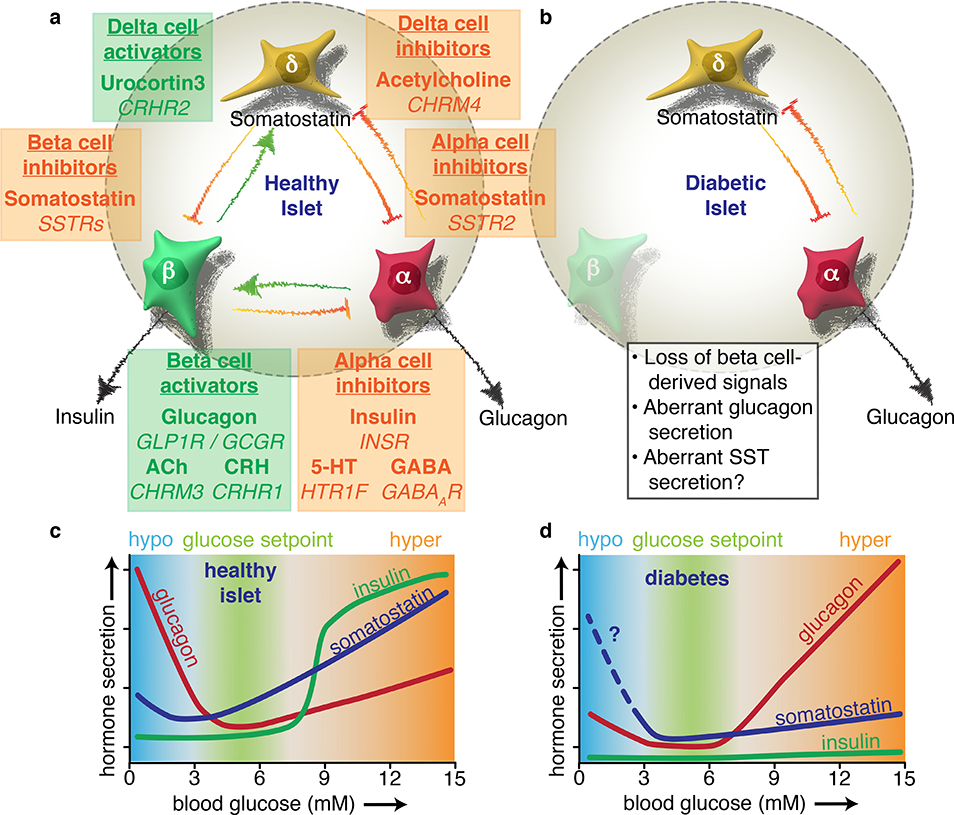
Figure 4. Diabetes disrupts the extensive paracrine signaling network of the islet.
Alpha, beta, and delta cells influence each other’s secretion via intra-islet crosstalk. a) Coloured text boxes (green for activating, orange for inactivating) denote the target cell type of paracrine signaling (underlined), the signal molecule involved (bold), and the target receptor gene (italicised). Each box is placed in between the target cell and the source of the signal. Beta cells initiate a negative feedback loop in high glucose whereby they release urocortin 3 to activate delta cells. The resulting somatostatin (SST) release feeds back to mediate insulin release, providing tonic inhibition that establishes the homeostatic glucose setpoint. Beta cells also experience paracrine activation from alpha cells, which release glucagon, acetylcholine (ACh), and corticotropin-releasing hormone (CRH), which all potentiate GSIS. Beta cell-derived products such as insulin, serotonin (5-HT), and GABA – in addition to urocortin 3-induced somatostatin release – all contribute to silence alpha cells during hyperglycemia. b) The onset of diabetes results in a loss of multiple paracrine signals. Due to autoimmune destruction, type 1 diabetic islets effectively lose all beta cell signals. In type 2 diabetes, urocortin 3 is severely downregulated in beta cells, blunting glucose-stimulated somatostatin secretion. c) The physiological impact of paracrine signaling can be visualised with glucose curves for each islet hormone. The homeostatic glucose set point is maintained by glucagon raising blood glucose during hypoglycaemia and insulin lowering glucose during hyperglycaemia. Somatostatin contributes as a fine-tuning mechanism via paracrine inhibition of both alpha and beta cells. d) The absence of beta cell-derived products in diabetes results in inappropriately high glucagon secretion during high glucose, which exacerbates hyperglycaemia. Glucagon counterregulation at low glucose is also impaired, possibly due to aberrant somatostatin secretion, although this is not yet fully understood. Based in part on Ref17.
The Traditional Incretin Effect
Incretins are classically defined as enteroendocrine hormones that potentiate GSIS. The two main incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). GLP-1 is produced by L-cells in the intestinal ileum and derives from the same precursor protein as the glucagon that is released by pancreatic alpha cells. In L-cells, proglucagon is cleaved by proprotein convertase 1/3 (PCSK1/3) to produce GLP-1, while alpha cells express PCSK2 that produces glucagon instead. GIP is released from K cells in the duodenum and jejunum86.
Together, incretins are believed to be responsible for as much as half of the insulin response to a carbohydrate meal87,88. Beta cells express relatively high levels of the GLP-1 receptor (GLP1R/Glp1r), while the GIP receptor (GIPR/Gipr) is more broadly expressed in multiple islet cells [Figure 3b]. Both receptors are Gαs-coupled GPCRs that potentiate insulin secretion primarily through adenylyl cyclase activation and the resulting cAMP-mediated signaling cascade86,89. In concert with the cAMP pathway, activation of the PLC/protein kinase C (PKC) may contribute to beta cell stimulation by incretins90. This potentiation of GSIS is known as the incretin effect, whereby oral glucose consumption results in a markedly higher insulin response compared to intravenous administration in which glucose bypasses the GI tract. Incretins also stimulate an expansion of beta cell mass via proliferation, which further augments total insulin secretory capacity91. However, the incretin effect is observed during the cephalic phase of a meal, before nutrients enter the gastro-intestinal tract. This constitutes a disconnect with regards to the source of the GLP-1 that potentiates GSIS long before food reaches the ileum where most L cells are located. A resolution of this conundrum may necessitate a re-evaluation of the physiological role of glucagon and incretin receptors and how they interact to potentiate GSIS.
Intra-Islet Glucagon Signaling
Glucagon has been known to be able to potentiate GSIS in a manner reminiscent of the incretin effect since the 1960s83, but the importance of this local action of glucagon for islet function is only now coming into full view. Mammalian beta cells express relatively high levels of glucagon receptor (GCGR/Gcgr) [Figure 3b], as well as GLP1R and GIPR. All these receptors belong to the same family of GPCRs that share similar cAMP-mediated downstream signaling mechanisms. Glucagon and GLP-1 are both derived from proglucagon, and signal via receptors that share 47% sequence homology92, which points to the potential for cross-reactivity between glucagon and GLP-1 and their receptors in the islet.
Indeed, multiple groups have now independently demonstrated that proglucagon-derived peptides produced by alpha cells activate beta cells via either GCGR or GLP1R and that these actions are required for normal GSIS in humans and mice93–98. The majority of circulating GLP-1 is derived from the GI tract, and knocking out gut-derived GLP-1 is impairs oral glucose tolerance99. However, islet-specific GLP-1 signaling, without any contribution from the gut, is shown to be necessary for normal glucose handling94. Separate studies have demonstrated that simultaneously knocking down or blocking both GLP1R and GIPR in beta cells severely reduces glucose-stimulated insulin secretion and glucose tolerance93,95. Glucagon is the predominant alpha cell ligand in mouse islets that engages both GCGR and GLP1R on beta cells to mediate most of this intra-islet signaling93,95. While alpha cells can produce GLP-1 under certain circumstances100, wild type mouse islets under normal circumstances secrete relatively little GLP-195.
