Abstract
Hemoglobin (Hb) North Manchester [β51(D2) Pro→His; HBB:c.155 C>A] is a rare Hb β‐globin gene variant that affects glycated Hb measurement values, such as ion‐exchange high‐performance liquid chromatography, in patients with diabetes. This variant was first detected in the UK in 1998. Here, we describe the first case involving Hb North Manchester detected incidentally in a patient with type 2 diabetes in Northern China. The Hb variant was discovered by ion‐exchange high‐performance liquid chromatography, yet capillary electrophoresis of both glycated Hb program and Hb program failed to detect it. Subsequently, Sanger sequencing was carried out to help identify the Hb variant.
Keywords: Capillary electrophoresis, Glycated hemoglobin, Hemoglobin variant
We report a hemoglobin (Hb) variant named Hb North Manchester, which was discovered by ion‐exchange high‐performance liquid chromatography, yet capillary electrophoresis of both glycated Hb program and Hb program failed to detect it. Sanger sequencing was carried out to help identify the Hb variant.
Introduction
Glycated hemoglobin (HbA1c) is widely used to monitor glucose control in patients with diabetes. Common methods available for HbA1c assays include ion‐exchange high‐performance liquid chromatography (HPLC), boronate affinity binding and HPLC, immunoassays, enzymatic assays and capillary electrophoresis1. HbA1c results can be affected when hemoglobin (Hb) variants are present, and boronate affinity methods have been shown to be minimally affected1. Here, we report a northern Chinese patient with type 2 diabetes showing abnormally low HbA1c values by ion‐exchange HPLC in whom the Hb North Manchester variant was detected using ion‐exchange HPLC, but was not detected by capillary electrophoresis.
Case Report
A 42‐year‐old man originally from Henan Province in northern China visited Peking University People’s Hospital in May 2018. He had an 11‐year history of type 2 diabetes, and had followed a diabetes‐specific diet and exercised regularly. He took metformin, acarbose and sitagliptin for glucose control during the previous year. Blood testing at multiple centers in February and August 2017 yielded low HbA1c levels of 4.3 and 4.4% by ion‐exchange HPLC (TOSOH HLC‐723 G8; Tosoh, Tokyo, Japan), and 5.1% in October 2017 by ion‐exchange HPLC (Variant II Turbo 2.0 [VII‐T 2.0], Bio‐Rad, Hercules, CA, USA). In March 2018, the fasting and 2‐h postprandial capillary blood glucose concentration derived from the patient’s home capillary measurement ranged 6.8–7.7 and 7.1–7.5 mmol/L, respectively. These relatively low HbA1c levels were not consistent with the patient’s blood glucose level; in order to determine the reason for this discrepancy, blood samples were collected to carry out hematological and serum biochemical measurements. The project was approved by the constituted ethics committee of the institution, and the patient had provided written informed consent. All hematological parameters and serum biochemical parameters remained within the reference ranges. The patient’s plasma glucose level was 5.97 mmol/L at fasting and 6.85 mmol/L at 2 h after breakfast with routine hypoglycemic agents. Enzymatic testing was used to detect serum levels of glycated albumin (Lucica GA‐L; Asahi‐Kasei, Tokyo, Japan) and yielded a normal value (12.45% [11–16%]).
HbA1c was also assayed using ion‐exchange HPLC (TOSOH HLC‐723 G8; Tosoh); ion‐exchange HPLC (VII‐T 2.0; Bio‐Rad), capillary electrophoresis (Capillarys 2 Flex Piercing [C2FP], HbA1c mode; Sebia, Lisses, France) and the boronate affinity HPLC method (Premier Hb 9210™; Trinity Biotech, Kansas City, MO, USA). Capillary electrophoresis of the Hb program (Minicap Flex Piercing, Phoresis Hb Variant mode V8.6.3; Sebia, Evry, France) was used to screen Hb variants. The TOSOH G8, VII‐T 2.0, C2FP and boronate affinity HPLC analyses yielded respective HbA1c values of 4.0% (20 mmol/mol), 3.7% (17 mmol/mol), 5.4% (36 mmol/mol) and 5.8% (40 mmol/mol). Unusual peaks were observed on the VII‐T 2.0 (Figure 1) and TOSOH G8 chromatogram (Figure 2), but not on the C2FP electropherogram (Figure 3). No unusual migration indicative of abnormal Hb was observed on the electropherogram using capillary electrophoresis of the Hb program.
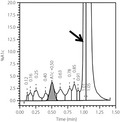
Figure 1.
