Abstract
Aims/Introduction
Iron metabolism can directly or indirectly affect the occurrence and development of type 2 diabetes. This meta‐analysis and systematic review aimed to analyze the association between serum iron metabolism indicators and type 2 diabetes.
Materials and Methods
The databases PubMed and Embase were searched for studies on the correlations between serum iron metabolism indicators (iron, ferritin, transferrin, hepcidin and soluble transferrin receptor) and type 2 diabetes since January 2006. Relevant data were extracted from the included studies, and meta‐analysis was carried out.
Results
A total of 12 case–control and cohort studies were analyzed. Of the 12 studies, 11 described the correlation between serum ferritin levels and type 2 diabetes. The median and high serum ferritin concentrations were significantly associated with the risks of type 2 diabetes (odds ratio [OR] 1.20, 95% confidence interval [CI] 1.08–1.33 and OR 1.43, 95% CI 1.29–1.59, respectively). However, the low concentration was not correlated with the risk of type 2 diabetes (OR 0.99, 95% CI 0.89–1.11). No significant association was observed between serum soluble transferrin receptor and type 2 diabetes, whereas the soluble transferrin receptor‐to‐ferritin ratio was significantly inversely related to the risk of type 2 diabetes in the median and high ratio subgroups (OR 0.71, 95% CI 0.51, 0.99 and OR 0.65, 95% CI 0.45–0.95).
Conclusions
The elevated serum ferritin was one of the risk factors for type 2 diabetes, and soluble transferrin receptor‐to‐ferritin ratio was inversely related to the risk of type 2 diabetes. A systematic review showed that serum transferrin and hepcidin might be directly or indirectly related to the development of diabetes.
Keywords: Ferritin, Soluble transferrin receptor, Type 2 diabetes
The present results showed that a higher level of serum ferritin was one of the risk factors for type 2 diabetes. Monitoring serum ferritin levels might be important in the diagnosis and treatment of type 2 diabetes. In addition, a systematic review showed that serum iron, transferrin, hepcidin and soluble transferrin receptor were also related to the development of diabetes.

Introduction
Diabetes is a common and frequent metabolic disease. Recently, the prevalence of diabetes has increased rapidly worldwide. The incidence rate of type 2 diabetes in Chinese adults aged >18 years has reached 11.6% (12.1% in men and 11% in women)1. Diabetes has become one of the most significant public health problems.
Iron, one of the essential trace mineral elements, is involved in regulating the differentiation and growth of living cells, and participates in electron transfer between cells, affecting the genomic synthesis. Furthermore, iron can combine and transport oxygen to various parts of the body, and participate in many metabolic processes essential for life2. The normal iron content in adults is approximately 60 g/dL. However, once the iron content in the body increases for various reasons, it might cause severe damage to the pancreatic cells through excessive oxidative stress. In addition, the ability to use insulin and gluconeogenesis in the liver are weakened, resulting in the occurrence and development of type 2 diabetes3, 4.
As a strong oxidant, iron can speed up the production of a large number of reactive oxygen radicals that participate in regulating the signal transduction process of islet β‐cells, thereby affecting the secretion of insulin and interfering with the glucose metabolism process5. Second, iron plays an important role in mitochondria, which can promote the synthesis of adenosine triphosphate and also affect the secretion levels of insulin, ultimately leading to glucose metabolism disorder6, 7.
The blood sugar levels are closely related to the iron contents in islet β‐cells. However, because of the higher expression levels of iron transport proteins in the islet β‐cells8 compared with other tissue cells, the islet β‐cells are more likely to accumulate iron. Too much iron might induce excessive oxidative stress, promote islet β‐cell apoptosis, and ultimately affect insulin secretion and increase the risk of insulin resistance.
Studies have shown that iron metabolism indicators (transferrin, ferritin, hepcidin, transferrin receptor and so on) can directly or indirectly affect the occurrence and development of type 2 diabetes. However, the results describing the correlation between iron metabolism and type 2 diabetes were inconsistent between different sex and ethnicity. Serum ferritin concentrations were significantly higher in women with diabetes than in women without diabetes in all racial/ethnic groups, but were significantly lower in Asian men with diabetes than in those without diabetes9. Therefore, the present meta‐analysis and systematic review was carried out on the relationship between serum iron metabolism indicators and type 2 diabetes.