The activation of GLP1R and other Gαs-coupled GPCRs such as GCGR and CRHR1 (see below) on beta cells in response to locally released, alpha cell-derived hormones represents a paradigm shift in our understanding of how insulin release can be potentiated. For instance, amino acids such as arginine and glutamine may elicit insulin secretion at least in part by stimulating glucagon release, which then indirectly promotes insulin release in a paracrine fashion, and not by direct stimulation of beta cells as was previously thought93. Such a scenario likely requires beta cell triggering by glucose, which is going to be present along with amino acids in most mixed-meal settings. A number of groups are pursuing GLP1R/GCGR dual agonists that may prove to be a superior method for amplifying insulin in treating type 2 diabetes given these mechanistic discoveries101,102. Together, these latest series of observations suggest that we need to reconsider our definition of GSIS: instead of reflecting the direct stimulatory effect of glucose on beta cells, GSIS from intact islets reflects the combined effects of glucose stimulation plus paracrine amplification via locally released glucagon and other alpha cell-derived products [Figure 4a]. These observations also reconcile the traditional view of glucagon as a counterregulatory hormone with the long-known ability of glucagon to potentiate GSIS83.
Corticotropin-Releasing Hormone
Corticotropin-releasing hormone (CRH/Crh), originally discovered as the principal hypothalamic factor to initiate ACTH release from the anterior pituitary, is also expressed abundantly in human and rat alpha cells32,103. Interestingly, mouse alpha cells do not express Crh peptide or mRNA32, although beta cells of mice, rats, and human all express corticotropin releasing hormone receptor 1 (CRHR1/Crhr1) [Figure 3b]104. CRHR1 is a Gαs-coupled GPCR that is related to the incretin receptors. Treating beta cells with CRH predictably induces a cAMP-mediated Ca2+ influx105, potentiates GSIS, protects against cytokine-induced beta cell apoptosis, promotes beta cell proliferation, and stimulates the expression of an immediate early gene signature106. While the physiological contribution of CRH derived from alpha cells remains untested, it is another alpha cell-derived peptide that is poised to potentiate GSIS.
Acetylcholine
In mice, acetylcholine originates from parasympathetic neurons innervating the islet19 [Figure 2]. In human islets, acetylcholine is released locally from alpha cells26,107 [Figure 4a]. Irrespective of whether acetylcholine is of neural or paracrine origins, acetylcholine potentiates insulin secretion from mouse and human islets via the Gαq-coupled muscarinic 3 cholinergic receptor (CHRM3/Chrm3) [Figure 3b]26,107–109. Acetylcholine’s effect on alpha cells is species-dependent. Human alpha cells do not respond to exogenous cholinergic as they synthesize their own acetylcholine107. Meanwhile, mouse alpha cells can be activated by cholinergic stimulation110, although the underlying mechanism may be indirect as cholinergic cholinergic receptors are enriched in beta and delta cells (Figure 3). In contrast to its stimulation of beta cells, acetylcholine inhibits glucose-induced somatostatin secretion from delta cells. Parasympathetic tone is known to inhibit somatostatin release24, and in mouse islets, cholinergic-mediated inhibition is prevented by pertussis toxin, suggesting the involvement of Gαi-mediated signaling111. This is in line with the selective expression by mammalian delta cells of the Gαi-coupled muscarinic 4 cholinergic receptor (CHRM4/Chrm4) [Figure 3b]. These observations support a model where cholinergic signals (derived from parasympathetic innervations or from alpha cells in humans islets) amplifies insulin release directly and indirectly by inhibiting delta cells [Figure 2, 4a]. It should be noted that, in direct contrast with acetylcholine-mediated delta cell inhibition, other groups have reported IP3-mediated stimulatory effects of cholinergic agonists on somatostatin secretion from mouse46 and human107 islets. Another way to use transcriptomes for decrypting signaling targets beyond just receptor profiles is to interrogate synthesis and degradation pathways for signaling molecules. Delta cells fit the profile of a target of cholinergic signaling given their receptor expression and the preferential expression of the enzyme acetylcholinesterase33, which, in neurons, breaks down acetylcholine at the postsynaptic membrane112. The coordinated actions of acetylcholine on beta and delta cells during normoglycemia may therefore contribute to the maintenance of basal insulin release between meals.
Beta Cell-Mediated Alpha Cell Inhibition
There is general agreement in the field that beta cell-derived products contribute to alpha cell silencing at high glucose. Insulin, serotonin, GABA, and zinc are some of the beta cell factors proposed to directly inhibit alpha cells. Beta cells also secrete the peptide hormone urocortin 3 at high glucose, which potentiates delta cell glucose-stimulated somatostatin secretion. Given that somatostatin is a powerful inhibitor of glucagon secretion, urocortin 3-mediated stimulation of somatostatin release represents an indirect mechanism by which beta cells may suppress glucagon release during high glucose. Many papers over the years have favoured one signal over the other for reasons we will review here. It is plausible that there is redundancy or additivity among these beta cell-derived signals in their ability to suppress alpha cells, or that they play similar roles in distinct physiological settings.
Insulin
While it is difficult to disentangle which of several beta cell-derived paracrine signal may be principally responsible for suppressing alpha cells at high glucose, multiple groups have reported decrements in glucagon upon direct insulin administration113–116. Human and mouse alpha cells express the insulin receptor (INSR/Insr)32,33, which can maintain KATP channels in the open configuration via PI3K/AKT signaling when activated. Open KATP channels drive alpha cells to a hyperpolarised state that prevents glucagon granule exocytosis [Figure 4a]115. Mice with an alpha cell-specific deletion of Insr exhibit increased glucagon secretion, and as a result develop hyperglycemia and glucose intolerance114. However, this effect is milder than expected given the amount of insulin present locally within the islet and is by itself insufficient to explain beta cell-mediated alpha cell inhibition. Indeed, knocking out the insulin receptor in delta cells results in lower somatostatin release and alpha cell insensitivity to insulin indicating 1) insulin also has a paracrine effect potentiating delta cells, and 2) that the inhibition of alpha cells by insulin is mediated at least in part indirectly via somatostatin117.
Serotonin
Serotonin is a neurotransmitter derived from tryptophan that regulates mood and anxiety in the brain. Beta cells express all of the components for serotonin production – tryptophan hydroxylase (TPH1,TPH2) and DOPA decarboxylase (DDC) – and vesicular loading – Vesicular monoamine transporter 1 and 2 (SLC18A1 and SLC18A2)118,119. Beta cell serotonin synthesis is more pronounced in females and is further enhanced during pregnancy and old age119,120. In humans, serotonin from beta cells is released at high glucose and acts in a paracrine manner, inhibiting neighbouring alpha cells via the Gαi-coupled serotonin receptor 1F (HTR1F)27,32. Early clinical studies where healthy human volunteers were administered serotonin antagonists reported increased glucagon secretion121.
