Abstract
Aims/Introduction
There is little evidence on the role of postprandial glycemia in the incidence of diabetic retinopathy (DR) in a real‐world setting. We aimed to assess the effect of postprandial hyperglycemia at clinic visits on the incidence of DR in patients with type 2 diabetes, and whether its effect differs depending on glycated hemoglobin (HbA1c) values and age.
Materials and Methods
Intrapersonal mean blood glucose levels at 1–2 h post‐breakfast (1–2h‐PBBG), post‐lunch (1–2 h‐PLBG) and both (1–2h‐PBLBG) during 2 years from the first visit were used as baseline data. This retrospective cohort study enrolled 487, 323 and 406 patients who had 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG measurements, respectively. These three groups were followed from 1999 up through 2017.
Results
DR occurred in 145, 92 and 126 patients in the 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG groups, respectively. Multivariate Cox regression analysis showed that the mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels were significant predictors of DR, independent of mean HbA1c. In patients with mean HbA1c <7.0% and those with a baseline age <60 years, the mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels were significant predictors.
Conclusions
Postprandial hyperglycemia at clinic visits might predict the incidence of DR, independent of HbA1c. The effect of postprandial hyperglycemia on DR is obvious in patients with well‐controlled HbA1c and in younger patients. Even with the lower HbA1c level, correcting postprandial hyperglycemia is important for preventing DR, especially in middle‐aged adults with type 2 diabetes.
Keywords: Diabetic retinopathy, Postprandial hyperglycemia, Type 2 diabetes
The present study shows that postprandial hyperglycemia at clinic visits is associated with the incidence of diabetic retinopathy, independent of glycated hemoglobin levels, in a real‐world setting in patients with type 2 diabetes. The effect of postprandial hyperglycemia on retinopathy is obvious in patients with well‐controlled glycated hemoglobin levels and in patients aged <60 years. Therefore, even with the lower glycated hemoglobin, correcting postprandial hyperglycemia is important for preventing retinopathy, especially in middle‐aged adults with type 2 diabetes.

Introduction
The risk factors for diabetic retinopathy (DR) have been identified, including hyperglycemia, duration of diabetes and hypertension, in many epidemiological studies and clinical trials1, 2, 3, 4, 5, 6. Chronic sustained hyperglycemia, as reflected by glycated hemoglobin (HbA1c), is considered to be the main determinant of DR. However, few studies have examined the relationship between transient hyperglycemia (i.e., postprandial hyperglycemia) and DR.
Shiraiwa et al. reported that postprandial hyperglycemia was a better predictor of progression of DR than HbA1c levels in Japanese patients with type 2 diabetes7, 8. In their studies, postprandial glycemia was measured 2 h after having an isocaloric mixed breakfast, representing a standard Japanese breakfast (10 kcal/kg bodyweight; 57% carbohydrate, 15% fat and 28% protein) on admission7, 8. However, to the best of our knowledge, no research has examined the relationship between postprandial glycemia at clinic visits and DR in real‐life conditions.
The contribution of postprandial glucose levels and basal glucose levels to overall glucose exposure, reflected by HbA1c levels, varies. This variation depends on glycemic control based on HbA1c categories9, which also differs between older and younger adults with type 2 diabetes10
Therefore, we aimed to assess the effect of postprandial hyperglycemia at clinic visits on the incidence of DR in a real‐world setting in patients with type 2 diabetes. We also examined whether the effect of postprandial hyperglycemia differs depending on HbA1c categories and age groups.
Methods
Assessment of postprandial glycemia
Capillary blood glucose levels were measured once at each visit using the glucose–oxidase method (Fuji DRI‐CHEM; Fuji Film, Tokyo, Japan), regardless of fasting or postprandial status, and are expressed as plasma equivalents. At each visit, a clinical laboratory technician checked the time when the patients began to eat their final meal, and recorded the postprandial time interval in 15‐min units. Blood glucose levels measured at 1–2 h after breakfast and after lunch, those after breakfast, and those after lunch were defined as 1–2 h post‐breakfast and post‐lunch blood glucose (1–2h‐PBLBG) levels, 1–2 h post‐breakfast blood glucose (1–2h‐PBBG) levels, and 1–2 h post‐lunch blood glucose (1–2h‐PLBG) levels, respectively. Intrapersonal mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels during 2 years from the first visit were used as baseline data.
Study participants
A flowchart of the three groups of patients who were included in the analyses is shown in Figure 1. A total of 1,149 patients with diabetes visited our clinic initially from 1997– 1999 and continued visiting our clinic for ≥1 year. Of these 1,149 patients, 549 were diagnosed with type 2 diabetes and were followed up for ≥2 years. Furthermore, these patients had one or more clinic visit per year, and had no DR at the first visit and during the first 2 years of their visits. Of these 549 patients, 487 had received 1–2h‐PBLBG measurements once or more during the first 2 years of their visits. Of these 487 patients, 323 had received 1–2h‐PBBG measurements and 406 had received 1–2h‐PLBG measurements once or more during the first 2 years of their visits. Analysis was carried out on three groups of these 487 patients (1–2h‐PBLBG group), 323 patients (1–2h‐PBBG group) and 406 patients (1–2h‐PLBG group).
