Abstract
Glucose is the most abundant monosaccharide, and an essential source of energy for most living cells. Glucose transport across the cell membrane is mediated by two types of transporters: facilitative glucose transporters (gene name: solute carrier 2A) and sodium–glucose cotransporters (SGLTs; gene name: solute carrier 5A). Each transporter has its own substrate specificity, distribution, and regulatory mechanisms. Recently, SGLT1 and SGLT2 have attracted much attention as therapeutic targets for various diseases. This review addresses the basal and functional properties of glucose transporters and SGLTs, and describes the pharmaceutical potential of SGLT1 and SGLT2.
Keywords: Antidiabetic drugs, Sodium–glucose cotransporter 1, Sodium–glucose cotransporter 2
Sodium–glucose cotransporter 1 and sodium–glucose cotransporter 2 have attracted much attention as therapeutic targets for various diseases.
Introduction
In mammals, glucose movement into and out of cells is achieved by glucose transporters (GLUTs) on the cell membrane. GLUTs are divided into two structurally and functionally distinct types: (i) GLUTs, which operate by facilitated diffusion 1 , 2 ; and (ii) sodium–glucose cotransporters (SGLTs), which actively transport glucose against the concentration gradient by coupling with sodium 3 , 4 . GLUTs are located in all body cells to facilitate transport of glucose into the cells, and the concentrations of glucose into and out of the cells become equal with GLUTs operation 1 . In the SGLTs, which comprise a family of at least six different isoforms in humans, glucose and sodium are simultaneously cotransported into the cells using the sodium concentration gradient 5 . Of these SGLTs, SGLT1 and SGLT2 have been frequently investigated, because they play key roles in the transport of glucose and sodium across the brush border membrane of intestinal and renal cells 3 , 6 .
In the intestinal epithelium, glucose influx into the epithelial cells is catalyzed by SGLT1 located in the apical membrane, and the glucose flows into the circulation through GLUT2 located in the basolateral membrane 5 , 7 . Also, the two types of transporter, GLUTs and SGLTs, work together in the renal tubular cells, with the SGLTs (SGLT2 and SGLT1) transporting glucose into the tubular cells across the apical membrane, and the GLUTs (GLUT2 and GLUT1) transporting the glucose across the basolateral membrane into the blood circulation 7 , 8 .
Recently, SGLT2 inhibitors have been developed, based on a new concept of antidiabetic action by inhibiting renal glucose reabsorption and increasing glucose excretion into urine. SGLT2 inhibitors reduce glucotoxicity by lowering blood glucose, and a decrease in cardiovascular death and the renal protective effects have been reported by large‐scale clinical trials 9 . Furthermore, SGLT1 is responsible for glucose absorption in the small intestine and for reabsorption of the part of the filtered glucose load in the kidney 10 , and might be an attractive target for the maintenance of good glycemic control and improvement of renal dysfunction 7 , 11 . In this review, we first present an outline summary of glucose transporters: GLUTs and SGLTs. We then focus on SGLT1 and SGLT2, and describe the functional properties and the pharmacological potential, including new insights.
Basal Properties in GLUTs
The facilitative glucose transporters, GLUTs, use the diffusion gradient of glucose or other sugars across cell membranes, each with unique substrate specificities, kinetic profile and expression profile in tissues 4 . GLUTs are divided into three classes by the similarity of amino acid sequence: class I facilitative transporters, GLUT1–4; class II facilitative transporters, GLUT5, 7, 9 and 11; and class III facilitative transporters, GLUT6, 8, 10, 12 and a proton myo‐inositol cotransporter/GLUT13 2 , 4 , 5 .
Among the class I facilitative transporters, GLUT1 has ubiquitous expression, with abundant expression in the brain and erythrocytes, and moderate expression in fat, muscle and the liver 4 , 12 , 13 , 14 . GLUT1 is responsible for constitutive or basal glucose uptake in the cells and can transport aldose, including pentose and hexose 1 , 15 . GLUT2 is expressed in pancreatic β‐cells, the liver, kidney and small intestine. In rodent β‐cells, GLUT2 plays a key role in the glucose‐sensing mechanism due to the low affinity and high capacity 16 , 17 . In contrast, in human β‐cells, GLUT1 is primarily expressed, and GLUT2 is expressed at an extremely low level 18 . In the liver, GLUT2 is expressed on the sinusoidal membrane and enables the bidirectional transport of glucose 1 . The GLUT2 in the kidney and small intestine is responsible for the movement of glucose out of absorptive epithelial cells into the blood circulation 16 . GLUT3 has a high affinity for glucose, and is known for its specific expression in neurons, in particular the brain. GLUT3 is also expressed in other cells having specific glucose requirements, such as sperm cells and embryos 5 , 19 , 20 . Insulin‐responsive glucose transporter GLUT4 is expressed in skeletal muscle, adipose tissue and the heart, and is also found in the brain 1 , 21 . When insulin binds to the insulin receptors, the GLUT4 moves to the cell membrane and facilitates glucose transport into the cells.
Among the class II facilitative transporters, GLUT5, which is the specific transporter for fructose, is predominantly expressed in the small intestine, testes and kidneys 4 , 22 . In the intestine, GLUT5 is responsible for transport of hexose from the villus epithelium with SGLT1. The hexose in the cell leaves the epithelium through GLUT2 located in the basolateral membrane 16 . GLUT7 is primarily expressed in the small intestine and colon, and works as a facilitated hexose transporter 23 . GLUT9 is highly expressed in the kidney and liver 2 . In the human kidney, GLUT9 is expressed in proximal convoluted tubules, and in rodents, it is expressed in distal connecting tubules 24 , 25 . GLUT9 is reportedly a urate transporter, and some mutations in its sequence induce hypouricemia 26 . GLUT9 is also known as voltage‐driven urate transporter 1 on the basolateral membrane 27 , 28 . Two splice variants of GLUT11, long and short forms (503 and 493 amino acid residues), are expressed in a tissue‐specific manner 29 , 30 , and hexose, glucose or fructose is transported. The short form of GLUT11 is predominantly expressed in heart and skeletal muscle, and the long form is detected in the liver, lung, trachea and brain 29 , 30 .
As for the class III facilitative transporters, GLUT6, which is a hexose transporter protein, is highly expressed in the brain, spleen and leukocytes 4 . GLUT8 is mainly expressed in the testis, and lower expressions of messenger ribonucleic acid (mRNA) were detected in other organs, including insulin‐sensitive tissues 5 . GLUT8 has been identified as an insulin‐responsive glucose transporter, and reportedly has a role in glucose uptake in the mammalian heart, along with GLUT4 31 . GLUT10 plays a role in glucose homeostasis control, and in humans, it has a higher mRNA level in the liver and pancreas 32 . GLUT12, which can facilitate transport of a variety of hexose, is expressed in the heart, small intestine, prostate and insulin‐sensitive tissues, including skeletal muscle and fat 33 . Proton myo‐inositol cotransporter/GLUT13 has been identified as a proton myo‐inositol cotransporter, and is highly expressed in glial cells and some neurons, suggesting that proton myo‐inositol cotransporter/GLUT13 might be responsible for myo‐inositol metabolism regulation in the brain 34 , 35 .
Overview of SGLTs
SGLTs constitute a large family of membrane proteins related to various transports of glucose, amino acids, vitamins and some ions across the apical membrane of the lumen side, including in the small intestine and renal tubules. In humans, six different isoforms have been reported, and two transporters, SGLT1 (solute‐carrier [SLC]5A1) and SGLT2 (SLC5A2) proteins, have been widely studied 36 . SGLT1 was discovered by expression cloning in 1987 37 , and SGLT2 was identified by homology screening in 1994 38 . GLUTs equilibrate the glucose levels on both sides of the plasma membrane, because the glucose gradient across the membrane is the driving force, whereas SGLT2 can exert differences in glucose concentration, because the transmembrane sodium gradient is the driving force for glucose uptake 3 , 4 , 5 .