Methods
Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) studies based on humans; (ii) studies on the correlations of serum iron, ferritin, transferrin, hepcidin and soluble transferrin receptor with type 2 diabetes mellitus; (iii) case–control studies or prospective cohort studies; and (iv) statistical indicators, such as odds ratio (OR) or relative risk values, and 95% confidence interval (CI), provided in the studies with adjustment.
The exclusion criteria were as follows: (i) studies based on animals; (ii) patients with type 2 diabetes having other complications; (iii) patients with type 2 diabetes and other diseases; (iv) case reports or review; and (v) poor quality or incomplete data.
Literature retrieval
Databases PubMed and Embase were searched for studies on the correlations between serum iron metabolism indicators (iron, ferritin, transferrin, hepcidin and soluble transferrin receptor) and type 2 diabetes since January 2006. The related references of the studies were traced back, and the accuracy and objectivity of the viewpoints of the retrieved studies were evaluated. According to the principle of patient intervention comparison outcome, the information (“Ferritins” [Mesh] OR “Iron” [Mesh] OR “Transferrin” [Mesh] OR “Ferroprotein” [Mesh]) AND (“Diabetes Mellitus, Type 2” [Mesh]) was retrieved by matching key words or free words with each other.
Data extraction
Two researchers independently carried out the retrieval of relevant studies to determine whether the included studies met the aforementioned inclusion criteria. Any disagreement between the two was solved by discussion. In the final included studies, the basic features were extracted as follows: first author’s name, year of publication, nationality of study, type of study, follow‐up years, selection criteria for type 2 diabetes and normal control groups, sample size of the study, average age of the participants, proportion of men in the normal control and type 2 diabetes groups, diagnostic criteria for patients with type 2 diabetes, iron metabolism indicators, and variables adjusted in the study.
Ethical approval
This study complied with the Declaration of Helsinki. Given this study was a meta‐analysis, no prior ethical approval was required.
Statistical analysis
First, the publication bias of the study was evaluated through Begg’s and Egger’s tests using the statistical analysis software Stata12.0 (StataCorp, College Station, TX, USA) Whether the distribution of funnel graphs in Begg’s or Egger’s test was approximately symmetric was used to subjectively evaluate the publication bias. The adjusted OR or relative risk values (or equivalent to the OR values) and 95% CI were extracted9, 10, 11, 12, 13. The combination of pure effect size and CI was used. Furthermore, a sensitivity analysis was carried out based on the heterogeneity index. P < 0.01 was considered statistically significant in the Begg’s and Egger’s test; however, in sensitivity analyses, P < 0.05 was considered as statistically significant.
Results
Screening literature results
A total of 168 studies in the English language, describing the correlations between serum iron metabolism indicators (iron, ferritin, transferrin and soluble transferrin receptor) and type 2 diabetes, were found from the two databases. Of these studies, 145 were from PubMed and 23 from Embase; 23 repeated studies were excluded. After skimming the titles and abstracts, followed by an intensive reading of the full text, 12 case–control studies and cohort studies were obtained10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, which included 6,516 patients with type 2 diabetes and 43,120 control individuals without type 2 diabetes. The details of the screening process are shown in Figure 1.
Figure 1.

Literature screening flowchart.
Basic features of the included studies
The basic characteristics and relevant outcome indicators of the studies were extracted, including the first author’s name, year of publication, country of study, type of study, follow‐up years, selection criteria for type 2 diabetes and normal control groups, sample sizes of the studies, average age of the participants, proportion of men in the normal control and type 2 diabetes groups, diagnostic criteria for patients with type 2 diabetes, iron metabolism indicators, and variables adjusted in the study. The detailed features of data extraction are shown in Table 1.
Table 1.
