Abstract
With an estimated 425 million diabetes patients worldwide in 2019, type 2 diabetes has reached a pandemic proportion and represents a major unmet medical need. A key determinant of the development and progression of type 2 diabetes is pancreatic ‐cell dysfunction, including the loss of cell mass, the impairment of insulin biosynthesis and inadequate exocytosis. Recent studies have shown that transient receptor potential vanilloid 4 (TRPV4), a Ca2+‐permeable non‐selective cation channel, is involved in ‐cell replication, insulin production and secretion. TRPV4 agonists have insulinotropic activity in pancreatic ‐cell lines, but the prolonged activation of TRPV4 leads to ‐cell dysfunction and death. In addition, TRPV4 is involved in a wide variety of pathophysiological activities, and has been reported to play an important role in diabetes‐related complications, such as obesity, cardiovascular diseases, diabetic retinopathy, nephropathy and neuropathy. In a rodent type 2 diabetes model, Trpv4 agonists promote vasodilation and improve cardiovascular function, whereas Trpv4 antagonists reduce high‐fat diet‐induced obesity, insulin resistance, diabetic nephropathy, retinopathy and neuropathy. These findings raise interest in using TRPV4 as a therapeutic target for type 2 diabetes. In this review, we intend to summarize the latest findings regarding the role of TRPV4 in diabetes as well as diabetes‐related conditions, and to evaluate its potential as a therapeutic target for diabetes and diabetes‐related diseases.
Keywords: Diabetes complications, Diabetes mellitus, Transient receptor potential vanilloid 4
Transient receptor potential vanilloid 4 is a Ca2+‐permeable non‐selective cation channel, which is involved in a wide variety of pathophysiological conditions. For example, ‐cell replication, insulin release and production are modulated by transient receptor potential vanilloid 4 protein. In addition, transient receptor potential vanilloid 4 had been reported to play an important role in diabetes‐related complications, such as obesity and cardiovascular disease, as well as diabetic retinopathy, nephropathy and neuropathy.
Introduction
According to the International Diabetes Federation, the prevalence of diabetes is increasing rapidly, and the number of patients will reach approximately 629 million by 2045 1 . Approximately 90% of all cases of diabetes are type 2 diabetes 2 , which is generally characterized by insulin resistance, during which the body does not fully respond to insulin for the proper control of blood glucose levels 3 . Insulin resistance triggers the exaggerated secretion of insulin to compensate for the insufficient metabolic actions of this hormone, and the persistence of this condition might lead to the “exhaustion” of pancreatic ‐cells. Such exhaustion ultimately results in the development of hyperglycemia. Various organs and tissues are damaged by prolonged exposure to high blood sugar levels, which leads to diabetes‐related complications 4 , 5 , such as cardiovascular disease (CVD), kidney disease, neuropathy, blindness and lower extremity amputation. These complications impact the quality of life of patients with diabetes 6 , and have become economic and healthcare burdens in many countries 7 .
Transient receptor potential vanilloid 4 (TRPV4) is a Ca2+‐permeable non‐selective cation channel 8 . There is increasing evidence for the involvement of TRPV4 in a variety of pathophysiological conditions. For example, TRPV4 is involved in pancreatic ‐cell replication and insulin production, and the activation of TRPV4 induces insulin secretion 9 . Furthermore, inhibiting or attenuating TRPV4 activity might reduce high‐fat diet (HFD)‐induced obesity and inflammation 10 . Table 1 summarizes recent findings on the involvement of TRPV4 in the pathogenesis of type 2 diabetes and diabetes‐related diseases 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 . Even though TRPV4 might play a significant role in the development of diabetes and related conditions, the underlying mechanism remains unclear. In this review, we intend to summarize the latest findings from the literature to explore the underlying mechanism of the role of TRPV4 in diabetes and evaluate the feasibility of using TRPV4 as a therapeutic target for diabetes and diabetes‐related diseases.
Table 1.
Involvement of transient receptor potential vanilloid 4 in a wide range of pathophysiological conditions in diabetes and diabetic complications
| Disease | Tissue/cell type | Species | Experiment | Effect | References |
|---|---|---|---|---|---|
| Type 2 diabetes | Pancreatic ‐cells | Murine | In vitro/in vivo | Modulation of insulin secretion; mediated ‐cell apoptosis | Casas 2008 17 , Skrzypski 2013 9 , Billert 2017 41 |
| Obesity | Adipocytes | Rat, murine, human | In vitro/in vivo | Adipogenesis and energy metabolism; adipose Ca2+ homeostasis and inflammation | Ye 2012 10 , Che 2014 29 , Chen 2015 31 , Janoschek 2016 37 , Sanchez 2016 38 , Sun 2017 44 |
| Skeletal muscle | Murine | In vitro/in vivo | Heightened metabolic capacity | Pritschow 2011 22 , Kusudo 2012 25 | |
| MSC, ASC | Murine | In vitro/in vivo | Increased obesity susceptibility | O’Conor 2013 28 | |
| Sebocyte | Human | In vivo | Influences glucose and lipid metabolism | Olah 2014 30 | |
| Blood | Human | Cross‐sectional studies | Increased genetic susceptibility to obesity | Duan 2015 32 , Tabur 2015 34 | |
| Diabetes‐related CVD | MAECs, Smooth muscle cells | Rat, murine | In vitro/in vivo | Vasodilator response | Earley 2009 19 , Mendoza 2010 20 , Ma 2013 27 , Ye 2018 47 |
| Mesenteric arteries | Rat | In vitro/in vivo | Endothelium‐dependent relaxation | Zou 2015 36 , Matsumoto 2017 43 , Bihzad 2017 40 | |
| CAECs | Rat | In vivo | Shear stress‐induced vasodilation | Kohler 2006 15 | |
| Diabetic nephropathy | HCD cells | Human | In vivo | Role in RVD | Hills 2006 14 , Hills 2012 12 |
| Collecting ducts, tubules | Murine | In vitro/in vivo | Control of mechanosensitivity | Berrout 2012 24 , Wu 2007 16 | |
| Diabetic retinopathy | RMECs, RPE | Rat, murine, human, bovine | In vitro/vivo | Water diffusion and BRB breakdown in the retina; endothelial dysfunction; role in RVD | Monaghan 2015 13 , Zhao 2015 35 , Arredondo 2017 39 , Orduna 2019 49 |
| HCECs | Human | In vivo | Role in RVD | Pan 2008 18 , Mergler 2011 21 | |
| RGCs, Müller cells | Murine | In vitro/in vivo | Polymodal sensory transduction; modulation of calcium flux and apoptosis | Ryskamp 2011 23 , Lakk 2017 42 , Lakk 2018 46 | |
| Painful diabetic neuropathy | DRGs, TGs | Rat, murine | In vitro/in vivo | Modulates mechanosensation; mechanical hyperalgesia | Alessandri 2008 11 , Alexander 2013 26 , Hinata 2018 45 |
| DRGs, sciatic nerve, hind paw plantar skin | Murine | In vitro/in vivo | Mechanical allodynia | Dias 2019 48 | |
| SGCs | Murine | In vitro/in vivo | Nociceptors for inflammatory pain | Rajasekhar 2015 33 |
ASC, subcutaneous adipose‐derived stem cells; BRB, blood–retina barrier; CAECs, carotid artery endothelial cells; CVD, cardiovascular disease; DRGs, dorsal root ganglia; HCD, human collecting duct; HCECs, human corneal endothelial cells; MAECs, mesenteric artery endothelial cells; MSC, bone marrow derived stem cells; RGCs, Retinal ganglion cells; RMECs, retinal microvascular endothelial cells; RPE, retinal pigment epithelium; RVD, regulatory volume decrease; SGCs, satellite glial cells; TGs, trigeminal ganglia.
Brief Introduction of TRPV4 Channel
TRPV4 belongs to the transient receptor potential vanilloid (TRPV) subfamily of transient receptor potential (TRP) cation channels, and is a vertebrate homologue of the Caenorhabditis elegans Osm‐9 gene 8 , 50 . To date, 28 TRP channels have been identified in mammals. Based on sequence homology, these proteins can be grouped into six subfamilies: TRPV, TRPC (transient receptor potential canonical), TRPM (transient receptor potential melastatin), TRPP (transient receptor potential polycystic), TRPML (transient receptor potential mucolipin) and TRPA (transient receptor potential ankyrin) 51 , 52 . TRPV4 is a widely expressed, polymodally gated, non‐selective cation channel for ions, such as calcium, sodium, potassium and magnesium 8 . Various stimulating factors, such as moderate heat, osmotic pressure, cell swelling, and endogenous and exogenous chemical compounds, can affect the activity of TRPV4 53 . TRPV4 has the capability of being activated without stimulation 54 , and is involved in various physiological functions, including osmoregulation 55 , Ca2+ homeostasis 56 , apoptosis and autophagy 57 . Furthermore, diseases, such as hyperalgesic, hypertensive, hypertrophic, degenerative, ischemic and metabolic disorders, can be attributed to the aberrant activity of TRPV4 58 .
