PREFACE
Objectives of the current guideline for the management of diabetes
The current guideline represents the 6th edition of the ‘Japanese Clinical Practice Guideline for Diabetes’ which has been revised every three years since its first appearance in 2004 to promote evidence‐based, rational, efficient and consistent clinical practice in diabetes.
Of note, dramatic progress has been made in recent years in diabetes research and clinical practice, which includes approval of antidiabetic agents with novel mechanisms of action along with publication of clinical trial results with these drugs, and novel diagnostic and therapeutic devices, such as continuous glucose monitoring (CGM) and sensor augmented pumps (SAP). Again, results from large‐scale clinical trials in Japan, such as J‐DOIT 1 to 3 and JDCP studies, have recently been reported. Further, in the last three years, new guidelines for lipid and blood pressure control have been released in a timely fashion from the Japan Atherosclerosis Society and the Japanese Society of Hypertension. Therefore, the current guideline has been compiled to include not only relevant advances in clinical practice but novel findings and new lines of evidence that have been made available to date.
While the current guideline has been organized along similar lines to those of the preceding 2016 edition and using the same clinical questions (CQs) and questions (Qs) format, each CQ or Q has been closely reviewed for revision and further CQs or Qs have been added as appropriate to further promote the use of the guidelines in clinical practice. Readers are therefore referred to the ‘Methods of developing the “Japanese Clinical Practice Guideline for Diabetes 2019”’ for a detailed account of the guideline development processes involved to make effective use of the current guideline.
It is hoped that the guideline will prove a helpful guide to evidence‐based medicine (EBM) in clinical settings thereby contributing not only to prolongation of healthy lifespan but to improved quality of life in patients with diabetes.
METHODS OF DEVELOPING THE ‘JAPANESE CLINICAL PRACTICE GUIDELINE FOR DIABETES 2019’
The guideline consists of general questions (cited as Qs) and clinical questions (cited as CQs) followed by explanations. Statements of recommendation were developed solely for CQs. Clinical guideline committee (CGC) members conducted systematic review (SR) of evidence from several resources to develop a statement of recommendation for CQs and presented a strength of recommendation rated as a grade. SR support team helped CGC members to make literature retrieval and confirm an evidence level for articles that they obtained. A brief criterion of the literature retrieval process was shown in this guideline. We referred to all the important articles necessary for the judgement of a statement and its strength of recommendation for CQs.
Abstract tables were constructed solely for the articles necessary to recommend a statement for CQs. They contained relevant articles with PICO (Populations, Interventions, Comparators, Outcomes of interest), study design, and evidence level as defined in Table 1. The quality of evidence was also summarized based on 5 items for meta‐analysis or systematic review, and 3 items for randomized controlled trial as shown in Table 1. The grade of recommendation was determined by each CGC member with consideration given to certainty of overall evidence, balance of benefits and harms, patient preferences/values, and costs (Table 2). Grades A and B stand for strong and weak recommendations, respectively. The CGC members reviewed and discussed all CQ guidelines. Votes were taken for each recommendation statement. A 75% agreement among eligible CGC members was required to approve each recommendation and its strength.
Table 1.
Study designs and their levels of evidence
| Study design | Level of evidence † |
|---|---|
| Meta‐analysis or systematic review (MA/SR) | |
| High‐quality | 1+ |
|
Satisfies all of the following 5 items:
|
|
| Low‐quality | 2 |
| Otherwise | |
| Randomized controlled trial (RCT) | |
| High‐quality | 1 |
|
Satisfies all of the following 3 items:
|
|
| Low‐quality | 2 |
| Otherwise | |
| Prospective cohort study | 2 |
| Pre‐specified sub‐analysis of RCT | 2 |
| Retrospective cohort study | 3 |
| Case‐control study | 3 |
| Post hoc sub‐analysis of RCT | 3 |
| Single‐arm trial | 3 |
| Cross‐sectional study | 3 |
| Case series or case report | 3 |
Level of evidence: 1+ (highest) to 3 (lowest).
Table 2.
Grading for the strength of recommendation
| Strength of recommendation | Grading | Note |
|---|---|---|
| Strongly recommended | Grade A | Positive rating is ahead for the 4 items below † |
| Weakly recommended | Grade B | Negative rating is ahead for the 4 items below † |
Certainty of overall evidence, balance of benefits and harms, patient preferences and values; and costs.
1. 1 GUIDELINE FOR THE DIAGNOSIS OF DIABETES MELLITUS
[Q1‐1] How is diabetes diagnosed? (Figure 1)
-
The diagnosis of diabetes mellitus should be as comprehensive as possible. It is confirmed by the presence of chronic hyperglycemia, and by the presence of other factors in each patient, such as associated symptoms, clinical laboratory findings, a family history of diabetes, and his/her body weight history 1 , 2 , 3 , 4 , 5 . For the diagnosis of diabetes, either of the following criteria is to be followed:
①Two assessments of the diabetic type in each patient (where one blood glucose test is mandatory).
②One assessment of the diabetic type (with mandatory blood glucose testing) along with the presence of typical symptoms of chronic hyperglycemia (e.g., dry mouth, polydipsia, polyuria, body weight loss, or diabetic retinopathy).
③Evidence of a prior diagnosis of ‘diabetes’.
Figure 1.
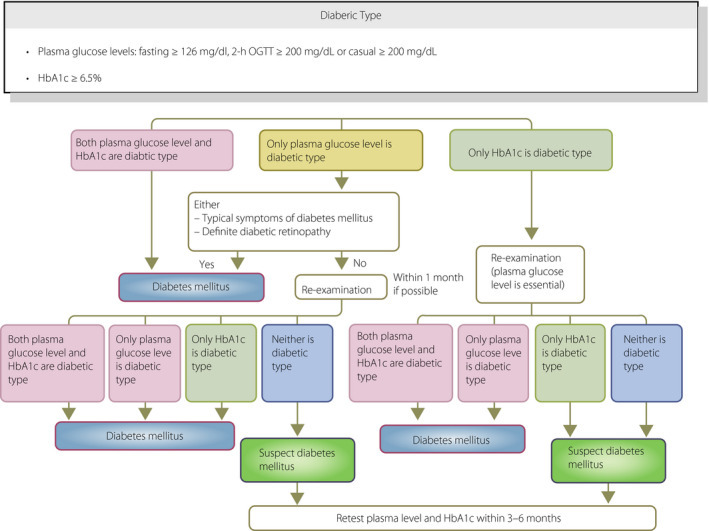
Flowchart outlining the steps in the clinical diagnosis of diabetes mellitus. OGTT, oral glucose tolerance test (Adapted from Seino Y et al. J Jpn Diabetes Soc 2012; 55: 485–504 4 ).
[Q1‐2] How is hyperglycemia assessed? (Figure 2)
Patients are to be classified into the normal type, borderline type, or diabetic type, based on the combination of fasting and 2‐h post‐75 g oral glucose tolerance test (OGTT) glucose values.
Patients whose fasting glucose values are 100–109 mg/dL are classified into the ‘high normal’ category as part of the normal type 6 .
The OGTT is to be proactively considered in high‐risk individuals (i.e., those who are suspected of having diabetes or the borderline type, those whose fasting glucose values are shown to be ‘high normal’, those with HbA1c values of ≥5.6%, those with obesity or dyslipidemia, and those with a strong family history of diabetes 4 ).
Measured venous plasma glucose values are to be used for the diagnosis of hyperglycemia, rather than those obtained with point of care testing (POCT) or a simple glucometer (including continuous glucose monitoring).
Figure 2.
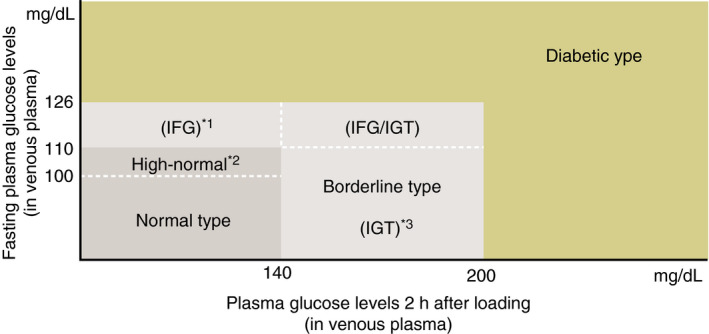
Categories of glycemia as indicated by fasting plasma glucose levels and 75 g OGTT results. *1 The impaired fasting glucose (IFG) category refers to individuals with fasting plasma glucose (FPG) levels of 110–125 mg/dL and 2‐h plasma glucose (PG) levels of <140 mg/dL in a 75 g OGTT (WHO), with the caveat, however, that IFG is defined as an FPG 100–125 mg/dL and only FPG is used in the diagnosis of IFG in the American Diabetes Association criteria. *2 Individuals with FPG 100–109 mg/dL are defined as the normal high FPG sub‐category as part of the normal FPG category. It is advisable to perform OGTTs in this population who are shown to be quite heterogeneous in their susceptibility to diabetes or the severity of IGT confirmed at OGTT. *3 As one of the definitions included in the diagnostic criteria proposed by the WHO, IGT is diagnosed in individuals with FPG <126 mg/dL or 2‐h 75 g OGTT PG ranging between 140 and 199 mg/dL.
[Q1‐3] How are individuals to be managed if they are shown to be the diabetic type in an initial glucose/HbA1c assessment but not on subsequent assessments?
When the diagnosis is not confirmed by repeated assessments, glucose measurements and OGTTs are to be performed every 3–6 months to monitor their clinical course 4 .
If the glucose value on the initial assessment was found to be ≥200 mg/dL on a casual blood glucose measurement, it would be preferable to use other tests on subsequent confirmatory assessments 4 .
In principle, confirmatory assessments are to involve both HbA1c and blood glucose measurements. The diagnosis must be made with close attention given to their blood glucose values, particularly in patients with any disease or condition that is likely to result in disparity between their HbA1c levels and mean glucose values 4 .
[Q1‐4] How is diabetes classified into its types? (Table 3)
The classifications of diabetes are to be primarily described according to the etiology (mechanism), and additionally according to the pathophysiological state (stage) based on the insufficiency of insulin action 4 (see Q1–7 for the relationship between their etiology and pathophysiology).
Diabetes and impaired glucose metabolism are to be classified into four categories: (I) type 1 diabetes, (II) type 2 diabetes, (III) other types due to specific pathophysiological mechanisms or diseases, and (IV) gestational diabetes (GDM). At present, all forms of diabetes or other glucose metabolic disorders that do not fall into as any of the above are to be classified as ‘unclassifiable’ 4 .
The etiological factors of patients should be assessed with attention to various types of clinical information such as the family history, age at the onset of diabetes and clinical course, physical characteristics, islet autoantibodies, human leukocyte antigen (HLA), insulin‐secretory capacity/severity of insulin resistance, and genetic test results 4 .
Individual patients may have multiple etiological factors 4 .
Table 3.
Etiological classification of diabetes and impaired glucose metabolism †
|
I. Type 1 (Characterized by pancreatic β‐cell destruction usually leading to absolute insulin deficiency)
|
| II. Type 2 (Characterized mainly by decreased insulin secretion or by the presence of insulin resistance, each possibly accompanied by relative insulin insufficiency) |
|
III. Diabetes due to some other specific mechanism or disease
|
| IV. Gestational diabetes |
All forms of diabetes that do not fall into either of the above classifications are handled as ‘unclassifiable’.
Include some impaired glucose metabolism that remain to be evaluated for their potential to lead to complications characteristic of diabetes. (Adapted from Seino Y et al. J Jpn Diabetes Soc 2012; 55: 485–504 4 ).
[Q1‐5] How is type 1 diabetes (including acute, slowly progressive, and fulminant forms of type 1 diabetes) to be diagnosed? (Table 4)
Type 1 diabetes is classified by etiology as (A) autoimmune and (b) idiopathic and also classified by manner of disease onset as acute, slowly progressive, and fulminant.
Patients with acute type 1 diabetes are generally likely to develop ketosis or ketoacidosis within 3 months of the onset of hyperglycemia and require insulin therapy immediately 7 .
Patients with slowly‐progressive (insulin‐dependent) type 1 diabetes do not develop ketosis or ketoacidosis and do not require insulin therapy immediately, although their diagnosis is established by a positive test for anti‐GAD antibodies or islet cell antibodies (ICA) 8 .
Patients with fulminant type 1 diabetes frequently develop ketosis or ketoacidosis within 1 week of the onset of hyperglycemia, require insulin therapy immediately, and are characterized as having lower HbA1c values relative to their glucose values 9 .
Table 4.
Diagnostic criteria for acute‐onset, slowly progressive, and fulminant type 1 diabetes (findings of relevance shown in square brackets)
| Criteria | Acute‐onset type 1 diabetes | Slowly‐progressive type 1 diabetes (SPIDDM) | Fulminant type 1 diabetes |
|---|---|---|---|
| 1. Symptoms of hyperglycemia and ketosis † | Affected individuals are expected to present with thirst, polydipsia, and polyuria, leading to the onset of ketosis or ketoacidosis within 3 months. | While affected individuals are expected to present with ketosis or ketoacidosis at disease onset or diagnosis, they do not require immediate insulin therapy. | Affected individuals are expected to present with the symptoms of hyperglycemia, e.g., thirst, polydipsia, and polyuria, leading to the onset of ketosis or ketoacidosis within about 1 week of onset of these symptoms; they are also expected to present with ketosis at initial consultation. |
| 2. Glycemic status/need for insulin therapy | Affected individuals are expected to require continuous insulin therapy from early after diagnosis of diabetes; they may also be expected to experience a transient ‘honeymoon phase’. ‡ | While favorable glycemic control can often be achieved without insulin therapy in individuals early after disease onset, insulin therapy is considered effective in delaying their progression to an insulin‐dependent state. | Affected individuals are expected to have casual blood glucose values 288 mg/dL or higher and HbA1c values <8.7% [thus necessitating initiation of insulin therapy]. |
| 3. Islet autoantibodies § | Affected individuals are expected to be confirmed positive for either GAD antibodies, IA‐2 antibodies, IAA, ZnT8 or ICA antibodies during their clinical course (where IAA positivity needs to be confirmed prior to initiation of insulin therapy). | Affected individuals are expected to be confirmed positive for either GAD antibodies or ICA during their clinical course. | [As a rule, affected individuals are expected to test negative for islet autoantibodies.] |
| 4. Endogenous insulin secretion | Affected individuals may not be confirmed positive for islet autoantibodies but are expected to have fasting serum C‐peptide values <0.6 ng/mL thus suggesting a deficit in endogenous insulin secretion. | [Some individuals may not show evidence of decreased endogenous insulin secretion, irrespective of their autoantibody values.] | Affected individuals are expected to have urinary C‐peptide values <10 μg/day at disease onset or fasting serum C‐peptide values <0.3 ng/mL and post‐glucagon load (or 2‐h postprandial) C‐peptide values <0.5 ng/mL. |
| Diagnosis |
Individuals who have met the above criteria 1–3 are to be diagnosed with acute‐onset (autoimmune) type 1 diabetes. Those who have met the above criteria 1, 2, and 4 are to be diagnosed with acute‐onset type 1 diabetes. Those who have met the above criteria 1 and 2 but not 3 and 4 are to be re‐evaluated after an interval with the diagnosis put on hold. Those who have met the criteria for fulminant type 1 diabetes are to be diagnosed as such. |
Individuals who have met the above criteria 1 and 3 are to be diagnosed with slowly‐progressive type 1 diabetes. | Individuals who have met the above criteria 1, 2 and 4 are to be diagnosed with fulminant type 1 diabetes. |
| Other relevant findings | Individuals with single‐gene disorders, such as HNF‐1α gene, mitochondrial gene, KCNJ11 gene mutations, are to be excluded from assessment. | Insulin therapy may be initiated in affected individuals from early after diagnosis while they are still not in an insulin‐dependent state. |
Some may lead to the onset of ketosis or ketoacidosis within about 1–2 weeks. The onset of fulminant type 1 diabetes may be associated with pregnancy. Exocrine pancreatic enzymes, e.g., amylase, lipase, and esterase 1, are shown to be elevated in 98% of affected individuals. Upper airway and gastrointestinal symptoms are noted in 70% of affected individuals. Fulminant type 1 diabetes is shown to be linked to HLA DRB1*04:05–DQB1*04:01. |
Ketosis, diagnosed when individuals are found positive for urinary ketone bodies or associated with increased serum ketone levels.
Honeymoon phase, defined as a phase during which glycemic control may be achieved without insulin therapy for months after initial insulin therapy implemented early after diagnosis.
Islet auto antibodies include glutamic acid decarboxylate (GAD) antibodies, insulinoma‐associated protein‐2 (IA‐2) antibodies, insulin autoantibodies (IAA), zinc transporter 8 (ZnT8) antibodies, and islet cell antibodies (ICA).
[Q1‐6] How are diabetes and impaired glucose metabolism due to other specific pathophysiological mechanisms or diseases diagnosed? (Table 5)
Recent advances in gene analysis techniques have led to a number of single‐gene abnormalities being identified as causes of diabetes. These are generally divided into: ① those related to the pancreatic β‐cell function and ② those related to the mechanisms of insulin action.
A diabetic condition may occasionally be a part of various diseases, syndromes and pathologies. Some of these were formerly called ‘secondary diabetes’ and include forms of diabetes associated with pancreatic, endocrine and hepatic diseases, drug use, exposure to chemicals, viral infections, and an array of genetic syndromes.
The diagnosis of these forms of diabetes requires a close review of relevant clinical data, which include: ① family history and mode of inheritance; ② age at onset of diabetes and clinical course; ③ other physical characteristics; and ④ islet autoantibodies.
Table 5.
Diabetes and impaired glucose metabolism† due to some other specific mechanisms or diseases
| A. Forms of diabetes for which responsible genetic alterations have been identified | B. Forms of diabetes associated with some other disease or condition |
|
|
Include some impaired glucose metabolism that remain to be evaluated for their potential to lead to complications characteristic of diabetes. (Adapted from Seino Y et al. J Jpn Diabetes Soc 2012; 55: 485–504 4 ).
[Q1‐7] How do the types of diabetes (their etiology) each relate to their respective pathophysiology (clinical stage)? (Figure 3)
Their etiology (mechanism) and pathophysiological states (stages) represent dimensions distinct from each other and both should be used to describe the condition in each individual patient.
Whatever the underlying etiology, diabetes may often develop through various conditions and its pathophysiology may change with the treatment.
Pathophysiological states (stages) of diabetes are to be classified into the following three stages based on the insufficiency of insulin action: (1) those not requiring insulin therapy; (2) those requiring insulin therapy for glycemic control; and (3) those requiring insulin therapy to prevent ketosis and to support/sustain life.
An insulin‐dependent state refers to a life‐threatening status in which patients who do not receive exogenous insulin are prone to ketosis. In contrast, a non‐insulin dependent state refers to a state in which insulin injection is required to ameliorate glycemic control but not to prevent ketosis or to support/sustain life. Thus, it should be noted that patients receiving insulin therapy are not always in an insulin‐dependent state.
Figure 3.
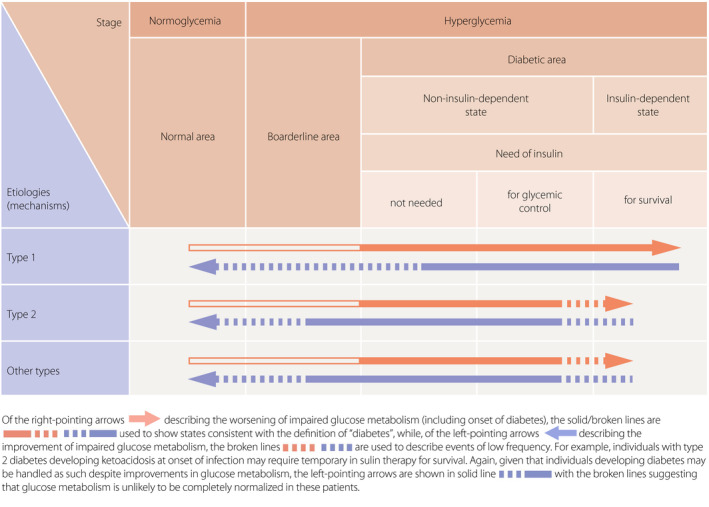
Schematic diagram showing the etiology (mechanisms of onset) and pathophysiological stages (phases) of diabetes mellitus (Adapted from Seino Y, et al. J Jpn Diabetes Soc 2012; 55: 485–504 4 ).
2. 2 GOALS AND STRATEGIES FOR DIABETES MANAGEMENT
[Q2‐1] What are the objectives of diabetes management?
The objectives of diabetes management are to improve metabolic dysfunctions resulting from hyperglycemia; to prevent the development or progression of diabetic complications and conditions associated with diabetes; and to enable affected individuals to maintain their quality of life (QOL) and life expectancy at a level comparable to those in healthy individuals.
[Q2‐2] How should a basic strategy for diabetes treatment be developed for each patient? (Figure 4)
The treatment strategy for diabetes may vary depending on the disease type, disease condition, patient age, metabolic abnormalities, and status of diabetic complications.
Insulin therapy is to be given not only to patients who are insulin‐dependent but also to pregnant patients, patients undergoing surgery that involves whole‐body management, and patients with severe infection, even if they are not insulin‐dependent. In addition, insulin therapy is to be given to those in whom glycemic control targets are not achievable with oral hypoglycemic agents (OHAs) or glucagon‐like peptide 1 (GLP‐1) receptor agonists.
OHA and/or GLP‐1 agonist therapy is to be given to non‐insulin‐dependent patients in whom favorable glycemic control is not achievable with adequate medical nutrition therapy (MNT) and physical activity/exercise continued for 2–3 months. OHA and/or GLP‐1 agonist therapy or insulin therapy may be given to these patients at the outset depending on the severity of the metabolic disorder involved.
Continued therapy is essential for patients with diabetes to prevent the onset or progression of complications. Team care‐based diabetes education for these patients forms the cornerstone of the diabetes treatment.
Figure 4.
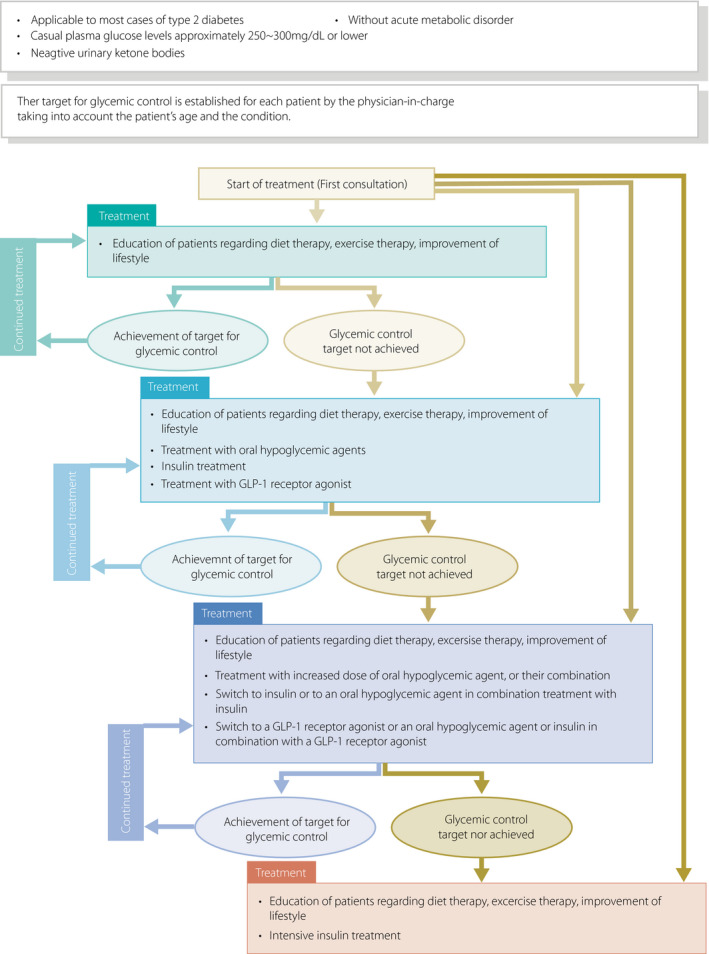
Treatment of type 2 diabetes patients in non‐insulin‐dependent state. This provides a guide to the management of patients without acute metabolic disorder [i.e., those who had a casual blood glucose level of 250–300 mg/dL or less than 250–300 mg/dL with a negative urinary ketone test]. The glycemic goal should be determined individually depending on the disease condition or age of the patient but is generally set at HbA1c <7.0%. ‘Diet therapy’ and ‘exercise therapy’ are referred to as ‘medical nutrition therapy (MNT)’ and ‘physical activity/exercise’, respectively, elsewhere in this guideline.
[Q2‐3] How is the glycemic goal to be set for each individual patient? (Figure 5)
Figure 5.

Glycemic control targets (see Figure 8 for those for patients 65 years of age or older). The glycemic control target should be determined for each individual in light of his/her age, duration of diabetes, presence of organ damage, risk of hypoglycemia, and access to any support available. *1 Intended for individuals capable of achieving glycemic control with appropriate diet therapy (MNT) or exercise therapy or those capable of achieving glycemic control while on pharmacotherapy without developing hypoglycemia. *2 Defined as HbA1c <7.0% for prevention of diabetic complications, which is assumed to correspond to fasting glucose <130 mg/dL and postprandial 2‐h glucose <180 mg/dL as measured glucose values. *3 Intended for individuals deemed less amenable to treatment intensification due to associated hypoglycemia or for some other reason. *4 All these targets are intended for use by adults except for pregnant women.
Glucose levels in affected individuals are to be controlled as close to normal as possible. Achieving and maintaining favorable glycemic control early after initiation of treatment is likely to lead to favorable long‐term outcomes in these individuals 1 .
[Q2‐4] How is the onset of chronic diabetic complications prevented or their progression delayed?
Diabetes management is aimed not merely at glycemic control 1 but also at ensuring continued smoking cessation and control of blood pressure and lipid levels, thereby preventing chronic diabetic complications or delaying their progression 2 , 3 , 4 , 5 .
3. 3 MEDICAL NUTRITION THERAPY (MNT)
[CQ3‐1] Is MNT effective in the management of diabetes?
In the management of diabetes, lifestyle modification centered on MNT is shown to be effective 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 (grade A: 100% agreement).
[CQ3‐2] Is MNT education by registered dieticians effective?
MNT education by registered dieticians is effective 10 , 11 (grade A: 95% agreement).
[Q3‐3] How is total energy intake to be determined in patients with diabetes?
The objective of MNT for type 2 diabetes is to maintain favorable systemic metabolism thereby preventing not only the onset but the progression of diabetic complications. While, to this end, total energy intake needs to be determined for each patient based on his/her body weight, this process is to be individualized with due consideration given to his/her target body weight that may vary depending on his/her age and disease condition, as follows: total energy intake is to be estimated at initiation of treatment in each patient but is to be modified, as required, with consideration given to changes in his/her disease condition, age, body composition, adherence and metabolic status.
As per the statement on target body weight vs total energy intake, for each patient, his/her target body weight and total energy intake is to be individually determined. Again, all values given below are primarily intended as suggested targets only and therefore need to be modified, as required, during patient consultation, in consideration of each patient’s current body weight, glycemic control and other parameters. There is also a need for accumulating evidence for body weight and total energy intake determination.
3.1. Target body weight (kg)
Given that the body mass index (BMI) value least associated with all‐cause mortality is expected to vary with age and fall within a certain range, the target body weight is to be calculated for each patient by the following formula:
*For elderly patients with diabetes aged ≥75 years, the target body weight should be determined based on their current body weight, with consideration also given to associated frailty, decreased (fundamental) ADL, concomitant disease(s), body composition, height shortening (loss), diet (nutritional) status, and metabolic status.
Level of physical activity and energy coefficient (kcal/kg) according to disease status
① Light exertion (static activity engaged mostly in the seated position): 25–30
② Ordinary exertion (mainly static activity engaged in the seated position including commuting, household chores, and light exercise): 30–35
③ Heavy exertion (heavy physical work or habitual, active exercise): 35~
For elderly patients, the coefficient could be made larger than that associated with their actual level of physical activity to prevent them from developing frailty. Conversely, for obese patients in a weight loss program, the coefficient could be made lower than that associated with their actual level of physical activity. In either case, individuals whose actual body weight widely differs from their target body weight, the coefficient could be flexibly determined with consideration given to the levels of physical activity and corresponding energy coefficients to given above.
