Abstract
The association of intra‐individual variability in insulin requirements with C‐reactive protein levels among acute phase patients remains unclear. This retrospective cohort study aimed to evaluate this association. Patients with type 2 diabetes undergoing surgery for lumbar spinal canal stenosis were included in the study. We analyzed 286 records of 49 patients using the linear mixed effects model. The model showed C‐reactive protein levels to be significantly associated with insulin requirements, with an effect size of 0.60 U/day for an elevation of 1 mg/dL. The effect size was increased in patients with higher hemoglobin A1c levels. Our findings imply that C‐reactive protein levels could be a useful clinical biomarker when blood glucose levels are controlled in acute phase patients.
Keywords: C‐reactive protein, Insulin, Linear mixed effects model
Our study aimed to show the association between C‐reactive protein levels and insulin requirement in acute phase patients, using longitudinal data and the linear mixed effects model. The model showed that C‐reactive protein levels were significantly associated with the amount of insulin administered in postoperative patients with lumbar spinal canal stenosis. Our findings showed that C‐reactive protein levels could be a metric for controlling insulin administration, which would help clinicians control blood glucose levels.
![]()
Introduction
Evidence from numerous studies suggests that insulin resistance increases during the acute phase in patients with type 2 diabetes mellitus; controlling blood glucose levels is therefore particularly important1, 2, 3. However, determining optimal insulin requirements for adequately controlling blood glucose levels without inducing hypoglycemia remains a particular challenge, even for experts4.
C‐reactive protein (CRP) is a frequently measured inflammatory marker. The association between CRP or high‐sensitivity CRP levels and insulin resistance in the chronic phase has been well‐studied5, 6, 7, 8. However, this association has not been adequately investigated in acute phase patients. In particular, intra‐individual variability in insulin requirements and its association with CRP levels remains unclear. Although the postoperative correlation between CRP levels and insulin resistance was evaluated in a cohort study, intra‐individual variability was not considered9.
Therefore, we aimed to determine the association between CRP levels and insulin requirements in acute phase patients. Understanding the intra‐individual correlation between CRP levels and insulin requirements in the acute phase might aid accurately estimating insulin requirements.
Methods
Ethics statement
The present study was approved by the institutional review board of Yokohama Rosai Hospital (No. 31‐25); the requirement for informed consent was waived, as the data were anonymous and the interventions were non‐invasive.
Participants
This retrospective cohort study carried out at a tertiary hospital included patients with type 2 diabetes mellitus admitted between April 2016 and March 2019 for lumbar spinal canal stenosis surgery; their blood glucose levels were controlled by endocrinologists. Patients with lumbar spinal canal stenosis were selected owing to the low postoperative infection risk10, high post‐surgery CRP levels11 and restricted physical activity, which impairs glucose tolerance12. Patients undergoing hemodialysis, receiving steroids and with postoperative infections were excluded13, 14, 15.
Statistical analysis
Owing to intra‐individual variability in serum CRP levels, we used the linear mixed effects model to analyze hierarchical data; that is, a nested dataset in which multiple recordings belonged to each participant16, 17, 18.
We analyzed records on days when CRP levels were measured after surgery. The difference between the daily amount of insulin administration and that of preoperative phases was used as the outcome (ΔInsulin). CRP levels, glucose intake, and oral hypoglycemic agent use were used as explanatory variables. These were time‐changing and had a temporal association with ΔInsulin. They were therefore incorporated in the level 1 equation. We also included hemoglobin A1c (HbA1c) levels, estimated glomerular filtration rate and body mass index for their importance in type 2 diabetes mellitus 19. We assumed that the values of these variables remained unchanged during the observational period, and accordingly incorporated them into the level 2 equation. To increase interpretability, we used them as the differential value from the mean of the study participants.