SGLT1 is responsible for glucose absorption in the small intestine, whereas SGLT2 is responsible for glucose reabsorption in the kidney (Table 1) 38 , 39 . Considering the physiological functions of SGLT1 and SGLT2, drug recovery research targeting the transporters is reasonable. In 1987, phlorizin, an inhibitor of SGLT1 and SGLT2, was reported to reverse experimental diabetes in partially pancreatectomized rats 40 . SGLT inhibitors have been developed using phlorizin as a lead compound 7 , 41 , 42 , resulting in the development of SGLT2 inhibitors, which have been successfully launched for the market 42 , 43 .
Table 1.
Tissue expression and biochemical characteristics of sodium–glucose cotransporter 1 and sodium–glucose cotransporter 2
| Characteristics | SGLT1 | SGLT2 |
|---|---|---|
| Site | Mostly in small intestine some kidney, heart, brain etc. | Mainly in kidney |
| Renal location | S3 segment of proximal tubules | S1 and S2 segments of proximal tubules |
| Sugar selectivity | Glucose = galactose | Glucose > galactose |
| Sodium/glucose stoichiometry | 1:2 | 1:1 |
| Affinity for glucose | High (0.5 mmol/L) | Low (2 mmol/L) |
| Glucose transport capacity | Low | High |
SGLT, sodium–glucose cotransporter.
As for the other SGLTs, SGLT3 (gene name: SLC5A4), which is expressed in the intestine, spleen, liver, kidney, skeletal muscle and cholinergic neurons, is not a functional SGLT, and seems to act as a glucose sensor in the plasma membrane of cholinergic neurons 44 . There are only a few reports on the other SGLTs: SGLT4, SGLT5 and SGLT6. SGLT4 (gene name: SLC5A9) is expressed in the small intestine, kidneys, liver, lung, brain, trachea, uterus and pancreas; SGLT5 (gene name: SLC5A10) is expressed only in the kidneys; and SGLT6 (gene name: SLC5A11) is considered to be a low‐affinity d‐glucose transporter in the small intestine 39 , 45 . Physiological roles of these SGLTs remain unknown.
Basal properties of SGLT1
The SGLT1 protein, encoded by the SLC5A gene on chromosome 22q13.1, is composed of 664 amino acids, comprising 14 transmembrane α‐helical domains, a single glycosylation site between transmembrane helices 5 and 6, and two phosphorylation sites, between transmembrane helices 6 and 7, and between 8 and 9 39 , 45 , 46 . The NH2 and COOH terminals are located in extracellular and intracellular membranes, respectively, and the glucose‐binding domain is supposed to include amino acid residues 457–460 45 , 47 . SGLT1 is a high‐affinity transporter for glucose (Michaelis–Menten constant [K m] = 0.4 mmol/L) and galactose, whereas fructose is not transported 39 , 48 , 49 . Two sodium ions are transported through the SGLT1 for each glucose molecule, and this cotransporter is allowed to transport glucose into the cells against its concentration gradient 4 .
SGLT1 mRNA expression has been detected by reverse transcription polymerase chain reaction in the following tissues in humans: small intestine, kidney, skeletal muscle, liver, lung, heart, trachea, prostate, testis, cervix of the uterus, stomach, mesenteric adipose tissue, pancreatic α‐cells, colon and brain 50 , 51 , 52 , 53 . SGLT1 protein expression has been localized to the apical brush border of the small intestine and the late proximal tubules, and has also been detected in the following tissues in humans: salivary gland, liver, lung, skeletal muscle, heart and pancreatic α‐cells 37 , 53 , 54 , 55 .
SGLT1 reportedly exerts the transport activity by many molecular regulations, including protein kinases. SGLT1 contains strain‐specific regulation sites by protein kinase A (PKA) and protein kinase C (PKC): one PKA site in humans and rabbit, none in rat; five consensus PKC sites in humans and rats, and four sites in rabbits 56 , 57 . PKA activation led to an increase in the number of SGLT1 proteins in the membrane of the small intestine in rats 58 , and PKA activator, 8‐bromo‐cyclic adenosine monophosphate, or forskolin increased the SGLT capacity and the SGLT1 activity in the plasma membrane 56 , 58 . The expression and activity of SGLT1 is positively regulated by PKA activity, and the effects on SGLT1 activation was inhibited by PKA inhibitor, H‐89 59 , 60 . PKC‐mediated effects on SGLT1 are also reported, but obvious species differences are admitted and the effects are controversial. PKC activation decreased the SGLT1 transport capacity in rats and rabbits, but increased the capacity in humans 56 .
In other reports, adenosine monophosphate‐activated protein kinase activation increased maximal sodium‐dependent glucose transport 61 , 62 , knockout of the serum‐ and glucocorticoid‐inducible kinase 3 caused a decrease of intestinal SGLT1 activity 63 , and Ste20p‐related proline alanine‐rich kinase caused a decrease of SGLT1 abundance in the plasma membrane 64 .
Intestinal SGLT1 activity and expression are regulated by dietary carbohydrate content. The SGLT1 activity and expression increased in mice, rats and sheep fed a high‐sugar diet 65 , and is maintained by the presence of luminal nutrients in the human intestine 66 . In addition, SGLT1 activity and expression are related to a diurnal rhythm that correlates waking hours with the highest expression of SGLT1 67 , 68 .
Basal properties of SGLT2
The SGLT2 protein, encoded by SLC5A2, is composed of 672 amino acids and its NH2 and COOH termini are extracellular 46 . The K m values in human SGLT2 for glucose and sodium are 2 and 25 mmol/L, respectively, and, differently from SGLT1, SGLT2 is a low‐affinity and high‐capacity glucose transporter 39 , 51 . SGLT2 is predominantly expressed in the kidney of rodents and humans, and low mRNA expressions were detected in the mammary glands, testis, liver, lung, intestine, skeletal muscle, spleen and cerebellum 39 , 51 , 52 , 69 , 70 . Also, SGLT2 is reportedly expressed in pancreatic α‐cells and related to glucagon secretion 53 . SGLT2 is localized in the luminal membrane of the segment (S)1 and S2 segments of renal proximal tubules in humans and rodents, whereas SGLT1 is localized in the luminal membrane of the S3 segment 39 , 52 , 70 , 71 . SGLT2 is mainly responsible for glucose reabsorption in nephron, and ≥80% of the filtered glucose is reabsorbed in the S1 and S2 segments of the proximal tubules through SGLT2 45 , 72 .
Protein kinase A and PKC activation increased glucose uptake by 225 and 150%, respectively, in human embryonic renal cells expressing SGLT2 73 . As for the mechanisms, the PKA‐mediated effect might be related to an increased rate of vesicle fusion with the membrane; however, no such mechanism was found on the PKC‐mediated effect. Also, SGLT2 expression reportedly increased through the activation of exchange protein directly activated by cyclic adenosine monophosphate/PKA through extracellular signal‐regulated kinase/p38 and mitogen‐activated protein kinase 73 , 74 . In the renal pig cell line, interleukin‐6 and tumor necrosis factor‐α increased SGLT2 mRNA and protein expressions 75 , and similarly, the phosphorylation of transforming growth factor‐β1 and the downstream transcription factor, smad3, increased the SGLT2 protein level in human renal proximal tubular cells 76 .
Functional properties of SGLTs in the small intestine
SGLT1 in the small intestine is localized in the apical cell membrane composing brush border (Figure 1) 6 , 52 , 54 . SGLT1 is responsible for the transport of glucose or galactose from the lumen into the epithelial cells, whereas the facilitative transporter, GLUT2, is subsequently responsible for the transport of glucose from the basolateral membrane into the blood circulation 77 , 78 .