Figure 1.
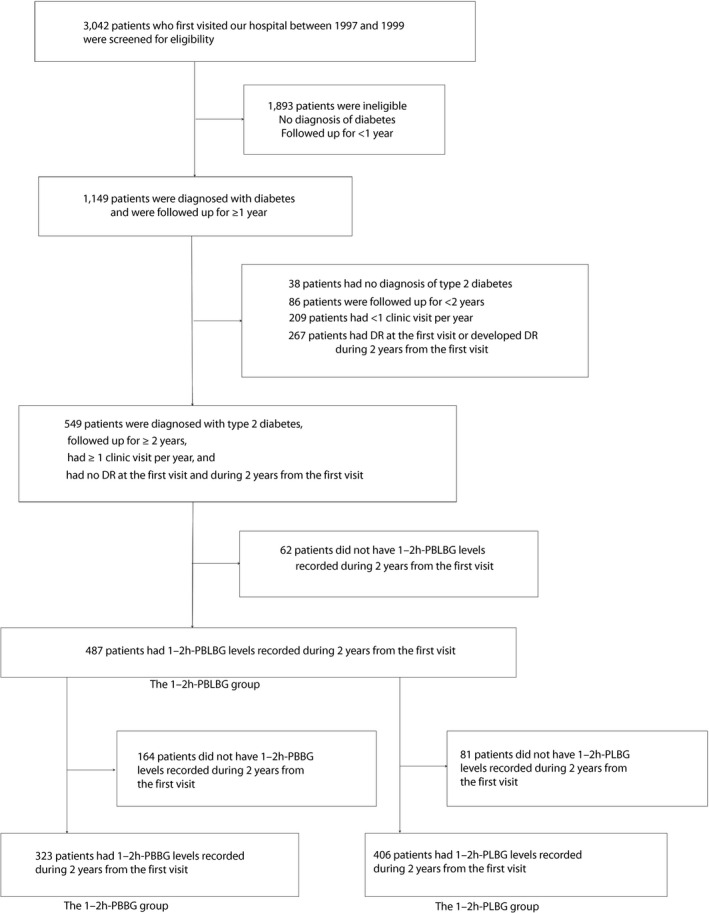
Flowchart of the three groups of patients included in the analyses. 1–2h‐PBBG, 1–2 h post‐breakfast blood glucose; 1–2h‐PBLBG, 1–2 h post‐breakfast and post‐lunch blood glucose; 1–2h‐PLBG, 1–2 h post‐lunch blood glucose; DR, diabetic retinopathy.
This study conformed to the Japanese Government’s Ethical Guidelines for Medical and Health Research Involving Human Subjects, and was approved by the ethics committee of the Institute for Adult Diseases, Asahi Life Foundation. The committee approved the use of the opt‐out approach for consent in the clinic.
End‐point definition
As described previously11, the end‐point was defined as the incidence of mild‐to‐moderate non‐proliferative DR12, which was diagnosed with a routine regular funduscopic examination by ophthalmologists who subspecialized in diabetes. In detail, the end‐point was the first time point when a microaneurysm, dot/blot hemorrhage or hard exudate was observed twice in succession at one or more sites in at least one eye. Patients who did not develop DR and did not complete the follow up were considered as censored cases.
Measurement of covariates
Clinical examinations and laboratory methods used in the present study have been described previously13, 14. To summarize, blood samples were collected regardless of fasting or postprandial status. HbA1c values were obtained once per visit. A diabetes analyzer (Tosoh Bioscience, Tokyo, Japan) was used to determine HbA1c values. The measurement method was a high‐performance liquid chromatography technique standardized by the Japan Diabetes Society. Using linear regression equations, recorded HbA1c values were converted to the Japan Diabetes Society standard HbA1c values. Subsequently, all previous HbA1c (%) values were converted to National Glycohemoglobin Standardization Program values (%)15. Lipid levels were measured once per few visits. The total cholesterol‐to‐high‐density lipoprotein cholesterol ratio was calculated on the basis of the finding that the total cholesterol‐to‐high‐density lipoprotein cholesterol ratio strongly predicts cardiovascular disease in patients with type 2 diabetes16, 17, 18. Blood pressure (BP) and body weight were usually assessed once each visit. An electronic sphygmomanometer (OMRON, Kyoto, Japan) was used to measure BP in a seated posture by a trained clinical laboratory technician. The intrapersonal mean values of these clinical examination data during the first 2 years of the patients’ visits were used as baseline data. Patients who showed fasting or casual blood glucose levels <70 mg/dL (3.9 mmol/L) once or more during the first 2 years of their visits were diagnosed with hypoglycemia. Patients who consumed ≥20 g of alcohol a day were defined as drinkers.
Statistical analysis
Data for continuous variables are shown as the mean ± standard deviation, and data for categorical variables as the number and percentages. Data on the follow‐up periods and the number of 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG measurements during the first 2 years of their visits were not normally distributed. Therefore, these data are expressed as the median and interquartile range (IQR). The baseline was set at 2 years after the first visit.