Basic characteristics of the included studies
| First author’s name | Publication year | Country | Type of study | Follow‐up years | Patient/control | Sample size (patient/control) | Average age, years (patient/control) | Outcome indicators | Variables adjusted |
|---|---|---|---|---|---|---|---|---|---|
| Xin Guo | 2013 | China | Case–control study | – | T2DM/normal control | 555/704 | 64/58 | SF, hepcidin | 1, 2, 6 |
| Chang Hee Jung | 2013 | Korea | Case–control study | 4 | T2DM/normal control | 186/1,843 | 54/50 | SF | 1, 5, 7, 8, 9, 11, 13, 14, 15, 16, 17, 18, 19 |
| Victoria Arija | 2014 | Caucasus | Case–control study | 6 | T2DM/normal control | 153/306 | 66/66 | SF, sTfR | 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 16, 19, 20 |
| Clemens Wittenbecher | 2015 | Potsdam | Cohort study | 7 | T2DM/normal control | 688/2,047 | 49/49 | SF | 1, 2, 3, 6, 7, 9, 10, 11, 12 |
| Sunyong Kim | 2015 | Korea | Cohort study | 3 | T2DM/normal control | 2,655/27,347 | 42/43 | SF | 1, 6, 7, 8, 9, 11, 14, 19, 22 |
| Jose C. Fernández‐Cao | 2016 | Spain | Case–control study | 6 | T2DM/normal control | 153/306 | 66/66 | sTfR | 3, 4, 5, 7, 9, 10, 13, 16, 19, 20, 21, 23, 24, 25 |
| Liang Sun | 2008 | China | Case–control study | – | T2DM/normal control | 440/2,812 | 50/50 | SF | 1, 2, 3, 6, 7, 8, 9, 10, 13, 16, 23, 25, 26 |
| DeChun Luan | 2008 | China | Case–control study | – | T2DM/normal control | 146/2,851 | 46/46 | SF | 1, 2, 5, 6, 7, 8, 10, 11, 12, 13, |
| N. G. Forouhi | 2007 | Norwich | Cohort Study | 5 | T2DM/normal control | 360/758 | 62/62 | SF | 1, 2, 6, 17, 18, 27 |
| J. Montonen H | 2012 | Potsdam | Cohort Study | 7 | T2DM/normal control | 849/2,500 | 50/50 | SF, sTfR | 1, 2, 3, 5, 6, 7, 8, 9, 12, 13, 16, 17, 18, 19, 20, 27 |
| S.N. Rajpathak | 2009 | USA | Case–control study | – | T2DM/normal control | 280/280 | 50/50 | SF | 1, 2, 6, 9, 13, 15 |
| Zumin Shi | 2006 | China | Cohort Study | – | T2DM/normal control | 51/1366 | 47/47 | SF | 1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 13, 23 |
Adjusted variables: 1: age; 2: sex; 3: education level; 4: marital status; 5: waist circumference; 6: body mass index; 7: smoking; 8: drinking alcohol; 9: sports; 10: dietary intake; 11: hypertension; 12: hyperlipidemia; 13: family history of diabetes; 14: homeostasis model of insulin resistance; 15: glycosylated hemoglobin; 16: high sensitivity C‐reactive protein; 17: glutamyl transpeptidase; 18: alanine aminotransferase; 19: triglyceride, high‐density lipoprotein, low‐density lipoprotein; 20: fasting blood glucose; 21: fasting insulin; 22: white blood cells; 23: saturated, monounsaturated and polyunsaturated fatty acids; 24: center (Pamplona/Barcelona/Tarragona); 25: intervention group (Mediterranean diet supplemented with nuts or olive oil/control group); 26: inflammatory factors (interleukin‐6, and tumor necrosis factor receptor 2); 27: adipokines (adiponectin, plasminogen activator inhibitor‐1 and retinol‐binding protein 4). SF, serum ferrtin; sTfR, soluble transferrin receptor‐to‐ferritin ratio; T2DM, type 2 diabetes.
The publication bias of the studies was evaluated by Begg’s and Egger’s tests. The distributions of funnel plots were approximately symmetric. The index of pr > |z| was 0.399 in Begg’s test and P > |t| was 0.49 in Egger’s test, showing no significant publication bias in these studies.
Serum ferritin levels and the risks of type 2 diabetes
Of the 12 studies, 11 described the correlation between serum ferritin levels and type 2 diabetes10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20. Eight studies were categorized into three subgroups (low, medium and high concentration) according to the quartiles or quintile of ferritin levels, respectively. The other three serum ferritin unstratified studies constituted an independent subgroup. Eventually, the effect sizes (adjusted OR or relative risk) and 95% CI were extracted. After taking the natural logarithm of the upper and lower limits of effect sizes and the 95% CI, the pure effect sizes and 95% CI were combined using the random effects models, and the forest maps were constructed.
The results showed that the median and high serum ferritin concentrations were significantly associated with the risks of type 2 diabetes (OR 1.20, 95% CI 1.08–1.33 and OR 1.43, 95% CI 1.29–1.59). However, no significant association was shown between the low serum ferritin concentration and the risks of type 2 diabetes (OR 0.99, 95% CI 0.89–1.11; Figure 2). In addition, serum ferritin levels remained a risk factor for type 2 diabetes in the unstratified subgroup (OR 1.35, 95% CI 1.23–1.48; 3).