Figure 1.
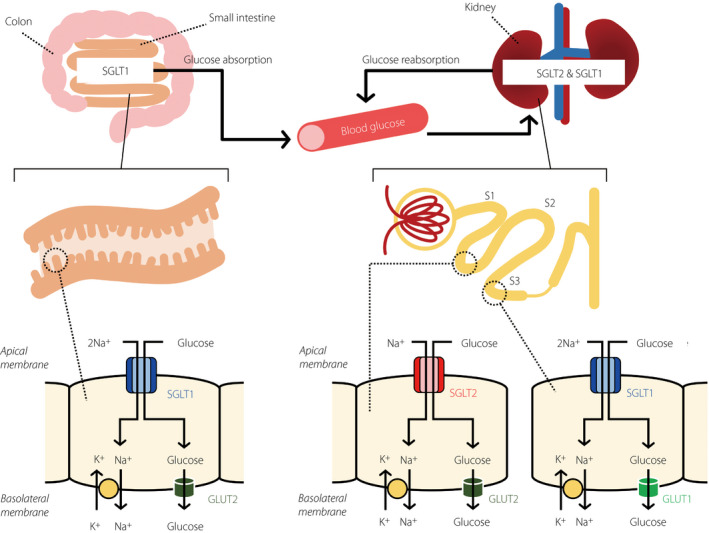
Glucose handling through sodium–glucose cotransporter (SGLT)1 and SGLT2. In the small intestine, dietary glucose is mainly absorbed by SGLT1 on the brush border membrane. SGLT1 has a high‐affinity (Michaelis–Menten constant [K m] = 0.4 mmol/L) for glucose, and transports sodium and glucose with a 2:1 stoichiometry. In the kidney, filtered glucose by the renal glomerulus is reabsorbed by SGLT2 and SGLT1 expressed in the luminal membrane of the segment (S)1 and S2 segments, and S3 segment of proximal tubules, respectively. The affinity of SGLT2 for glucose is lower (K m = 2 mmol/L), and transport of sodium and glucose by SGLT2 occurs with a 1:1 stoichiometry. GLUT, glucose transporter.
The level of SGLT1 expression provides the capacity for glucose absorption and undergoes short‐term and long‐term regulations depending on the luminal nutrients 65 , 66 . A high‐glucose diet or a high‐sodium diet reportedly increases the level of SGLT1 expression in the small intestine 65 , 66 . Also, an increase in the luminal glucose concentrations induces GLUT2 translocation to the brush border membrane 78 , 79 .
The SGLT1 expression in the small intestine is reportedly increased in diabetes, which is considered to be related to the response to greater dietary glucose intake. Intestinal SGLT1 mRNA expression increased in diabetic animal models, such as streptozotocin‐induced diabetic models and Otsuka Long‐Evans Tokushima Fatty rats 80 , 81 . In type 2 diabetes patients, the intestinal SGLT1 mRNA and protein expressions in the brush border membrane were higher, and also the intestinal glucose uptake was elevated 82 . The upregulation of SGLT1‐mediated glucose uptake in the small intestine is considered to induce the rapid postprandial hyperglycemia in diabetes 83 , 84 .
Functional properties of SGLTs in the kidney
In the kidney, glucose is transported through the apical membrane of the proximal convoluted tubule by SGLT2 and SGLT1, and exits through the basolateral membrane of the proximal tubule by the facilitative transporters GLUT2 and GLUT1 39 , 85 . SGLT2 is expressed in the upper part of the proximal tubule, S1 and S2 segments, whereas SGLT1 is expressed in the downward part of the proximal tubule, the S3 segment in humans and rodents (Figure 1) 52 , 71 , 86 .
In the capacity of filtered glucose reabsorption in euglycemia, SGLT2 exerts the main function, showing the aforementioned ≥80% glucose reabsorption, whereas SGLT1 reabsorbs the remaining glucose or approximately 5% of the filtered glucose 77 , 86 , 87 . As a point to be noted, the coupling ratio of glucose and sodium is different between the two cotransporters: SGLT2 transports glucose and sodium in a 1:1 ratio, whereas SGLT1 transports glucose and sodium in a 1:2 ratio 38 , 39 . The transport property of SGLT2 enhances the concentrating power to reabsorb the glucose delivered to the distal part S3 segment of the proximal tubule 49 . Furthermore, it is reported that SGLT1 prepares the highly reserved ability of glucose reabsorption 72 , 87 , 88 . When pharmacological SGLT2 inhibition induces the glucose flow downstream in the distal proximal tubule, SGLT1 can compensate for the reabsorption of glucose. As a result, euglycemic humans treated with SGLT2 inhibitors maintained a fractional glucose reabsorption of 40–50% 72 , 87 , and the mean value of fractional glucose reabsorption in euglycemic SGLT2 knockout (KO) mice was 36% 86 . In wild mice, SGLT2 inhibitor, empagliflozin, dose‐dependently increased the urinary glucose excretion, whereas the dose–response curve was shifted leftward and the maximum response doubled in SGLT1 KO mice 87 . The compensatory effect of SGLT1 is also supported by studies in SGLT1/SGLT2 double KO mice 89 , 90 and SGLT1 KO mice treated with SGLT2 inhibitor 87 . Sustained hyperglycemia, which induces exceeding of the transport capacity of the proximal SGLT2, increased the glucose flow to the distal proximal tubule and enhanced the SGLT1‐mediated glucose reabsorption 7 . The reserved ability of glucose reabsorption and compensatory effect of SGLT1 are notable properties in consideration of the physiological function.
In type 1 and type 2 diabetes animal models, the renal SGLT2 protein level was reportedly increased 42 , 91 , whereas the reported results for renal SGLT1 levels are controversial. Streptozotocin rats showed increased mRNA and protein expressions of SGLT1 in the renal cortex 92 , 93 . Also, renal SGLT1 mRNA expression in Zucker fatty rats was increased 94 . In ob/ob mice, the renal membrane SGLT1 protein level was increased, but the mRNA expression was decreased 95 . In contrast, it was reported that the renal membrane SGLT1 protein level was decreased in diabetic Akita mice 96 . SGLT2 and SGLT1 properties in renal glucose reabsorption in euglycemic condition are well understood; however, those properties in the diabetic state remain poorly understood, and in particular, a better understanding of the physiological significance in the renal SGLT1 regulation is a pivotal subject for the future.
Functional properties of SGLTs in the heart
The localization of SGLT1 protein was found in capillaries of the heart in humans and rats 52 , 97 , whereas the expression was not found in capillaries of the small intestine 97 . Also, SGLT1 was reportedly expressed in the cell membrane of cardiomyocytes in humans and mice 98 , 99 . Thus, cardiac SGLT1 might be involved in glucose transport from capillaries into the cardiomyocytes. In contrast, SGLT2 is not expressed in the heart. In the heart, two facilitated glucose transporters, GLUT1 and GLUT4, play a primary role in glucose uptake: GLUT1 for basal glucose uptake, and GLUT4 for insulin‐dependent glucose uptake 100 . In consideration of the physiological roles of SGLT1 in the heart, the involvement with the facilitated glucose transporters is essential and cannot be bypassed.
Cardiac SGLT1 mRNA expression is reportedly increased in patients with type 2 diabetes and diabetic cardiomyopathy 101 . In streptozotocin diabetic rats, GLUT4 mRNA and protein expressions were decreased, whereas GLUT1 mRNA expression was not significantly changed 102 , 103 . The reduction of cardiac GLUT4 activity led to a decrease of glucose uptake and development of diabetic cardiomyopathy, whereas the physiological roles of GLUT1 in the heart remain unclear 104 , 105 , 106 .
