Abstract
Neuropathic pain is a chronic pain state characterized by nerve damage, inflammation, and nociceptive neuron hyperactivity. As the underlying pathophysiology is complex, a more effective therapy for neuropathic pain would be one that targets multiple elements. Here, we generated recombinant adeno-associated viruses (AAVs) encoding three therapeutic genes, namely, glutamate decarboxylase 65, glial cell-derived neurotrophic factor, and interleukin-10, with various combinations. The efficacy for pain relief was evaluated in a rat spared nerve injury model of neuropathic pain. The maximal analgesic effect was achieved when the AAVs expressing all three genes were administered to rats with neuropathic pain. The combination of two virus constructs expressing the three genes was named KLS-2031 and evaluated as a potential novel therapeutic for neuropathic pain. Single transforaminal epidural injections of KLS-2031 into the intervertebral foramen to target the appropriate dorsal root ganglion produced notable long-term analgesic effects in female and male rats. Furthermore, KLS-2031 mitigated the neuroinflammation, neuronal cell death, and dorsal root ganglion hyperexcitability induced by the spared nerve injury. These results suggest that KLS-2031 represents a promising therapeutic option for refractory neuropathic pain.
Keywords: Adeno-associated virus, Neuropathic pain, Gene therapy, glutamate decarboxylase 65, glial cell-derived neurotrophic factor, interleukin-10
Graphical Abstract
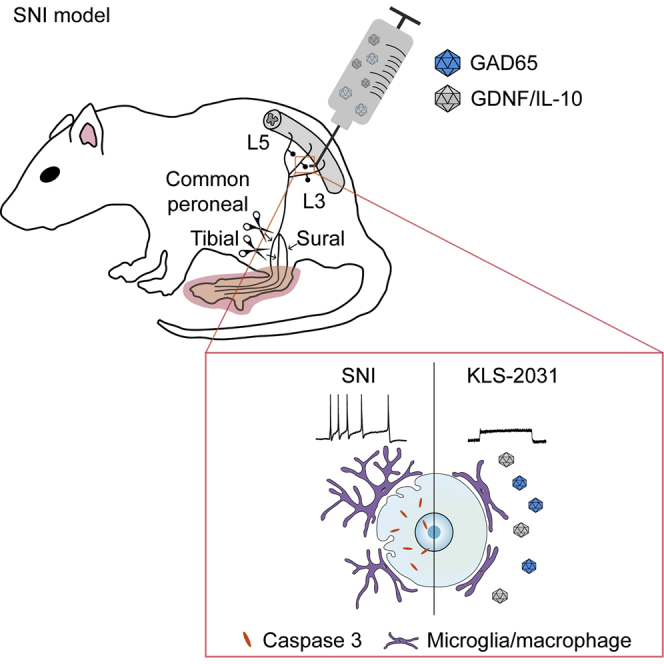
Kim and colleagues demonstrated that single transforaminal injection of KLS-2031 (AAV vectors containing GAD65, GDNF, and IL-10) could produce long-term analgesic effects in rats with neuropathic pain by diminishing the neuroinflammation, neuronal cell death, and hyperexcitability. These observations suggest that KLS-2031 could be a promising treatment option for neuropathic pain.
Introduction
Neuropathic pain (NP) results from a lesion or disease affecting the somatosensory system, according to the International Association for the Study of Pain (IASP). Current NP treatment regimens with anticonvulsants, antidepressants, and opioids focus primarily on managing the symptoms. First-line treatments include gabapentinoids and serotonin-norepinephrine reuptake inhibitors or tricyclic antidepressants, followed by weak opioids, and finally strong opioids and botulinum toxin A.1 However, all of these have limited efficacy, highlighting an urgent need to develop new pain medications for the management of NP. As various factors contribute to the pathophysiology of NP, it is thought that simultaneous targeting of multiple elements is required to develop effective medications.
In NP, persistent neuroinflammation and cellular stress consequent to nerve damage result in neuronal hypersensitivity and hyperactivity.2,3 Hyperactivated sensory neurons release excessive amounts of neurotransmitters, further activating the pain pathway in a phenomenon known as sensitization, which affects both pre- and postsynaptic neurons.4,5 The sensitization of sensory neurons accounts for the hyperalgesia, allodynia, and spontaneous pain associated with NP.6,7 For individuals with NP, the sensation of pain is no longer beneficial because of this overstimulation and sensitization of nociceptors.
To address the major pathological features of NP, namely, nerve injury, neuroinflammation, and abnormal pain signal transmission, we selected the following respective therapeutic targets: glial cell-derived neurotrophic factor (GDNF), interleukin-10 (IL-10), and glutamate decarboxylase 65 (GAD65). GDNF is a neurotrophic factor that protects neurons from various stresses.8 GDNF is released into the extracellular space and acts on nonpeptidergic neurons that express the GDNF family receptor alpha-1 (GFRα1) receptor,9 activating the rearranged during transfection (RET)-tyrosine kinase (RET-Tk) signaling pathway that prevents neuronal death and reduced pain signaling in NP.10,11 IL-10 is a major anti-inflammatory cytokine that attenuates neuroinflammation in the dorsal root ganglion (DRG) and spinal cord.12 GAD65 is a key enzyme in the synthesis of the major inhibitory neurotransmitter gamma-aminobutyric acid (GABA).13, 14, 15 GABA, released from presynaptic vesicles, binds with GABAA receptors on sensory neurons and reduces pain signal transduction.16,17 Additionally, GDNF also enhances GABA signaling and reduces excitatory glutamate signaling to suppress the pain pathway, thereby restoring homeostatic synaptic transmission.18,19 We hypothesized that these three factors, namely, GDNF, IL-10, and GAD65, perform independent and complimentary functions in NP and that long-term expression of the genes encoding these would be effective in chronic NP.
To this end, we induced expression of the corresponding genes via adeno-associated viruses (AAVs), small single-stranded DNA viruses that have been used in many clinical trials and shown to be safe.16,18, 19, 20 Furthermore, the expression of human genes from AAV vectors is long lasting.21,22 Thus, we anticipated that a therapy incorporating this vector would provide a long-term analgesic effect. Our data demonstrate that direct application of a combinatorial AAV-mediated gene therapy to the DRG in a rat model of spared nerve injury (SNI) relieves NP by modulating neurogenic inflammation, nerve damage, and neuronal excitability.
Results
Expression of Therapeutic Genes in the DRG after Transforaminal Epidural (TF) Injection
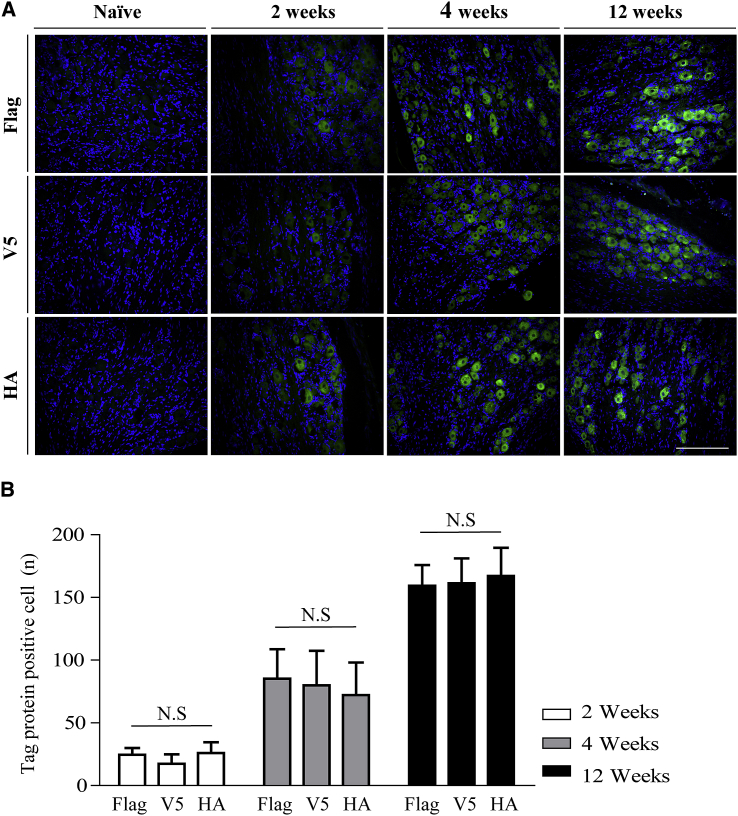
To deliver genes to DRGs, we utilized a TF injection method, which is widely used to deliver drugs into the epidural space.23 AAV serotype 5 (AAV5) vectors harboring either FLAG-tagged GAD65 or V5-tagged GDNF and hemagglutinin (HA)-tagged IL-10 were injected into the left fourth lumbar (L4) DRGs of 8-week-old uninjured (naïve) rats. Immunohistochemistry (IHC), performed 2, 4, and 12 weeks later, demonstrated successful transduction of DRG cells (Figure 1A), with increasing numbers of cells expressing the tagged proteins with time (Figure 1B). There were no significant differences in the expression of FLAG-, V5-, and HA-tagged proteins at each time point (Figure 1B). As shown in Figure 1A, all three genes were clearly expressed in both large-sized and small-sized neurons. To clarify the types of neurons that expressed the tagged proteins, AAV5-green fluorescence protein (GFP) was injected into 8-week-old naïve rats via TF injections, and transduced cell types were studied by NeuN and isolectin B4 (IB4) immunostaining. GFP was detected in both IB4-positive and -negative neurons (Figure S4). By contrast, very few cells in the contralateral DRGs expressed the tagged proteins 4 weeks after TF injection, and no expression was detected in L4 or L1 spinal cord (Figure S5). These results show that the TF injection method can be used to deliver AAV vectors successfully to induce long-term gene expression in the targeted DRG.