Figure 2.

The association between serum ferritin and type 2 diabetes in ferritin concentration stratified group. CI, confidence interval; OR, odds ratio.
Figure 3.

The association between serum soluble transferrin receptor and type 2 diabetes in the ferritin unstratified group. CI, confidence interval; OR, odds ratio.
Sensitivity analysis
Figure 2 showed that in the high concentration subgroup, I 2 was 81.2% (P = 0.000), suggesting high heterogeneity among the eight included studies. The sensitivity analysis was carried out to explore the source of heterogeneity.
The natural logarithms of the corresponding effect sizes were taken, and the standard error of effect size logarithms was calculated. The results showed that the effect and 95% CI were evenly distributed on both sides of the baseline in each study. However, one study13 deviated from the baseline level further, as compared with the other seven studies, showing distinct heterogeneity, and hence, was eliminated.
After removing a reference with obvious heterogeneity, the effect size and 95% CI were again combined by the same method, and the forest map was drawn. As shown in Figure 4, the high serum ferritin concentrations were significantly associated with the risk of type 2 diabetes (OR 2.32, 95% CI 1.90–2.83). Furthermore, compared with the statistical variables (I 2 = 81.2%, P = 0.000) in the eight studies, the I 2 values were significantly decreased (I 2 = 1.1%, P = 0.416), which suggested that the excluded study was the main source of heterogeneity in the high concentration subgroup.
Figure 4.

The association between serum ferritin and type 2 diabetes, excluding one study. CI, confidence interval; OR, odds ratio.
Serum soluble transferrin receptor levels and the risk of type 2 diabetes
Of the 12 studies, three described the correlations between serum soluble transferrin receptor and type 2 diabetes12, 18, 21. The effect size OR values and 95% CI were extracted from these two studies. As shown in Figure 5, no significant correlation was found between serum soluble transferrin receptor levels and the risk of type 2 diabetes in the low concentration (OR 1.36, 95% CI 0.90–2.07), median concentration (OR 1.10, 95% CI 0.79–1.52) and high concentration (OR 0.95, 95% CI 0.67–1.34) subgroups.
Figure 5.
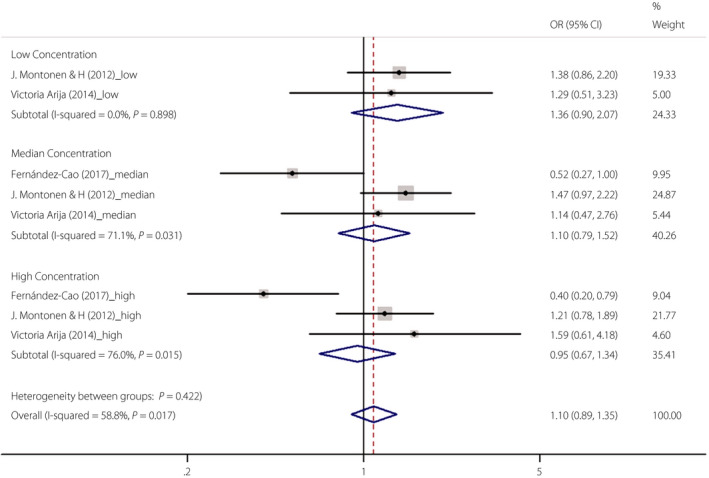
The association between serum soluble transferrin receptor and type 2 diabetes. CI, confidence interval; OR, odds ratio.
Soluble transferrin receptor‐to‐ferritin ratio and the risks of type 2 diabetes
Of the 12 studies, two described the correlations between serum soluble transferrin receptor‐to‐ferritin ratio and type 2 diabetes12, 18. The effect size OR values and 95% CI were extracted from these two studies. As shown in Figures 5, 6, soluble transferrin receptor‐to‐ferritin ratios were significantly inversely related to the risk of type 2 diabetes in median (OR 0.71, 95% CI 0.51–0.99) and high ratio subgroups (OR 0.65, 95% CI 0.45–0.95); however, no correlation was observed in the low soluble transferrin receptor‐to‐ferritin ratios subgroup (OR 0.87, 95% CI 0.63–1.20).
Figure 6.

The association between serum soluble transferrin receptor to ferritin ratio and type 2 diabetes. CI, confidence interval; OR, odds ratio.