A number of endogenous small molecules have been shown to affect TRPV4 activity. These compounds include the arachidonic acid metabolite 5,6‐epoxyeicosatrienoic acid (5,6‐EET), acetylcholine, dimethylallyl pyrophosphate, and the endocannabinoid anandamide 59 , 60 , 61 , 62 . In addition, chemicals, such as gadolinium, lanthanum and ruthenium red, botanical extracts, such as bisandrographolide and apigenin 63 , 64 , and synthetic compounds, including phorbol ester 4α‐phorbol 12,13‐didecanoate (4αPDD), can also affect TRPV4 activity 48 , 65 , 66 , 67 , 68 , 69 . TRPV4 antagonists have been successfully used in vivo for pulmonary edema induced by heart failure, and one of them, GSK2798745, is currently being evaluated in a clinical trial for heart failure 58 , 70 .
TRPV4 and Type 2 Diabetes
A critical determinant of type 2 diabetes is pancreatic ‐cell dysfunction, including ‐cell deficiency, impaired insulin biosynthesis and insufficient exocytosis 71 . It has been shown that the intracellular Ca2+ level ([Ca2+]i) in pancreatic ‐cells affects insulin secretion 72 , 73 . Adenosine triphosphate (ATP)‐sensitive K+ (KATP) channels and voltage‐gated Ca2+ channels (VGCCs) are considered mediators of glucose‐stimulated insulin secretion 74 , 75 . High blood glucose concentrations increase glucose metabolism in pancreatic ‐cells and lead to a higher cellular ATP/adenosine diphosphate ratio. A high ATP/adenosine diphosphate ratio induces the closure of KATP channels followed by the depolarization of the membrane and the opening of VGCCs to facilitate Ca2+ influx, thereby elevating the [Ca2+]i and stimulating insulin secretion.
A KATP channel‐independent mechanism of glucose‐induced insulin secretion has also been proposed 76 , 77 . Takii et al. 78 showed the secretion of insulin through hypotonic‐induced ‐cell swelling, which activates Gd3+‐sensitive cation channels followed by membrane depolarization, the activation of VGCCs and an increased [Ca2+]i. Trpv4 is expressed in the ‐cell lines, MIN6 and INS‐1E, as well as in the rodent pancreas 9 , 17 , 41 . Immunohistochemistry has also shown a high abundance of TRPV4 protein in human islets 79 . TRPV4 can act as a mechano‐ and osmosensor; for example, TRPV4 activity can be induced by hypotonic stress or moderate heat, leading to the influx of extracellular Ca2+ and an increased [Ca2+]i 9 . Therefore, TRPV4‐mediated intracellular Ca2+ concentration changes might be involved in the regulation of glucose‐induced ‐cell insulin secretion. Skrzypski et al. 9 found that 4αPDD‐induced Trpv4 activation results in an increase in the intracellular Ca2+ concentration and insulin secretion in rat INS‐1E cells. The induction of this effect by 4αPDD can be eliminated by TRPV4 inhibitors. However, Sawatani et al. 80 found that 4αPDD and GSK1016790A show no apparent effect on the [Ca2+]i in isolated mouse ‐cells or MIN6 cells. The authors speculated that the inconsistencies in the findings were caused by differences between mice and rats, as well as differences in experimental temperature; room temperature was used in the study by Skrzypski et al., and 37°C was used by Sawatani et al. As TRPV4 can be activated by a warm temperature (>27°C), the lack of effects of 4α‐PDD and GSK1016790A on the [Ca2+]i might have been caused by the higher temperature used by Sawatani et al.
The activation of TRPV4 is not only associated with insulin secretion, but also affects the level of insulin messenger ribonucleic acid (mRNA) in ‐cells. Billert et al. 41 found that the GSK1016790A‐induced activation of TRPV4 promotes the expression of insulin mRNA after 1 and 3 h of treatment. In contrast, when cells are incubated with GSK1016790A for 24 h, the mRNA expression of insulin and ‐cell specific genes, such as Ins1, Ins2, Pdx1 and Gck, are suppressed, and this suppression is accompanied by an increase in cell death 41 . Extracellular signal‐related kinase 1 and 2 (ERK1/2) are activated by glucose in a Ca2+‐dependent manner, and ERK1/2 regulates other transcription factors associated with insulin gene expression and ‐cell survival 81 , 82 , 83 . GSK1016790A can promote ERK1/2 phosphorylation in INS‐1E cells, and the pharmacological blockade of ERK1/2 weakens insulin mRNA expression induced by GSK1016790A over time 41 . Therefore, Billert et al. proposed that TRPV4 stimulates insulin mRNA expression through ERK1/2 activation; however, the involvement of ERK1/2 in TRPV4 activation‐induced insulin mRNA expression has yet to be confirmed. In contrast, TRPV4 activation can stimulate nitric oxide (NO) production and inducible NO synthase mRNA expression, resulting in NO‐induced endoplasmic reticulum stress and the suppression of insulin mRNA expression in ‐cells 41 . Casas et al. 17 also found that the activation of TRPV4 by human islet amyloid polypeptide in mouse MIN6 ‐cells induces apoptosis (Figure 1).
Figure 1.
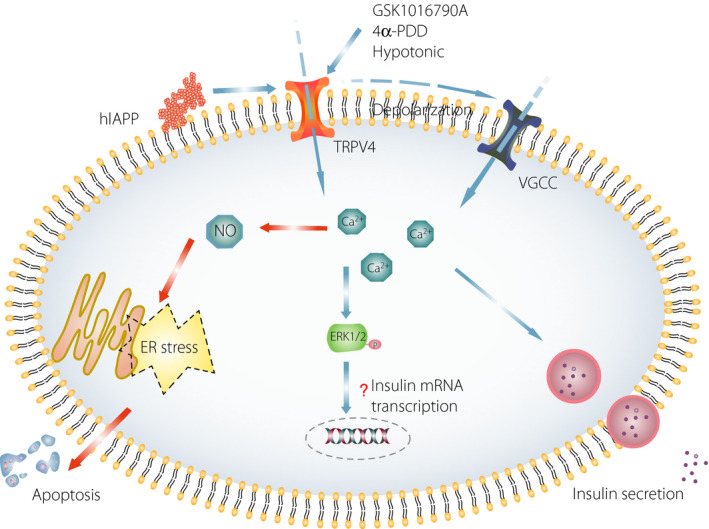
Transient receptor potential vanilloid 4 (TRPV4) activation affects insulin release and apoptosis from pancreatic ‐cells. In pancreatic ‐cells, the activation of TRPV4 can be involved in the regulation of insulin release and apoptosis through different mechanisms. In contrast, the activation of TRPV4 induced by hypotonia and agonists (GSK1016790A and phorbol ester 4α‐phorbol 12,13‐didecanoate [4α‐PDD]) can promote Ca2+ inflow, leading to plasma membrane depolarization, which elevates intracellular Ca2+ levels by activating voltage‐gated Ca2+ channels (VGCCs) in ‐cells, ultimately leading to insulin secretion. In addition, elevated intracellular Ca2+ levels promote Extracellular signal‐related kinase 1 and 2 (ERK1/2) phosphorylation, which is involved in the regulation of insulin messenger ribonucleic acid (mRNA) transcription. However, few studies have reported that TRPV4 activation is involved in the regulation of insulin mRNA expression through the ERK1/2 pathway, and the mechanism has not yet been elucidated. In contrast, protracted or human islet amyloid polypeptide‐induced TRPV4 activation can stimulate nitric oxide (NO) production in ‐cells, resulting in endoplasmic reticulum (ER) stress and the promotion of cell apoptosis. hIAPP, human islet amyloid polypeptide; P, phosphorylation.