A recent study reported that chronic cardiac overexpression of SGLT1 in mice led to pathological cardiac hypertrophy and left ventricular failure, and cardiac knockdown of SGLT1 attenuated the disease phenotype 107 . In contrast, a recent study also reported that dual SGLT1/SGLT2 inhibitor exacerbated cardiac dysfunction after experimental myocardial infarction in rats 108 . Considering that SGLT2 is not expressed in the heart, this effect might be linked to SGLT1 inhibition. Whether cardiac SGLT1 inhibition exerts protective effects on cardiovascular disease still remains unclear. Further research is required.
Functional properties of SGLTs in the brain
SGLT1 mRNA expression was found in the brains of humans, rabbits, pigs and rodents 109 , 110 , 111 . In rabbits and pigs, SGLT1 mRNA expression was found in neurons of the frontal cortex, Purkinje cells of the cerebellum and neurons of the hippocampus 50 . In rodents, SGLT1 mRNA expression was found in neurons of the brain cortex, hippocampus, hypothalamus, corpus striatum and cerebellum 50 , 111 . The SGLT1 protein was reportedly expressed in small vessels of the rodent brain 109 . Also, a radioactively labeled SGLT1 selective glucose analog could not pass the blood–brain barrier, suggesting that SGLT1 is only localized in the luminal membrane of endothelial cells 50 . In consideration of the localization and function of SGLT1, SGLT1 in the brain might play a key role as an energy supply source for neurons on increased glucose demand, such as in hypoxemia and hypoglycemia.
Functional properties of SGLTs in other organs
There are some reports of SGLT1 in the lung, liver, pancreas and T lymphocytes. SGLT1 mRNA was detected in the trachea, bronchi and lung tissue in humans 51 , 52 , and SGLT1 protein was detected in alveolar type 2 cells, and in the luminal membrane of Clara cells in bronchioles in humans and rats 52 . SGLT1‐mediated glucose uptake might be responsible for fluid absorption, and provides energy for the production of surfactants in alveolar type 2 cells, and for mucin and surfactants in Clara cells.
SGLT1 mRNA was detected in the liver and gallbladder in humans 51 , and SGLT1 protein was detected in the apical membrane of bile duct epithelial cells in humans and rats 52 , 71 .
Small amounts of SGLT1 mRNA were detected in the pancreas of humans, and SGLT1 mRNA and protein expressions were found in pancreatic α‐cells of humans and mice 51 , 53 . Also, SGLT1 mRNA expression was found in activated T lymphocytes of mice 112 . Physiological roles of SGLT1 in the liver, pancreas and T lymphocytes are not well understood.
Therapeutic Potential of SGLT1 and SGLT2 Inhibition
The therapeutic potential of selective SGLT2 inhibitors as an antihyperglycemic strategy has been well established. In contrast, the therapeutic potential, including efficacy and safety, of dual SGLT2/SGLT1 inhibitor or selective SGLT1 inhibitor remains less clear (Table 2).
Table 2.
Preclinical and clinical sodium–glucose cotransporter 1, sodium–glucose cotransporter 2 and dual sodium–glucose cotransporter 1/2 inhibitors
| Selective SGLT1 inhibitors | Selective SGLT2 inhibitors | Dual SGLT1/2 inhibitors |
|---|---|---|
| KGA‐2727 | Dapagliflozin | Sotagliflozin |
| GSK‐1614235 (mizagliflozin) | Canagliflozin | Licogliflozin |
| LX2761 | Empagliflozin | |
| JTT‐662 | Ipragliflozin | |
| Luseogliflozin | ||
| Tofogliflozin | ||
| Ertugliflozin |
SGLT, sodium–glucose cotransporter.
SGLT2 inhibitors
Selective SGLT2 inhibitors – dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, luseogliflozin and tofogliflozin – have been approved for the treatment of type 2 diabetes 113 . These SGLT2 inhibitors reduce plasma glucose levels by a different mechanism than other antidiabetic drugs, involving an increase of the renal glucose excretion through SGLT2 in the proximal tubule leading to diminished glucose toxicity. In contrast, mechanisms of other drugs are as for metformin: inhibition of gluconeogenesis in the liver; sulfonylurea derivatives, glucagon‐like peptide (GLP)‐1 analogs and dipeptidyl peptide‐4 inhibitors: increase of insulin secretion in the pancreas; and thiazolidinediones: enhancement of insulin sensitivity. These SGLT2 inhibitors have different selectivity for inhibition of SGLT2 versus SGLT1. SGLT2/SGLT1 selectivity is ≥1,000‐fold higher in dapagliflozin, empagliflozin, luseogliflozin and tofogliflozin, whereas the selectivity of canagliflozin and ipragliflozin is lower, at 190‐ and 250‐fold, respectively 113 .
In preclinical studies in diabetic animal models, the SGLT2 inhibitors decreased fasting and non‐fasting glucose levels, hemoglobin A1c levels, and blood pressure, and improved glucose intolerance 114 , 115 , 116 , 117 . Furthermore, SGLT2 inhibitors have a different mechanism from the other antidiabetic drugs, as described above, and can be used in combination with those drugs, as well as in monotherapy for the treatment of type 2 diabetes 118 , 119 , 120 , 121 , 122 .
Recent studies reported that SGLT2 inhibitors had a renal protective effect in animal models of diabetic nephropathy 122 , 123 , 124 . The renal protective effects of SGLT2 inhibitors also have been shown in clinical trials 9 , 125 , 126 . The mechanism of action is speculated as follows: SGLT2 inhibitor increases the amount of sodium delivery to the distal tubule by suppressing sodium absorption in the proximal tubule. As a result, the tubuloglomerular feedback through the macula densa is activated, and this allows afferent arteriolar contraction and normalizes the glomerular filtration rate 127 .
SGLT1 inhibitors
Postprandial hyperglycemia is a risk factor for cardiovascular failure and diabetic microangiopathy, including retinopathy 128 , 129 , 130 . As glucose absorption from the small intestine is mostly mediated by SGLT1, an improvement of postprandial hyperglycemia with SGLT1 inhibitor would definitely be a useful therapy. In diabetic rats, a single dose of KGA‐2727, a selective SGLT1 inhibitor, improved postprandial hyperglycemia, and its chronic administration reduced hemoglobin A1c levels 131 , suggesting that SGLT1 inhibition might maintain good glycemic control for the long term. In an oral glucose tolerance test with KGA‐2727, plasma insulin levels, as well as plasma glucose levels, were reduced, and protective effects on the pancreas are also expected.
Recent studies reported that SGLT1 KO mice and mice treated with phloridzin had lower plasma total GLP‐1 levels 5 min after glucose challenge than wild‐type mice and control mice, respectively 77 , 132 . This result suggests that SGLT1 is required to trigger GLP‐1 secretion in the early phase after glucose stimulation. In contrast, another study reported that SGLT1 KO mice had high plasma total GLP‐1 levels from 30 min to 6 h after a glucose‐containing meal 89 . The increase of plasma total GLP‐1 in the late phase after a meal was also observed in healthy humans treated with SGLT1 inhibitor 84 and patients with type 2 diabetes treated with SGLT1/2 inhibitor 83 . One possible mechanism of delayed GLP‐1 release is fermentation of glucose to short‐chain fatty acids (SCFAs). SGLT1 inhibition in the early intestine reduces glucose absorption and thereby increases glucose delivery to the more distal parts of the small intestine, where glucose is used by the microbiome to form SCFAs. SCFAs induce glucagon‐like peptide‐1 secretion through G protein‐coupled receptors, including G protein‐coupled receptor 41 and G protein‐coupled receptor 43 133 . From the above, although SGLT1 inhibition reduces glucose‐stimulated GLP‐1 release in the early phase, SCFAs generated by fermentation of glucose induce GLP‐1 release in the late phase, suggesting that SGLT1 inhibitor increases net circulating GLP‐1 levels.