GABA
GABA is the classic inhibitory neurotransmitter of the CNS, and while it plays a role in islet signaling, neurogenic GABA’s contribution appears to be minor. Beta cells are able to synthesise GABA at some of the highest concentrations outside the CNS, with islet tissue content measurements in the millimolar range; comparable to local insulin concentrations at basal glucose25,122. Glutamic acid decarboxylase 1 (GAD1), the enzyme that synthesises GABA, is highly enriched in beta cells and is a major Type 1 Diabetes autoantigen32,33. In rodents, GABA inhibits alpha cells through ionotropic GABA A receptors123–125 [Figure 3b], although metabotropic GABA B1 receptor expression is also detectable. GABA from beta cells has been proposed to be the reason alpha cells are silenced at high glucose concentrations124, but GABA treatment by itself does not fully suppress rodent glucagon secretion126. The fact that GABA A receptors are difficult to detect on human alpha cells and that the application of GABA elicits little electrophysiological response127 argue against GABA as a significant contributor to alpha cell inhibition during hyperglycemia [Figure 4a].
Zinc
Insulin secretory granules in the beta cells are highly enriched for zinc ions (Zn2+) to facilitate the formation of the insulin crystal128. Zn2+ co-released with insulin has therefore been considered as another beta cell product poised to inhibit alpha cells129,130. Zn2+ is taken up by the zinc transporter Znt8 (SLC30A8), which is abundantly expressed by both alpha and beta cells32,33,131 [Figure 3c]. However, whole body, beta-specific, and alpha-specific Znt8 deletion does not affect glucagon secretion131,132, indicating that Zn2+ is unlikely to contribute to beta cell-dependent inhibition of glucagon under high glucose.
Delta Cell-Mediated Islet Hormone Coordination
The delta cells have emerged as important inhibitory modulators of alpha and beta cell activity and physiological metabolism. Somatostatin is an important local factor controlling and coordinating the amount and timing of insulin and glucagon release from the islet, which ultimately contributes to setting homeostatic blood glucose levels.
Somatostatin
Somatostatin has important inhibitory functions in the GI tract as well as the anterior pituitary gland. Since delta cell-derived somatostatin accounts for only 5–10% of systemic circulating somatostatin content, its predominant function is likely as a paracrine regulator15,16. Delta cells are active throughout the majority of the physiological range of glucose with secretion starting as low as 3 mM glucose and increasing in a linear, dose-dependent manner towards 20 mM12,15,133. This large range of activity may have direct implications in the inhibition of both alpha and beta cells.
There are five somatostatin receptor (SSTR/Sstr) isoforms – all of which are Gαi-coupled GCPRs134. The most abundant form expressed by mouse beta cells is Sstr3 [Figure 3b]31,33,135. The predominant SSTR(s) on human beta cells remains unclear as some combination of SSTR1, SSTR2, SSTR3 and SSTR5 are expressed32 and impact insulin secretion to some degree136,137. In addition to preventing hormone secretion by decreasing adenylyl cyclase activity, somatostatin receptor signaling simultaneously activates G protein-gated inwardly-rectifying K+ (GIRK) channels that can counteract glucose-mediated membrane depolarisation137 and inactivates VGCCs that are critical to insulin release138. Somatostatin released during high glucose provides inhibitory feedback that – under physiological conditions – does not fully shut down beta cells, but rather provides tonic inhibition. We have proposed that this arrangement is likely instrumental in preventing insulin release in excess of what is required to restore normoglycemia, and in doing so prevents insulin-induced hypoglycemia17. The local release of somatostatin during high blood glucose thus provides an additional layer of control to establish and stabilise blood glucose around its homeostatic setpoint.
Alpha cell activity is controlled by delta cells as the majority of the glucagonostatic effect of high glucose requires the paracrine actions of somatostatin. Somatostatin inhibits alpha cells primarily via SSTR2 in mouse and human islets with additional contribution of SSTR1 in humans31,33,137,139. While somatostatin-independent factors such as glucose contribute to inhibition, alpha cells are under constant tonic inhibition from delta cells. In mice, glucagon output increases across the full range of physiological blood glucose levels when either somatostatin is knocked out140 or when islets are treated with Sstr2 antagonists or inhibitors of downstream somatostatin signaling12,51,141.
Based on our understanding of the inhibition provided by delta cells, it is clear that somatostatin as a paracrine regulator ultimately dictates the total alpha and beta cell output. However, delta cell activity itself is dependent on paracrine inputs as well, chief among these the beta cell hormone urocortin 3.
Urocortin 3
The peptide urocortin 3 (UCN3/Ucn3) is the third most abundant hormone produced by beta cells, and is a member of the same peptide hormone family as CRH14. Urocortin 3 is packaged in the same secretory granules as insulin and co-released with insulin during GSIS. Delta cell GSSS is potentiated by urocortin 3 via the Gαs-coupled GPCR, corticotropin releasing hormone receptor 2 (CRHR2/Crhr2)14, which is selectively expressed by delta cells [Figure 3b]. Beta cell activation is required for full delta cell activity at high glucose as demonstrated by impaired somatostatin release in urocortin 3 knockout mice and islets treated with a Crhr2 antagonist14. Additionally, these same conditions – urocortin 3 knockout and Crhr2 antagonism – both demonstrate markedly increased GSIS, demonstrating that urocortin 3 attenuates insulin release by potentiating GSSS from delta cells in a classic negative feedback loop.
The physiological contribution of urocortin 3 to glucose homeostasis is best illustrated by the timing of its expression during development. Ucn3 is one of the last beta cell genes that is turned on during beta cell maturation; full expression does not occur until around day 14 postpartum in 142 and at the end of the first trimester in human pancreas development143. The onset of urocortin 3 expression coincides with a general increase in plasma glucose levels that is correlated with a drop in circulating insulin levels144. Premature induction of urocortin 3 specifically in beta cells of transgenic mice from embryonic day 10.5 onwards results in prematurely elevated blood glucose relative to control littermates14 demonstrating causality between the onset of urocortin 3 and the rise in blood glucose. Urocortin 3 thus establishes the homeostatic glucose setpoint by activating somatostatin-mediated feedback inhibition of insulin [Figure 4c].
Islet signaling changes in diabetes
The balance between insulin and glucagon release that is so effectively maintained by the integration of a multitude of signals that converge on healthy islets is severely disrupted in diabetes. Autoimmune attack in type 1 diabetes, or peripheral insulin resistance in type 2 diabetes, ultimately leads to beta dysfunction and death6. Alpha cells in type 1 diabetes exhibit an impaired counterregulatory response to hypoglycemia145, and conversely in type 2 diabetes they aggravate hyperglycemia by inappropriate post-prandial glucagon secretion [Figure 4d]146.
There is evidence that these many clinical manifestations are tied to a breakdown of the paracrine crosstalk that so tightly regulates islet function in healthy islets. The impaired alpha cell counterregulatory response has been attributed to autonomic failure, where adrenergic stimulation that assists in potentiating hypoglycemic glucagon secretion is lost147,148. A paracrine explanation (that need not be mutually-exclusive) is that somatostatin is elevated in response to hypoglycemia in STZ-induced diabetic rats149, likely contributing to impaired counterregulatory glucagon release. Indeed, blockade of the Sstr2 that is selectively expressed by alpha cells suffices to restore counterregulation in rats150.