Figure 1.
Expression of Genes after TF Injection
AAV5-FLAG-GAD65 and AAV5-GDNF-V5/IL-10-HA were administered to the left L4 DRGs of naïve 8-week-old rats via TF injection. Ipsilateral DRGs were harvested 2, 4, and 12 weeks later. (A) FLAG-, V5-, and HA-tagged proteins (green) were visualized via immunostaining; nuclear counterstaining is in blue. Representative images for ipsilateral DRG at each time point are shown. Images were acquired with a 20× lens objective. Scale bar, 200 μm. (B) The numbers of immunopositive cells were counted using NIS-Elements BR software; n = 18 slices from six animals for each time point. Data are presented as mean ± SEM. Statistical differences among groups were assessed using Kruskal-Wallis one-way ANOVA, followed by Dunnett’s post hoc test. See also Figures S4 and S5.
Optimal Combination of Therapeutic Genes
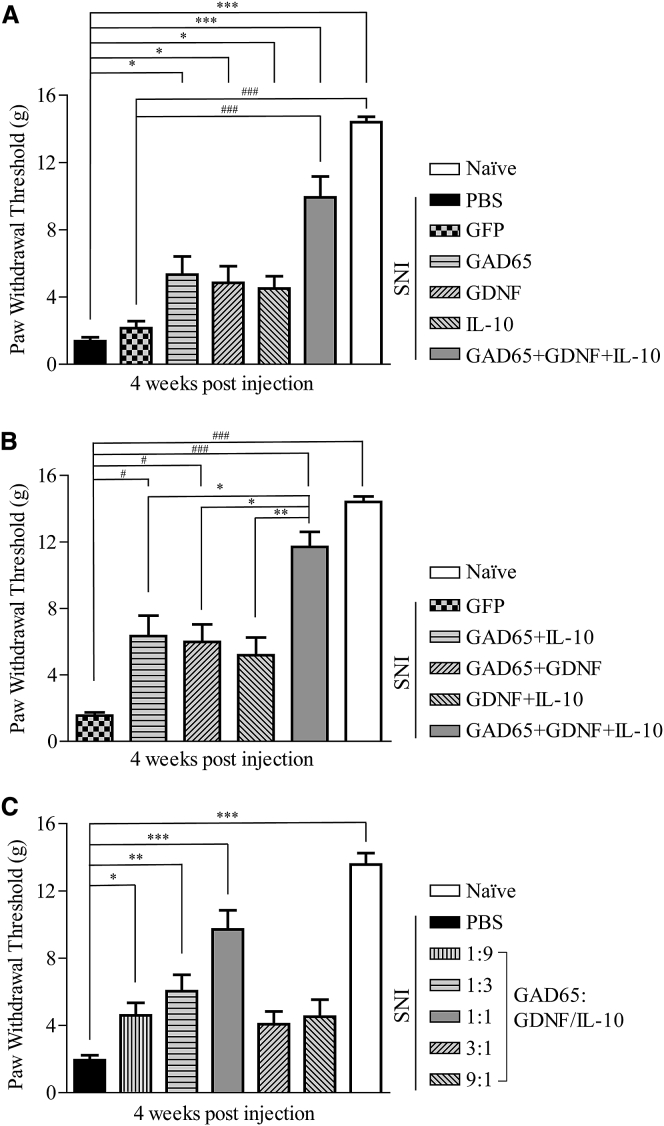
We first generated three AAV vectors, expressing GDNF, IL-10, or GAD65, which have demonstrated analgesic effects in previous studies, to determine the effects on neuroprotection, neuroinflammation, and neural hyperactivity, respectively. We utilized the TF injection method to administer these alone and in various combinations to rats 2 weeks after SNI, which models the mechanical and thermal sensitivities observed in individuals with NP. This model is appropriate for the study of long-term analgesic efficacy, as the pain is maintained stably for an extended period.24 We investigated the analgesic efficacy of three genes for the treatment of NP. AAV vectors containing single genes (AAV5-GAD65, AAV1-GDNF, AAV5-IL-10), or a combination of three AAV vectors (AAV5-GAD65 + AAV1-GDNF + AAV5-IL-10) were administered by TF injection to rats with NP. Injection of PBS or AAV5-GFP was used as a control. 4 weeks after administration, the analgesic efficacy for mechanical pain was assessed with the von Frey filament (vF) test. The vF test is a widely used method to evaluate mechanical pain sensation in rodents.25 Rats receiving single- or triple-gene treatment had significantly higher withdrawal thresholds than those in the PBS- or AAV5-GFP-injected SNI groups (Figure 2A). Notably, injection with AAV vectors encoding all three genes resulted in pain thresholds similar to those of naïve rats. Although a significant analgesic effect was observed when AAV vectors encoding two of the three genes were administered, the expression of all three genes had a synergistic effect that was significantly greater than any of the two-gene combinations (Figure 2B). These data suggest that maximal analgesic efficacy is achieved with AAV-mediated expression of GAD65, IL-10, and GDNF in the DRG. These recombinant viruses resulted in expression of active proteins, as verified via western blotting, ELISA, and GABA production assays in vitro (Figure S3).
Figure 2.
Analgesic Efficacy of Three Therapeutic Genes
Male rats received TF injections with the respective AAVs or PBS, 2 weeks after SNI. (A) Mechanical allodynia for rats receiving AAVs containing single genes (AAV5-GAD65, AAV1-GDNF, or AAV5-IL-10; 9 × 108 VG) or a combination of three AAVs (AAV5-GAD65 + AAV1-GDNF + AAV5-IL-10; 3 × 108 VG for each) was measured with vF tests, 4 weeks after administration. AAV5-GFP was used as control; n = 5 animals per group. #versus GFP; ∗versus PBS-treated SNI group. (B) Mechanical allodynia for rats receiving a combination of two AAVs (4.5 × 108 VG for each) or three AAVs (3 × 108 VG for each) was measured with vF tests, 4 weeks after administration. AAV5-GFP was used as control; n = 6 animals per group. #versus AAV5-GFP-treated SNI; ∗versus AAV5-GAD65 + GDNF + IL-10-treated SNI. (C) Mechanical allodynia for rats receiving the various virus ratios (1:9, 1:3, 1:1, 3:1, 9:1; total dose, 1 × 109 VG) of KLS-2031 was measured with the vF test, 4 weeks after administration; n = 6 animals per group. ∗versus PBS-injected SNI group. Data are presented as mean ± SEM. Statistical differences among groups were assessed using Kruskal-Wallis one-way ANOVA, followed by Dunnett’s post hoc test.
AAV is a small virus with limited capacity to load transgenes into the virus genome. Therefore, we loaded three transgenes into two AAV vectors (AAV5-GAD65 and AAV5-GDNF/IL-10) (Figure S2). To determine the optimal gene-expression ratio, two AAV5 vectors were mixed at various ratios (1:9, 1:3, 1:1, 3:1, and 9:1). The analgesic effects of viruses with these combinations on mechanical pain were assessed using the vF test in rats with SNI. Viral ratios of 1:9, 1:3, and 1:1 had increasing effects on alleviating pain, whereas virus combinations at ratios of 3:1 and 9:1 did not induce effects that were significantly different from the PBS-injected SNI group (Figure 2C). Thus, we utilized a 1:1 ratio of AAV5-GAD65 and AAV5-GDNF/IL-10, which we named KLS-2031, for subsequent studies.