The baseline characteristics of the patients who developed DR were compared with those who did not develop DR using the Student’s t‐test, Wilcoxon rank sum test and the χ2‐test. Multivariate Cox proportional hazard models were used to calculate the hazard ratios (HRs) for intrapersonal mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels during the first 2 years for the incidence of DR. The number of 1–2h‐PBLBG measurements, intrapersonal mean HbA1c level, intrapersonal mean systolic BP (SBP), the presence or absence of hypoglycemia during the first 2 years, age, sex and duration of diabetes were used as covariates in model 1. Model 2 included intrapersonal mean body mass index and mean total cholesterol‐to‐high‐density lipoprotein cholesterol ratio during the first 2 years, and ever smoking in addition to model 1. Model 3 included use of oral antidiabetic agents, insulin, antihypertensive agents and lipid‐lowering agents at 2 years after the first visits in addition to model 2. Models 4–6 included the number of 1–2h‐PBBG measurements instead of the number of 1–2h‐PBLBG measurements in models1–3. Similarly, models 7–9 included the number of 1–2h‐PLBG measurements instead of the number of 1–2h‐PBLBG measurements in models 1–3. In all models, the number of measurements of 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG was ln‐transformed. Furthermore, stratified analyses were carried out by the mean HbA1c level of 7.0% and age at baseline of 60 years.
All analyses were carried out using SAS software (version 9.4; SAS Institute, Cary, NC, USA). A two‐sided P‐value <0.05 was considered significant.
Results
Baseline characteristics of the 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG groups according to the incidence of DR
Table 1 shows baseline characteristics of the total patients and those who did or did not develop DR in each of the 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG groups. Oral antidiabetic agents mainly used at baseline included sulfonylureas, alpha‐glucosidase inhibitors, low‐dose biguanides and glinides.
Table 1.
Baseline characteristics of the patients classified according to the incidence of diabetic retinopathy
| 1–2h‐PBLBG group | 1–2h‐PBBG group | 1–2h‐PLBG group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | DR onset (–) | DR onset (+) | Total | DR onset (–) | DR onset (+) | Total | DR onset (–) | DR onset (+) | |
| n | 487 | 342 | 145 | 323 | 231 | 92 | 406 | 280 | 126 |
| Men (%) | 382 (78.4) | 275 (80.4) | 107 (73.8) | 257 (79.6) | 188 (81.4) | 69 (75.0) | 316 (77.8) | 225 (80.4) | 91 (72.2) |
| Age (years) | 59.2 ± 8.6 | 59.4 ± 8.9 | 58.8 ± 7.9 | 58.8 ± 8.7 | 59.1 ± 8.9 | 58.1 ± 8.1 | 59.2 ± 8.6 | 59.4 ± 9.0 | 58.7 ± 7.8 |
| Duration of diabetes (years) | 7.1 ± 5.6 | 6.5 ± 5.5 | 8.3 ± 5.8* | 6.8 ± 5.3 | 6.5 ± 5.4 | 7.5 ± 4.9* | 7.2 ± 5.8 | 6.8 ± 5.7 | 8.3 ± 5.8* |
| Mean HbA1c (%) | 6.8 ± 0.8 | 6.6 ± 0.7 | 7.1 ± 0.7* | 6.7 ± 0.7 | 6.6 ± 0.7 | 7.1 ± 0.7* | 6.8 ± 0.8 | 6.6 ± 0.7 | 7.1 ± 0.8* |
| (mmol/mol) | 50 ± 8 | 49 ± 8 | 54 ± 8* | 50 ± 8 | 48 ± 8 | 54 ± 8* | 51 ± 8 | 49 ± 8 | 54 ± 8* |
| Mean 1–2h‐PBLBG (mmol/L) | 10.3 ± 2.6 | 9.9 ± 2.5 | 11.1 ± 2.7* | – | – | – | – | – | – |
| Mean 1–2h‐PBBG (mmol/L) | – | – | – | 9.7 ± 2.9 | 9.2 ± 2.7 | 11.0 ± 3.2* | – | – | – |
| Mean 1–2h‐PLBG (mmol/L) | – | – | – | – | – | – | 10.7 ± 3.1 | 10.4 ± 2.9 | 11.5 ± 3.4* |
| Mean SBP (mmHg) | 125.5 ± 14.8 | 124.1 ± 14.9 | 128.6 ± 14.1* | 125.4 ± 14.7 | 124.1 ± 14.7 | 128.9 ± 14.1* | 125.7 ± 14.6 | 124.4 ± 14.8 | 128.5 ± 13.8* |
| Mean DBP (mmHg) | 72.3 ± 9.1 | 71.6 ± 9.2 | 74.1 ± 8.7* | 72.4 ± 8.9 | 71.4 ± 9.1 | 74.7 ± 8.0* | 72.4 ± 9.1 | 71.7 ± 9.1 | 74.0 ± 8.9* |