TRPV4 and Diabetes‐Related Complications
Diabetes patients have an increased risk of developing heart, blood vessel, eye, tooth, kidney and nerve conditions as a result of prolonged high blood glucose levels 1 . Hyperglycemia‐associated conditions are often accompanied by dysregulated metabolic processes of carbohydrates, fats, proteins and electrolytes, all of which have significant impacts on the normal function of the cardiovascular system 84 . Furthermore, endothelial capillary cells, including those in the retina and renal glomerulus, are damaged by an excessive accumulation of glucose in cells 85 . The consequent complications are termed “microvascular diseases” and “macrovascular diseases” owing to damage to small blood vessels and the arteries, respectively 4 . One of the common comorbidities of type 2 diabetes is obesity caused by calorie overload, a lack of physical activity and insulin resistance, which all contribute to the vascular complications associated with diabetes 4 , 86 .
Several studies have shown an association between TRPV4 activity and obesity 87 and the progression of diabetic macrovascular and microvascular complications, such as diabetes‐related cardiovascular diseases, diabetic retinopathy, nephropathy and neuropathy (Figure 2) 8 , 88 , 89 , 90 . In type 2 diabetes rodent models, TRPV4 agonists promote vasodilation and improve cardiovascular function 43 . In addition, TRPV4 antagonists reduce HFD‐induced obesity and insulin resistance, and alleviate the progression of diabetic nephropathy, retinopathy and neuropathy 10 , 12 , 39 , 48 .
Figure 2.
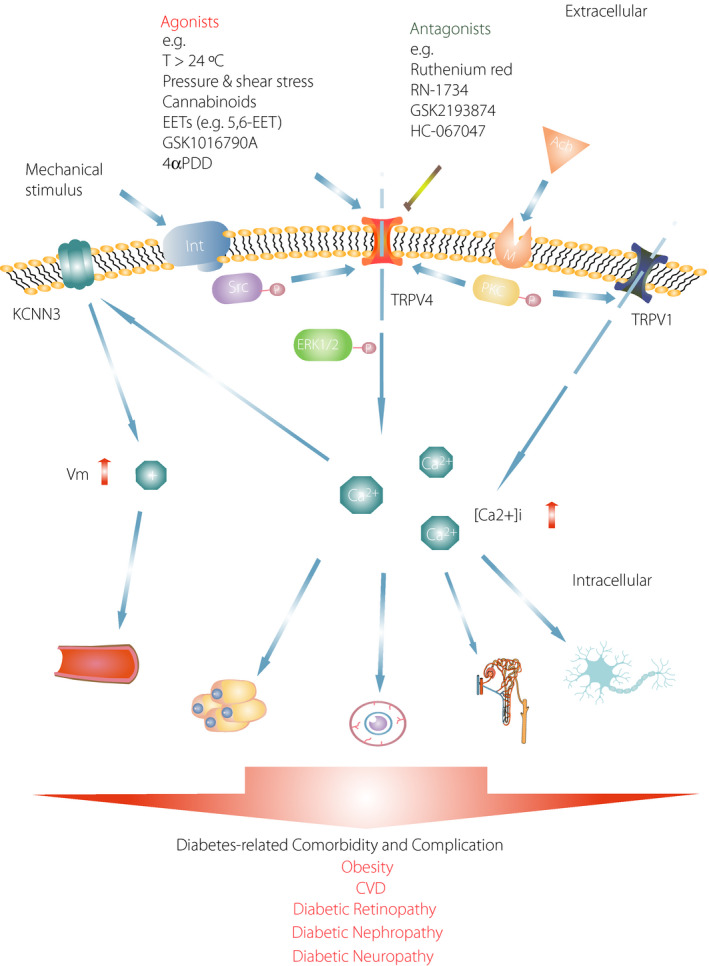
Diabetes leads to cellular damage mediated by transient receptor potential vanilloid 4 (TRPV4) activity. Pressure and shear stress activate TRPV4, which is a mechanosensitive channel. Integrin (Int) signaling is stimulated by the mechanical activation of TRPV4 through Src tyrosine kinase (Src). The stimulation of muscarinic (M) receptors by acetylcholine (ACh) also activates the colocalization of TRPV4 and TRPV1 through protein kinase C (PKC). TRPV4 and TRPV1 activation increase the intracellular Ca2+ concentrations ([Ca2+]i), which can result in damage to various cell types, such as artery cells, retinal microvascular endothelial cells, collecting duct cells, Müller cells and satellite glial cells. In addition, the activation of TRPV4 promotes extracellular signal‐regulated kinase 1/2 (ERK1/2) phosphorylation, and an elevated [Ca2+]i in adipocytes can lead to inflammation and insulin resistance. However, an increase in the [Ca2+]i can activate the KCNN3 channel, which can result in an increase in membrane potential and promote vasodilation. In diabetes, the dysfunction of TRPV4 can result in diabetes‐related diseases such as obesity, cardiovascular disease (CVD), diabetic retinopathy, nephropathy and neuropathy. KCNN3, potassium calcium‐activated channel subfamily N member 3; P, phosphorylation; Vm, membrane potential.
TRPV4 and Obesity
Obesity is caused by an imbalance between energy intake and consumption, and is a common comorbidity in individuals with type 2 diabetes. Compared with individuals in the healthy weight range, obese men have a sevenfold higher risk of developing type 2 diabetes, whereas obese women have a 12‐fold higher risk 91 . Adipose tissue (AT) was previously thought to be a passive tissue that preserves excessive energy in the form of lipids, and modulates the distribution of lipids in the body 92 . It is now becoming clear that AT can also serve as an endocrine organ, releasing many biologically active factors, such as adipokines, to communicate with different organs and regulate metabolic pathways 93 . In addition, brown and beige AT have been found to sustain euthermia by diffusing energy in the form of heat 94 .
TRPV4 is highly expressed in human preadipocytes, and is involved in lipogenesis through the phosphorylation of protein kinase B. In addition, TRPV4 activation affects ERK1/2 activity, which disrupts normal glucose and lipid metabolism 29 , 30 . Ye et al. 10 showed that Trpv4 deficiency positively regulates white adipocyte “browning” and beige adipocyte differentiation, and TRPV4 knockdown has been found to cause the elevated expression of thermogenic genes, including Ppargc1a and Ucp1, in 3T3‐F442A adipocytes. Furthermore, the activation of TRPV4 has been found to cause the rapid phosphorylation of ERK1/2 and c‐Jun N terminal kinase 1/2, which inhibits thermogenic gene expression. As found in a study carried out in cells 10 , Trpv4 –/– mice have elevated levels of thermogenic genes and lower bodyweight when fed a HFD 25 . Kusudo et al. reported that skeletal muscle metabolic capacity is increased in Trpv4 –/– mice, resulting in resistance to HFD‐induced obesity 25 . The activation of TRPV4 controls resting Ca2+ influx in skeletal muscle, which regulates skeletal muscle contraction 22 . However, O’Conor et al. 28 found that HFD‐fed Trpv4 −/− mice are prone to obesity, which is contradictory to the findings of Kusudo et al. 25 . This inconsistency was probably caused by differences in diet and animal age. In the report by Kusudo et al., mice were fed a HFD with 41.9% kcal for 16 weeks beginning at 4 months‐of‐age (approximately 12 weeks) 25 , whereas a HFD with 60% kcal was administered for 22 weeks beginning at 10 weeks‐of‐age in the study by O’Conor et al. 28 . Nevertheless, the pathophysiological role of TRPV4 in obesity is complicated and further study is warranted. In humans, TRPV4 mRNA levels in peripheral blood leukocytes are markedly reduced in patients with metabolic syndrome 34 .
Studies have shown that the Trpv4 level is significantly increased in the white AT and brown AT of HFD‐induced obese mice and diabetes (db/db) mice 31 , 37 , 44 . In addition, maternal obesity causes a high level of Trpv4 in the white AT of offspring on postnatal day 21 37 . Increased Trpv4 mRNA levels might be the result of macrophage infiltration in AT, as macrophages also express Trpv4, promoting the expression of pro‐inflammatory genes in adipocytes 10 , 95 . Chronic low‐grade inflammation of AT is a trigger for systemic inflammation and insulin resistance 96 . Weight loss procedures, such as exercise and dietary intervention, are able to revert the Trpv4 level in AT back to the normal level 31 , 37 .
The relationship between TRPV4 and obesity‐associated pathologies is still unclear, as very few studies have reported the role of TRPV4 in human adipocytes. Sanchez et al. 38 first carried out an electrophysiological study of TRPV4 in human adipocytes, and confirmed that the cation current in cultured human adipocytes can be activated under hypotonic conditions. This current is mediated by TRPV4 channels and can depolarize cells, thereby increasing Ca2+ levels in adipocytes. In addition, Duan et al. 32 also reported the impact of TRPV4 gene polymorphisms on BMI and body fat mass.