Dose-Dependent Effect of KLS-2031 on Pain Alleviation in Rats with SNI
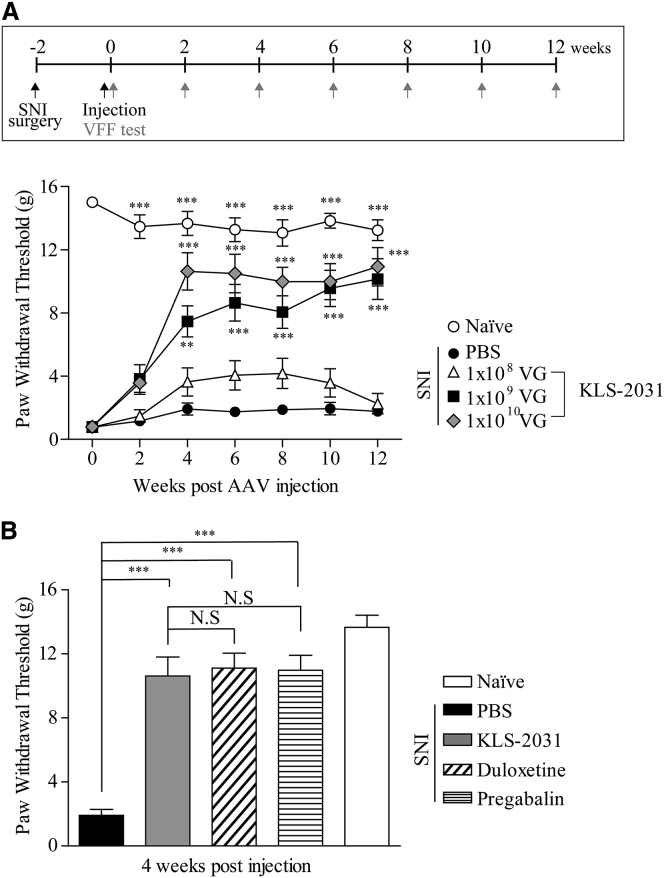
We assessed the analgesic efficacy of different doses of KLS-2031 (108, 109, and 1010 viral genomes [VGs]) over time in rats with SNI. vF tests revealed that the lowest dose, 108 VG, did not alleviate mechanical pain (Figure 3A). However, KLS-2031 doses of 109 VG and 1010 VG significantly increased the paw withdrawal thresholds, beginning 4 weeks after administration. The analgesic efficacy of these doses was maintained up to 12 weeks after administration (Figure 3A).
Figure 3.
Effect of KLS-2031 on Pain Alleviation in Rats with SNI
(A, top) Experiment schedule is shown. (A, bottom) 2 weeks after rats underwent SNI surgery, they received TF injections of KLS-2031 at different doses (108, 109, or 1010 VG) or PBS (5 μL), and vF tests were conducted at the indicated time points; n = 6 animals per group. Statistical differences among groups were assessed for each time point using Kruskal-Wallis one-way ANOVA, followed by Dunnett’s post hoc test. ∗versus PBS-injected SNI group. (B) 2 weeks after rats underwent SNI surgery, they received TF injections of KLS-2031 (1010 VG) or PBS (5 μL). vF tests were conducted 4 weeks later. The PBS-injected SNI rats were orally administered duloxetine (30 mg/kg) or pregabalin (30 mg/kg), dissolved in 1 mL saline at 2 h and 1 h, respectively, prior to the vF test. The same quantity of saline was orally administered to the PBS- and KLS-2031-injected SNI groups; n = 6 animals per group. ∗versus PBS-injected SNI group. Data are presented as mean ± SEM. Statistical differences among groups were assessed using Kruskal-Wallis one-way ANOVA, followed by Dunnett’s post hoc test.
We also compared the analgesic efficacy of KLS-2031 with that of medications approved as first-line treatments for NP, such as pregabalin and duloxetine. The administration doses of pregabalin and duloxetine (30 mg/kg of body weight for both) were calculated according to the body surface area ratio and bioavailability to provide maximum plasma concentrations at 1 and 2 h, respectively, for vF testing.26, 27, 28, 29 For the PBS- and KLS-2031-injected SNI groups, the same quantity of saline (1 mL) was orally administered 1 h and 30 min prior to the vF test. The analgesic effect of KLS-2031 (1 × 1010 VG) was similar to that of the pregabalin- and duloxetine-treated SNI groups (Figure 3B). Remarkably, a single injection of KLS-2031 provided long-lasting analgesia, whereas conventional NP treatments require daily dosing.
No Sex Difference for Analgesic Efficacy of KLS-2031
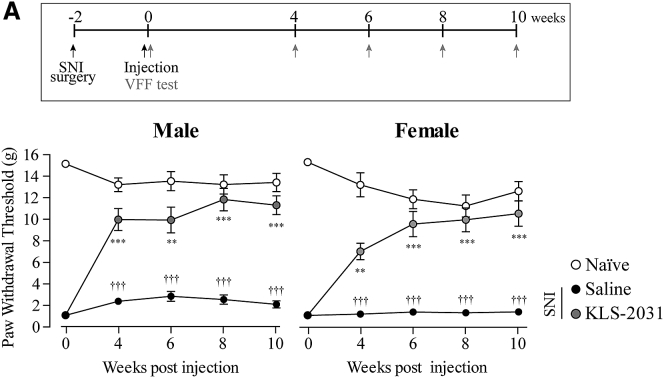
Recent nonclinical and clinical studies reported sex differences regarding the responsiveness to analgesics and sensitivity to pain.30,31 Although most studies have shown that analgesic effects are higher in female subjects than in their male counterparts, others have reported the opposite.31, 32, 33, 34 In addition, the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) guidance, which suggests what to consider in nonclinical studies of analgesic development, also recommends that experiments assess drug responses in both sexes.35 Therefore, we compared the efficacy of KLS-2031 in male and female rats with SNI. vF testing revealed that the analgesic effects of KLS-2031 were comparable between the sexes and were similarly maintained for up to 10 weeks (Figure 4).
Figure 4.
Equivalent Analgesic Efficacy of KLS-2031 in Male and Female Rats with SNI
(Top) Experiment schedule is shown. (Bottom) 2 weeks after rats underwent SNI surgery, they received TF injections of KLS-2031 (1 × 1010 VG) or saline (5 μL), and vF tests were conducted at the indicated time points; n = 6 animals per group. ∗versus saline-injected SNI groups; †versus naïve. Data are presented as mean ± SEM. Statistical differences among groups were assessed for each time point using Kruskal-Wallis one-way ANOVA, followed by Dunnett’s post hoc test.