In another example where paracrine crosstalk breaks down in diabetes, impaired urocortin 3 signaling likely contributes to dysregulated insulin, somatostatin, secretion in diabetes. In Type 1 Diabetes, the majority of beta cells are destroyed and no longer can serve as a source of local urocortin 3 [Figure 4b]. Pre-diabetic (type 2) human, NHP, and mouse beta cells selectively down regulate urocortin 3 expression14,151,152. The ensuing effects of the loss of urocortin 3 on GSSS in human and NHP islets have not been established, but islets from moderately diabetic leptin-deficient mice demonstrate a loss of urocortin 3 and consequently release little somatostatin at high glucose, despite the fact that delta cells remain in normal, or even increased numbers in diabetes14. Restoring urocortin 3 expression in diabetic mice exacerbates hyperglycemia, in line with the re-activation of beta cell inhibition by somatostatin and similar to the premature embryonic urocortin 3 induction14. The mechanism responsible for the loss of urocortin 3 expression that precipitates the breakdown of local crosstalk early in diabetes is not known, which illustrates that a better understanding of paracrine communication between islet cells is vital to improved therapeutic options.
At the nutrient level, elevated blood glucose in diabetes provokes many functional changes in beta cells. Hyperglycemia contributes to beta cell proliferation in mice and ultimately leads to glucotoxicity and beta cell dysfunction. In the dysfunctional state, the loss of urocortin 3 likely removes much of the somatostatin inhibition, as stated above14. Unrestrained from paracrine signals including somatostatin, alpha cells release glucagon that stimulates hepatic glucose production and aggravates hyperglycemia [Figure 4d]. With hepatic insulin resistance, individuals can also develop impaired glucagon signaling, which inhibits gluconeogenesis and leads to an accumulation of circulating amino acids. This sets off a vicious cycle of hyperaminoacidemia and hyperglucagonemia, with neither signal efficiently correcting the other64. Initial insulin compensation in type 2 diabetes can lead to fatty infiltration where the islet microenvironment becomes filled with adipocytes23. While fatty acids will contribute to the compensatory increase in insulin release via potentiating secretion and increasing beta cell mass, extended exposure to fatty acids can lead to lipotoxic stress, beta cell dysfunction and decreased insulin secretion153. Without intervention, these initially compensatory mechanisms to increase insulin output cause a state of partial or complete beta cell dysfunction.
The value of transcriptomics and pathways for drug discovery
Transcriptomics supported by rigourous validation experiments via imaging and hormone secretion have rapidly advanced our grasp of islet function. Such increased mechanistic understanding can inform direct translational progress. RNA-Seq performed on islet cells from diabetic donors has allowed for direct comparisons between the healthy and diseased islet 34,154. Two examples of the insights generated by these islet cell transcriptomes are clarifications of the islet mechanisms of action of the hunger hormone, ghrelin33, and, separately, the receptor GPR119:
The mechanism of ghrelin-mediated insulin suppression
Ghrelin release occurs in the fasted state and inhibits insulin release from rodent and human pancreata155–157. For years, these insulinostatic actions had been attributed to direct inhibition of beta cells by ghrelin155,158, even though the growth hormone secretagogue receptor (GHSR/Ghsr) that mediates ghrelin’s signal is a GPCR normally associated with an activating Gαq subunit159. As ghrelin’s inhibitory actions were sensitive to pertussis toxin, it was proposed that GHSR couples to an inhibitory Gαi subunit in beta cells160. However, islet cell transcriptomes from multiple groups resolved this conundrum by demonstrating that GHSR is selectively expressed by delta cells [Figure 3b], and the resulting Gαq-mediated somatostatin release from delta cells silences beta cells in a Gαi-mediated fashion31,33,161. Coupling transcriptomics to functional assays has expanded our ability to interrogate which islet cell type expresses the requisite receptors in order to respond to each respective signal that impinges on the islet.
GPR119 stimulates insulin secretion indirectly via alpha cells
GPR119 is a Gαs-coupled GPCR that binds lipids and lipid metabolites. The receptor has been a drug target of interest for diabetes since the discovery that GPR119 activation enhances both GSIS and incretin release162,163. Early GPR119 research found its expression to be largely limited to the pancreatic islets and GI tract, and inaccurately built a case for GPR119 as a beta cell-specific insulinotropic receptor. Following these observation, multiple companies generated small molecule agonists for GPR119164. The receptor proved to be highly druggable with a number of molecules eliciting improved glucose clearance, but efforts have stalled recently in the face of common challenges related to safety and efficacy. Moreover, drug developers may have been targeting the wrong cell type – comprehensive islet transcriptomes clearly demonstrate relatively selective expression of GPR119 by alpha cells [Figure 3b]33,40. This has been validated by a subsequent study demonstrating that GPR119 activation in mouse and human islets improves glucagon release during hypoglycemia165. The improved GSIS that is observed in response to GPR119 agonism is likely due to the actions of glucagon potentiating GSIS from beta cells that we reviewed earlier.
Conclusions
While many aspects that contribute to the regulation of pancreatic islets in health and disease remain unresolved, a brief survey of recent trends in islet research shows that in taking a small step back to appreciate the islet beyond the beta cells and glucose, the diabetes field is taking significant strides forward towards a more comprehensive understanding. Shining a light on alpha and delta cells, the intra-islet crosstalk they engage in, and non-canonical nutrient signaling may turn out to be key in tackling diabetes. Especially in the context of drug discovery, these interactions cannot be ignored. The availability of comprehensive bulk and single cell transcriptomes for each islet cell type will continue to facilitate the delineation between direct and indirect effects of hormones, nutrients, and neurotransmitters. Similarly, much of the work done to date on stem cells for a cure for Type 1 Diabetes has focused on generating insulin-secreting beta cell-like cells. However, it has become clear that beta cells require input from neighboring alpha and delta cells for mature beta cell function. Future studies should be cognizant of these interactions as Occam’s Razor (the simplest explanation is likely correct) applied to the pancreatic islet must account for the islet as an interactive unit where multiple endocrine and non-endocrine cell types coordinate the overall release of insulin and glucagon from the islet.
Acknowledgements
We apologise to those whose work we were not able to discuss due to limitations in space and scope. Work discussed in this Review was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-110276), American Diabetes Association (#1-19-IBS-078), and the Juvenile Diabetes Research Foundation (2-SRA-2019-700-S-B). G.M.N. was supported by a NIGMS-funded Pharmacology Training Program (T32GM099608). We thank Alex Mawla for assistance in bioinformatics analysis and data presentation and Dr. Talitha van der Meulen for a critical reading of the manuscript.
Competing Interests
M.O.H. receives funding from Crinetics Inc. to study somatostatin analogues. None of this work is discussed in this review.
Footnotes
Data availability Statement
The dataset analysed in this study is available in the GEO repository under accession number GSE90766.
