Abstract
We previously reported that co-stimulation blockade by abatacept limits the decline of β-cell function and the frequency of circulating CD4+ central memory (CD45RO+CD62L+) T (TCM) cells in new-onset type 1 diabetes. In human subjects receiving placebo we found a significant association between increase in CD4+ TCM cells and decline of β-cell function. To extend and refine these findings, we examined changes in human CD4+ and CD8+ naïve and memory T-cell subsets at greater resolution using polychromatic flow and mass cytometry. In the placebo group we successfully reproduced the original finding of a significant association between TCM and β-cell function, and extended this to other T-cell subsets. Furthermore, we show that abatacept treatment significantly alters the frequencies of a majority of CD4+ conventional and regulatory T-cell subsets; in general, antigen-naïve subsets increase and antigen-experienced decrease, while CD8+ T-cell subsets are relatively resistant to drug effects, indicating a lesser reliance on CD28-mediated co-stimulation. Importantly, abatacept uncouples the relationship between changes in T-cell subsets and β-cell function that is a component of the natural history of the disease. Although these data suggest immunological markers for predicting change in β-cell function in type 1 diabetes, the finding that abatacept blunts this relationship renders the biomarkers non-predictive for this type of therapy. In sum, our findings point to a novel mechanism of action for this successful immunotherapy that may guide other disease-modifying approaches for type 1 diabetes.
Introduction
Type 1 diabetes is an autoimmune disease characterised by gradual loss of pancreatic islet β-cell function/mass mediated by immune cells, especially autoreactive CD4+ and CD8+ T cells (1, 2).
During a T-cell mediated immune response, naïve (antigen-inexperienced) T cells encounter their cognate target and under conditions in which co-stimulatory signals are provided (e.g. via CD80/CD86 interaction with CD28) become activated, antigen-experienced cells. There follows a sequential differentiation process that may involve further exposure to autoantigens, resulting in T-cell subsets with memory properties (rapid response to the same antigen), effector phenotypes (e.g. cytokine secretion, tissue homing properties) and distinct functional characteristics and longevity (3, 4).
In particular, the critical role of the co-stimulation process in T-cell activation and acquisition of memory/effector function led to the development of therapeutic strategies aimed at blocking key molecular interactions (5). The immunomodulatory drug cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)Ig (abatacept) prevents full activation of subsets of T cells requiring co-stimulation, by blocking the binding of CD80/CD86 expressed on antigen-presenting cells with the co-stimulatory molecule CD28 on the T cell (6).
In a clinical trial (“Effects of CTLA-4 Ig (abatacept) on the Progression of Type 1 Diabetes in New Onset Subjects (TN-09)”, (NCT00505375)) in patients with new-onset type 1 diabetes, abatacept treatment for two years significantly reduced the rate of loss of β-cell function, an effect sustained one year after completion of treatment, indicating that co-stimulation dependent immune pathways play a role in disease progression after diagnosis (7, 8). In the same study, we reported that in the placebo arm a significant association existed between an increase in the frequency of CD4+ central memory T (TCM) cells (CD45RO+CD62L+) from baseline and loss of β-cell function (9). Importantly, during abatacept treatment this relationship was no longer observed. These findings suggested that co-stimulation blockade has the effect of limiting CD4+ T-cell differentiation pathways that are a key pathobiological component of disease progression. These original findings were made using a fresh whole blood flow cytometry analysis with a limited panel of surface phenotypic markers (n=5), including those (e.g. CD62L) that are difficult to stain on cryopreserved cells. The objective of the current study is to characterise, in-depth, the effect of co-stimulation modulation, by abatacept, on T-cell subsets to expand and refine the previous finding of a link between immunological change and metabolic change. This may be important in the interpretation of outcomes of ongoing clinical trials such as the TrialNet study TN-18 examining the effects of abatacept in delaying disease progression in high-risk subjects with multiple autoantibodies (NCT01773707).
Materials and Methods
Samples
Cryopreserved peripheral blood mononuclear cells (PBMC) were provided by Type 1 Diabetes TrialNet from the clinical trial NCT00505375 (7). Samples from 40 abatacept- and 19 placebo-treated individuals were analysed at baseline, year 1 and year 2. Samples were provided blinded to treatment and time of sampling; each subject’s samples from the three collection times were stained and analysed on the same day. TrialNet Coordinating Centre provided clinical and demographic metadata following submission of raw data files. PBMCs were thawed at 37°C, washed in pre-warmed basal media (RPMI1640 Glutamax with HEPES, containing 100U/mL penicillin and 100μg/mL streptomycin (all Gibco)) containing 5% human heat-inactivated AB serum (Gibco), 20mM tumour necrosis factor (TNF)-α Protease Inhibitor-2 (TAPI-2) (Calbiochem) and 50U/mL Benzonase (Millipore). Cells were rested for 1 hour at 37°C/5% CO2 in basal media containing 10% human AB serum and 20mM TAPI-2 before washing in 1X basal media and 1X DPBS (Gibco) and staining of 2×106 PBMC each for flow and mass cytometry.
Sample staining and acquisition
For flow cytometry PBMCs were stained with LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit (Invitrogen) and subsequently with antibodies against CD197 (clone: G043H7, BV421, Biolegend) for 10 minutes at 37°C/5% CO2 followed by staining for CD14 (clone: M5E2, V500, BD), CD16 (clone: 3G8, V500, BD), CD19 (clone: HIB 19, V500, BD), CD3 (clone: SK7, APC-H7, BD), CD4 (clone: OKT4, BV785, BL), CD8 (clone: RPA-T8, FITC, BL), CD27 (clone: O323, BV605, BL), CD45RA (clone: HI100, APC, BD), CD95 (clone: DX2, PE, BL), CD45R0 (clone: UCHL1, PerCPCy5.5, BL), CD62L (clone: DREG-55, PE-Cy7, BL) and CD31 (clone: WM59, PE-CF594, BD) for 25 minutes on ice. A BD LSRFortessa set up with CS&T Beads (BD Biosciences) and application settings was used for acquisition. Where available conjugated CyTOF-ready antibodies were used; unconjugated Maxpar-ready antibodies were conjugated in-house using the Maxpar antibody labelling kit (Fluidigm). PBMCs were stained in 1X Rh-Intercalator (Fluidigm) for 15 minutes at room temperature (RT), before blocking FcR with TruStain FcX (Biolegend) for 10 minutes at RT. Cells were stained with antibodies against CD196 [CCR6] (clone: G034E3, 141Pr, Fluidigm), CD11a (clone: HI111, 142Nd, Fluidigm), CD45RA (clone: HI100, 143Tm, Fluidigm), CD195 [CCR5] (clone: NP-6G4, 144Nd, Fluidigm), CD4 (clone: RPA-T4, 145Nd, Fluidigm), IgD (clone: IA6-2, 147Sm, BD), CD14 (clone: RMO52, 148Nd, Fluidigm), CD16 (clone: 3G8, 148Nd, Fluidigm), CD25 (clone: 2A3, 149Sm, Fluidigm; clone: M-A251, Biolegend), CD31 (clone: WM59, 150Nd, eBioscience), CD49d (clone: 9F10, 151Eu, eBioscience), CD62L (clone: DREG-56, 153Eu, Fluidigm), CD45 (clone: HI30, 154Sm, Fluidigm), CD183[CXCR3] (clone: G025H7, 156Gd, Fluidigm), CD194 [CCR4] (clone: 205410, 158Gd, Fluidigm), CD197 (CCR7) (clone: G043H7, 159Tb, Fluidigm), CD28 (clone: CD28.2, 160Gd, Fluidigm), CD39 (clone: eBioA1, 161Dy, eBiosience), CD69 (clone: FN50, 162Dy, Fluidigm), CD161 (clone: HP-3G10, 163Dy, Biolegend), CD95 [FAS] (clone: DX2, 164Dy, Fluidigm), CD45RO (clone: UCHL1, 165Ho, Fluidigm), CD44 (clone: BJ18, 166Er, Fluidigm), CD27 (clone: L128, 167Er, FLUIDIGM), CD8a (clone: RPA-T8, 168Er, Fluidigm), Integrin beta7 (clone: FIB504, 169Tm, BD), CD3 (clone: UCHT1), 170Er, FLUIDIGM), CD19 (clone: HIB19, 171Yb, Biolegend), CD57 (clone: HCD57, 172Yb, Fluidigm), CD38 (clone: HIT2, 173Yb, Biolegend), HLA-DR (clone: L243, 174Yb, Fluidigm), CD279/PD-1 (clone: EH12.2H7, 175Lu, Fluidigm) and CD127 (clone: A019D5, 176Yb, Fluidigm) for 30 minutes at RT. After washing cells were fixed in 2% paraformaldehyde (Electron Microscopy Sciences) overnight at 4°C. Cells were washed and stained with 1X lr-intercalator for 20 minutes at RT, washed extensively in Milli-Q water (Millipore) and analysed by CyTOF (Fluidigm). CyTOF data were normalised using EQ-Four-Element-Calibration Beads (Fluidigm). T-cell subsets in flow and mass cytometry were gated in FlowJo (version 10, FlowJo). T cell subsets were defined accordingly; naïve (TNV), recent thymic emigrant (TRTE), stem-cell-memory (TSCM), central memory (TCM), transitional memory (TTM), effector memory (TEM), late-effector (TLE); by two markers: pre-TNV/RTE/SCM # (CD45RO−/CD27+), pre-TCM/TM # (CD45RO+/CD27+), pre-TEM # (CD45RO+/CD27−), pre-TLE # (CD45RO−/CD27−); by four markers: pre-TNV/RTE #.# (CD45RO−/CD27+/CD95−/CCR7+), pre-TSCM #.# (CD45RO−/CD27+/CD95+/CCR7+), pre-TCM #.# (CD45RO+/CD27+/CD95+/CCR7+), pre-TTM #.# (CD45RO+/CD27+/CD95+/CCR7−), pre-TEM #.# (CD45RO+/CD27−/CD95+/CCR7−), pre-TLE #.# (CD45RO−/CD27−/CD95+/CCR7−); by six markers: TNV (CD45RO−/CD27+/CD95−/CCR7+/CD45RA+/CD31−), TRTE (CD45RO−/CD27+/CD95−/CCR7+/CD45RA+/CD31+), TSCM-CD31 (CD45RO−/CD27+/CD95+/CCR7+/CD45RA+/CD31−), TSCM+CD31 (CD45RO−/CD27+/CD95+/CCR7+/CD45RA+/CD31+), TCM-CD31 (CD45RO+/CD27+/CD95+/CCR7+/CD45RA+/CD31−), TCM+CD31 (CD45RO+/CD27+/CD95+/CCR7+/CD45RA+/CD31+), TTM-CD31 (CD45RO+/CD27+/CD95+/CCR7−/CD45RA+/CD31−), TTM+CD31 (CD45RO+/CD27+/CD95+/CCR7− /CD45RA+ /CD31+), TEM-CD31 (CD45RO+ /CD27− /CD95+ /CCR7−/CD45RA+/CD31−), TEM+CD31 (CD45RO+/CD27−/CD95+/CCR7−/CD45RA+/CD31+), TLE-CD31 (CD45RO−/CD27−/CD95+ /CCR7−/CD45RA+/CD31−) TLE+CD31 (CD45RO−/CD27−/CD95+/CCR7−/CD45RA+/CD31+). Unsupervised clustering algorithm FlowSOM is part of Cytobank Enterprise platform (Cytobank) (10). For FlowSOM analysis, which relies on consistent inter-sample staining profiles for clustering, we analysed a subcohort of 29 individuals (20 abatacept- and 9 placebo-treated) that exhibited consistent staining profile between individuals. FlowSOM settings used were: 50 meta-cluster derived from 256 clusters and 10 iterations.
Statistical analysis
All statistical analysis, except for mixed linear modelling, were performed in Prism (v8, GraphPad) or in R Studio using packages (all available via CRAN; cran.r-project.org) FactoMineR (v1.42) (11) and Factoextra (v1.0.5) (12) for PCA, ComplexHeatmap (v2.1.0) (13) and Circlize (v0.4.7) (14) for Heatmaps, and ggplot2 (v3.2.0) (15) for visualisation. Generalized linear mixed modelling was conducted with SAS (V.9.4, Cary, NC). Statistical significance in T-cell subset frequencies was calculated using Mann-Whitney U-test or Wilcoxon signed-rank test as indicated; adjustment for multiple comparison testing by Benjamini-Hochberg and considered significant at p<0.05. β-cell function and mass was measured with stimulated C-peptide from a mixed meal tolerance test (MMTT). The area under the stimulated C-peptide curve (AUC) over the 2-hour MMTT was computed using the trapezoidal rule and divided by the duration of the test to estimate the average C-peptide level during stimulation (“average C-peptide”). For analysis of the relationship between percent change from baseline in C-peptide and in T-cell subsets the log (natural) of the percents were analysed with mixed-effects general linear models (GLMs) having subject as a random effect and adjusting for baseline T-cell level. Treatment-related alterations in the slope of the relationship between T-cell subset change and C-peptide change were declared statistically significant whenever the type 3 p-value for testing differences between treatment-specific slopes was significant after False Discovery Rate (FDR) adjustment set to yield a 5% FDR. A False Discovery Rate of 10% was used to select T-cell subsets as having a significant difference between treatments. Parameter estimates from the GLM analysis were used to estimate the retention of C-peptide level per unit of change in T-cell subset. The 2 year change models also considered the “lagged” T-cell value (i.e. the value of the T cell at the first visit after baseline) versions of the T-cell variables. Pearson correlations between change from baseline in T-cell subsets at time points (year 1 and 2) were computed and considered statistically significant at P=0.01.
Study approval
Protocol and consent documents were approved by appropriate Independent Ethics Committees or Institutional Review Boards for participating TrialNet centres. All subjects or their parents gave written informed consent and assent as appropriate before study participation.
Data and Resource Availability
The datasets generated during the current study are available from the corresponding author upon reasonable request. Clinical data and patient characteristics can be requested from the NIDDK public repository at https://repository.niddk.nih.gov/studies/trialnet/.
Results
Study cohort
Flow and mass cytometry were performed on a randomly sampled, representative subset of the original study cohort comprising 59 individuals (placebo n=19, abatacept n=40) (7, 9). The year 2 abatacept-induced reduction in C-peptide loss was comparable to that observed in the original study cohort. Mean baseline-adjusted C-peptide level was 75% higher in the treatment arm of our study (0.323 nmol/L compared to 0.185 nmol/L for abatacept and placebo, respectively) compared to 59% higher in the original study (0.378 nmol/L and 0.238 nmol/L) (data not shown).
Defining TCM cells in cryopreserved peripheral blood mononuclear cells
In the original study, flow cytometry was performed on fresh whole blood and CD4+ TCM were defined as CD45RO+CD62L+. CD62L is known to be shed in cryopreserved samples. We added the metalloprotease tumour necrosis factor alpha protease inhibitor 2 (TAPI-2) during thawing and cell recovery to limit CD62L shedding (16) but find that prevention of CD62L shedding is inconsistent. This leads to a marked underrepresentation of CD4+ TCM (CD45RO+CD62L+) in cryopreserved samples and a modest correlation with the data from fresh staining (Figure 1). However, we observed a stronger and highly significant correlation between CD4+ TCM (CD45RO+CD62L+) in fresh staining and on cryopreserved samples using other memory markers (Figure 1), with CD45RO+CD27+ being close to equivalence (slope=1.1, R=0.82). Subsequently, therefore, CD27 was used as a surrogate for CD62L when defining central memory subsets.