Effects of KLS-2031 on Neurogenic Inflammation in DRG
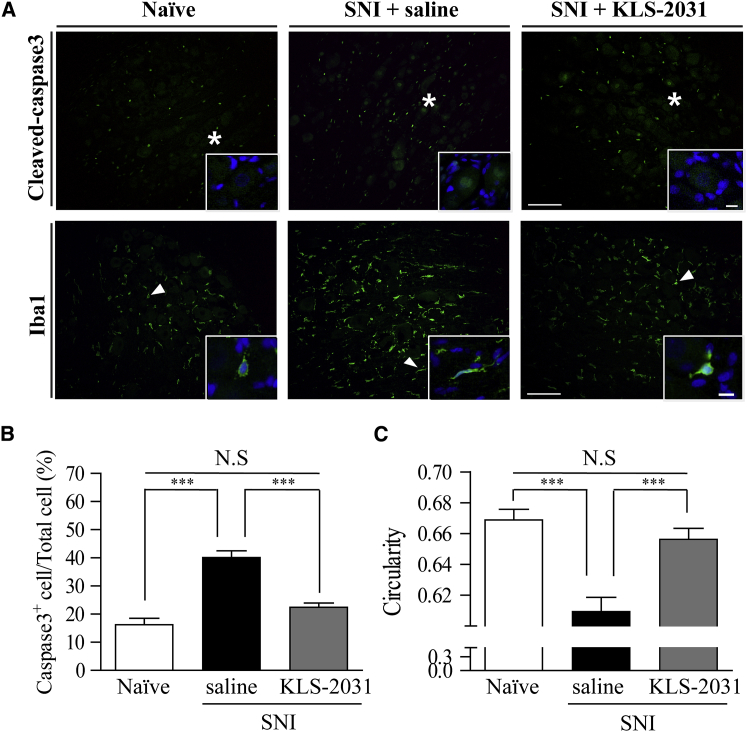
As KLS-2031 treatment regulated the expression of the therapeutic genes, we expected to observe amelioration of nerve injury, neuroinflammation, and neuronal hyperexcitability in rats with NP induced by SNI. Following nerve injury, the caspase 3 expression was dramatically increased in the saline-injected SNI group (Figure 5A, top, and Figure 5B). IHC of DRGs harvested 4 weeks after the TF injections of KLS-2031 revealed a reduction in the expression of cleaved caspase 3, a marker of apoptosis, to levels similar to those in the naïve group (Figure 5A, top, and Figure 5B). To identify which genes are responsible for this neuroprotection, we performed similar experiments but with AAV5s each containing only one of the genes. AAV5-GDNF and AAV5-IL-10 were protective against apoptosis, although not as effective as KLS-2031 (Figures S7A and S7B). Immunostaining for Iba1, a marker of microglia, revealed that SNI altered the morphology of microglia in the DRG, as quantified by their circularity, measured using NIS-Elements Basic Research (BR) software (Figure 5A, bottom, and Figure 5C). The morphological alterations of microglia in response to injury or inflammation are known to be maintained long term.36, 37, 38 The observed decrease in circularity was ameliorated by KLS-2031 (Figure 5A, bottom, and Figure 5C). Additional experiments with single-gene AAV5s indicated that GAD65 expression by itself induced a significant anti-inflammatory effect, although IL-10 expression contributed, in part, to this effect (Figure S7C). These data indicate that KLS-2031 improves multiple aspects (i.e., apoptosis and neuroinflammation) contributing to the pathophysiology of NP.
Figure 5.
Neuroprotective Effect of KLS-2031 in DRG
Ipsilateral DRGs (L4) from rats with SNI were harvested 4 weeks after TF injection of KLS-2031 (1 × 109 VG). (A) Immunohistochemistry shows that apoptotic cells (cleaved caspase-3 marker; green [top]) were detected in the cytosol and nuclei (blue), and microglia (Iba1 marker; green [bottom]) formed clusters around neurons. Insets show enlargements of cleaved caspase 3- and Iba1-positive cells, indicated by asterisks and arrowheads, respectively. Images were acquired with a 20× lens objective. Scale bars, 100 μm (10 μm for insets). (B and C) For quantitative analysis, the proportions of caspase 3-positive cells (B) and circularity of microglia cells (C) were calculated; n = 6 animals per group; ∗versus saline-injected SNI group. Data are presented as mean ± SEM. Statistical differences among groups were assessed using Kruskal-Wallis one-way ANOVA, followed by Dunnett’s post hoc test. See also Figure S7.
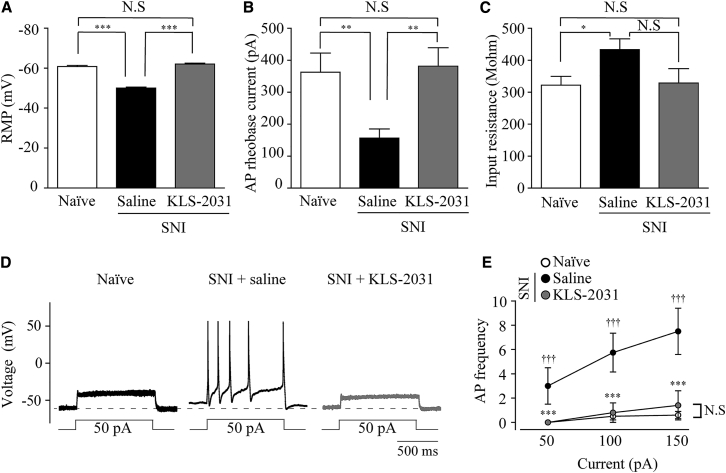
Effects of KLS-2031 on Excitability of Small-Sized L4 DRG Neurons
To examine the effect of KLS-2031 on DRG neuron hyperactivity, we performed patch-clamp electrophysiology on small-sized DRG neurons (electrical capacitance of ≤25 pF), which are the cell bodies of c-fibers that deliver pain signals, as opposed to A-fibers.39,40 We measured the resting membrane potential (RMP), the minimum current threshold value required to evoke an action potential (AP) (rheobase current), and the AP firing frequency. The RMPs of neurons in the saline-treated SNI group were significantly depolarized relative to those from the naïve group (Figure 6A), indicating that the c-fiber neurons of the NP model show increased excitability of the corresponding pain fibers. Notably, RMPs were normalized in small-sized DRGs from animals receiving TF injections of KLS-2031 (Figure 6A). The rheobase current was significantly decreased in the saline-treated SNI group compared with that in the naïve group and was restored by KLS-2031 administration (Figure 6B). Input resistance was higher in the saline-treated SNI group, which was also reduced by TF injections of KLS-2031 (Figure 6C). Furthermore, the frequencies of APs in DRG neurons, evoked by depolarizing current injections (50, 100, and 150 pA, 1 s duration), were dramatically and significantly decreased in the saline-treated SNI group, observed as an upward shift representing a decreased gain in the input-output relationship (Figures 6D and 6E). This hyperactivity was ameliorated in the KLS-2031-treated SNI group. These data suggest that KLS-2031 suppresses pain signals by reversing the hyperexcitability of pain-conducting nerve fibers.40,41
Figure 6.
Effects of KLS-2031 on the Small-Sized DRG Excitability
Electrophysiology was performed in L4 DRGs via the whole-cell patch-clamp method. (A) RMPs were recorded in a whole-cell configuration within 10 s after patch break-in; n = 8 cells for naïve, SNI + saline, and SNI + KLS-2031 groups. (B) The rheobase currents of APs were elicited by a series of depolarizing currents from 0 to 700 pA (10 ms) in 50 pA step increments under current-clamp mode; n = 8 cells for naïve, SNI + saline, and SNI + KLS-2031 groups. (C) Input resistances were measured by membrane potential changes at a given hyperpolarizing current input (−200 pA, 200 ms); n = 8 cells for naïve, SNI + saline, and SNI + KLS-2031 groups. (D) Representative traces for current-clamp recordings from small-sized DRG neurons from naïve, SNI + saline, and SNI + KLS-2031 groups in response to prolonged (1 s), depolarizing 50 pA current injections. Dashed lines represent the RMP in naïve, small-sized L4 DRG neurons. (E) AP frequencies in response to 1 s current injections of various amplitudes in naïve rats (n = 8 cells), saline-injected SNI group (n = 8 cells), and KLS-2031-injected SNI group (n = 8 cells). ∗versus saline-injected SNI group; †versus naïve. Data are presented as mean ± SEM. Statistical differences among groups were assessed with Mann-Whitney U test.
Discussion
The results of the present study demonstrate that combinatorial gene therapy targeting GAD65, GDNF, and IL-10 expression in DRGs provides long-lasting analgesia for NP. The efficacy of this treatment, referred to as KLS-2031, was attributed to reduced cell death, inflammation, and pain fiber hyperexcitability. These findings demonstrate the potential success of treatment of NP by targeting multiple facets of the complex underlying pathophysiology. Indeed, the combination, including all three targets, was significantly more effective than single- or dual-gene therapies. In addition, we found that the optimal AAV5-GAD65 to AAV5-GDNF/IL-10 vector ratio was 1:1, and the analgesic effect disappeared at higher ratios. This suggests that the analgesic effect of increasing the amount of GABA is limited in a chronic NP environment. As a result, long-term analgesia requires reduced inflammation, as well reduced neuronal hyperactivity in the DRG. The therapeutic genes delivered by KLS-2031 not only reversed these neuropathological changes but also normalized the excitability of peripheral nerves, indicating that this treatment controls pain sensation while promoting a favorable environment for nerve function.