TRPV4 and Diabetes‐Related Cardiovascular Diseases
CVD is one of the major morbidities associated with diabetes. Compared with individuals without diabetes, diabetes patients are two‐ to fourfold more likely to be hospitalized for CVD or CVD‐related clinical events 6 . In addition, CVD is one of the leading causes of death in diabetes patients 97 , and the incidence of cardiovascular mortality in diabetes patients is three‐ to fivefold higher than that in individuals without diabetes 98 . It is now known that endothelial dysfunction in resistance vessels is a hallmark of conditions, such as hypertension and vascular complications, associated with diabetes 99 .
TRPV4 is expressed in the endothelium of small mesenteric arteries and is involved in the regulation of endothelial Ca2+ levels 8 , 20 . In vitro experiments have shown that the activation of TRPV4 by mild hypothermia leads to the production of acetylcholine by endothelial cells, which activates muscarinic receptors in adjacent cells to promote endothelium‐dependent relaxation 36 . In addition, in Trpv4 –/– mice, acetylcholine‐induced hyperpolarization and vasodilation are reduced by approximately 75% in mesenteric resistance arteries 19 . Vascular smooth muscle tone is dynamically regulated by vasoactive substances secreted by the endothelium 10 . Luminal flow in normal animals results in endothelial‐dependent dilation through the release of NO and endothelium‐derived hyperpolarizing factor; however, in Trpv4 –/– mice, these responses are significantly reduced 15 , 101 . Although the basal blood pressure of wild‐type and Trpv4 –/– mice is comparable, transient hypertension induced t the suppression of NO synthase is higher in Trpv4 –/– mice, suggesting that the activation of Trpv4 counteracts hypertensive stimuli in vivo 19 . In normal mice, 11,12‐EET (11,12‐epoxyeicosatrienoic acid) induces the hyperpolarization of vascular smooth muscle cell membranes and the vasodilation of mesenteric arteries, but after the activity of Trpv4 in the endothelium is disrupted, hyperpolarization and vasodilation induced by 11,12‐EET are decreased by half 19 . Likewise, the 11,12‐EET‐induced vasodilation response is also markedly reduced in the tissues of diabetic animals or in tissues incubated with the TRPV4 inhibitor ruthenium red 40 .
Ma et al. 27 showed that the colocalization of Trpv4 with Ca2+‐sensitive K+ channels 2.3 (potassium calcium‐activated channel subfamily N member 3 [Kcnn3]), which induces membrane hyperpolarization and vascular relaxation in smooth muscle, occurred in mesenteric artery endothelial cells in rats. However, the endothelial expression of Trpv4 and the downstream Kcnn3 is decreased in the mesenteric arteries from streptozotocin‐induced diabetic rats, and Trpv4 expression is also decreased in microvascular endothelium cultured in a hyperglycemic environment 27 . Furthermore, Matsumoto et al. 43 found that vasodilation responses induced by various TRPV4 activating factors, such as acetylcholine, GSK1016790A and NS309, are impaired in the superior mesenteric arteries of female Otsuka Long‐Evans Tokushima Fatty rats, a diabetic rat strain. TRPV4 interacts with the protein fibronectin type III domain‐containing 5 47 , an exercise‐induced myokine with vasoprotective effects on endothelial function; however, its endothelium‐dependent vasodilation can be abolished by TRPV4 antagonist 102 , 103 . These findings suggest that the dysregulation of TRPV4 activity contributes to vasodilation dysfunction in cardiovascular diseases.
TRPV4 and Diabetic Nephropathy
Approximately 20–40% of diabetes patients develop microalbuminuria, a precursor to diabetic nephropathy 14 , and approximately 30–40% of them further develop diabetic nephropathy 15 , which is the main cause of end‐stage renal disease 16 . In diabetes patients, the elevated blood glucose tends to be excreted into the urine, which induces osmotic drag to increase tubular flow, and increases the formation of hyperosmotic urine. These physical alterations induce renal epithelial cell contractions and eventually trigger compensatory mechanisms to restore cell volume 12 . TRPV4 is widely distributed in the renal vasculature, and is considered to be a sensor or transducer that senses the mechanical stress caused by the flow of liquid into the collecting duct of the kidney 16 , 24 , 43 . The rise of the cytosolic Ca2+ concentration through TRPV4 activation after hypo‐osmotic fluid‐induced cell swelling has been shown in various volume‐regulating cells, such as bladder urothelial cells, chondrocytes and bronchial epithelial cells 107 , 108 , 109 . Therefore, TRPV4 is a potential mediator of osmoreceptors, which play a role in cell volume recovery 24 .
Mechanosensitive TRPV4 channels regulate cell volume by Ca2+‐dependent mechanisms 110 . Human collecting duct (HCD) cells express TRPV4 14 , and mechanical stimulation activates TRPV4, which leads to the elevation of the Ca2+ level in HCD cells to induce a regulatory volume decrease 111 . Recent studies have shown a decrease in TRPV4 levels in HCD cells when they are cultivated in a high‐glucose environment, undermining TRPV4‐mediated regulatory volume decrease 12 . Furthermore, Trpv4 knockdown eliminates changes in cellular Ca2+ levels caused by mechanical stimulation and inhibits the ability of HCD cells to respond to membrane deformation caused by osmotic or mechanical stress 12 . Cell volume regulation plays an important role in maintaining the overall integrity and function of nephrons. Failure to properly respond to osmotic stimuli might have adverse outcomes for fluid and electrolyte balance in the kidney, which eventually leads to various kidney conditions, such as diabetic nephropathy and end‐stage renal disease.
TRPV4 and Diabetic Retinopathy
Approximately one‐third of diabetes patients might develop diabetic retinopathy, which is the primary reason for blindness associated with diabetes 112 . Non‐proliferative diabetic retinopathy and proliferative diabetic retinopathy are the two main categories of diabetes‐associated retinopathy 113 , and they have incidences of approximately 27.9% and 7.5%, respectively, among diabetes patients 114 . Non‐proliferative diabetic retinopathy is a disorder without neovascularization, and might progress to proliferative diabetic retinopathy with neovascularization. Macular edema with swelling or thickening of the macula was attributed to sub‐ and intraretinal fluid accumulation in the macula triggered by the breakdown of the blood–retinal barrier (BRB), which can occur in non‐proliferative diabetic retinopathy 115 , is the most common cause of vision loss 116 .
The BRB is formed by vascular endothelial (inner layer) and retinal pigment epithelial (outer layer) cells 117 . Under normal circumstances, the inner layer of the BRB is formed by close‐fitting intraretinal vascular endothelial cells and thereby restricts the entry of liquid. High‐glucose conditions damage the integrity of vascular endothelial and retinal pigment epithelial cells, which increases the permeability of the retinal barrier and leads to protein leakage into the interstitial retinal tissue 118 , 119 , 120 , 121 . Recent studies have found that TRPV4 participates in regulating BRB permeability in retinal microvascular endothelial cells and retinal pigment epithelial cells 13 , 35 , 39 . For example, Arredondo et al. 39 observed that BRB breakdown can be attenuated by Trpv4‐selective antagonists, including RN‐1734 and GSK2193874, in diabetic rats. Under diabetic conditions, BRB breakdown is aggravated by increased water diffusion into the retina, which can be stopped by inhibiting TRPV4 activity 49 . These results suggest that TRPV4 is involved in maintaining the homeostasis and structural integrity of the retina 49 . In hyperglycemia and diabetes, TRPV4 activity is decreased in retinal microvascular endothelial cells 13 . The activation of TRPV4 induces inflammatory responses that result in edema 122 , 123 , 124 , 125 , which can be resolved by using TRPV4 inhibitors 126 , 127 . TRPV4 is expressed in human corneal epithelial cells 21 , and the activation of TRPV4 results in Ca2+ influx, which might induce regulatory volume decrease 18 . Therefore, a decrease in TRPV4 levels during hyperglycemia and diabetes might be a compensatory mechanism aimed at maintaining retinal homeostasis.