Figure 1: Relationship between fresh and cryopreserved sample staining to identify TCM.
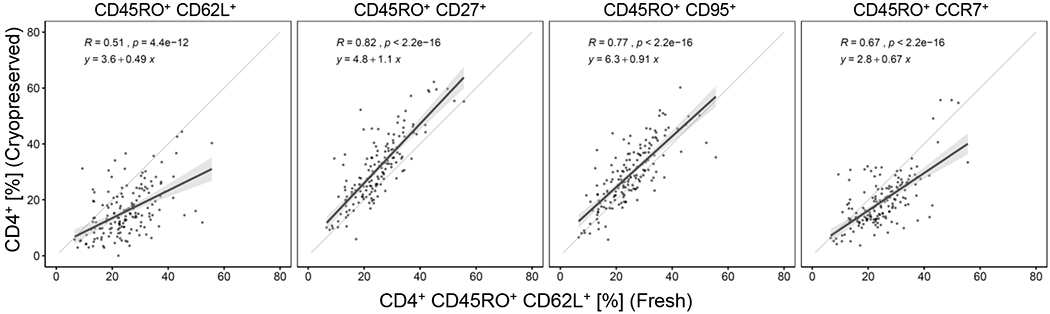
The frequency of CD4+ TCM (CD45RO+CD62L+) in fresh whole blood (on the x-axis) is compared to the frequency of CD4+ TCM in the cryopreserved sample defined using the markers indicated. Frequency is calculated as proportion of total CD4+ T cells; each data point is a single subject at one timepoint. Linear regression analysis = black line; 95% CI = grey shaded area; equality = grey line.
Abatacept treatment decreases memory and increases naïve CD4 T-cell subsets
We next examined abatacept-induced changes in memory and naïve CD4+ T-cell subsets with this refined flow cytometry panel to replicate and extend our original findings (gating strategy in Supplemental Figure 1) (17). We identified 12 traditionally defined T-cell subsets by sequential quadrant gating and performed principal component analysis (PCA) on the change of T-cell subsets from baseline to year 1 and year 2 (defined as “% of baseline”; change in frequency of a specific T-cell subset, over time (i.e. from baseline to year 1 or year 2), represented as proportion of baseline frequency”).
PCA separate individuals by treatment arm at year 1 and year 2, indicating that changes to CD4+ T-cell subsets are induced by abatacept (Figure 2a, b). Dimension 1 (accounting for ~47.2% of the variance in year 1 and 43% in year 2) is mainly driven by changes to CD4+ T cells with phenotypes corresponding to naïve (TNV), transitional memory (TTM), effector memory (TEM) and late-stage effector (TLE) cells; whereas dimension 2 (accounting for ~19% in year 1/ 21% in year 2 of the variance) is driven by changes to CD4+ T cells with stem-cell-memory-like (TSCM) and central memory (TCM) phenotypes (Supplementary Figure 2a, b).
Figure 2: Change in CD4+ T-cell subsets in treatment groups over time.
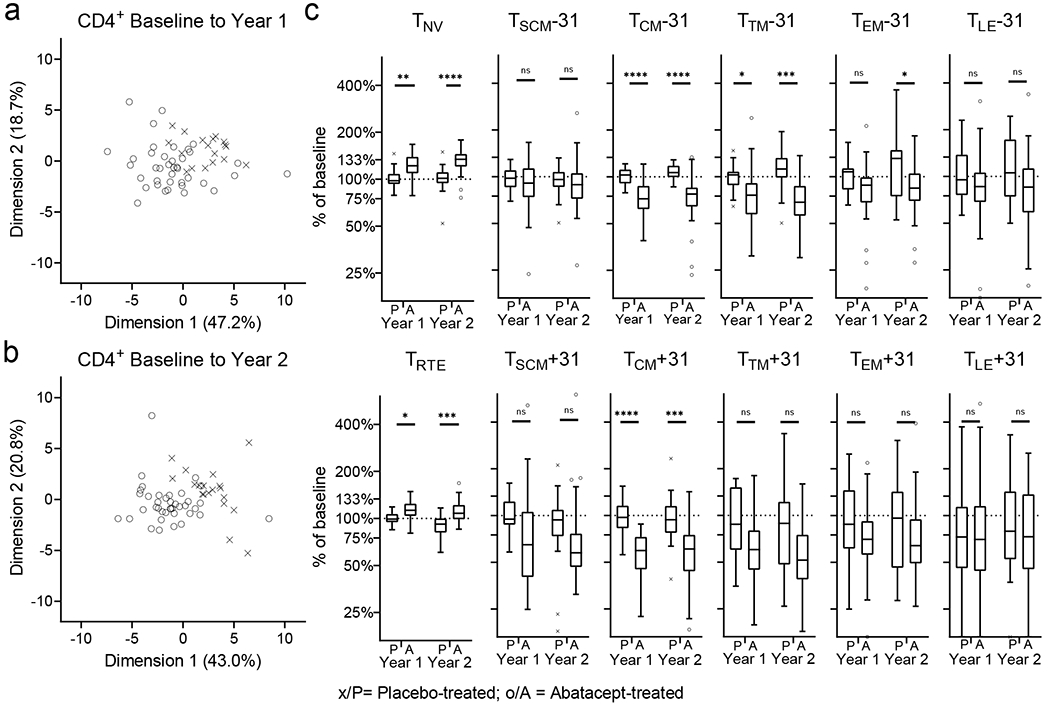
Change in CD4+ T-cell subsets from baseline to year 1 and to year 2 in treatment groups is analysed. Principal component analysis of individuals from treatment groups are shown for baseline to year 1 (a) and to year to 2 (b). Change (as percentage from baseline) for all CD4+ T-cell subsets is shown (c). Mann-Whitney U-test with Benjamini-Hochberg correction; * p<0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001; The horizontal line in the middle of each box indicates the median; top and bottom borders mark the 75th and 25th percentiles, respectively; whiskers according to Tukey. See Material & Methods for definition of T-cell subsets. % of baseline; change in frequency of a specific T-cell subset, over time (i.e. from baseline to year 1 or year 2), represented as proportion of baseline frequency.
Within these subsets we observed a treatment-associated increase from baseline for antigen-inexperienced (i.e. CD45RO−CD95−) TNV and recent thymic emigrant (TRTE) and a decrease for several antigen-experienced (CD95+) T-cell subsets (i.e. TSCM, TCM, TTM, TEM, TLE,) (Figure 2c). For the majority of subsets the change from baseline in the treatment group is significantly different from the placebo group, including after correcting for multiple comparison testing at both timepoints (TNV, TRTE, TCM, TTM; for CD31+/−) or year 2 only (TEM, CD31− only); some subsets (i.e. TSCM, TLE; for CD31+/−) are not significantly different between the treatment groups at either timepoint. We also noted that, in addition to changes in T-cell subset frequency, abatacept treatment is associated with reduced cell surface expression of markers within the first hierarchy gate (CD45RO/CD27). We observed a significant reduction in the median fluorescence intensity (MFI) of CD45RO and CD95 in the pre-CD4+ TCM/EM (CD45RO+CD27+) T-cell subset (Figure 3).
Figure 3: Reduction in expression levels of phenotypic markers during treatment.