Therapeutic approaches for NP at the level of the peripheral nervous system are a viable alternative to systemic application of therapeutic agents. Similar to other studies in which gene delivery to the DRG was reported,42, 43, 44 we targeted the DRGs for AAV transduction. After peripheral nerve injury, DRGs synthesize and release excessive amounts of neurotransmitters, and activated glial cells release inflammatory mediators.45 With the delivery of the therapeutic agent directly to the cell bodies responsible for NP, unpredictable adverse effects that occur with systemically applied drugs can be minimized.46 DRGs represent an ideal target for localized AAV-mediated gene delivery because there is no overlying dura mater, and these cell body-rich areas are devoid of tight junctions.47 With regard to NP treatment, such as in our SNI model, DRG neurons at the level of L4 give rise to the sciatic nerve. Segmentalized treatment is also applicable to DRGs depending on the location of the pain.42 Although we initially considered directly injecting AAVs into the DRGs via intraganglionic injections, which result in efficient gene transfer to the primary sensory neurons and long-term stable transgene expression,42,43 the procedure itself can induce nerve injury and activate microglia and astrocytes in the spinal cord.45 Thus, we chose the TF injection method, in which the AAVs were administered into the opening at the side of the spine known as the foramen, where the nerve roots reside. TF injections have been widely used to treat patients with leg and back pain without involving invasive surgery.23 To our knowledge, this is the first report of the delivery of large molecules, such as AAVs, to DRGs via TF injections (Figures 1, S4, and S5). Our results demonstrate that this method was effective at inducing long-term gene expression in the target cells.
KLS-2031, based on AAV5, is expected to follow the tropism of AAV5, which infects and transduces both large-sized and small-sized neurons in the DRG.48, 49, 50 Our study confirmed this, as GFP was detected in both IB4-positive and IB4-negative neurons when AAV5-GFP was administered to the DRG via TF injection (Figure S4). We therefore expect that the therapeutic proteins of KLS-2031 are expressed in both large- and small-sized neurons in the DRG.
Previous studies have shown that cell death and neuroinflammation occur in the DRGs and spinal cords of animal models of NP.51, 52, 53 Accordingly, we observed an increase in cleaved caspase 3 expression and altered morphology of Iba1-positive microglia in the saline-injected SNI group (Figure 5). KLS-2031 treatment reduced the amount of apoptosis in affected DRGs, observed as reduced immunoreactivity for cleaved caspase 3, likely reflecting the contributions of IL-10 and GDNF (Figures 5 and S7). IL-10 reduces cell death by inhibiting the production of activated caspase 3,54 and GDNF, a ligand of the GFRα1 receptor, inhibits cell death via the RET signaling pathway.55 Similarly, KLS-2031 treatment restored the morphological appearance of microglia, the cells responsible for the innate immune response in nervous tissues. Specifically, KLS-2031 restored microglial circularity, which resulted in a ring-like appearance and wrapping around cells of the DRG in NP models, consistent with the findings of other researchers.37,56 Microglia contribute to analgesic efficacy through IL-10/STAT3 signaling in the spinal cords of NP models.57 Among the three transgenes, IL-10 and GAD65 showed anti-inflammatory effects in the NP model (Figure S7). Surprisingly, GAD65 was a major factor in the stabilization of microglial activity (Figure S7C). A previous study showed that nocifensive behavior and mechanical allodynia were mitigated when GABA was administered to DRGs, which synthesize and are responsive to GABA.58 Microglia express GABAA and GABAB receptors, and GABA may alter their shape and function.59 Thus, the functional changes of microglia in DRG are attributed to the effects of GABA produced by GAD65. Our findings show that the synergistic actions of GAD65 and IL-10, as well as GDNF, on inflammation and apoptosis in the DRG are therapeutically efficacious in alleviating NP. Future studies should evaluate whether the beneficial effects of KLS-2031 also occur in the spinal cord, where the synaptic targets of DRG neurons reside.
The third therapeutic effect of KLS-2031 was on neuronal excitability in the DRG (Figure 6). Although the precise mechanism by which neurons become hyperactive in NP is not yet known, it is thought to reflect a change in the number and/or activity of ion channels in the membrane, such as voltage-gated potassium (Kv) and sodium (Nav) channels.60,61 Primary afferent somatosensory neurons express multiple types of Nav and Kv channels with distinct voltage dependence and kinetic properties.60,62 It was reported that Kv channels are pivotal in the control of neuronal excitability, including for the RMP and AP frequency.63 In particular, the transient inactivating A-type Kv channel (IA) with slower inactivation kinetics is predominantly found in small-diameter nociceptors, which play a key role regulating neuronal excitability in various brain regions.64 In the present study, we found that DRG neuronal excitability was significantly increased in the SNI model and recovered by KLS-2031 treatment, suggesting changes in Kv or Nav channel activation (Figure 6). Future studies are required to determine the precise mechanism by which neuronal excitability is modulated by KLS-2031.
There is an urgent need to develop new treatment options for people suffering from severe pain. This study shows that KLS-2031 has the potential to resolve such an unmet medical need. Moreover, we demonstrated that a single administration of KLS-2031 has long-term efficacy and the potential to be a disease-modifying agent. An investigational new drug study of KLS-2031 was approved by the US Food and Drug Administration (FDA), and the first-in-human study (ClinicalTrials.gov: NCT04238793) was initiated.
Materials and Methods
Plasmid Preparation
Transgenes for GFP, human (h)GAD65, hIL-10, rat (r)IL-10, hGDNF, or tagged (FLAG, V5, HA) versions were cloned into the AAV plasmid containing the cytomegalovirus (CMV) or CMV enhancer/chicken β-actin (CAG) promoter and simian virus 40 poly A (SV40pA) or bovine growth hormone poly A (bGHpA) cassette that was flanked by AAV2 inverted terminal repeats. Prepared AAV plasmids were used to generate single-stranded AAV (ssAAV) vectors. Information on the construct of AAV vectors used in the experiment is provided in Figure S2. cDNAs corresponding to respective amino acid sequences (NCBI: NP_000809.1, hGAD65; NP_000563, hIL-10; NP_036986.2, rIL-10; NP_954701.1, hGDNF) were ordered from Bioneer (Daejeon, Korea). Tagging sequences (FLAG, 5′-GATTACAAGGATGACGACGATAAG-3′; V5, 5′-GGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACG-3′; HA, 5′-TACCCATACGATGTTCCAGATTACGCT-3′) were synthesized at Bioneer and inserted at the N- or C-terminal regions of transgenes. All elements comprising expression vectors were cloned into the pVAX1 vector (Invitrogen, Carlsbad, CA, USA), except for GFP, which was purchased from the University of North Carolina Vector Core (UNC; Chapel Hill, NC, USA).
Recombinant AAV Production
All of the AAV vectors used in this study were produced using an AAV helper-free system.65 Briefly, HEK293 cells were transiently transfected with three plasmids (pRep/Cap, pHelper, and pTransgene). Following transfection, the cells were harvested and lysed. Next, cell lysates were purified by CsCl or iodixanol gradient ultracentrifugation or column chromatography. The manufacturers of each vector are as follows: AAV5-GFP, AAV5-hGAD65 (batch 1), AAV5-hIL-10, and AAV5-rIL-10 by UNC Vector Core; AAV1-hGDNF by SCT (Cheongju, Korea); AAV5-hGDNF, AAV5-FLAG-hGAD65, and AAV5-hGDNF-V5/hIL-10-HA by CdmoGen (Cheongju, Korea); and AAV5-hGAD65 (batch 2) and AAV5-hGDNF/hIL-10 by Research Institute at Nationwide Children’s Hospital (RINCH; Columbus, OH, USA). The VG titer was measured by quantitative polymerase chain reaction (qPCR). There was no difference in efficacy when comparing AAV1-hGDNF and AAV5-hGDNF (Figure S6A), and AAV5-rIL-10 and AAV5-hIL-10 (Figure S6B) in vF experiments. The AAV vectors used for each experiment are shown in Table S1.
Animals
Sprague-Dawley (SD) rats (Koatech, Pyeongtaek, Korea) were maintained in a temperature- and humidity-controlled room on a 12-h light/dark cycle during the whole study, including 7 days of acclimation. Rats were given a regular laboratory rodent diet and water ad libitum. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 80-23), revised 1996, and under appropriate veterinary supervision at the animal facility of Kolon Life Science (Seoul, Korea) with approval of the Institutional Animal Care and Use Committee (IACUC; numbers KLS IACUC-2016-35, KLS IACUC-2016-37, KLS IACUC-2017-52, KLS IACUC-2017-58, KLS IACUC-2017-62, KLS IACUC-2017-63, KLS IACUC-2017-64, KLS IACUC-2017-72, KLS IACUC-2018-54, KLS IACUC-2018-55, and KLS IACUC-2019-52). Age-matched naïve rats did not receive surgical operation and treatment. SNI surgery, TF injection, and cardiac perfusion fixation processes for IHC and electrophysiology were performed under 3% isoflurane (Hana Pharm, Hwaseong, Korea) anesthetization under sterile conditions.