| Mean BMI (kg/m2) | 22.7 ± 2.8 | 22.5 ± 2.8 | 23.1 ± 2.7* | 22.7 ± 2.6 | 22.5 ± 2.6 | 23.2 ± 2.6* | 22.7 ± 2.8 | 22.5 ± 2.9 | 23.0 ± 2.7 |
| Mean TC (mmol/L) | 5.5 ± 0.8 | 5.5 ± 0.8 | 5.5 ± 0.8 | 5.5 ± 0.8 | 5.5 ± 0.8 | 5.6 ± 0.8 | 5.5 ± 0.8 | 5.4 ± 0.8 | 5.5 ± 0.8 |
| Mean HDLC (mmol/L) | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.4 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 |
| Mean eGFR (mL/min/1.73 m2) | 78.9 ± 13.8 | 78.4 ± 12.9 | 79.9 ± 15.7 | 78.4 ± 14.1 | 77.7 ± 12.9 | 80.1 ± 16.8 | 79.1 ± 13.7 | 78.8 ± 12.9 | 80.0 ± 15.4 |
| Hypoglycemia† | 27 (5.5) | 18 (5.3) | 9 (6.2) | 13 (4.0) | 11 (4.8) | 2 (2.2) | 25 (6.2) | 17 (6.1) | 8 (6.4) |
| Ever smoker | 319 (65.5) | 227 (66.4) | 92 (63.5) | 212 (65.6) | 153 (66.2) | 59 (64.1) | 269 (66.3) | 189 (67.5) | 80 (63.5) |
| Alcohol intake | 277 (56.9) | 200 (58.5) | 77 (53.1) | 183 (50.2) | 135 (58.4) | 48 (52.2) | 225 (55.4) | 160 (57.1) | 65 (51.6) |
| Oral antidiabetic agents alone | 250 (51.3) | 158 (46.2) | 92 (63.5)* | 162 (56.7) | 101 (43.7) | 61 (66.3)* | 215 (53.0) | 134 (47.9) | 81 (64.3)* |
| Insulin‡ | 57 (11.7) | 34 (9.9) | 23 (15.9) | 39 (12.1) | 25 (10.8) | 14 (15.2) | 51 (12.6) | 30 (10.7) | 21 (16.7) |
| Antihypertensive agents | 120 (24.6) | 74 (21.6) | 46 (31.7)* | 79 (24.5) | 50 (21.7) | 29 (31.5) | 99 (24.4) | 58 (20.7) | 41 (32.5)* |
| Lipid‐lowering agents | 110 (22.6) | 61 (17.8) | 49 (33.8)* | 69 (21.4) | 38 (16.5) | 31 (33.7)* | 93 (22.9) | 51 (18.2) | 42 (33.3)* |
Values are n (%) or mean ± standard deviation. Intrapersonal mean values during the 2‐year period from the first visit were used as baseline data.
P < 0.05 versus diabetic retinopathy (DR) onset (–).
Presence of fasting or casual blood glucose levels <70 mg/dL (3.9 mmol/L) at least once during the 2‐year period from the first visit.
Including patients treated with both oral antidiabetic agents and insulin. 1–2h‐PBBG, 1–2 h post‐breakfast blood glucose; 1–2h‐PBLBG, 1–2 h post‐breakfast and post‐lunch blood glucose; 1–2h‐PLBG, 1–2 h post‐lunch blood glucose; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDLC, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
A total of 145 out of 487 patients in the 1–2h‐PBLBG group developed DR. The median of the follow‐up period and the number of 1–2h‐PBLBG measurements during the first 2 years were 6.9 years (IQR 3.3–13.9) and five times (IQR 2–8), respectively. Patients who developed DR had a longer duration of diabetes (P = 0.0001), a higher mean body mass index (P = 0.014), a higher mean HbA1c level (P < 0.0001), a higher mean 1–2h‐PBLBG level (P < 0.0001), higher mean SBP and DBP (P = 0.002 and P = 0.006, respectively), and higher percentages of users of oral antidiabetic agents (P = 0.0005), antihypertensive agents (P = 0.018) and lipid‐lowering agents (P = 0.0001) compared with those who did not develop DR.
A total of 92 out of 323 patients in the 1–2h‐PBBG group developed DR. The median of the follow‐up period and the number of 1–2h‐PBBG measurements during the first 2 years were 7.5 years (IQR 3.3–14.2 years) and two times (IQR 1–6), respectively. Patients who developed DR had a longer duration of diabetes (P = 0.014), a higher mean body mass index (P = 0.040), a higher mean HbA1c level (P < 0.0001), a higher mean 1–2h‐PBBG level (P < 0.0001), higher mean SBP and diastolic BP (DBP; P = 0.008 and P = 0.002, respectively), and higher percentages of users of oral antidiabetic agents (P = 0.0002) and lipid‐lowering agents (P = 0.0006) compared with those who did not develop DR.