3.2. Target total energy intake
The target total energy intake is to be calculated by the following formula:
** As a rule, the target body weight is to be determined based on consideration of patient age.
[CQ3‐4] How are the dietary nutrient ratios to be determined?
There is no clear evidence available for determining ideal energy‐producing nutrient ratios toward the prevention and management of diabetes 12 .
The dietary nutrient ratios for each patient should be flexibly determined, with consideration given to patient factors, such as his/her level of physical activity, concomitant disease(s), age and preferences.
Given that insulin is shown to have a wide‐ranging action affecting not only glucose metabolism but lipid and protein metabolism, all of which are closely linked, energy‐producing nutrients as components of MNT must be assessed for their balance and validity against each patient’s disease condition, as well as associated risks including hyperglycemia. Furthermore, consideration is to be given not only to the safety of the dietary components but to Japanese cuisine culture and patient preferences, to ensure long‐term implementation of MNT. However, there is no evidence available to support the effectiveness of any particular dietary nutrient ratios that contribute to long‐term management of diabetes.
To ensure long‐term implementation of MNT in patients with diabetes, priority is to be given to honoring their eating habits and preferences thus allowing them to enjoy their meals as far as they do not defeat the purpose of MNT medically, while at the same time giving consideration to any potential risks associated with their individual diet regimens.
[Q3‐5] How does dietary carbohydrate intake affect diabetic management?
To date, no correlation has been shown between dietary carbohydrate intake and risk of diabetes or diabetic control status 13 , 14 .
Patients may be encouraged to take up to one unit of fructose (fruit), given that the intake of fructose up to a certain amount is not shown to affect diabetes. However, they should abstain from sucrose‐rich sweets and juices, which are thought likely to worsen glycemic control and promote the metabolic syndrome 15 , 16 .
Instructing patients on carbohydrate counting (CC) during insulin therapy is effective in achieving glycemic control.
Glycemic index (GI)‐guided food choices have not been proved to be useful in the management of diabetes.
[Q3‐6] How does the dietary protein intake affect diabetes management?
There is no evidence to demonstrate that an increased protein intake is associated with an increased risk of diabetic nephropathy 17 .
An intake of protein that accounts for ≥20% of the total energy intake may increase the risk of mortality from any causes including atherosclerosis. No evidence is available to support the long‐term safety of the practice 18 .
[Q3‐7] How does dietary fat intake affect diabetes management?
While no clear relationship has been shown between total dietary fat intake and the risk of diabetes, an increased animal fat (saturated fatty acid [SFA]) intake has been shown to be associated with the risk of diabetes 19 , 20 , 21 , 22 , 23 .
No evidence is available to support the benefits of n‐3 fatty acids in diabetes management.
[Q3‐8] How does the dietary fiber intake affect diabetes management?
Given that dietary fiber has been shown to be effective in improving diabetic states, patients are encouraged to consume ≥20 g of dietary fiber daily, irrespective of their carbohydrate intake.
[Q3‐9] How does the dietary vitamin and mineral intake affect diabetes management?
No clear relationship has been shown between the dietary vitamin and mineral intake and the management of diabetes.
[Q3‐10] How does dietary salt intake affect diabetic management?
The target salt intake recommended is less than 7.5 g/day and 6.5 g/day for men and women, as well as less than 6.0 g/day for those with hypertension.
[Q3‐11] How does alcohol intake affect diabetic management?
Alcohol intake is to be individually determined for each patient depending on his/her drinking habit, with up to a maximum of 25 g/day as a guide. While it remains unclear how alcohol intake may vary in its impact on diabetic control depending on its kind, attention is also to be given to the amount of energy taken through carbohydrate‐containing drinks such as low‐malt beer. Again, hypoglycemia is to be watched for as an acute effect of alcohol intake in patients receiving insulin therapy. Patients may be allowed to take alcohol, provided that these factors are thought to be readily manageable.
[CQ3‐12] How does sweetener intake affect diabetic control?
While sucrose intake is a risk factor for diabetes, the influence of artificial sweeteners on the risk of diabetes and glycemic control has not been sufficiently elucidated.
[CQ3‐13] How do each patient’s eating patterns affect his/her diabetic control?
In light of his/her current eating patterns, each patient with diabetes is to be encouraged to consistently choose appropriate foodstuffs. Regularly eating three meals a day is shown to be effective in preventing the onset of diabetes.
4. 4 PHYSICAL ACTIVITY/EXERCISE
[CQ4‐1] Is physical activity/exercise effective in diabetic control?
Physical activity/exercise involving aerobic exercise, resistance exercise or their combination is shown to improve glycemic control 1 , 2 , 3 , 4 , 5 , 6 and risk factors for cardiovascular disease 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 . Aerobic exercise and resistance exercise are shown to be effective individually, and more in combination in improving glycemic control in patients with type 2 diabetes 1 , 6 , 8 , 14 (grade A 100% agreement).
Despite lack of consensus on its role in improving glycemic control in patients with type 1 diabetes, long‐term exercise is shown to reduce risk factors for cardiovascular disease and improve quality of life (QOL) 15 , 16 , 17 , 18 (grade B: 100% agreement).
[Q4‐2] Is a medical check‐up required before implementing physical activity/exercise?
Prior to initiation of physical activity/exercise, patients with diabetes are to be examined for concomitant diseases, such as retinopathy, nephropathy, and neuropathy, and for physical abnormalities, such as orthopedic diseases, to see if exercise needs to be restricted 19 .
In general, screening for cardiovascular disease is not necessary in asymptomatic patients implementing light‐ to moderate‐intensity exercise (e.g., one that can be implemented as a daily activity, e.g., brisk walking) 19 . However, screening by physicians, as well as exercise stress testing, may be considered in patients implementing higher‐than‐usual‐intensity exercise or those at high risk of cardiovascular disease 20 .
[Q4‐3] How is physical activity/exercise regimen to be implemented?
Patients with diabetes are recommended to implement: moderate‐intensity aerobic exercise 150 min or longer in total at a frequency of 3 or more days a week without taking more than consecutive 2 days or more off from exercise; resistance exercise 2 to 3 times a week or every other day of the week; or both if not contraindicated 19 , 21 .
Patients with diabetes are recommended to break up their sitting times with light activity to avoid prolonged periods of sitting 19 , 21 .
5. 5 TREATMENT WITH GLUCOSE‐LOWERING AGENTS (EXCLUDING INSULIN)
[Q5‐1] What are the indications for glucose‐lowering agents?
Glucose‐lowering agents are indicated for patients with non‐insulin‐dependent stage of diabetes who fail to achieve favorable glycemic control despite 2–3 months of sufficient MNT and physical activity/exercise 1 , 2 , 3 , and the timing of initiation of these agents is to be determined for each eligible patient with consideration also given to his/her current disease condition, history of prior treatment, and target of glycemic control. Early use of glucose‐lowering agents including insulin may be indicated in patients requiring immediate resolution of glucotoxicity.
Glucose‐lowering agents are not to be initiated, and insulin therapy immediately implemented, in patients who represent absolute indications for insulin therapy: those with insulin‐dependent stages (including type 1 diabetes), acute metabolic derangement (e.g., diabetic ketoacidosis, hyperosmolar hyperglycemic state, and lactic acidosis), severe hepatic/renal impairment making glycemic control with MNT difficult, hyperglycemic disorders in pregnancy requiring intervention other than MNT, and severe infection, as well as those undergoing surgery who require whole‐body management and those who require glycemic control during intravenous alimentation.
[Q5‐2] How are glucose‐lowering agents chosen for use in diabetes treatment?
Currently available glucose‐lowering agents are classified into the following seven categories: insulin secretagogues sulfonylureas (SUs); another type of insulin secretagogues, rapid‐acting insulin secretagogues (i.e., glinides); dipeptidyl‐peptidase‐4 (DPP‐4) inhibitors; insulin‐sensitizers, biguanides; another type of insulin‐sensitizers, thiazolidinediones (TZDs); α‐glucosidase inhibitors which improve postprandial hyperglycemia by delaying glucose uptake; and sodium‐glucose cotransporter 2 (SGLT2) inhibitors which facilitate glucose excretion by inhibiting renal glucose reuptake; and non‐insulin injectable glucagon‐like peptide 1 (GLP1) receptor agonists.
Glucose‐lowering agents are to be chosen in light of their pharmacological and side effect profiles to address each patient’s disease condition. With the patient’s informed consent, treatment should be initiated with a single agent and at a low dose. Whenever feasible, it should also be considered to titrate its dose upwards, to combine it with another agent with a different mechanism of action or insulin, or to switch to insulin therapy, as required.
[Q5‐3] What are the characteristics of sulfonylureas (SUs)?
Sulfonylureas (SUs) potently lower blood glucose level through their ability to promote the secretion of insulin from pancreatic β cells. Current evidence demonstrates their usefulness in reducing microangiopathy 3 . SUs have been shown to exert their effects immediately in patients with preserved insulin capacity; however, they have often been shown to be associated with the side effect of hypoglycemia. SUs are also associated with weight gain in patients who are less adherent to MNT and/or physical activity/exercise 4 .
[Q5‐4] What are the characteristics of biguanides?
Biguanides are currently used as first‐line glucose‐lowering agents in Western countries. Biguanides exert their effect by inhibiting hepatic glucose production as well as by improving peripheral insulin sensitivity. Current evidence demonstrates their usefulness in reducing macroangiopathy in patients with type 2 diabetes 5 , 6 , 7 , 8 . Although they are rarely associated with lactic acidosis, caution needs to be taken to determine whether the patient can be safely treated with biguanides.
[Q5‐5] What are the characteristics of α‐glucosidase inhibitors?
α‐Glucosidase inhibitors, which inhibit intestinal glycolysis and delay intestinal glucose absorption and suppress postprandial hyperglycemia and hyperinsulinemia, are to be taken immediately before meals; they are also often associated with flatus and diarrhea. Hypoglycemia in patients treated with these agents can be effectively improved with the ingestion of only glucose.
[Q5‐6] What are the characteristics of thiazolidinediones (TZDs)?
Thiazolidinediones (TZDs) are shown to improve glycemic control by promoting peripheral insulin sensitivity and inhibiting hepatic glucose release; they are also often associated with weight gain due to their ability to promote fluid retention and adipocyte differentiation. Patients receiving TZDs require monitoring for edema, anemia and fracture associated with the use of TZDs 9 , 10 , 11 , 12 , 13 .
[Q5‐7] What are the characteristics of glinides?
Glinides are shown to correct postprandial hyperglycemia by immediately promoting insulin secretion, with their action diminishing in such a short time that they are less associated with the risk of hypoglycemia.
[Q5‐8] What are the characteristics of DPP‐4 inhibitors?
DPP‐4 inhibitors glucose‐dependently promote postprandial insulin secretion while at the same time inhibiting glucagon secretion, thus improving both fasting and postprandial hyperglycemia. While the risk of hypoglycemia with DPP‐4 inhibitor monotherapy is small, combination therapy with an SU or insulin often increases the risk of hypoglycemia, suggesting the rationale for reducing the dose of either partnering agent 14 , 15 , 16 , 17 , 18 .
They are not associated with an increased risk of macroangiopathy 21 , 22 , 23 . Thus, at present, DPP‐4 inhibitors appear to have a favorable safety profile 19 , 20 , 21 , 22 , while attention needs to be given to the potential onset of acute pancreatitis and bullous pemphigoid with these agents.
[Q5‐9] What are the characteristics of GLP‐1 receptor agonists?
GLP‐1 receptor agonists, which are available as injectable agents, promote postprandial insulin secretion in a glucose‐dependent manner while at the same time inhibiting glucagon secretion; thus they improve both fasting and postprandial hyperglycemia and are less associated with a risk of hypoglycemia. While these agents have also been shown to exert their glucose‐lowering effect in combination with an SU or insulin, this combination therapy is shown to be associated with an increased risk of hypoglycemia, suggesting the rationale for reducing the dose of either partnering agent 23 , 24 .
GLP‐1 receptor agonists are noted for their gastrointestinal adverse effects. Thus, a GLP‐1 receptor agonist is to be initiated at a low dose, with its dose titrated upwards as appropriate. The association between the use of GLP‐1 receptor agonists and the risk of acute pancreatitis has been shown to be negative 25 , 26 , 27 , 28 , 29 . On the other hand, liraglutide, as well as duraglutide although given at a higher dose than that approved for use in Japan, is shown to significantly suppress the onset of macroangiopathy in patients at high risk of cardiovascular events 30 , 31 .
[Q5‐10] What are the characteristics of SGLT2 inhibitors?
SGLT2 inhibitors inhibit glucose reabsorption in the proximal renal tubule and promote urinary glucose excretion, thus exerting their glucose‐lowering effect; they not only improve glycemic control independently of insulin‐mediated mechanisms but also associated with body weight reduction.
Empagliflozin and canagliflozin (the latter given at a higher dose than that approved for use in Japan) are shown to significantly reduce the risk of macroangiopathy in patients at high risk of cardiovascular events 32 , 33 .
SGLT2 inhibitors tend to be associated with such adverse effects as an increased frequency of genital infection and fluid loss‐related events 32 , 34 . Attention is also to be given to potential occurrence of acute renal impairment and ketone body‐related events in patients receiving SGLT2 inhibitors.
[Q5‐11] Is combination therapy with glucose‐lowering agents effective?
In patients failing to achieve their glycemic target while on monotherapy with a first‐line agent, consideration may be given to increasing the dose of the first‐line agent, switching to a more potent glucose‐lowering agent, or combining the first‐line agent with another glucose‐lowering agent with a different mechanism of action. No clear synergistic effect has been demonstrated between agents used in combination, and no guidelines have been established for combination therapy with glucose‐lowering agents.
In patients with inadequate glycemic control despite monotherapy with a first‐line agent, combination therapy with another glucose‐lowering agent with a different mechanism of action is usually chosen. While combination therapy with any two agents has been shown to be effective for lowering glucose levels 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , combination therapy with three or more agents (other than SU/glinide or DPP‐4 inhibitor/GLP‐1 receptor agonist combinations) has also been shown to be effective for lowering glucose levels 47 , 48 , 49 , 50 , 51 .
[Q5‐12] How are patients to be managed if they have inadequate glycemic control despite treatment with glucose‐lowering agents?
In patients with inadequate glycemic control despite combination therapy with glucose‐lowering agents, consideration needs to be given to reassessing MNT and/or physical activity/exercise as well as to adding basal insulin therapy or switching to intensive insulin therapy.
6. 6 INSULIN THERAPY
[Q6‐1] What types of insulin formulation are available?
The currently available insulin formulations are classified based on their onset/duration of action into rapid‐acting insulin, regular insulin, intermediate‐acting (neutral protamine Hagedorn, NPH) insulin, long‐acting insulin, premixed regular/intermediate‐acting, premixed rapid‐/intermediate‐acting (or biphasic) insulin, and rapid‐acting and long‐acting insulin combination formulations.
Intermediate‐ or long‐acting insulin formulations are used to supplement basal insulin secretion, while regular or rapid‐acting insulin formulations are used to supplement bolus insulin secretion.
[Q6‐2] What are the indications for insulin therapy?
Absolute indications for insulin therapy include insulin‐dependent states irrespective of disease type, hyperglycemic coma (diabetic ketoacidosis, hyperosmolar hyperglycemic state, lactic acidosis), and pregnancy complicated by diabetes that is not adequately controlled by MNT alone. Insulin therapy is also recommended for use in serious infections and surgery requiring systemic management.
Insulin therapy is also implemented in patients with type 2 diabetes having inadequate glycemic control despite MNT, increased physical activity/exercise and therapy with non‐insulin glucose‐lowering agents, or when hyperglycemia‐associated glucose toxicity must be eliminated.
[Q6‐3] What are the adverse reactions that occur in association with insulin therapy?
Insulin therapy may be associated with hypoglycemia as well as a transient worsening of retinopathy or neuropathy in some patients 1 , 2 . Patients receiving insulin therapy need to be monitored for long‐term risks associated with insulin therapy, such as weight gain 3 .
[Q6‐4] What approaches are available for insulin therapy in type 1 diabetes?
Multiple insulin injection therapy (3–4 injections/day) or continuous subcutaneous insulin infusion (CSII) are available to optimize glycemic control in type 1 diabetes 4 .
[CQ6‐5] Is intensive insulin therapy effective in suppressing microangiopathy in type 1 diabetes?
Intensive insulin therapy, which combines multiple insulin injections or CSII and self‐monitoring of blood glucose (SMBG) has been shown to be effective in preventing the onset of microangiopathy (retinopathy, nephropathy and neuropathy) and in suppressing their progression 4 , 5 (grade A: 100% agreement).
[CQ6‐6] Is intensive insulin therapy effective in suppressing macroangiopathy in type 1 diabetes?
Intensive insulin therapy that combines multiple insulin injection therapy and SMBG has been shown to also be effective in suppressing the progression of macroangiopathy (coronary artery disease, cerebrovascular disease, and peripheral artery disease) 6 , 7 (grade A: 100% agreement).
[Q6‐7] What are the indications/approaches for insulin therapy in type 2 diabetes?
Insulin therapy is to be implemented in patients with type 2 diabetes having inadequate glycemic control despite MNT, increased physical activity/exercise and treatment with non‐insulin glucose‐lowering agents 3 , 8 , 9 , 10 .
While injection of once‐daily long‐acting insulin or twice‐daily premixed insulin (morning and evening) may be sufficient to provide favorable glycemic control in patients with mild diabetes, intensive insulin therapy with multiple insulin injections is to be implemented in those with moderate‐to‐severe diabetes 8 , 11 , 12 .
Combination therapy with insulin and oral glucose‐lowering agents (SUs 13 , 14 , fast‐acting insulin secretagogues [glinides] 15 , 16 , 17 ), biguanides 18 , 19 , 20 , 21 , α‐glucosidase inhibitors 22 , 23 , insulin sensitizers 24 , 25 , 26 , 27 , and, DPP‐4 inhibitors 28 , and SGLT2 inhibitors 29 or GLP‐1 receptor agonists 30 are shown to improve glycemic control and reduce the insulin dose being used in patients with type 2 diabetes.
[CQ6‐8] Is intensive insulin therapy effective in suppressing microangiopathy in type 2 diabetes?
Strict glycemic control with intensive insulin therapy has been shown to be effective in preventing the onset of microangiopathy (retinopathy, nephropathy, and neuropathy) as well as in suppressing the progression of microangiopathy 8 , 9 (grade A: 94% agreement).
[Q6‐9] Is intensive insulin therapy effective in suppressing macroangiopathy in type 2 diabetes?
7. 7 DIABETES SELF‐MANAGEMENT EDUCATION AND SUPPORT FOR THE SELF‐MANAGEMENT OF DIABETES
[CQ7‐1] Are organized support and education for the self‐management of diabetes useful for the management of diabetes?
Organized education and support for the self‐management of diabetes have been shown to be useful for diabetes management 1 , 2 , 3 , 4 (grade A: 100% agreement).
[CQ7‐2] Is the group and individualized education useful for the diabetes management?
Both group and individualized education has been shown to be useful for diabetes management 5 , 6 , 7 , 8 (grade A: 95% agreement).
[CQ7‐3] Is the self‐monitoring of blood glucose (SMBG) useful for diabetes management?
SMBG has been shown to be useful for patients with type 1 diabetes 9 , 10 , 11 and for patients with type 2 diabetes receiving insulin therapy 12 (grade A: 95% agreement).
[Q7‐4] In which respects is continuous glucose monitoring (CGM) useful in diabetes control?
A number of reports in the literature suggest that real‐time continuous glucose monitoring (rt‐CGM) may be more effective than self‐monitoring of blood glucose (SMBG) in improving glycemic control not only in pediatric and adult patients with type 1 diabetes but in adult patients with type 2 diabetes 13 , 14 , 15 .
There are some reports in the literature suggesting that intermittently viewed CGM (i‐CGM) may be more effective than SMBG in shortening the hypoglycemic durations in patients with type 1 and type 2 diabetes 16 , 17 .
[Q7‐5] What are the psychological issues in diabetes management and treatment?
Diabetes is often associated with depressive symptoms and anxiety disorders specific to the disease 18 , 19 , 20 , leading to suboptimal self‐care, worsening of glycemic control, an increased risk of diabetic complications, and an impaired QOL, thus adversely affecting the prognosis of affected patients 21 , 22 . Intervention that addresses both depressive symptoms and diabetes‐related mental distress and anxiety is required to improve the self‐care abilities and glycemic control of affected patients 23 .
[CQ7‐6] Are psychological/behavioral approaches effective in diabetes management?
Psychological/behavioral approaches have been shown to be effective in diabetes management 24 , 25 (grade A: 95% agreement).
[Q7‐7] Is depression screening/treatment important in diabetes management?
After at‐risk patients with diabetes are screened for depression, systematically coordinated care is essential for both diabetes and depression 26 , 27 .
[Q7‐8] How are the available guidelines and practice manuals to be used in practice?
Practice manuals are guides to apply in clinical practice the treatment policies recommended in clinical practice guidelines constructed through systematic reviews of available evidences. Practice manuals are also intended to promote information sharing between healthcare provider teams and their patients as well as delivery of personalized care to address the disease condition and the needs of each patient.
8. 8 DIABETIC RETINOPATHY
[CQ8‐1] Is a routine ophthalmologic check‐up useful for preventing the onset/progression of diabetic retinopathy?
A routine ophthalmologic check‐up has been shown to be useful for preventing the onset/progression of diabetic retinopathy 1 , 2 , 3 , 4 (grade A: 95% agreement).
[CQ8‐2] Is glycemic control useful for the management of diabetic retinopathy?
Glycemic control has been shown to be useful for suppressing the onset/progression of diabetic retinopathy in patients with type 1 and type 2 diabetes 5 , 6 , 7 , 8 (grade A: 100% agreement).
[CQ8‐3] Is blood pressure control useful for the management of diabetic retinopathy?
Blood pressure control has been shown to be useful for suppressing the onset/progression of diabetic retinopathy in patients with type 2 diabetes 9 , 10 , 11 (grade A: 100% agreement).
[CQ8‐4] Is lipid control useful for the management of diabetic retinopathy?
Fenofibrates have been shown to have the potential to suppress the progression of diabetic retinopathy in patients with type 2 diabetes complicated by dyslipidemia 7 , 12 (grade B: 85% agreement).
[Q8‐5] Can the onset/progression of retinopathy be prevented with medical therapy, other than glucose, blood pressure and lipid lowering?
There is no clinical evidence to suggest the usefulness of antiplatelet agents for suppressing the onset/progression of diabetic retinopathy.
[CQ8‐6] Is ophthalmologic treatment useful for preventing the progression of retinopathy?
Ophthalmologic treatment such as retinal photocoagulation has been shown to be useful for suppressing the progression of retinopathy 13 , 14 (grade A: 95% agreement).
[Q8‐7] Is diabetic retinopathy a risk factor for the onset of other diabetes‐associated complications?
9. 9 DIABETIC NEPHROPATHY
[CQ9‐1] Is the measurement of urinary albumin useful for the early diagnosis of diabetic nephropathy?
The measurement of urinary albumin has been shown to be useful in the early diagnosis of diabetic nephropathy 1 , 2 (grade A: 95% agreement).
[Q9‐2] What parameters are used to assess renal function?
It is recommended that, for ease of use, estimated glomerular filtration rate (eGFR), calculated based on serum creatinine (Scr) values obtained through an enzyme‐based method, be used to assess renal function in daily clinical practice 3 , while inulin clearance, creatinine clearance or eGFRcys‐c calculated based on serum cysteine C values may also be used, as required.
(1) eGFR: Intended to estimate renal function using a serum creatinine‐based equation 3
Advantage: This Scr‐based formula offers convenience by allowing renal function to be estimated with a blood test alone. With this formula, eGFR is likely to fall ± 30% of measured GFR (mGFR) in 75% of patients.
Disadvantage: Adjusted for average body surface area (BSA) (1.73 m2), the formula is likely to be associated with a greater estimation error in patients of large and small build. The formula is also associated with overestimated values in patients with low muscle mass.
(2) eGFRcys‐c: Intended to estimate renal function using serum cysteine C values 4
Advantage: Secreted from all nucleated cells, cysteine C is thought less likely to be influenced by muscle mass or dietary content.
Disadvantage: Adjusted for average BSA (1.73 m2), the formula is also likely to be associated with a greater estimation error in patients of large and small build.
[CQ9‐3] Is glycemic control effective for the management of diabetic nephropathy?
Glycemic control is shown to be effective in inhibiting the onset of diabetic nephropathy as well as in inhibiting the progression of early‐stage nephropathy 5 , 6 , 7 , 8 , 9 , 10 (grade A: 100% agreement).
[CQ9‐4] Is blood pressure control effective for the management of diabetic nephropathy?
Blood pressure control is shown to be effective in inhibiting the onset/progression of diabetic nephropathy 11 , 12 , 13 (grade A: 100% agreement).
[CQ9‐5] Is lipid control effective for the management of diabetic nephropathy?
Anti‐dyslipidemic agents (e.g., fibrates, statins) are shown likely to be effective in inhibiting the progression of diabetic nephropathy in patients with intact renal function 14 , 15 , 16 (grade B: 81% agreement).
[CQ9‐6] Are angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) recommended as first‐line medications for blood pressure control in patients with diabetic nephropathy?
Angiotensin‐converting enzyme (ACE) inhibitors or angiotensin II receptor blocker (ARB) is recommended as a first‐line medication for blood pressure control in patients with diabetic nephropathy 17 , 18 , 19 (grade A: 93% agreement).
[CQ9‐7] Is dietary salt restriction recommended for the management of diabetic nephropathy?
Dietary salt restriction is recommended for the management of diabetic nephropathy 20 (grade B: 95% agreement).
[Q9‐8] Is protein restriction effective for the management of diabetic nephropathy?
While protein restriction is thought likely to be effective in inhibiting the progression of diabetic nephropathy in patients with overt or more advanced nephropathy, there is a paucity of clinical evidence to support its implementation 21 , 22 .
[Q9‐9] Is the treatment of anemia effective for suppressing the progression of diabetic nephropathy?
It remains unclear if the treatment of anemia may have a role in suppressing the progression of diabetic nephropathy 23 .
[Q9‐10] Is diabetic nephropathy a risk factor for other complications in patients with diabetes?
Diabetic nephropathy frequently occurs concomitantly with cardiovascular diseases. Patients with diabetic nephropathy show a high rate of cardiovascular disease‐related mortality.
A decreased GFR and the occurrence of albuminuria are independent risk factors for cardiovascular disease 24 .
10. 10 DIABETIC NEUROPATHY
[Q10‐1] How is diabetic neuropathy diagnosed? (Table 6)
Diabetic neuropathy is one of the most common complications associated with diabetes, and therefore, patients are to be assessed for neuropathy on a regular basis during the course of their treatment.
In diagnosing diabetic neuropathy, patients are to be interviewed about subjective symptoms of neuropathy and assessed for sensory functions, such as pain and vibratory sensations, as well as for Achilles tendon reflex. All abnormal sensory findings must necessarily be characterized as being distal and symmetric.
Nerve conduction examinations are essential for the definitive diagnosis of diabetic neuropathy and are useful in the early diagnosis of diabetic neuropathy including asymptomatic neuropathy.
Coefficient of variation R‐R interval (CVR‐R) testing is a convenient and useful test to assess autonomic nerve function.
Table 6.
Proposed simplified diagnostic criteria for diabetic polyneuropathy
| Prerequisite conditions (the following two must be met) |
|
| Criteria (any two of the following three must be met) |
|
| Note |
|
Subjective symptoms of diabetic polyneuropathy are characterized as:
|
|
Findings of interest (diabetic neuropathy is to be confirmed if one of the following two has been met, despite failure to meet the criteria described above)
|
Diagnostic criteria proposed by the Conference on Diabetic Polyneuropathy (revised January 18, 2002).
[Q10‐2] How is diabetic neuropathy classified?
Diabetic neuropathy is classified into distal symmetric polyneuropathies and focal mononeuropathies 1 , 2 . The former, including sensorimotor and autonomic neuropathies, are the most frequent of all diabetic neuropathies.
[Q10‐3] What are the risk factors for the onset/progression of diabetic neuropathy?
The risk factors for the onset/progression of diabetic neuropathy include: ① poor glycemic control, ② duration of diabetes, ③ hypertension, ④ dyslipidemia, ⑤ smoking, and ⑥ obesity 3 , 4 , 5 .