Induction of SNI in Rats
Surgical procedures for SNI were performed as previously described,66 with minor modifications. Briefly, the SNI procedure consisted of ligation and axotomy of the tibial and common peroneal nerves, leaving the sural nerve intact. The lateral part of the skin and biceps femoris of the left thigh were incised to expose the common peroneal and tibial nerves. After two ligations were made, 5 mm apart on each nerve, with 7.0 silk (Ailee, Busan, Korea), nerves were incised in the middle of two ties. After the incision was sutured, rats were allowed to recover on a heating pad and returned to their cages. 2 weeks after the surgery, outliers, based on pain behavioral testing, were removed, and the remaining animals were randomly divided into the experimental groups to minimize differences in the mean pain threshold value for each group. The AAV or vehicle control was administered by TF injection at 2 weeks after the SNI surgery.
TF Injection
The TF injection method in rats was established with reference to the previously described methods.67, 68, 69 Briefly, to produce the microneedle catheter, a 31-G, blunt-end, L-shaped, stainless-steel needle (customized; Jeung Do Bio & Plant, Seoul, Korea) was connected to the 0.4 × 0.8-mm polythene tubing (Harvard, Holliston, MA, USA). The test materials were pulled to 5 μL into this microneedle catheter using a 10-μL syringe (Hamilton, Reno, NV, USA). The surgical microscope (World Precision Instruments [WPI], Sarasota, FL, USA) was used during the surgical procedure. The skin and muscles from the L4 to L5 level, 5 mm left of the midline, were incised to the depth of transverse processes. The muscles were retracted so that the L4 and L5 transverse processes were exposed. To secure the sight of the neural foramen, the intervertebral ligament and muscle were removed. The needle was inserted just below the facet joint to avoid nerve damage. If there was resistance to the facet joint, then the inserted needle was slightly lifted upward, and there was no pressure when administering the test substance; the test drug was considered to be injected around the DRG. After injection, the muscles and skin were sutured using 4.0 nylon thread (Ailee) and a medical stapler (Covidien, Dublin, Ireland). An image of the L-shaped microneedle catheter and the dose for each of the animal studies can be found in Figure S1 and Table S1, respectively.
Oral Administration of Analgesics
Duloxetine (Sigma-Aldrich, St. Louis, MO, USA) and pregabalin (Sigma-Aldrich) were dissolved in saline solution and administrated orally (p.o., an injection volume of 1 mL). The doses of the two analgesics were 30 mg/kg, which was an equivalent dose of the maximum daily dose in humans, adjusted by the difference of bioavailability between two species.26,27,29 Duloxetine and pregabalin were administered 2 h and 1 h, respectively, prior to vF testing, considering the time to reach maximum blood concentration of each analgesic.28
vF Test
The vF test was performed to assess pain during the light cycle. Chaplan’s 50% up-down threshold method was used in this study. In short, rats were placed on a metallic mesh floor covered with a transparent plastic box, which permitted easy access to their paws, and were allowed to adapt to the testing environment for at least 5 min.25 The filaments (Stoelting, Wood Dale, IL, USA) were applied in an ascending order of force to the lateral side of the left hind paw; the series of monofilaments ranged from 0.4 to 15 g. A positive response was defined as a rapid withdrawal or licking of the paw. Testing was begun with the 2-g monofilament. If the rat responded to the first filament, then the next-lower filament was used until the rat stopped emitting a positive response or response to the lowest filament (0.4 g). If a rat did not show a positive response, then the next-higher filament in the sequence was tested until the rat showed a positive response or response to the highest filament (15 g). Threshold was determined according to the standard provided by Chaplan.25 Each test was conducted by three independent observers blinded to experimental conditions.
IHC
Rats were sacrificed through a cardiac perfusion fixation process. Initial perfusion solution was saline (Dai Han Pharm, Seoul, Korea), followed by a 2% paraformaldehyde (PFA; Wako, Osaka, Japan). DRG and the spinal cord were isolated from the rat and postfixed in a 2% PFA solution overnight at 4°C, followed by immersion in 15% and 30% sucrose solutions (Sigma-Aldrich) at 4°C for 48 h to achieve cryoprotection. Tissues were mounted in optimal cutting temperature (O.C.T.) compound (Sakura, Tokyo, Japan) and sliced with a cryostat (Thermo Fisher Scientific, Waltham, MA, USA) to obtain sections at a 10-μm thickness for the DRG and a 30 μm thickness for the spinal cord. The sections were incubated in 1 M PBS for 5 min to remove O.C.T. compound. Sections were incubated in blocking solution (10% normal goat serum [Abcam, Cambridge, UK], 3% bovine serum albumin [Sigma-Aldrich], 0.4% Triton X-100 [Amresco, Solon, OH, USA], 1% glycine [Sigma-Aldrich], and 10% Tris-buffered saline [TBS; GenDEPOT, Katy, TX, USA] in 1 M PBS) for 1 h, followed by primary antibodies with blocking solution overnight at 4°C (primary antibody information is provided in Table S2). After incubation with primary antibodies, sections were washed with 1 M TBS, 3 times for 5 min at room temperature (RT), and incubated in secondary antibody with blocking solution for 1 h at RT (secondary antibody information is provided in Table S2). After incubation with secondary antibodies, sections were washed with 1 M TBS. Sections were again incubated in 1 M TBS for 5 min and then incubated with 4′,6-diamidino-2-phenylindole (DAPI) solution (0.1 μg/μL in 1 M PBS) (Sigma-Aldrich) for 5 min at RT. Finally, sections were washed with 1 M TBS, 3 times for 5 min at RT, and then mounted on silane 3-coated slide glass (Muto Pure Chemicals, Tokyo, Japan) using fluorescence mounting medium (Dako, Glostrup, Denmark).
Fluorescence images were acquired in proper exposure by an Axio Scope A1 microscope (Zeiss, Oberkochen, Germany) and a confocal microscope (Zeiss 700 and 880). Images were analyzed with NIS-Elements BR software (Nikon, Tokyo, Japan), ZEN 2 (Zeiss), and ImageJ software. To quantify the number of fluorescent cells, stained cells were counted manually. For circularity analysis, stained cells were automatically detected by setting a proper intensity range. Then, noise signals, such as dust or cellular membrane, were eliminated manually. Circularity of each cell was calculated automatically by NIS-Elements BR software.
Whole-Cell Patch-Clamp Recording
Ipsilateral L4 DRG was excised and maintained in a PBS solution at 4°C. After removal of sheaths and connective tissues, it was cut into small pieces, and they were stirred for 45 min at 35°C in 5 mL modified Earle’s balanced salt solution (EBSS; pH 7.4) containing 0.7 mg/mL collagenase (type IA) and 0.3 mg/mL papain. After culturing, they were shaken vigorously to separate neurons, centrifuged at 1,000 rpm, and resuspended in DMEM (HyClone, Logan, UT, USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). Neurons were transferred to poly-l-lysine-coated cover glasses (12 mm; Marienfeld Superior, Lauda-Königshofen, Germany) in a 24-well plate and cultured in a 37°C humidified incubator (95% O2, 5% CO2). All cells were used within 12 h after separation. Only small-sized cells were examined (cell capacitance ≤25 pF), most of which are nociceptors.40,41 With a brief application of strong suction, the cell membrane was disrupted. The interior of the pipette can be filled with a solution matching the cytoplasm for a whole-cell recording. The internal pipette solution contained the following (in mM): 140 KCl, 1.2 MgCl2, 4 MgATP, 0.4 Na2-GTP, 10 phosphocreatine, 10 HEPES, 0.5 EGTA (pH 7.2, with KOH and 298 mOsm/kg H2O). Extracellular solution (artificial cerebrospinal fluid [aCSF]) contained the following (in mM): 145 NaCl, 2.5 CaCl2, 5.4 KCl, 1.2 MgCl2, 10 glucose, 10 HEPES (pH 7.4, with NaOH and 320 mOsm/kg H2O). Flow rate was set at 1–2 mL/min. Recordings were made using standard whole-cell techniques. Electrodes were pulled from borosilicate glass microcapillary tubes (Sutter Instrument, Novato, CA, USA) and had resistances from 1.5 to 2.5 MΩ when filled with internal solution. Series resistance (Rs), after establishing a whole-cell configuration, was between 4.1 and 5.5 MΩ. Cells were discarded when the Rs changed by >20% of the baseline value. Recordings were obtained using a patch-clamp amplifier (MultiClamp 700A; Molecular Devices, San Jose, CA, USA). Voltage and current commands and digitization of membrane voltages and currents were controlled using a Digidata 1322A interfaced with Clampex 10 of the pClamp software package (version 10; Molecular Devices). Currents were low-pass filtered at 2 kHz. Capacitance (Cm) values were taken from automatically calculated recordings by pClamp 10 software. APs were recorded in the current-clamp mode. Membrane potential measurement was low-pass filtered at 10 kHz. All experiments were conducted at RT. All reagents used in the study were purchased from Sigma-Aldrich, and data were recorded and analyzed using the pClamp software.