As a concerning point, in the small intestine, the SGLT1 inhibitor is considered to induce gastrointestinal side‐effects, including diarrhea, but no serious gastrointestinal side‐effects were observed in the treatment of selective SGLT1 inhibitors, GSK‐1614235 and KGA‐2727, or a dual SGLT1/SGLT2 inhibitor, sotagliflozin 83 , 84 , 134 .
The SGLT1 inhibitors induce a delay of absorption of monosaccharides and thus their retention, and the SGLT1 inhibitors might improve the intestinal condition in diabetes patients through changes in gut microbiota. An increase in colonic microbiol production of propionate with increased glucose exposure reportedly contributed to positive intestinal metabolic effects 10 .
SGLT1 is expressed in the brush border membrane of the S3 segment of proximal tubule in the kidney, and reabsorbs glucose that escapes from SGLT2‐mediated reabsorption in the S1 and S2 segments 39 , 52 . Studies on SGLT2 KO mice and selective SGLT2 inhibitors described the renal transport capacity of SGLT1, showing that the SGLT1‐mediated glucose reabsorption is maintained at 40–50% on inhibition of SGLT2 under euglycemic conditions (Figure 2) 87 . Inhibition of SGLT2 under the conditions of prolonged and severe hyperglycemia that exceeds the transport capacity of SGLT2 activates the full renal transport capacity of SGLT1, and SGLT1 exerts a compensatory function in renal reabsorption of glucose. Therefore, the combination therapy of an SGLT1 inhibitor and an SGLT2 inhibitor or a dual SGLT1/SGLT2 inhibitor is expected to induce significantly greater glucosuria and glycemic control than either an SGLT1 or SGLT2 inhibitor alone 87 , 89 , 135 . Also, a stronger effect of dual SGLT1/SGLT2 inhibition on blood glucose levels was observed in mice with modest hyperglycemia, as well as those with euglycemia 87 , 89 . Thus, the combined effects of dual SGLT1/SGLT2 inhibition might induce synergistic effects on the early and distal proximal tubules.
Figure 2.
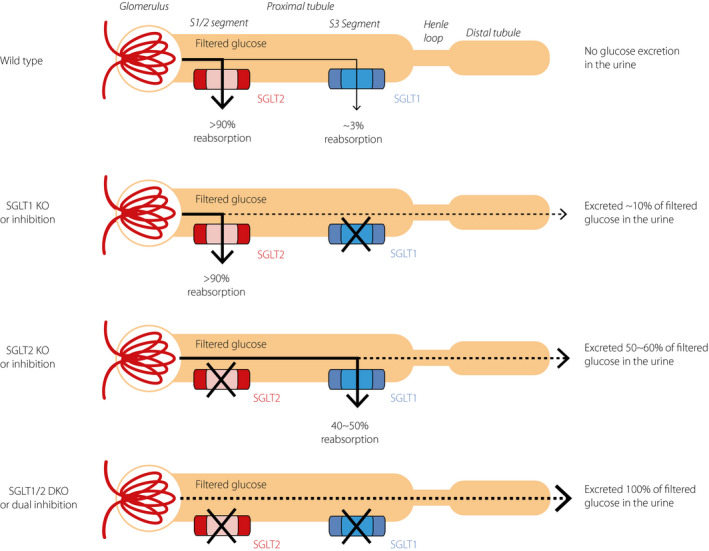
Capacity of sodium–glucose cotransporter (SGLT)1 and SGLT2 for filtered glucose reabsorption under euglycemic conditions. Under euglycemic conditions, most filtered glucose is reabsorbed by SGLT2 expressed in the segment (S)1 and S2 segments of proximal tubules, and the remaining is reabsorbed by SGLT1 expressed in the S3 segment of proximal tubules, resulting in no glucose being detected in the urine. Complete suppression of transport activity of SGLT1 (e.g., SGLT1 knockout [KO] or inhibition) only slightly increases the urinary glucose excretion, because most filtered glucose is reabsorbed by SGLT2. If SGLT2 is absent (e.g., SGLT2 KO or inhibition), SGLT1 reabsorbs 40–50% of filtered glucose. If both SGLT1 and SGLT2 are absent (e.g., SGLT1/2 double KO [DKO] or dual inhibition), almost all of the filtered glucose is excreted in the u‐rine.
Although selective SGLT1 inhibitors are not on the market yet, some compounds (e.g., LX2761 and JTT‐662) are under development for the treatment of diabetes.
Conclusion and Perspective
In this review, we described the basal properties of GLUTs and SGLTs, and also the functional properties of SGLT1 and SGLT2, and focused on the pharmacological potential of SGLT1 or SGLT2 inhibition alone, and the dual inhibition of SGLT1 and SGLT2. These glucose transporters have diverse multiple functions, and are attractive as therapeutic targets for metabolic diseases.
In basic studies of the kidney in SGLT2 KO mice and using an SGLT2 inhibitor, a high reserved ability of glucose reabsorption has been disclosed. Six selective SGLT2 inhibitors have been approved for treatment of diabetes, and the usefulness is widely admitted.
Based on the phenotype of loss‐of‐function of the SGLT1 gene in humans and mice, it is clear that SGLT1 is the main transporter of glucose absorption in the small intestine. As described above, it is expected that SGLT1 inhibitors would improve postprandial hyperglycemia in diabetes patients by reducing glucose absorption in the small intestine. This mechanism of action would be beneficial, particularly in diabetes patients with declining renal function, because SGLT2 inhibitors are less effective in such patients.
A dual SGLT1/SGLT2 inhibitor or a combination of an SGLT1 inhibitor and SGLT2 inhibitor might be good option for the treatment of diabetes, because dual inhibition leads to blockade of both intestinal and renal glucose absorption, thus lowering blood glucose levels robustly. Combined treatment of SGLT1 inhibitor and dipeptidyl peptide‐4 inhibitor might also be a good strategy, because the combination could effectively increase active GLP‐1 levels.
Diabetes is a leading cause of end‐stage kidney disease and cardiovascular disease. Despite the emergence of a large variety of antihyperglycemic agents, it is still difficult to maintain good glycemic control with monotherapy over a long‐term period. These agents also have potential risks and side‐effects (e.g., hypoglycemia, ketoacidosis and more). For these reasons, other antihyperglycemic agents with different mechanisms of action are required. Inhibition of SGLT1 or dual inhibition of SGLT1/2 are novel therapeutic strategies for glycemic control in diabetes patients. However, further studies are required to confirm the long‐term efficiency and safety of these strategies.
Disclosure
Ryuhei Sano and Yuichi Shinozaki are employees of Japan Tobacco Inc. Takeshi Ohta declares no conflict of interest.
Acknowledgment
The authors thank Dr Tomohiko Sasase for suggestions on writing this manuscript.