[CQ10‐4] Is glycemic control effective for the management of diabetic neuropathy?
Strict glycemic control has been shown to suppress the onset/progression of diabetic neuropathy 6 , 7 , 8 (grade A: 90% agreement).
[Q10‐5] How is pharmacotherapy to be implemented in patients with neurosensory damage?
Epalrestat has been shown to suppress the progression of diabetic neuropathy in some patients.
Neurosensory damage often resolves with improved glycemic control and lifestyle modification in patients with mild painful neuropathy. Non‐steroidal anti‐inflammatory drugs (NSAIDs) have only been shown to be effective in mild cases.
Tricyclic antidepressants 9 , pregabalin 10 , 11 , and duloxetine 12 , 13 are recommended as first‐line medications for patients with moderate‐to‐severe painful neuropathy.
[Q10‐6] How is autonomic nerve damage to be treated?
Autonomic nerve damage often improves with improved glycemic control and lifestyle modification in patients with mild autonomic neuropathy. However, symptom‐specific pharmacotherapy is required for patients whose activities of daily living (ADL) are impaired in association with advanced neuropathy.
[Q10‐7] How is mononeuropathy to be treated?
Mononeuropathy has been shown to resolve often spontaneously, independently of glycemic control.
[Q10‐8] Is diabetic neuropathy a risk factor for other complications in patients with diabetes?
Diabetic neuropathy has been shown to be a risk factor for diabetic retinopathy and nephropathy 14 .
11. 11 DIABETIC FOOT
[Q11‐1] What is diabetic foot?
Diabetic foot is globally defined as ‘infections, ulcers and destructive lesions occurring on the lower limb tissue of patients with diabetes in association with ongoing neuropathy and peripheral artery disease’ 1 .
Diabetic foot occurs in response to external factors in the presence of hypoesthesia due to neuropathy, foot deformities, dry or keratinized skin, and decreased blood flow due to peripheral artery disease. When diabetic foot is complicated by infection, it is likely to become severe, leading not only to lower limb amputation, but also to a worse prognosis 1 , 2 .
[CQ11‐2] Is a routine foot examination effective for the prevention of diabetic foot?
While there is a paucity of evidence to support the effectiveness of routine foot examinations in the prevention of diabetic foot, the incidence of lower limb amputations has been shown to decrease following the introduction of foot care, including foot examinations, in clinical practice 3 . Foot examinations are essential for the early detection of diabetic foot and the implementation of foot care and are thus thought to be effective for the prevention of diabetic foot (grade A by consensus: 80% agreement).
[CQ11‐3] Is foot care education effective for the prevention of diabetic foot?
Foot care education is thought to promote the acquisition of relevant knowledge and improve self‐care activities and is thus thought likely to be effective for achieving the prevention of diabetic foot 4 , 5 (grade B by consensus: 80% agreement).
[CQ11‐4] Is glycemic control effective for preventing the onset of foot lesions and sparing lower limbs?
Glycemic control is shown to be effective not only for preventing the onset of foot lesions and sparing lower limb amputation 6 , 7 but also for preventing neuropathy as a risk factor for foot lesions 7 (grade A: 85% agreement).
[CQ11‐5] Is foot care effective for the prevention of foot ulcers or limb salvage in high‐risk patients?
Foot care is shown to be effective for preventing foot ulcers or limb salvage in high‐risk patients 8 , 9 . [grade A: 100% agreement]
[Q11‐6] How are foot ulcers to be treated?
The treatment of diabetic foot in patients with diabetes entails a wide array of interventions, which include control of their general condition, local procedures (i.e., debridement), the treatment of infectious disease, revascularization for severe lower limb ischemia, the use of non‐weight bearing/off‐loading devices and specially prepared shoes, walking rehabilitation, nutritional education, and care support, in which multidisciplinary team‐based care involving diverse specialists and practitioners remains the cornerstone 1 .
Infections, abscesses or necrotizing fasciitis associated with the presence of gas in the deep tissues is an indication for emergency surgery. While no established criteria are available for indications for amputation, the blood flow of the prospective amputation site must be evaluated prior to amputation 10 , 11 .
[CQ11‐7] Is team‐based care effective in preventing diabetic foot and treating foot ulcers?
Team‐based care is shown to be effective for prevention of foot lesions and treatment of foot ulcers 12 , 13 (grade B: 90% agreement).
[CQ11‐8] Is foot ulcer treatment effective in maintaining the quality of life (QOL) of affected patients?
Foot ulcer treatment has been shown to be effective in maintaining the QOL of affected patients 14 , 15 (grade A: 100% agreement).
[Q11‐9] Is diabetic foot a risk factor for other complications in patients with diabetes?
Patients with diabetic foot are significantly associated with high all‐cause mortality 16 , as well as a high incidence of cardiovascular/cerebrovascular diseases, depression 17 , and cognitive impairment 18 , suggesting that diabetic foot lesions likely represent a risk factor for mortality and these diseases.
12. 12 DIABETIC MACROANGIOPATHY
[Q12‐1] When and how should risk management be initiated to prevent diabetic macroangiopathy?
It is recommended that established risk factors for diabetic macroangiopathy, such as impaired glucose tolerance (IGT), hypertension, dyslipidemia, obesity, and chronic kidney disease (CKD), should be detected at an early stage, and comprehensively managed 1 , 2 , 3 , 4 .
[Q12‐2] For which patient with diabetes is risk management beneficial in preventing diabetic macroangiopathy?
All patients with diabetes represent candidates for angiopathy risk management. In elderly patients and patients with advanced angiopathy, careful monitoring for hypoglycemia and hypotension is required 4 , 5 , 6 .
[CQ12‐3] Are the modification of lifestyle habits and the correction of obesity effective in preventing diabetic macroangiopathy?
Conditions, such as IGT, hypertension, dyslipidemia, obesity, and CKD, and lifestyle habits, such as physical inactivity, excessive salt intake, and smoking, both represent risk factors for cardiovascular events. The modification of lifestyle habits and the correction of obesity are recommended in patients with diabetes, given that these measures are shown to be associated with the amelioration of these risk factors 7 , 8 , 9 (grade A: 90% agreement).
[CQ12‐4] Is glycemic control effective against diabetic macroangiopathy?
Tight glycemic control, initiated early after the onset of diabetes, has been shown to be effective in suppressing the risk of diabetic macroangiopathy 10 , 11 (grade A: 95% agreement).
[CQ12‐5] Is blood pressure control effective in preventing diabetic macroangiopathy?
Tight blood pressure control has been shown to be effective in suppressing the risk of diabetic macroangiopathy 12 , 13 (grade A: 100% agreement).
[CQ12‐6] Is lipid control effective in preventing diabetic macroangiopathy?
Lipid control has been shown to be effective in the primary and secondary prevention of diabetic macroangiopathy 14 , 15 (grade A: 100% agreement).
[CQ12‐7] Are antiplatelet agents effective in preventing diabetic macroangiopathy?
The use of antiplatelet agents has been shown to be effective in the secondary prevention of diabetic macroangiopathy 16 (grade A: 100% agreement).
13. 13 DIABETES AND PERIODONTITIS
[Q13‐1] What is periodontal disease?
Periodontal disease is an inflammatory disease involving plaque bacteria and is broadly classified into gingivitis in which inflammation is confined to the gingiva, and periodontitis which involves a loss of supporting tissue.
Periodontal disease is a disease of the oral cavity that is reported to affect approximately 80% of the Japanese individuals of middle age or older and is the foremost cause of dental extraction.
The treatment of periodontal disease entails not only establishing plaque control in affected patients but also improving inflammation through plaque and calculus removal from periodontal pockets and ensuring routine post‐removal periodontal maintenance care aimed at preventing a relapse of the disease.
[Q13‐2] Does diabetes influence the onset/progression of periodontal disease?
Periodontal disease has been shown to occur more frequently among patients with type 1 diabetes in comparison to young healthy individuals 1 .
The risk of the onset of periodontal disease and the progression of alveolar bone resorption is significantly increased in patients with type 2 diabetes and an HbA1c value of ≥6.5% 2 .
[CQ13‐3] Is diabetes treatment effective in improving periodontal disease?
Diabetes treatment may lead to the improvement of periodontal tissue inflammation 3 (grade B: 95% agreement).
[Q13‐4] Does periodontal disease affect glycemic control?
Periodontal disease as an inflammatory disease has been epidemiologically shown to adversely affect glycemic control 4 .
As periodontal disease becomes more severe, it becomes more difficult to achieve glycemic control in affected patients 5 .
[CQ13‐5] Is treating periodontal disease effective in improving glycemic control?
14. 14 DIABETES COMPLICATED BY OBESITY (INCLUDING METABOLIC SYNDROME)
[Q14‐1] What are the causes of obesity?
Obesity is classified into secondary obesity (i.e., obesity with clear underlying causes), and primary obesity (i.e., obesity with no clear causes but which is associated with lifestyle habits such as physical inactivity) 1 .
While primary obesity is most frequent of all forms of obesity, secondary obesity includes endocrinologically induced obesity, inherited obesity, hypothalamic obesity and drug‐induced obesity 1 .
[Q14‐2] How is obesity diagnosed?
In Japan, obesity is defined by a body mass index (BMI) of 25 kg/m2 or higher according to the Japan Society for the Study of Obesity 1 .
Obesity is to be handled as a disease in patients with obesity‐induced or obesity‐associated health problems or in patients who are likely to have obesity‐associated health problems and for whom weight loss is medically indicated 1 .
There are two categories of patients with obesity disease: ① patients with a health problem due to/related to obesity requiring weight loss (which is expected to be improved or arrested with appropriate weight loss) ②those with visceral obesity (i.e., those with no existing health problem who are deemed to be at high risk of developing one, such as diabetes and thus represent targets for lifestyle intervention 1 , where an umbilical‐level CT‐measured visceral fat area (VFA) of 100 cm2 or higher is used for the diagnosis of visceral obesity in both males and females. Note that the use of non‐CT (e.g., BIA)‐measured VFA remains controversial, given that the evidence to support its use is not necessarily sufficient 2 , 3 , 4 , 5 .
[Q14‐3] How is obesity‐associated diabetes to be managed?
Secondary obesity is to be carefully ruled out in patients with type 2 diabetes and obesity, and those who are thought to be likely to have primary obesity are to be interviewed about their living environmental and psychological factors. Attention is to be paid to the discontinuation or modification of any lifestyle habits that cause obesity 6 . This is to entail, first, instructing patients on lifestyle modification including MNT and/or physical activity/exercise, stress management and a regular lifestyle to lose weight 6 . Pharmacotherapy is to be considered for patients whose glycemic control is inadequate despite maintaining lifestyle modifications over a certain period 6 .
Left untreated, obesity often becomes more severe in patients with diabetes and obesity receiving exclusive therapy for hyperglycemia 7 . Attention needs to be focused on ensuring that these patients proactively modify their lifestyles to achieve favorable glycemic control without weight gain 8 .
[Q14‐4] Is behavioral therapy effective in reducing body weight and achieving glycemic control in patients with type 2 diabetes and obesity?
Behavioral therapy needs to be combined with lifestyle modification to achieve and maintain weight reduction over the long term in patients with type 2 diabetes and obesity 9 . Obesity is associated with abnormal eating behavior, such as speed eating characterized by an excessive intake of energy over a short time, impulse eating, and eating between meals from post‐lunch to nighttime, can be problematic in many of these patients. Thus, when their treatment targets have been determined, their overeating behavior should be evaluated through diet journals and body weight measurements to establish a favorable eating behavior. Behavioral enhancement through routine motivation measures is thought to be effective in maintaining desired behavioral changes. However, no clear evidence is currently available to support the effectiveness of behavioral therapy in the achievement of glycemic control in patients with type 2 diabetes and obesity.
[Q14‐5] Is pharmacotherapy effective for achieving glycemic control in patients with type 2 diabetes and obesity?
The use of insulin or SUs should be minimized in patients with type 2 diabetes and obesity, given that their uncritical use may promote obesity 7 .
SGLT2 inhibitor is shown to be associated with a weight loss of about 3 kg in obese patients with type 2 diabetes. SGLT2 inhibitor monotherapy may be effective for glycemic control while being less likely associated with hypoglycemia 10 .
The appetite‐inhibitory and weight‐reducing properties of glucagon‐like peptide 1 (GLP‐1) receptor agonists may improve glycemic control in patients with type 2 diabetes and obesity 7 . Some of GLP‐1 receptor agonists are currently used to treat obesity overseas.
[CQ14‐6] Is surgical therapy effective for patients with type 2 diabetes and high‐degree obesity?
If appropriate perioperative support and safety are ensured, surgical therapy for obesity is shown to be effective in patients with type 2 diabetes and high‐degree obesity who have difficulty losing weight 11 , 12 , 13 , 14 (grade B: 90% agreement).
[Q14‐7] What is metabolic syndrome?
Metabolic syndrome is defined as a condition that involves any two of the following conditions, in addition to visceral fat accumulation (visceral fat area ≥100 m2 on CT measurement at the level of the umbilicus): fasting hyperglycemia ≥110 mg/dL, dyslipidemia such as hypertriglyceridemia (≥150 mg/dL), hypo‐high‐density‐lipoprotein (HDL)‐cholesterolemia (<40 mg/dL), and high blood pressure (≥130/85 mmHg) 15 .
15. 15 HYPERTENSION ASSOCIATED WITH DIABETES
[Q15‐1] Is hypertension a risk factor for macroangiopathy in patients with diabetes?
Both diabetes and hypertension are established risk factors for atherosclerosis‐associated macroangiopathy; patients with diabetes and hypertension have a higher incidence of macroangiopathy and a poorer prognosis 1 .
[Q15‐2] Is hypertension a risk factor for microangiopathy in patients with diabetes?
Concomitant hypertension in patients with diabetes is a risk factor for microangiopathy, such as diabetic neuropathy, diabetic retinopathy, and diabetic nephropathy (see also relevant pages for diabetic neuropathy, diabetic retinopathy, and diabetic nephropathy).
[Q15‐3] What is the office blood pressure threshold for initiating antihypertensive therapy in patients with diabetes? (Table 7, Figure 6)
Table 7.
Classification of blood pressure levels in adults
| Classification | Office blood pressure (mmHg) | Home blood pressure (mmHg) | ||||
|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |||
| Normal blood pressure | <120 | and | <80 | <115 | and | <75 |
| High normal blood pressure | 120–129 | and | <80 | 115–124 | and | <75 |
| Elevated blood pressure | 130–139 | and/or | 80–89 | 125–134 | and/or | 75–84 |
| Grade I hypertension | 140–159 | and/or | 90–99 | 135–144 | and/or | 85–89 |
| Grade II hypertension | 160–179 | and/or | 100–109 | 145–159 | and/or | 90–99 |
| Grade III hypertension | ≥180 | and/or | ≥110 | ≥160 | and/or | ≥100 |
| (Isolated) systolic hypertension | ≥140 | and | <90 | ≥135 | and | <85 |
Cited from Umemura, S., Arima, H., Arima, S. et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019) Hypertens Res. 2019 Sep;42(9):1254. https://doi.org/10.1038/s41440‐019‐0284‐9, with the permission of the JSH.
Figure 6.
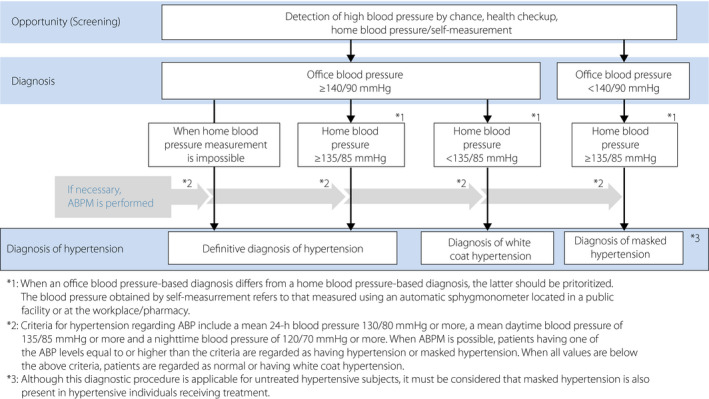
Blood pressure measurement and procedure for hypertension diagnosis. (Cited from Umemura, S., Arima, H., Arima, S. et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019) Hypertens Res. 2019 Sep;42(9):1256. https://doi.org/10.1038/s41440‐019‐0284‐9, with the permission of the JSH).
The initiation of antihypertensive therapy is deemed appropriate in patients with an office blood pressure of ≥130/80 mmHg 1 .
Intervention with antihypertensive agents should be immediately initiated in patients with an office blood pressure of ≥140/90 mmHg 1 .
Lifestyle modification (lasting no more than 3 months) may be indicated for patients with diabetes and an office blood pressure of 130–139/80–89 mmHg if such modification is expected to achieve the patient’s blood pressure target; if not, antihypertensive agents should be initiated immediately 1 .
[CQ15‐4] Is controlling office blood pressure to <130/80 mmHg effective in preventing the onset of complications in patients with diabetes and hypertension? (Figure 7)
Figure 7.
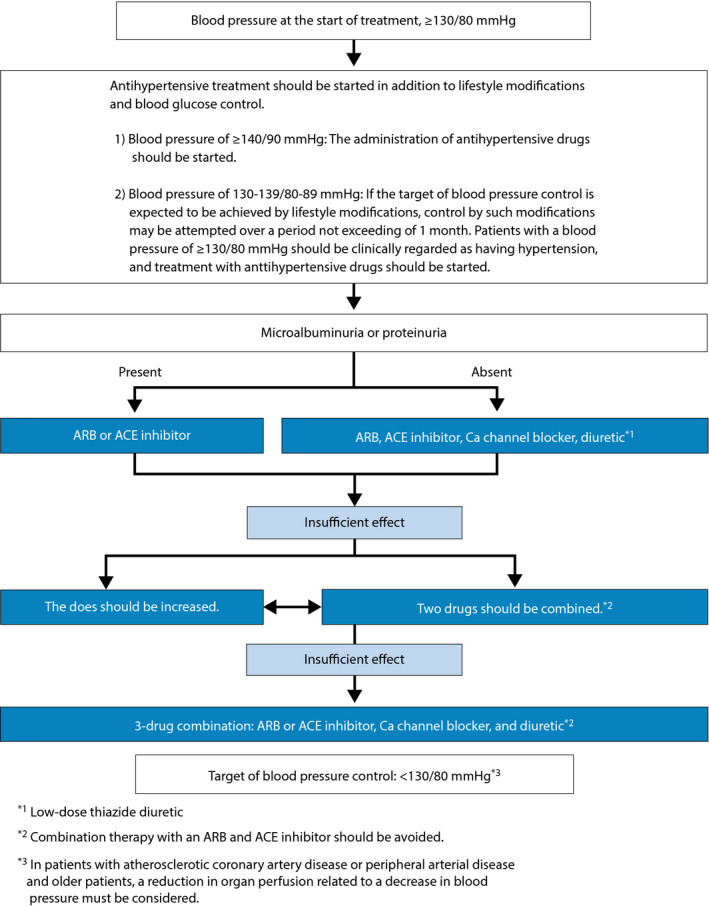
Treatment plan for hypertension complicated by diabetes mellitus. (Cited from Umemura, S., Arima, H., Arima, S. et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019) Hypertens Res. 2019 Sep;42(9):1356. https://doi.org/10.1038/s41440‐019‐0284‐9, with the permission of the JSH).
A blood pressure of <130/80 mmHg is deemed appropriate as the office blood pressure target for preventing complications in patients with diabetes and hypertension 2 (grade B: 90% agreement).
[CQ15‐5] Should angiotensin‐converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) be used as first‐line antihypertensive medications for patients with diabetes and hypertension?
Not only ACE inhibitors/ARBs but calcium channel blockers (CCBs) and thiazide diuretics are recommended for use in hypertensive patients with diabetes as antihypertensive agents of first choice 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 . In patients with microalbuminuria or proteinuria, priority should be given to ACE inhibitors or ARBs (grade B: 93% agreement).
[Q15‐6] Which is preferable, a calcium channel blocker (CCB) or a diuretic, as an add‐on agent in patients with diabetes and hypertension?
In patients with inadequate blood pressure control despite treatment with an ACE inhibitor/ARB, a CCB or low‐dose thiazide diuretic should be given as an add‐on agent. Triple antihypertensive therapy with an ACE inhibitor/ARB, a CCB and a thiazide diuretic should be given when an additional agent is required (consensus between the Japanese Society of Hypertension and the Japan Diabetes Society).
16. DYSLIPIDEMIA ASSOCIATED WITH DIABETES
[Q16‐1] Is dyslipidemia a risk factor for macroangiopathy in diabetes?
Dyslipidemia is a risk factor for macroangiopathy 1 .
Hyper‐low‐density‐lipoprotein (LDL)‐cholesterolemia is a strong risk factor for coronary artery disease 2 .
[Q16‐2] Is dyslipidemia a risk factor for microangiopathy in diabetes?
Hypertriglyceridemia is a risk factor for microangiopathy 3 .
Hypo high‐density‐lipoprotein (HDL)‐cholesterolemia is a risk factor for microangiopathy 4 .
[Q16‐3] What are the thresholds for initiating antidyslipidemic therapy and its control targets in diabetes? (Tables 8 and 9)
The primary goal of antidyslipidemic therapy is to control the LDL‐cholesterol level to <100 mg/dL in patients with a history of coronary artery disease and to <120 mg/dL in patients without a history of coronary artery disease.
For the secondary prevention of coronary artery disease, consider to ensure the control of LDL‐cholesterol <70 mg/dL for stricter‐than‐usual lipid control when patients with diabetes have at least one of the following high‐risk factors, namely, familial hypercholesterolemia, non‐cardiogenic cerebral infarction, peripheral artery disease (PAD), microangiopathy, metabolic syndrome, persistently unfavorable glycemic control, multiple major risk factors, or smoking (Table 9).
The control goal for fasting triglyceride (TG) is <150 mg/dL.
The control goal for HDL cholesterol is ≥40 mg/dL.
Table 8.
Lipid control targets for dyslipidemia in diabetes
| History of coronary artery disease | Lipid control target (mg/dL) | |||
|---|---|---|---|---|
| LDL‐C | HDL‐C | TG | non‐HDL‐C | |
| No | <120 | ≥40 | <150 | <150 |
| Yes | <100 (<70) † | <130 (<100) † | ||
For patients who are also suffering from high‐risk conditions such as FH, ACS, and diabetes complicated by other high‐risk conditions (Noncardiogenic cerebral infarction, Peripheral artery disease (PAD), Chronic kidney disease (CKD), Metabolic syndrome, Overlap of major risk factors and smoking), stricter LDL‐C control should be considered, with a level of <70 mg/dL as the target.
Although non‐drug therapy is used as a standard means for achieving the management target in primary prevention, drug therapy should be considered for patients with low risk if the LDL‐C level is ≥180 mg/dL. The possibility of FH should also be considered.
Achieving the LDL‐C management target should be the first goal, and reaching the non‐HDL‐C management target should be the next goal after the first goal has been achieved. Managing the TG and HDL‐C levels is important during this process.
These values are challenging goals by utmost effort; a 20–30% reduction in LDL‐C levels for primary prevention (low or moderate risk) and a decrease of ≥50% for secondary prevention are also possible targets.
For elderly patients (aged ≥75 years), refer to Chapter 7 in ‘JAS Guidelines’. (Adapted from Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017 J Atheroscler Thromb 2018; 25(9): 853–855. https://doi.org/10.5551/jat.GL2017, with the permission of JAS).
Table 9.
High risk factors for coronary artery disease
|
Adapted from Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017, J Atheroscler Thromb 2018; 25(9): 908. https://doi.org/10.5551/jat.GL2017, with the permission of JAS.
[CQ16‐4] Is MNT effective against dyslipidemia in patients with diabetes?
MNT has been shown to be effective against dyslipidemia in patients with diabetes 5 , 6 , 7 (grade A: 100% agreement).
The intake of polyunsaturated fatty acids (PUFA) is recommended 8 (grade A: 90% agreement).
[CQ16‐5] Is physical activity/exercise effective against dyslipidemia in patients with diabetes?
Physical activity/exercise has been shown to be effective against dyslipidemia in patients with diabetes 9 , 10 (grade A: 100% agreement).
[CQ16‐6] Is statin therapy effective in reducing the risk of cardiovascular disease (CVD) or mortality in patients with diabetes and dyslipidemia?
The use of statins has been shown to reduce the risk of CVD and mortality in patients with diabetes and dyslipidemia 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 (grade A: 100% agreement).
Statins are the agents of choice for hyper‐LDL‐cholesterolemia in patients with diabetes 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 (grade A: 100% agreement).
[CQ16‐7] Is the use of non‐statin agents effective in reducing the risk of CVD or mortality in patients with diabetes and dyslipidemia?
The use of fibrates has been shown to reduce the risk of non‐fatal CVD in patients with diabetes and dyslipidemia 24 , 25 , 26 , 27 , 28 (grade B: 88% agreement).
Ezetimibe or a PCSK9 inhibitor as add‐on to statin therapy is shown to reduce the incidence of CVD in patients with diabetes and hyper‐LDL‐cholesterolemia 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 (grade B: 88% agreement).
17. 17 HYPERGLYCEMIC DISORDERS IN PREGNANCY
[CQ17‐1] Does glycemic control before and during pregnancy lead to improvements in the maternal and neonatal prognosis?
While poor glycemic control before and during early phase pregnancy has been shown to be associated with an increased incidence of congenital anomalies and fetal death, strict glycemic control from well before pregnancy has been shown to be associated with a reduced incidence of these complications 1 , 2 (grade A: 100% agreement).
While poor glycemic control during pregnancy has been shown to be associated with an increased risk of perinatal complications, strict glycemic control during pregnancy has been shown to be associated with a reduction in the risk of these complications 3 , 4 (grade A: 100% agreement).
[Q17‐2] How are hyperglycemic disorders diagnosed in pregnancy? (Table 10)
Hyperglycemic disorders in pregnancy include: ① gestational diabetes mellitus (GDM), ② overt diabetes in pregnancy, and ③ pregestational diabetes mellitus, and are diagnosed based on 75 g oral glucose tolerance tests (OGTTs), HbA1c values and the clinical findings.
Table 10.
Gestational diabetes mellitus: its definition and diagnostic criteria
| Definition | Gestational diabetes mellitus (GDM) is defined as a state of pre‐diabetic impaired glucose tolerance (IGT) identified or occurring for the first time during pregnancy, but it does not include overt diabetes in pregnancy or pre‐gestational diabetes mellitus. |
| Gestational diabetes mellitus (GDM) |
GDM is diagnosed if one or more of the following criteria have been met in a 75 g OGTT: ① Fasting blood glucose value ≥92 mg/dL ② 1‐h post‐OGTT glucose value ≥180 mg/dL ③ 2‐h post‐OGTT glucose value ≥153 mg/dL |
| Diagnostic criteria for overt diabetes in pregnancy (ODM)*1 |
Overt diabetes in pregnancy is diagnosed if ① or ② below has been met: ① Fasting blood glucose value ≥126 mg/dL ② HbA1c ≥6.5%
|
| Pre‐gestational diabetes mellitus |
① Diabetes mellitus diagnosed before pregnancy ② Pregnancy associated with unequivocal evidence of diabetic retinopathy |
*1 Overt diabetes in pregnancy includes diabetes overlooked before pregnancy, impaired glucose tolerance due to changes in glucose metabolism during pregnancy and type 1 diabetes occurring during pregnancy. In either case, the diagnosis needs to be confirmed in affected women after delivery.
*2 Women are expected to show higher post‐OGTT glucose values during pregnancy, particularly later pregnancy, than usual, reflecting increased physiological insulin resistance during pregnancy. Thus, the casual blood glucose and 75 g OGTT values defined in the diagnostic criteria for diabetes mellitus are not readily applicable.
These diagnostic criteria are intended for use during pregnancy and any diagnosis made based on these criteria requires to be assessed after delivery based on the ‘diagnostic criteria for diabetes’ 15 .
[Q17‐3] How are pregnant women screened for impaired glucose metabolism?
It is preferable that all pregnant women be screened based on a glucose‐based assessment including casual glucose measurements and a glucose challenge test (GCT) 5 .
Ideally, at first consultation and at between 24 and 28 weeks of gestation.
[Q17‐4] How are patients with diabetes to be managed before pregnancy?