Statistical Analysis
All statistical parameters were calculated using Sigma Plot (version 13.0; Systat Software, San Jose, CA, USA) and R-package (version 3.6.2; R Studio, Boston, MA, USA). Significant differences were verified by one-way ANOVA, followed by Dunnett’s post hoc test or Kruskal-Wallis one-way ANOVA, followed by Dunnett’s or Dunn’s post hoc test or Mann-Whitney U test and t test. Differences with a p value less than 0.05 were considered statistically significant. Values are expressed as the mean ± standard errors of the mean (SEM).
Author Contributions
Conceived and Designed the Experiments, K.-R.K., M.K., Y.K., H.C., and S.K.; Executed Experiments, M.-J.K., D.K., J.-J.P., J.-H.C., Y.S., and H.J; Analyzed the Data, K.-R.K., Y.S., and H.J.; Interpreted the Data, K.-R.K., Y.K., H.C., and S.K.; Writing – Review & Editing, K.-R.K., H.J., M.K., Y.K., and S.K. All authors reviewed and approved the final manuscript.
Conflicts of Interest
All authors are employees of Kolon Life Science (KLS). KLS is a publicly traded Korean biopharmaceutical company.
Acknowledgments
We thank BnH Research for valuable technical support. This research was co-supported by the Global High-Tech Biomedicine Technology Development Program of the National Research Foundation (NRF) and Korea Health Industry Development Institute (KHIDI), funded by the Korean government Ministry of Science and ICT (MSIT) and Ministry of Health and Welfare (MOHW); numbers 2015M3D6A1065094 and HI15C3518.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.06.018.
Contributor Information
Heonsik Choi, Email: heonsik@kolon.com.
Sujeong Kim, Email: sujeong@kolon.com.
Supplemental Information
References
- 1.Baron R., Binder A., Attal N., Casale R., Dickenson A.H., Treede R.D. Neuropathic low back pain in clinical practice. Eur. J. Pain. 2016;20:861–873. doi: 10.1002/ejp.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasingam V., Yong V.W. Attenuation of astroglial reactivity by interleukin-10. J. Neurosci. 1996;16:2945–2955. doi: 10.1523/JNEUROSCI.16-09-02945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González A., Ugarte G., Restrepo C., Herrera G., Piña R., Gómez-Sánchez J.A., Pertusa M., Orio P., Madrid R. Role of the Excitability Brake Potassium Current IKD in Cold Allodynia Induced by Chronic Peripheral Nerve Injury. J. Neurosci. 2017;37:3109–3126. doi: 10.1523/JNEUROSCI.3553-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Sandoval A., Chai N., Nutile-McMenemy N., Deleo J.A. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L.J., Xu H., Ko S.W., Yoshimura M., Zhuo M. Feed-forward inhibition: a novel cellular mechanism for the analgesic effect of substance P. Mol. Pain. 2005;1:34. doi: 10.1186/1744-8069-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randić M., Jiang M.C., Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J. Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell J.N., Meyer R.A. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha S.M., Cristovão A.C., Campos F.L., Fonseca C.P., Baltazar G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol. Dis. 2012;47:407–415. doi: 10.1016/j.nbd.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Hunt S.P., Koltzenburg M. Oxford University Press; 2005. The Neurobiology of Pain: (Molecular and Cellular Neurobiology) [Google Scholar]
- 10.Bennett D.L., Michael G.J., Ramachandran N., Munson J.B., Averill S., Yan Q., McMahon S.B., Priestley J.V. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Airaksinen M.S., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 12.Milligan E.D., Sloane E.M., Langer S.J., Cruz P.E., Chacur M., Spataro L., Wieseler-Frank J., Hammack S.E., Maier S.F., Flotte T.R. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol. Pain. 2005;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue M., Arikawa T., Chen Y.H., Moriwaki Y., Price M., Brown M., Perfect J.R., Shinohara M.L. T cells down-regulate macrophage TNF production by IRAK1-mediated IL-10 expression and control innate hyperinflammation. Proc. Natl. Acad. Sci. USA. 2014;111:5295–5300. doi: 10.1073/pnas.1321427111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yilma A.N., Singh S.R., Fairley S.J., Taha M.A., Dennis V.A. The anti-inflammatory cytokine, interleukin-10, inhibits inflammatory mediators in human epithelial cells and mouse macrophages exposed to live and UV-inactivated Chlamydia trachomatis. Mediators Inflamm. 2012;2012:520174. doi: 10.1155/2012/520174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagvadorj J., Naiki Y., Tumurkhuu G., Hassan F., Islam S., Koide N., Mori I., Yoshida T., Yokochi T. Interleukin-10 inhibits tumor necrosis factor-alpha production in lipopolysaccharide-stimulated RAW 264.7 cells through reduced MyD88 expression. Innate Immun. 2008;14:109–115. doi: 10.1177/1753425908089618. [DOI] [PubMed] [Google Scholar]
- 16.Vit J.P., Ohara P.T., Sundberg C., Rubi B., Maechler P., Liu C., Puntel M., Lowenstein P., Castro M., Jasmin L. Adenovector GAD65 gene delivery into the rat trigeminal ganglion produces orofacial analgesia. Mol. Pain. 2009;5:42. doi: 10.1186/1744-8069-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrant M., Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 18.Salio C., Ferrini F., Muthuraju S., Merighi A. Presynaptic modulation of spinal nociceptive transmission by glial cell line-derived neurotrophic factor (GDNF) J. Neurosci. 2014;34:13819–13833. doi: 10.1523/JNEUROSCI.0808-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koelsch A., Feng Y., Fink D.J., Mata M. Transgene-mediated GDNF expression enhances synaptic connectivity and GABA transmission to improve functional outcome after spinal cord contusion. J. Neurochem. 2010;113:143–152. doi: 10.1111/j.1471-4159.2010.06593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller C., Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 21.Piguet F., Alves S., Cartier N. Clinical Gene Therapy for Neurodegenerative Diseases: Past, Present, and Future. Hum. Gene Ther. 2017;28:988–1003. doi: 10.1089/hum.2017.160. [DOI] [PubMed] [Google Scholar]
- 22.Mittermeyer G., Christine C.W., Rosenbluth K.H., Baker S.L., Starr P., Larson P., Kaplan P.L., Forsayeth J., Aminoff M.J., Bankiewicz K.S. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum. Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchikanti L., Buenaventura R.M., Manchikanti K.N., Ruan X., Gupta S., Smith H.S., Christo P.J., Ward S.P. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician. 2012;15:E199–E245. [PubMed] [Google Scholar]
- 24.Low L.A., Millecamps M., Seminowicz D.A., Naso L., Thompson S.J., Stone L.S., Bushnell M.C. Nerve injury causes long-term attentional deficits in rats. Neurosci. Lett. 2012;529:103–107. doi: 10.1016/j.neulet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 26.European Medicines Agency (EMEA). Duloxetine–scientific discussion. 2005. https://www.ema.europa.eu/en/documents/scientific-discussion/cymbalta-epar-scientific-discussion_en.pdf
- 27.European Medicines Agency (EMEA). Pregabalin–scientific discussion. 2004. https://www.ema.europa.eu/en/documents/scientific-discussion/lyrica-epar-scientific-discussion_en.pdf
- 28.Miyazaki R., Yamamoto T. The efficacy of morphine, pregabalin, gabapentin, and duloxetine on mechanical allodynia is different from that on neuroma pain in the rat neuropathic pain model. Anesth. Analg. 2012;115:182–188. doi: 10.1213/ANE.0b013e31824f94ca. [DOI] [PubMed] [Google Scholar]
- 29.Shin J.-W., Seol I.-C., Son C.-G. Interpretation of Animal Dose and Human Equivalent Dose for Drug Development. J. Korean Oriental Med. 2010;31:1–7. [Google Scholar]
- 30.Fillingim R.B., Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum. 1995;4:209–221. [Google Scholar]
- 31.Averbuch M., Katzper M. A search for sex differences in response to analgesia. Arch. Intern. Med. 2000;160:3424–3428. doi: 10.1001/archinte.160.22.3424. [DOI] [PubMed] [Google Scholar]