References
- 1.Collaboration, N. C. D. R. F. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530, doi: 10.1016/S0140-6736(16)00618-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unger RH & Cherrington AD Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 122, 4–12, doi: 10.1172/JCI60016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera O et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103, 2334–2339, doi: 10.1073/pnas.0510790103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner DJ, Kim A, Miller K & Hara M Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2, 135–145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brissova M et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53, 1087–1097, 10.1369/jhc.5C6684.2005 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Keane K & Newsholme P Metabolic regulation of insulin secretion. Vitam Horm 95, 1–33, doi: 10.1016/B978-0-12-800174-5.00001-6 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Grodsky GM et al. Effects of Carbohydrates on Secretion of Insulin from Isolated Rat Pancreas. Am J Physiol 205, 638–644, doi: 10.1152/ajplegacy.1963.205.4.638 (1963). [DOI] [PubMed] [Google Scholar]
- 8.Jensen MV et al. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295, E1287–1297, doi: 10.1152/ajpendo.90604.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimitriadis G, Mitrou P, Lambadiari V, Maratou E & Raptis SA Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract 93 Suppl 1, S52–59, doi: 10.1016/S0168-8227(11)70014-6 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Hart NJ & Powers AC Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia 62, 212–222, doi: 10.1007/s00125-018-4772-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briant L, Salehi A, Vergari E, Zhang Q & Rorsman P Glucagon secretion from pancreatic alpha-cells. Ups J Med Sci 121, 113–119, doi: 10.3109/03009734.2016.1156789 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira E, Salehi A & Gylfe E Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50, 370–379, doi: 10.1007/s00125-006-0511-1 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Walker JN et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab 13 Suppl 1, 95–105, doi: 10.1111/j.1463-1326.2011.01450.x (2011). [DOI] [PubMed] [Google Scholar]
- 14.van der Meulen T et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med 21, 769–776, doi: 10.1038/nm.3872 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rorsman P & Huising MO The somatostatin-secreting pancreatic delta-cell in health and disease. Nat Rev Endocrinol 14, 404–414, doi: 10.1038/s41574-018-0020-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessio DA & Ensinck JW Fasting and postprandial concentrations of somatostatin-28 and somatostatin-14 in type II diabetes in men. Diabetes 39, 1198–1202 (1990). [DOI] [PubMed] [Google Scholar]
- 17.Huising MO, van der Meulen T, Huang JL, Pourhosseinzadeh MS & Noguchi GM The Difference delta-Cells Make in Glucose Control. Physiology (Bethesda) 33, 403–411, doi: 10.1152/physiol.00029.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rorsman P & Ashcroft FM Pancreatic beta-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol Rev 98, 117–214, doi: 10.1152/physrev.00008.2017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Diaz R & Caicedo A Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 28, 745–756, doi: 10.1016/j.beem.2014.05.002 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Amisten S et al. A comparative analysis of human and mouse islet G-protein coupled receptor expression. Sci Rep 7, 46600, doi: 10.1038/srep46600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amisten S, Salehi A, Rorsman P, Jones PM & Persaud SJ An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther 139, 359–391, doi: 10.1016/j.pharmthera.2013.05.004 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Atanes P et al. Defining G protein-coupled receptor peptide ligand expressomes and signalomes in human and mouse islets. Cell Mol Life Sci 75, 3039–3050, doi: 10.1007/s00018-018-2778-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang SC et al. Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia 61, 168–181, doi: 10.1007/s00125-017-4409-x (2018). [DOI] [PubMed] [Google Scholar]
- 24.Brunicardi FC, Shavelle DM & Andersen DK Neural regulation of the endocrine pancreas. Int J Pancreatol 18, 177–195, doi: 10.1007/BF02784941 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Ling Z & Pipeleers D Comparison of cellular and medium insulin and GABA content as markers for living beta-cells. Am J Physiol Endocrinol Metab 288, E307–313, doi: 10.1152/ajpendo.00222.2004 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Diaz R et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 17, 888–892, doi: 10.1038/nm.2371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almaca J et al. Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells. Cell reports 17, 3281–3291, doi: 10.1016/j.celrep.2016.11.072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Diaz R et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14, 45–54, doi:S1550–4131(11)00212–9 [pii] 10.1016/j.cmet.2011.05.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen TW et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300, doi: 10.1038/nature12354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorrell C et al. Human islets contain four distinct subtypes of beta cells. Nat Commun 7, 11756, doi: 10.1038/ncomms11756 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adriaenssens AE et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 59, 2156–2165, doi: 10.1007/s00125-016-4033-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benner C et al. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15, 620, doi: 10.1186/1471-2164-15-620 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiGruccio MR et al. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab 5, 449–458, doi: 10.1016/j.molmet.2016.04.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segerstolpe A et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab 24, 593–607, doi: 10.1016/j.cmet.2016.08.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briant LJ et al. Functional identification of islet cell types by electrophysiological fingerprinting. J R Soc Interface 14, doi: 10.1098/rsif.2016.0999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadwell CR et al. Multimodal profiling of single-cell morphology, electrophysiology, and gene expression using Patch-seq. Nat Protoc 12, 2531–2553, doi: 10.1038/nprot.2017.120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camunas-Soler J et al. Pancreas patch-seq links physiologic dysfunction in diabetes to single-cell transcriptomic phenotypes. BioRxiv, doi:doi: 10.1101/555110 (2019). [DOI] [Google Scholar]
- 38.Bonner-Weir S, Sullivan BA & Weir GC Human Islet Morphology Revisited: Human and Rodent Islets Are Not So Different After All. J Histochem Cytochem 63, 604–612, doi: 10.1369/0022155415570969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilimnik G, Jo J, Periwal V, Zielinski MC & Hara M Quantification of islet size and architecture. Islets 4, 167–172, doi: 10.4161/isl.19256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benner C et al. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15, 620, doi: 10.1186/1471-2164-15-620 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skelin Klemen M, Dolensek J, Slak Rupnik M & Stozer A The triggering pathway to insulin secretion: Functional similarities and differences between the human and the mouse beta cells and their translational relevance. Islets 9, 109–139, doi: 10.1080/19382014.2017.1342022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longuet C et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab 8, 359–371, doi: 10.1016/j.cmet.2008.09.008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boden G, Rezvani I & Owen OE Effects of glucagon on plasma amino acids. J Clin Invest 73, 785–793, doi: 10.1172/JCI111272 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rorsman P & Braun M Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 75, 155–179, doi: 10.1146/annurev-physiol-030212-183754 (2013). [DOI] [PubMed] [Google Scholar]