The main pathogenesis of diabetic retinopathy involves not only structural alterations in retinal blood vessels, but also the dysfunction of perivascular neurons or glial tissue 128 . Using continuous fixed‐focus electroretinography, retinal ganglion cells (RGCs) and bipolar cells were shown to exhibit abnormal activity in early diabetic retinopathy. In addition, RGCs and Müller cells, a type of retinal glial cell, show increased apoptosis 129 . Trpv4 is expressed in RGCs, Müller cells and the optic nerve head in mice 42 , 46 . Cholesterol molecules act as sentinels for metabolic, osmotic, mechanical and inflammatory signals within the retina, and are involved in regulating a range of pathophysiological functions, such as the loss of RGCs and photoreceptors, the hypertrophy and pathological swelling of Müller cells, and the maintenance of the BRB. Lakk et al. 42 reported that Trpv4 can mediate cholesterol‐dependent multimodal transduction in Müller cells. Furthermore, hypercholesterolemic retinas show similar pathologies, including reactive gliosis, elevated retinal microvascular endothelial barrier permeability, RGC degeneration and pathological glial swelling, with excessive TRPV4 activation 42 . A study by Ryskamp et al. 23 showed that TRPV4 activation in RGCs mediates responses to membrane stretching, resulting in elevated Ca 2 + levels in the cells and augmented excitability. Sustained exposure to a TRPV4 agonist leads to excessive Ca2+ influx, which might activate Ca2+‐dependent pro‐apoptotic signaling pathways and induce time‐ and dose‐dependent apoptosis in RGCs 23 .
TRPV4 and Diabetic Neuropathic Pain
Diabetic sensorimotor polyneuropathy is the most common diabetes‐associated microvascular complication, and develops in 10–54% of diabetes patients 130 , approximately one‐third of which will develop diabetic neuropathy 131 . Patients with neuropathic pain can experience spontaneous and stimulus‐induced pain, including hyperalgesia and allodynia 132 . Mechanical allodynia often manifests as an adverse reaction to innocuous stimuli, including the touch of bedsheets, clothing and shoes, or can be activated by slight motion, such as walking and position adjustment 133 . Therefore, diabetic neuropathic pain has a markedly negative impact on quality of life 134 .
A cluster of primary somatosensory neurons, also called nociceptors, originating from the trigeminal ganglion and the dorsal root ganglion (DRG) are responsible for regulating the ability to perceive pain 133 . TRPV4 is expressed in the DRG and trigeminal ganglion, and serves as a detector and transducer in nociceptive neurons 135 . TRPV4 has been reported to maintain neuropathic pain induced by alcohol, vincristine, paclitaxel, 2'‐3'‐dideoxycytidine and the chronic compression of the DRG 11 , 136 , 137 . Dias et al. 48 showed that HC‐067047, a TRPV4 inhibitor, can hinder the progression of mechanical allodynia in diabetic mice induced by streptozotocin. Similarly, streptozotocin‐induced mechanical hyperalgesia is also weakened in Trpv4 –/– mice 11 . These findings suggest that the reduced TRPV4 activity can modulate the hyperactivity of mechanosensitive afferent nerves and effectively prevent diabetes‐induced mechanical allodynia.
Immunohistochemical analysis shows no difference in the level of Trpv4 in the hind paw skin, sciatic nerve, and DRG between diabetic and non‐diabetic mice 48 . As TRPV4 is constitutively expressed and capable of spontaneous activation in the absence of agonist stimulation, Dias et al. 48 suggested that TRPV4 can transduce neuropathic pain independent of the TRPV4 level. In fact, TRPV4 has been shown to be constitutively active, and might be involved in controlling neuronal excitability 138 . Furthermore, sensory nerve damage, inflammation, swelling and mechanical stimulation can trigger an integrin signal cascade transmitted by SRC family tyrosine kinases that causes the membrane insertion and activation of TRPV4 without changing the TRPV4 level 11 , 45 . Furthermore, protein kinase C is activated in the DRG neurons of monoiodoacetate‐induced arthritis rats, causing the sensitization of Trpv4 and Trpv1, which are coexpressed in the DRG, thereby aggravating pain signaling 139 , 140 , 141 , 142 . However, Alexander et al. 26 showed by immunohistochemical analysis that TRPV4 is not expressed in cultured DRG and trigeminal ganglion neurons, and that there is no difference in the Ca2+ concentration induced by hypo‐osmotic solution or 4αPDD between neurons from wild‐type and Trpv4 –/– mice. Approximately 73% of Trpv4 expression in mouse dorsal root ganglia is derived from satellite glial cells, which form a sheath around the neurons of the sensory ganglia, and can regulate pain and inflammation‐related neuronal excitability 33 . TRPV4 is also expressed in other nonneuronal cells, such as skin keratinocytes, and regulates nociception, itching and inflammation 33 , 143 , 144 , 145 . Collectively, these findings imply that TRPV4 plays an important role in diabetic neuropathic pain.
Conclusion and Perspective
TRPV4 has been shown to play important roles in various diseases. TRPV4 is constitutively expressed and capable of spontaneous activation without stimulation, which is in accordance with its participation in controlling homeostatic functions through the regulation of cellular Ca2+ levels, and intracellular and systemic water balance. TRPV4 is expressed in different cell types throughout the body, which indicates its diverse activities under normal physiological conditions. For example, TRPV4 is highly expressed in the cilia of the bronchial epithelium in the lung, and is involved in mucociliary transport and ciliary beat frequency 146 . In addition, it is also found in osteoclasts, osteoblasts and chondrocytes, and is probably involved in bone remodeling and development 147 . It has been shown that TRPV4 activity is increased in hippocampal astrocytes after ischemia and hypoxia, which suggests its involvement in poststroke oxidative stress‐associated cell activities 148 , 149 .
Regarding its involvement in type 2 diabetes, TRPV4 is expressed in pancreatic ‐cells, and is involved in insulin secretion and ‐cell apoptosis 9 , 41 . In addition, it is abundant in the vascular endothelium and smooth muscle of arteries, which suggests its involvement in vascular tone 19 , 20 . TRPV4 is also expressed in epithelial cells of the nephron and urothelial cells of the bladder, where it plays a role in osmosensation 107 , 150 . In sensory neurons, TRPV4 is expressed in the DRG and is involved in pain perception 135 . Accordingly, TRPV4 seems to be an important protein involved in type 2 diabetes and type 2 diabetes‐associated complications.
It seems difficult to develop TRPV4 as a therapeutic target for specific diseases, such as type 2 diabetes, because of its ubiquitous expression and involvement in various pathophysiological processes. However, there are several clinical trials investigating TRPV4 antagonists for different conditions, including a trial studying the use of GSK2798745 for congestive heart failure. The results of these trials will allow us to evaluate the feasibility of using TRPV4 as a therapeutic target for specific diseases and the implications of its off‐target effects 70 .
Based on the current findings, TRPV4 agonists have insulinotropic activities, but the continuous activation of TRPV4 leads to ‐cell dysfunction and death 9 , 41 . TRPV4 reduces obesity and insulin resistance induced by HFD, and alleviates the development of diabetic complications, including diabetic nephropathy, retinopathy and neuropathy 10 , 11 , 25 , 39 . Therefore, TRPV4 has apparent clinical potential for managing diabetes and diabetic complications. However, the development of TRPV4 as a therapeutic target for type 2 diabetes might face multiple challenges associated with its interesting biological properties, including its wide expression profile and some contradictory indications.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
National Science Foundation of China (No. 81773312) (YH), Natural Science Foundation of Guangdong Province (No. 2015A030313517) (YH), Talents Recruitment Grant of “Yangfan Plan” of Guangdong (No. 201433005) (YH), Science & Technology Program of Guangdong Province (No. c173191900055) (YH), International Science & Technology Cooperation Program of Guangzhou Development District (No. 2017GH17) (YH), Science & Technology Program of Guangzhou (No. 201704030073) (Y.H.), Science and Technology Basic Research Program of Guangdong (No. 2017A020215061) (DY) and “Group‐type” Special Supporting Project for Educational Talents in Universities (No. 4SG19046G).