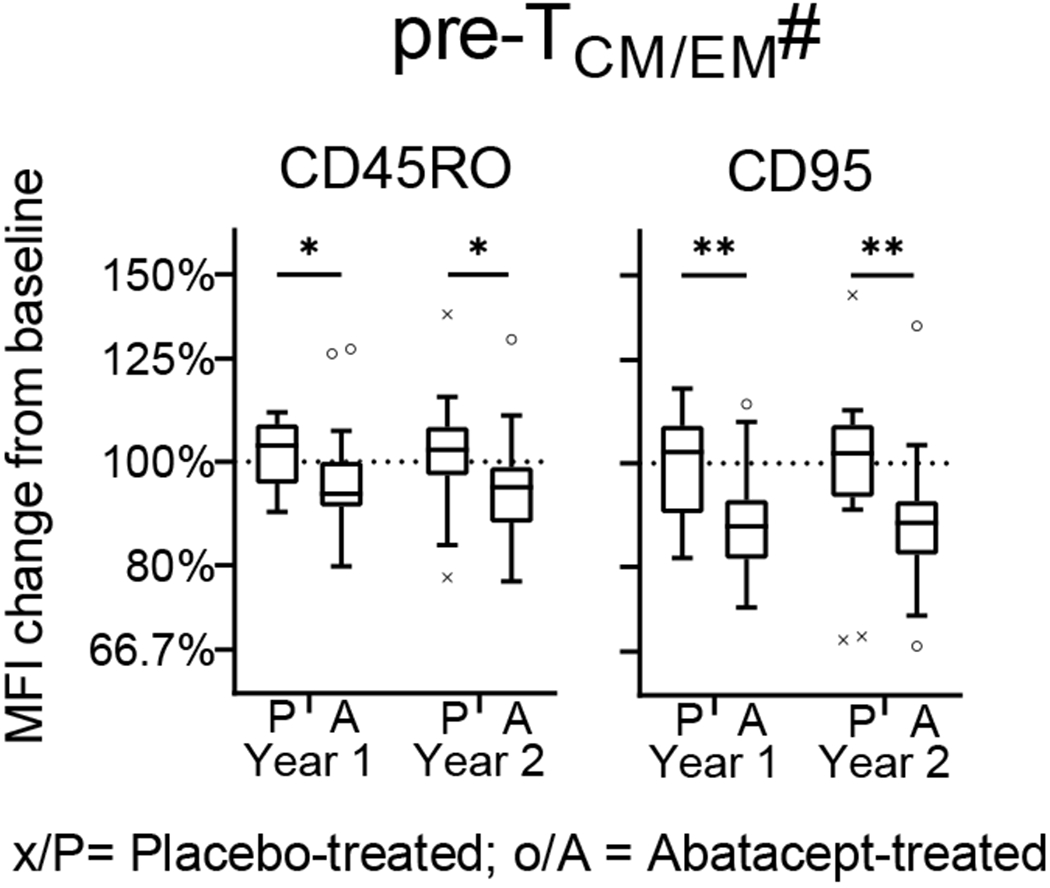
The change of expression level (as median fluorescence intensity) of 6 phenotypic markers was assessed on pre-T-cell subsets (#; defined by CD45RO and CD27) from baseline to year 1 and to year 2. Mann-Whitney U-test with Benjamini-Hochberg correction; * p<0.05, ** p≤0.01; The horizontal line in the middle of each box indicates the median; top and bottom borders mark the 75th and 25th percentiles, respectively; whiskers according to Tukey; see Material & Methods for definition of T-cell subsets.
In contrast, treatment effects of abatacept on CD8+ T-cell subsets were minor. Individuals from treatment groups do not separate in PCA and a significantly different change in frequency between treatment groups was found only in TEM and TLE at baseline to year 2 (Supplementary Figure 3).
Treatment with abatacept uncouples the correlation between changes in T-cell subsets and C-peptide
In the original mechanistic study we reported that, as part of the natural history of type 1 diabetes post-diagnosis, a relationship between changes in naïve/memory T-cell subsets and changes in β-cell function exists. Specifically, in the placebo arm, the decline from baseline value of average secreted C-peptide during a mixed meal tolerance test (MMTT) was correlated with an increase from baseline in CD4+ TCM (CD45RO+CD62L+) when measured at a preceding timepoint. Here, we extend this analysis to other T-cell subsets and identify, within the placebo arm, several immune changes that correlate with C-peptide change over time (Figure 4). As examples, higher rates of C-peptide retention from baseline to year 1 are associated with an increased frequency of CD4+ pre-TNV/SCM (CD45RO−CD27+) (slope of linear association = 5.75) (Figure 4a) and conversely a decreased frequency of CD4+ pre-TCM/TM (CD45RO+CD27+) (slope of linear association = −5.89) (Figure 4b).
Figure 4: Relationship of change in T-cell subsets and β-cell function.
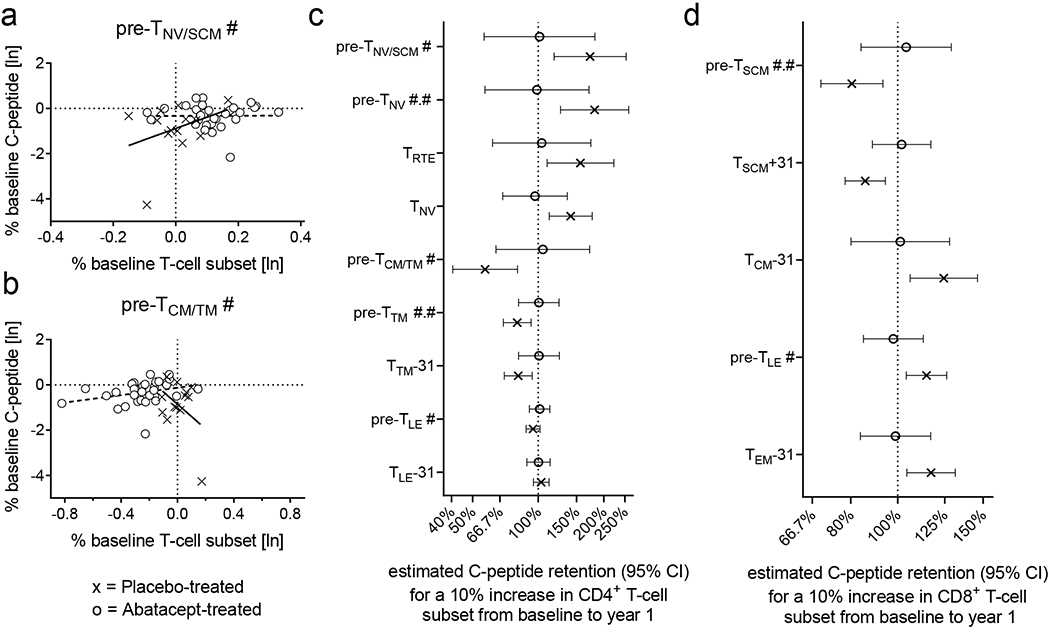
Panels a and b: Analysis of the linear association between a change in a T-cell subset and β-cell function from baseline for both treatment groups. In the placebo group, CD4+ pre-TNV/SCM # have a positive (a) and CD4+ pre-TCM/TM # have a negative (b) linear association with β-cell function as indicated by treatment-specific regression lines. Panels c and d: C-peptide retention (95% CI) estimated by the linear association models. Only T-cell subsets having significantly different rates of C-peptide retention between treatment groups are shown. Linear models that estimate the retention of baseline C-peptide for a 10% increase of specified for CD4+ and CD8+ T-cell subset. See Material & Methods for definition of T-cell subsets.
We used the linear model to estimate the retention of baseline C-peptide at year 1 for a 10% increase of a specified T-cell subset from baseline to year 1 (as by our prior analysis (9)). For example, a 10% increase in CD4+ TNV (CD45RO−CD27+CD95−CCR7+CD45RA+CD31−) is associated with a C-peptide retention of 141% (95% CI, 112%−176%). In contrast, a 10% increase in CD4+ pre-TCM/TM (CD45RO+CD27+) is associated with C-peptide retention of 57% (CI 41%−80%) (Figure 4c).