- 45.De Vos A et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 96, 2489–2495, doi: 10.1172/JCI118308 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q et al. R-type Ca(2+)-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nature cell biology 9, 453–460, doi: 10.1038/ncb1563 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Briant LJB et al. delta-cells and beta-cells are electrically coupled and regulate alpha-cell activity via somatostatin. J Physiol 596, 197–215, doi: 10.1113/JP274581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopel SO, Kanno T, Barg S & Rorsman P Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets. J Physiol 528, 497–507 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salehi A, Qader SS, Grapengiesser E & Hellman B Pulses of somatostatin release are slightly delayed compared with insulin and antisynchronous to glucagon. Regul Pept 144, 43–49, doi: 10.1016/j.regpep.2007.06.003 (2007). [DOI] [PubMed] [Google Scholar]
- 50.MacDonald PE et al. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 5, e143, doi:06-PLBI-RA-1371R2 [pii] 10.1371/journal.pbio.0050143 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai BK et al. Somatostatin Is Only Partly Required for the Glucagonostatic Effect of Glucose but Is Necessary for the Glucagonostatic Effect of KATP Channel Blockers. Diabetes 67, 2239–2253, doi: 10.2337/db17-0880 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Smith PA et al. Electrogenic arginine transport mediates stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol 499 (Pt 3), 625–635, doi: 10.1113/jphysiol.1997.sp021955 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunne MJ, Yule DI, Gallacher DV & Petersen OH Effects of alanine on insulin-secreting cells: patch-clamp and single cell intracellular Ca2+ measurements. Biochim Biophys Acta 1055, 157–164, doi: 10.1016/0167-4889(90)90116-u (1990). [DOI] [PubMed] [Google Scholar]
- 54.Henquin JC & Meissner HP Effects of amino acids on membrane potential and 86Rb+ fluxes in pancreatic beta-cells. Am J Physiol 240, E245–252, doi: 10.1152/ajpendo.1981.240.3.E245 (1981). [DOI] [PubMed] [Google Scholar]
- 55.Newsholme P, Bender K, Kiely A & Brennan L Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans 35, 1180–1186, doi: 10.1042/BST0351180 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Yan-Do R et al. A Glycine-Insulin Autocrine Feedback Loop Enhances Insulin Secretion From Human beta-Cells and Is Impaired in Type 2 Diabetes. Diabetes 65, 2311–2321, doi: 10.2337/db15-1272 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Rocha DM, Faloona GR & Unger RH Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest 51, 2346–2351, doi: 10.1172/JCI107046 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ang T, Bruce CR & Kowalski GM Postprandial Aminogenic Insulin and Glucagon Secretion Can Stimulate Glucose Flux in Humans. Diabetes 68, 939–946, doi: 10.2337/db18-1138 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Rorsman P & Hellman B Voltage-activated currents in guinea pig pancreatic alpha 2 cells. Evidence for Ca2+-dependent action potentials. J Gen Physiol 91, 223–242, doi: 10.1085/jgp.91.2.223 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C et al. Regulation of glucagon secretion in normal and diabetic human islets by gamma-hydroxybutyrate and glycine. J Biol Chem 288, 3938–3951, doi: 10.1074/jbc.M112.385682 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dean ED et al. Interrupted Glucagon Signaling Reveals Hepatic alpha Cell Axis and Role for L-Glutamine in alpha Cell Proliferation. Cell Metab 25, 1362–1373 e1365, doi: 10.1016/j.cmet.2017.05.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gelling RW et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 100, 1438–1443, doi: 10.1073/pnas.0237106100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic alpha Cell Hyperplasia in Mice. Cell Metab 25, 1348–1361 e1348, doi: 10.1016/j.cmet.2017.05.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wewer Albrechtsen NJ et al. Evidence of a liver-alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 61, 671–680, doi: 10.1007/s00125-017-4535-5 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Wewer Albrechtsen NJ et al. Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Am J Physiol Gastrointest Liver Physiol 314, G91–G96, doi: 10.1152/ajpgi.00216.2017 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Itoh Y et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422, 173–176, doi: 10.1038/nature01478 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Pujol JB et al. Coordination of GPR40 and Ketogenesis Signaling by Medium Chain Fatty Acids Regulates Beta Cell Function. Nutrients 10, doi: 10.3390/nu10040473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML & Prentki M Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 55 Suppl 2, S16–23, doi: 10.2337/db06-s003 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Kristinsson H, Bergsten P & Sargsyan E Free fatty acid receptor 1 (FFAR1/GPR40) signaling affects insulin secretion by enhancing mitochondrial respiration during palmitate exposure. Biochim Biophys Acta 1853, 3248–3257, doi: 10.1016/j.bbamcr.2015.09.022 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Nagasumi K et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 58, 1067–1076, doi: 10.2337/db08-1233 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haber EP et al. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol 194, 1–12, doi: 10.1002/jcp.10187 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Ferdaoussi M et al. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 55, 2682–2692, doi: 10.1007/s00125-012-2650-x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kristinsson H, Smith DM, Bergsten P & Sargsyan E FFAR1 is involved in both the acute and chronic effects of palmitate on insulin secretion. Endocrinology 154, 4078–4088, doi: 10.1210/en.2013-1352 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Opara EC, Garfinkel M, Hubbard VS, Burch WM & Akwari OE Effect of fatty acids on insulin release: role of chain length and degree of unsaturation. Am J Physiol 266, E635–639, doi: 10.1152/ajpendo.1994.266.4.E635 (1994). [DOI] [PubMed] [Google Scholar]
- 75.Hoppa MB et al. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab 10, 455–465, doi: 10.1016/j.cmet.2009.09.011 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olofsson CS, Salehi A, Holm C & Rorsman P Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol 557, 935–948, doi: 10.1113/jphysiol.2004.066258 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerich JE, Langlois M, Schneider V, Karam JH & Noacco C Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. J Clin Invest 53, 1284–1289, doi: 10.1172/JCI107675 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kristinsson H et al. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Sci Rep 7, 4657, doi: 10.1038/s41598-017-04730-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olofsson CS, Salehi A, Gopel SO, Holm C & Rorsman P Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes 53, 2836–2843 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Guettet C, Mathe D, Navarro N & Lecuyer B Effects of chronic glucagon administration on rat lipoprotein composition. Biochim Biophys Acta 1005, 233–238 (1989). [DOI] [PubMed] [Google Scholar]
- 81.Xiao C, Pavlic M, Szeto L, Patterson BW & Lewis GF Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60, 383–390, doi: 10.2337/db10-0763 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stone VM et al. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia 57, 1182–1191, doi: 10.1007/s00125-014-3213-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samols E, Marri G & Marks V Promotion of Insulin Secretion by Glucagon. Lancet 2, 415–416 (1965). [DOI] [PubMed] [Google Scholar]
- 84.Watts M, Ha J, Kimchi O & Sherman A Paracrine regulation of glucagon secretion: the beta/alpha/delta model. Am J Physiol Endocrinol Metab 310, E597–E611, doi: 10.1152/ajpendo.00415.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez-Diaz R et al. Paracrine Interactions within the Pancreatic Islet Determine the Glycemic Set Point. Cell Metab 27, 549–558 e544, doi: 10.1016/j.cmet.2018.01.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauritsen KB, Moody AJ, Christensen KC & Lindkaer Jensen S Gastric inhibitory polypeptide (GIP) and insulin release after small-bowel resection in man. Scand J Gastroenterol 15, 833–840 (1980). [DOI] [PubMed] [Google Scholar]
- 87.Elrick H, Stimmler L, Hlad CJ Jr. & Arai Y Plasma Insulin Response to Oral and Intravenous Glucose Administration. J Clin Endocrinol Metab 24, 1076–1082, doi: 10.1210/jcem-24-10-1076 (1964). [DOI] [PubMed] [Google Scholar]
- 88.Nauck MA et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 63, 492–498, doi: 10.1210/jcem-63-2-492 (1986). [DOI] [PubMed] [Google Scholar]
- 89.MacDonald PE et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51 Suppl 3, S434–442 (2002). [DOI] [PubMed] [Google Scholar]