Other iron metabolism indicators and the risks of type 2 diabetes
In addition, during the process of retrieving and selecting the studies, just one study described the correlation between serum hepcidin level and type 2 diabetes10. No related studies on the correlation between serum transferrin and type 2 diabetes were found; therefore, it was impossible to extract and analyze relevant data. Hence, the relationships of serum transferrin and hepcidin with type 2 diabetes were not evaluated by the meta‐analysis, and only discussed in the systematic review.
Discussion
Iron is one of the essential trace elements for the human body. The body contains 3–5 g iron. The body regulates the amount of iron mainly through absorption. When iron is deficient or excessive, it causes dysfunction of the body. Excessive iron stores have been suggested to be associated with a high risk of type 2 diabetes by causing damage to the pancreatic β‐cells and insulin resistance through increased oxidative stress22.
Ferritin, a key protein regulating iron homeostasis, is the main form of iron storage in the body. The level of ferritin might be increased due to the injury of non‐stored iron organs, which could be used as a sensitive indicator of excessive iron load in the body. Some studies have shown that serum ferritin levels are elevated in type 2 diabetes patients, and increased serum ferritin levels in Western and Asian populations are associated with an increased risk of diabetes10, 11, 12, 13, 14.
In the present study, OR for the risk of type 2 diabetes was significant in the median and high concentration subgroups. However, no correlation was established between the low concentration subgroup and type 2 diabetes. The results showed that the risk of type 2 diabetes increased with the increase in serum ferritin concentration. Consistent with the current results, three meta‐analyses evaluated the correlations between serum ferritin levels and the risk of type 2 diabetes, suggesting that high ferritin levels were associated with the risk of type 2 diabetes23, 24, 25. Therefore, Jung et al.11 speculated a threshold between serum ferritin and the risk of type 2 diabetes, above which, the risk is increased.
Serum ferritin levels were a risk factor for type 2 diabetes in the unstratified subgroup, which might be related to the high average serum ferritin levels of participants in these three studies.
The sensitivity analysis showed that a study might be the main resource of high heterogeneity in the serum ferritin high concentration subgroup13. The reason might be related to the following factors. First, the participants were young compared with the participants of the other studies. Age has a significant effect on ferritin level, which is related to the weakening of the body protection mechanism and the decline in the metabolic capacity after aging26. Second, each study had different variables to adjust, and as compared with the other three studies, the present study adjusted relatively few parameters. Third, serum ferritin concentrations varied according to sex and ethnicity27. A previous study identified innate biological differences in serum ferritin levels between races. Asian men and women were reported to have higher adjusted mean serum ferritin concentrations compared with white men and women27. In addition, the body composition of different races had different effects on the levels of serum ferritin28. The levels of the high ferritin subgroup (the 4th quartiles, ≥149.2 mg/mL) in the present study were lower than the levels in the other seven studies.
The mechanisms underlying the relationship between serum ferritin and type 2 diabetes might be as follows. First, the elevated serum ferritin might interact with other kinds of pathogenic factors, impair the function of islet β‐cells, affect the secretion of insulin and increase the risks of type 2 diabetes29. Second, insulin is involved in regulating the transcription of serum ferritin and increasing the use of iron in the peripheral tissues. When the body iron content is in excess, insulin secretion is affected, and the iron use in peripheral tissues is reduced. The overload iron causes excessive oxidative stress to influence normal physiological functions of various tissues and organs30. Third, serum ferritin is also considered as an acute‐phase reaction marker. When the body is affected by factors, such as inflammation, the serum ferritin levels increase, thereby affecting the insulin secretion and disrupting the normal glucose metabolism process. This leads to insulin resistance, which increases the risk of type 2 diabetes. Fourth, the elevated ferritin levels leading to an increased risk of type 2 diabetes are associated with oxidative stress. This phenomenon is caused by an increase in iron‐catalyzed hydroxyl radicals, which leads to systemic insulin resistance and hyperglycemia31.
Soluble transferrin receptor and type 2 diabetes
Serum soluble transferrin receptor, an indicator reflecting the intracellular iron storage, often binds to serum transferrin, which can transport iron from outside into the cell32. Soluble transferrin receptor, as a truncated form of transferrin receptor, is located in the serum proportional to the transmembrane protein, and therefore, to cell iron demands. Soluble transferrin receptor is considered as an optimal biomarker of iron deficiency. Hitherto, just a few studies have evaluated the association between soluble transferrin receptor and the risk of type 2 diabetes.