We next examined the effect of abatacept treatment on this relationship, since its therapeutic effect is retention of C-peptide (7), in association with changes to several T-cell subsets (Figure 2 and Supplementary Figure 3). Using the same model, all linear associations (slope of the relationship) between T-cell subsets and C-peptide attenuate to zero (Figure 4c). For example, in the treatment arm, a 10% increase of CD4+ TNV (CD45RO−CD27+CD95−CCR7+CD45RA+CD31−) is associated with C-peptide retention of 97% (CI 69%−136%); similarly, a 10% increase in CD4+ pre-TCM/TM (CD45RO+CD27+) is associated with C-peptide retention of 105% (CI 64%−172%). Thus abatacept significantly decreases, i.e. uncouples, the relationship between specific T-cell subsets and β-cell function observed in the placebo arm. Similarly, the uncoupling effect was also observed for CD8+ T-cell subsets (Figure 4d).
Abatacept-treatment induced changes to TCONV and TREG subsets
We analysed the samples by mass cytometry to examine activation, differentiation and homing in memory/naïve subsets in CD4+ TCONV (i.e. non-TREG) and TREG (Supplementary Figure 1 for gating strategy). There was a good correlation between identical subsets (See Material & Methods for definition of T-cell subsets.) identified by flow and mass cytometry (data not shown), yet some attenuation is observed for large flow values.
For TCONV, PCA separates the treatment groups at year 1 and year 2 (Figure 5a, b and Supplementary Figure 2 e, f), similar to that seen by flow cytometry. In year 1, dimension 1 (accounting for 42.9% of the variance) is mainly driven by changes to TCM and TTM (Supplementary Figure 2e) whereas dimension 2 (accounting for 15.8% of the variance) is mainly driven by changes to TEM. In year 2 (Supplementary Figure 2f) dimension 1 (accounting for 44.3% of the variance) is mainly driven by changes to TCM, whereas dimension 2 (accounting for ~17.4% of the variance) is mainly driven by changes to TEM, TTM and TNV (Figure 5c).
Figure 5: Change in TCONV subsets in treatment groups.
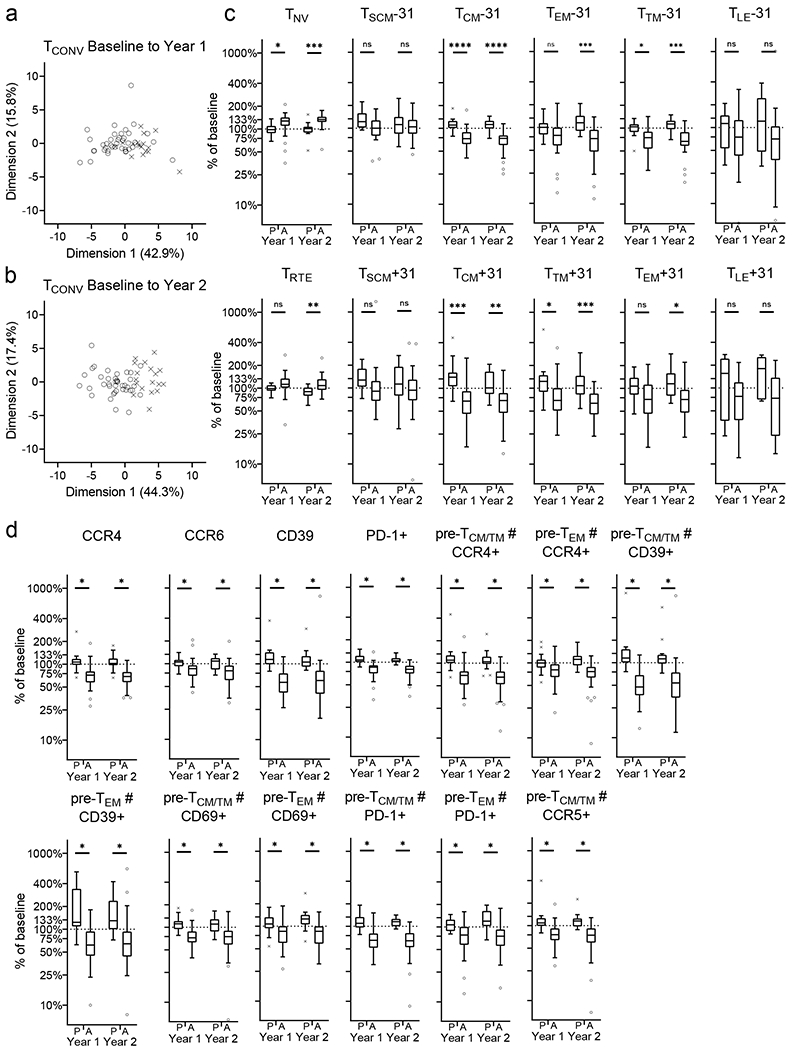
Change in TCONV subsets from baseline to year 1 and to year 2 in treatment groups is analysed (a, b and c). Principal component analysis of individuals from treatment groups are shown for baseline to year 1 (a) and to year to 2 (b). Change (as percentage from baseline) for TCONV subsets is shown (c). TCONV subsets (in depth phenotyping) with a significant abatacept treatment effect are shown (d). Mann-Whitney U-test with Benjamini-Hochberg correction; * p<0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001; The horizontal line in the middle of each box indicates the median; top and bottom borders mark the 75th and 25th percentiles, respectively; whiskers according to Tukey; See Material & Methods for definition of T-cell subsets. % of baseline; change in frequency of a specific T-cell subset, over time (i.e. from baseline to year 1 or year 2), represented as proportion of baseline frequency.
We then further defined the “standard” T-cell subsets (defined by CD45RO and CD27) with additional markers (i.e. CCR4, CCR5, CCR6, CXCR3, CD69, PD-1, CD39) and tested for abatacept-induced change. We found that abatacept treatment reduces TCONV expressing CCR4, CCR6, CD39 or PD-1, early-stage memory (pre-TCM/TM) TCONV expressing CCR5, and early and late-stage memory (pre-TCM/TM and pre-TEM) TCONV expressing CCR4, CD39, CD69 or PD-1 (Figure 5d).
For TREG, PCA again separates the treatment groups at year 1 and year 2, in a similar fashion to that observed for the TCONV, indicating that changes to TREG subsets are likewise induced by abatacept treatment (Figure 6a, b).
Figure 6: Change in TREG subsets in treatment groups.
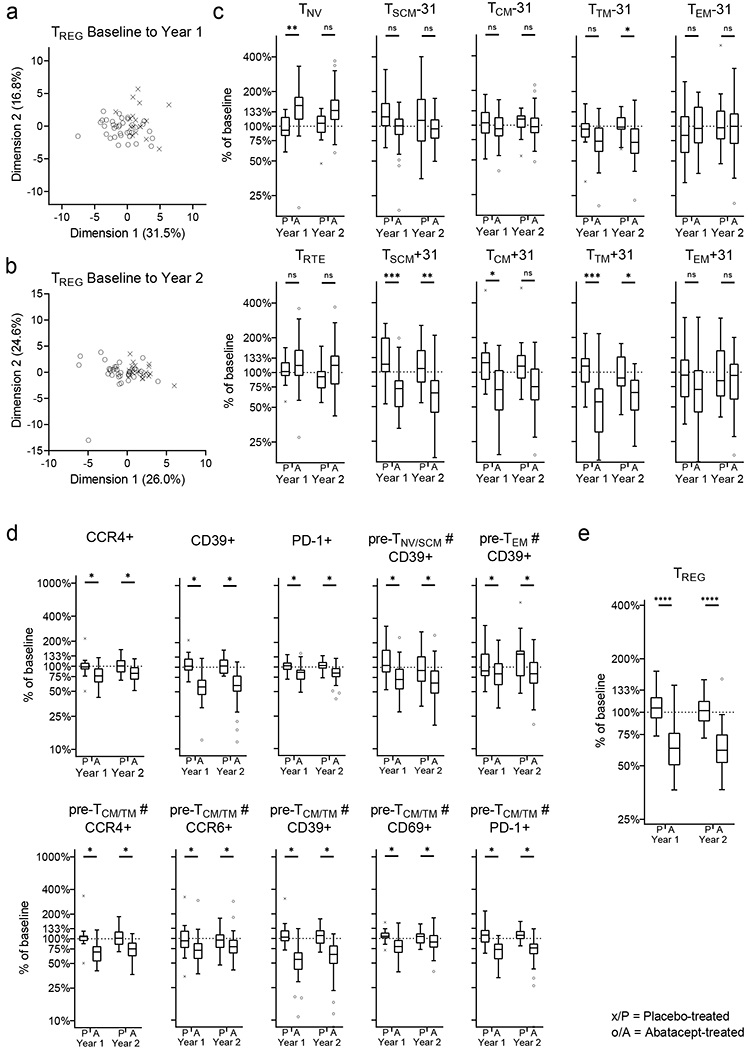
Change in TREG subsets from baseline to year 1 and to year 2 in treatment groups is analysed (a, b and c). Principal component analysis of individuals from treatment groups are shown for baseline to year 1 (a) and to year to 2 (b). Change (as percentage from baseline) for TCONV subsets is shown (c). TCONV subsets (in depth phenotyping) with a significant abatacept treatment effect are shown (d). Abatacept treatment effect on total TREG (e) Mann-Whitney U-test with Benjamini-Hochberg correction; * p<0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001; The horizontal line in the middle of each box indicates the median; top and bottom borders mark the 75th and 25th percentiles, respectively; whiskers according to Tukey; See Material & Methods for definition of T-cell subsets. % of baseline; change in frequency of a specific T-cell subset, over time (i.e. from baseline to year 1 or year 2), represented as proportion of baseline frequency.