- 90.Shigeto M et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest 125, 4714–4728, doi: 10.1172/JCI81975 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoffers DA et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49, 741–748 (2000). [DOI] [PubMed] [Google Scholar]
- 92.Yang DH, Zhou CH, Liu Q & Wang MW Landmark studies on the glucagon subfamily of GPCRs: from small molecule modulators to a crystal structure. Acta Pharmacol Sin 36, 1033–1042, doi: 10.1038/aps.2015.78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Capozzi ME et al. beta-Cell tone is defined by proglucagon peptides through cyclic AMP signaling. JCI Insight, doi: 10.1172/jci.insight.126742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chambers AP et al. The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice. Cell Metab 25, 927–934 e923, doi: 10.1016/j.cmet.2017.02.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Svendsen B et al. Insulin Secretion Depends on Intra-islet Glucagon Signaling. Cell Rep 25, 1127–1134 e1122, doi: 10.1016/j.celrep.2018.10.018 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Zhu L et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 5, doi: 10.1172/jci.insight.127994 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huypens P, Ling Z, Pipeleers D & Schuit F Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43, 1012–1019, doi: 10.1007/s001250051484 (2000). [DOI] [PubMed] [Google Scholar]
- 98.Kawai K, Yokota C, Ohashi S, Watanabe Y & Yamashita K Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia 38, 274–276 (1995). [DOI] [PubMed] [Google Scholar]
- 99.Song Y et al. Gut-Proglucagon-Derived Peptides Are Essential for Regulating Glucose Homeostasis in Mice. Cell Metab, doi: 10.1016/j.cmet.2019.08.009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whalley NM, Pritchard LE, Smith DM & White A Processing of proglucagon to GLP-1 in pancreatic alpha-cells: is this a paracrine mechanism enabling GLP-1 to act on beta-cells? J Endocrinol 211, 99–106, doi: 10.1530/JOE-11-0094 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Tillner J et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab 21, 120–128, doi: 10.1111/dom.13494 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Zhou J et al. A novel glucagon-like peptide-1/glucagon receptor dual agonist exhibits weight-lowering and diabetes-protective effects. Eur J Med Chem 138, 1158–1169, doi: 10.1016/j.ejmech.2017.07.046 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Petrusz P, Merchenthaler I, Maderdrut JL, Vigh S & Schally AV Corticotropin-releasing factor (CRF)-like immunoreactivity in the vertebrate endocrine pancreas. Proc Natl Acad Sci U S A 80, 1721–1725 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huising MO et al. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc Natl Acad Sci U S A 107, 912–917, doi: 10.1073/pnas.0913610107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanno T, Suga S, Nakano K, Kamimura N & Wakui M Corticotropin-releasing factor modulation of Ca2+ influx in rat pancreatic beta-cells. Diabetes 48, 1741–1746 (1999). [DOI] [PubMed] [Google Scholar]
- 106.Blaabjerg L et al. CRFR1 activation protects against cytokine-induced beta-cell death. J Mol Endocrinol 53, 417–427, doi: 10.1530/JME-14-0056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Molina J et al. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 63, 2714–2726, doi: 10.2337/db13-1371 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gautam D et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 3, 449–461, doi: 10.1016/j.cmet.2006.04.009 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Gilon P & Henquin JC Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 22, 565–604, doi: 10.1210/edrv.22.5.0440 (2001). [DOI] [PubMed] [Google Scholar]
- 110.Duttaroy A et al. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes 53, 1714–1720, doi: 10.2337/diabetes.53.7.1714 (2004). [DOI] [PubMed] [Google Scholar]
- 111.Hauge-Evans AC, Anderson RL, Persaud SJ & Jones PM Delta cell secretory responses to insulin secretagogues are not mediated indirectly by insulin. Diabetologia 55, 1995–2004, doi: 10.1007/s00125-012-2546-9 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Martyn JA, Fagerlund MJ & Eriksson LI Basic principles of neuromuscular transmission. Anaesthesia 64 Suppl 1, 1–9, doi: 10.1111/j.1365-2044.2008.05865.x (2009). [DOI] [PubMed] [Google Scholar]
- 113.Ostenson CG Regulation of glucagon release: effects of insulin on the pancreatic A2-cell of the guinea pig. Diabetologia 17, 325–330 (1979). [DOI] [PubMed] [Google Scholar]
- 114.Kawamori D et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 9, 350–361, doi: 10.1016/j.cmet.2009.02.007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Franklin I, Gromada J, Gjinovci A, Theander S & Wollheim CB Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54, 1808–1815 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Ravier MA & Rutter GA Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 54, 1789–1797 (2005). [DOI] [PubMed] [Google Scholar]
- 117.Vergari E et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat Commun 10, 139, doi: 10.1038/s41467-018-08193-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohta Y et al. Convergence of the insulin and serotonin programs in the pancreatic beta-cell. Diabetes 60, 3208–3216, doi:db10–1192 [pii] 10.2337/db10-1192 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim H et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 16, 804–808, doi:nm.2173 [pii] 10.1038/nm.2173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim YG et al. beta-cell serotonin production is associated with female sex, old age, and diabetes-free condition. Biochem Biophys Res Commun 493, 1197–1203, doi: 10.1016/j.bbrc.2017.09.130 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Marco J, Hedo JA, Martinell J, Calle C & Villanueva ML Potentiation of glucagon secretion by serotonin antagonists in man. J Clin Endocrinol Metab 42, 215–221, doi: 10.1210/jcem-42-2-215 (1976). [DOI] [PubMed] [Google Scholar]
- 122.Michalik M & Erecinska M GABA in pancreatic islets: metabolism and function. Biochem Pharmacol 44, 1–9 (1992). [DOI] [PubMed] [Google Scholar]
- 123.Jin Y, Korol SV, Jin Z, Barg S & Birnir B In intact islets interstitial GABA activates GABA(A) receptors that generate tonic currents in alpha-cells. PLoS One 8, e67228, doi: 10.1371/journal.pone.0067228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rorsman P et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341, 233–236, doi: 10.1038/341233a0 (1989). [DOI] [PubMed] [Google Scholar]
- 125.Wendt A et al. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes 53, 1038–1045 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C & Henquin JC The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 129, 2521–2529, doi: 10.1210/endo-129-5-2521 (1991). [DOI] [PubMed] [Google Scholar]
- 127.Braun M et al. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 59, 1694–1701, doi: 10.2337/db09-0797 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baker EN et al. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci 319, 369–456, doi: 10.1098/rstb.1988.0058 (1988). [DOI] [PubMed] [Google Scholar]
- 129.Ishihara H, Maechler P, Gjinovci A, Herrera PL & Wollheim CB Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nature cell biology 5, 330–335, doi: 10.1038/ncb951 (2003). [DOI] [PubMed] [Google Scholar]
- 130.Zhou H, Zhang T, Harmon JS, Bryan J & Robertson RP Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes 56, 1107–1112, doi: 10.2337/db06-1454 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Nicolson TJ et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58, 2070–2083, doi: 10.2337/db09-0551 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wijesekara N et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53, 1656–1668, doi: 10.1007/s00125-010-1733-9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramracheya R et al. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes 59, 2198–2208, doi: 10.2337/db09-1505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patel YC, Greenwood MT, Warszynska A, Panetta R & Srikant CB All five cloned human somatostatin receptors (hSSTR1–5) are functionally coupled to adenylyl cyclase. Biochem Biophys Res Commun 198, 605–612, doi: 10.1006/bbrc.1994.1088 (1994). [DOI] [PubMed] [Google Scholar]