J Diabetes Investig 2020; 11: 770–782
References
- 1. Mueckler M. Facilitative glucose transporters. Eur J Biochem 1994; 219: 713–725. [DOI] [PubMed] [Google Scholar]
- 2. Joost HG, Thorens B. The extended GLUT‐family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 2001; 18: 247–256. [DOI] [PubMed] [Google Scholar]
- 3. Wright EM. Renal Na(+)‐glucose cotransporters. Am J Physiol Renal Physio 2001; 280: F10–F18. [DOI] [PubMed] [Google Scholar]
- 4. Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 2003; 89: 3–9. [DOI] [PubMed] [Google Scholar]
- 5. Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr 2004; 28: 364–371. [DOI] [PubMed] [Google Scholar]
- 6. Hirayama BA, Wong HC, Smith CD, et al Intestinal and renal Na+/glucose cotransporters share common structures. Am J Physiol 1991; 261: C296–C304. [DOI] [PubMed] [Google Scholar]
- 7. Song P, Onishi A, Koepsell H, et al Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin Ther Targets 2016; 20: 1109–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 2010; 5: 133–141. [DOI] [PubMed] [Google Scholar]
- 9. Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 10. Lehmann A, Hornby PJ. Intestinal SGLT1 in metabolic health and disease. Am J Physiol Gastrointest Liver Physiol 2016; 310: G887–G898. [DOI] [PubMed] [Google Scholar]
- 11. Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol 2013; 1: 140–151. [DOI] [PubMed] [Google Scholar]
- 12. Guo X, Geng M, Du G. Glucose transporter 1, distribution in the brain and in neural disorders: its relationship with transport of neuroactive drugs through the blood‐brain barrier. Biochem Genet 2005; 43: 175–187. [DOI] [PubMed] [Google Scholar]
- 13. Aoyama M, Maejima Y, Suzuki T, et al Androgen suppresses corticotropin‐induced increase in plasma cortisol level but enhances the increase in plasma aldosterone level in goats. J Vet Med Sci 2009; 71: 281–285. [DOI] [PubMed] [Google Scholar]
- 14. Jurcovicova J. Glucose transport in brain ‐ effect of inflammation. Endocr Regul 2014; 48: 35–48. [DOI] [PubMed] [Google Scholar]
- 15. Mueckler M, Makepeace C. Transmembrane segment 6 of the Glut1 glucose transporter is an outer helix and contains amino acid side chains essential for transport activity. J Biol Chem 2008; 283: 11550–11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bell GI, Kayano T, Buse JB, et al Molecular biology of mammalian glucose transporters. Diabetes Care 1990; 13: 198–208. [DOI] [PubMed] [Google Scholar]
- 17. Leturque A, Brot‐Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab 2009; 296: E985–E992. [DOI] [PubMed] [Google Scholar]
- 18. De Vos A, Heimberg H, Quartier E, et al Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Investig 1995; 96: 2489–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haber RS, Weinstein SP, O’Boyle E, et al Tissue distribution of the human GLUT3 glucose transporter. Endocrinology 1993; 132: 2538–2543. [DOI] [PubMed] [Google Scholar]
- 20. Carayannopoulos MO, Xiong F, Jensen P, et al GLUT3 gene expression is critical for embryonic growth, brain development and survival. Mol Genet Metab 2014; 111: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rayner DV, Thomas ME, Trayhurn P. Glucose transporters (GLUTs 1–4) and their mRNAs in regions of the rat brain: insulin‐sensitive transporter expression in the cerebellum. Can J Physiol Pharmacol 1994; 72: 476–479. [DOI] [PubMed] [Google Scholar]
- 22. Rand EB, Depaoli AM, Davidson NO, et al Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol 1993; 264: G1169–G1176. [DOI] [PubMed] [Google Scholar]
- 23. Cheeseman C. GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrinol Metab 2008; 295: E238–E241. [DOI] [PubMed] [Google Scholar]
- 24. Augustin R, Carayannopoulos MO, Dowd LO, et al Identification and characterization of human glucose transporter‐like protein‐9 (GLUT9): alternative splicing alters trafficking. J Biol Chem 2004; 279: 16229–16236. [DOI] [PubMed] [Google Scholar]
- 25. Keembiyehetty C, Augustin R, Carayannopoulos MO, et al Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up‐regulated in diabetes. Mol Endocrinol 2006; 20: 686–697. [DOI] [PubMed] [Google Scholar]
- 26. Matsuo H, Chiba T, Nagamori S, et al Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Human Genet 2008; 83: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anzai N, Ichida K, Jutabha P, et al Plasma urate level is directly regulated by a voltage‐driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 2008; 283: 26834–26838. [DOI] [PubMed] [Google Scholar]
- 28. Doblado M, Moley KH. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am J Physiol Endocrinol Metab 2009; 297: E831–E835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doege H, Bocianski A, Scheepers A, et al Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar‐transport facilitator specifically expressed in heart and skeletal muscle. Biochem J 2001; 359: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu X, Li W, Sharma V, et al Cloning and characterization of glucose transporter 11, a novel sugar transporter that is alternatively spliced in various tissues. Mol Genet Metab 2002; 76: 37–45. [DOI] [PubMed] [Google Scholar]
- 31. Maria Z, Campolo AR, Lacombe VA. Diabetes alters the expression and translocation of the insulin‐sensitive glucose transporters 4 and 8 in the atria. PLoS ONE 2015; 10: e0146033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dawson PA, Mychaleckyj JC, Fossey SC, et al Sequence and functional analysis of GLUT10: a glucose transporter in the Type 2 diabetes‐linked region of chromosome 20q12‐13.1. Mol Genet Metab 2001; 74: 186–199. [DOI] [PubMed] [Google Scholar]
- 33. Rogers S, Macheda ML, Docherty SE, et al Identification of a novel glucose transporter‐like protein‐GLUT‐12. Am J Physiol Endocrinol Metab 2002; 282: E733–E738. [DOI] [PubMed] [Google Scholar]
- 34. Uldry M, Ibberson M, Horisberger JD, et al Identification of a mammalian H(+)‐myo‐inositol symporter expressed predominantly in the brain. EMBO J 2001; 20: 4467–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Di Daniel E, Mok MH, Mead E, et al Evaluation of expression and function of the H+/myo‐inositol transporter HMIT. BMC Cell Biol 2009; 10: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poulsen SB, Fenton RA, Rieg T. Sodium‐glucose cotransport. Curr Opin Nephrol Hypertens 2015; 24: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hediger MA, Coady MJ, Ikeda TS, et al Expression cloning and cDNA sequencing of the Na+/glucose co‐transporter. Nature 1987; 330: 379–381. [DOI] [PubMed] [Google Scholar]
- 38. Kanai Y, Lee WS, You G, et al The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D‐glucose. J Clin Investig 1994; 93: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91: 733–794. [DOI] [PubMed] [Google Scholar]
- 40. Rossetti L, Smith D, Shulman GI, et al Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Investig 1987; 79: 1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012; 14: 5–14. [DOI] [PubMed] [Google Scholar]
- 42. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 2015; 66: 255–270. [DOI] [PubMed] [Google Scholar]
- 43. Abdul‐Ghani MA, Norton L, DeFronzo RA. Renal sodium‐glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol 2015; 309: F889–F900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diez‐Sampedro A, Hirayama BA, Osswald C, et al A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci USA 2003; 100: 11753–11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wright EM, Loo DD, Hirayama BA, et al Surprising versatility of Na+‐glucose cotransporters: SLC5. Physiology 2004; 19: 370–376. [DOI] [PubMed] [Google Scholar]
- 46. Turk E, Wright EM. Membrane topology motifs in the SGLT cotransporter family. J Membr Biol 1997; 159: 1–20. [DOI] [PubMed] [Google Scholar]
- 47. Tyagi NK, Kumar A, Goyal P, et al D‐Glucose‐recognition and phlorizin‐binding sites in human sodium/D‐glucose cotransporter 1 (hSGLT1): a tryptophan scanning study. Biochemistry 2007; 46: 13616–13628. [DOI] [PubMed] [Google Scholar]
- 48. Hediger MA, Rhoads DB. Molecular physiology of sodium‐glucose cotransporters. Physiol Rev 1994; 74: 993–1026. [DOI] [PubMed] [Google Scholar]