Montonen et al. 18 reported that no significant association was observed between the serum levels of soluble transferrin receptor and the risk of type 2 diabetes. In the present meta‐analysis, three studies described the correlations between serum soluble transferrin receptor and type 2 diabetes12, 15, and no significant correlation was established between the levels of serum soluble transferrin receptor and the risk of type 2 diabetes. To date, the independent association between serum soluble transferrin receptor and risk of diabetes was reported in only one study19; the elevated levels of soluble transferrin receptor levels were associated with an increased risk of diabetes among overweight and obese persons with impaired glucose tolerance. The study suggested that iron overload in the body leads to an excessive oxidative stress response; free iron is consumed, and in turn, serum soluble transferrin receptor increases the risk of patients with type 2 diabetes having abnormal glucose metabolism19. In addition, serum soluble transferrin receptor might be a biomarker of some other factor (such as obesity, insulin resistance or inflammation etc.) that is causally related to the development of diabetes and possibly unrelated to the iron load.
Soluble transferrin receptor‐to‐ferritin ratio and type 2 diabetes
Soluble transferrin receptor levels might be affected by other factors, such as the degree of glucose tolerance, insulin resistance, hyperinsulinemia, inflammation and obesity. Recent studies showed that the ratio of serum soluble transferrin receptor‐to‐ferritin could be used as an indicator to evaluate the iron content in vivo 18. Arija et al.12 reported a low soluble transferrin receptor‐to‐ferritin ratio level and the incidence of type 2 diabetes, but no association with soluble transferrin receptor. In addition, the present study showed serum soluble transferrin receptor‐to‐ferritin ratios were significantly inversely related to the risk of type 2 diabetes in median (OR 0.71, 95% CI 0.51–0.99) and high ratio subgroups (OR 0.65, 95% CI 0.45–0.95). However, as relatively few studies have been included to date, there might be some bias in the results; hence, additional studies are required to further confirm the correlation between the soluble transferrin receptor‐to‐ferritin ratio level and type 2 diabetes. At the same time, no association was observed in the low ratio subgroup, and whether the negative correlation between soluble transferrin receptor‐to‐ferritin ratio and type 2 diabetes in median and high ratio subgroups was related to increased serum ferritin levels might require further confirmation.
Hepcidin and type 2 diabetes
Only one study on the relationship between serum hepcidin and type 2 diabetes was included10. A previous study showed that serum hepcidin concentrations correlated with serum hemoglobin and ferritin concentrations. No difference was found in serum hepcidin concentrations between diabetes and control groups. The serum hepcidin concentrations were not significantly correlated with the risk factors for type 2 diabetes (body mass index, glycosylated hemoglobin, fasting blood glucose levels etc.).
Although no accurate evidence of a direct correlation between serum hepcidin and the risks of type 2 diabetes was found, serum hepcidin, as an important negative regulator of iron homeostasis in the body, might directly regulate the expressional levels of iron. Serum hepcidin might be mediated by the interaction between serum transferrin and cell membrane. On the one hand, with the increase in the body’s iron levels, iron levels in the liver also increase, inhibit the transcription and expression of iron transport protein on the cell membrane of the duodenum, and prevent the transfer of iron from the cells into circulation, ultimately reducing the absorption of iron in the gut. On the other hand, serum hepcidin can also act on liver cells and macrophages, and reduce the release of iron from the tissues, thereby decreasing the levels of iron in the body33, 34. Serum hepcidin is involved in the balance of iron by preventing the absorption, use and release of iron from the tissues35. The animal experiments confirmed that insulin might directly induce serum hepcidin production36. Under the influence of dietary factors, gluconeogenesis is continuously activated and serum hepcidin levels also rise, which induce iron accumulation and excessive oxidative stress, and interfere with glucose metabolism by activating p38 mitogen‐activated protein kinase and peroxisome proliferator‐activated receptor‐γ coactivator‐1α signaling pathways and CCAAT/enhancer‐binding protein alpha phosphorylation37. In addition, serum hepcidin might also affect the function of mitochondria and participate in glycogen synthesis. The excessive expression of serum hepcidin causes iron accumulation in the liver and an increase in active oxygen free radicals. The excessive oxidative stress eventually damages pancreatic cells, affects insulin secretion and increases the occurrence of type 2 diabetes38.