A total of 126 out of 406 patients in the 1–2h‐PLBG group developed DR. The median of the follow‐up period and the number of 1–2h‐PLBG measurements during the first 2 years were 6.7 years (IQR 3.3–13.7 years) and three times (IQR 1–5), respectively. Patients who developed DR had a longer duration of diabetes (P = 0.002), a higher mean HbA1c level (P < 0.0001), a higher mean 1–2h‐PLBG level (P = 0.0005), higher mean SBP and DBP (P = 0.010 and P = 0.018, respectively), and higher percentages of users of oral antidiabetic agents (P = 0.002), antihypertensive agents (P = 0.010) and lipid‐lowering agents (P = 0.0008) compared with those who did not develop DR.
Postprandial glycemia and the risk of the incidence of DR
Table 2 shows the HRs for mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels for the incidence of DR that were estimated by multivariate Cox proportional hazard models. In models 1–9, mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels significantly predicted the incidence of DR. In all models, the mean HbA1c level was a strong predictor of the incidence of DR, whereas postprandial glycemia (i.e., mean levels of 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG) was also significant, independent of the mean HbA1c level.
Table 2.
Multivariate Cox proportional hazard models for the incidence of diabetic retinopathy
| HR (95%CI) | P‐value | |
|---|---|---|
| 1–2h‐PBLBG group events/patients | 145/487 | |
| Model 1 | ||
| Mean 1–2h‐PBLBG (1 mmol/L) | 1.10 (1.03–1.18) | 0.007 |
| Mean HbA1c (%) | 1.94 (1.51–2.49) | <0.0001 |
| Model 2 | ||
| Mean 1–2h‐PBLBG (1 mmol/L) | 1.10 (1.03–1.19) | 0.008 |
| Mean HbA1c (%) | 1.85 (1.43–2.39) | <0.0001 |
| Model 3 | ||
| Mean 1–2h‐PBLBG (1 mmol/L) | 1.08 (1.01–1.17) | 0.033 |
| Mean HbA1c (%) | 1.70 (1.28–2.26) | 0.0003 |
| 1–2h‐PBBG group events/patients | 92/323 | |
| Model 4 | ||
| Mean 1–2h‐PBBG (1 mmol/L) | 1.09 (1.01–1.18) | 0.029 |
| Mean HbA1c (%) | 2.22 (1.57–3.13) | <0.0001 |
| Model 5 | ||
| Mean 1–2h‐PBBG (1 mmol/L) | 1.09 (1.01–1.18) | 0.031 |
| Mean HbA1c (%) | 2.19 (1.54–3.10) | <0.0001 |
| Model 6 | ||
| Mean 1–2h‐PBBG (1 mmol/L) | 1.08 (1.00–1.17) | 0.047 |
| Mean HbA1c (%) | 1.73 (1.17–2.56) | 0.006 |
| 1–2h‐PLBG group events/patients | 126/406 | |
| Model 7 | ||
| Mean 1–2h‐PLBG (1 mmol/L) | 1.08 (1.02–1.14) | 0.007 |
| Mean HbA1c (%) | 1.94 (1.51–2.49) | <0.0001 |
| Model 8 | ||
| Mean 1–2h‐PLBG (1 mmol/L) | 1.08 (1.02–1.14) | 0.007 |
| Mean HbA1c (%) | 1.88 (1.46–2.44) | <0.0001 |
| Model 9 | ||
| Mean 1–2h‐PLBG (1 mmol/L) | 1.07 (1.01–1.13) | 0.026 |
| Mean HbA1c (%) | 1.71 (1.29–2.29) | 0.0002 |
Intrapersonal mean values during the 2‐year period from the first visit were used as baseline data. Model 1 included the number of 1–2 h post‐breakfast and post‐lunch blood glucose (1–2h‐PBLBG) measurements (ln‐transformed), age, sex, duration of diabetes, mean systolic blood pressure and hypoglycemia as covariates. Hypoglycemia was defined as the presence of fasting or casual blood glucose levels <70 mg/dL (3.9 mmol/L) at least once during the 2‐year period from the first visit. Model 2 included mean body mass index, mean total cholesterol‐to‐high‐density lipoprotein cholesterol ratio and ever smoking in addition to model 1. Model 3 included use of oral antidiabetic agents, insulin, antihypertensive agents and lipid‐lowering agents in addition to model 2. Models 4–6 included the number of 1–2 h post‐breakfast blood glucose (1–2h‐PBBG) measurements (ln‐transformed) instead of the number of 1–2h‐PBLBG measurements (ln‐transformed) in models 1–3. Models 7–9 included the number of 1–2 h post‐lunch blood glucose (1–2h‐PLBG) measurements (ln‐transformed) instead of the number of 1–2h‐PBLBG measurements (ln‐transformed) in models 1–3. CI, confidence interval; HbA1c, glycated hemoglobin; HR, hazard ratio.
Additionally, severe hypoglycemia was defined as fasting or casual blood glucose levels <54 mg/dL (3.0 mmol/L) once or more during 2 years from the first visits. Severe hypoglycemia occurred in five (1.0%) patients in the 1–2h‐PBLBG group, in two (0.6%) patients in the 1–2h‐PBBG group and in five (1.2%) patients in the 1–2h‐PLBG group. When severe hypoglycemia was used as a covariate instead of hypoglycemia, similar results were obtained (data not shown).