In year 1, dimension 1 (accounting for 31.5% of the variance) is mainly driven by changes to TREG with phenotypes corresponding to TNV, TCM, TTM whereas dimension 2 (accounting for 16.8% of the variance) is mainly driven by changes to TSCM, TEM. In year 2 dimension 1 (accounting for 26% of the variance) is mainly driven by changes to TNV and TCM whereas dimension 2 (accounting for 24.6% of the variance) is mainly driven by changes to TEM and TNV (Figure 6c and Supplementary Figure 2g, h).
We further defined the activation, differentiation and homing state of TREG with additional markers (i.e. CCR4, CCR5, CCR6, CXCR3, CD69, PD-1, and CD39) within the T-cell subsets (defined by CD45RO and CD27) and tested for abatacept-induced change. Abatacept treatment reduces TREG expressing CCR4, CD39 or PD-1, naïve (pre-TNV/SCM) TREG expressing CD39, early and late-stage memory (pre-TCM/TM and pre-TEM) TREG expressing CD39, CCR4, CCR6, CD69 or PD-1 and late-stage memory (pre-TEM) TREG expressing CD39 (Figure 6d). Additionally, abatacept treatment leads to a significant reduction of the frequency of TREG within CD4+ T cells over time (Figure 6e).
Abatacept treatment selectively reduces a diverse subset of antigen-experienced CD4+ TCONV
To study changes in CD4+ TCONV at greater depth, we used the unsupervised clustering algorithm FLowSOM. Performing clustering on 18 phenotypic markers, FlowSOM identifies 50 subsets of TCONV providing an in-depth characterisation of the effect of abatacept treatment on the phenotypic landscape. The median baseline frequency of subsets ranged from 0.02% (range 0%−0.28%) to 55% (range 26%−81%). In 28 unique subsets abatacept treatment significantly alters the frequency from baseline to year 1 (22 subsets) or to year 2 (23 subsets) (Supplementary Figure 4).
PCA of the change in frequency from baseline to year 1 and to year 2 of the 50 subsets clearly separates treatment groups at both timepoints (Figure 7a, b), and achieves better visual separation than is seen analysing CD4+ T cells, TREG or TCONV (Figures 2, 5, 6). A total of 17 subsets are significantly affected in the treatment group compared to the placebo group (one increases and 16 decrease) at both timepoints and these subsets drive separation in the PCA (Figure 7c, d and Supplementary Figure 2i, j). Hierarchical clustering of the 17 significantly affected subsets identifies the major groups, namely TNV, TCM, TTM and TEM (Figure 7c). Within the 17 significantly affected subsets, absent or low levels of CD127 (IL-7 receptor) expression on antigen experienced cells marks a subset of TCONV subsets that actually decrease, particularly those expressing PD-1 (subsets 5, 6, 34, 44), CD38 (5, 6, 10), CD39 (35, 36) and homing makers beta-7 (2), CXCR3 (4), CCR4 (36, 38) CCR6 (7) (Figure 7c, d). A decrease in cell frequency is also observed for subsets expressing medium to high levels of CD127 in conjunction with expression of CD25 (8, 18, 23, 33), CD161 (8, 18, 19, 23) or homing makers CCR4 (33) and CCR6 (8, 10). This suggests that abatacept treatment specifically targets antigen experienced T-cell subsets with a distinct and diverse differentiation, homing and activation status.
Figure 7: Change in FlowSOM-identified TCONV subsets in treatment groups.
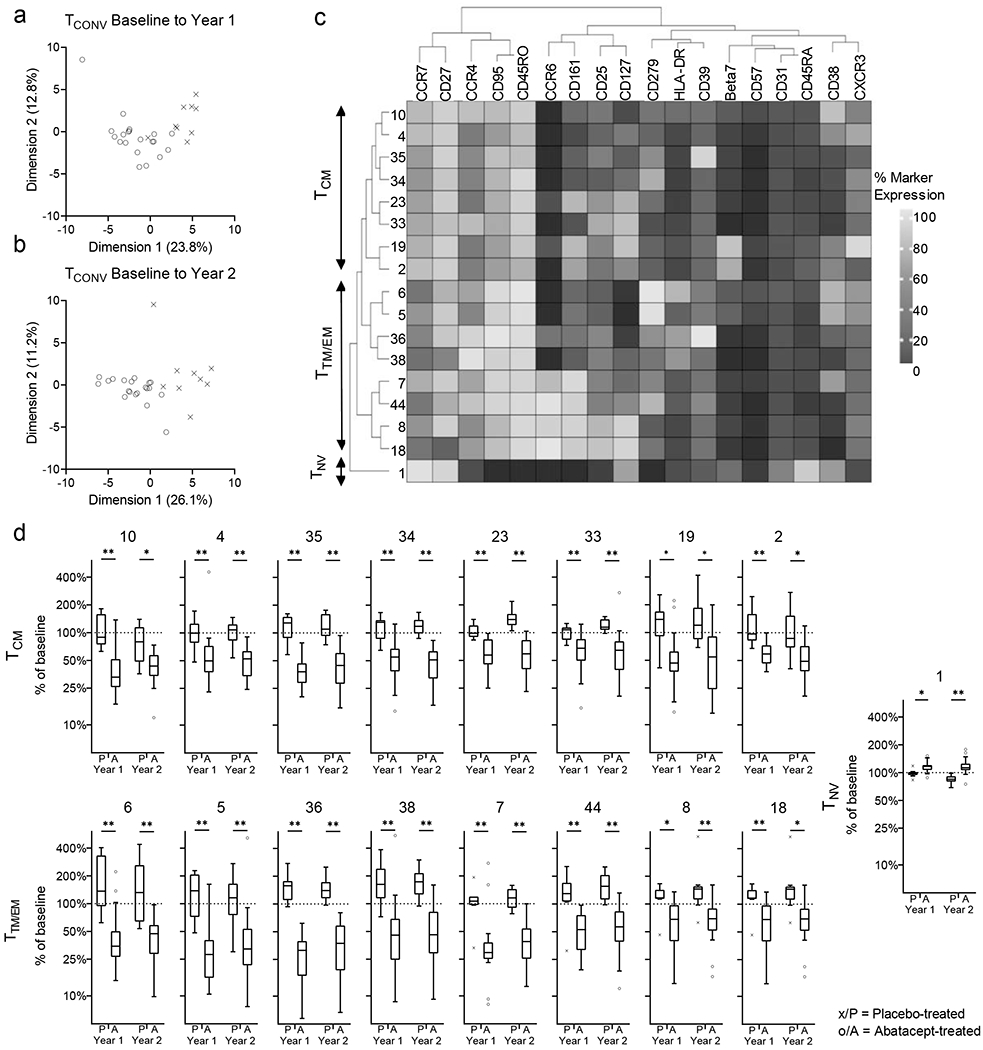
Principal component analysis of changes to the 50 FlowSOM-identified TCONV subsets are shown for baseline to year 1 (a) and to year 2 (b) (see also Supplementary Figure 3i, j). For the 17 FlowSOM-identified TCONV subsets that are significantly different between the treatment groups for change from baseline to year 1 and year 2 hierarchical clustering (c) and change in frequency of subset from baseline (d) are shown. Mann-Whitney U-test with Benjamini-Hochberg correction; * p<0.05, ** p≤0.01; The horizontal line in the middle of each box indicates the median; top and bottom borders mark the 75th and 25th percentiles, respectively; whiskers according to Tukey.