The chromatogram of ion‐exchange high‐performance liquid chromatography (Variant II Turbo 2.0; Bio‐Rad, Hercules, CA, USA). The arrow indicates an unusual peak corresponding to the hemoglobin North Manchester variant. A1c, glycated hemoglobin.
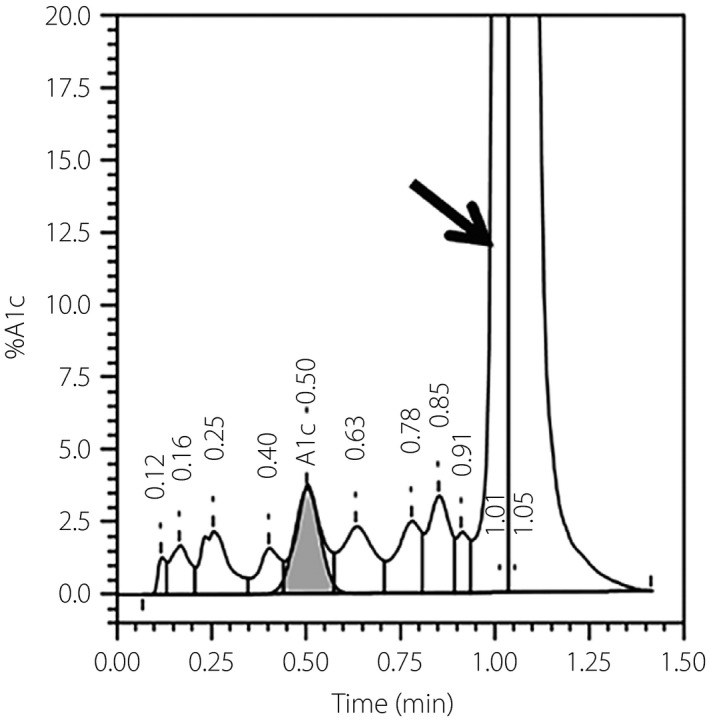
Figure 2.
The chromatogram of ion‐exchange high‐performance liquid chromatography (TOSOH G8; Tosoh, Tokyo, Japan). The arrow indicates an unusual peak corresponding to the hemoglobin North Manchester variant. A1A, HbA1a, glycated hemoglobin with fructose‐1,6‐diphosphate or glucose‐6‐phosphate to the amino terminus of the HbA β chain; A1B, HbA1b, glycated hemoglobin with a pyruvate to the amino terminus of the HbA β chain; A0, HbA0, non‐glycated hemoglobin of the HbA β chain; FP, front peak; F, HbF, fetal hemoglobin; HbA1, total glycated hemoglobin, include HbA1a and HbA1b and HbA1c; HbA1C, glycated hemoglobin (HbA1c), nonenzymatic addition of glucose to the amino‐terminal valine residues of the HbA β chains; HbF, fetal hemoglobin; LA1C+, labile glycated hemoglobin; SA1C: stable glycated hemoglobin; P00, Hb variant.
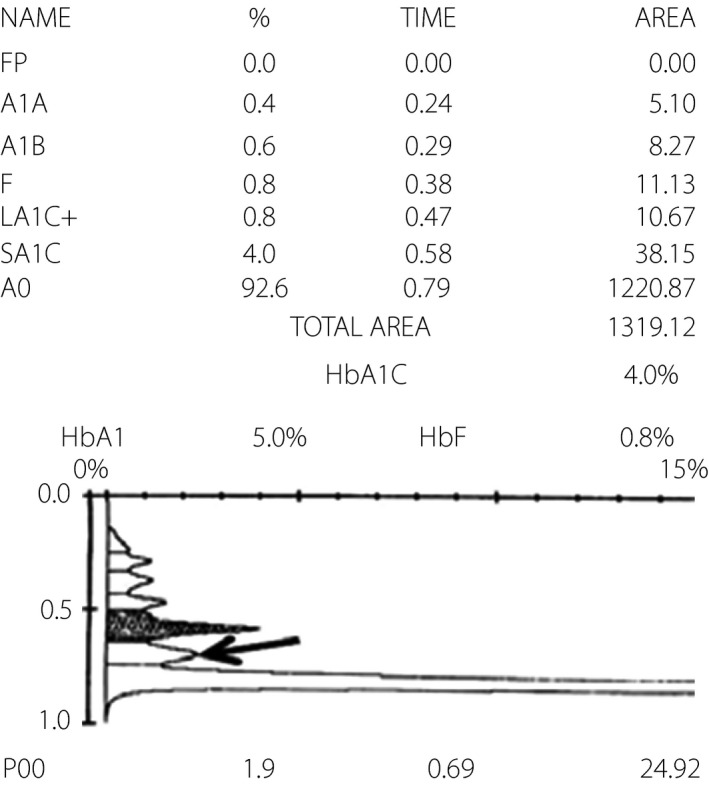
Figure 3.