We used two‐thirds and one‐third of patients’ data as the training and test datasets, respectively. Using the training dataset, we developed the linear mixed effects model by full information maximum likelihood. By calculating the Pearson’s correlation coefficient between the predicted and actual amounts of ΔInsulin using fivefold cross‐validation20, 21, 22, we determined to add each variable or random effect. During cross‐validation, the model was evaluated using the data of patients not included in model development, similar to leave‐subject‐out cross‐validation23, 24. We also validated the model on the independent test dataset and finally calculated the effect size using the entire dataset.
To confirm sample size sufficiency, we calculated the performance in cross‐validation by changing the sample size from small to entire datasets25, and also carried out power analysis for the significance of the CRP coefficient.
All analyses were carried out using open source software R (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria) with a library “lmerTest” (version 0.3.1, https://cran.r-project.org/web/packages/lmerTest/citation.html) and “simr” (version 1.0.5, https://cran.r-project.org/web/packages/simr/citation.html). P < 0.05 was considered significant.
Measurements
Serum CRP levels were measured using latex coagulation (Nittobo Medical Co., Ltd., Tokyo, Japan). The estimated glomerular filtration rate was calculated using serum creatinine levels measured by the enzyme method (Serotec Co., Ltd., Sapporo, Japan). Serum HbA1c levels were measured using high‐performance liquid chromatography (Tosoh Co., Ltd., Tokyo, Japan).
Results
Overall, 286 records were obtained from 49 patients; their descriptive data are shown in Table 1. We used 190 records from 32 patients as the training dataset and 96 records from 17 patients as the test dataset. The final model achieved an average Pearson’s correlation coefficient of 0.429 on cross‐validation and achieved a significantly positive Pearson’s correlation coefficient of 0.444 (95% confidence interval [CI] 0.267–0.592) in the test dataset.
Table 1.
Characteristics of study participants
| Patients (n = 49) | ||
|---|---|---|
| Continuous variables | Mean (SD) | Median (IQR) |
| Age (years) | 74.04 (7.37) | 74 (11) |
| HbA1c (%) | 7.19 (1.01) | 6.9 (1.0) |
| eGFR (mL/min/1.73 m2) | 71.42 (15.91) | 72.70 (21.21) |
| BMI (kg/m2) | 24.58 (4.41) | 23.95 (3.97) |
| Glucose intake (g/day) | 190.67 (48.33) | 198.00 (65.90) |
| Peak CRP (mg/dL) | 7.14 (5.04) | 6.65 (7.74) |
| Peak ΔInsulin (U/day) | 7.61 (10.76) | 4 (8) |
| Categorical variables | n (%) |
|---|---|
| Male sex | 26 (53) |
| OHA use | 39 (80) |
No patient used glucagon‐like peptide‐1 receptor agonists. Δ, Differential value from the mean; BMI, body mass index; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IQR, interquartile range; OHA, oral hypoglycemic agent; SD, standard deviation.
The coefficients of the model are shown in Table 2 (see Appendix S1 for the equation). The intercept, CRP, and interaction terms of CRP and HbA1c were statistically significant. The intercept showed that patients required an average of 3.11 U/day (95% CI 1.14–5.07 U/day) of additional insulin than the preoperative state. The model also showed that an increase in CRP levels to 1 mg/dL increased the amount of insulin to 0.60 U/day (95% CI 0.33–0.86 U/day), and 1% differences in HbA1c levels increased the effect size to 0.91 (95% CI 0.52–1.30). Other variables were not significant, but improved model performance. The model and data are shown in Figure 1.
Table 2.
Coefficients of the linear mixed‐effect model
| Total n = 286 | Mean (95% CI) effect | P‐value |
|---|---|---|
| Independent effects | ||
| Intercept | 3.11 (1.14 to 5.07) | 0.0026 |
| ΔHbA1c (%) | 1.91 (−2.48 to 6.30) | 0.39 |
| CRP (mg/dL) | 0.60 (0.33 to 0.86) | <0.001 |
| OHA | −1.32 (−3.11 to 0.47) | 0.15 |
| Interactive effects | ||
| CRP × ΔHbA1c | 0.91 (0.52 to 1.30) | <0.001 |
| Glu (g/day) × ΔHbA1c | 0.0042 (−0.015 to 0.024) | 0.67 |
| OHA × ΔeGFR (mL/min/1.73 m2) | −0.089 (−0.18 to 0.0004) | 0.053 |
Bold values indicates P < 0.05.