Contribution of postprandial hyperglycemia to the incidence of DR stratified by the mean HbA1c level
Table 3 shows the HRs for mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels for the incidence of DR stratified by the mean HbA1c level of 7.0%. The covariates in models 1, 4 and 7 in Table 3 are the same as those in Table 2. In models 1, 4 and 7, for the stratum of patients with a mean HbA1c value <7.0%, mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels were significant predictors of the incidence of DR. For the stratum of patients with a mean HbA1c value ≥7.0%, neither the mean 1–2h‐PBLBG, 1–2h‐PBBG nor 1–2h‐PLBG level was significant, whereas the mean HbA1c level remained significant. There was no significant interaction in all models. When severe hypoglycemia was used as a covariate instead of hypoglycemia, similar results were obtained (data not shown).
Table 3.
Hazard ratios for the incidence of diabetic retinopathy stratified by the mean glycated hemoglobin level and age at baseline
| Mean HbA1c <7.0% | Mean HbA1c ≥7.0% | Interaction P | Age at baseline <60 years | Age at baseline ≥60 years | Interaction P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| Model 1 (events/patients) | 75/327 | 70/160 | 83/270 | 62/217 | ||||||
| Mean 1–2h‐PBLBG (1 mmol/L) | 1.12 (1.02–1.23) | 0.023 | 1.11 (0.99–1.24) | 0.066 | 0.56 | 1.12 (1.02–1.22) | 0.014 | 1.09 (0.94–1.27) | 0.27 | 0.74 |
| Mean HbA1c (%) | 3.46 (1.76–6.81) | 0.0003 | 1.99 (1.26–3.14) | 0.003 | 1.65 (1.14–2.39) | 0.009 | 2.31 (1.56–3.44) | <0.0001 | ||
| Model 4 (events/patients) | 47/220 | 45/103 | 55/185 | 37/138 | ||||||
| Mean 1–2h‐PBBG (1 mmol/L) | 1.12 (1.00–1.24) | 0.042 | 1.05 (0.94–1.18) | 0.39 | 0.73 | 1.12 (1.02–1.23) | 0.023 | 1.07 (0.93–1.22) | 0.35 | 0.92 |
| Mean HbA1c (%) | 3.12 (1.37–7.13) | 0.007 | 2.48 (1.27–4.84) | 0.008 | 1.66 (1.06–2.60) | 0.027 | 3.13 (1.73–5.68) | 0.0002 | ||
| Model 7 (events/patients) | 65/270 | 61/136 | 73/226 | 53/180 | ||||||
| Mean 1–2h‐PLBG (1 mmol/L) | 1.12 (1.03–1.21) | 0.011 | 1.07 (0.99–1.16) | 0.11 | 0.25 | 1.08 (1.01–1.15) | 0.023 | 1.12 (0.96–1.30) | 0.15 | 0.32 |
| Mean HbA1c (%) | 3.27 (1.60–6.68) | 0.001 | 2.24 (1.40–3.58) | 0.0008 | 1.69 (1.17–2.43) | 0.005 | 2.24 (1.48–3.39) | 0.0001 | ||
Intrapersonal mean values during the 2‐year period from the first visit were used as baseline data. Model 1 included the number of 1–2 h post‐breakfast and post‐lunch blood glucose (1–2h‐PBLBG) measurements (ln‐transformed), age, sex, duration of diabetes, mean systolic blood pressure and hypoglycemia as covariates. Model 4 included the number of 1–2 h post‐breakfast blood glucose (1–2h‐PBBG) measurements (ln‐transformed), age, sex, duration of diabetes, mean systolic blood pressure and hypoglycemia as covariates. Model 7 included the number of 1–2 h post‐lunch blood glucose (1–2h‐PLBG) measurements (ln‐transformed), age, sex, duration of diabetes, mean systolic blood pressure and hypoglycemia as covariates. Hypoglycemia was defined as the presence of fasting or casual blood glucose levels <70 mg/dL (3.9 mmol/L) at least once during the 2‐year period from the first visit. CI, confidence interval; HbA1c, glycated hemoglobin; HR, hazard ratio.
Contribution of postprandial hyperglycemia to the incidence of DR stratified by age at baseline
Table 3 shows the HRs for mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels for the incidence of DR stratified by age at baseline of 60 years. In models 1, 4 and 7, for the stratum of patients with age at baseline <60 years, mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels were significant predictors of the incidence of DR. For the stratum of patients with age at baseline ≥60 years, neither the mean 1–2h‐PBLBG, 1–2h‐PBBG nor 1–2h‐PLBG level was significant, whereas the mean HbA1c level remained significant. There was no significant interaction in all models. When severe hypoglycemia was used as a covariate instead of hypoglycemia, similar results were obtained (data not shown).
Discussion
The present study showed that postprandial glycemia, which was expressed as mean 1–2h‐PBLBG, 1–2h‐PBBG and 1–2h‐PLBG levels at clinic visits during 2 years after the first visit, was a predictor of the incidence of DR in patients with type 2 diabetes. This finding was independent of the mean HbA1c level. Additionally, the effect of postprandial hyperglycemia on the incidence of DR was obvious in well‐controlled patients with an HbA1c value <7.0% and in patients aged <60 years.