The electropherogram derived from capillary electrophoresis (C2FP; Sebia, Lisses, France). HbA1c, glycated hemoglobin.
The presence of the Hb variant was probable, as both ion‐exchange HPLC methods showed unusual peaks. Sanger sequencing was carried out for confirmation. All exons and intron–exon junctions of the genes encoding Hb α (HBA1 and HBA2) and β (HBB) were polymerase chain reaction‐amplified, sequenced and compared with corresponding reference sequences (GeneIDs: 3039, 3040 and 3043, respectively) obtained from the NCBI GenBank (https://www.ncbi.nlm.nih.gov/gene/) to identify the Hb variant.
Sanger sequencing analysis showed no HBA gene variants, and a heterozygous HBB mutation, β51(D2) Pro→His; HBB:c.155 C>A, was revealed and identified according to the Database of Human Hemoglobin Variants and Thalassemias nucleotide and amino acid numbering system2. This variant was previously detected through electrospray mass spectrometry in a man with diabetes from Manchester, the UK, in 1998, and was named Hb North Manchester3. This single nucleotide substitution (C>A) at codon 51 in HBB (Figure 4) causes a change from proline to histidine in the β‐globin chain, leading to a 40‐Da increase in the β‐chain mass3.
Figure 4.
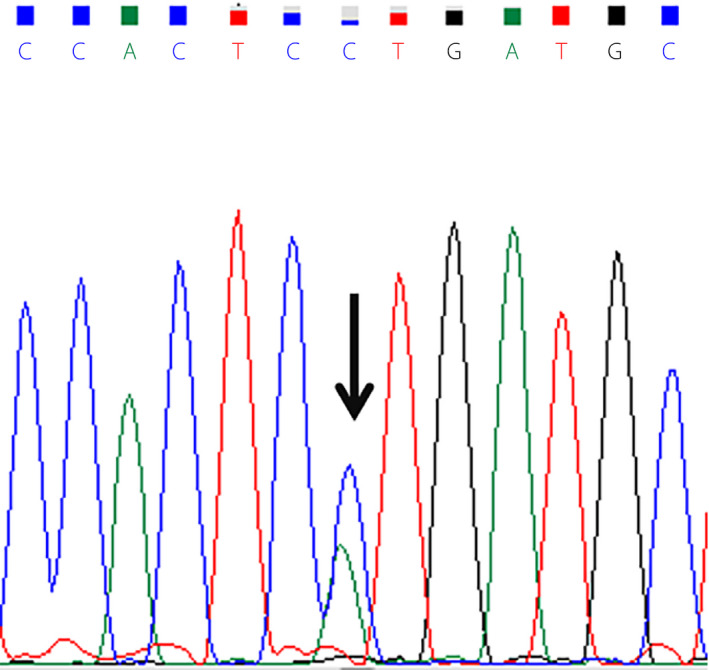
Sanger sequencing results of the HBB gene. The arrow indicates the hemoglobin North Manchester variant, β51(D2) Pro→His; HBB:c.155 C>A.
Discussion
The patient’s plasma glucose, glycated albumin and the HbA1c level detected using the boronate affinity HPLC method were all within the normal range. However, those HbA1c values obtained using ion‐exchange HPLC methods were abnormally low. Because the boronate affinity HPLC method is generally least likely to be affected by Hb variants, it directly measures total HbA1c as a fraction of total Hb. According to the Standards of Medical Care in Diabetes of 2018, discordance between the HbA1c and plasma glucose values might suggest an Hb variant4.
Here, the unusual peaks on the VII‐T 2.0 and TOSOH G8 chromatograms, but not the C2FP electropherogram, indicate the differing specificities of ion‐exchange HPLC and capillary electrophoresis for the Hb variant. The ion‐exchange HPLC method separates HbA1c based on charge differences; many variant molecules carry different charges and thus move at different speeds, yielding different retention times and aberrant chromatography peaks5. Accordingly, it might be helpful to review the chromatogram for additional peaks, especially when the HbA1c level is abnormally low or high. In the present patient, the Hb North Manchester variant led to a falsely low HbA1c result on ion‐exchange HPLC. Additionally, the chromatogram contained an unusual peak close to the HbA0 peak. It could be inferred that because the Hb variant has a different retention time with HbA0, the Hb variant could be separated from the HbA0 peak.