Patients with diabetes who wish to become pregnant, as well as their families, are to be fully informed about the importance of strict glycemic control being implemented from well before pregnancy to prevent congenital anomalies, fetal death and miscarriage due to poor glycemic control during early pregnancy 1 , 2 .
Every effort should be made to achieve glycemic control that is as close to normal as possible while at the same time avoiding hypoglycemia 1 , 2 .
Oral glucose‐lowering agents are not recommended in patients who wish to become pregnant. Insulin therapy is to be implemented in patients whose glycemic control is deemed inadequate despite MNT and physical activity/exercise.
The presence of concomitant disease, such as diabetic complications, obesity and hypertension, is shown to adversely affect the health status of mothers and their pregnancy outcomes. Patients are to be assessed for presence of these concomitant diseases and treated before they become pregnant 6 , 7 , 8 .
Patients should be instructed on the importance of pregnancy planning (pre‐pregnancy lifestyle management) as well as on effective contraception measures.
[Q17‐5] How are glycemic control targets to be determined for pregnant women with hyperglycemic disorders? (Table 11)
Glycemic control should be as close to normal as possible while at the same time avoiding hypoglycemia.
Ideally, the patients are to be assessed for early‐morning fasting and postprandial glucose values 8 , 9 , 10 .
Table 11.
| Japan Diabetes Society (JDS) | American Diabetes Association (ADA) | National Institute for Health and Care Excellence (NICE) | |
|---|---|---|---|
| Fasting plasma glucose (FPG) | <95 mg/dL*1 | <95 mg/dL |
<5.3 mmol/L*4 (<95 mg/dL) |
| Postprandial plasma glucose (PPG) |
1‐h PPG <140 mg/dL Or 2‐h PPG <120 mg/dL |
1‐h PPG <140 mg/dL Or 2‐h PPG <120 mg/dL |
1‐h PPG <7.8 mmol/L (<140 mg/dL) Or 2‐h PPG <6.4 mmol/L (115 mg/dL) |
| HbA1c | <6.0–6.5%*2 | <6.0%*3 | <6.5% |
*1 In patients at high risk of severe hypoglycemia, such as hypoglycemia unawareness, consideration is to be given to measuring blood glucose at different time points and to relaxing the glycemic control targets.
*2 Given that HbA1c is subject to the influence of iron metabolism in pregnant women, priority should be given to self‐monitoring of blood glucose (SMBG)‐based targets for glycemic control in these women. Again, the HbA1c target is to be individually determined for each pregnant woman, which vary depending on her gestational age (weeks) and risk of hypoglycemia.
*3 HbA1c control targets may be relaxed and set at <7.0% to avoid onset of hypoglycemia for pregnant women in whom hypoglycemia is an issue.
*4 Care is to be given to ensuring that FPG is maintained at >4.0 mmol/L (72 mg/dL) in women receiving insulin therapy.
[Q17‐6] How are pregnant women with hyperglycemic disorder to be managed during pregnancy? (Table 12)
MNT in pregnant women with hyperglycemic disorders not only provides necessary and sufficient nutrition for healthy fetal development, but also ensures strict glycemic control and appropriate weight gain 11 .
While there is a paucity of evidence to support the usefulness of increased physical activity/exercise in the management of hyperglycemic disorders in pregnancy, increased physical activity/exercise may have a role to promote health with improving maternal glycemic control, and suppressing excessive weight gain 12 .
Before the instruction of increased physical activity/exercise, it should be examined whether or not it is contraindicated in the patients.
Insulin therapy is to be implemented in pregnant women with hyperglycemic disorders if their glycemic control target is not achievable with MNT and increased physical activity/exercise. Intensive insulin therapy, combines with self‐monitoring of blood glucose (SMBG), is to be employed to better ensure sustained glycemic control 13 .
Table 12.
Appropriate weight gain in pregnant women
| Physique | Appropriate weight gain |
| BMI < 18.5 | 9–12 kg |
| 18.5 ≤ BMI < 25 | 7–12 kg |
| 25 ≤ BMI | Range to be determined individually for each woman (with 5 kg as a guide) |
BMI, body mass index. Ministry of Health, Labor and Welfare: Recommended diets for expectant and nursing mothers. Report of the Commission for Promotion of the ‘Healthy Mother‐Child Policy 21’ 2006. Source: http://www.mhlw.go.jp/houdou/2006/02/h0201‐3a.html 18 .
[Q17‐7] How are women with hyperglycemic disorders to be managed after delivery?
Since patients with gestational diabetes are shown to be at high risk of developing impaired glucose tolerance even after delivery 14 , they are to be re‐assessed for glucose metabolism from early after delivery. Thus, these women are to undergo 75 g oral glucose tolerance tests (OGTTs) 6 to 12 weeks after delivery, receive follow‐up care on a regular basis after an initial OGTT, and continue to receive instructions on MNT and physical activity/exercise.
18. 18 PEDIATRIC/ADOLESCENT DIABETES
[Q18‐1] What is the basic treatment policy for pediatric/adolescent diabetes?
The treatment policy for pediatric/adolescent patients with diabetes is to accommodate age‐specific differences in development/growth and comprehension, with sufficient consideration given to the patient’s mental immaturity 1 , 2 .
[Q18‐2] How is pediatric/adolescent type 1 diabetes diagnosed?
The diagnosis of pediatric/adolescent type 1 diabetes consists of demonstrating evidence of progressively declining endogenous insulin secretion or its depletion; islet‐specific autoantibodies have been shown to be present in the majority (70–90%) of patients 3 .
[Q18‐3] How are pediatric/adolescent patients with type 1 diabetes to be treated? (Table 13)
In pediatric/adolescent patients with type 1 diabetes, insulin injection therapy is indispensable and is therefore to be initiated immediately after the diagnosis has been established 1 , 2 .
Intensive insulin therapy represents the cornerstone of therapy for pediatric/adolescent patients with type 1 diabetes 4 .
MNT in pediatric/adolescent patients with type 1 diabetes is not primarily intended to restrict the energy intake but rather to ensure the age‐ and gender‐specific intake of energy that is necessary and sufficient for their normal development and growth 1 , 2 .
All types of sport are recommended as physical activity/exercise for pediatric/adolescent patients with type 1 diabetes as long as they have no advanced complications and their glycemic control remains stable 1 , 2 .
Hypoglycemia is likely to be associated with cognitive impairment. Hypoglycemia may not be recognized in patients below 6–7 years of age and may therefore become severe. Thus, countermeasures are to be taken against hypoglycemia in these patients. It is also to be noted that persistent hyperglycemia is associated with cognitive impairment.
Table 13.
Glycemic control targets
| Level of control | Ideal (non‐diabetes) | Appropriate | Inappropriate (intervention suggested) | High risk (intervention required) |
|---|---|---|---|---|
| Clinical evaluation | ||||
| Hyperglycemia | Not present | Asymptomatic | Polydipsia, polyuria and/or enuresis | Visual impairment, poor weight gain, growth impairment, late puberty, poor school attendance, skin or pudendal infection, and/or angiopathy |
| Hypoglycemia | Not present | No severe hypoglycemia | Severe hypoglycemia (impaired consciousness, convulsion) | |
| Biochemical evaluation | ||||
| SMBG value (mg/dL) | ||||
| Early morning/preprandial value | 65–100 | 90–145 | >145 | >162 |
| PG* value (mg/dL) | ||||
| PPG** | 80–126 | 90–180 | 180–250 | >250 |
| Bedtime PG | 80–100 | 120–180 | <120 or 180–200 | <80 or >200 |
| Nighttime PG | 65–100 | <80–161 | <75 or >162 | <70 or >200 |
| HbA1c (%) | <6.5 | <7.5 | 7.5–9.0 | >9.0 |
*PG, plasma glucose; **PPG, postprandial plasma glucose.
(1) All values given above are intended as a guide only and the glycemic control target should be determined individually to ensure it will not be associated with severe hypoglycemia or frequent mild‐to‐moderate hypoglycemia and will help achieve glycemic control as near‐normal as possible in each patient. (2) All values given above should be modified for each patient depending on whether he/she has a prior history of severe hypoglycemia or hypoglycemia unawareness. (3) PG value is given as plasma glucose value in self‐monitoring of blood glucose (SMBG). (Adapted from Rewers M et al. Pediatr Diabetes 2014 16 ).
[Q18‐4] How is type 2 diabetes diagnosed in pediatric/adolescent patients?
An oral glucose tolerance test (OGTT) using glucose (body weight × 1.75 g) (ideal body weight may also be used; up to a maximum of 75 g) is to be performed in pediatric/adolescent patients and their diagnosis is to be made according to the same glucose categories and diagnostic criteria that are used in adult patients 1 .
A family history of obesity or type 2 diabetes provides a credible clue to help establish the diagnosis of type 2 diabetes in pediatric/adolescent patients 5 .
[Q18‐5] How are pediatric/adolescent patients with type 2 diabetes to be treated?
As in adult patients with type 2 diabetes, MNT and physical activity/exercise are the mainstay of therapy in pediatric/adolescent patients with type 2 diabetes 1 , 6 .
MNT in pediatric/adolescent patients with type 2 diabetes is not primarily intended to restrict their energy intake but rather to ensure age‐ and gender‐specific intake of energy that is necessary and sufficient for their normal development and growth 1 , 7 . In obese individuals, however, their energy intake is to be limited to 90–95% of that required for their ideal body weight and to be nutritionally well‐balanced, while increased physical activity/exercise in these individuals is to primarily involve aerobic exercise, thus increasing both their physical activity levels and energy consumption 1 , 7 .
Pharmacotherapy is to be initiated 1 , 6 , with metformin as the first choice 8 , 9 , 10 in pediatric/adolescent patients with type 2 diabetes with suboptimal glycemic control despite MNT and physical activity/exercise.
In patients with ketoacidosis or those with inadequate glycemic control despite administration of oral glucose‐lowering agents, insulin therapy is to be initiated 1 , 6 .
[Q18‐6] How is neonatal diabetes to be diagnosed and treated?
Neonatal diabetes is broadly classified into transient and persistent phenotypes; their diagnosis entails testing for the respective responsible genes 11 .
Sulfonylureas (SUs) have been shown to be effective in treating patients with KCNJ11/ABCCB8 gene mutations and to allow these patients to discontinue insulin therapy 12 .
[Q18‐7] How are pediatric/adolescent patients and their families to be supported?
Pediatric/adolescent patients are to be given optimal therapy, even at school 1 , 13 .
Pediatric/adolescent patients are to participate in all school events and school administrators are to ensure that their schools provide support for their participation 13 .
Given that mental/psychological factors have been shown to significantly affect the patient’s diabetes management and prognosis, mental/psychological counseling is to be offered with sufficient care given to addressing individual differences in mental/psychological maturity 14 , 15 .
Immediately after affected patients have been diagnosed, their families are to be fully instructed on their diabetes as well as the treatment policy decided on to address their individual maturity 13 .
Diabetes camps are intended to offer support for pediatric patients to grow into independent adults and include medically designed and recreational programs 1 .
19. 19 SYNOPSIS OF THE JGS/JDS CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT OF DIABETES IN THE ELDERLY (Clinical questions [CQ], summaries and grades of recommendation)
I. Background and characteristics of diabetes in the elderly
-
1
Aging and glucose tolerance
[I‐CQ‐1] Is there any relationship between aging and impaired glucose tolerance?
Summary
Glucose tolerance deteriorates, and the frequency of occurrence of diabetes increases with aging.
-
2
Characteristics of diabetes in the elderly
[I‐CQ‐2] What are the characteristics of diabetes in the elderly?
Summary
-
Elderly patients with diabetes are mainly characterized as:
① Being susceptible to postprandial hyperglycemia and hypoglycemia (see II‐CQ‐2) and as being vulnerable to hypoglycemia (see II‐CQ‐4);
② Being susceptible to drug‐related adverse effects due to such factors as impaired renal function (see II‐CQ‐5);
③ Being likely to be associated with atherosclerotic complications (see II‐CQ‐6); and
④ Being likely to be associated with geriatric syndrome, e.g., dementia, cognitive impairment, depression, decreased activities of daily living (ADL), and sarcopenia (see II‐CQ‐8 to II‐CQ‐10).
-
3
Complications of diabetes in the elderly
[I‐CQ‐3] What are the complications associated with diabetes in the elderly?
Summary
In elderly patients with diabetes as well, hyperglycemia represents a risk factor for diabetic retinopathy, diabetic nephropathy, coronary artery disease, stroke, and cardiac failure.
Patients with diabetes 75 years old or older are particularly highly likely to be associated with geriatric syndrome, e.g., dementia, decreased ADL, sarcopenia, falls/fractures, frailty, urinary incontinence, and undernutrition.
II. Diagnosis/pathophysiology of diabetes in the elderly
-
1
Diagnosis of diabetes in the elderly
[II‐CQ‐1] Are the diagnostic criteria employed for diabetes in the elderly similar to those used for diabetes in adults?
Summary
Similar diagnostic criteria are employed for diabetes in the elderly to those used for diabetes in adults.
-
2
Hyperglycemia in diabetes in the elderly
[II‐CQ‐2] Are elderly patients with diabetes susceptible to postprandial hyperglycemia?
Summary
Elderly patients with diabetes are susceptible to postprandial hyperglycemia.
[II‐CQ‐3] Are elderly patients with diabetes susceptible to hyperosmolar hyperglycemic state (HHS)?
Summary
Elderly patients with diabetes are susceptible to HHS.
-
3
Hypoglycemia in diabetes in the elderly
[II‐CQ‐4] How is hypoglycemia characterized in elderly patients with diabetes?
Summary
Hypoglycemia in the elderly is characterized as being associated with vagueness of autonomic symptoms, e.g., perspiration, palpitation and hand tremor. They are likely to develop into hypoglycemia unawareness (asymptomatic hypoglycemia) and severe hypoglycemia. Thus, hypoglycemia is likely to adversely affect the elderly.
-
4
Diabetes in the elderly and changes associated with aging
[II‐CQ‐5] Are elderly patients with diabetes associated with drug‐related adverse events?
Summary
Elderly patients with diabetes are associated with impairment of renal and hepatic function in many cases and are therefore susceptible to drug‐related adverse events.
[II‐CQ‐6] Is diabetes in the elderly associated with an increased incidence of atherosclerotic disease?
Summary
Diabetes in the elderly is associated with many complications which result from atherosclerosis as an underlying disease and may remain asymptomatic in many cases.
[II‐CQ‐7] Is the risk of mortality increased in elderly patients with diabetes compared to that in those without?
Summary
Elderly patients with diabetes are associated with a higher risk of mortality than those without diabetes.
While poor glycemic control is associated with a risk for mortality in the elderly, this association becomes weaker in those aged 75 years old or older.
[II‐CQ‐8] Are elderly patients with diabetes likely to be associated with cognitive impairment or dementia?
Summary
Elderly patients with diabetes are likely to be associated with cognitive impairment or dementia (see III‐CQ‐2 to III‐CQ‐4 and V).
[II‐CQ‐9] What psychological states need to be watched for in elderly patients with diabetes?
Summary
Elderly patients with diabetes need to be watched for depression (depressive tendency or depression) and decreased quality of life (QOL) to which they are susceptible.
[II‐CQ‐10] Are elderly patients with diabetes associated with impairment of physical function?
Summary
Elderly patients with diabetes are associated with impairment of physical function leading to decreased ADL, falls/fractures, sarcopenia, and frailty.
III. Comprehensive geriatric assessment in elderly patients with diabetes
[III‐CQ‐1] What do elderly patients with diabetes need to be assessed for?
Summary
Comprehensive geriatric assessment (CGA) is intended to assess physical and cognitive functions, psychological states, nutritional status, drug use and socioeconomic status in the elderly as well as to implement various measures required based on this assessment.
Given its proven efficacy in decreasing the number of institutionalized patients and in decreasing mortality, multidisciplinary CGA is to be implemented in elderly patients with diabetes (grade of recommendation: A)
[III‐CQ‐2] Why do elderly patients with diabetes need to be assessed for cognitive function?
Summary
Elderly patients with diabetes need to be assessed for cognitive function, as cognitive impairment in these patients leads to decreased adherence to their self‐management (self‐care) resulting in an increased risk of severe hypoglycemia.
[III‐CQ‐3] How are elderly patients with diabetes screened for cognitive impairment?
Summary
Screening tests for cognitive impairment include Mini‐Mental State Examination (MMSE), Revised Hasegawa’s Dementia Scale (HDS‐R), Dementia Assessment Sheet in Community‐based Integrated Care System‐21 items (DASC‐21), and Montreal Cognitive Assessment (MoCA).
Screening tests for impaired performance, with which elderly patients with diabetes are likely to be associated, include a simply scored clock drawing test and the Mini‐Cog.
[III‐CQ‐4] How are elderly patients with diabetes assessed for physical function?
Summary
Elderly patients with diabetes are assessed for physical function using instrumental ADL (IADL) (e.g., shopping, food preparation, ability to handle drugs, ability to handle finances), as well as basic ADL (BADL) (e.g., dressing, bathing, toileting, mobility).
IV. Assessment of complications in elderly patients with diabetes
[IV‐CQ‐1] What complications should elderly patients with diabetes be assessed for?
Summary
Elderly patients with diabetes should be assessed for diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, diabetic foot lesions, macroangiopathy, infections, periodontal disease, and dementia.
1. Diabetic retinopathy
[IV‐CQ‐2] Should elderly patients with diabetes be referred to an ophthalmologist for assessment of diabetic retinopathy?
Summary
Given that hyperglycemia is a risk factor for the onset or progression of diabetic retinopathy in the elderly as well, elder patients with diabetes need to be regularly referred to an ophthalmologist to be assessed for diabetic retinopathy and other relevant factors (grade A).
2. Diabetic nephropathy
[IV‐CQ‐3] Should elderly patients with diabetes be assessed regularly for urinary albumin/protein values and estimated glomerular filtration rates (eGFR)?
Summary
Given that hyperglycemia and hypertension are both risk factors for the onset or progression of diabetic nephropathy in the elderly as well, elderly patients with diabetes should be assessed for diabetic nephropathy based on regular assessment of their urinary albumin/protein values and eGFR (grade A).
3. Diabetic neuropathy
[IV‐CQ‐4] How are elderly patients with diabetes assessed for diabetic neuropathy and cared for?
Summary
While diabetic neuropathy is usually diagnosed based on the bilateral absence of Achilles tendon reflex, decreased vibratory sensation in the lower extremities and subjective symptoms, it should be kept in mind that vibratory sensation becomes attenuated with aging.
Foot care should be given to prevent diabetic foot lesions in patients with diabetic neuropathy.
4. Diabetes in the elderly and infections
[IV‐CQ‐5] What infections are elderly patients with diabetes susceptible to?
Summary
Elderly patients with diabetes are susceptible to such infections as pneumonia, urinary tract infections, septicemia and tuberculosis.
[IV‐CQ‐6] Are these infections amenable to prevention with pneumococcal and influenza vaccines?
Summary
It is preferable that pneumococcal and influenza vaccination be implemented in the elderly to prevent infections (grade B).
V. Glycemic control and dementia
[V‐CQ‐1] Is diabetes or hyperglycemia a likely risk factor for cognitive impairment or onset of dementia in the elderly?
Summary
Diabetes is a likely risk factor for cognitive impairment or onset of dementia in the elderly.
Hyperglycemia, too, is a likely risk factor but requires to be examined in greater detail for its association with dementia.
[V‐CQ‐2] Is severe hypoglycemia a likely risk factor for cognitive impairment or onset of dementia in the elderly?
Summary
Severe hypoglycemia is a likely risk factor for cognitive impairment or onset of dementia in the elderly.
[V‐CQ‐3] Is tight glycemic control effective in reducing cognitive impairment or dementia in elderly patients with diabetes?
Summary
Very few randomized controlled trials (RCTs) have demonstrated a clear role for favorable glycemic control in reducing the onset or progression of dementia.
It remains unclear whether tight glycemic control is effective in preventing cognitive impairment or onset of dementia.
VI. Glycemic control and decreased physical function
[VI‐CQ‐1] Is hyperglycemia a likely risk factor for decreased ADL, sarcopenia, and falls/fractures in elderly patients with diabetes?
Summary
Hyperglycemia is a likely risk factor for decreased ADL, sarcopenia, and falls/fractures in elderly patients with diabetes.
[VI‐CQ‐2] Is low HbA1c or hypoglycemia a likely risk factor for falls/fractures or frailty in elderly patients with diabetes?
Summary
Low HbA1c or hypoglycemia is a risk factor for falls/fractures or frailty in elderly patients with diabetes.
[VI‐CQ‐3] Is glycemic control effective in maintaining ADL in elderly patients with diabetes?
Summary
While hyperglycemia is a risk factor for decreased ADL or decreased physical function, there is little clear evidence to demonstrate that ADL deterioration may be prevented by improving glycemic control.
[VI‐CQ‐4] Is diabetes or hypoglycemia a likely risk factor for depression (depression or depressive tendency) in the elderly?
Summary
Diabetes is a risk factor for depression in the elderly.
Hypoglycemia is associated with depression or decreased QOL in elderly patients with diabetes.
VII. Glycemic control goals in elderly patients with diabetes
[VII‐CQ‐1] Is glycemic control effective in inhibiting the onset or progression of complications in elderly patients with diabetes?
Summary
Favorable and appropriate glycemic control should be ensured for elderly patients with diabetes, given that hyperglycemia is a risk factor for diabetic microangiopathy, macroangiopathy, infections (see VII‐CQ‐2), mortality, cognitive impairment (see V‐CQ‐1), decreased ADL, sarcopenia, frailty, and falls/fractures (see VI‐CQ‐1) in elderly patients with diabetes (grade A).
[VII‐CQ‐2] Is glycemic control effective in preventing infections in elderly patients with diabetes?
Summary
Favorable glycemic control is effective for preventing infections in elderly patients with diabetes (grade A).
[VII‐CQ‐3] Is there any relationship between HbA1c values and the onset of macroangiopathy or mortality?
Summary
Given that there is a J‐curve phenomenon between HbA1c values and the onset of macroangiopathy or mortality, not only high but low HbA1c values need to be watched for.
[VII‐CQ‐4] Should tight glycemic control be implemented in elderly patients with diabetes?
Summary
Appropriate glycemic control focused on ensuring safety, rather than tight glycemic control, should be implemented in elderly patients with diabetes (grade A).
[VII‐CQ‐5] What are the considerations to be kept in mind in determining the glycemic control goal for elderly patients with diabetes?
Summary
The glycemic control goal should be individually determined for each patient not only in light of the Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee’s ‘Glycemic control (HbA1c) goals in elderly patients with diabetes’ (Figure 8), which recommends that careful consideration be given to IADL, BADL, cognitive function, coexisting diseases/functional impairment, and the risk for severe hypoglycemia, but also with due consideration given to his/her psychology, QOL, socioeconomic status, and his/her and family preferences (grade A).
Figure 8.
Glycemic control targets (HbA1c values) for elderly patients with diabetes. The glycemic target is to be determined for each patient by taking into account his/her age, duration of diabetes, risk for hypoglycemia, and any support available to the patient, as well as the patient’s cognitive function, basic/instrumental ADL, and comorbidities/functional impairments, while noting the potential risk of hypoglycemia that increases with age in each patient. Note 1: Refer to the Japan Geriatrics Society website (https://www.jpn‐geriat‐soc.or.jp/tool/index.html), for the evaluation of the cognitive function, basic ADL (e.g. self‐care abilities such as dressing, mobility, bathing, and toileting), and instrumental ADL (e.g. abilities to maintain an independent household such as shopping, meal preparation, taking medication, and handling finances). In end‐of‐life care, priority is to be given to preventing significant hyperglycemia and subsequent dehydration and acute complications through appropriate therapeutic measures. Note 2: As in other age groups, the glycemic target is set at <7.0% in the elderly for preventing diabetic complications. However, this could be set at <6.0% for those likely to achieve glycemic control through diet and exercise therapy alone or those likely to achieve glycemic control with drug therapy without adverse reactions, or 8.0% for those in whom intensifying therapy may prove difficult. In either case, no lower limit is specified for the glycemic target. A glycemic target of <8.5% may be allowed in patients thought to be in category III and therefore at risk of developing adverse reactions to multi‐drug combination therapy or in those with serious comorbidities or poor social support. Note 3: In patients in whom priority should be given to preventing the onset/progression of diabetic complications due to their duration of disease, the glycemic control target or its lower limit may be set for each elderly patient with appropriate measures in order to prevent severe hypoglycemia. In patients in whom any of these agents was initiated before the age of 65 and whose HbA1c values are shown to fall below their glycemic control targets described above, current treatments are to be continued, with utmost care being taken to avoid potential severe hypoglycemia. Glinides may be classified as drugs unlikely to be associated with severe hypoglycemia, as the onset of severe hypoglycemia varies depending on the type and amount of glinide used in a particular patient relative to the patient’s glucose level. (Cited from Haneda, M., Inagaki, N., Suzuki, R. et al. Glycemic targets for elderly patients with diabetes. Diabetol Int 7, 331–333 (2016). https://doi.org/10.1007/s13340‐016‐0293‐8).
VIII. MNT for elderly patients with diabetes
[VIII‐CQ‐1] Is MNT as effective for elderly patients with diabetes as for non‐elderly patients?
Summary
MNT aimed at ensuring appropriate overall energy intake and a balanced diet is effective in correcting hyperglycemia, dyslipidemia or obesity in elderly patients with diabetes as well (grade A).
[VIII‐CQ‐2] What are the considerations to be kept in mind in determining adequate energy intake at initiation of therapy for elderly patients with diabetes?
Summary
The amount of energy to be taken (adequate energy intake) per 1 kg/target body weight (TBD)* may be approximately defined as ranging between 25 and 30 kcal in elderly patients engaged in low‐level activities.
A relatively greater, well‐balanced, intake of energy appears to be desirable in elderly patients with sarcopenia, frailty, and undernutrition or at risk of any of these conditions.
Energy intake needs to be altered for each patient, as required, in light of changes in body weight, body mass index (BMI), muscle mass, muscular strength, mental/psychological examinations.
* While the term ‘ideal body weight’ was used in this section of the Japanese version of the current Guidelines in reference to the Clinical Practice Guidelines for the Management of Diabetes in the Elderly 2017, the term ‘target body weight’ is used, instead of ‘ideal body weight’, to ensure consistency with that used in the Chapter 3 on Medical nutrition therapy (MNT) of the current English‐language version.
[VIII‐CQ‐3] What are the considerations to be kept in mind in determining adequate carbohydrate, protein and lipid intakes for elderly patients with diabetes?
Summary
In MNT for diabetes, it is generally assumed that carbohydrates and proteins should account for 50 to 60% and up to 20%, respectively, of the diet being given, with lipids accounting for the remaining 20% or less. When lipids account for more than 25% of the diet, care needs to be given to ensuring an appropriate fatty acid composition, for instance, by reducing saturated fatty acids.
Care needs to be taken to ensure that carbohydrate intake is not inadequate or excessive in elderly patients.
Elderly patients should take an adequate amount of proteins to protect against frailty and sarcopenia unless they have severe renal impairment.
[VIII‐CQ‐4] Is dietary sodium (salt) restriction effective for elderly patients?
Summary
Salt restriction improves blood pressure in elderly patients as well (grade A).
Salt restriction may reduce the onset of cardiovascular disease in elderly patients with diabetes.
It is recommended that salt restriction be implemented with a focus on maintaining an adequate dietary intake and QOL in elderly patients.
[VIII‐CQ‐5] Is there any relationship between vitamin/fatty acid intake and cognitive impairment in elderly patients with diabetes?
Summary
An inadequate intake of vitamin Bs/As and vegetables may be linked to cognitive impairment.
[VIII‐CQ‐6] Is there any dietary pattern recommended for elderly patients with diabetes?
Summary
A well‐balanced dietary pattern, characterized by an adequate intake of vegetables and fish, is recommended (grade B).
[VIII‐CQ‐7] Is there any relationship between inadequate vitamin D/calcium intake and bone mineral density (BMD)?
Summary
Inadequate calcium intake is associated with decreases in BMD.
There is no consensus as to whether there is any relationship between vitamin D intake and BMD.
[VIII‐CQ‐8] How are elderly patients with diabetes assessed for undernutrition?
Summary
While assessments of undernutrition in elderly patients include Subjective Global Assessment (SGA), Mini Nutritional Assessment (MNA), MNA‐Short Form (MNA‐SF), and Malnutrition Universal Screening Tool (MUST), weight loss and decreased dietary intake also serve as clues as to the presence of undernutrition in elderly patients.