Δ, Differential value from the mean; CI, confidence interval; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; Glu, glucose intake; HbA1c, hemoglobin A1c; OHA, oral hypoglycemic agent use.
Figure 1.
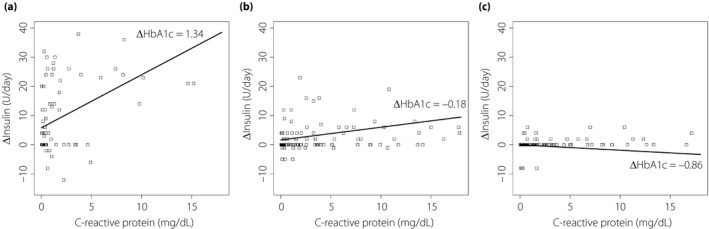
Representation of the model and data. ΔInsulin represents the differential value of the doses of insulin administered compared to preoperative requirements. ΔHbA1c represents the remainder after subtracting the mean HbA1c of all patients (7.19%) from the HbA1c of each patient. The coefficients of the model are shown in Table 2. The data have been categorized into three groups based on hemoglobin A1c (HbA1c) levels, namely, high, moderate and low, with approximately one‐third of patients in each. The graphs have been drawn using the mean value in each group. (a) The mean ΔHbA1c of the high HbA1c group is 1.34; the graph has a steep slope. (b) The mean ΔHbA1c of the moderate HbA1c group is −0.18; the graph has a shallow slope. (c) The mean ΔHbA1c of the low HbA1c group is −0.86; the slope of the graph is almost flat.
The relationship between the accuracy and sample size is shown in Figure 2. The accuracy was almost saturated on achieving sample sizes of 30. The power for significance of the CRP coefficient was 0.989. Both results implied that the sample size was almost sufficient for analysis.
Figure 2.
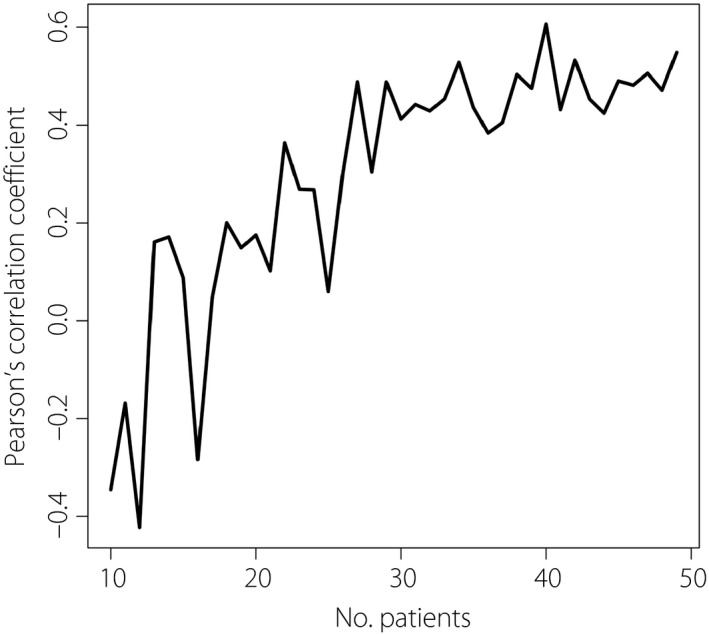
The relationship between the performance of the model and sample size. The model performance was evaluated based on the Pearson’s correlation coefficient between the predicted and actual amounts of ΔInsulin on fivefold cross‐validation, by increasing the study sample from small (n = 10) to the current entire cohort size (n = 49). The graph shows that after attaining a sample size of 30, the accuracy was almost saturated.
Discussion
Using longitudinal data of diabetes patients who had undergone lumbar spinal canal stenosis surgery, we found that CRP levels were significantly associated with insulin requirements during the postoperative acute phase.