J Diabetes Investig 2020; 11: 757–769
References
- 1. Cho NH, Shaw JE, Karuranga S, et al IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018; 138: 271–281. [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Gao P, Zhang M, et al Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32(Suppl 1): S62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013; 93: 137–188. [DOI] [PubMed] [Google Scholar]
- 5. Lotfy M, Adeghate J, Kalasz H, et al Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev 2017; 13: 3–10. [DOI] [PubMed] [Google Scholar]
- 6. Harding JL, Pavkov ME, Magliano DJ, et al Global trends in diabetes complications: a review of current evidence. Diabetologia 2019; 62: 3–16. [DOI] [PubMed] [Google Scholar]
- 7. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White JP, Cibelli M, Urban L, et al TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 2016; 96: 911–973. [DOI] [PubMed] [Google Scholar]
- 9. Skrzypski M, Kakkassery M, Mergler S, et al Activation of TRPV4 channel in pancreatic INS‐1E beta cells enhances glucose‐stimulated insulin secretion via calcium‐dependent mechanisms. FEBS Lett 2013; 587: 3281–3287. [DOI] [PubMed] [Google Scholar]
- 10. Ye L, Kleiner S, Wu J, et al TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 2012; 151: 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alessandri‐Haber N, Dina OA, Joseph EK, et al Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 2008; 28: 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hills CE, Bland R, Squires PE. Functional expression of TRPV4 channels in human collecting duct cells: implications for secondary hypertension in diabetic nephropathy. Exp Diabetes Res 2012; 2012: 936518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monaghan K, McNaughten J, McGahon MK, et al Hyperglycemia and diabetes downregulate the functional expression of TRPV4 channels in retinal microvascular endothelium. PLoS One 2015; 10: e0128359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hills CE, Bland R, Wheelans DC, et al Glucose‐evoked alterations in connexin43‐mediated cell‐to‐cell communication in human collecting duct: a possible role in diabetic nephropathy. Am J Physiol Renal Physiol 2006; 291: F1045–1051. [DOI] [PubMed] [Google Scholar]
- 15. Kohler R, Heyken WT, Heinau P, et al Evidence for a functional role of endothelial transient receptor potential V4 in shear stress‐induced vasodilatation. Arterioscler Thromb Vasc Biol 2006; 26: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 16. Wu L, Gao X, Brown RC, et al Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 2007; 293: F1699–F1713. [DOI] [PubMed] [Google Scholar]
- 17. Casas S, Novials A, Reimann F, et al Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 2008; 51: 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan Z, Yang H, Mergler S, et al Dependence of regulatory volume decrease on transient receptor potential vanilloid 4 (TRPV4) expression in human corneal epithelial cells. Cell Calcium 2008; 44: 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Earley S, Pauyo T, Drapp R, et al TRPV4‐dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 2009; 297: H1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mendoza SA, Fang J, Gutterman DD, et al TRPV4‐mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 2010; 298: H466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mergler S, Valtink M, Taetz K, et al Characterization of transient receptor potential vanilloid channel 4 (TRPV4) in human corneal endothelial cells. Exp Eye Res 2011; 93: 710–719. [DOI] [PubMed] [Google Scholar]
- 22. Pritschow BW, Lange T, Kasch J, et al Functional TRPV4 channels are expressed in mouse skeletal muscle and can modulate resting Ca2+ influx and muscle fatigue. Pflugers Arch 2011; 461: 115–122. [DOI] [PubMed] [Google Scholar]
- 23. Ryskamp DA, Witkovsky P, Barabas P, et al The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci 2011; 31: 7089–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berrout J, Jin M, Mamenko M, et al Function of transient receptor potential cation channel subfamily V member 4 (TRPV4) as a mechanical transducer in flow‐sensitive segments of renal collecting duct system. J Biol Chem 2012; 287: 8782–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kusudo T, Wang Z, Mizuno A, et al TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet‐induced obesity. J Appl Physiol 1985; 2012(112): 1223–1232. [DOI] [PubMed] [Google Scholar]
- 26. Alexander R, Kerby A, Aubdool AA, et al 4alpha‐phorbol 12,13‐didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br J Pharmacol 2013; 168: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma X, Du J, Zhang P, et al Functional role of TRPV4‐KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin‐induced diabetic rats. Hypertension 2013; 62: 134–139. [DOI] [PubMed] [Google Scholar]
- 28. O’Conor CJ, Griffin TM, Liedtke W, et al Increased susceptibility of Trpv4‐deficient mice to obesity and obesity‐induced osteoarthritis with very high‐fat diet. Ann Rheum Dis 2013; 72: 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Che H, Yue J, Tse HF, et al Functional TRPV and TRPM channels in human preadipocytes. Pflugers Arch 2014; 466: 947–959. [DOI] [PubMed] [Google Scholar]
- 30. Olah A, Toth BI, Borbiro I, et al Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest 2014; 124: 3713–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen N, Cheng J, Zhou L, et al Effects of treadmill running and rutin on lipolytic signaling pathways and TRPV4 protein expression in the adipose tissue of diet‐induced obese mice. J Physiol Biochem 2015; 71: 733–742. [DOI] [PubMed] [Google Scholar]
- 32. Duan DM, Wu S, Hsu LA, et al Associations between TRPV4 genotypes and body mass index in Taiwanese subjects. Mol Genet Genomics 2015; 290: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 33. Rajasekhar P, Poole DP, Liedtke W, et al P2Y1 receptor activation of the TRPV4 Ion channel enhances purinergic signaling in satellite glial cells. J Biol Chem 2015; 290: 29051–29062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tabur S, Oztuzcu S, Duzen IV, et al Role of the transient receptor potential (TRP) channel gene expressions and TRP melastatin (TRPM) channel gene polymorphisms in obesity‐related metabolic syndrome. Eur Rev Med Pharmacol Sci 2015; 19: 1388–1397. [PubMed] [Google Scholar]
- 35. Zhao PY, Gan G, Peng S, et al TRP channels localize to subdomains of the apical plasma membrane in human fetal retinal pigment epithelium. Invest Ophthalmol Vis Sci 2015; 56: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zou Q, Leung SW, Vanhoutte PM. Transient receptor potential channel opening releases endogenous acetylcholine, which contributes to endothelium‐dependent relaxation induced by mild hypothermia in spontaneously hypertensive rat but not Wistar‐Kyoto rat arteries. J Pharmacol Exp Ther 2015; 354: 121–130. [DOI] [PubMed] [Google Scholar]
- 37. Janoschek R, Bae‐Gartz I, Vohlen C, et al Dietary intervention in obese dams protects male offspring from WAT induction of TRPV4, adiposity, and hyperinsulinemia. Obesity (Silver Spring) 2016; 24: 1266–1273. [DOI] [PubMed] [Google Scholar]
- 38. Sanchez JC, Rivera RA, Munoz LV. TRPV4 channels in human white adipocytes: electrophysiological characterization and regulation by insulin. J Cell Physiol 2016; 231: 954–963. [DOI] [PubMed] [Google Scholar]
- 39. Arredondo Zamarripa D, Noguez Imm R, Bautista Cortes AM, et al Dual contribution of TRPV4 antagonism in the regulatory effect of vasoinhibins on blood‐retinal barrier permeability: diabetic milieu makes a difference. Sci Rep 2017; 7: 13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bihzad SM, Yousif MH. 11,12‐Epoxyeicosatrienoic acid induces vasodilator response in the rat perfused mesenteric vasculature. Auton Autacoid Pharmacol 2017; 37: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Billert M, Skrzypski M, Sassek M, et al TRPV4 regulates insulin mRNA expression and INS‐1E cell death via ERK1/2 and NO‐dependent mechanisms. Cell Signal 2017; 35: 242–249. [DOI] [PubMed] [Google Scholar]
- 42. Lakk M, Yarishkin O, Baumann JM, et al Cholesterol regulates polymodal sensory transduction in Muller glia. Glia 2017; 65: 2038–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matsumoto T, Kobayashi S, Ando M, et al Impaired endothelium‐derived hyperpolarization‐type relaxation in superior mesenteric arteries isolated from female Otsuka Long‐Evans Tokushima Fatty rats. Eur J Pharmacol 2017; 807: 151–158. [DOI] [PubMed] [Google Scholar]
- 44. Sun W, Li C, Zhang Y, et al Gene expression changes of thermo‐sensitive transient receptor potential channels in obese mice. Cell Biol Int 2017; 41: 908–913. [DOI] [PubMed] [Google Scholar]
- 45. Hinata M, Imai S, Sanaki T, et al Sensitization of transient receptor potential vanilloid 4 and increasing its endogenous ligand 5,6‐epoxyeicosatrienoic acid in rats with monoiodoacetate‐induced osteoarthritis. Pain 2018; 159: 939–947. [DOI] [PubMed] [Google Scholar]