Undernutrition should be suspected in patients with unintentional weight loss and those exhibiting decreased dietary intake and these patients should be assessed for BMI and body composition and closely examined for the underlying cause, including the potential presence of malignancy.
IX. Physical activity/exercise for elderly patients with diabetes
[IX‐CQ‐1] Is physical activity/exercise effective in improving glycemic control, cognitive function, ADL, depression and QOL in elderly patients with diabetes?
Summary
Physical activity/exercise, e.g., regular physical activity and walking, is effective not only in correcting metabolic derangement but also in maintaining life prognosis and ADL and reducing cognitive impairment in elderly patients with diabetes as well (grade A)
Resistance training improves glycemic control and increases lean body mass and muscle strength in elderly patients with type 2 diabetes (grade B).
X. Oral hypoglycemic agents and GLP‐1 receptor agonists in elderly patients with diabetes
[X‐CQ‐1] What are the precautions to be kept in mind when implementing glucose‐lowering therapy in elderly patients with diabetes?
Summary
Glucose lowering therapy should be implemented in elderly patients with diabetes with care to avoid hypoglycemia and other adverse events, leading to impairment of cognitive function, ADL and QOL with due consideration given to each patient’s psychosomatic function and disease condition as well as to the pharmacological profile of each hypoglycemic agent being used (grade A).
[X‐CQ‐2] Is the use of sulfonylureas (SUs) likely to cause hypoglycemia in elderly patients with diabetes?
Summary
The use of SU is a risk factor for hypoglycemia in elderly patients with diabetes.
Each SU should be initiated at its minimum dose and titrated upward or downward for each patient depending on his/her renal function, HbA1c and hypoglycemic symptoms.
[X‐CQ‐3] Does metformin reduce cardiovascular death in elderly patients with diabetes?
Summary
Metformin may reduce the risk of cardiovascular death in the elderly as well.
[X‐CQ‐4] Is metformin a risk factor for lactic acidosis in elderly patients with diabetes?
Summary
The Cochrane reviews demonstrate that lactic acidosis occurs only very rarely in elderly patients receiving metformin, with its incidence shown to be not significantly higher than that in those not receiving metformin.
Given that no data is currently available on metformin‐associated lactic acidosis from large‐scale clinical studies involving elderly patients with diabetes alone, however, the JDS ‘Committee on the Proper Use of Biguanides’ recommends that metformin be used carefully in elderly patients and even more carefully in those aged 75 years old or older.
Elderly patients receiving metformin or any metformin‐containing combination agent should be regularly assessed for renal function using eGFR.
[X‐CQ‐5] What are the precautions to be kept in mind when using oral hypoglycemic agents other than SUs or metformin, as well as GLP‐1 receptor agonists, in elderly patients with diabetes?
Summary
While glinides are suitable for use in improving postprandial hyperglycemia characteristic of diabetes in the elderly, the risk of hypoglycemia associated with their use, as well as their dosing frequency and timings, is likely to increase the patient’s burden.
Attention needs to be given to gastrointestinal symptoms and dosing frequency with α‐glucosidase inhibitors (α‐GIs), the risk of cardiac failure and fracture with thiazolidinediones (TZDs), and dehydration and urogenital infections with SGLT2 inhibitors.
DPP‐4 inhibitors are less likely to cause hypoglycemia when used as monotherapy but may cause severe hypoglycemia when used in combination with SU. Thus, SU needs to be used at a reduced dose in combination therapy with a DPP‐4 inhibitor.
Attention needs to be given to gastrointestinal symptoms (e.g., nausea and vomiting) and weight loss with GLP‐1 receptor agonists.
[X‐CQ‐6] Is multi‐drug combination therapy a risk factor for hypoglycemia or falls in elderly patients with diabetes?
Summary
Multi‐drug combination therapy is a risk factor for hypoglycemia or falls in elderly patients with diabetes.
XI. Insulin therapy in elderly patients with diabetes
[XI‐CQ‐1] What are the precautions to be kept in mind when implementing insulin therapy in elderly patients with diabetes?
Summary
Given that insulin therapy is likely to cause severe hypoglycemia in elderly patients with diabetes, measures need to be taken to protect them against hypoglycemia and to ensure that these patients and their caregivers are well informed about the measures being taken.
XII. Countermeasures against hypoglycemia and sick days in elderly patients with diabetes
[XII‐CQ‐1] Are hypoglycemic symptoms in elderly patients with diabetes similar to those in younger adults?
Summary
It should be noted that typical autonomic symptoms of hypoglycemia (e.g., palpitation, perspiration and tremor) are likely to become attenuated, and atypical symptoms (e.g., dizziness and shakiness) are likely to become more frequent, with advancing years.
[XII‐CQ‐2] What are the risk factors for hypoglycemia in elderly patients with diabetes?
Summary
Risk factors for hypoglycemia in elderly patients with diabetes include: insulin therapy; use of SUs; low/high HbA1c values; prolonged duration of diabetes; history of coronary artery disease or stroke; advanced age (75–80 years); cognitive impairment or dementia; depression; decreased ADL; renal impairment; liver disease; decreased dietary intake; infections; multi‐drug combination therapy; early post‐discharge phase; and nursing home institutionalization.
[XII‐CQ‐3] What are the precautions against diabetes sick days characterized by the onset of fever, diarrhea, vomiting and decreased appetite?
Summary
Care needs to be taken to supplement each patient’s water and food (carbohydrate) intake on sick days.
Consideration needs to be given to reducing or interrupting the oral hypoglycemic agents being used in each patient on sick days, as a rule, and precautions should be taken against hypoglycemia associated with SUs, lactic acidosis associated with metformin, and dehydration associated with SGLT2 inhibitors.
Long‐ and intermediate‐acting insulin formulations should not be discontinued, as a rule, in elderly patients with diabetes, even on sick days.
Precautions should be taken against hypoglycemia associated with insulin therapy.
XIII. Hypertension and dyslipidemia in elderly patients with diabetes
[XIII‐CQ‐1] Is antihypertensive management effective in reducing the onset or progression of diabetic microangiopathy and macroangiopathy in elderly patients with diabetes?
Summary
Antihypertensive management is effective in reducing the onset or progression of diabetic microangiopathy and macroangiopathy in elderly patients with diabetes (grade A).
[XIII‐CQ‐2] Is antidyslipidemic management effective in reducing the onset or progression of macroangiopathy in elderly patients with diabetes?
Summary
Antidyslipidemic management is effective in reducing the onset or progression of macroangiopathy in elderly patients with diabetes (grade A).
XIV. Diabetes in institutionalized elderly patients
[XIV‐CQ‐1] Is diabetes in elderly patients a likely risk factor for nursing home institutionalization?
Summary
Diabetes in elderly patients is a likely risk factor for nursing home institutionalization.
[XIV‐CQ‐2] What are the characteristics of institutionalized elderly patients with diabetes?
Summary
Institutionalized elderly patients with diabetes are characterized as frequently requiring emergency room visits or hospital admissions and as being frequently associated with bedsore and hypoglycemia.
XV. Terminal care for elderly patients with diabetes
[XV‐CQ‐1] What are the precautions to be kept in mind in providing terminal care for elderly patients with diabetes?
Summary
Care for elderly patients with diabetes in the terminal phase should be focused on decreasing symptoms of marked hyperglycemia and hypoglycemia and alleviating pain while honoring their preferences, thus enabling them to live out their life with dignity.
20. 20 ACUTE METABOLIC COMPLICATIONS OF DIABETES, SICK DAYS, AND INFECTIOUS DISEASES
[Q20‐1] How is diabetic ketoacidosis (DKA) diagnosed and treated?
Diabetic ketoacidosis (DKA) is defined as a state that occurs as a consequence of inadequate insulin action and increased insulin‐counterregulatory hormone secretion and which requires emergency attention due to associated hyperglycemia (>250 mg/dL), ketosis (increased β‐hydroxybutyric acid), acidosis (arterial blood pH, ≤7.30; bicarbonate ion [HCO3−], ≤18 mEq/L) 1 , 2 .
Patients presenting with DKA are to be appropriately managed with normal saline‐based fluid and electrolyte (e.g., sodium chloride and potassium) replacement as required 1 .
As a rule, acidosis is not to be corrected in patients with DKA 1 , 2 .
Patients presenting with DKA are to be given regular insulin as continuous intravenous insulin infusions 1 , 2 .
The use of bolus insulin injection in children is associated with the risk of cerebral edema and is not recommended 3 .
[Q20‐2] How is a hyperosmolar hyperglycemic state (HHS) diagnosed and treated?
A hyperosmolar hyperglycemic state (HHS) is associated with hyperglycemia (>600 mg/dL) and hyperosmolarity (effective osmolality, >320 mOsm/L) and potentially mild ketosis (if present), but not severe ketoacidosis (arterial blood pH >7.30; HCO3−, ≤18 mEq/L) 2 .
Patients presenting with HHS are to be appropriately managed with normal saline‐based fluid and electrolyte replacement as required 2 .
As with patients with DKA, patients presenting with HHS are to be given regular insulin as continuous intravenous insulin infusions 2 .
[Q20‐3] How is lactic acidosis (LA) diagnosed and treated?
Lactic acidosis (LA) is defined as a state of metabolic acidosis (arterial blood pH, <7.35) due to the presence of a markedly increased lactic acid concentration (≥5.0 mmol/L) resulting from the overproduction or metabolic dysregulation of lactic acid and requires emergency attention 4 .
Although LA is reported in patients receiving biguanides, the majority of these cases occur in patients for whom biguanides should have been contraindicated or used with caution.
Patients with LA should be treated for any underlying disease 4 .
In patients with LA, sufficient tissue blood flow and oxygenation should be ensured with oxygen supplementation, artificial respiration, extracellular fluid replacement or vasopressor therapy, as required 4 .
[Q20‐4] How is hypoglycemia managed?
Patients exhibiting hypoglycemic symptoms, such as palpitation, sweating, weakness or a decreased level of consciousness, or those with a usual glucose level of <70 mg/dL should be diagnosed as having hypoglycemia and managed accordingly 5 .
Patients with hypoglycemia should be managed with oral carbohydrates (equivalent to glucose 5–10 g), intravenous glucose infusion (equivalent to glucose 10–20 g), or muscular glucagon injection. Hypoglycemia may recur or be prolonged, even after the resolution of symptoms and therefore needs to be closely monitored and managed 6 .
[Q20‐5] Are any infections typically associated with diabetes?
Infections, such as emphysematous cholecystitis, organ or soft tissue abscesses, rhinocerebral mucormycosis, malignant external otitis, emphysematous cystitis, emphysematous pyelitis, necrotizing fasciitis and Fournier’s gangrene, tend to have diabetes as an underlying disease 7 .
[Q20‐6] How is glycemic control managed during infection?
Diabetes is associated with decreased multinuclear neutrophil migration, adhesion, phagocytic and bactericidal capacity. Thus, infections tend to persist and become severe in patients with poor glycemic control.
Hyperglycemia should be treated with insulin therapy in patients with a severe infection 5 , 8 , 9 .
These patients must also be managed not only with fluid replacement and continuous intravenous insulin infusion, but also with immediate treatment of any underlying disease responsible for hyperglycemia from an early stage onwards (the primary infection site and the causative bacteria are to be identified and appropriate agents are to be chosen for the pathogen) 9 .
[Q20‐7] Is vaccination recommended in patients with diabetes?
Influenza vaccination is recommended for patients with diabetes 10 , 11 .
Pneumococcal vaccination is recommended for patients with diabetes 12 .
[Q20‐8] How are sick days to be managed?
Patients with diabetes should be encouraged to establish a connection with healthcare facilities ahead of time to ensure that they will be available for consultation during sick days 2 , 13 .
Patients with diabetes are to be instructed not to discontinue oral hypoglycemic agents or insulin without their physicians’ instruction 2 , 13 .
When they have any problems with eating, patients with diabetes are to be encouraged to consult healthcare facilities early and to receive appropriate instructions 2 , 13 .
Care is to be taken to make sure that patients with diabetes have a sufficient water intake to prevent potential dehydration and that they consume a sufficient amount of easily digestible carbohydrates (e.g., porridge, noodles and fruit juice) to ensure a sufficient intake of energy 2 , 13 during sick days.
Patients with diabetes are to be instructed to self‐monitor their glucose levels and to have their ketone body levels measured as frequently as possible during sick days 2 , 13 .
21. 21 PREVENTION OF TYPE 2 DIABETES
[Q21‐1] How are patients assessed to determine their risk of type 2 diabetes?
Various risk factors have been identified for type 2 diabetes, and a risk model (risk scores) is currently being developed for type 2 diabetes in Japanese 1 , 2 , 3 .
[Q21‐2] How much does obesity or body weight change contribute to the onset of type 2 diabetes?
There is a strong relationship between the extent of obesity and the onset of type 2 diabetes 4 . Body mass index (BMI) cut‐off for onset of type 2 diabetes is shown to be lower in Asians than in Westerners 5 , 6 .
Overweight during childhood to early adulthood is shown to increase the future risk of type 2 diabetes. Overweight during early adulthood is particularly important 7 . Overweight in childhood does not increase the risk, if it is resolved before adolescence 7 .
A 2 kg weight loss with lifestyle modification is associated with a reduced risk of type 2 diabetes 8 , 9 , 10 . Weight loss surgery markedly reduces the risk of type 2 diabetes in highly obese individuals 11 .
[Q21‐3] Are physical activity and exercise habits associated with the risk of type 2 diabetes?
There is a negative dose‐response relationship between physical activity level and risk of type 2 diabetes, while overexercise is not associated with the risk of type 2 diabetes 12 , 13 .
Not only aerobic exercise but muscle training is associated with a reduced risk of type 2 diabetes, with their combination shown to markedly decrease the risk further 14 , 15 .
Longer time spent in watching television or working in a sitting position is associated with an increased risk of type 2 diabetes 16 , 17 .
[Q21‐4] How much do energy intakes or dietary nutrient ratios contribute to the onset of type 2 diabetes?
Dietary modification aimed at ensuring an optimal total energy intake plays an important role in preventing type 2 diabetes 8 , 9 , 10 .
There is a positive dose‐response relationship between qualitative markers of carbohydrate, i.e., glycemic index (GI) or glycemic load (GL), and the risk of type 2 diabetes 18 , 19 .
Intake of dietary fibers 20 , 21 and dietary magnesium 20 , 22 , 23 is associated with a reduced risk of type 2 diabetes.
[Q21‐5] How much does intake of alcohol or other beverages contribute to the onset of type 2 diabetes?
Drinking alcohol is not recommended as a preventive measure against type 2 diabetes in Asians in whom no U‐shaped relationship has been shown between alcohol intake and the risk of type 2 diabetes 24 .
Addition of not only sugar but artificial sweeteners in drinks is associated with an increased risk of type 2 diabetes 25 .
Coffee or tea intake is shown to be a preventive factor against type 2 diabetes 26 , 27 .
[Q21‐6] Do smoking and smoking cessation affect the risk of type 2 diabetes?
Smoking is an established risk factor for diabetes 28 .
Smoking cessation is temporarily associated with increased risk of diabetes due to associated weight gain, but is associated with a decreased risk of diabetes over the long term 28 .
[Q21‐7] How much does sleep contribute to the onset of type 2 diabetes?
Sleeping hours are associated with the risk of type 2 diabetes. Both short and long sleeping hours are associated with an increased risk 29 , 30 .
Decreased quality of sleep 30 and excessive daytime naps 31 are associated with an increased risk of type 2 diabetes.
[Q21‐8] How much do psychosocial factors, such as stress and working environments, contribute to the onset of type 2 diabetes?
Mental stress 32 and depressive tendencies (depression) 33 , 34 are associated with an increased risk of diabetes.
Poor working environments 35 , 36 , 37 , 38 , 39 or social environments 40 , 41 may constitute an important risk factor for type 2 diabetes.
[CQ21‐9] Does intervention with lifestyle modification prevents type 2 diabetes?
Lifestyle intervention focused on dietary and/or exercise modification is shown to delay the onset of type 2 diabetes 8 , 9 , 10 , with its effects shown to last even after completion of the intervention 42 , 43 , 44 (grade A: 100% agreement).
[Q21‐10] Is the onset of type 2 diabetes preventable with pharmacotherapy?
Biguanides 45 , α‐glucosidase inhibitors 46 , 47 , 48 , thiazolidinediones (TZDs) 49 , basal insulin formulations 50 , and GLP‐1 receptor agonists (GLP‐1RA) 51 are shown to have inhibitory effects on the onset of type 2 diabetes.
It is shown that angiotensin II receptor blockers (ARBs) and angiotensin‐converting enzyme (ACE) inhibitors decrease, and thiazide diuretics increase, the risk of type 2 diabetes 52 , 53 .
While statins are shown to be associated with an increased risk of type 2 diabetes 54 , this disadvantage does not outweigh the benefits of their cardiovascular event‐inhibitory effects 54 , 55 .
DISCLOSURE
Eiichi Araki received honoraria from AstraZeneca, Daiichi Sankyo, Kowa, Mitsubishi Tanabe Pharma, MSD, Novo Nordisk, Ono Pharmaceutical and Sanofi, also received subsidies or donations from Astellas Pharma, Bayer Yakuhin, Daiichi Sankyo, Eli Lilly Japan, Kowa, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk, Pfizer Japan, Sanofi, Sumitomo Dainippon Pharma, Taisho Pharmaceutical and Takeda Pharmaceutical, and belongs to endowed departments by MSD, Ono Pharmaceutical and Terumo. Mitsuhiko Noda received subsidies or donations from Astellas Pharma, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly Japan, Mitsubishi Tanabe Pharma, MSD, Novo Nordisk Pharma, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical and Teijin Pharma. Hiroshi Noto received honoraria from Eli Lilly Japan and MSD. Haruhiko Osawa received research funding from Daiichi Sankyo, Ono Pharmaceutical, Sysmex, Taisho Toyama Pharmaceutical and Takeda Pharmaceutical. Yukio Tanizawa received honoraria from Astellas Pharma, MSD, Novo Nordisk Pharma, Ono Pharmaceutical and Takeda Pharmaceutical, also received research funding from Seastar, also received subsidies or donations from Astellas Pharma, Daiichi Sankyo, Eli Lilly Japan, Kyowa Kirin, Mitsubishi Tanabe Pharma, MSD, Nippon Boehringer Ingelheim, Sanofi, Sumitomo Dainippon Pharma and Takeda Pharmaceutical. Kazuyuki Tobe received honoraria from Novo Nordisk Pharma, Kowa Pharmaceutical and Astellas Pharma, also received research funding from The Uehara Memorial Foundation and The Naito Foundation, also received subsidies or donations from Mitsubishi Tanabe Pharma, Takeda Pharmaceutical, Daiichi Sankyo, MSD, Asahi Kasei Pharma, Teijin Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Novo Nordisk Pharma, Eli Lilly Japan, Fuji Chemical Industries and Arkray. Narihito Yoshioka received honoraria from Novo Nordisk Pharma and Takeda Pharmaceutical. Atsushi Goto, Tatsuya Kondo, Hideki Origasa, Akihiko Taguchi have nothing to declare.
The Japan Diabetes Society: Organizational Conflict of Interest
Co‐sponsored seminar: Abbott Diagnostics Medical, Abbott Japan, Abbott Vascular Japan, Aegerion Pharmaceuticals, Ajinomoto, AR Brown, Arkray, Arkray Global Business, Asahi Kasei Pharma, ASKA Pharmaceutical, Astellas Pharma, AstraZeneca, Bayer Yakuhin, Cosmic Corporation, Covidien Japan, Daiichi Sankyo, Eiken Chemical, Eizai, Eli Lilly Japan, Fujifilm Pharma, Fujifilm Toyama Chemical, Fukuda Colin, Fukuda Denshi, Gilead Sciences, Hakubaku, Healthy Network, Hitachi Chemical Diagnostics Systems, Horiba, InBody Japan, Johnson & Johnson, Kaken Pharmaceutical, Kissei Pharmaceutical, Kotobuki Pharmaceutical, Kowa, Kracie Pharmaceutical, Kyowa Kirin, LifeScan Japan, LSI Medience, Medtronic Japan, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Mylan EPD, Nikkiso, Nippon Becton Dickinson, Nippon Boehringer Ingelheim, Nipro, Novartis Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Otsuka Pharmaceutical, Rizap Group, Roche DC Japan, Sanofi, Santen Pharmaceutical, Sanwa Kagaku Kenkyusho, SRL, Sumitomo Dainippon Pharma, Taisho Pharma, Taisho Pharmaceutical, Takeda Pharmaceutical, Terumo, Unex, Welby.
Supporting member: Abbott Japan, Arkray Global Business, Astellas Pharma, AstraZeneca, Bunkodo, Chugai Pharmaceutical, Daiichi Sankyo, EA Pharma, Eizai, Eli Lilly Japan, H + B Life Science, Horiba, Japan Tobacco, Johnson & Johnson, Kaken Pharmaceutical, Kissei Pharmaceutical, Kowa, Kyowa Kirin, LifeScan Japan, Medtronic Japan, Mitsubishi Tanabe Pharma, MSD, Nippon Boehringer Ingelheim, Nipro, Novo Nordisk Pharma, Ono Pharmaceutical, PHC, Roche DC Japan, Sanofi, Sanwa Kagaku Kenkyusho, Sekisui Medical, Shionogi, SRL, Sumitomo Dainippon Pharma, Sysmex, Taisho Pharma, Taisho Pharmaceutical, Takeda Pharmaceutical, Terumo, Tosoh.
Research grant: Abbott Japan, Eli Lilly Japan, MSD, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Sanofi, Takeda Pharmaceutical.
Award system: Eli Lilly Japan, Novo Nordisk Pharma, Sanofi.
Funding statement: The society received no specific funding for this work.
Ethics Policy
The article does not contain any studies with human or animal subjects performed by any of the authors.
Appendix 1.
Diabetes and cancer
1. Report of the Committee on Diabetes and Cancer I/II
In recent years, clear evidence has emerged from multiple meta‐analyses of the available data including those from the Japanese population to demonstrate the association between diabetes and cancer risk 1 , 2 . In 2010, the American Diabetes Association and the American Cancer Society jointly released their consensus report on the relationship between diabetes and cancer 3 . Experts from the Japan Diabetes Society and the Japanese Cancer Association launched a Joint Committee, published the first report in 2013, which provided its recommendations for physicians and healthcare providers as well as for the general public (including patients) 4 . The Committee published its second report, Report of the Joint Committee on Diabetes and Cancer II in 2016 5 .
2. Cancer risk in patients with diabetes
To date, a number of studies have reported the association between diabetes and cancer risk 6 , 7 . Generally, diabetes (mainly the type 2 variety) is reported to be associated with an increased risk of colonic, hepatic, pancreatic, breast, endometrial, and bladder cancers, as well as a reduced risk of prostate cancer.
Assumed mechanisms of oncogenesis in diabetes include insulin resistance and associated hyperinsulinemia, hyperglycemia and chronic inflammation. However, whether diabetes is a causal risk factor for cancer remains to be elucidated.
3. Glucose‐lowering agents and cancer risk
At present, the association between glucose‐lowering agents and the cancer risk remains to be fully clarified. Thus, it is thought to be preferable that priority be given to maximizing the benefits of favorable glycemic control with these agents, with due attention given to the warnings contained in their package inserts.
4. Glycemic control and cancer risk in patients with diabetes
The ‘Report of the Committee on Diabetes and Cancer II’ addressed this issue and examined the impact of glycemic control on the subsequent risk of cancer in patients with diabetes 5 , demonstrating that there is no high‐quality evidence available, at present, to clarify the association between glycemic control and cancer risk in patients with diabetes.
5. Management and prognosis of patients with cancer and diabetes
It is reported that patients with cancer and diabetes are associated with poorer short‐term and long‐term life prognosis than patients with cancer without diabetes 8 , 9 . It is also shown that diabetic patients with cancer are less likely to receive aggressive cancer therapy than patients with cancer with diabetes 10 and thatpatients with pancreatic cancer and diabetes whose HbA1c is 9.0% or higher have a lower survival rate than those whose HbA1c is less than 9.0% 11 .
Appendix 2.
Diabetes and bone mineral metabolism
1. Bone fracture risk of patients with diabetes
Bone strength is determined by bone mineral density and bone quality. The former is determined by the amount of bone mineral in bone tissue, and the latter is determined by various factors including bone composition and structure. Any decrease in bone strength is associated with an increased risk of bone fracture 1 . The relative risk of developing proximal femoral fractures is increased by about 3‐ to 7‐fold in patients with type 1 diabetes 2 , 3 , 4 , 5 . These patients are generally characterized by decreased bone mineral density, but the risk of bone fracture is disproportionately high. It is assumed that deteriorated bone quality as well as decreased bone mineral density accounts for decreased bone strength.
The impact of bone quality is more apparent in patients with type 2 diabetes than those with type 1 diabetes. The relative risk of developing proximal femoral fractures is shown to be increased to 1.3‐ to 2.8‐fold in those with type 2 diabetes 2 , 3 , 6 , although they have significantly greater bone mineral density than those with type 1 diabetes 7 .
2. Antidiabetic agents and bone metabolism
A meta‐analysis of 10 randomized controlled trials (RCTs) comparing patients with type 2 diabetes receiving and those not receiving thiazolidinediones (TZDs; e.g., rosiglitazone, pioglitazone) demonstrated that the relative risk of fracture is increased to 1.45‐fold among those receiving TZDs 8 . The relative risk is shown to be increased to 2.23‐fold among women but not men receiving TZDs 8 .
At present, no consensus has been reached about the risk of fracture associated with the use of insulin, DPP‐4 inhibitors, GLP‐1 receptor agonists, metformin, or SGLT2 inhibitors.
3. Use of osteoporosis agents in patients with diabetes
A post hoc analysis of data from RCTs demonstrated no significantly different increase in lumbar vertebra or femoral neck with the use of alendronate between patients with type 2 diabetes and controls 9 .
Appendix 3.
Pancreas/islet transplantation
1. Pancreas transplantation
Pancreas transplantation is broadly divided into simultaneous pancreas and kidney transplantation (SPK), pancreas‐after‐kidney transplantation (PAK), and pancreas transplantation alone (PTA). SPK accounts for >80% of all pancreas transplants performed in Japan and the rest of the world.
Data from the 361 brain‐dead and non‐heart beating donor pancreas transplants, performed in Japan as of the end of 2014, demonstrated a 5‐year graft survival rate of 94.9%, with the 5‐year pancreas and kidney survival rates of 76.0% and 91.4%, respectively.
2. Islet transplantation
Islet transplantation is a form of tissue transplantation that involves transplanting islets isolated from a donor pancreas into the portal vein of a recipient.
Islet transplantation is performed on insulin‐depleted patients with diabetes shown to have severe hypoglycemia repeatedly despite receiving specialist diabetes care.
While, unlike pancreas transplantation, islet transplantation may not allow its recipients to remain off insulin therapy for prolonged periods of time, it is expected to reduce the frequency of hypoglycemia and mean glucose values by stabilizing glycemic variations.
Islets transplantation from non‐heart‐beating donors were conducted 34 times to a total of 18 patients (male/female, 5/13) with the modified Edmonton protocol between 2004 and 2007 in Japan 1 .
As in Western studies, HbA1c was improved, and severe hypoglycemia resolved, among those with successful islet engraftment 1 .
While the need for multiple transplants and improvement of long‐term prognosis were among the challenges with islet transplantation, the University of Minnesota protocol, which consists of induction immunotherapy with antithymocyte globulin (thymoglobulin) or an anti‐TNF‐α receptor antibody followed by maintenance therapy with a low‐dose calcineurin inhibitor (tacrolimus) and an mTOR inhibitor (sirolimus) or an anti‐metabolic agent (mycofenolate mofetil), was reported to lead to the secession from insulin therapy in all 8 patients with type 1 diabetes receiving islet transplants from each single donor 2 .
In Japan, from 2012 onwards, islet transplantation was resumed as a part of advanced medical care B program in insulin‐depleted patients presenting with severe hypoglycemic episodes, employing a similar protocol to that of University of Minnesota and is currently being implemented as First‐class Regenerative Medicine according to the ‘Act on Securing Safety of Regenerative Medicine’.
Appendix 4.