Intra‐individual correlation observed in the postoperative state might improve the current understanding regarding the association between CRP levels and insulin resistance5, 6, 7, 8, 9. There are two potential explanations for our findings. First, CRP is a proxy of other inflammatory cytokines, such as interleukin‐6 or tumor necrosis factor‐α, which have been reported to induce insulin resistance26, 27. Second, CRP directly impairs glucose tolerance, as shown in an in vivo study28. Further studies are required to identify such potential underlying mechanisms.
As shown by the significant interaction between CRP and HbA1c, the degree of association between CRP levels and insulin resistance was higher in patients with higher HbA1c levels. Although previous studies have identified preoperative HbA1c levels to be associated with postoperative glycemic control29, they only examined the independent effect. The present findings probably indicate the synergistic effects of postoperative inflammation and preoperative glycemic control on insulin resistance.
As evident from the significantly positive intercept of the model, patients in this cohort required more insulin postoperatively. This might reflect the activity of pathways associated with hormones other than inflammatory cytokines, such as glucagon or catecholamine30.
The present study had several limitations. First, as we used insulin requirements as the outcome, the maintenance of blood glucose levels within the optimal range was not validated; however, the lack of insulin was partially compensated for by the sliding scale. Second, there was no specified protocol for controlling blood glucose levels; therefore, the insulin doses might have varied among physicians. Third, we could not incorporate C‐peptide or immunoreactive insulin owing to missing data. Fourth, the types and amounts of oral hypoglycemic agents were not considered to streamline the analysis. Fifth, although CRP elevation is delayed by 24–72 h after inflammation11, the time lag was not considered. Finally, elevation of CRP levels strongly depends on bacterial species15; therefore, the effect size would not be generalizable to other diseases.
In conclusion, we found an association between postoperative CRP levels and the doses of insulin administered. The findings led us to speculate that CRP levels could be a metric for determining insulin requirements. Further prospective multicenter studies are required to validate the present findings.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1 | The equation of the linear mixed effects model.
J Diabetes Investig 2020; 11: 980–984
References
- 1. Qaseem A, Humphrey LL, Chou R, et al Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 154: 260–267. [DOI] [PubMed] [Google Scholar]
- 2. Mifsud S, Schembri EL, Gruppetta M. Stress‐induced hyperglycaemia. Br J Hosp Med (Lond) 2018; 79: 634–639. [DOI] [PubMed] [Google Scholar]
- 3. Raju TA, Torjman MC, Goldberg ME. Perioperative blood glucose monitoring in the general surgical population. J Diabetes Sci Technol 2009; 3: 1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umpierrez GE, Smiley D, Jacobs S, et al Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Bao W, Liu J, et al Inflammatory markers and risk of type 2 diabetes: a systematic review and meta‐analysis. Diabetes Care 2013; 36: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dehghan A, Kardys I, de Maat MP, et al Genetic variation, C‐reactive protein levels, and incidence of diabetes. Diabetes 2007; 56: 872–878. [DOI] [PubMed] [Google Scholar]
- 7. Lee CC, Adler AI, Sandhu MS, et al Association of C‐reactive protein with type 2 diabetes: prospective analysis and meta‐analysis. Diabetologia 2009; 52: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 8. Uemura H, Katsuura‐Kamano S, Yamaguchi M, et al Relationships of serum high‐sensitivity C‐reactive protein and body size with insulin resistance in a Japanese cohort. PLoS ONE 2017; 12: e0178672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Micić D, Lalić N, Djukić V, et al Influence of IL‐6, TNF‐α and Hs‐CRP on insulin sensitivity in patients after laparoscopic cholecystectomy or open hernia repair. J Med Biochem 2018; 37: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arinzon Z, Adunsky A, Fidelman Z, et al Outcomes of decompression surgery for lumbar spinal stenosis in elderly diabetic patients. Eur Spine J 2004; 13: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kraft CN, Krüger T, Westhoff J, et al CRP and leukocyte‐count after lumbar spine surgery: fusion vs. nucleotomy. Acta Orthop 2011; 82: 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim KT, Cho DC, Sung JK, et al Changes in HbA1c levels and body mass index after successful decompression surgery in patients with type 2 diabetes mellitus and lumbar spinal stenosis: results of a 2‐year follow‐up study. Spine J 2017; 17: 203–210. [DOI] [PubMed] [Google Scholar]