- 46. Lakk M, Young D, Baumann JM, et al Polymodal TRPV1 and TRPV4 sensors colocalize but do not functionally interact in a subpopulation of mouse retinal ganglion cells. Front Cell Neurosci 2018; 12: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ye L, Xu M, Hu M, et al TRPV4 is involved in irisin‐induced endothelium‐dependent vasodilation. Biochem Biophys Res Commun 2018; 495: 41–45. [DOI] [PubMed] [Google Scholar]
- 48. Dias FC, Alves VS, Matias DO, et al The selective TRPV4 channel antagonist HC‐067047 attenuates mechanical allodynia in diabetic mice. Eur J Pharmacol 2019; 856: 172408. [DOI] [PubMed] [Google Scholar]
- 49. Orduna Rios M, Noguez Imm R, Hernandez Godinez NM, et al TRPV4 inhibition prevents increased water diffusion and blood‐retina barrier breakdown in the retina of streptozotocin‐induced diabetic mice. PLoS One 2019; 14: e0212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liedtke W, Choe Y, Marti‐Renom MA, et al Vanilloid receptor‐related osmotically activated channel (VR‐OAC), a candidate vertebrate osmoreceptor. Cell 2000; 103: 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol 2014; 171: 2474–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han H, Yi F. New insights into TRP channels: Interaction with pattern recognition receptors. Channels (Austin) 2014; 8: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Darby WG, Grace MS, Baratchi S, et al Modulation of TRPV4 by diverse mechanisms. Int J Biochem Cell Biol 2016; 78: 217–228. [DOI] [PubMed] [Google Scholar]
- 54. Strotmann R, Harteneck C, Nunnenmacher K, et al OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2000; 2: 695–702. [DOI] [PubMed] [Google Scholar]
- 55. Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4‐/‐ mice. Proc Natl Acad Sci U S A 2003; 100: 13698–13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hill‐Eubanks DC, Gonzales AL, Sonkusare SK, et al Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 2014; 29: 343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhan L, Yang Y, Ma TT, et al Transient receptor potential vanilloid 4 inhibits rat HSC‐T6 apoptosis through induction of autophagy. Mol Cell Biochem 2015; 402: 9–22. [DOI] [PubMed] [Google Scholar]
- 58. Grace MS, Bonvini SJ, Belvisi MG, et al Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol Ther 2017; 177: 9–22. [DOI] [PubMed] [Google Scholar]
- 59. Bang S, Yoo S, Yang TJ, et al Nociceptive and pro‐inflammatory effects of dimethylallyl pyrophosphate via TRPV4 activation. Br J Pharmacol 2012; 166: 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adapala RK, Talasila PK, Bratz IN, et al PKCalpha mediates acetylcholine‐induced activation of TRPV4‐dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol 2011; 301: H757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vriens J, Owsianik G, Fisslthaler B, et al Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 2005; 97: 908–915. [DOI] [PubMed] [Google Scholar]
- 62. Watanabe H, Vriens J, Prenen J, et al Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003; 424: 434–438. [DOI] [PubMed] [Google Scholar]
- 63. Ma X, He D, Ru X, et al Apigenin, a plant‐derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br J Pharmacol 2012; 166: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smith PL, Maloney KN, Pothen RG, et al Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem 2006; 281: 29897–29904. [DOI] [PubMed] [Google Scholar]
- 65. Cheung M, Bao W, Behm DJ, et al Discovery of GSK2193874: an orally active, potent, and selective blocker of transient receptor potential vanilloid 4. ACS Med Chem Lett 2017; 8: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mola MG, Sparaneo A, Gargano CD, et al The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: A different point of view on the role of aquaporins. Glia 2016; 64: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vincent F, Acevedo A, Nguyen MT, et al Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 2009; 389: 490–494. [DOI] [PubMed] [Google Scholar]
- 68. Nilius B, Vriens J, Prenen J, et al TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol 2004; 286: C195–205. [DOI] [PubMed] [Google Scholar]
- 69. Thorneloe KS, Sulpizio AC, Lin Z, et al N‐((1S)‐1‐{[4‐((2S)‐2‐{[(2,4‐dichlorophenyl)sulfonyl]amino}‐3‐hydroxypropanoyl)‐1 ‐piperazinyl]carbonyl}‐3‐methylbutyl)‐1‐benzothiophene‐2‐carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther 2008; 326: 432–442. [DOI] [PubMed] [Google Scholar]
- 70. Goyal N, Skrdla P, Schroyer R, et al Clinical pharmacokinetics, safety, and tolerability of a novel, first‐in‐class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects. Am J Cardiovasc Drugs 2019; 19: 335–342. [DOI] [PubMed] [Google Scholar]
- 71. Marrif HI, Al‐Sunousi SI. Pancreatic beta cell mass death. Front Pharmacol 2016; 7: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic alpha‐ and beta‐cells in health and disease. Cell Calcium 2012; 51: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shen W, Taylor B, Jin Q, et al Inhibition of DYRK1A and GSK3B induces human beta‐cell proliferation. Nat Commun 2015; 6: 8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jacobson D, Shyng SL. Ion Channels of the Islets in Type 2 Diabetes. J Mol Biol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang SN, Berggren PO. Beta‐cell CaV channel regulation in physiology and pathophysiology. Am J Physiol Endocrinol Metab 2005; 288: E16–E28. [DOI] [PubMed] [Google Scholar]
- 76. Komatsu M, Takei M, Ishii H, et al Glucose‐stimulated insulin secretion: a newer perspective. J Diabetes Investig 2013; 4: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Davies SL, Brown PD, Best L. Glucose‐induced swelling in rat pancreatic alpha‐cells. Mol Cell Endocrinol 2007; 264: 61–67. [DOI] [PubMed] [Google Scholar]
- 78. Takii M, Ishikawa T, Tsuda H, et al Involvement of stretch‐activated cation channels in hypotonically induced insulin secretion in rat pancreatic beta‐cells. Am J Physiol Cell Physiol 2006; 291: C1405–1411. [DOI] [PubMed] [Google Scholar]
- 79. Islam MS. Calcium signaling in the islets. Adv Exp Med Biol 2010; 654: 235–259. [DOI] [PubMed] [Google Scholar]
- 80. Sawatani T, Kaneko YK, Doutsu I, et al TRPV2 channels mediate insulin secretion induced by cell swelling in mouse pancreatic beta‐cells. Am J Physiol Cell Physiol 2019; 316: C434–C443. [DOI] [PubMed] [Google Scholar]
- 81. Leduc M, Richard J, Costes S, et al ERK1 is dispensable for mouse pancreatic beta cell function but is necessary for glucose‐induced full activation of MSK1 and CREB. Diabetologia 2017; 60: 1999–2010. [DOI] [PubMed] [Google Scholar]
- 82. Lawrence MC, McGlynn K, Shao C, et al Chromatin‐bound mitogen‐activated protein kinases transmit dynamic signals in transcription complexes in beta‐cells. Proc Natl Acad Sci U S A 2008; 105: 13315–13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lawrence M, Shao C, Duan L, et al The protein kinases ERK1/2 and their roles in pancreatic beta cells. Acta Physiol (Oxf) 2008; 192: 11–17. [DOI] [PubMed] [Google Scholar]
- 84. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 85. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med 2012; 2012: 918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yeung DC, Xu A, Tso AW, et al Circulating levels of adipocyte and epidermal fatty acid‐binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care 2009; 32: 132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Uchida K, Sun W, Yamazaki J, et al Role of thermo‐sensitive transient receptor potential channels in brown adipose tissue. Biol Pharm Bull 2018; 41: 1135–1144. [DOI] [PubMed] [Google Scholar]
- 88. Moore C, Gupta R, Jordt SE, et al Regulation of pain and itch by TRP channels. Neurosci Bull 2018; 34: 120–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Darby WG, Potocnik S, Ramachandran R, et al Shear stress sensitizes TRPV4 in endothelium‐dependent vasodilatation. Pharmacol Res 2018; 133: 152–159. [DOI] [PubMed] [Google Scholar]
- 90. Marko L, Mannaa M, Haschler TN, et al Renoprotection: focus on TRPV1, TRPV4, TRPC6 and TRPM2. Acta Physiol (Oxf) 2017; 219: 589–612. [DOI] [PubMed] [Google Scholar]
- 91. Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract 2014; 68: 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol 2016; 231: R77–R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol 2007; 2: 31–56. [DOI] [PubMed] [Google Scholar]
- 94. Cypess AM, Lehman S, Williams G, et al Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Khalil M, Alliger K, Weidinger C, et al Functional role of transient receptor potential channels in immune cells and epithelia. Front Immunol 2018; 9: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity‐associated low‐grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med 2013; 71: 174–187. [PubMed] [Google Scholar]
- 97. Abi Khalil C, Roussel R, Mohammedi K, et al Cause‐specific mortality in diabetes: recent changes in trend mortality. Eur J Prev Cardiol 2012; 19: 374–381. [DOI] [PubMed] [Google Scholar]