Large‐scale clinical trials in Japan
1. J‐DOIT1
Between March 2007 and March 2012, the Japan Diabetes Outcome Intervention Trial 1 (J‐DOIT1) was conducted to investigate the effectiveness of non‐face‐to‐face, telephone‐based intervention in individuals at high risk of diabetes in preventing incident diabetes.
Of the people undergoing health check‐ups in the fiscal year 2006, high‐risk individuals (i.e., those with impaired fasting glucose [IFG]) aged 20–65 years were identified and allocated to the intervention group (n = 1,367) and the self‐management group (n = 1,240).
After completion of one‐year intervention, the study followed up all subjects for 5.5 years on average by way of annual health check‐ups and questionnaires and found no significant difference in cumulative incidence of diabetes between the intervention and self‐management groups but did find a significantly lower incidence (−41%) among those receiving telephone counselling 10 times per year than among those receiving such counselling 3 or 6 times per year in the intervention group when analyzed at each study site.
2. J‐DOIT 2 (Japan Diabetes Outcome Intervention Trial 2)
The ‘Japan Diabetes Outcome Intervention Trial 2 (J‐DOIT 2)’ was an interventional study intended to address how to decrease consultation interruptions by patients with type 2 diabetes. The interventional measures implemented in the study included encouraging patients who were being treated by their family physicians to continue treatment/consultation, providing healthcare instructions, and assisting their family physicians in their treatment/consultations.
The results of the study demonstrated that treatment/consultation interruptions decreased by 63%, suggesting that the interventional measures were significantly effective.
3. J‐DOIT 3 (the Japan Diabetes Optimal Integrated Treatment study for 3 major risk factors of cardiovascular diseases (J‐DOIT 3)
In J‐DOIT3, a total of 2,542 patients with type 2 diabetes and hypertension/dyslipidemia aged 45 to 69 years were randomly assigned to receive current guideline‐consistent treatment (conventional therapy group; targets, HbA1c <6.9%, blood pressure 130/80 mmHg, LDL‐cholesterol <120 mg/dL [or <100 mg/dL in those with a history of cardiovascular disease]) or to receive treatment aimed at more stringent control (intensive therapy group; targets, HbA1c <6.2%, blood pressure 120/75 mmHg, LDL‐cholesterol <80 mg/dL [or <70 mg/dL in those with a history of cardiovascular disease]).
At median follow‐up of 8.5 years, the primary endpoints of the study (i.e., all‐cause mortality, myocardial infarction, stroke, coronary/cerebral artery revascularization) were reduced by 19% in the intensive therapy group, while this reduction was not significantly different from that in the conventional therapy group (P = 0.094) but were significantly reduced by 24% after adjustment for all pre‐specified factors, such as smoking (P = 0.042), compared to that in the conventional therapy group 1 .
4. JDCP study
The JDCP study was a large‐scale prospective observational study of Japanese patients with type 1 and type 2 diabetes. The study was conducted to identify the risk factors for diabetes‐related comorbidities that they develop during follow‐up.
The JDCP study enrolled a total of 6,338 patients, 40–74 years of age who were being treated at participating sites nationwide between June 2007 and November 2009. The primary endpoints of the study included the onset/progression of nephropathy, retinopathy, neuropathy, macroangiopathy, and periodontal disease.
All events observed in the course of the study are currently being reviewed by the 8 subspecialty working groups involved in the study.
5. J‐DREAMS
A large‐scale registry needs to be built in an attempt to clarify how patients with diabetes are being currently treated and how diabetic complications may occur as a result, as well as to provide recommendations toward improved diabetes care and healthcare policy. Thus, with these objectives in mind, a large‐scale registry has been built since 2015 as a joint project between the Japan Diabetes Society (JDS) and the National Center for Global Health and Medicine, solicitating the participation of JDS‐accredited diabetes education facilities.
At the end of 2018, a total of 51 university and other facilities have participated in the project, with the number of patients registered totaling some 54,000, of whom 1,900 or more patients have type 1 diabetes.
J Diabetes Investig 2020; 11: 1020–1076
This article is based on the ‘Japanese Clinical Practice Guideline for Diabetes 2019’ (ISBN:978‐4‐524‐24148‐4), which was published in Japanese by Nankodo Co., Ltd. (© The Japan Diabetes Society (JDS), 2019) and has been jointly published in Diabetology International (the official English journal of JDS: https://doi.org/10.1007/s13340‐020‐00439‐5) and Journal of Diabetes Investigation (the official journal of the Asian Association for the Study of Diabetes).
References
References
1 GUIDELINE FOR THE DIAGNOSIS OF DIABETES MELLITUS
- 1. Kosaka K, Akanuma Y, Goto Y, et al Report of Committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 1982; 25: 859–866 (Japanese). [Google Scholar]
- 2. World Health Organization . Report of a WHO Consultation: Definition, Diagnosis and Classification of Diabetes MELLITUS and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization Department of Noncommunicable Disease Surveillance, 1999. Available from: http://www.staff.ncl.ac.uk/philip.home/who_dmc.htm. [Google Scholar]
- 3. Kuzuya T, Nakagawa S, Satoh J, et al Report of the Committee of Japan Diabetes Society on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 1999; 42: 385–404 (Japanese). [Google Scholar]
- 4. Seino Y, Nanjo K, Tajima N, et al Report of the Committee on the classification and diagnostic criteria of diabetes mellitus: The Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Diabetol Int 2010; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(Suppl 1): S13–S27. [DOI] [PubMed] [Google Scholar]
- 6. Kadowaki T, Haneda M, Tominaga M, et al Report of the Japan Diabetes Society’s Committee on the diagnostic criteria for diabetes mellitus and glucose metabolism disorder—a new category of fasting plasma glucose values : “high‐normal”. J Jpn Diabetes Soc 2008; 51: 281–283 (Japanese). [Google Scholar]
- 7. Kawasaki E, Maruyama T, Imagawa A, et al Diagnostic criteria for acute‐onset type 1 diabetes mellitus (2012): Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute‐onset Type 1 Diabetes Mellitus. Diabetol Int 2013; 4: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka S, Ohmori M, Awata T, et al Erratum to: Diagnostic criteria for slowly progressive insulin‐dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the Committee on Slowly Progressive Insulin‐Dependent (Type 1) Diabetes Mellitus of the Japan Diabetes Society. Diabetol Int 2015; 6: 149. [Google Scholar]
- 9. Imagawa A, Hanafusa T, Awata T, et al Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute‐onset Type 1 Diabetes Mellitus: New Diagnostic Criteria of Fulminant Type 1 Diabetes Mellitus (2012). Diabetol Int 2012; 3: 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
2 GOALS AND STRATEGIES FOR DIABETES MANAGEMENT
- 1. United Kingdom Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 2. Schauer PR, Bhatt DL, Kirwan JP, et al Bariatric surgery versus intensive medical therapy for diabetes–3‐year outcomes. N Engl J Med 2014; 370: 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Look ARG, Gregg EW, Jakicic JM, et al Association of the magnitude of weight loss and changes in physical fitness with long‐term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post‐hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016; 4: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sone H, Tanaka S, Tanaka S, et al Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab 2011; 96: 3448–3456. [DOI] [PubMed] [Google Scholar]
- 5. Ueki K, Sasako T, Okazaki Y, et al Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 951–964. [DOI] [PubMed] [Google Scholar]
3 MEDICAL NUTRITION THERAPY (MNT)
- 1. Tuomilehto J, Lindström J, Eriksson JG, et al Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350 [level 1]. [DOI] [PubMed] [Google Scholar]
- 2. Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369: 145–154 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terranova CO, Brakenridge CL, Lawler SP, et al Effectiveness of lifestyle‐based weight loss interventions for adults with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab 2015; 17: 371–378 [level 1]. [DOI] [PubMed] [Google Scholar]
- 5. Chen L, Pei JH, Kuang J, et al Effect of lifestyle intervention in patients with type 2 diabetes: a meta‐analysis. Metabolism 2015; 64: 338–347 [level 1]. [DOI] [PubMed] [Google Scholar]
- 6. Huang XL, Pan JH, Chen D, et al Efficacy of lifestyle interventions in patients with type 2 diabetes: a systematic review and meta‐analysis. Eur J Intern Med 2016; 27: 37–47 [level 1]. [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Devlin HM, Smith B, et al Effect of lifestyle interventions on cardiovascular risk factors among adults without impaired glucose tolerance or diabetes: a systematic review and meta‐analysis. PLoS One 2017; 12: e0176436 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franz MJ, Boucher JL, Rutten‐Ramos S, et al Lifestyle weight‐loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta‐analysis of randomized clinical trials. J Acad Nutr Diet 2015; 115: 1447–1463 [level 1]. [DOI] [PubMed] [Google Scholar]
- 9. Fu S, Li L, Deng S, et al Effectiveness of advanced carbohydrate counting in type 1 diabetes mellitus: a systematic review and meta‐analysis. Sci Rep 2017; 6: 37067 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Møller G, Andersen HK, Snorgaard O. A systematic review and meta‐analysis of nutrition therapy compared with dietary advice in patients with type 2 diabetes. Am J Clin Nutr 2017; 106: 1394–1400 [level 1]. [DOI] [PubMed] [Google Scholar]
- 11. Huang MC, Hsu CC, Wang HS, et al Prospective randomized controlled trial to evaluate effectiveness of registered dietitian‐led diabetes management on glycemic and diet control in a primary care setting in Taiwan. Diabetes Care 2010; 33: 233–239 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emadian A, Andrews RC, England CY, et al The effect of macronutrients on glycaemic control: a systematic review of dietary randomised controlled trials in overweight and obese adults with type 2 diabetes in which there was no difference in weight loss between treatment groups. Br J Nutr 2015; 114: 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmadi‐Abhari S, Robert N, et al Dietary intake of carbohydrates and risk of type 2 diabetes: The European Prospective Investigation into Cancer‐Norfolk study. Br J Nutr 2014; 111: 342–352. [DOI] [PubMed] [Google Scholar]
- 14. Noto H, Goto A, Tsujimoto T, et al Long‐term low‐carbohydrate diets and Type 2 diabetes risk: a systematic review and meta‐analysis of observational studies. J Gen Fam Med 2016; 17: 60–70. [Google Scholar]
- 15. Muraki I, Imamura F, Manson JE, et al Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ 2013; 347: f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bazzano LA, Li TY, Joshipura KJ, et al Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008; 31: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halbesma N, Bakker SJ, Jansen DF, et al High protein intake associates with cardiovascular events but not with loss of renal function. J Am Soc Nephrol 2009; 20: 1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen AN, Kondrup J, Børsheim E. Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res 2013; 57: 21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guasch‐Ferré M, Becerra‐Tomás N, Ruiz‐Canela M, et al Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr 2017; 105: 723–735. [DOI] [PubMed] [Google Scholar]
- 20. Tobias DK, Chen M, Hu FB, et al Effect of low‐fat diet interventions versus other diet interventions on long‐term weight change in adults: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015; 3: 968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L, Folsom AR, Eckfeldt JH, et al. ARIC Study Investigators. Plasma fatty acid composition and incidence of diabetes in middle‐aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003; 78: 91–98. [DOI] [PubMed] [Google Scholar]
- 22. Hodge AM, English DR, Giles GG, et al Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007; 86: 189–197. [DOI] [PubMed] [Google Scholar]
- 23. Harding AH, Day NE, Wareham NJ, et al Dietary fat and the risk of clinical type 2 diabetes: the European prospective investigation of Cancer‐Norfolk. Am J Epidemiol 2004; 159: 73–82. [DOI] [PubMed] [Google Scholar]
4 PHYSICAL ACTIVITY/EXERCISE
- 1. Umpierre D, Ribeiro PA, Kramer CK, et al Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta‐analysis. JAMA 2011; 305: 1790–1799 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 2. Boulé NG, Haddad E, Kenny GP, et al Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. JAMA 2001; 286: 1218–1227 [level 2]. [DOI] [PubMed] [Google Scholar]
- 3. Pai LW, Li TC, Hwu YJ, et al The effectiveness of regular leisure‐time physical activities on long‐term glycemic control in people with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2016; 113: 77–85 [level 2]. [DOI] [PubMed] [Google Scholar]
- 4. Boniol M, Dragomir M, Autier P, et al Physical activity and change in fasting glucose and HbA1c: a quantitative meta‐analysis of randomized trials. Acta Diabetol 2017; 54: 983–991 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 5. MacLeod SF, Terada T, Chahal BS, et al Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: a meta‐analysis of studies using continuous glucose monitoring. Diabetes Metab Res Rev 2013; 29: 593–603 [level 2]. [DOI] [PubMed] [Google Scholar]
- 6. Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta‐analysis. Diabetes Care 2006; 29: 2518–2527 [level 2]. [DOI] [PubMed] [Google Scholar]
- 7. Qiu S, Cai X, Schumann U, et al Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta‐analysis. PLoS One 2014; 9: e109767 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwingshackl L, Missbach B, Dias S, et al Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetologia 2014; 57: 1789–1797 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 9. Figueira FR, Umpierre D, Cureau FV, et al Association between physical activity advice only or structured exercise training with blood pressure levels in patients with type 2 diabetes: a systematic review and meta‐analysis. Sports Med 2014; 44: 1557–1572 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 10. Boulé NG, Kenny GP, Haddad E, et al Meta‐analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 2003; 46: 1071–1081 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 11. Kelley GA, Kelley KS. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: a meta‐analysis of randomized‐controlled trials. Public Health 2007; 121: 643–655 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006; CD002968 [level: 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashino Y, Jackson JL, Fukumori N, et al Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta‐analysis of randomized controlled trials. Diabetes Res Clin Pract 2012; 98: 349–360 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 14. Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta‐analysis. Diabetes Care 2011; 34: 1228–1237 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ostman C, Jewiss D, King N, et al Clinical outcomes to exercise training in Type 1 diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2018; 139: 380–391 [level 2]. [DOI] [PubMed] [Google Scholar]
- 16. Yardley JE, Hay J, Abou‐Setta AM, et al A systematic review and meta‐analysis of exercise interventions in adults with type 1 diabetes. Diabetes Res Clin Pract 2014; 106: 393–400 [level 2]. [DOI] [PubMed] [Google Scholar]
- 17. Tonoli C, Heyman E, Roelands B, et al Effects of different types of acute and chronic (training) exercise on glycaemic control in type 1 diabetes mellitus: a meta‐analysis. Sports Med 2012; 42: 1059–1080 [level 2]. [DOI] [PubMed] [Google Scholar]
- 18. Kennedy A, Nirantharakumar K, Chimen M, et al Does exercise improve glycaemic control in type 1 diabetes? A systematic review and meta‐analysis. PLoS One 2013; 8: e58861 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colberg SR, Sigal RJ, Yardley JE, et al Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016; 39: 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colberg SR, Sigal RJ, Fernhall B, et al Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010; 33: e147–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes A . 4. Lifestyle Management: Standards of Medical Care in Diabetes‐2018. Diabetes Care 2018; 41: S38–S50. [DOI] [PubMed] [Google Scholar]
5 TREATMENT WITH GLUCOSE‐LOWERING AGENTS (EXCLUDING INSULIN)
- 1. United Kingdom Prospective Diabetes Study (UKPDS) . 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non‐insulin dependent diabetes followed for three years. BMJ 1995; 310: 83–88. [PMC free article] [PubMed] [Google Scholar]
- 2. Stratton IM, Adler AI, Neil HA, et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 4. Bennett WL, Wilson LM, Bolen S, et al Oral diabetes medications for adults with type 2 diabetes: an update. Rockville, MD: Agency for Healthcare Research and Quality (US), 2011. [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 6. Selvin E, Bolen S, Yeh HC, et al Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med 2008; 168: 2070–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaku K, Tajima N, Kawamori R, et al Melbin Observational Research (MORE) study of metformin therapy in patients with type 2 diabetes mellitus. J Jpn Diabetes Soc 2006; 49: 325–331 (in Japanese). [Google Scholar]
- 8. Davies MJ, D'Alessio DA, Fradkin J, et al Management of hyperglycemia in type 2 diabetes, 2018: a Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meier C, Kraenzlin ME, Bodmer M, et al Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168: 820–825. [DOI] [PubMed] [Google Scholar]
- 10. Loke YK, Singh S, Furberg CD. Long‐term use of thiazolidinediones and fractures in type 2 diabetes: a meta‐analysis. CMAJ 2009; 180: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Home PD, Pocock SJ, Beck‐Nielsen H, et al Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet 2009; 373: 2125–2135. [DOI] [PubMed] [Google Scholar]
- 12. Colhoun HM, Livingstone SJ, Looker HC, et al Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose‐lowering drugs. Diabetologia 2012; 55: 2929–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nissen SE, Nicholls SJ, Wolski K, et al Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008; 299: 1561–1573. [DOI] [PubMed] [Google Scholar]
- 14. Tajima N, Kadowaki T, Odawara M, et al Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int 2011; 2: 32–44. [Google Scholar]
- 15. Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled study with an open‐label, long‐term extension. Diabetes Obes Metab 2014; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- 16. Hermansen K, Kipnes M, Luo E, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9: 733–745. [DOI] [PubMed] [Google Scholar]
- 17. Iwakura T, Fujimoto K, Tahara Y, et al A case of severe hypoglycemia induced by sitagliptin added to ongoing glimepiride therapy in patients with type 2 diabetes. J Jpn Diabetes Soc 2010; 53: 505–508 (Japanese). [Google Scholar]
- 18. Kadowaki T, Tajima N, Odawara M, et al Efficacy and safety of sitagliptin add‐on therapy in Japanese patients with type 2 diabetes on insulin monotherapy. Diabetol Int 2013; 7: 160–172. [Google Scholar]
- 19. Scirica BM, Bhatt DL, Braunwald E, et al Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 20. White WB, Cannon CP, Heller SR, et al Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 21. Green JB, Bethel MA, Armstrong PW, et al Effect of sitagliptin on cardiovascular outcomes in type 2 dibetes. N Engl J Med 2015; 373: 232–242. [DOI] [PubMed] [Google Scholar]
- 22. Rosenstock J, Perkovic V, Johansen OE, et al Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA Randomized Clinical Trial. JAMA 2019; 321: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaku K, Rasmussen MF, Clauson P, et al Improved glycaemic control with minimal hypoglycaemia and no weight change with the once‐daily human glucagon‐like peptide‐1 analogue liraglutide as add‐on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 341–347. [DOI] [PubMed] [Google Scholar]
- 24. Seino Y, Min KW, Niemoeller E. Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab 2012; 14: 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Storgaard H, Cold F, Gluud LL, et al Glucagon‐like peptide‐1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab 2017; 19: 906–908. [DOI] [PubMed] [Google Scholar]
- 26. Nauck MA, Frossard JL, Barkin JS, et al Assessment of pancreas safety in the development program of once‐weekly GLP‐1 receptor agonist dulaglutide. Diabetes Care 2017; 40: 647–654. [DOI] [PubMed] [Google Scholar]
- 27. Pfeffer MA, Claggett B, Diaz R, et al Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 28. Steinberg WM, Buse JB, Ghorbani MLM, et al Amylase, lipase, and acute pancreatitis in people with type 2 diabetes treated with liraglutide: results from the LEADER randomized trial. Diabetes Care 2017; 40: 966–972. [DOI] [PubMed] [Google Scholar]
- 29. Marso SP, Holst AG, Vilsboll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376: 891–892. [DOI] [PubMed] [Google Scholar]
- 30. Marso SP, Daniels GH, Brown‐Frandsen K, et al Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerstein HC, Colhoun HM, Dagenais GR, et al Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet 2019; 394: 121–130. [DOI] [PubMed] [Google Scholar]
- 32. Neal B, Perkovic V, Mahaffey KW, et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 33. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 34. Li DD, Wang TS, Shen S, et al Urinary tract and genital infections in patients with type 2 diabetes treated with sodium‐glucose co‐transporter 2 inhibitors: a meta‐analysis of randomized controlled trials. Diabetes Obes Metab 2017; 19: 348–355. [DOI] [PubMed] [Google Scholar]
- 35. Bolinder J, Ljunggren O, Johansson L, et al Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014; 16: 159–169. [DOI] [PubMed] [Google Scholar]
- 36. Kaku K, Sumino S, Katou M, et al Randomized, double‐blind, phase III study to evaluate the efficacy and safety of once‐daily treatment with alogliptin and metformin hydrochloride in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2017; 19: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ono Y, Nakamura A, Cho KY, et al The glycemic/metabolic responses to meal tolerance tests at breakfast, lunch and dinner, and effects of the mitiglinide/voglibose fixed‐dose combination on postprandial profiles in Japanese patients with type 2 diabetes mellitus. Expert Opin Pharmacother 2014; 15: 311–324. [DOI] [PubMed] [Google Scholar]
- 38. Aoki C, Suzuki K, Kuroda H, et al Fixed‐dose combination of alogliptin/pioglitazone improves glycemic control in Japanese patients with type 2 diabetes mellitus independent of body mass index. Nagoya J Med Sci 2017; 79: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kadowaki T, Inagaki N, Kondo K, et al Efficacy and safety of canagliflozin as add‐on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: results of a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2017; 19: 874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaku K, Inagaki N, Kobayashi N. Long‐term effects of mitiglinide in Japanese diabetics inadequately controlled with DPP‐4 inhibitor or biguanide monotherapy. Diabetes Ther 2014; 5: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Charpentier G, Fleury F, Kabir M, et al Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med 2001; 18: 828–834. [DOI] [PubMed] [Google Scholar]
- 42. Moses R, Slobodniuk R, Boyages S, et al Effect of repaglinide addition to metformin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 1999; 22: 119–124. [DOI] [PubMed] [Google Scholar]
- 43. Van Gaal L, Maislos M, Schernthaner G, et al Miglitol combined with metformin improves glycaemic control in type 2 diabetes. Diabetes Obes Metab 2001; 3: 326–331. [DOI] [PubMed] [Google Scholar]
- 44. Einhorn D, Rendell M, Rosenzweig J, et al Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo‐controlled study. Clin Ther 2000; 22: 1395–1409. [DOI] [PubMed] [Google Scholar]
- 45. Taskinen MR, Rosenstock J, Tamminen I, et al Safety and efficacy of linagliptin as add‐on therapy to metformin in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2011; 13: 65–74. [DOI] [PubMed] [Google Scholar]
- 46. DeFronzo RA, Ratner RE, Han J, et al Effects of exenatide (exendin‐4) on glycemic control and weight over 30 weeks in metformin‐treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 47. Derosa G, Salvadeo SA, D'Angelo A, et al Metabolic effect of repaglinide or acarbose when added to a double oral antidiabetic treatment with sulphonylureas and metformin: a double‐blind, cross‐over, clinical trial. Curr Med Res Opin 2009; 25: 607–615. [DOI] [PubMed] [Google Scholar]
- 48. Scheen AJ, Tan MH, Betteridge DJ, et al Long‐term glycaemic control with metformin‐sulphonylurea‐pioglitazone triple therapy in PROactive (PROactive 17). Diabet Med 2009; 26: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 49. Lukashevich V, Del Prato S, Araga M, et al Efficacy and safety of vildagliptin in patients with type 2 diabetes mellitus inadequately controlled with dual combination of metformin and sulphonylurea. Diabetes Obes Metab 2014; 16: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kendall DM, Riddle MC, Rosenstock J, et al Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 51. Wilding JP, Charpentier G, Hollander P, et al Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract 2013; 67: 1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
6 INSULIN THERAPY
- 1. The Diabetes Control and Complications Trial (DCCT) Research Group . Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol 1998; 116: 874–886. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi Y, Takayama S, Ito T, et al Clinical features of eighty‐six diabetic patients with post‐treatment painful neuropathy. J Jpn Diabetes Soc 1998; 41: 165–170 (Japanese). [Google Scholar]
- 3. United Kingdom Prospective Diabetes Study (UKPDS) Group . United Kingdom Prospective Diabetes Study 24: a 6‐year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med 1998; 128: 165–175. [DOI] [PubMed] [Google Scholar]
- 4. The Diabetes Control and Complications Trial (DCCT) Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [level 1]. [DOI] [PubMed] [Google Scholar]
- 5. The Diabetes Control and Complications Trial (DCCT) Research Group . The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998; 41: 416–423 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kahler P, Grevstad B, Almdal T, et al Targeting intensive versus conventional glycemic control for type 1 diabetes mellitus: a systematic review with meta‐analyses and trial sequential analysis of randomized clinical trials. BMJ Open 2014; 4: e004806 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan DM, Cleary PA, Backlund JY, et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohkubo Y, Kishikawa H, Araki E, et al Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [level 2]. [DOI] [PubMed] [Google Scholar]
- 9. United Kingdom Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [level 1]. [PubMed] [Google Scholar]
- 10. Shichiri M, Kishikawa H, Ohkubo Y, et al Long‐term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(Suppl 2): B21–B29. [PubMed] [Google Scholar]
- 11. Holman RR, Thorne KI, Farmer AJ, et al Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007; 357: 1716–1730. [DOI] [PubMed] [Google Scholar]