Capillary electrophoresis is a precise and robust HbA1c measurement method that can screen most common Hb variants6. Capillary electrophoresis of the Hb program, can screen Hb variants, and detect most common and some clinically silent Hb variants. In the present case, the HbA1c value determined through capillary electrophoresis with the HbA1c program was slightly lower than that obtained with the boronate affinity HPLC method. It is difficult to tell the HbA1c value using capillary electrophoresis method would not be interfered, unless a sample with the same Hb variant in the higher glucose level shows an abnormal HbA1c range. However, a review of the electropherograms of both the HbA1c program and Hb program did not show additional Hb peaks. As capillary electrophoresis separates Hb molecules according to electrophoretic mobility in an electric field7, we speculated that the Hb variant in this case would show similar or identical electrophoretic mobilities and co‐migrate with HbA0, so that additional Hb peaks did not appear on the electropherograms of both the HbA1c program and Hb program. It has been reported that the Hb Silver Springs variant similarly yielded an unusual peak on the ion‐exchange HPLC chromatogram, but was not detected by capillary electrophoresis of the HbA1c program8. By contrast, the Hb New York variant was detected by capillary electrophoresis of the Hb program, but not ion‐exchange HPLC, indicating that the latter could not separate the variant from HbA9. Furthermore, Hb Melusine yielded normal HPLC and capillary electrophoresis of Hb program profiles, and only yielded an unusual peak through capillary electrophoresis of the HbA1c program10. In summary, a normal capillary electropherogram by HbA1c or Hb program or chromatogram by ion‐exchange HPLC cannot exclude the presence of an Hb variant.
In conclusion, this is the first reported case involving the Hb North Manchester variant in a Chinese patient. The present results show that the Hb North Manchester variant can affect the results of HbA1c analysis through ion‐exchange HPLC, but not that obtained from the boronate affinity HPLC method. The inclusion of plasma glucose and glycated albumin levels can assist with the interpretation of the HbA1c values. Furthermore, only ion‐exchange HPLC suggested the presence of the Hb variant in this case, suggesting that a careful review of the resulting chromatogram might reveal a potential variant. Finally, a genetic evaluation, such as Sanger sequencing, can confirm an Hb variant.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work is supported by grants 2016YFC1305600 and 2016YFC1305603 from the Major Chronic Non‐communicable Disease Prevention and Control Research, National Key R&D Program of China. The authors thank the Sebia Shanghai Representative office for providing technical support.
J Diabetes Investig 2020; 11: 1014–1017
References
- 1. Rhea JM, Molinaro R. Pathology consultation on HbA(1c) methods and interferences. Am J Clin Pathol 2014; 141: 5–16. [DOI] [PubMed] [Google Scholar]
- 2. A Database of Human Hemoglobin Variants and Thalassemias. Available from http://globin.bx.psu.edu/hbvar/menu.html. Accessed March 26, 2019.
- 3. Wiener K, Roberts NB, Green BN. The effect of an unusual haemoglobin variant (beta 51Pro–>His) on haemoglobin A1c measurement. Ann Clin Biochem 1998; 35: 321–323. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes‐2018. Diabetes Care 2018; 41: S13‐S27. [DOI] [PubMed] [Google Scholar]
- 5. Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001; 47: 153–163. [PubMed] [Google Scholar]
- 6. Riou J, Szuberski J, Godart C, et al Precision of CAPILLARYS 2 for the detection of hemoglobin variants based on their migration positions. Am J Clin Pathol 2018; 149: 172–180. [DOI] [PubMed] [Google Scholar]
- 7. Gordon MJ, Huang X, Pentoney SL Jr, et al Capillary electrophoresis. Science 1988; 242: 224–228. [DOI] [PubMed] [Google Scholar]
- 8. Little RR, La’ulu SL, Hanson SE, et al Effects of 49 different rare Hb variants on HbA1c measurement in eight methods. J Diabetes Sci Technol 2015; 9: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. You‐Qiong L, Hui‐Ping H, Zhi‐Zhong C, et al Comparison of capillary electrophoresis and high performance liquid chromatography for detection and quantification of hemoglobin New York. Clin Chem Lab Med 2016; 54: 91–95. [DOI] [PubMed] [Google Scholar]
- 10. Peeters B, Brandt I, Desmet K, et al Hb Melusine and Hb Athens‐Georgia: potentially underreported in the Belgian population? Four cases demonstrating the lack of detection using common CE‐HPLC methods either for glycated hemoglobin (HbA1C) analysis or Hb variant screening. Acta Clin Belg 2016; 71: 458–461. [DOI] [PubMed] [Google Scholar]