- 98. Taylor KS, Heneghan CJ, Farmer AJ, et al All‐cause and cardiovascular mortality in middle‐aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care 2013; 36: 2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014; 2014: 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Triggle CR, Samuel SM, Ravishankar S, et al The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol 2012; 90: 713–738. [DOI] [PubMed] [Google Scholar]
- 101. Hartmannsgruber V, Heyken WT, Kacik M, et al Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2007; 2: e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hou N, Han F, Sun X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin Endocrinol (Oxf) 2015; 83: 339–343. [DOI] [PubMed] [Google Scholar]
- 103. Xiang L, Xiang G, Yue L, et al Circulating irisin levels are positively associated with endothelium‐dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis 2014; 235: 328–333. [DOI] [PubMed] [Google Scholar]
- 104. Mora‐Fernandez C, Dominguez‐Pimentel V, de Fuentes MM, et al Diabetic kidney disease: from physiology to therapeutics. J Physiol 2014; 592: 3997–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tesch GH. Diabetic nephropathy ‐ is this an immune disorder? Clin Sci (Lond) 2017; 131: 2183–2199. [DOI] [PubMed] [Google Scholar]
- 106. Persson F, Rossing P. Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int Suppl 2011; 2018(8): 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gevaert T, Vriens J, Segal A, et al Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 2007; 117: 3453–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yellowley CE, Hancox JC, Donahue HJ. Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J Cell Biochem 2002; 86: 290–301. [DOI] [PubMed] [Google Scholar]
- 109. Fernandez‐Fernandez JM, Nobles M, Currid A, et al Maxi K+ channel mediates regulatory volume decrease response in a human bronchial epithelial cell line. Am J Physiol Cell Physiol 2002; 283: C1705–1714. [DOI] [PubMed] [Google Scholar]
- 110. Oku H, Kodama T, Sakagami K, et al Diabetes‐induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci 2001; 42: 1915–1920. [PubMed] [Google Scholar]
- 111. Pasantes‐Morales H, Morales Mulia S. Influence of calcium on regulatory volume decrease: role of potassium channels. Nephron 2000; 86: 414–427. [DOI] [PubMed] [Google Scholar]
- 112. Liew G, Wong VW, Ho IV. Mini review: changes in the incidence of and progression to proliferative and sight‐threatening diabetic retinopathy over the last 30 years. Ophthalmic Epidemiol 2017; 24: 73–80. [DOI] [PubMed] [Google Scholar]
- 113. Solomon SD, Chew E, Duh EJ, et al Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yau JW, Rogers SL, Kawasaki R, et al Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci 2018; 19: E1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Romero‐Aroca P, Baget‐Bernaldiz M, Pareja‐Rios A, et al Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res 2016; 2016: 2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood‐retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013; 34: 19–48. [DOI] [PubMed] [Google Scholar]
- 118. Xu HZ, Le YZ. Significance of outer blood‐retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci 2011; 52: 2160–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Simo R, Villarroel M, Corraliza L, et al The retinal pigment epithelium: something more than a constituent of the blood‐retinal barrier–implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol 2010; 2010: 190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Romero‐Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care 2010; 33: 2484–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes 2018; 67: 1729–1741. [DOI] [PubMed] [Google Scholar]
- 122. Matsumoto K, Yamaba R, Inoue K, et al Transient receptor potential vanilloid 4 channel regulates vascular endothelial permeability during colonic inflammation in dextran sulphate sodium‐induced murine colitis. Br J Pharmacol 2018; 175: 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Okada Y, Shirai K, Miyajima M, et al Loss of TRPV4 function suppresses inflammatory fibrosis induced by alkali‐burning mouse corneas. PLoS One 2016; 11: e0167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vergnolle N, Cenac N, Altier C, et al A role for transient receptor potential vanilloid 4 in tonicity‐induced neurogenic inflammation. Br J Pharmacol 2010; 159: 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Reiter B, Kraft R, Gunzel D, et al TRPV4‐mediated regulation of epithelial permeability. FASEB J 2006; 20: 1802–1812. [DOI] [PubMed] [Google Scholar]
- 126. Zhao H, Zhang K, Tang R, et al TRPV4 blockade preserves the blood‐brain barrier by inhibiting stress fiber formation in a rat model of intracerebral hemorrhage. Front Mol Neurosci 2018; 11: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lu KT, Huang TC, Tsai YH, et al Transient receptor potential vanilloid type 4 channels mediate Na‐K‐Cl‐co‐transporter‐induced brain edema after traumatic brain injury. J Neurochem 2017; 140: 718–727. [DOI] [PubMed] [Google Scholar]
- 128. Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Palmowski AM, Sutter EE, Bearse MA Jr, et al Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci 1997; 38: 2586–2596. [PubMed] [Google Scholar]
- 130. Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ 2014; 348: g1799. [DOI] [PubMed] [Google Scholar]
- 131. Abbott CA, Malik RA, van Ross ER, et al Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care 2011; 34: 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gandhi RA, Selvarajah D. Understanding and treating painful diabetic neuropathy: time for a paradigm shift. Diabet Med 2015; 32: 771–777. [DOI] [PubMed] [Google Scholar]
- 133. Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest 2010; 120: 3760–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vinik AI, Nevoret ML, Casellini C, et al Diabetic neuropathy. Endocrinol Metab Clin North Am 2013; 42: 747–787. [DOI] [PubMed] [Google Scholar]
- 135. Mickle AD, Shepherd AJ, Mohapatra DP. Sensory TRP channels: the key transducers of nociception and pain. Prog Mol Biol Transl Sci 2015; 131: 73–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Qu YJ, Zhang X, Fan ZZ, et al Effect of TRPV4‐p38 MAPK pathway on neuropathic pain in rats with chronic compression of the dorsal root ganglion. Biomed Res Int 2016; 2016: 6978923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen Y, Yang C, Wang ZJ. Proteinase‐activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel‐induced neuropathic pain. Neuroscience 2011; 193: 440–451. [DOI] [PubMed] [Google Scholar]
- 138. Shibasaki K, Sugio S, Takao K, et al TRPV4 activation at the physiological temperature is a critical determinant of neuronal excitability and behavior. Pflugers Arch 2015; 467: 2495–2507. [DOI] [PubMed] [Google Scholar]
- 139. Koda K, Hyakkoku K, Ogawa K, et al Sensitization of TRPV1 by protein kinase C in rats with mono‐iodoacetate‐induced joint pain. Osteoarthritis Cartilage 2016; 24: 1254–1262. [DOI] [PubMed] [Google Scholar]
- 140. Kim S, Barry DM, Liu XY, et al Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci Signal 2016; 9: ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem 2009; 284: 27884–27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cao DS, Yu SQ, Premkumar LS. Modulation of transient receptor potential Vanilloid 4‐mediated membrane currents and synaptic transmission by protein kinase C. Mol Pain 2009; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Basbaum AI, Bautista DM, Scherrer G, et al Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chung MK, Lee H, Mizuno A, et al TRPV3 and TRPV4 mediate warmth‐evoked currents in primary mouse keratinocytes. J Biol Chem 2004; 279: 21569–21575. [DOI] [PubMed] [Google Scholar]
- 145. Mihara H, Boudaka A, Sugiyama T, et al Transient receptor potential vanilloid 4 (TRPV4)‐dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol 2011; 589: 3471–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lorenzo IM, Liedtke W, Sanderson MJ, et al TRPV4 channel participates in receptor‐operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A 2008; 105: 12611–12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Masuyama R, Vriens J, Voets T, et al TRPV4‐mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab 2008; 8: 257–265. [DOI] [PubMed] [Google Scholar]
- 148. Bai JZ, Lipski J. Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress‐induced cell death in organotypic hippocampal culture. Neurotoxicology 2010; 31: 204–214. [DOI] [PubMed] [Google Scholar]
- 149. Butenko O, Dzamba D, Benesova J, et al The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 2012; 7: e39959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tian W, Salanova M, Xu H, et al Renal expression of osmotically responsive cation channel TRPV4 is restricted to water‐impermeant nephron segments. Am J Physiol Renal Physiol 2004; 287: F17–F24. [DOI] [PubMed] [Google Scholar]