Serum transferrin and type 2 diabetes
Very few studies reported on the direct link between serum transferrin and type 2 diabetes. Because of the molecular weight and negative charges, serum transferrin is susceptible to leakage from glomeruli. Therefore, monitoring urinary transferrin levels might help in the early evaluation of the progression of diabetic nephropathy39. At the same time, serum transferrin can also induce lipolysis of the body’s fat cells, cause an increase in free fatty acids and affect the secretion function of the pancreas, ultimately leading to insulin resistance and an increased risk of type 2 diabetes40.
In conclusion, the elevated level of serum ferritin is one of the risk factors for type 2 diabetes. The serum soluble transferrin receptor‐to‐ferritin ratio was inversely related to the risk of type 2 diabetes. Serum soluble transferrin receptors might not be associated with the risk of type 2 diabetes. A systematic review showed that serum transferrin and hepcidin might be directly or indirectly related to the development of diabetes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Dr Song Xuping, School of public Health, Lanzhou University, for providing help for meta‐analysis and manuscript revision.
J Diabetes Investig 2020; 11: 946–955
References
- 1. Xu Y, Wang L, He J, et al Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 2. Hershko C, Peto TE, Weatherall DJ. Iron and infection. BMJ 1988; 296: 660–664. [PMC free article] [PubMed] [Google Scholar]
- 3. Fernández‐Real JM, Ricart‐Engel W, Arroyo E, et al Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care 1998; 21: 62–68. [DOI] [PubMed] [Google Scholar]
- 4. Wilson JG, Lindquist JH, Grambow SC, et al Potential role of increased iron stores in diabetes. Am J Med Sci 2003; 325: 332–339. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Yun JW, Lei XG. Glutathione peroxidase mimic ebselen improves glucose‐stimulated insulin secretion in murine islets. Antioxid Redox Signal 2014; 20: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ashcroft F, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell 2012; 148: 1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tarasov A, Dusonchet J, Ashcroft F. Metabolic regulation of the pancreatic beta‐cell ATP‐sensitive K+ channel: a pas de deux. Diabetes 2004; 53(Suppl 3): S113–S122. [DOI] [PubMed] [Google Scholar]
- 8. Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol 1999; 31: 991–994. [DOI] [PubMed] [Google Scholar]
- 9. Acton RT, Barton JC, Passmore LV, et al Relationships of serum ferritin, transferring saturation, and HFE mutations and self‐reported diabetes in the Hemochromatosis and Iron Overload Screening (HEIRS) study. Diabetes Care 2006; 29: 2084–2089. [DOI] [PubMed] [Google Scholar]
- 10. Guo X, Zhou D, An P, et al Associations between serum hepcidin, ferritin and Hb concentrations and type 2 diabetes risks in a Han Chinese population. Br J Nutr 2013; 110: 2180–2185. [DOI] [PubMed] [Google Scholar]
- 11. Jung CH, Lee MJ, Hwang JY, et al Elevated serum ferritin level is associated with the incident type 2 diabetes in healthy Korean men: a 4 year longitudinal study. PLoS ONE 2013; 8: e75250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arija V, Fernández‐Cao JC, Basora J, et al Excess body iron and the risk of type 2 diabetes mellitus: a nested case–control in the PREDIMED (PREvention with MEDiterranean Diet) study. Br J Nutr 2014; 112: 1896–1904. [DOI] [PubMed] [Google Scholar]
- 13. Kim S, Park SK, Ryoo JH, et al Incidental risk for diabetes according to serum ferritin concentration in Korean men. Clin Chim Acta 2015; 451: 165–169. [DOI] [PubMed] [Google Scholar]
- 14. Wittenbecher C, Mühlenbruch K, Kröger J, et al Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am J Clin Nutr 2015; 101: 1241–1250. [DOI] [PubMed] [Google Scholar]
- 15. Sun L, Franco OH, Hu FB, et al Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle‐aged and elderly Chinese. J Clin Endocrinol Metab 2008; 93: 4690–4696. [DOI] [PubMed] [Google Scholar]
- 16. de Luan C, Li H, Li SJ, et al Body iron stores and dietary iron intake in relation to diabetes in adults in North China. Diabetes Care 2008; 31: 285–286. [DOI] [PubMed] [Google Scholar]
- 17. Forouhi NG, Harding AH, Allison M, et al Elevated serum ferritin levels predicts new‐onset type 2 diabetes:results from the EPIC‐Norfolk prospective study. Diabetologia 2007; 50: 949–956. [DOI] [PubMed] [Google Scholar]