Discussion
Taking advantage of the setting of a phase 2 therapeutic intervention trial in patients with type 1 diabetes, our study was able to address two major aspects, namely the impact of co-stimulation blockade on peripheral T-cell compartments, and its effect on immune changes that are associated with the natural history of the disease. We define these two aspects, at unprecedented depth and resolution.
Defining antigen experience in T-cell compartments we see pronounced abatacept-induced changes for CD4+ T cells (both CD4+ TCONV and TREG), but only limited changes in CD8+ T cells, in line with these being less dependent on CD28-mediated co-stimulation for proliferation and activation (5, 18). This apparent difference in effects on distinct T-cell differentiation pathways (CD4+ and CD8+) may be an important consideration for building on the deployment of abatacept beyond its use as a monotherapy in type 1 diabetes intervention. An attractive approach, for example, might be to use abatacept in combination with a CD8-targeted therapy.
Our data demonstrate that abatacept affects CD4+ T cells according to their state of antigen experience: in general antigen-naïve subsets increase, whereas antigen-experienced subsets decrease. In particular, the frequency of TNV and TRTE increases significantly, while early memory TCM, TTM and to a lesser extent late memory TEM decrease; we observe little or no change in the frequency for TSCM. Several subsets with immunological memory are affected, indicating that treatment is acting on the generation and/or maintenance of specific cell subsets. According to the two main models of phenotypic maturation of T cells: (i) following activation and effector T-cell generation, there is a contraction phase after which T cells die or transition into a memory phenotype; or (ii) T cells progress gradually through the memory phenotypes upon every consecutive round of antigen stimulation (3, 19). Regardless of the model that may apply, our data showing increased frequencies in TNV after abatacept provide evidence that treatment is limiting the activation and recruitment of naïve CD4+ T cells into memory phenotypes. Both models suggest that we should observe a reduction in the frequency of other memory T-cell subsets (downstream of TCM in the case of model (ii)), which is indeed what we observe at year 2. The frequency of TSCM, a T-cell subset defined by its longevity, to provide long-term memory (20) hardly changes, indicating that abatacept has a limited effect on cells with high capacity for homeostatic proliferation.
Besides interfering with memory pathways abatacept directly targets T-cell subset. Within the pre-TCM/TM (CD45RO+CD27+), we find that the expression level of CD95 is significantly reduced following treatment. CD95 (Fas receptor) is a death receptor from the tumour necrosis factor receptor superfamily associated with programmed cell death, cell growth and proliferation (21). The data suggest that pre-TCM/TM expressing high levels of CD95 are preferentially deleted during treatment, potentially through increased apoptotic signalling (22).
Our deep-phenotyping approach supports the notion of selective loss of T-cell subsets. We find significant treatment-induced reduction in frequency in subsets expressing homing markers CCR4, CCR6, and alpha-4 beta-7 and activation markers HLA-DR, CD38, CD39, and PD-1. Abatacept predominantly affects T-cell subsets with low levels of CD127 (IL-7 receptor). Affected subsets expressing higher levels of CD127 typically also express CD25 (IL-2 receptor alpha chain) indicating a reliance on IL-2 signalling. Abatacept prevents T-cell activation which in turn leads to lower IL-2 secretion(23). Given that TREG, a lineage reliant on IL-2 for optimal function and survival, are significantly reduced by abatacept, also previously observed by us and others (9, 24), strengthens the notion of reduced IL-2 levels during therapy. Again, in TREG we observe significant reduction during therapy in subsets associated with activation and homing. The potentially negative effects of reduced TREG frequency during treatment seem to be off-set by the beneficial effects of modulating TCONV activation and proliferation. In addition, a recent study shows that CTLA-4 Ig can replace TREG function in promoting memory CD8+ T cells in a quiescent state (25).
Overall our findings therefore suggest that co-stimulation blockade targets some T-cell subsets more effectively than others, and it appears that the least affected cells possess a higher capacity for homeostatic proliferation and longevity. It is thus likely that not all diabetogenic cells (e.g. TSCM and late effectors) are targeted by treatment.
Co-stimulation blockade with abatacept profoundly affects the phenotypic landscape of peripheral T cells by operating largely in relation to the antigen-experience and co-stimulation dependence of a cell. A notable outcome of this treatment effect in terms of the natural history of type 1 diabetes is that it uncouples the relationship between acquisition of CD4+ T-cell memory and loss of β-cell function. Abatacept treatment uncoupled this link in all instances where we observed a significant relationship between change in a specific cell subset and change in β-cell function Thus, natural history markers such as change in T-cell memory will have only limited utility as tools for monitoring disease progression in the setting of studies on co-stimulation blockade since such a therapy renders the biomarker non-predictive in the group receiving treatment.
We have not fully explored the explanation of attenuation in our analysis and this remains for the future. Nevertheless, abatacept-induced changes to the subset landscape are vast and of variable magnitude, and mostly uniform in direction within the study cohort. We could not detect a correlation between the magnitude of change in any T-cell subset and β-cell function, suggesting that a beneficial outcome is achieved by crossing a certain threshold and/or that we do not asses the relevant T-cell subset. Given the depth of analysis it is unlikely that such a subset was missed albeit it is conceivable that the vast changes induced by the treatment hide such a subset. However, it is more likely that a relationship between change in T-cell subsets and β-cell function persists when focusing on type 1 diabetes-associated antigen specific T cells or specific phenotypes thereof (26).
Due to the difficulty to access pancreatic tissue for sampling during clinical trials, we sample peripheral blood to assess the effect of co-stimulation modulation on T-cell subset. At present, there is no study either confirming or challenging the notion that monitoring peripheral blood allows insight into the immunological situation in the pancreatic islets of patients with type 1 diabetes. Nevertheless, several recent reports correlate changes in peripheral immune-cell subset to changes in β-cell function and/or type 1 diabetes progression (26–28). These data suggest that one can utilise the immunological activity in peripheral blood as a proxy for pathological activity within the pancreatic islets. It remains for future studies to establish whether the changes in immune subset in peripheral blood (or draining lymph nodes (29), as a closer proxy) are identical to those in in the pancreatic islets.
We have confirmed and extended our initial finding of a relationship between changes in T-cell subsets and β-cell function as part of the natural course of type 1 diabetes pathology. Abatacept treatment uncoupled this link in all instances where we observed a significant relationship between change in a specific cell subset and change in β-cell function. Thus, natural history markers such as change in T-cell memory will have only limited utility as tools for monitoring disease progression in the setting of studies on co-stimulation blockade since such therapies render the biomarker non-predictive in the group receiving treatment. In a larger context, an important lesson from our analysis is that every biomarker needs to be validated in treated populations as well as control populations.
Supplementary Material
Key points.