- 49. Hummel CS, Lu C, Loo DD, et al Glucose transport by human renal Na+/D‐glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 2011; 300: C14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poppe R, Karbach U, Gambaryan S, et al Expression of the Na+‐D‐glucose cotransporter SGLT1 in neurons. J Neurochem 1997; 69: 84–94. [DOI] [PubMed] [Google Scholar]
- 51. Chen J, Williams S, Ho S, et al Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 2010; 1: 57–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vrhovac I, Balen Eror D, Klessen D, et al Localizations of Na(+)‐D‐glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Archiv 2015; 467: 1881–1898. [DOI] [PubMed] [Google Scholar]
- 53. Bonner C, Kerr‐Conte J, Gmyr V, et al Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015; 21: 512–517. [DOI] [PubMed] [Google Scholar]
- 54. Lee WS, Kanai Y, Wells RG, et al The high affinity Na+/glucose cotransporter. Re‐evaluation of function and distribution of expression. J Biol Chem 1994; 269: 12032–12039. [PubMed] [Google Scholar]
- 55. Sabino‐Silva R, Freitas HS, Lamers ML, et al Na+‐glucose cotransporter SGLT1 protein in salivary glands: potential involvement in the diabetes‐induced decrease in salivary flow. J Membr Biol 2009; 228: 63–69. [DOI] [PubMed] [Google Scholar]
- 56. Hirsch JR, Loo DD, Wright EM. Regulation of Na+/glucose cotransporter expression by protein kinases in Xenopus laevis oocytes. J Biol Chem 1996; 271: 14740–14746. [DOI] [PubMed] [Google Scholar]
- 57. Wright EM, Hirsch JR, Loo DD, et al Regulation of Na+/glucose cotransporters. J Exp Biol 1997; 200: 287–293. [DOI] [PubMed] [Google Scholar]
- 58. Subramanian S, Glitz P, Kipp H, et al Protein kinase‐A affects sorting and conformation of the sodium‐dependent glucose co‐transporter SGLT1. J Cell Biochem 2009; 106: 444–452. [DOI] [PubMed] [Google Scholar]
- 59. Ishikawa Y, Eguchi T, Ishida H. Mechanism of beta‐adrenergic agonist‐induced transmural transport of glucose in rat small intestine. Regulation of phosphorylation of SGLT1 controls the function. Biochim Biophys Acta 1997; 1357: 306–318. [DOI] [PubMed] [Google Scholar]
- 60. Aschenbach JR, Borau T, Gabel G. Glucose uptake via SGLT‐1 is stimulated by beta(2)‐adrenoceptors in the ruminal epithelium of sheep. J Nutr 2002; 132: 1254–1257. [DOI] [PubMed] [Google Scholar]
- 61. Sopjani M, Bhavsar SK, Fraser S, et al Regulation of Na+‐coupled glucose carrier SGLT1 by AMP‐activated protein kinase. Mol Membr Biol 2010; 27: 137–144. [DOI] [PubMed] [Google Scholar]
- 62. Banerjee SK, Wang DW, Alzamora R, et al SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J Mol Cell Cardiol 2010; 49: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sandu C, Rexhepaj R, Grahammer F, et al Decreased intestinal glucose transport in the sgk3‐knockout mouse. Pflugers Archiv 2005; 451: 437–444. [DOI] [PubMed] [Google Scholar]
- 64. Elvira B, Blecua M, Luo D, et al SPAK‐sensitive regulation of glucose transporter SGLT1. J Membr Biol 2014; 247: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 65. Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev 1997; 77: 257–302. [DOI] [PubMed] [Google Scholar]
- 66. Dyer J, Hosie KB, Shirazi‐Beechey SP. Nutrient regulation of human intestinal sugar transporter (SGLT1) expression. Gut 1997; 41: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tavakkolizadeh A, Berger UV, Shen KR, et al Diurnal rhythmicity in intestinal SGLT‐1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol 2001; 280: G209–G215. [DOI] [PubMed] [Google Scholar]
- 68. Pan X, Terada T, Okuda M, et al The diurnal rhythm of the intestinal transporters SGLT1 and PEPT1 is regulated by the feeding conditions in rats. J Nutr 2004; 134: 2211–2215. [DOI] [PubMed] [Google Scholar]
- 69. Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genom 2007; 8: 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sabolic I, Vrhovac I, Eror DB, et al Expression of Na+‐D‐glucose cotransporter SGLT2 in rodents is kidney‐specific and exhibits sex and species differences. Am J Physiol Cell Physiol 2012; 302: C1174–C1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Balen D, Ljubojevic M, Breljak D, et al Revised immunolocalization of the Na+‐D‐glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 2008; 295: C475–C489. [DOI] [PubMed] [Google Scholar]
- 72. Abdul‐Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 2013; 62: 3324–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ghezzi C, Wright EM. Regulation of the human Na+‐dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 2012; 303: C348–C354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sunilkumar S, Ford SM. Elevated glucose concentration in culture media decreases membrane trafficking of SGLT2 in LLC‐PK1 cells via a cAMP/PKA‐dependent pathway. Am J Physiol Cell Physiol 2019; 316: C913–C924. [DOI] [PubMed] [Google Scholar]
- 75. Maldonado‐Cervantes MI, Galicia OG, Moreno‐Jaime B, et al Autocrine modulation of glucose transporter SGLT2 by IL‐6 and TNF‐alpha in LLC‐PK(1) cells. J Physiol Biochem 2012; 68: 411–420. [DOI] [PubMed] [Google Scholar]
- 76. Panchapakesan U, Pegg K, Gross S, et al Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLoS ONE 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gorboulev V, Schurmann A, Vallon V, et al Na(+)‐D‐glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose‐dependent incretin secretion. Diabetes 2012; 61: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roder PV, Geillinger KE, Zietek TS, et al The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014; 9: e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kellett GL, Brot‐Laroche E, Mace OJ, et al Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr 2008; 28: 35–54. [DOI] [PubMed] [Google Scholar]
- 80. Miyamoto K, Hase K, Taketani Y, et al Diabetes and glucose transporter gene expression in rat small intestine. Biochem Biophys Res Commun 1991; 181: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 81. Fujita Y, Kojima H, Hidaka H, et al Increased intestinal glucose absorption and postprandial hyperglycaemia at the early step of glucose intolerance in Otsuka Long‐Evans Tokushima Fatty rats. Diabetologia 1998; 41: 1459–1466. [DOI] [PubMed] [Google Scholar]
- 82. Dyer J, Wood IS, Palejwala A, et al Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 2002; 282: G241–248. [DOI] [PubMed] [Google Scholar]
- 83. Zambrowicz B, Freiman J, Brown PM, et al LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo‐controlled trial. Clin Pharmacol Ther 2012; 92: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dobbins RL, Greenway FL, Chen L, et al Selective sodium‐dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose‐dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol 2015; 308: G946–954. [DOI] [PubMed] [Google Scholar]
- 85. Vallon V, Sharma K. Sodium‐glucose transport: role in diabetes mellitus and potential clinical implications. Curr Opin Nephrol Hypertens 2010; 19: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vallon V, Platt KA, Cunard R, et al SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011; 22: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rieg T, Masuda T, Gerasimova M, et al Increase in SGLT1‐mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 2014; 306: F188–F193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu JJ, Lee T, DeFronzo RA. Why Do SGLT2 inhibitors inhibit only 30–50% of renal glucose reabsorption in humans? Diabetes 2012; 61: 2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Powell DR, DaCosta CM, Gay J, et al Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab 2013; 304: E117–E130. [DOI] [PubMed] [Google Scholar]
- 90. Powell DR, DaCosta CM, Smith M, et al Effect of LX4211 on glucose homeostasis and body composition in preclinical models. J Pharmacol Exp Ther 2014; 350: 232–242. [DOI] [PubMed] [Google Scholar]
- 91. Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 2012; 74: 351–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vestri S, Okamoto MM, de Freitas HS, et al Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol 2001; 182: 105–112. [DOI] [PubMed] [Google Scholar]