There is little evidence on the role of postprandial glycemia in the incidence of DR. Shiraiwa et al. reported that postprandial hyperglycemia on admission is a stronger predictor of the progression of DR than HbA1c levels in Japanese patients with type 2 diabetes7, 8. In the present study of real‐life conditions, the mean HbA1c level was the most important predictor of the incidence of DR among traditional risk factors, whereas postprandial glycemia had an additional value of predicting DR.
The relative contribution of postprandial glycemia is predominant in patients with type 2 diabetes who show a satisfactory level of diabetes control based on HbA1c, whereas basal glycemia becomes the major contributor in poorly controlled patients9. This finding could explain our result that postprandial hyperglycemia was associated with the incidence of DR in patients with well‐controlled HbA1c levels, but not in those with poorly controlled HbA1c levels.
The Wisconsin Epidemiological Study of Diabetes Retinopathy reported that the severity of DR was related to a younger age at diagnosis1, 19. Cross‐sectional studies have reported that a younger age is associated with an increased risk of DR in type 2 diabetes20, 21, 22. The relative contribution of postprandial hyperglycemia to total glycemic exposure, as reflected by HbA1c levels, is greater in older patients than in younger patients with type 2 diabetes10. However, the present study showed that postprandial hyperglycemia was obviously associated with the incidence of DR, independent of HbA1c levels, in patients aged <60 years. The effect of a greater contribution of postprandial hyperglycemia on HbA1c levels in older patients might be negligible compared with the higher susceptibility to DR in younger patients. Furthermore, visit‐to‐visit variability in fasting blood glucose levels is greater in younger patients than in older patients23, and is associated with the incidence of DR24. This finding might partly explain the difference in the incidence of DR according to age groups.
Several biochemical pathways that are overactivated in diabetes are based on one common abnormality, mitochondrial overproduction of reactive oxygen species, which is caused by intracellular excess glucose flux25. Intermittent high glucose levels enhance cellular proliferation and overexpression of vascular endothelial growth factor through reactive oxygen species overproduction at the mitochondrial transport chain level in human retinal endothelial cells. This suggests that glucose variability has an important pathological effect on the development of DR that is dependent on mitochondrial reactive oxygen species26. In a cross‐sectional study of patients with type 2 diabetes, the time that an individual was within their target glucose range (usually 3.9–10.0 mmol/L) was associated with the prevalence of DR27. This metric (i.e., time in range) derived from continuous glucose monitoring provides a great deal of information about the frequency and duration of hyperglycemia or hypoglycemia over time. The relationship between short‐term glucose variability and DR needs to be clarified in the future.
The strengths of the present study are the long‐term observational period, the use of real‐world data and the various measures of postprandial glycemia monitored at the clinic. However, several limitations should be mentioned in this study. First, the design of this study was retrospective. No direct causality was proven. Changes in the laboratory measurement methods, self‐reporting of postprandial time intervals and differences in the number of postprandial blood glucose measurements among patients were considered as potential sources of information bias. However, linear regression equations that were obtained from duplicate assays were used to transform the data acquired by different laboratory techniques. Postprandial time intervals were carefully confirmed by experienced laboratory technicians. Data that were derived from having a snack between meals or from feeding after hypoglycemia were excluded. Furthermore, the number of intrapersonal measurements of postprandial blood glucose levels, which were ln‐transformed, was added to the models as a covariate for adjustment. Second, the number of study participants and events was relatively small. Larger studies are required to confirm the results of the present study. Finally, the participants of this study were treated at a single Japanese clinic. Therefore, there is a limit to the generalizability of our findings to other ethnic groups.