- 18. Montonen J, Boeing H, Steffen A, et al Body iron stores and risk of type 2 diabetes:results from the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Potsdam study. Diabetologia 2102; 55: 2613–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajpathak SN, Wylie‐Rosett J, Gunter MJ, et al Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes Metab. 2009; 11: 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi Z, Hu X, Yuan B, et al Association between serum feritin, hemoglobin, iron intake, and diabetes in adults in Jiangsu, China. Diabetes Care 2006; 29: 1878–1883. [DOI] [PubMed] [Google Scholar]
- 21. Fernández‐Cao JC, Arija V, Aranda N, et al Soluble transferrin receptor and risk of type 2 diabetes in the obese and nonobese. Eur J Clin Invest 2017; 47: 221–230. [DOI] [PubMed] [Google Scholar]
- 22. Bertelsen M, Anggard EE, Carrier MJ. Oxidative stress impairs insulin internalization in endothelial cells in vitro. Diabetologia 2001; 44: 605–613. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Z, Li S, Liu G, et al Body iron stores and heme‐iron intake in relation to risk of type 2 diabetes: a systematic review and meta‐analysis. PLoS ONE 2012; 7: e41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bao W, Rong Y, Rong S, et al Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta‐analysis. BMC Med 2012; 10: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kunutsor SK, Apekey TA, Walley J, et al Ferritin levels and risk of type 2 diabetes mellitus: an updated systematic review and meta‐analysis of prospective evidence. Diabetes Metab Res Rev 2013; 29: 308–318. [DOI] [PubMed] [Google Scholar]
- 26. Jia R, Wang Y, Ma AG, et al An epidemiological study of the relationship of serum ferritin level between age and body mass index among mid‐old women. Progress Modern Biomed 2016; 16: 3100–3103. [Google Scholar]
- 27. Harris EL, McLaren CE, Reboussin DM, et al Serum ferritin and transferrin saturation in Asians and Pacific Islanders. Arch Intern Med 2007; 167: 722–726. [DOI] [PubMed] [Google Scholar]
- 28. Sheu WH, Chen YT, Lee WJ, et al A relationship between serum ferritin and the insulin resistance syndrome is present in non‐diabetic women but not in non‐diabetic men. Clin Endocrinol (Oxf) 2003; 58: 380–385. [DOI] [PubMed] [Google Scholar]
- 29. Cooksey RC, Jouihan HA, Ajioka RS, et al Oxidative stress, beta‐cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004; 145: 5305–5312. [DOI] [PubMed] [Google Scholar]
- 30. Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem 1986; 261: 8708–8711. [PubMed] [Google Scholar]
- 31. Zhuang T, Han H, Yang Z. Iron, oxidative stress and gestational diabetes. Nutrients 2014; 6: 3968–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. García Y, Díaz‐Castro J. Advantages and disadvantages of the animal models v. in vitro studies in iron metabolism: a review. Animal 2013; 7: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 33. Justyna P, ZekanowskaEwa. The role of hepcidin, ferroportin, HCP1, and DMT1 protein in iron absorption in the human digestive tract. Gastroenterol Rev 2014; 4: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rishi G, Wallace D, Subramaniam V. Hepcidin: regulation of the master iron regulator. Biosci Rep 2015; 35: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ganz T. Systemic Iron Homeostasis. Physiol Rev 2013; 93: 1721. [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Li H, Jiang X, et al Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin‐induced diabetic rats. Diabetes 2014; 63: 1506–1518. [DOI] [PubMed] [Google Scholar]
- 37. Podmore C, Meidtner K, Schulze MB, et al The association of multiple biomarkers of iron metabolism and type 2 diabetes: the EPIC‐InterAct Study. Diabetes Care 2016; 39: 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee HJ, Choi JS, Lee HJ, et al Effect of excess iron on oxidative stress and gluconeogenesis through hepcidin during mitochondrial dysfunction. J Nutr Biochem 2015; 1414–1423. [DOI] [PubMed] [Google Scholar]
- 39. Kazumi T, Hozumi T, Ishida Y, et al Increased urinarytransferrin excretion predicts microalbuminuria in patients with type 2 diabetes. Diabetes Care 1999; 22: 1176–1180. [DOI] [PubMed] [Google Scholar]
- 40. Huth C, Beuerle S, Zierer A, et al Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. Eur J Endocrinol 2015; 173: 643–653. [DOI] [PubMed] [Google Scholar]