- 13. Abe M, Kaizu K, Matsumoto K. Evaluation of the hemodialysis‐induced changes in plasma glucose and insulin concentrations in diabetic patients: comparison between the hemodialysis and non‐hemodialysis days. Ther Apher Dial 2007; 11: 288–295. [DOI] [PubMed] [Google Scholar]
- 14. Liu D, Ahmet A, Ward L, et al A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurisu K, Yoshiuchi K, Ogino K, et al Peak C‐reactive protein levels do not predict 30‐day mortality for bacteremia: a retrospective cohort study. J Infect Chemother 2020; 26: 23–27. [DOI] [PubMed] [Google Scholar]
- 16. Yoshiuchi K, Cook DB, Ohashi K, et al A real‐time assessment of the effect of exercise in chronic fatigue syndrome. Physiol Behav 2007; 92: 963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panisch S, Johansson T, Flamm M, et al The impact of a disease management programme for type 2 diabetes on health‐related quality of life: multilevel analysis of a cluster‐randomised controlled trial. Diabetol Metab Syndr 2018; 10: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sivaprasad S, Prevost AT, Vasconcelos JC, et al Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single‐blinded, randomised, controlled, phase 2b, non‐inferiority trial. Lancet 2017; 389: 2193–2203. [DOI] [PubMed] [Google Scholar]
- 19. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management (NICE Guideline 28). Available from: https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-pdf-1837338615493. Accessed September 25, 2019.
- 20. Guan Y, Zhang H, Quang D, et al Machine learning to predict anti‐tumor necrosis factor drug responses of rheumatoid arthritis patients by integrating clinical and genetic markers. Arthritis Rheumatol 2019; 71: 1987–1996. [DOI] [PubMed] [Google Scholar]
- 21. Lind AP, Anderson PC. Predicting drug activity against cancer cells by random forest models based on minimal genomic information and chemical properties. PLoS ONE 2019; 14: e0219774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen K, Lu Y, Zhao H, et al. Predicting the change of exon splicing caused by genetic variant using support vector regression. Hum Mutat 2019; 40: 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaume A, Dreyfus G, Vialatte FB. A cognitive brain‐computer interface monitoring sustained attentional variations during a continuous task. Cogn Neurodyn 2019; 13: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan F, Liu M, Ding C. Driving style recognition based on electroencephalography data from a simulated driving experiment. Front Psychol 2019; 10: 1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gulshan V, Peng L, Coram M, et al Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016; 316: 2402–2410. [DOI] [PubMed] [Google Scholar]
- 26. Senn JJ, Klover PJ, Nowak IA, et al Interleukin‐6 induces cellular insulin resistance in hepatocytes. Diabetes 2002; 51: 3391–3399. [DOI] [PubMed] [Google Scholar]
- 27. Plomgaard P, Bouzakri K, Krogh‐Madsen R, et al Tumor necrosis factor‐alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005; 54: 2939–2945. [DOI] [PubMed] [Google Scholar]
- 28. Xi L, Xiao C, Bandsma RH, et al C‐reactive protein impairs hepatic insulin sensitivity and insulin signaling in rats: role of mitogen‐activated protein kinases. Hepatology 2011; 53: 127–135. [DOI] [PubMed] [Google Scholar]
- 29. Sato H, Carvalho G, Sato T, et al The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 2010; 95: 4338–4344. [DOI] [PubMed] [Google Scholar]
- 30. Dagogo‐Jack S, Alberti KG. Management of diabetes mellitus in surgical patients. Diabetes Spectrum 2002; 15: 44–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | The equation of the linear mixed effects model.