In conclusion, postprandial hyperglycemia at clinic visits might predict the incidence of DR, independent of HbA1c levels, in a real‐world clinical setting among patients with type 2 diabetes. The effect of postprandial hyperglycemia on the incidence of DR is obvious in patients with HbA1c levels of <7.0% and in patients aged <60 years. Therefore, even with well‐controlled HbA1c levels, correcting postprandial hyperglycemia is important for preventing DR, especially in middle‐aged adults with type 2 diabetes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank Ms Kumiko Kimura at the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan, for her excellent assistance with collection of research data. We thank Ellen Knapp, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
J Diabetes Investig 2020; 11: 930–937
References
- 1. Klein R, Klein BEK, Moss SE, et al The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984; 102: 527–532. [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Klein BEK, Moss SE, et al The Beaver Dam Eye Study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology 1992; 99: 58–62. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell P, Smith W, Wang JJ, et al Prevalence of diabetic retinopathy in an older community. The Blue Mountains Eye Study. Ophthalmology 1998; 105: 406–411. [DOI] [PubMed] [Google Scholar]
- 4. Klein R, Klein BEK, Moss SE, et al Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA 1988; 260: 2864–2871. [PubMed] [Google Scholar]
- 5. Klein R, Klein BEK, Moss SE, et al Relationship of hyperglycemia to the long‐term incidence and progression of diabetic retinopathy. Arch Intern Med 1994; 154: 2169–2178. [PubMed] [Google Scholar]
- 6. van Leiden HA, Dekker JM, Moll AC, et al Blood pressure, lipids, and obesity are associated with retinopathy: the Hoorn Study. Diabetes Care 2002; 25: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 7. Shiraiwa T, Kaneto H, Miyatsuka T, et al Post‐prandial hyperglycemia is an important predictor of the incidence of diabetic microangiopathy in Japanese type 2 diabetic patients. Biochem Biophys Res Commun 2005; 336: 339–345. [DOI] [PubMed] [Google Scholar]
- 8. Shiraiwa T, Kaneto H, Miyatsuka T, et al Postprandial hyperglycemia is a better predictor of the progression of diabetic retinopathy than HbA1c in Japanese type 2 diabetic patients. Diabetes Care 2005; 28: 2806–2807. [DOI] [PubMed] [Google Scholar]
- 9. Monnier L, Colette C, Owens D. Postprandial and basal glucose in type 2 diabetes: Assessment and respective impacts. Diabetes Technol Ther 2011; 13: S‐25–S‐32. [DOI] [PubMed] [Google Scholar]
- 10. Munshi MN, Pandya N, Umpierrez GE, et al Contributions of basal and prandial hyperglycemia to total hyperglycemia in older and younger adults with type 2 diabetes mellitus. J Am Geriatr Soc 2013; 61: 535–541. [DOI] [PubMed] [Google Scholar]
- 11. Takao T, Suka M, Yanagisawa H, et al Predictive ability of visit‐to‐visit variability in HbA1c and systolic blood pressure for the development of microalbuminuria and retinopathy in people with type 2 diabetes. Diabetes Res Clin Pract 2017; 128: 15–23. [DOI] [PubMed] [Google Scholar]
- 12. Wilkinson CP, Ferris FL III, Klein RE, et al Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 13. Takao T, Suka M, Yanagisawa H, et al Impact of postprandial hyperglycemia at clinic visits on the incidence of cardiovascular events and all‐cause mortality in patients with type 2 diabetes. J Diabetes Investig 2017; 8: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takao T, Takahashi K, Suka M, et al Association between postprandial hyperglycemia at clinic visits and all‐cause and cancer mortality in patients with type 2 diabetes: A long‐term historical cohort study in Japan. Diabetes Res Clin Pract 2019; 148: 152–159. [DOI] [PubMed] [Google Scholar]
- 15. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sone H, Tanaka S, Tanaka S, et al Comparison of various lipid variables as predictors of coronary heart disease in Japanese men and women with type 2 diabetes: Subanalysis of the Japan Diabetes Complications Study. Diabetes Care 2012; 35: 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang R, Schulze MB, Li T, et al Non‐HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care 2004; 27: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 18. Holman RR, Coleman RL, Shine BSF, et al Non‐HDL cholesterol is less informative than the total‐to‐HDL cholesterol ratio in predicting cardiovascular risk in type 2 diabetes. Diabetes Care 2005; 28: 1796–1797. [DOI] [PubMed] [Google Scholar]
- 19. Klein R, Klein BEK, Moss SE, et al The Wisconsin epidemiologic study of diabetic retinopathy XIV. Ten‐year incidence and progression of diabetic retinopathy. Arch Ophthalmol 1994; 112: 1217–1228. [DOI] [PubMed] [Google Scholar]
- 20. Lim MCC, Lee SY, Cheng BCL, et al Diabetic retinopathy in diabetics referred to a tertiary centre from a nationwide screening programme. Ann Acad Med Singapore 2008; 37: 753–759. [PubMed] [Google Scholar]
- 21. Raman R, Vaitheeswaran K, Vinita K, et al Is prevalence of retinopathy related to the age of onset of diabetes? Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Report No. 5. Ophthalmic Res 2011; 45: 36–41. [DOI] [PubMed] [Google Scholar]
- 22. Zou W, Ni L, Lu Q, et al Diabetes onset at 31–45 years of age is associated with an increased risk of diabetic retinopathy in type 2 diabetes. Sci Rep 2016; 6: 38113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takao T, Okayasu M, Yanagisawa H, et al Influence of plasma glucose variability and age on onset of diabetic retinopathy in diabetic patients: Analysis of results of long‐term outpatient follow‐up for 30 years or more. Nihon Ronen Igakkai Zasshi. 2009; 46: 528–536. (Japanese). [DOI] [PubMed] [Google Scholar]
- 24. Takao T, Ide T, Yanagisawa H, et al The effect of fasting plasma glucose variability on the risk of retinopathy in type 2 diabetic patients: Retrospective long‐term follow‐up. Diabetes Res Clin Pract 2010; 89: 296–302. [DOI] [PubMed] [Google Scholar]
- 25. Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia 2018; 61: 29–38. [DOI] [PubMed] [Google Scholar]
- 26. Sun J, Xu Y, Sun S, et al Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol Cell Biochem 2010; 343: 27–35. [DOI] [PubMed] [Google Scholar]
- 27. Lu J, Ma X, Zhou J, et al Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018; 41: 2370–2376. [DOI] [PubMed] [Google Scholar]