- 93. Vidotti DB, Arnoni CP, Maquigussa E, et al Effect of long‐term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol 2008; 28: 107–114. [DOI] [PubMed] [Google Scholar]
- 94. Tabatabai NM, Sharma M, Blumenthal SS, et al Enhanced expressions of sodium‐glucose cotransporters in the kidneys of diabetic Zucker rats. Diabetes Res Clin Pract 2009; 83: e27–e 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gembardt F, Bartaun C, Jarzebska N, et al The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 2014; 307: F317–F325. [DOI] [PubMed] [Google Scholar]
- 96. Vallon V, Gerasimova M, Rose MA, et al SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014; 306: F194–F204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Elfeber K, Stumpel F, Gorboulev V, et al Na(+)‐D‐glucose cotransporter in muscle capillaries increases glucose permeability. Biochem Biophys Res Commun 2004; 314: 301–305. [DOI] [PubMed] [Google Scholar]
- 98. Zhou L, Cryan EV, D’Andrea MR, et al Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem 2003; 90: 339–346. [DOI] [PubMed] [Google Scholar]
- 99. Kashiwagi Y, Nagoshi T, Yoshino T, et al Expression of SGLT1 in human hearts and impairment of cardiac glucose uptake by phlorizin during ischemia‐reperfusion injury in mice. PLoS ONE 2015; 10: e0130605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schwenk RW, Luiken JJ, Bonen A, et al Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res 2008; 79: 249–258. [DOI] [PubMed] [Google Scholar]
- 101. Banerjee SK, McGaffin KR, Pastor‐Soler NM, et al SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res 2009; 84: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Garvey WT, Hardin D, Juhaszova M, et al Effects of diabetes on myocardial glucose transport system in rats: implications for diabetic cardiomyopathy. Am J Physiol 1993; 264: H837–H844. [DOI] [PubMed] [Google Scholar]
- 103. Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007; 115: 3213–3223. [DOI] [PubMed] [Google Scholar]
- 104. Liao R, Jain M, Cui L, et al Cardiac‐specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation 2002; 106: 2125–2131. [DOI] [PubMed] [Google Scholar]
- 105. Pereira RO, Wende AR, Olsen C, et al Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J Am Heart Assoc 2013; 2: e000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pereira RO, Wende AR, Olsen C, et al GLUT1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J Mol Cell Cardiol 2014; 72: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ramratnam M, Sharma RK, D’Auria S, et al Transgenic knockdown of cardiac sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2 cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1 causes pathologic hypertrophy and dysfunction in mice. J Am Heart Assoc 2014; 3: e000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Connelly KA, Zhang Y, Desjardins JF, et al Dual inhibition of sodium‐glucose linked cotransporters 1 and 2 exacerbates cardiac dysfunction following experimental myocardial infarction. Cardiovasc Diabetol 2018; 17: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Elfeber K, Kohler A, Lutzenburg M, et al Localization of the Na+‐D‐glucose cotransporter SGLT1 in the blood‐brain barrier. Histochem Cell Biol 2004; 121: 201–207. [DOI] [PubMed] [Google Scholar]
- 110. Kang L, Routh VH, Kuzhikandathil EV, et al Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 2004; 53: 549–559. [DOI] [PubMed] [Google Scholar]
- 111. O’Malley D, Reimann F, Simpson AK, et al Sodium‐coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes 2006; 55: 3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bhavsar SK, Singh Y, Sharma P, et al Expression of JAK3 Sensitive Na+ Coupled Glucose Carrier SGLT1 in Activated Cytotoxic T Lymphocytes. Cell Physiol Biochem 2016; 39: 1209–1228. [DOI] [PubMed] [Google Scholar]
- 113. Koepsell H. The Na(+)‐D‐glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer. Pharmacol Therap 2017; 170: 148–165. [DOI] [PubMed] [Google Scholar]
- 114. Tahara A, Kurosaki E, Yokono M, et al Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol 2013; 715: 246–255. [DOI] [PubMed] [Google Scholar]
- 115. Michel MC, Mayoux E, Vallon V. A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans. Naunyn Schmiedebergs Arch Pharmacol 2015; 388: 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Qiang S, Nakatsu Y, Seno Y, et al Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr 2015; 7: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Komiya C, Tsuchiya K, Shiba K, et al Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS ONE 2016; 11: e0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Luippold G, Klein T, Mark M, et al Empagliflozin, a novel potent and selective SGLT‐2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin‐induced diabetic rats, a model of type 1 diabetes mellitus. Diabetes Obes Metab 2012; 14: 601–607. [DOI] [PubMed] [Google Scholar]
- 119. Kojima N, Williams JM, Takahashi T, et al Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Therap 2013; 345: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kern M, Kloting N, Mark M, et al The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice both as monotherapy and in combination with linagliptin. Metabolism 2016; 65: 114–123. [DOI] [PubMed] [Google Scholar]
- 121. Akahane K, Ojima K, Yokoyama A, et al Effects of combination of mitiglinide with various oral antidiabetic drugs in streptozotocin‐nicotinamide‐induced type 2 diabetic rats and Zucker fatty rats. Clin Exp Pharmacol Physiol 2017; 44: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 122. Abdel‐Wahab AF, Bamagous GA, Al‐Harizy RM, et al Renal protective effect of SGLT2 inhibitor dapagliflozin alone and in combination with irbesartan in a rat model of diabetic nephropathy. Biomed Pharmacother 2018; 103: 59–66. [DOI] [PubMed] [Google Scholar]
- 123. Terami N, Ogawa D, Tachibana H, et al Long‐term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014; 9: e100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shin SJ, Chung S, Kim SJ, et al Effect of sodium‐glucose co‐transporter 2 inhibitor, dapagliflozin, on renal renin‐angiotensin system in an animal model of type 2 diabetes. PLoS ONE 2016; 11: e0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wiviott SD, Raz I, Bonaca MP, et al Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl j Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 126. Perkovic V, Jardine MJ, Neal B, et al Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 127. Ito M, Tanaka T. The anticipated renoprotective effects of sodium‐glucose cotransporter 2 inhibitors. Intern Med 2018; 57: 2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Williams SB, Goldfine AB, Timimi FK, et al Acute hyperglycemia attenuates endothelium‐dependent vasodilation in humans in vivo . Circulation 1998; 97: 1695–1701. [DOI] [PubMed] [Google Scholar]
- 129. Shiraiwa T, Kaneto H, Miyatsuka T, et al Post‐prandial hyperglycemia is an important predictor of the incidence of diabetic microangiopathy in Japanese type 2 diabetic patients. Biochem Biophys Res Commun 2005; 336: 339–345. [DOI] [PubMed] [Google Scholar]
- 130. O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 2007; 100: 899–904. [DOI] [PubMed] [Google Scholar]
- 131. Shibazaki T, Tomae M, Ishikawa‐Takemura Y, et al KGA‐2727, a novel selective inhibitor of a high‐affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Therap 2012; 342: 288–296. [DOI] [PubMed] [Google Scholar]
- 132. Moriya R, Shirakura T, Ito J, et al Activation of sodium‐glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 2009; 297: E1358–E1365. [DOI] [PubMed] [Google Scholar]
- 133. Tolhurst G, Heffron H, Lam YS, et al Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes 2012; 61: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cefalo CMA, Cinti F, Moffa S, et al Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives. Cardiovascu Diabetol 2019; 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Powell DR, Smith MG, Doree DD, et al LP‐925219 maximizes urinary glucose excretion in mice by inhibiting both renal SGLT1 and SGLT2. Pharmacol Res Perspect 2015; 3: e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]