- 135.Blodgett DM et al. Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets. Diabetes 64, 3172–3181, doi: 10.2337/db15-0039 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh V et al. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. J Clin Endocrinol Metab 92, 673–680, doi: 10.1210/jc.2006-1578 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Kailey B et al. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic beta- and alpha-cells. Am J Physiol Endocrinol Metab 303, E1107–1116, doi: 10.1152/ajpendo.00207.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hsu WH, Xiang HD, Rajan AS, Kunze DL & Boyd AE 3rd. Somatostatin inhibits insulin secretion by a G-protein-mediated decrease in Ca2+ entry through voltage-dependent Ca2+ channels in the beta cell. J Biol Chem 266, 837–843 (1991). [PubMed] [Google Scholar]
- 139.Kumar U et al. Subtype-selective expression of the five somatostatin receptors (hSSTR1–5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48, 77–85 (1999). [DOI] [PubMed] [Google Scholar]
- 140.Cheng-Xue R et al. Tolbutamide controls glucagon release from mouse islets differently than glucose: involvement of K(ATP) channels from both alpha-cells and delta-cells. Diabetes 62, 1612–1622, doi: 10.2337/db12-0347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li J et al. Submembrane ATP and Ca2+ kinetics in alpha-cells: unexpected signaling for glucagon secretion. FASEB J 29, 3379–3388, doi: 10.1096/fj.14-265918 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van der Meulen T et al. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells. PLoS One 7, e52181, doi: 10.1371/journal.pone.0052181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van der Meulen T & Huising MO Maturation of Stem Cell-Derived Beta cells Guided by the Expression of Urocortin 3. Rev. Diabet. Stud. 11, 115–132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blum B et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 30, 261–264, doi: 10.1038/nbt.2141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cryer PE, Davis SN & Shamoon H Hypoglycemia in diabetes. Diabetes Care 26, 1902–1912 (2003). [DOI] [PubMed] [Google Scholar]
- 146.Shah P et al. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85, 4053–4059, doi: 10.1210/jcem.85.11.6993 (2000). [DOI] [PubMed] [Google Scholar]
- 147.Ma Y, Wang Q, Joe D, Wang M & Whim MD Recurrent hypoglycemia inhibits the counterregulatory response by suppressing adrenal activity. J Clin Invest 128, 3866–3871, doi: 10.1172/JCI91921 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cryer PE Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 369, 362–372, doi: 10.1056/NEJMra1215228 (2013). [DOI] [PubMed] [Google Scholar]
- 149.Yue JT et al. Somatostatin receptor type 2 antagonism improves glucagon and corticosterone counterregulatory responses to hypoglycemia in streptozotocin-induced diabetic rats. Diabetes 61, 197–207, doi: 10.2337/db11-0690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karimian N et al. Somatostatin receptor type 2 antagonism improves glucagon counterregulation in biobreeding diabetic rats. Diabetes 62, 2968–2977, doi: 10.2337/db13-0164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rui J et al. beta Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice. Cell Metab 25, 727–738, doi: 10.1016/j.cmet.2017.01.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blum B et al. Reversal of beta cell de-differentiation by a small molecule inhibitor of the TGFbeta pathway. eLife 3, e02809, doi: 10.7554/eLife.02809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou YP & Grill VE Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 93, 870–876, doi: 10.1172/JCI117042 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lawlor N et al. Single cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res, doi: 10.1101/gr.212720.116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Egido EM, Rodriguez-Gallardo J, Silvestre RA & Marco J Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 146, 241–244 (2002). [DOI] [PubMed] [Google Scholar]
- 156.Reimer MK, Pacini G & Ahren B Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 144, 916–921, doi: 10.1210/en.2002-220819 (2003). [DOI] [PubMed] [Google Scholar]
- 157.Tong J et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59, 2145–2151, doi: 10.2337/db10-0504 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Date Y et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 51, 124–129 (2002). [DOI] [PubMed] [Google Scholar]
- 159.Damian M et al. Ghrelin receptor conformational dynamics regulate the transition from a preassembled to an active receptor:Gq complex. Proc Natl Acad Sci U S A 112, 1601–1606, doi: 10.1073/pnas.1414618112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dezaki K, Kakei M & Yada T Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes 56, 2319–2327, doi: 10.2337/db07-0345 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Lawlor N et al. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res 27, 208–222, doi: 10.1101/gr.212720.116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chu ZL et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 148, 2601–2609, doi: 10.1210/en.2006-1608 (2007). [DOI] [PubMed] [Google Scholar]
- 163.Soga T et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun 326, 744–751, doi: 10.1016/j.bbrc.2004.11.120 (2005). [DOI] [PubMed] [Google Scholar]
- 164.Ritter K, Buning C, Halland N, Poverlein C & Schwink L G Protein-Coupled Receptor 119 (GPR119) Agonists for the Treatment of Diabetes: Recent Progress and Prevailing Challenges. J Med Chem 59, 3579–3592, doi: 10.1021/acs.jmedchem.5b01198 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Li NX et al. GPR119 Agonism Increases Glucagon Secretion During Insulin-Induced Hypoglycemia. Diabetes 67, 1401–1413, doi: 10.2337/db18-0031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harding SD et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46, D1091–D1106, doi: 10.1093/nar/gkx1121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brereton MF, Vergari E, Zhang Q & Clark A Alpha-, Delta- and PP-cells: Are They the Architectural Cornerstones of Islet Structure and Co-ordination? J Histochem Cytochem 63, 575–591, doi: 10.1369/0022155415583535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Adrian TE et al. Distribution and release of human pancreatic polypeptide. Gut 17, 940–944, doi: 10.1136/gut.17.12.940 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Batterham RL et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 88, 3989–3992 (2003). [DOI] [PubMed] [Google Scholar]
- 170.Andralojc KM et al. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 52, 486–493, doi: 10.1007/s00125-008-1238-y (2009). [DOI] [PubMed] [Google Scholar]
- 171.Brissova M et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes 55, 2974–2985, doi: 10.2337/db06-0690 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Narayanan S et al. Intra-islet endothelial cell and beta-cell crosstalk: Implication for islet cell transplantation. World J Transplant 7, 117–128, doi: 10.5500/wjt.v7.i2.117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tang SC, Chiu YC, Hsu CT, Peng SJ & Fu YY Plasticity of Schwann cells and pericytes in response to islet injury in mice. Diabetologia 56, 2424–2434, doi: 10.1007/s00125-013-2977-y (2013). [DOI] [PubMed] [Google Scholar]
- 174.Almaca J, Weitz J, Rodriguez-Diaz R, Pereira E & Caicedo A The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab 27, 630–644 e634, doi: 10.1016/j.cmet.2018.02.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sasson A et al. Islet Pericytes Are Required for beta-Cell Maturity. Diabetes 65, 3008–3014, doi: 10.2337/db16-0365 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Mwangi S et al. Glial cell line-derived neurotrophic factor increases beta-cell mass and improves glucose tolerance. Gastroenterology 134, 727–737, doi: 10.1053/j.gastro.2007.12.033 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Geutskens SB, Otonkoski T, Pulkkinen MA, Drexhage HA & Leenen PJ Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol 78, 845–852, doi: 10.1189/jlb.1004624 (2005). [DOI] [PubMed] [Google Scholar]
- 178.Weitz JR et al. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 61, 182–192, doi: 10.1007/s00125-017-4416-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zang G et al. Activated pancreatic stellate cells can impair pancreatic islet function in mice. Ups J Med Sci 120, 169–180, doi: 10.3109/03009734.2015.1032453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Barreto SG, Carati CJ, Toouli J & Saccone GT The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol 299, G10–22, doi: 10.1152/ajpgi.00077.2010 (2010). [DOI] [PubMed] [Google Scholar]