Co-stimulation blockade reduces the loss of β-cell function in type 1 diabetes
CB effects peripheral regulatory and conventional CD4+, but not CD8+ T-cell subsets
CB blunts relationship between disease progression and CD4+ memory T-cell subsets
Acknowledgements
M.E. and R.B. performed experiments. S.H. and R.J.E. were instrumental in setting up mass cytometry experiments. M.E., R.B., M.P. and C.A.B. analysed results, applied statistical analysis and edited the manuscript. M.E., C.A.B. and M.P. wrote the manuscript. M.P. and C.A.B. conceived and designed the study.
We are grateful to Cynthia Bishop, Rianne Wester, Anna Rose, PJ Chana, Nedyalko Petrov, all members of the Guy’s and St Thomas’ Biomedical Research Council flow cytometry core.
Parts of this study have been presented in poster format at the 2019 FOCIS Annual Meeting, Boston, U.S.A.
Funding:
This work was supported by the US National Institutes of Health (NIH) via DP3DK101109 to C.A.B and M.P.
The Type 1 Diabetes TrialNet Study is a clinical trials network currently funded by the NIH through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development; through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085461, U01 DK085465, U01 DK085466, U01 DK085476, U01 DK085499, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, and UC4 DK106993; and through JDRF International.
We acknowledge financial support from the UK Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Gu’s & St Thoma’ NHS Foundation Trust in partnership with King’ College London and King’ College Hospital NHS Foundation Trust.
We also acknowledge support from the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement 115797 INNODIA, which receives support from the European Union’s Horizon 2020 Research and Innovation Programme, and the European Federation of Pharmaceutical Industries and Associations, JDRF International, and the Leona M. and Harry B. Helmsley Charitable Trust to M.P.
Funders were not involved in the following: in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript. As such the content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Abbreviations used in this article:
- AUC
area under the curve
- CI
confidence interval
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- FDR
false discovery rate
- GLMs
general linear models
- MFI
median fluorescence intensity
- MMTT
mixed meal tolerance test
- PBMC
peripheral blood mononuclear cell
- PCA
principal component analysis
- TAPI-2
tumour necrosis factor (TNF)-α Protease Inhibitor-2
- TCM
central memory T cell
- TCONV
conventional T cell
- TEM
effector memory T cell
- TLE
late-effector T cell
- TNV
naïve T cell
- TREG
regulatory T cell
- TRTE
recent thymic emigrant T cell
- TSCM
stem-cell-memory T cell
- TTM
transitional memory T cell
Footnotes
The authors declare no financial conflicts of interest.
References
- 1.Roep BO, and Peakman M. 2011. Diabetogenic T lymphocytes in human Type 1 diabetes. Curr Opin Immunol 23: 746–753. [DOI] [PubMed] [Google Scholar]
- 2.Roep BO, and Peakman M. 2012. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med 2: a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaech SM, and Cui W. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jameson SC, and Masopust D. 2018. Understanding Subset Diversity in T Cell Memory. Immunity 48: 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fife BT, and Bluestone JA. 2008. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 224: 166–182. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, St Clair EW, and Turka LA. 2006. CTLA4Ig: bridging the basic immunology with clinical application. Immunity 24: 233–238. [DOI] [PubMed] [Google Scholar]
- 7.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS, and G. Type 1 Diabetes TrialNet Abatacept Study. 2011. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Peakman M, Raskin P, Russell WE, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS, and G. Type 1 Diabetes TrialNet Abatacept Study. 2014. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 37: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orban T, Beam CA, Xu P, Moore K, Jiang Q, Deng J, Muller S, Gottlieb P, Spain L, Peakman M, and G. Type 1 Diabetes TrialNet Abatacept Study. 2014. Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes 63: 3449–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Gassen S, Callebaut B, Van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, and Saeys Y. 2015. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 87: 636–645. [DOI] [PubMed] [Google Scholar]
- 11.Lê S, Josse J, and Husson F. 2008. FactoMineR: An R Package for Multivariate Analysis. Journal of Statistical Software 25: 1–18. [Google Scholar]
- 12.A K, and M F 2017. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. [Google Scholar]
- 13.Gu Z, Eils R, and Schlesner M. 2016. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32: 2847–2849. [DOI] [PubMed] [Google Scholar]
- 14.Gu Z, Gu L, Eils R, Schlesner M, and Brors B. 2014. circlize Implements and enhances circular visualization in R. Bioinformatics 30: 2811–2812. [DOI] [PubMed] [Google Scholar]
- 15.Wickham H 2009. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York. [Google Scholar]
- 16.Jabbari A, and Harty JT. 2006. Simultaneous assessment of antigen-stimulated cytokine production and memory subset composition of memory CD8 T cells. J Immunol Methods 313: 161–168. [DOI] [PubMed] [Google Scholar]
- 17.Mahnke YD, Brodie TM, Sallusto F, Roederer M, and Lugli E. 2013. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 43: 2797–2809. [DOI] [PubMed] [Google Scholar]
- 18.Chan DV, Gibson HM, Aufiero BM, Wilson AJ, Hafner MS, Mi QS, and Wong HK. 2014. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun 15: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jameson SC, and Masopust D. 2009. Diversity in T cell memory: an embarrassment of riches. Immunity 31: 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, Price DA, Waldmann TA, Restifo NP, Franchini G, and Roederer M. 2013. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 123: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi F, Frazzette N, Cruz AC, Klebanoff CA, and Siegel RM. 2018. Beyond Cell Death: New Functions for TNF Family Cytokines in Autoimmunity and Tumor Immunotherapy. Trends Mol Med 24: 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouillet P, and O’Reilly LA. 2009. CD95, BIM and T cell homeostasis. Nat Rev Immunol 9: 514–519. [DOI] [PubMed] [Google Scholar]
- 23.Malek TR 2008. The biology of interleukin-2. Annu Rev Immunol 26: 453–479. [DOI] [PubMed] [Google Scholar]
- 24.Langdon K, and Haleagrahara N. 2018. Regulatory T-cell dynamics with abatacept treatment in rheumatoid arthritis. Int Rev Immunol 37: 206–214. [DOI] [PubMed] [Google Scholar]
- 25.Kalia V, Penny LA, Yuzefpolskiy Y, Baumann FM, and Sarkar S. 2015. Quiescence of Memory CD8(+) T Cells Is Mediated by Regulatory T Cells through Inhibitory Receptor CTLA-4. Immunity 42: 1116–1129. [DOI] [PubMed] [Google Scholar]
- 26.Yeo L, Woodwyk A, Sood S, Lorenc A, Eichmann M, Pujol-Autonell I, Melchiotti R, Skowera A, Fidanis E, Dolton GM, Tungatt K, Sewell AK, Heck S, Saxena A, Beam CA, and Peakman M. 2018. Autoreactive T effector memory differentiation mirrors beta cell function in type 1 diabetes. J Clin Invest 128: 3460–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habib T, Long SA, Samuels PL, Brahmandam A, Tatum M, Funk A, Hocking AM, Cerosaletti K, Mason MT, Whalen E, Rawlings DJ, Greenbaum C, Buckner JH, and G. Type 1 Diabetes TrialNet Study. 2019. Dynamic Immune Phenotypes of B and T Helper Cells Mark Distinct Stages of T1D Progression. Diabetes 68: 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiedeman AE, Muir VS, Rosasco MG, DeBerg HA, Presnell S, Haas B, Dufort MJ, Speake C, Greenbaum CJ, Serti E, Nepom GT, Blahnik G, Kus AM, James EA, Linsley PS, and Long SA. 2020. Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J Clin Invest 130: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang JHM, Khatri L, Mickunas M, Williams E, Tatovic D, Alhadj Ali M, Young P, Moyle P, Sahni V, Wang R, Kaur R, Tannahill GM, Beaton AR, Gerlag DM, Savage COS, Napolitano Rosen A, Waldron-Lynch F, Dayan CM, and Tree TIM. 2019. Phenotypic Analysis of Human Lymph Nodes in Subjects With New-Onset Type 1 Diabetes and Healthy Individuals by Flow Cytometry. Front Immunol 10: 2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