- 32.Chen E.H., Shofer F.S., Dean A.J., Hollander J.E., Baxt W.G., Robey J.L., Sease K.L., Mills A.M. Gender disparity in analgesic treatment of emergency department patients with acute abdominal pain. Acad. Emerg. Med. 2008;15:414–418. doi: 10.1111/j.1553-2712.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 33.Packiasabapathy S., Sadhasivam S. Gender, genetics, and analgesia: understanding the differences in response to pain relief. J. Pain Res. 2018;11:2729–2739. doi: 10.2147/JPR.S94650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartley E.J., Fillingim R.B. Sex differences in pain: a brief review of clinical and experimental findings. Br. J. Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews N.A., Latrémolière A., Basbaum A.I., Mogil J.S., Porreca F., Rice A.S., Woolf C.J., Currie G.L., Dworkin R.H., Eisenach J.C. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain. 2016;157:901–909. doi: 10.1097/j.pain.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graeber M.B., Streit W.J. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 37.Walker F.R., Nilsson M., Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr. Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streit W.J., Walter S.A., Pennell N.A. Reactive microgliosis. Prog. Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 39.Cummins T.R., Black J.A., Dib-Hajj S.D., Waxman S.G. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J. Neurosci. 2000;20:8754–8761. doi: 10.1523/JNEUROSCI.20-23-08754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold M.S. Spinal nerve ligation: what to blame for the pain and why. Pain. 2000;84:117–120. doi: 10.1016/s0304-3959(99)00309-7. [DOI] [PubMed] [Google Scholar]
- 41.Cummins T.R., Rush A.M., Estacion M., Dib-Hajj S.D., Waxman S.G. Voltage-clamp and current-clamp recordings from mammalian DRG neurons. Nat. Protoc. 2009;4:1103–1112. doi: 10.1038/nprot.2009.91. [DOI] [PubMed] [Google Scholar]
- 42.Fischer G., Kostic S., Nakai H., Park F., Sapunar D., Yu H., Hogan Q. Direct injection into the dorsal root ganglion: technical, behavioral, and histological observations. J. Neurosci. Methods. 2011;199:43–55. doi: 10.1016/j.jneumeth.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu H., Fischer G., Ferhatovic L., Fan F., Light A.R., Weihrauch D., Sapunar D., Nakai H., Park F., Hogan Q.H. Intraganglionic AAV6 results in efficient and long-term gene transfer to peripheral sensory nervous system in adult rats. PLoS ONE. 2013;8:e61266. doi: 10.1371/journal.pone.0061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pleticha J., Maus T.P., Christner J.A., Marsh M.P., Lee K.H., Hooten W.M., Beutler A.S. Minimally invasive convection-enhanced delivery of biologics into dorsal root ganglia: validation in the pig model and prospective modeling in humans. Technical note. J. Neurosurg. 2014;121:851–858. doi: 10.3171/2014.6.JNS132364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Julius D., Basbaum A.I. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 46.Chang M.F., Hsieh J.H., Chiang H., Kan H.W., Huang C.M., Chellis L., Lin B.S., Miaw S.C., Pan C.L., Chao C.C., Hsieh S.T. Effective gene expression in the rat dorsal root ganglia with a non-viral vector delivered via spinal nerve injection. Sci. Rep. 2016;6:35612. doi: 10.1038/srep35612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirakawa H., Okajima S., Nagaoka T., Kubo T., Takamatsu T., Oyamada M. Regional differences in blood-nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion. Neuroreport. 2004;15:405–408. doi: 10.1097/00001756-200403010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Vulchanova L., Schuster D.J., Belur L.R., Riedl M.S., Podetz-Pedersen K.M., Kitto K.F., Wilcox G.L., McIvor R.S., Fairbanks C.A. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol. Pain. 2010;6:31. doi: 10.1186/1744-8069-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu D., Klaw M.C., Kholodilov N., Burke R.E., Detloff M.R., Côté M.P., Tom V.J. Expressing Constitutively Active Rheb in Adult Dorsal Root Ganglion Neurons Enhances the Integration of Sensory Axons that Regenerate Across a Chondroitinase-Treated Dorsal Root Entry Zone Following Dorsal Root Crush. Front. Mol. Neurosci. 2016;9:49. doi: 10.3389/fnmol.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Q., Chou B., Fitzsimmons B., Miyanohara A., Shubayev V., Santucci C., Hefferan M., Marsala M., Hua X.Y. In vivo gene knockdown in rat dorsal root ganglia mediated by self-complementary adeno-associated virus serotype 5 following intrathecal delivery. PLoS ONE. 2012;7:e32581. doi: 10.1371/journal.pone.0032581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krames E.S. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014;15:1669–1685. doi: 10.1111/pme.12413. [DOI] [PubMed] [Google Scholar]
- 52.Tsuda M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016;7:17–26. doi: 10.1111/jdi.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteside G.T., Munglani R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J. Neurosci. Res. 2001;64:168–173. doi: 10.1002/jnr.1062. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z., Peng X., Insolera R., Fink D.J., Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp. Neurol. 2009;220:183–190. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 56.Katoh M., Wu B., Nguyen H.B., Thai T.Q., Yamasaki R., Lu H., Rietsch A.M., Zorlu M.M., Shinozaki Y., Saitoh Y. Polymorphic regulation of mitochondrial fission and fusion modifies phenotypes of microglia in neuroinflammation. Sci. Rep. 2017;7:4942. doi: 10.1038/s41598-017-05232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu H.Y., Mao X.F., Tang X.Q., Ali U., Apryani E., Liu H., Li X.Y., Wang Y.X. Spinal interleukin-10 produces antinociception in neuropathy through microglial β-endorphin expression, separated from antineuroinflammation. Brain Behav. Immun. 2018;73:504–519. doi: 10.1016/j.bbi.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Du X., Hao H., Yang Y., Huang S., Wang C., Gigout S., Ramli R., Li X., Jaworska E., Edwards I. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J. Clin. Invest. 2017;127:1741–1756. doi: 10.1172/JCI86812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vélez-Fort M., Audinat E., Angulo M.C. Central role of GABA in neuron-glia interactions. Neuroscientist. 2012;18:237–250. doi: 10.1177/1073858411403317. [DOI] [PubMed] [Google Scholar]
- 60.Devor M. Sodium channels and mechanisms of neuropathic pain. J. Pain. 2006;7(1, Suppl 1):S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Gonçalves T.C., Benoit E., Partiseti M., Servent D. The NaV1.7 Channel Subtype as an Antinociceptive Target for Spider Toxins in Adult Dorsal Root Ganglia Neurons. Front. Pharmacol. 2018;9:1000. doi: 10.3389/fphar.2018.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett D.L., Clark A.J., Huang J., Waxman S.G., Dib-Hajj S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019;99:1079–1151. doi: 10.1152/physrev.00052.2017. [DOI] [PubMed] [Google Scholar]
- 63.Du X., Gamper N. Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr. Neuropharmacol. 2013;11:621–640. doi: 10.2174/1570159X113119990042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman D.A., Magee J.C., Colbert C.M., Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 65.Wright J.F. Transient transfection methods for clinical adeno-associated viral vector production. Hum. Gene Ther. 2009;20:698–706. doi: 10.1089/hum.2009.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pertin M., Gosselin R.D., Decosterd I. The spared nerve injury model of neuropathic pain. Methods Mol. Biol. 2012;851:205–212. doi: 10.1007/978-1-61779-561-9_15. [DOI] [PubMed] [Google Scholar]
- 67.Kim N.H., Lee S.H., Lee S.J. Percutaneous transforaminal epidural injection method in an experimental rat: minimally invasive drug delivery method to spinal epidural space. Ann. Rehabil. Med. 2012;36:640–647. doi: 10.5535/arm.2012.36.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeon Y.-T., Hwang J.-W., Lim Y.-J., Kim Y.-C., Lee S.-C. Epidural Hyaluronic Acid in a Rat Model of Chronic Dorsal Root Ganglion Compression. Korean J. Anesthesiol. 2004;46:462–466. [Google Scholar]
- 69.Hu S.J., Xing J.L. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.