- 12. Liebl A, Prager R, Binz K, et al Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab 2009; 11: 45–52. [DOI] [PubMed] [Google Scholar]
- 13. Feinglos MN, Thacker CR, Lobaugh B, et al Combination insulin and sulfonylurea therapy in insulin‐requiring type 2 diabetes mellitus. Diabetes Res Clin Pract 1998; 39: 193–199. [DOI] [PubMed] [Google Scholar]
- 14. Wright A, Burden AC, Paisey RB, et al. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002; 25: 330–336. [DOI] [PubMed] [Google Scholar]
- 15. Ozbek M, Erdogan M, Karadeniz M, et al Preprandial repaglinide decreases exogenous insulin requirements and HbA1c levels in type 2 diabetic patients taking intensive insulin treatment. Acta Diabetol 2006; 43: 148–151. [DOI] [PubMed] [Google Scholar]
- 16. De Luis DA, Aller R, Cuellar L, et al Effect of Repaglinide addition to NPH insulin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2001; 24: 1844–1855. [DOI] [PubMed] [Google Scholar]
- 17. Yamada S, Watanabe M, Funae O, et al Effect of combination therapy of a rapid‐acting insulin secretagogue (Glinide) with premixed insulin in type 2 diabetes mellitus. Intern Med 2007; 46: 1893–1897. [DOI] [PubMed] [Google Scholar]
- 18. Avilés‐Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin‐treated type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled trial. Ann Intern Med 1999; 131: 182–188. [DOI] [PubMed] [Google Scholar]
- 19. Relimpio F, Pumar A, Losada F, et al Adding metformin versus insulin dose increase in insulin‐treated but poorly controlled type 2 diabetes mellitus: an open‐label randomized trial. Diabet Med 1998; 15: 997–1002. [DOI] [PubMed] [Google Scholar]
- 20. Yki‐Järvinen H, Ryysy L, Nikkilä K, et al Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med 1999; 130: 389–396. [DOI] [PubMed] [Google Scholar]
- 21. Ponssen HH, Elte JW, Lehert P, et al Combined metformin and insulin therapy for patients with type 2 diabetes mellitus. Clin Ther 2000; 22: 709–718. [DOI] [PubMed] [Google Scholar]
- 22. Juntti‐Berggren L, Pigon J, Hellström P, et al Influence of acarbose on post‐prandial insulin requirements in patients with type 1 diabetes. Diabetes Nutr Metab 2000; 13: 7–12. [PubMed] [Google Scholar]
- 23. Han A, Katoh S, Nemoto M, et al Effect of combination therapy of premixtured 50 R and voglibose in patients with type 2 diabetes. J Jpn Diabetes Soc 2004; 47: 137–140 (Japanese). [Google Scholar]
- 24. Schwartz S, Raskin P, Fonseca V, et al Effect of troglitazone in insulin‐treated patients with typeII diabetes mellitus: Troglitazone and Exogenous Insulin Study Group. N Engl J Med 1998; 338: 861–866. [DOI] [PubMed] [Google Scholar]
- 25. Mattoo V, Eckland D, Widel M, et al Metabolic effects of pioglitazone in combination with insulin in patients with type 2 diabetes mellitus whose disease is not adequately controlled with insulin therapy: results of a six‐month, randomized, double‐blind, prospective, multicenter, parallel‐group study. Clin Ther 2005; 27: 554–567. [DOI] [PubMed] [Google Scholar]
- 26. Bhat R, Bhansali A, Bhadada S, et al Effect of pioglitazone therapy in lean type 1 diabetes mellitus. Diabetes Res Clin Pract 2007; 78: 349–354. [DOI] [PubMed] [Google Scholar]
- 27. Raskin P, Rendell M, Riddle MC, et al A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin‐treated type 2 diabetes. Diabetes Care 2001; 24: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 28. Vilsbøll T, Rosenstock J, Yki‐Järvinen H, et al Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 167–177. [DOI] [PubMed] [Google Scholar]
- 29. Terauchi Y, Tamura M, Senda M, et al Long‐term safety and efficacy of tofogliflozin as add‐on to insulin in patients with type 2 diabetes: Results from a 52‐week, multicenter, randomized, double‐blind, open‐label extension, Phase 4 study in Japan (J‐STEP/INS). Diabetes Obes Metab 2018; 1–10: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eng C, Kramer CK, Zinman B, et al Glucagon‐like peptide‐1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta‐analysis. Lancet 2014; 384: 2228–2234. [DOI] [PubMed] [Google Scholar]
- 31. Holman RR, Paul SK, Bethel MA, et al 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 32. The ACCORD Study Group . Long‐term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364: 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
7 DIABETES SELF‐MANAGEMENT EDUCATION AND SUPPORT FOR THE SELF‑MANAGEMENT OF DIABETES
- 1. He X, Li J, Wang B, et al Diabetes self‐management education reduces risk of all‐cause mortality in type 2 diabetes patients: a systematic review and meta‐analysis. Endocrine 2017; 55: 712–731 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 2. Ismail K, Winkley K, Rabe‐Hesketh S. Systematic review and meta‐analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004; 363, 1589–1597 [level 2]. [DOI] [PubMed] [Google Scholar]
- 3. Pillay J, Armstrong MJ, Butalia S, et al Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta‐analysis. Ann Intern Med 2015; 163: 848–860 [level 2]. [DOI] [PubMed] [Google Scholar]
- 4. Pillay J, Armstrong MJ, Butalia S, et al Behavioral programs for type 1 diabetes mellitus: a systematic review and meta‐analysis. Ann Intern Med 2015; 163: 836–847 [level 2]. [DOI] [PubMed] [Google Scholar]
- 5. Rickheim PL, Weaver TW, Flader JL, et al Assessment of group versus individual diabetes education: a randomized study. Diabetes Care 2002; 25: 269–274 [level 1]. [DOI] [PubMed] [Google Scholar]
- 6. Trento M, Gamba S, Gentile L, et al Rethink Organization to iMprove Education and Outcomes (ROMEO): a multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care 2010; 33: 745–747 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duke S‐AS, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009; 21: CD005268 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Odgers‐Jewell K, Ball LE, Kelly JT, et al Effectiveness of group‐based self‐management education for individuals with Type 2 diabetes: a systematic review with meta‐analyses and meta‐regression. Diabet Med 2017; 34: 1027–1039 [level 2]. [DOI] [PubMed] [Google Scholar]
- 9. Diabetes Control Complications Trial Research Group , Nathan DM, Genuth S, et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [level 1]. [DOI] [PubMed] [Google Scholar]
- 10. Miller KM, Beck RW, Bergenstal RM, et al Evidence of a strong association between frequency of self‐monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013; 36: 2009–2014 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziegler R, Heidtmann B, Hilgard D, et al Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes 2011; 12: 11–17 [level 2]. [DOI] [PubMed] [Google Scholar]
- 12. Murata GH, Shah JH, Hoffman RM, et al Intensified blood glucose monitoring improves glycemic control in stable, insulin‐treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES). Diabetes Care 2003; 26: 1759–1763 [level 2]. [DOI] [PubMed] [Google Scholar]
- 13. Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta‐analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr 2013; 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lind M, Polonsky W, Hirsch IB, et al Continuous glucose monitoring vs conventional therapy for glycemic control in adults with Type 1 diabetes treated with multiple daily insulin injections: the GOLD Randomized Clinical Trial. JAMA 2017; 317: 379–387. [DOI] [PubMed] [Google Scholar]
- 15. Beck RW, Riddlesworth T, Ruedy K, et al Effect of continuous glucose monitoring on glycemic control in adults with Type 1 diabetes using insulin injections: the DIAMOND Randomized Clinical Trial. JAMA 2017; 317: 371–378. [DOI] [PubMed] [Google Scholar]
- 16. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, et al Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet 2016; 388: 2254–2263. [DOI] [PubMed] [Google Scholar]
- 17. Haak T, Hanaire H, Ajjan R, et al Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated Type 2 Diabetes: a Multicenter Open‐Label Randomized Controlled Trial. Diabetes Ther 2017; 8: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fisher L, Hessler D, Polonsky W, et al Diabetes distress in adults with type 1 diabetes: prevalence, incidence and change over time. J Diabetes Complications 2016; 30: 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perrin NE, Davies MJ, Robertson N, et al The prevalence of diabetes‐specific emotional distress in people with Type 2 diabetes: a systematic review and meta‐analysis. Diabet Med 2017; 34: 1508–1520. [DOI] [PubMed] [Google Scholar]
- 20. Li C, Barker L, Ford ES, et al Diabetes and anxiety in US adults: findings from the 2006 Behavioral Risk Factor Surveillance System. Diabetic Med 2008; 25: 878–881. [DOI] [PubMed] [Google Scholar]
- 21. Hessler DM, Fisher L, Polonsky WH, et al Diabetes distress is linked with worsening diabetes management over time in adults with Type 1 diabetes. Diabet Med 2017; 34: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitz N, Gariépy G, Smith KJ, et al Recurrent subthreshold depression in type 2 diabetes: an important risk factor for poor health outcomes. Diabetes Care 2014; 37: 970–978. [DOI] [PubMed] [Google Scholar]
- 23. Chew BH, Vos RC, Metzendorf MI, et al Psychological interventions for diabetes‐related distress in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2017; 9: CD011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avery L, Flynn D, van Wersch A, et al Changing physical activity behavior in type 2 diabetes: a systematic review and meta‐analysis of behavioral interventions. Diabetes Care 2012; 35: 2681–2689 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cradock KA, ÓLaighin G, Finucane FM, et al Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta‐analysis. Int J Behav Nutr Phys Act 2017; 14: 18 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katon WJ, Lin EH, Von Korff M, et al Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010; 363: 2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang Y, Wei X, Wu T, et al Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta‐analysis. BMC Psychiatry 2013; 13: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
8 DIABETIC RETINOPATHY
- 1. Klein R, Klein BE, Moss SE, et al The Wisconsin Epidemiologic Study of Diabetic Retinopathy: IX. Four‐year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1989; 107: 237–243 [level 2]. [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Klein BE, Moss SE, et al Ⅹ. Four‐year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol 1989; 107: 244–249 [level 2]. [DOI] [PubMed] [Google Scholar]
- 3. Younis N, Broadbent DM, Vora JP, et al Incidence of sight‐threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet 2003; 361: 195–200 [level 2]. [DOI] [PubMed] [Google Scholar]
- 4. Misra A, Bachmann MO, Greenwood RH, et al Trends in yield and effects of screening intervals during 17 years of a large UK community‐based diabetic retinopathy screening programme. Diabet Med 2009; 26: 1040–1047 [level 2]. [DOI] [PubMed] [Google Scholar]
- 5. Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [level 1]. [DOI] [PubMed] [Google Scholar]
- 6. United Kingdom Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [level 1]. [PubMed] [Google Scholar]
- 7. ACCORD Study Group , Chew EY, Ambrosius WT, et al Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010; 363: 233–244 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zoungas S, Arima H, Gerstein HC, et al Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta‐analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017; 5: 431–437 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 9. United Kingdom Prospective Diabetes Study (UKPDS) Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713 [level 1]. [PMC free article] [PubMed] [Google Scholar]
- 10. UK Prospective Diabetes Study (UKPDS) Group . Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Opthalmol 2004; 122: 1631–1640 [level 1]. [DOI] [PubMed] [Google Scholar]
- 11. Wang B, Wang F, Zhang Y, et al Effects of RAS inhibitors on diabetes retinopathy: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015; 3: 263–274 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 12. Keech AC, Mitchell P, Summanen PA, et al Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007; 370: 1687–1697 [level 1]. [DOI] [PubMed] [Google Scholar]
- 13. The Diabetic Retinopathy Study Research Group . Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978; 85: 82–106 [level 1]. [DOI] [PubMed] [Google Scholar]
- 14. Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2014; 11: CD011234 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10‐year prospective observational study. Diabetes Care 2002; 25: 859–864. [DOI] [PubMed] [Google Scholar]
- 16. Cheung N, Wang JJ, Klein R, et al Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care 2007; 30: 1742–1746. [DOI] [PubMed] [Google Scholar]
- 17. Cheung N, Rogers S, Couper DJ, et al Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007; 38: 398–401. [DOI] [PubMed] [Google Scholar]
- 18. Gerstein HC, Ambrosius WT, Danis R, et al Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care 2013; 36: 1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kramer CK, Rodrigues TC, Canani LH, et al Diabetic retinopathy predicts all‐cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta‐analysis of observational studies. Diabetes Care 2011; 34: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawasaki R, Tanaka S, Tanaka S, et al Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan Diabetes Complications Study. Ophthalmology 2013; 120: 574–582. [DOI] [PubMed] [Google Scholar]
9 DIABETIC NEPHROPATHY
- 1. Katayama S, Moriya T, Tanaka S, et al Low transition rate from normo‐ and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals: the Japan Diabetes Complications Study (JDCS). Diabetologia 2011; 54: 1025–1031 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moriya T, Tanaka S, Kawasaki R, et al Diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan Diabetes Complications Study. Diabetes Care 2013; 36: 2803–2809 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 4. Horio M, Imai E, Yasuda Y, et al GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis 2013; 61: 197–203. [DOI] [PubMed] [Google Scholar]
- 5. Diabetes C , Complications Trial Research Group , Nathan DM, et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [level 1]. [DOI] [PubMed] [Google Scholar]
- 6. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [level 1]. [PubMed] [Google Scholar]
- 7. Ohkubo Y, Kishikawa H, Araki E, et al Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [level 1]. [DOI] [PubMed] [Google Scholar]
- 8. Ismail‐Beigi F, Craven T, Banerji MA, et al Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–430 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duckworth W, Abraira C, Moritz T, et al Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139 [level 1]. [DOI] [PubMed] [Google Scholar]
- 10. ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [level 1]. [DOI] [PubMed] [Google Scholar]
- 11. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713 [level 1]. [PMC free article] [PubMed] [Google Scholar]
- 12. Makino H, Haneda M, Babazono T, et al Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 2007; 30: 1577–1578 [level 1]. [DOI] [PubMed] [Google Scholar]
- 13. Lewis EJ, Hunsicker LG, Clarke WR, et al Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [level 1]. [DOI] [PubMed] [Google Scholar]
- 14. Davis TM, Ting R, Best JD, et al Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 2011; 54: 280–290 [level 3]. [DOI] [PubMed] [Google Scholar]
- 15. Colhoun HM, Betteridge DJ, Durrington PN, et al Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54: 810–819 [level 3]. [DOI] [PubMed] [Google Scholar]
- 16. Shen X, Zhang Z, Zhang X, et al Efficacy of statins in patients with diabetic nephropathy: a meta‐analysis of randomized controlled trials. Lipids Health Dis 2016; 15: 179 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haneda M, Kikkawa R, Sakai H, et al Antiproteinuric effect of candesartan cilexetil in Japanese subjects with type 2 diabetes and nephropathy. Diabetes Res Clin Pract 2004; 66: 87–95 [level 1]. [DOI] [PubMed] [Google Scholar]
- 18. Casas JP, Chua W, Loukogeorgakis S, et al Effect of inhibitors of the renin‐angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta‐analysis. Lancet 2005; 366: 2026–2033 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 19. Palmer SC, Mavridis D, Navarese E, et al Comparative efficacy and safety of blood pressure‐lowering agents in adults with diabetes and kidney disease: a network meta‐analysis. Lancet 2015; 385: 2047–2056 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 20. Suckling RJ, He FJ, MacGregor GA. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev 2010; 12: CD006763 [level 2]. [DOI] [PubMed] [Google Scholar]
- 21. Hansen HP, Tauber‐Lassen E, Jensen BR, et al Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int 2002; 62: 220–228. [DOI] [PubMed] [Google Scholar]
- 22. Nezu U, Kamiyama H, Kondo Y, et al. Effect of low‐protein diet on kidney function in diabetic nephropathy: meta‐analysis of randomised controlled trials. BMJ Open 2013; 3: e002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfeffer MA, Burdmann EA, Chen CY, et al A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032. [DOI] [PubMed] [Google Scholar]
- 24. Afkarian M, Sachs MC, Kestenbaum B, et al Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013; 24: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
10 DIABETIC NEUROPATHY
- 1. Hotta N, Toyoda R. Diabetic Neuropathy. Kanehara, Tokyo, 1996. p. 145–154 (Japanese). [Google Scholar]
- 2. Boulton AJ, Vinik AI, Arezzo JC, et al Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962. [DOI] [PubMed] [Google Scholar]
- 3. Tesfaye S, Chaturvedi N, Eaton SE, et al Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
- 4. Clair C, Cohen MJ, Eichler F, et al The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta‐analysis. J Gen Intern Med 2015; 30: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaiswal M, Divers J, Dabelea D, et al Prevalence of and risk factors for diabetic peripheral neuropathy in youth with Type 1 and Type 2 diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017; 40: 1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [level 1]. [DOI] [PubMed] [Google Scholar]
- 7. Ohkubo Y, Kishikawa H, Araki E, et al Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [level 2]. [DOI] [PubMed] [Google Scholar]
- 8. Ismail‐Beigi F, Craven T, Banerji MA, et al Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–430 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Max MB, Culnane M, Schafer SC, et al Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987; 37: 589–596. [DOI] [PubMed] [Google Scholar]
- 10. Freeman R, Durso‐Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care 2008; 31: 1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Satoh J, Yagihashi S, Baba M, et al Efficacy and safety evaluation of pregabalin treatment over 52 weeks in patients with diabetic neuropathic pain extended after a double‐blind placebo‐controlled trial. J Diabetes Investig 2011; 2: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pritchett YL, McCarberg BH, Watkin JG, et al Duloxetine for the management of diabetic peripheral neuropathic pain: response profile. Pain Med 2007; 8: 397–409. [DOI] [PubMed] [Google Scholar]
- 13. Yasuda H, Hotta N, Nakao K, et al Superiority of duloxetine to placebo in improving diabetic neuropathic pain: Results of a randomized controlled trial in Japan. J Diabetes Investig 2011; 2: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charles M, Soedamah‐Muthu SS, Tesfaye S, et al Low peripheral nerve conduction velocities and amplitudes are strongly related to diabetic microvascular complications in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2010; 33: 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
11 DIABETIC FOOT
- 1. IWGDF Guidelines on the prevention and management of diabetic foot disease. https://iwgdfguidelines.org/wp‐content/uploads/2019/05/IWGDF‐Guidelines‐2019.pdf.
- 2. Frykberg RG, Zgonis T, Armstrong DG, et al Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg 2006; 45: S1–S66. [DOI] [PubMed] [Google Scholar]
- 3. Krishinan S, Nash F, Baker N et al Reduction in diabetic amputations over 11 years in a defined U.K. population benefits of multidisciplinary team work and continuous prospective audit. Diabetes Care 2008; 31: 99–101 [level 2]. [DOI] [PubMed] [Google Scholar]
- 4. Malone JM, Snyder M, Anderson G, et al Prevention of amputation by diabetic education. Am J Surg 1989; 158: 520–524 [level 1]. [DOI] [PubMed] [Google Scholar]
- 5. Bonner T, Foster M, Spears‐Lanoix E. Type 2 diabetes related foot care knowledge and foot self‐care practice interventions in the United States: a systematic review of the literature. Diabet Foot Ankle 2016; 7: 29758 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stratton IM, Adler A, Meil HAW, et al Association of glycaemia with macrovascular and microvascular complications of type 2diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasan R, Firwana B, Elraiyah T, et al A systematic review and meta‐analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg 2016; 63: 22S–28S [level 1]. [DOI] [PubMed] [Google Scholar]
- 8. Netten JJV, Price PE, Lavery LA, et al Prevention of foot ulcers in the at‐risk patient with diabetes: a systematic review. Diabetes Metab Res Rev 2016; 32 (Suppl 1): 84–98 [level 1]. [DOI] [PubMed] [Google Scholar]
- 9. Weck M, Slesaczeck T, Hartmut Paetzold H, et al Structured health care for subjects with diabetic foot ulcers results in a reduction of major amputation rates. Cardiovasc Diabetol 2013; 12: 45 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rooke TW, Hirsch AT, Misra S, et al 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58: 2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lipsky BA, Berendt AR, Cornia PB, et al 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54: e132–e173. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong DG, Bharara M, White M, et al The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev 2012; 28: 514–518 [level 3]. [DOI] [PubMed] [Google Scholar]
- 13. Paisey RB, Abbott A, Levenson R, et al Diabetes‐related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the South‐West of England. Diabet Med 2018; 35: 53–62 [level 3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hogg FRA, Peach G, Price P, et al Measures of health‐related quality of life in diabetes‐related foot disease: a systematic review. Diabetologia 2012; 55: 552–565 [level 1]. [DOI] [PubMed] [Google Scholar]
- 15. Siersma V, Thorsen H, Holstein PF, et al Diabetic complications do not hamper improvement of health‐related quality of life over the course of treatment of diabetic foot ulcers ‐ the Eurodiale study. J Diabetes Complications 2017; 31: 1145–1151 [level 2]. [DOI] [PubMed] [Google Scholar]
- 16. Brownrigg JR, Davey J, Holt PJ, et al The association of ulceration of the foot with cardiovascular and all‐cause mortality in patients with diabetes. Diabetologia 2012; 55: 2906–2912. [DOI] [PubMed] [Google Scholar]
- 17. Ismail K, Winkley K, Stahl D, et al A Cohort Study of People with diabetes and their first foot ulcer. Diabetes Care 2007; 30: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 18. Natovich R, Kushnir T, Harman‐Boehm I, et al Cognitive Dysfunction: Part and parcel of the diabetic foot. Diabetes Care 2016; 39: 1202–1207. [DOI] [PubMed] [Google Scholar]
12 DIABETIC MACROANGIOPATHY
- 1. Gaede P, Vedel P, Larsen N, et al Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. [DOI] [PubMed] [Google Scholar]
- 2. Gaede P, Lund‐Andersen H, Parving HH, et al Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. [DOI] [PubMed] [Google Scholar]
- 3. Gaede P, Oellgaard J, Carstensen B, et al Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia 2016; 59: 2298–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ueki K, Sasako T, Okazaki Y, et al Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 951–964. [DOI] [PubMed] [Google Scholar]
- 5. Miller ME, Williamson JD, Gerstein HC, et al ACCORD Investigators: Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care 2014; 37: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odden MC, Paralta CA, Haan MN, et al Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172: 1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wing RR, Look AHEAD Research Group . Long‐term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four‐year results of the Look AHEAD trial. Arch Intern Med 2010; 170: 1566–1575 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Metz JA, Stern JS, Kris‐Etherton P, et al A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med 2000; 160: 2150–2158 [level 1]. [DOI] [PubMed] [Google Scholar]
- 9. Esposito K, Maiorino MI, Ciotola M et al Effects of a Mediterranean‐style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med 2009; 151: 306–314 [level 1]. [DOI] [PubMed] [Google Scholar]
- 10. Roussel R, Steg PG, Mohammedi K, et al Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: A perspective on glucose‐lowering interventions. Diabetes Obes Metab 2018; 20: 238–244 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 11. Stratton IM, Adler AI, Neil HA, et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adler AI, Stratton IM, Neil HA, et al Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000; 321: 412–419 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emdin CA, Rahimi K, Neal B, et al Blood pressure lowering in type 2 diabetes: a systematic review and meta‐analysis. JAMA 2015; 313: 603–615 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 14. de Vries FM, Denig P, Pouwels KB, et al Primary prevention of major cardiovascular and cerebrovascular events with statins in diabetic patients: a meta‐analysis. Drugs 2012; 72: 2365–2373 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 15. de Vries FM, Kolthof J, Postma MJ, et al Efficacy of standard and intensive statin treatment for the secondary prevention of cardiovascular and cerebrovascular events in diabetes patients: a meta‐analysis. PLoS One 2014; 9: e111247 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simpson SH, Gamble JM, Mereu L, et al Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta‐analysis. J Gen Intern Med 2011; 26: 1336–1344 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
13 DIABETES AND PERIODONTITIS
- 1. Takahashi K, Nishimura F, Kurihara M, et al Subgingival microflora and antibody responses against periodontal bacteria of young Japanese patients with type 1 diabetes mellitus. J Int Acad Periodontol 2001; 3: 104–111. [PubMed] [Google Scholar]
- 2. Morita I, Inagaki K, Nakamura F, et al Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res 2012; 91: 161–166. [DOI] [PubMed] [Google Scholar]
- 3. Katagiri S, Nitta H, Nagasawa T, et al Effect of glycemic control on periodontitis in type 2 diabetic patients with periodontal disease. J Diabetes Investig 2013; 4: 320–325 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Demmer RT, Jacobs DR, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow‐up study. Diabetes Care 2008; 31: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graziani F, Gennai S, Solini A, et al A systematic review and meta‐analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP‐AAP review. J Clin Periodontol 2018; 45: 167–187. [DOI] [PubMed] [Google Scholar]
- 6. Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta‐analysis. J Periodontol 2013; 84: S153–S169 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simpson TC, Weldon JC, Worthington HV, et al Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev 2015; 11: CD004714 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
14 DIABETES COMPLICATED BY OBESITY (INCLUDING METABOLIC SYNDROME)
- 1. Matsuzawa Y, Sakata T, Ikeda Y, et al Guidelines for the management of obesity disease 2006. 2006: 1–91 (Japanese).
- 2. Examination Committee of Criteria for ‘Obesity Disease’ in Japan , Japan Society for the Study of Obesity . New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992. [DOI] [PubMed] [Google Scholar]
- 3. Hayashi T, Boyko EJ, McNeely MJ, et al Minimum waist and visceral fat values for identifying Japanese Americans at risk for the metabolic syndrome. Diabetes Care 2007; 30: 120–127. [DOI] [PubMed] [Google Scholar]
- 4. Kashihara H, Lee JS, Kawakubo K, et al Criteria of waist circumference according to computed tomography‐measured visceral fat area and the clustering of cardiovascular risk factors. Circ J 2009; 73: 1881–1886. [DOI] [PubMed] [Google Scholar]
- 5. Hiuge‐Shimizu A, Kishida K, Funahashi T, et al Absolute value of visceral fat area measured on computed tomography scans and obesity‐related cardiovascular risk factors in large‐scale Japanese general population (the VACATION‐J study). Ann Med 2012; 44: 82–92. [DOI] [PubMed] [Google Scholar]
- 6. Saito Y, Shirai A, Nakamura M, et al Diagnostic criteria for obesity disease 2011. Obes Res 2011; 17: 1–78 (Japanese). [Google Scholar]
- 7. Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial: a prospective controlled intervention study of bariatric surgery. J Intern Med 2013; 273: 219–234. [DOI] [PubMed] [Google Scholar]
- 8. The Japan Diabetes Society . Treatment Guide for Diabetes 2018–2019. Bunkodo, 2018. (Japanese). [Google Scholar]
- 9. Madigan CD, Aveyard P, Jolly K, et al Regular self‐weighing to promote weight maintenance after intentional weight loss: a quasi‐randomized contorolled trail. J Public Health (Oxf) 2014; 36: 259–267. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Q, Dou J, Lu J. Combinational therapy with metformin and sodium‐glucose cotransporter inhibitors in management of type 2 diabetes: systematic review and meta‐analyses. Diabetes Res Clin Pract 2014; 105: 313–321. [DOI] [PubMed] [Google Scholar]
- 11. Shoar S, Saber AA. Long‐term and midterm outcomes of laparoscopic sleeve gastrectomy versus roux‐en‐y gastric bypass: a systematic review and meta‐analysis of comparative studies. Surg Obes Relat Dis 2017; 13: 170–180 [level 2]. [DOI] [PubMed] [Google Scholar]
- 12. Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta‐analysis. Obes Surg 2014; 24: 437–455 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang SH, Stoll CR, Song J, et al The effectiveness and risks of bariatric surgery: an updated systematic review and meta‐analysis, 2003–2012. JAMA Surg 2014; 149: 275–287 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan Y, Sha Y, Yao G, et al Roux‐en‐Y gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients: a systematic review and meta‐analysis of randomized controlled trials. Medicine (Baltimore) 2016; 95: e3462 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Committee on Diagnostic Criteria for Metabolic Syndrome . Metabolic syndrome: its definition and diagnostic criteria. J Jpn Soc Intern 2005; 94: 794–799 (Japanese). [Google Scholar]
15 HYPERTENSION ASSOCIATED WITH DIABETES
- 1. Umemura S, Arima H, Arima S, et al The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res 2019; 42: 1235–1481. [DOI] [PubMed] [Google Scholar]
- 2. Ueki K, Sasako T, Okazaki Y, Kato M, et al Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabet Endcrinol 2017; 5: 951–964 [level 1]. [DOI] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study Group . Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317: 713–720 [level 1]. [PMC free article] [PubMed] [Google Scholar]
- 4. Baba S. Nifedipine and enalapril equally reduce the progression of nephropathy in hypertensive type 2 diabetics. Diabetes Res Clin Pract 2001; 54: 191–201 [level 1]. [DOI] [PubMed] [Google Scholar]
- 5. Berl T, Hunsicker LG, Lewis JB, et al Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med 2003; 138: 542–549 [level 1]. [DOI] [PubMed] [Google Scholar]
- 6. Chan JC, Ko GT, Leung DH et al Long‐term effects of angiotensin‐converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int 2000; 57: 590–600 [level 1]. [DOI] [PubMed] [Google Scholar]
- 7. Estacio RO, Jeffers BW, Hiatt WR, et al The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. N Engl J Med 1998; 338: 645–652 [level 2]. [DOI] [PubMed] [Google Scholar]
- 8. Lewis EJ, Hunsicker LG, Clarke WR, et al Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [level 1]. [DOI] [PubMed] [Google Scholar]
- 9. Lindholm LH, Hansson L, Ekbom T, et al Comparison of antihypertensive treatments in preventing cardiovascular events in elderly diabetic patients: results from the Swedish Trial in Old Patients with Hypertension‐2. STOP Hypertension‐2 Study Group. J Hypertens 2000; 18: 1671–1675 [level 1]. [DOI] [PubMed] [Google Scholar]
- 10. Lindholm LH, Ibsen H, Dahlöf B, et al Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 1004–1010 [level 1]. [DOI] [PubMed] [Google Scholar]
- 11. Marre M, Puig JG, Kokot F, et al Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes: the NESTOR Study. J Hypertens 2004; 22: 1613–1622 [level 1]. [DOI] [PubMed] [Google Scholar]
- 12. Nakao K, Hirata M, Oba K, et al Role of diabetes and obesity in outcomes of the candesartan antihypertensive survival evaluation in Japan (CASE‐J) trial. Hypertens Res 2010; 33: 600–606 [level 2]. [DOI] [PubMed] [Google Scholar]
- 13. Niskanen L, Hedner T, Hansson L, et al Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first‐line therapy with an ACE inhibitor compared with a diuretic/beta‐blocker‐based treatment regimen: a subanalysis of the Captopril Prevention Project. Diabetes Care 2001; 24: 2091–2096 [level 1]. [DOI] [PubMed] [Google Scholar]
- 14. Rahman M, Pressel S, Davis BR, et al Renal outcomes in high‐risk hypertensive patients treated with an angiotensin‐converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2005; 165: 936–994 [level 2]. [DOI] [PubMed] [Google Scholar]
- 15. Tatti P, Pahor M, Byington RP, et al Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care 1998; 21: 597–603 [level 1]. [DOI] [PubMed] [Google Scholar]
- 16. Whelton PK, Barzilay J, Cushman WC, et al Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 2005; 165: 1401–1409 [level 1]. [DOI] [PubMed] [Google Scholar]
- 17. Yamashita K, Kondo T, Muramatsu T, et al Effects of valsartan versus amlodipine in diabetic hypertensive patients with or without previous cardiovascular disease. Am J Cardiol 2013; 112: 1750–1756 [level 1]. [DOI] [PubMed] [Google Scholar]
- 18. Yui Y, Sumiyoshi T, Kodama K, et al Nifedipine retard was as effective as angiotensin converting enzyme inhibitors in preventing cardiac events in high‐risk hypertensive patients with diabetes and coronary artery disease: the Japan Multicenter Investigation for Cardiovascular Diseases‐B (JMIC‐B) subgroup analysis. Hypertens Res 2004; 27: 449–456 [level 1]. [DOI] [PubMed] [Google Scholar]
16 DYSLIPIDEMIA ASSOCIATED WITH DIABETES
- 1. Wang Y, Lammi‐Keefe CJ, Hou L, et al Impact of low‐density lipoprotein cholesterol on cardiovascular outcomes in people with type 2 diabetes: a meta‐analysis of prospective cohort studies. Diabetes Res Clin Pract 2013; 102: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner RC, Millns H, Neil HA, et al Risk factors for coronary artery disease in non‐insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS 23). BMJ 1998; 316: 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacks FM, Hermans MP, Fioretto P, et al Association between plasma triglycerides and high‐density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case‐control study in 13 countries. Circulation 2014; 129: 999–1008. [DOI] [PubMed] [Google Scholar]
- 4. Toth PP, Simko RJ, Palli SR, et al The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2012; 11: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heilbronn LK, Noakes M, Clifton PM. Effect of energy restriction, weight loss, and diet composition on plasma lipids and glucose in patients with type 2 diabetes. Diabetes Care 1999; 22: 889–895 [level 2]. [DOI] [PubMed] [Google Scholar]
- 6. Huang XL, Pan JH, Chen D, et al Efficacy of lifestyle interventions in patients with type 2 diabetes: a systematic review and meta‐analysis. Eur J Intern Med 2016; 27: 37–47 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 7. Esposito K, Maiorino MI, Bellastella G, et al A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta‐analyses. BMJ Open 2015; 5: e008222 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hartweg J, Perera R, Montori V, et al Omega‐3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008; CD003205 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayashino Y, Jackson JL, Fukumori N, et al Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta‐analysis of randomized controlled trials. Diabetes Res Clin Pract 2012; 98: 349–360 [level 2]. [DOI] [PubMed] [Google Scholar]
- 10. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006; CD002968 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamura H, Arakawa K, Itakura H, et al Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368: 1155–1163 [level 1]. [DOI] [PubMed] [Google Scholar]
- 12. Cholesterol Treatment Trialists C , Kearney PM, Blackwell L, et al Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet 2008; 371: 117–125 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 13. Colhoun HM, Betteridge DJ, Durrington PN, et al Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet 2004; 364: 685–696 [level 1]. [DOI] [PubMed] [Google Scholar]
- 14. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–1389 [level 1]. [PubMed] [Google Scholar]
- 15. Collins R, Armitage J, Parish S, et al MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet 2003; 361: 2005–2016 [level 1]. [DOI] [PubMed] [Google Scholar]
- 16. Sever PS, Poulter NR, Dahlof B, et al Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo‐Scandinavian Cardiac Outcomes Trial–lipid‐lowering arm (ASCOT‐LLA). Diabetes Care 2005; 28: 1151–1157 [level 2]. [DOI] [PubMed] [Google Scholar]
- 17. Gaede P, Lund‐Andersen H, Parving HH, et al Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591 [level 2]. [DOI] [PubMed] [Google Scholar]
- 18. Ueki K, Sasako T, Okazaki Y, et al J‐DOIT3 Study Group. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 951–964 [level 1]. [DOI] [PubMed] [Google Scholar]
- 19. Knopp RH, d'Emden M, Smilde JG, et al Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non‐insulin‐dependent diabetes mellitus (ASPEN). Diabetes Care 2006; 29: 1478–1485 [level 2]. [DOI] [PubMed] [Google Scholar]
- 20. Brugts JJ, Yetgin T, Hoeks SE, et al The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta‐analysis of randomised controlled trials. BMJ 2009; 338: b2376 [level 1+]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pyörälä K, Pedersen TR, Kjekshus J, et al Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20: 614–620 [level 3]. [DOI] [PubMed] [Google Scholar]
- 22. Shepherd J, Barter P, Carmena R, et al Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care 2006; 29: 1220–1226 [level 3]. [DOI] [PubMed] [Google Scholar]
- 23. Taguchi I, Iimuro S, et al High‐dose versus low‐dose pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL‐CAD). Circulation 2018; 137: 1997–2009 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keech A, Simes RJ, Barter P, et al Effects of long‐term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366: 1849–1861 [level 1]. [DOI] [PubMed] [Google Scholar]
- 25. ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1563–1574 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgess DC, Hunt D, Li L, et al Incidence and predictors of silent myocardial infarction in type 2 diabetes and the effect of fenofibrate: an analysis from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Eur Heart J 2010; 31: 92–99 [level 3]. [DOI] [PubMed] [Google Scholar]
- 27. Allemann S, Diem P, Egger M, et al Fibrates in the prevention of cardiovascular disease in patients with type 2 diabetes mellitus: meta‐analysis of randomised controlled trials. Curr Med Res Opin 2006; 22: 617–623 [level 1]. [DOI] [PubMed] [Google Scholar]
- 28. Effect of fenofibrate on progression of coronary‐artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 2001; 357: 905–910 [level 2]. [PubMed] [Google Scholar]
- 29. Leiter LA, Betteridge DJ, Farnier M, et al Lipid‐altering efficacy and safety profile of combination therapy with ezetimibe/statin vs. statin monotherapy in patients with and without diabetes: an analysis of pooled data from 27 clinical trials. Diabetes Obes Metab 2011; 13: 615–628 [level 2]. [DOI] [PubMed] [Google Scholar]
- 30. Baigent C, Landray MJ, Reith C, et al The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet 2011; 377: 2181–2192 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cannon CP, Blazing MA, Giugliano RP, et al Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387–2397 [level 1]. [DOI] [PubMed] [Google Scholar]
- 32. Giugliano RP, Cannon CP, Blazing MA, et al IMPROVE‐IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators: Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients With vs. Without Diabetes: Results from IMPROVE‐IT. Circulation 2018; 137: 1571–1582 [level 2]. [DOI] [PubMed] [Google Scholar]
- 33. Sattar N, Preiss D, Robinson JG, et al Lipid‐lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta‐analysis of individual patient data. Lancet Diabetes Endocrinol 2016; 4: 403–410 [level 1+]. [DOI] [PubMed] [Google Scholar]
- 34. Sabatine MS, Giugliano RP, Keech AC, et al FOURIER Steering Committee and Investigators: Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722 [level 1]. [DOI] [PubMed] [Google Scholar]
- 35. Sabatine MS, Leiter LA, Wiviott SD, et al Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 941–950 [level 2]. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz GG, Steg PG, Szarek M, et al ODYSSEY OUTCOMES Committees and Investigators: Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med 2018; 379: 2097–2107 [level 1]. [DOI] [PubMed] [Google Scholar]
17 IMPAIRED GLUCOSE METABOLISM IN PREGNANCY
- 1. Ray JG, O'Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta‐analysis. QJM 2001; 94: 435–444 [level 2]. [DOI] [PubMed] [Google Scholar]
- 2. Jensen DM, Korsholm L, Ovesen P, et al Peri‐conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care 2009; 32: 1046–1048 [level 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database Syst Rev 2009; 8: CD003395 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nachum Z, Ben‐Shlomo I, Weiner E, et al Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy: randomised controlled trial. BMJ 1999; 319: 1223–1227 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffin ME, Coffey M, Johnson H, et al Universal vs. risk factor‐based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabet Med 2000; 17: 26–32. [DOI] [PubMed] [Google Scholar]
- 6. Ekbom P, Damm P, Feldt‐Rasmussen B, et al Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care 2001; 24: 1739–1744. [DOI] [PubMed] [Google Scholar]
- 7. Liu P, Xu L, Wang Y, et al Association between perinatal outcomes and maternal pre‐pregnancy body mass index. Obes Rev 2016; 17: 1091–1102. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association . Management of diabetes in pregnancy. Diabetes Care 2019; 42: S165–S172. [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence (NICE) guideline. Available from https://www.nice.org.uk/. Accessed February 21, 2018.
- 10. Hiramatsu Y. Workshop “Gestational diabetes mellitus and self monitoring of blood glucose (SMBG)”. Diabetes Pregnancy 2015; 15: 89–90 (Japanese). [Google Scholar]
- 11. Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within‐family comparison. Lancet 2010; 376: 984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev 2017; 6: CD012202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langer O, Rodriguez DA, Xenakis EM, et al Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol 1994; 170: 1036–1046; discussion 1046–1047. [DOI] [PubMed] [Google Scholar]
- 14. Bellamy L, Casas JP, Hingorani AD, et al Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta‐analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 15. Hiramatsu Y, Haneda M, et al The joint committee with the Japan Society of Diabetes and Pregnancy and the Japan Diabetes Society “An abnormal glucose metabolism during pregnancy and the standardization of its diagnostic criteria”. J Jpn Diabetes Soc 2015; 58: 801–803 (Japanese). [Google Scholar]
- 16. de Veciana M, Major CA, Morgan MA, et al Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med 1995; 333: 1237–1241. [DOI] [PubMed] [Google Scholar]
- 17. Manderson JG, Patterson CC, Hadden DR, et al Preprandial versus postprandial blood glucose monitoring in type 1 diabetic pregnancy: a randomized controlled clinical trial. Am J Obstet Gynecol 2003; 189: 507–512. [DOI] [PubMed] [Google Scholar]
- 18. Ministry of Health, Labor and Welfare . Recommended diets for expectant and nursing mothers. Report of the Commission for Promotion of the ‘Healthy Mother‐Child Policy 21’, 2006. Source: http://www.mhlw.go.jp/houdou/2006/02/h0201‐3a.html. Accessed February 1, 2018.
18 PEDIATRIC/ADOLESCENT DIABETES
- 1. ISPAD Clinical Practice Consensus Guidelines for Pediatric and Adolescent Diabetes 2014. Nankodo, Tokyo, 2015.
- 2. Ascerini C, Craig ME, de Beaufort C, et al ISPAD clinical practice consensus guidelines 2014 compendium. Introduction. Pediatr Diabetes 2014; 15(Suppl 20): 1–3. [DOI] [PubMed] [Google Scholar]
- 3. Craig ME, Jefferies C, Dabelea D, et al ISPAD clinical practice consensus guidelines 2014 compendium. Definition, epidemiology, and classification of diabetes in children and adlescents. Pediatr Diabetes 2014; 15(Suppl 20): 4–17. [DOI] [PubMed] [Google Scholar]
- 4. White NH, Cleary PA, Dahms W, et al Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001; 139: 804–812. [DOI] [PubMed] [Google Scholar]
- 5. Urakami T, Morimoto S, Nitadori Y, et al Urine glucose screening program at schools in Japan to detect children with diabetes and its outcome: Incidence and clinical characteristics of childhood type 2 diabetes in Japan. Pediatr Res 2007; 61: 141–145. [DOI] [PubMed] [Google Scholar]
- 6. Zeitler P, Fu J, Tandon N, et al ISPAD clinical practice consensus guidelines 2014 compendium. Type 2 diabetes in the child and adolescent. Pediatr Diabetes 2014; 15(Suppl 20): 26–46. [DOI] [PubMed] [Google Scholar]
- 7. Urakami T, Suzuki J, Mugishima H, et al. Screening and treatment of childhood type 1 and type 2 diabetes mellitus in Japan. Pediatr Endocrinol Rev 2012; 10(Suppl 1): 51–61. [PubMed] [Google Scholar]
- 8. Laffel L, Chang N, Grey M, et al Metformin monotherapy in youth recent‐onset type 2 diabetes: experience from the prerandomization run‐in phase of the TODAY study. Pediatr Diabetes 2012; 13: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones KL, Arslanian S, Peterokova VA, et al Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002; 25: 89–94. [DOI] [PubMed] [Google Scholar]
- 10. Matsuura N, Takeuchi M, Amemiya S, et al Trial of Metformin Clinical Trial of Metformin Monotherapy in Children and Adolescents with Type 2 diabetes mellitus in Japan. J Japan Diabet Soc 2008; 51: 427–434. [Google Scholar]
- 11. Rubio‐Cabezas O, Hattersley AT, Njölstad PR, et al ISPAD clinical practice consensus guidelines 2014 compendium. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes 2014; 15 (Suppl 20): 47–64. [DOI] [PubMed] [Google Scholar]
- 12. Rafiq M, Flanagan SE, Patch AM, et al Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care 2008; 31: 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lange K, Swift P, Pankowska E, et al ISPAD clinical practice consensus guidelines 2014 compendium. Diabetes education. Pediatr Diabetes 2014; 15(Suppl 20): 77–85. [DOI] [PubMed] [Google Scholar]
- 14. Winkley K, Landau S, Eisler I, et al Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta‐analysis of randomized controlled trials. BMJ 2006; 333: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delamater AM, de Wit M, McDarby V, et al ISPAD clinical practice consensus guidelines 2014 compendium. Psychological issues. Pediatr Diabetes 2014; 15(Suppl 20): 232–244. [DOI] [PubMed] [Google Scholar]
- 16. Rewers M, Pillay K, de Beaufort C, et al ISPAD clinical practice consensus guidelines 2014 compendium. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2014; 15 (Suppl 20): 102–114. [DOI] [PubMed] [Google Scholar]
20 ACUTE METABOLIC COMPLICATIONS OF DIABETES, SICK DAYS, AND INFECTIOUS DISEASES
- 1. Nyenwe EA, Kitabchi AE. Evidence‐based management of hyperglycemic emergencies in diabetes mellitus. Diabetes Res Clin Pract 2011; 94: 340–351. [DOI] [PubMed] [Google Scholar]
- 2. Kitabchi AE, Umpierrez GE, Miles JM, et al Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009; 32: 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfsdorf JI, Allgrove J, Craig ME, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 2014; 15(Suppl 20): 154–179. [DOI] [PubMed] [Google Scholar]
- 4. Jeffrey A, Kraut MD, Nicolaos E, et al Lactic acidosis. N Engl J Med 2014; 371: 2309–2319. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Standards of medical care in diabetes–2018. Diabetes Care 2018; 41: S1–S157. [DOI] [PubMed] [Google Scholar]
- 6. Seaquist ER, Anderson J, Childs B, et al Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab 2013; 98: 1845–1859. [DOI] [PubMed] [Google Scholar]
- 7. Nirmal J, Gregory M, Caputo GM, et al Infections in patients with diabetes mellitus. N Engl J Med 1999; 341: 1906–1912. [DOI] [PubMed] [Google Scholar]
- 8. NICE‐SUGAR Study Investigators , Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283–1297. [DOI] [PubMed] [Google Scholar]
- 9. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2016 (J‐SSCG 2016). J Intensive Care 2018; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Remschmidt C, Wichmann O, Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: systematic review and meta‐analysis. BMC Med 2015; 13: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vamos EP, Pape UJ, Curcin V, et al Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ 2016; 188: E342–E351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moberley SA, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; CD000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brink S, Joel D, Laffel L, et al Sick day management in children and adolescents with diabetes. Pediatr Diabetes 2014; 15: 193–202. [DOI] [PubMed] [Google Scholar]
21 PREVENTION OF TYPE 2 DIABETES
- 1. Doi Y, Ninomiya T, Hata J, et al Two risk score models for predicting incident type 2 diabetes in Japan. Diabet Med 2012; 29: 107–114. [DOI] [PubMed] [Google Scholar]
- 2. Nanri A, Nakagawa T, Kuwahara K, et al Development of risk score for predicting 3‐year incidence of type 2 diabetes: Japan Epidemiology Collaboration on Occupational Health study. PLoS One 2015; 10: e0142779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heianza Y, Arase Y, Hsieh SD, et al Development of a new scoring system for predicting the 5 year incidence of type 2 diabetes in Japan: the Toranomon Hospital Health Management Center Study 6 (TOPICS 6). Diabetologia 2012; 55: 3213–3223. [DOI] [PubMed] [Google Scholar]
- 4. Maskarinec G, Erber E, Grandinetti A, et al Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes 2009; 58: 1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu M, Austin PC, Manuel DG, et al Deriving ethnic‐specific BMI cutoff points for assessing diabetes risk. Diabetes Care 2011; 34: 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu WC, Araneta MR, Kanaya AM, et al BMI cut points to identify at‐risk Asian Americans for type 2 diabetes screening. Diabetes Care 2015; 38: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjerregaard LG, Jensen BW, Angquist L, et al Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med 2018; 378: 1302–1312. [DOI] [PubMed] [Google Scholar]
- 8. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005; 67: 152–162 [level 1]. [DOI] [PubMed] [Google Scholar]
- 9. Kawahara T, Takahashi K, Inazu T, et al Reduced progression to type 2 diabetes from impaired glucose tolerance after a 2‐day in‐hospital diabetes educational program: the Joetsu Diabetes Prevention Trial. Diabetes Care 2008; 31: 1949–1954 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saito T, Watanabe M, Nishida J, et al Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011; 171: 1352–1360 [level 1]. [DOI] [PubMed] [Google Scholar]
- 11. Booth H, Khan O, Prevost T, et al Incidence of type 2 diabetes after bariatric surgery: population‐based matched cohort study. Lancet Diabetes Endocrinol 2014; 2: 963–968. [DOI] [PubMed] [Google Scholar]
- 12. Aune D, Norat T, Leitzmann M, et al Physical activity and the risk of type 2 diabetes: a systematic review and dose‐response meta‐analysis. Eur J Epidemiol 2015; 30: 529–542. [DOI] [PubMed] [Google Scholar]
- 13. Smith AD, Crippa A, Woodcock J, et al Physical activity and incident type 2 diabetes mellitus: a systematic review and dose‐response meta‐analysis of prospective cohort studies. Diabetologia 2016; 59: 2527–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grontved A, Pan A, Mekary RA, et al Muscle‐strengthening and conditioning activities and risk of type 2 diabetes: a prospective study in two cohorts of US women. PLoS Medicine 2014; 11: e1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grontved A, Rimm EB, Willett WC, et al A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med 2012; 172: 1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all‐cause mortality: a meta‐analysis. JAMA 2011; 305: 2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biswas A, Oh PI, Faulkner GE, et al Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta‐analysis. Ann Intern Med 2015; 162: 123–132. [DOI] [PubMed] [Google Scholar]
- 18. Greenwood DC, Threapleton DE, Evans CE, et al Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose‐response meta‐analysis of prospective studies. Diabetes Care 2013; 36: 4166–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhupathiraju SN, Tobias DK, Malik VS, et al Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta‐analysis. Am J Clin Nutr 2014; 100: 218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulze MB, Schulz M, Heidemann C, et al Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta‐analysis. Arch Intern Med 2007; 167: 956–965. [DOI] [PubMed] [Google Scholar]
- 21. Yao B, Fang H, Xu W, et al Dietary fiber intake and risk of type 2 diabetes: a dose‐response analysis of prospective studies. Eur J Epidemiol 2014; 29: 79–88. [DOI] [PubMed] [Google Scholar]
- 22. Hata A, Doi Y, Ninomiya T, et al. Magnesium intake decreases type 2 diabetes risk through the improvement of insulin resistance and inflammation: the Hisayama study. Diabet Med 2013; 30: 1487–1494. [DOI] [PubMed] [Google Scholar]
- 23. Fang X, Han H, Li M, et al Dose‐response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: a systematic review and meta‐regression analysis of prospective cohort studies. Nutrients 2016; 8: E739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose‐response meta‐analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 2015; 38: 1804–1812. [DOI] [PubMed] [Google Scholar]
- 25. Imamura F, O'Connor L, Ye Z, et al Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta‐analysis, and estimation of population attributable fraction. BMJ 2015; 351: h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA 2005; 294: 97–104. [DOI] [PubMed] [Google Scholar]
- 27. Yang WS, Wang WY, Fan WY, et al Tea consumption and risk of type 2 diabetes: a dose‐response meta‐analysis of cohort studies. Br J Nutr 2014; 111: 1329–1339. [DOI] [PubMed] [Google Scholar]
- 28. Pan A, Wang Y, Talaei M, et al Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015; 3: 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shan Z, Ma H, Xie M, et al Sleep duration and risk of type 2 diabetes: a meta‐analysis of prospective studies. Diabetes Care 2015; 38: 529–537. [DOI] [PubMed] [Google Scholar]
- 30. Cappuccio FP, D’Elia L, Strazzullo P, et al Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta‐analysis. Diabetes Care 2010; 33: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo VY, Cao B, Wong CKH, et al The association between daytime napping and risk of diabetes: a systematic review and meta‐analysis of observational studies. Sleep Med 2017; 37: 105–112. [DOI] [PubMed] [Google Scholar]
- 32. Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta‐analysis of longitudinal studies. J Clin Psychiatry 2013; 74: 31–37. [DOI] [PubMed] [Google Scholar]
- 33. Kato M, Noda M, Inoue M, et al Psychological factors, coffee and risk of diabetes mellitus among middle‐aged Japanese: a population‐based prospective study in the JPHC study cohort. Endocr J 2009; 56: 459–468. [DOI] [PubMed] [Google Scholar]
- 34. Vancampfort D, Rosenbaum S, Ward PB, et al Type 2 diabetes among people with posttraumatic stress disorder: systematic review and meta‐analysis. Psychosom Med 2016; 78: 465–473. [DOI] [PubMed] [Google Scholar]
- 35. Vetter C, Dashti HS, Lane JM, et al Night shift work, genetic risk, and type 2 diabetes in the UK Biobank. Diabetes Care 2018; 41: 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vetter C, Devore EE, Ramin CA, et al Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 2015; 38: 1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu T, Magnusson Hanson LL, Lange T, et al Workplace bullying and violence as risk factors for type 2 diabetes: a multicohort study and meta‐analysis. Diabetologia 2018; 61: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kivimaki M, Virtanen M, Kawachi I, et al Long working hours, socioeconomic status, and the risk of incident type 2 diabetes: a meta‐analysis of published and unpublished data from 222,120 individuals. Lancet Diabetes Endocrinol 2015; 3: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrie JE, Virtanen M, Jokela M, et al Job insecurity and risk of diabetes: a meta‐analysis of individual participant data. CMAJ 2016; 188: E447–E455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ludwig J, Sanbonmatsu L, Gennetian L, et al Neighborhoods, obesity, and diabetes–a randomized social experiment. N Engl J Med 2011; 365: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Christine PJ, Auchincloss AH, Bertoni AG, et al Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the Multi‐Ethnic Study of Atherosclerosis (MESA). JAMA Intern Med 2015; 175: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diabetes Prevention Program Research Group . Long‐term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15‐year follow‐up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015; 3: 866–875 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lindstrom J, Peltonen M, Eriksson JG, et al Improved lifestyle and decreased diabetes risk over 13 years: long‐term follow‐up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013; 56: 284–293 [level 1]. [DOI] [PubMed] [Google Scholar]
- 44. Gong Q, Zhang P, Wang J, et al Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30‐year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol 2019; 7: 452–461 [level 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Knowler WC, Barrett‐Connor E, Fowler SE, et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiasson JL, Josse RG, Gomis R, et al Acarbose for prevention of type 2 diabetes mellitus: the STOP‐NIDDM randomised trial. Lancet 2002; 359: 2072–2077. [DOI] [PubMed] [Google Scholar]
- 47. Padwal R, Majumdar SR, Johnson JA, et al A systematic review of drug therapy to delay or prevent type 2 diabetes. Diabetes Care 2005; 28: 736–744. [DOI] [PubMed] [Google Scholar]
- 48. Kawamori R, Tajima N, Iwamoto Y, et al Voglibose for prevention of type 2 diabetes mellitus: a randomised, double‐blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009; 373: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 49. DeFronzo RA, Tripathy D, Schwenke DC, et al Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364: 1104–1115. [DOI] [PubMed] [Google Scholar]
- 50. Gerstein HC, Bosch J, Dagenais GR, et al Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 51. le Roux CW, Astrup A, Fujioka K, et al 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double‐blind trial. Lancet 2017; 389: 1399–1409. [DOI] [PubMed] [Google Scholar]
- 52. Li Z, Li Y, Liu Y, et al Comparative risk of new‐onset diabetes mellitus for antihypertensive drugs: a network meta‐analysis. J Clin Hypertens 2017; 19: 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet 2007; 369: 201–207. [DOI] [PubMed] [Google Scholar]
- 54. Sattar N, Preiss D, Murray HM, et al Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet 2010; 375: 735–742. [DOI] [PubMed] [Google Scholar]
- 55. Preiss D, Seshasai SR, Welsh P, et al Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA 2011; 305: 2556–2564. [DOI] [PubMed] [Google Scholar]
APPENDIX 1. DIABETES AND CANCER
- 1. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta‐analysis. J Natl Cancer Inst 2005; 97: 1679–1687. [DOI] [PubMed] [Google Scholar]
- 2. Larsson SC, Orsini N, Brismar K, et al Diabetes mellitus and risk of bladder cancer: a meta‐analysis. Diabetologia 2006; 49: 2819–2823. [DOI] [PubMed] [Google Scholar]
- 3. Giovannucci E, Harlan DM, Archer MC, et al Diabetes and cancer: a consensus report. CA Cancer J Clin 2010; 60: 207–221. [DOI] [PubMed] [Google Scholar]
- 4. Kasuga M, Ueki K, Tajima N, et al Report of the JDS/JCA Joint Committee on Diabetes and Cancer. Diabetol Int 2013; 2: 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goto A, Noto H, Noda M, et al Report of the Japan Diabetes Society (JDS)/Japanese Cancer Association (JCA) Joint Committee on Diabetes and Cancer, Second Report. Diabetol Int 2016; 7: 12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue M, Iwasaki M, Otani T, et al Diabetes mellitus and the risk of cancer: results from a large‐scale population‐based cohort study in Japan. Arch Intern Med 2006; 166: 1871–1877. [DOI] [PubMed] [Google Scholar]
- 7. Sasazuki S, Charvat H, Hara A, et al Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci 2013; 104: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barone BB, Yeh HC, Snyder CF, et al Long‐term all‐cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta‐analysis. JAMA 2008; 300: 2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barone BB, Yeh HC, Snyder CF, et al Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta‐analysis. Diabetes Care 2010; 33: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Poll‐Franse LV, Houterman S, Janssen‐Heijnen ML, et al Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer 2007; 120: 1986–1992. [DOI] [PubMed] [Google Scholar]
- 11. Lee W, Yoon YS, Han HS, et al Prognostic relevance of preoperative diabetes mellitus and the degree of hyperglycemia on the outcomes of resected pancreatic ductal adenocarcinoma. J Surg Oncol 2016; 113: 203–208. [DOI] [PubMed] [Google Scholar]
APPENDIX 2. DIABETES AND BONE MINERAL METABOLISM
- 1. Guidelines for Prevention and Treatment of Osteoporosis. Life Science Publishing. [Google Scholar]
- 2. Janghorbani M, Van Dam RM, Willett WC, et al Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495–505. [DOI] [PubMed] [Google Scholar]
- 3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta‐analysis. Osteoporos Int 2007; 18: 427–444. [DOI] [PubMed] [Google Scholar]
- 4. Weber DR, Haynes K, Leonard MB, et al Type 1 diabetes is associated with an increased risk of fracture across the life span: a population‐based cohort study using The Health Improvement Network (THIN). Diabetes Care 2015; 38: 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hothersall EJ, Livingstone SJ, Looker HC, et al Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res 2014; 29: 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melton LJ 3rd, Leibson CL, Achenbach SJ, et al Fracture risk in type 2 diabetes: update of a population‐based study. J Bone Miner Res 2008; 23: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma L, Oei L, Jiang L, et al Association between bone mineral density and type 2 diabetes mellitus: a meta‐analysis of observational studies. Eur J Epidemiol 2012; 27: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loke YK, Singh S, Furberg CD. Long‐term use of thiazolidinediones and fractures in type 2 diabetes: a meta‐analysis. CMAJ 2009; 180: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keegan TH, Schwartz AV, Bauer DC, et al Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care 2004; 27: 1547–1553. [DOI] [PubMed] [Google Scholar]
APPENDIX 3. PANCREAS/ISLET TRANSPLANTATION
- 1. Saito T, Gotoh M, Satomi S, et al Islet transplantation using donors after cardiac death: report of the Japan Islet Transplantation Registry. Transplantation 2010; 90: 740–747. [DOI] [PubMed] [Google Scholar]
- 2. Hering BJ, Kandaswamy R, Ansite JD, et al Single‐donor, marginal‐dose islet transplantation in patients with type 1 diabetes. JAMA 2005; 293: 830–835. [DOI] [PubMed] [Google Scholar]
APPENDIX 4. LARGE‐SCALE CLINICAL TRIALS IN JAPANESE PATIENTS WITH DIABETES
- 1. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol 2017. [DOI] [PubMed] [Google Scholar]


